Abstract
Background:
A novel inflammation-related biomarker, the monocyte to high-density lipoprotein cholesterol ratio (MHR), had a great relation to the development and prognosis of coronary atherosclerotic heart disease. Current study was to investigate whether the MHR was a potential tool in predicting the mortality and major adverse cardiac events (MACEs) in patients suffering coronary heart disease (CHD) by meta-analysis.
Methods:
The Cochrane Library, PubMed, MEDLINE, Scopus, EMBASE, and Web of science were searched for relevant cohort studies published prior to February 10, 2022. The association between MHR and mortality/MACEs was analyzed in patients with CHD. Hazard ratios (HR) with 95% confidence interval (CI) were calculated to estimate the strength of association.
Results:
In the meta-analysis, a total of 9 studies of 11,345 patients with CHD were included. Compared with the low level of MHR group, the high MHR value was associated with higher long-term MACEs (HR = 1.72 95% CI 1.36–2.18, P < .001), long-term mortality (HR = 1.71, 95% CI 1.10–2.66, P = .017), and in-hospital mortality/MACEs (HR = 2.82, 95% CI = 1.07–7.41, P = .036).
Conclusions:
This study suggested that increased MHR value might be associated with higher long-term mortality and long-term MACEs in CHD patients. MHR might serve as a potential prognostic indicator for risk stratification in patients with CHD.
Keywords: coronary heart disease, major adverse cardiac events, monocyte to high-density lipoprotein ratio, mortality
1. Introduction
Accumulating evidence suggests that coronary heart disease (CHD) is one of the most prevalent and chronic life-threatening disease.[1,2] Patients of CHD are frequently hospitalized and at high risk for death and major adverse cardiac events (MACEs), which would increase the medical burden.[3,4] The current dilemma for clinical physician is figuring out a simple and powerful prognostic biomarker to identify the high-risk CHD patients.[5,6]
Clinical and experimental evidences found that inflammation played a critical role in the development and progression of CHD.[7] Previous studies showed that some laboratory markers, such as white blood cells and its subtypes including monocyte, had been reported as prognostic indicators in patients with CHD.[8] Monocytes have been established as a predictor of coronary events.[9] Monocytes and their descendant macrophages were of great importance to the progression of atherosclerotic plaque.[10] Except functioning inside the arterial wall, the monocytes extended to the circulation contributed to the pathogenesis of CHD and its complications.[11]
High-density lipoprotein (HDL) represents a variety of lipoproteins and HDL-cholesterol (HDL-C) is the most widely used biomarker about HDL. Although the causal relationship between HDL and atherosclerosis was uncertain, previous studies from different racial and ethnic groups worldwide had showed that HDL-C and the risk of CHD were of negative correlation. What’s more, it was also reported that the HDL-C had a protective effect on the process of atherosclerosis.[11,12]
Previous researches assessed the value of a novel biomarker, named as monocyte to HDL-C ratio (MHR), in predicting mortality and MACEs in patients with CHD.[13–21] MHR has showed to be an inexpensive and convenient method for predicting the prognosis of CHD, but the outcomes were diverse. Therefore, we carried out a meta-analysis to evaluate the relationship between MHR and the risk of mortality and MACEs in CHD patients.
2. Material and Methods
2.1. Search strategy
This research was carried out according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for meta-analyses of observational studies.[22] A systematic literature search for prospective cohort studies was conducted in the Cochrane Library, PubMed, MEDLINE, Scopus, EMBASE, and Web of science. The latest update was performed in February 10, 2022. We used the following terms: “coronary heart disease”, “acute coronary syndrome”, “myocardial infraction”, “monocyte to high-density lipoprotein ratio”, “monocyte count/HDL cholesterol ratio”, “monocyte/high-density lipoprotein ratio”, “monocyte/HDL ratio”. The reference list of all included articles was scrutinized to identify additional eligible studies.
2.2. Study selection
Original studies were included if they met the following inclusion criteria: (1) Prospective clinical studies with follow-up time. (2) Adults patients (over 18 years old) diagnosed as CHD, including acute coronary syndrome (ACS) and chronic coronary artery disease (CAD). (3) The outcomes were associated with mortality or MACEs. (4) peer-reviewed publications in English journals.
The following 4 points were exclusion criteria: (1) Overlapping or duplicate publications. (2) Absence of available data or their corresponding 95% CI. (3) Nonhuman studies. (4) Absence of MHR or mortality/MACEs. If any of the above 4 characteristics were met, the study was excluded. If duplicate studies were from the same cohort, only the latest published study was included in our meta-analysis.
2.3. Data extraction and collection
Two investigators (Hong-Tao Liu, Zhong-Hui Jiang) independently extracted data from all of the included studies using a standardized data collection form for analysis. Disagreements were solved by discussion with other investigators (Xiao-Qing Quan, Zhong-Bin Yang). For each selected study, the following data were extracted: authors, year of publication, study region, sample size and gender of patients, the mean age, disease subtypes, duration of follow-up, HRs and 95% CIs, and end points. It should be noted that the MACEs was defined as cardiovascular death, acute left ventricular failure, ventricular arrhythmia, nonfatal myocardial infarction, cardiogenic shock and nonfatal ischemic stroke.
2.4. Quality analysis of study
The quality of each study was evaluated by the New Castle-Ottawa scale (NOS) system.[23] If the score was >6, we considered the study to be of high quality.
2.5. Ethics
In this study, ethical approval was not necessary because the included data was based on previous published articles, and no original clinical data was collected or utilized.
2.6. Statistical analysis
All data analyses were performed with STATA 15.1 statistical software. For stabilizing the variances and normalizing the distributions, before pooling the data, adjusted HR from were converted to lnHR. The heterogeneity among studies was evaluated by using Cochran Q test and the I2 statistic, whereby I² > 50%, P < .05 indicated significant heterogeneity. The random-effects model was used to minimize inter-study heterogeneity when there was significant heterogeneity between various studies. The fixed effect model was used if there was no significant heterogeneity between studies. If not, the random effects model was used.
3. Results
3.1. Study identification and selection
The details of how the studies were selected were showed in the flow diagram (Fig. 1). Initially, a total of 2302 potential articles were found in the electronic databases, of which 1715 duplicates were removed. The remaining 587 articles were reviewed in titles and abstracts, of which 549 were excluded. The remaining 38 articles were acquired for full article access. Of these, 29 articles were excluded and 9 studies were chosen for this meta-analysis.[13–21] Details of study selection and the flowchart of the literature search were depicted in Figure 1.
Figure 1.
Flow chart for selection of published eligible studies for the meta-analysis.
3.2. Characteristics of included studies
The basic information of the 9 studies was shown in Table 1. A total of 11,345 patients with 8054 (70.99%) males were included, and the mean ages of them were among 55.9–64.6 years old. All of the end points selected were analyzed by COX proportional hazard model, and we chose the HR and 95% CI of the adjusted analyses. These studies were all observation researches and were conducted in China,[15,17–20] and Turkey.[13,14,16,21] One study included CAD patients,[15] 3 studies included ST-segment elevation myocardial infarction (STEMI) patients,[14,16,19] and 5 studies ACS patients.[13,17,18,20,21]
Table 1.
Characteristics of studies included in meta-analyses.
| Study & year | Country | Follow-up (mo) | Sample size | Mean Age (yr) |
Male (%) |
Disease subtype |
End-point | Quality (NOS) |
|---|---|---|---|---|---|---|---|---|
| Cetin M 2016[13] | Turkey | 31.6 | 2661 | 59.8 | 66.4 | ACS | Long term MACEs Long term mortality In-hospital MACEs |
7 |
| Karata M 2016[14] | Turkey | 12.0 | 513 | 56.4 | 71.8 | STEMI | Long term MACEs Long term mortality |
7 |
| Wu T 2019[15] | China | 60.0 | 673 | 59.1 | 80.7 | CAD | Long term mortality Long term MACEs |
7 |
| Acikgoz S 2016[16] | Turkey | 23.4 | 1598 | 55.9 | 83.9 | STEMI | Long term mortality In-hospital mortality |
9 |
| Zhang Y 2016[17] | China | 24.6 | 3630 | 57.9 | 70.4 | ACS | Long term MACEs | 7 |
| Chen L 2021[18] | China | 12.0 | 1405 | 64.6 | 69.2 | ACS | Long term MACEs In-hospital MACEs |
8 |
| Hu J 2018[19] | China | 6.0 | 278 | 58.6 | 67.4 | STEMI | Long term MACEs | 7 |
| Mao Q 2019[20] | China | 12.0 | 435 | 63.0 | 67.4 | ACS | Long term MACEs | 7 |
| Oylumlu M 2020[21] | Turkey | 39.0 | 825 | 62.4 | 71.3 | ACS | Long-term mortality | 7 |
ACS = Acute coronary syndrome, CAD = coronary artery disease, MACEs = Major adverse cardiac events, NOS = New Caslte-Ottawa scale, STEMI = ST-segment elevation myocardial infarction.
3.3. Incidence of long-term mortality/MACEs
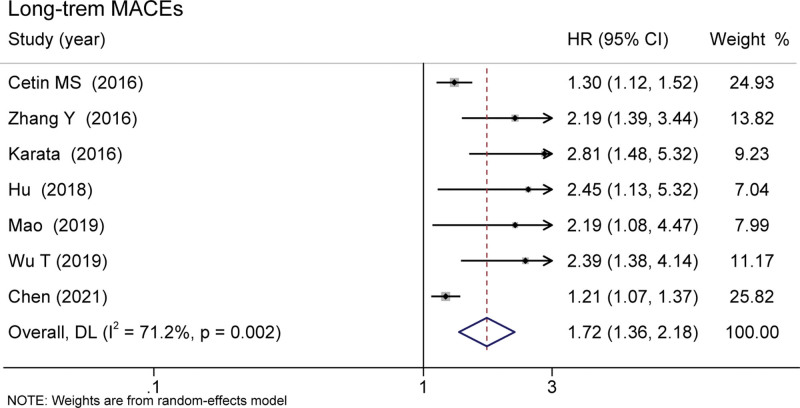
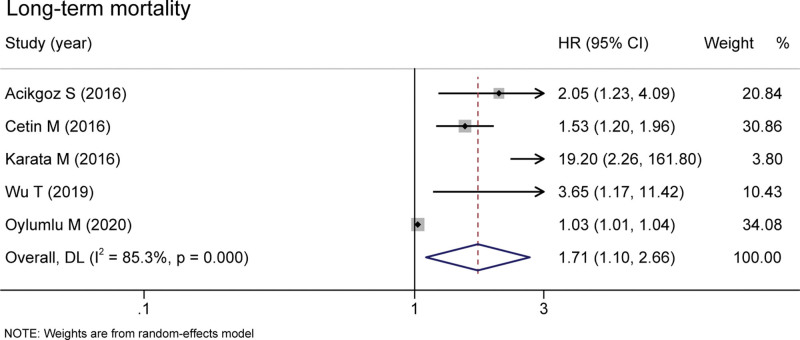
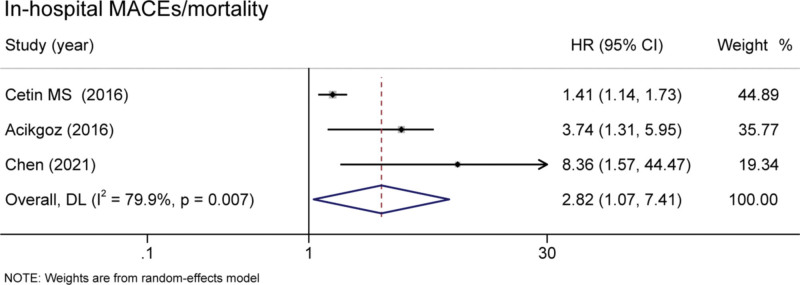
Six studies covering 9595 patients documented the relationship between MHR and long-term MACEs. Elevated MHR was associated with an increased risk of long-term MACEs in patients with CHD (HR = 1.72 95% CI 1.36–2.18, P < .001, Fig. 2).[13–15,17–20] Besides, the pooled result of 5 studies including 6270 patients found that the high MHR value was associated with higher long-term mortality (HR = 1.71, 95% CI 1.10–2.66, P = .017, Fig. 3).[13–16,21] These were 3 studies included the data of in-hospital mortality/MACEs. The meta-analysis analysis results show that compared with the low level of MHR group, the risk of in-hospital mortality/MACEs was 2.82-fold in high MHR patients (95% CI 1.07-7.41, P = .036, Fig. 4).[13,16,18]
Figure 2.
Pooled risk of MHR and long-term MACEs of the included studies. MACEs = major adverse cardiac events, MHR = monocyte to high-density lipoprotein ratio.
Figure 3.
Pooled risk of MHR and long-term mortality of the included studies. MHR = monocyte to high-density lipoprotein ratio.
Figure 4.
Pooled risk of MHR and in-hospital mortality/MACEs of the included studies. MACEs = major adverse cardiac events, MHR = monocyte to high-density lipoprotein.
3.4. Subgroup analyses
In the subgroup analysis, we grouped the studies by country, disease subtype, sample size, mean age, and follow-up. Analysis showed that the risk of long-term MACEs in high MHR group was significantly higher than patients with low MHR in population of Turkey (HR = 1.90, 95% CI 1.27–2.85, P = .002)[13,14] and age > 60 years (HR = 2.02, 95% CI 1.38–2.96, P < .001).[18,20] Subgroup analysis according to sample size, and follow-up showed pooled HR estimates with similar results to that of the original analysis. Interestingly, STEMI patients (HR = 2.66, 95% CI 1.62–4.35, P < .001)[14,19] with high MHR tend to have a higher risk of developing long-term MACEs than ACS patients (HR = 1.41, 95% CI 1.15–1.73, P = .001).[13,17,18,20] The subgroup analysis results of HR values for long-term MACEs were shown in Table 2.
Table 2.
The subgroup analysis of the association of MHR and long-term MACEs for CHD patients.
| Subgroup | Study (no.) | I2(%) | P(I2) | HR | P(HR) | |
|---|---|---|---|---|---|---|
| Country | China[15, 17–20] | 5 | 80.9 | 0.022 | 1.79 (0.85–3.76) | 0.123 |
| Turkey[13, 14] | 2 | 74.2 | 0.004 | 1.90 (1.27–2.85) | 0.002 | |
| Disease subtype | ACS[13, 17, 18, 20] | 4 | 64.3 | 0.039 | 1.41 (1.15–1.73) | 0.001 |
| STEMI[14, 19] | 2 | 0.0 | 0.789 | 2.66 (1.62–4.35) | <0.001 | |
| CAD[15] | 1 | NA | NA | 2.39 (1.38–4.14) | 0.046 | |
| Sample size | > 1000[13,17,18] | 3 | 67.8 | 0.045 | 1.36 (1.11–1.65) | 0.002 |
| ≤ 1000[14,15,19,20] | 4 | 0.0 | 0.964 | 2.46 (1.77–3.41) | <0.001 | |
| Mean Age | > 60 years[18, 20] | 2 | 71.3 | 0.007 | 2.02 (1.38–2.96) | <0.001 |
| ≤ 60 years[13–15, 17, 19] | 5 | 61.6 | 0.107 | 1.46 (0.85–2.51) | 0.169 | |
| Follow-up | > 12 months[13, 15, 17] | 3 | 75.5 | 0.017 | 1.79 (1.15–2.78) | 0.009 |
| ≤ 12 months[14, 18–20] | 4 | 73.9 | 0.009 | 1.21 (1.07–1.37) | 0.013 |
CHD = coronary heart disease, CI = confidence interval, HR = hazard ratio, MACEs = major adverse cardiac events, MHR = monocyte to high-density lipoprotein cholesterol ratio.
4. Discussion
As one of the most widespread chronic diseases, CHD is associated with tremendous social and economic costs because of its high morbidity and mortality rate. Early assessment of the condition can provide better guidance for CHD patients. This was a meta-analysis drawn from 9 studies involving 11,345 patients reported that elevated MHR level was related to mortality and MACEs. The aggregated results showed that higher MHR had an increased risk of poor prognosis. MHR might be a potential biomarker of risk stratification in patients with CHD.
The present meta-analysis indicated that MHR might be a predictor for long-term MACEs and long-term mortality in patients with CHD. CHD patients with the high MHR respectively had 1.72-fold and 1.71-fold risk of long-term MACEs and long-term mortality. The exact mechanism underlying elevated MHR and poor clinical outcome in CHD are not fully understood. One possible explanation for these findings is that combination of monocyte and HDL-cholesterol may reflect the level of the systemic inflammation. CHD is related to atherosclerosis, which is accompanied with the infiltration of inflammatory/immunocompetent cells.
It is known that inflammation has an essential role in the progression of atherosclerosis.[24] Monocytes are pivotal immune cells and play an important role in inflammatory response and atherosclerosis development. Experimental and clinical data supports the notion that monocytes, representing the innate immune system, play a crucial role in the occurrence and development of atherosclerotic lesions.[25] Activated by different factors, peripheral blood mononuclear cells cross the surface of endothelium and differentiate into macrophages.[26,27] After that, macrophages in the arterial wall ingest oxidized low-density lipoprotein and assume a foamy appearance.[28] Several kinds of pro-inflammatory cytokines, growth factors, and tissue factor are secreted by foam cells.[29] They can promote the formation of large lipid core and accelerate the rupture of plaque in patients with CHD.[30]
Previously, the linkage between aberrant level of blood HDL-C and CHD has been noted.[31,32] HDL-C, the so-called “good cholesterol”, has been recognized as an antioxidant and protective component in the circulation.[33] Low HDL-C level increases the risk of cardiovascular death and recurrent myocardial infarction, whereas high HDL-C level reduces the risk of MACEs.[34] However, some studies have come to different conclusions. Among patients with atherosclerotic vascular disease, niacin (added to statin therapy) failed to reduce cardiovascular risk despite a significant increase in HDL-C levels.[35]
HDL-C is well-known as an antiinflammatory indicator which protects endothelial cells by attenuating inflammation.[36] HDL-C reduces the endothelial adhesion molecules expression and controls the activation of monocytes.[37] As well, HDL-C also inhibits the oxidation of LDL-C which is also a contributor to the atherosclerosis.[38] Thus, MHR, which is derived from the numbers of monocytes and HDL-C, is a novel indicator of inflammatory and pro-thrombotic status. The data of present study suggests that MHR can potentially be used as a long-term prognostic indicator for CHD. MHR might be used as an inexpensive and convenient marker in assessment of patients with CHD.
There were some following limitations in the present study. Firstly, there were no similar definite cutoff values for MHR of the 9 studies, which might exert substantial heterogeneity. Second, the follow-up duration and the difference in the study population may lead to heterogeneity to some extent. Thirdly, only studies conducted in China and Turkey were included in this research, therefore, further meta-analysis is required and should include are more studies from more regions. Finally, substantial heterogeneity was present in the total pooled analysis of mortality and MACEs. Our meta-analysis results should be interpreted cautiously.
In conclusion, MHR value could be an effective indicator of the risks of long-term mortality/MACEs in the patients with CHD. Further studies are needed to pay more attention on the cut off value of MHR in the predicting of prognosis of CHD.
Acknowledgments
The authors thank all participants who contribute to this meta-analysis.
Author contributions
Hong-Tao Liu and Zhong-Hui Jiang: Design of the study, acquisition and interpretation of data, manuscript preparation and the initial draft, accountable for all aspects of the work. Zhong-Hui Jiang and Zhong-Bin Yang: statistical analysis, analysis and interpretation of data, accountable for all aspects of the work. Xiao-Qing Quan and Hong-Tao Liu: Design of the study, critical review of the draft and contribution to the writing of the manuscript, final approval of the version to be published, and accountable to the accuracy or integrity of the work.
Abbreviations:
- CAD =
- coronary artery disease
- CHD =
- coronary heart disease
- CI =
- confidence interval
- HDL =
- High-density lipoprotein
- HDL-C =
- HDL-cholesterol
- HR =
- Hazard ratios
- MACEs =
- major adverse cardiac events
- MHR =
- monocyte to high-density lipoprotein cholesterol ratio
- NOS =
- New Castle-Ottawa scale
- PRISMA =
- preferred reporting items for systematic reviews and meta-analyses
- STEMI =
- ST-segment elevation myocardial infarction
Hong-Tao Liu and Zhong-Hui Jiang contributed equally to this work.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Liu H-T, Jiang Z-H, Yang Z-B, Quan X-Q. Monocyte to high-density lipoprotein ratio predict long-term clinical outcomes in patients with coronary heart disease: a meta-analysis of 9 studies. Medicine 2022;101:33(e30109).
Funding: This work is supported by the Guangdong Basic and Applied Basic Research Foundation (2021A1515220123) and Shenzhen Key Medical Discipline Construction Fund (SZXK063).
The authors have no conflicts of interest to disclose.
Contributor Information
Hong-Tao Liu, Email: 18071596345@163.com.
Zhong-Hui Jiang, Email: 13277356550@163.com.
Zhong-Bin Yang, Email: 2591784066@qq.com.
References
- [1].Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bauersachs R, Zeymer U, Brière JB, et al. Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther. 2019;2019:8295054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen H, Chen C, Fang J, et al. Efficacy and safety of antiplatelet therapy plus Xa factor inhibitors in patients with coronary heart disease: a meta-analysis. Med Sci Monit. 2019;25:5473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Emery C, Torreton E, Briere JB, et al. Economic burden of coronary artery disease or peripheral artery disease in patients at high risk of ischemic events in the French setting: a claims database analysis. J Med Econ. 2020;23:513–20. [DOI] [PubMed] [Google Scholar]
- [5].Zhao Y, Meng S, Liu T, et al. Economic analysis of surgical and interventional treatments for patients with complex coronary artery disease: insights from a one-year single-center study. Med Sci Monit. 2020;26:e919374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu S, Yang G, Huang Y, et al. Predictive value of LncRNA on coronary restenosis after percutaneous coronary intervention in patients with coronary heart disease: a protocol for systematic review and meta-analysis. Medicine. 2021;100:e24114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- [8].Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–43. [DOI] [PubMed] [Google Scholar]
- [9].Olivares R, Ducimetière P, Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol. 1993;137:49–53. [DOI] [PubMed] [Google Scholar]
- [10].Tani S, Matsumoto M, Anazawa T, et al. Development of a model for prediction of coronary atherosclerotic regression: evaluation of high-density lipoprotein cholesterol level and peripheral blood monocyte count. Heart Vessels. 2012;27:143–50. [DOI] [PubMed] [Google Scholar]
- [11].Gratchev A, Sobenin I, Orekhov A, et al. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology. 2012;217:476–82. [DOI] [PubMed] [Google Scholar]
- [12].Toth PP, Barter PJ, Rosenson RS, et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:484–525. [DOI] [PubMed] [Google Scholar]
- [13].Cetin MS, Ozcan Cetin EH, Kalender E, et al. Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome. Heart Lung Circ. 2016;25:1077–86. [DOI] [PubMed] [Google Scholar]
- [14].Karataş MB, Çanga Y, Özcan KS, et al. Monocyte to high-density lipoprotein ratio as a new prognostic marker in patients with STEMI undergoing primary percutaneous coronary intervention. Am J Emerg Med. 2016;34:240–4. [DOI] [PubMed] [Google Scholar]
- [15].Wu TT, Zheng YY, Chen Y, et al. Monocyte to high-density lipoprotein cholesterol ratio as long-term prognostic marker in patients with coronary artery disease undergoing percutaneous coronary intervention. Lipids Health Dis. 2019;18:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Açikgöz SK, Açikgöz E, Şensoy B, et al. Monocyte to high-density lipoprotein cholesterol ratio is predictive of in-hospital and five-year mortality in ST-segment elevation myocardial infarction. Cardiol J. 2016;23:505–12. [DOI] [PubMed] [Google Scholar]
- [17].Zhang Y, Li S, Guo YL, et al. Is monocyte to HDL ratio superior to monocyte count in predicting the cardiovascular outcomes: evidence from a large cohort of Chinese patients undergoing coronary angiography. Ann Med. 2016;48:305–12. [DOI] [PubMed] [Google Scholar]
- [18].Li C, Fan H, Liu Y, et al. The monocyte to high-density lipoprotein cholesterol ratio and outcomes in type 2 diabetes mellitus patients with non-ST-segment elevation acute coronary syndrome. Ann Transl Med. 2021;9:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu R, Hou R, Wang T, et al. Correlation between monocyte to high-density lipoprotein ratio and major adverse cardiovascular events in patients with acute coronary syndrome after percutaneous coronary intervention. Pak J Med Sci. 2021;37(3):885–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mao Q, Xiang C, Wang Y, et al. Effect of ratio of monocyte to high-density lipoprotein cholesterol on prognosis of patients with non-ST-segment elevation acute coronary syndrome. Acta Acad Med Mil Tert. 2019;41:454–0. [Google Scholar]
- [21].Oylumlu M, Oylumlu M, Arik B, et al. Monocyte to high-density lipoprotein cholesterol and lymphocyte to monocyte ratios are predictors of in-hospital and long-term mortality in patients with acute coronary syndrome. Int J Clin Pract. 2021;75:e13973. [DOI] [PubMed] [Google Scholar]
- [22].Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (Clinical research ed. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [24].Wang J, Dong PK, Xu XF, et al. Identification of tRNA-derived fragments and their potential roles in atherosclerosis. Curr Med Sci. 2021;41:712–21. [DOI] [PubMed] [Google Scholar]
- [25].Mahemuti A, Abudureheman K, Schiele F, et al. Association between inflammatory markers, hemostatic markers, and traditional risk factors on coronary artery spasm in patients with normal coronary angiography. J Interv Cardiol. 2014;27:29–35. [DOI] [PubMed] [Google Scholar]
- [26].Kottke BA, Subbiah MT. Pathogenesis of atherosclerosis. Concepts based on animal models. Mayo Clin Proc. 1978;53:35–48. [PubMed] [Google Scholar]
- [27].Yang LY, Wu YS, Dai BB, et al. sPLA2-IB level correlates with hyperlipidemia and the prognosis of idiopathic membranous nephropathy. Curr Med Sci. 2020;40:683–90. [DOI] [PubMed] [Google Scholar]
- [28].Ghattas A, Griffiths HR, Devitt A, et al. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62:1541–51. [DOI] [PubMed] [Google Scholar]
- [29].Moreno PR, Purushothaman KR, Fuster V, et al. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: implications for plaque vulnerability. Circulation. 2002;105:2504–11. [DOI] [PubMed] [Google Scholar]
- [30].Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Distelmaier K, Wiesbauer F, Blessberger H, et al. Impaired antioxidant HDL function is associated with premature myocardial infarction. Eur J Clin Invest. 2015;45:731–8. [DOI] [PubMed] [Google Scholar]
- [32].Xu RX, Zhang Y, Zhang Y, et al. Effects of pitavastatin on lipoprotein subfractions and oxidized low-density lipoprotein in patients with atherosclerosis. Curr Med Sci 2020;40:879–84. [DOI] [PubMed] [Google Scholar]
- [33].Navab M, Reddy ST, Van Lenten BJ, et al. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–32. [DOI] [PubMed] [Google Scholar]
- [34].Park JS, Cha KS, Lee HW, et al. Predictive and protective role of high-density lipoprotein cholesterol in acute myocardial infarction. Cardiol J. 2019;26:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12. [DOI] [PubMed] [Google Scholar]
- [36].Tran-Dinh A, Diallo D, Delbosc S, et al. HDL and endothelial protection. Br J Pharmacol. 2013;169:493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen HC, Lee WC, Fang HY, et al. Impact of high triglyceride/high-density lipoprotein cholesterol ratio (insulin resistance) in ST-segment elevation myocardial infarction. Medicine. 2020;99:e22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Navab M, Reddy ST, Van Lenten BJ, et al. High-density lipoprotein and 4F peptide reduce systemic inflammation by modulating intestinal oxidized lipid metabolism: novel hypotheses and review of literature. Arterioscler Thromb Vasc Biol. 2012;32:2553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]