Abstract
Background:
Increasing clarithromycin resistance has led to changes in several guidelines for treatment of Helicobacter pylori infections. We compared the H. pylori eradication rates of the empirical concomitant therapy (CoT) and a tailored therapy (TaT) using dual-priming oligonucleotide-based polymerase chain reaction to detect mutations in the 23S rRNA gene that are related to clarithromycin resistance.
Methods:
Between June 2020 and May 2021, 290 patients were enrolled and randomly assigned to 2 groups. In the CoT group, the patients received rabeprazole 20 mg, amoxicillin 1 g, clarithromycin 500 mg, and metronidazole 500 mg twice daily for 14 days. In the TaT group, point mutation-negative patients received rabeprazole 20 mg, amoxicillin 1 g, and clarithromycin 500 mg twice daily for 14 days and point mutation-positive patients received rabeprazole 20 mg twice daily, metronidazole 500 mg thrice daily, and bismuth 120 mg and tetracycline 500 mg 4 times daily for 14 days.
Results:
A total of 290 and 261 patients were included in the intention-to-treat (ITT) and per-protocol (PP) analyses, respectively. A2142G and/or A2143G point mutations were identified in 28.6% of the patients. No significant difference in eradication rates were observed between the 2 groups as per ITT (CoT, 82.8% and TaT, 85.5%, P = .520) and PP (CoT, 88.6% and TaT, 94.6%, P = .084) analyses. In point mutation-positive patients, the eradication rates in the CoT group were lower than those in the TaT group as per ITT (69.8% and 87.5%, respectively, P = .050) and PP (76.9% and 97.1%, respectively, P = .011) analyses.
Conclusion:
CoT and TaT showed similar overall eradication rates for H. pylori. However, CoT eradication rate was suboptimal, especially in point mutation-positive patients.
Keywords: clarithromycin resistance, concomitant therapy, eradication rate, Helicobacter pylori, polymerase chain reaction, tailored therapy
1. Introduction
Multiantibiotic regimens containing antisecretory agents are used to eradicate Helicobacter pylori infections.[1–6] Triple therapy, which involves a proton-pump inhibitor (PPI), amoxicillin, and clarithromycin, was used as the first-line treatment for H. pylori infections in Korea and Japan.[7,8] However, growing resistance to antibiotics, such as clarithromycin, metronidazole, and levofloxacin, has decreased the eradication rates.[9,10] In particular, the eradication rate of the triple therapy declined significantly for strains exhibiting clarithromycin resistance.[11] Moreover, increasing the duration of clarithromycin treatment cannot improve the eradication rate of triple therapy in case of clarithromycin-resistant strains.[12,13] Therefore, several European and American guidelines recommend discontinuing the use of triple therapy without antibiotic susceptibility testing in regions where clarithromycin resistance is >15%.[1–3] Concomitant therapy (CoT) is the preferred regimen among nonbismuth quadruple regimens because it shows higher eradication rates than sequential therapy in case of clarithromycin or metronidazole resistance.[14,15]
The culture-based antimicrobial susceptibility test is an ideal method for selecting a regimen to successfully eradicate H. pylori. However, it is time consuming, expensive, and often difficult to perform.[16] Dual-priming oligonucleotide-based polymerase chain reaction (DPO-PCR) and sequencing tests can rapidly and conveniently detect the A2142G and/or A2143G point mutations in 23S ribosomal RNA (rRNA) that are related to clarithromycin resistance.[17] Tailored therapy (TaT) based on DPO-PCR for point mutations related to clarithromycin resistance is helpful for clinicians in deciding the first-line treatment regimen for patients in areas with high clarithromycin resistance. A revised Korean guideline recommends a 7-day standard triple therapy as the first-line treatment if mutations in the 23S rRNA gene related to clarithromycin resistance are detected through PCR or sequencing.[6] In patients infected with H. pylori strains harboring A2142G and/or A2143G point mutations, PPI, amoxicillin, metronidazole (PAM), or bismuth quadruple therapy (BQT) are recommended.[5,6]
However, a recent prospective multicenter study failed to show the superiority of eradication rate of TaT (14-day triple therapy in patients without point mutation and 14-day PAM therapy in patients with point mutation) compared with that of CoT, based on detection of 23S rRNA gene point mutations, and the eradication rate of 14-day PAM therapy was 70.5% in the per-protocol (PP) analysis.[18] In addition, in a previous retrospective study on patients with point mutations, a higher eradication rate was reported for TaT, composed of a BQT, compared with that for PAM.[19] Therefore, we aimed to compare the eradication rates of the empirical CoT and TaT using BQT instead of PAM in patients with point mutations in the 23S rRNA gene.
2. Materials and Methods
2.1. Participants and inclusion criteria
This was a prospective, randomized controlled, multicenter study to compare the eradication rates of CoT and DPO-PCR-guided TaT comprising BQT and triple therapy. Patients who underwent endoscopy and were diagnosed with H. pylori infection were recruited at 6 tertiary care hospitals (Busan Paik Hospital, Kosin University College of Medicine, Pusan National University Hospital, Haeundae Paik Hospital, Dong-A University Hospital, and Pusan National University Yangsan Hospital) in Busan and Geyongnam-do, South Korea, from June 2020 to May 2021. Eligible patients were randomly assigned to either CoT or TaT groups using a randomization table stratified by age, sex, and eradication indication by the computer. Allocation ratio was 1:1 between the groups. After assignment, participants and physicians were no longer blinded to the eradication regimens.
Participants who were (i) younger than 18 years of age, (ii) had undergone H. pylori eradication treatment, (iii) had a history of gastric surgery, (iv) had severe comorbidity, (v) had a history of antibiotic allergy, (vi) had a history of antibiotic use within 4 weeks of study commencement, and (vii) were pregnant or lactating were excluded from the study.
This study was approved by the ethics committee of each hospital participating in the study (Pusan National University Yangsan Hospital 05-2020-018, Busan Paik Hospital 2020-01-022, Kosin University College of Medicine 2020-01-006, Pusan National University Hospital 2020-036-089, Haeundae Paik Hospital 2020-01-020-001, and Dong-A University Hospital 2020-20-116). All procedures followed the ethical standards prescribed by the responsible committee on human experimentation (institutional and national) and the Helsinki Declaration of 1964 and its later versions. This randomized trial was registered with the Clinical Research Information Service of the Korea National Institute of Health (KCT0006120).
2.2. Diagnosis of H. pylori infection
Two biopsy specimens were taken from the antrum and corpus of patients during endoscopy. To diagnose H. pylori infection, DPO-PCR (Seeplex H. pylori-ClaR ACE Detection; Seegene Inc., Seoul, Korea) was performed using DNA extracted from the gastric biopsy specimens. PCR was performed to amplify a portion of the 23S rRNA gene to identify A2142G and/or A2143G mutations associated with clarithromycin resistance.
The success of H. pylori eradication was evaluated using the 13C-urea breath test (UBiT-IR300; Otsuka Electronics Co. Ltd., Tokyo, Japan) at least 4 weeks after the treatment. All patients swallowed a 100 mg tablet of 13C-urea (UBiTkit; Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan) with 100 mL water after 4 hours of fasting. Two breath samples were collected before and 20 minutes after swallowing the tablet. A cutoff value of 13CO2 < 2.5% was considered successful eradication of H. pylori.[20]
2.3. Eradication regimens
All patients in the CoT group were treated with CoT for 14 days. CoT consisted of rabeprazole 20 mg, amoxicillin 1 g, clarithromycin 500 mg, and metronidazole 500 mg twice daily. In the TaT group, patients without A2142G and A2143G point mutations were treated with standard triple therapy for 14 days, and patients with A2142G and/or A2143G point mutations were treated with BQT for 14 days. Standard triple therapy included rabeprazole 20 mg, amoxicillin 1 g, and clarithromycin 500 mg twice daily. BQT consisted of rabeprazole 20 mg twice daily, metronidazole 500 mg thrice daily, and tetracycline 500 mg and bismuth 300 mg 4 times daily.
After completing the eradication process, 13C-urea breath tests were performed to assess the success rate of H. pylori eradication. Patients in whom H. pylori eradication failed after the completion of CoT or TaT were recommended to take BQT for 14 days as salvage therapy. In the TaT group, the patients in whom BQT failed as the first-line therapy were recommended to take levofloxacin triple therapy for 14 days. The levofloxacin triple therapy included rabeprazole 20 mg and amoxicillin 1 g twice daily and levofloxacin 500 mg once daily. The eradication success rate of the salvage therapies was also determined using the 13C-urea breath test.
2.4. Assessment of drug compliance and treatment-related adverse events
All patients were instructed on how to record adverse events and drug compliance. The patients visited the hospitals and underwent the urea breath test after 4 weeks of finishing the eradication therapies and reported drug compliance and drug-related adverse events. Drug compliance was assessed according to the consumption of the prescribed medication. Noncompliance was defined as the consumption of < 80% of the total medication based on the count of remaining pills. Adverse reactions were graded as mild, moderate, or severe. Mild adverse reactions were defined as > 80% drug compliance and side effects that did not disrupt daily activities. Moderate adverse reactions were defined as < 80% drug compliance and side effects that affected daily activities. Severe adverse reactions were defined as < 50% drug compliance or side effects that limited daily activities.
2.5. Sample size calculation and statistical analysis
Eradication rates obtained in previous studies on CoT and TaT were set at 78.3% and 91.8%, respectively.[21,22] The minimal number of participants required in each group to obtain statistical power with an α-error of 0.05 and a β error of 0.20 was calculated to be 123. Considering a withdrawal rate of 10%, at least 145 participants in each group were required; therefore, the target sample size was calculated as 290 patients in total.
The intention-to-treat (ITT) and PP analyses were performed to evaluate the eradication rates in the CoT and TaT groups. ITT analysis included all enrolled study population after excluding patients meeting the exclusion criteria. PP analysis excluded the patients who were lost to follow-up or had poor drug compliance (consumed < 80% of the prescribed medications).
Categorical variables were analyzed using the Chi-square test or Fisher exact test, whereas continuous variables were analyzed using Student t-test. A P value < 0.05 was considered statistically significant. Statistical calculations were performed using the Statistical Package for the Social Sciences version 22.0 for Windows (IBM Corp., Armonk, NY).
3. Results
3.1. Characteristics of the participants
The 290 patients enrolled in this study were randomly assigned to either the CoT (n = 145) or the TaT group (n = 145) between June 2020 and May 2021. Using DPO-PCR test, A2142G, and/or A2143G point mutations were identified in 78 patients (28.6%) who were classified as infected with clarithromycin-resistant H. pylori. Among them, 43 (29.7%) patients were assigned to the CoT group and 35 (27.6%) patients were assigned to the TaT group. Clinical characteristics including age, sex, presence of hypertension, and/or diabetic mellitus, and reason of eradication therapy were not significantly different between the 2 groups (Table 1).
Table 1.
Baseline characteristics of patients.
| Concomitant therapy (n = 145) | Tailored therapy (n = 145) | P | |
|---|---|---|---|
| Mean age, yrs | 59.5 | 57.1 | .079 |
| Sex | .212 | ||
| Male, n (%) | 92 (63.4) | 81 (56.3) | |
| Female, n (%) | 53 (36.6) | 64 (43.7) | |
| Smoking, n (%) | 36 (24.8) | 30 (20.8) | .419 |
| Alcohol, n (%) | 50 (53.2) | 44 (46.8) | .476 |
| Cause of treatment, n (%) | .299 | ||
| Peptic ulcer | 46 (31.7) | 57 (39.6) | |
| ER for EGC or adenoma | 52 (35.9) | 39 (27.1) | |
| MALT lymphoma | 1 (0.7) | 4 (2.8) | |
| Gastritis | 44 (0.5) | 46 (31.7) |
ER = endoscopic resection; EGC = early gastric cancer; MALT = mucosa-associated lymphoid tissue.
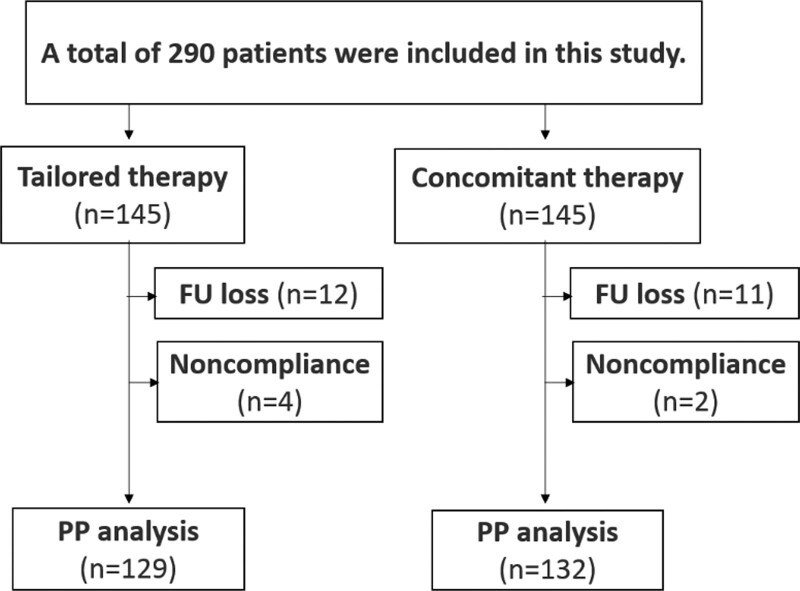
The eradication success was not evaluated for 23 patients (11 in the CoT group and 12 in the TaT group) because of follow-up loss after eradication. Two patients in the CoT group and 4 in the TaT group failed to take > 80% of the total medication. Therefore, 290 (145 in the CoT group and 145 in the TaT group) and 261 (132 in the CoT group and 129 in the TaT group) patients were included in ITT and PP analyses, respectively (Fig. 1).
Figure 1.
Flowchart of the study.
3.2. Eradication rates of H. pylori
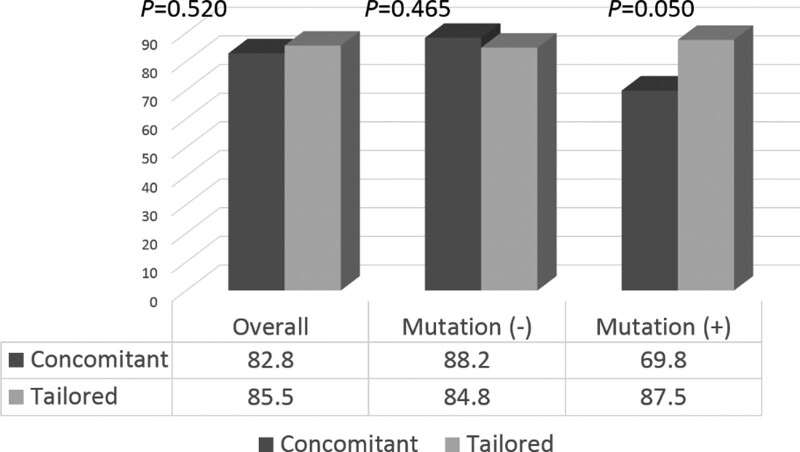
No significant differences in the eradication rates were observed between the CoT (82.8%) and TaT (85.5%) groups (P = .520) based on the ITT analysis. In the subgroup of patients infected with H. pylori without 23S rRNA point mutations, the eradication rates were 88.2% in the CoT group and 84.8% in the TaT group, and the difference was not statistically significant (P = .465). For patients infected with H. pylori harboring A2142G and/or A2413G mutations, the eradication rate in the CoT group was significantly lower than that in the TaT group (69.8% and 87.5%, respectively, P = .050) (Fig. 2).
Figure 2.
Overall and subgroup eradication rates according to the presence of point mutations as per the intention-to-treat analysis.
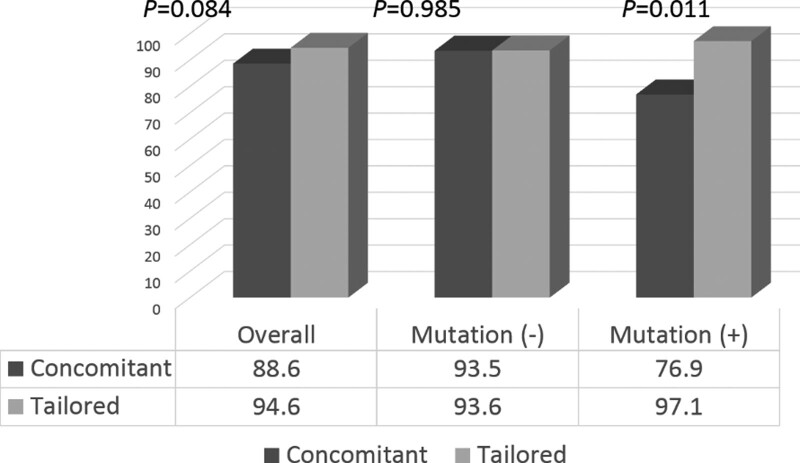
The eradication rates were 88.6% in the CoT group and 94.6% in the TaT group based on the PP analysis but the difference was not statistically significant (P = .084). In the subgroup of patients infected with H. pylori without 23S rRNA point mutations, there was no significant difference in the eradication rates between the CoT (93.5%) and TaT (93.6%) groups (P = .985). For patients infected with H. pylori harboring A2142G and/or A2413G mutations, the CoT group showed significantly lower eradication rate than the TaT group (76.9% and 97.1%, respectively, P = .011) (Fig. 3).
Figure 3.
Overall and subgroup eradication rates according to the presence of point mutations, using per-protocol analysis.
3.3. Adverse events
Adverse events including metallic taste, nausea or vomiting, diarrhea, abdominal discomfort or pain, and urticaria were reported in 18.7% (25/134) of the patients in the CoT group and 23.3% (31/133) of the patients in the TaT group (P = .351) (Table 2). Among these 56 patients, 2 patients in the CoT group and 4 in the TaT group (4 triple therapy cases) showed severe adverse reactions; the 2 patients in the CoT group reported nausea and/or vomiting, whereas 3 patients in the TaT group reported abdominal pain with diarrhea and 1 patient reported dyspnea and urticaria. These 6 patients were classified as noncompliant because their medication consumption was < 80%. The symptoms of all 6 patients who experienced adverse events disappeared after stopping the eradication medications and taking symptom-control treatment, without hospitalization.
Table 2.
Adverse events associated with the 2 therapies.
| Concomitant therapy (n = 134) | Tailored therapy (n = 133) | P | |
|---|---|---|---|
| Metallic taste, n (%) | 5 (3.7) | 7 (5.3) | .546 |
| Nausea, n (%) | 6 (4.5) | 6 (4.5) | .989 |
| Diarrhea and/or pain, n (%) | 11 (8.2) | 14 (10.5) | .516 |
| Headache, n (%) | 2 (1.5) | 2 (1.5) | 1.000 |
| Allergy, n (%) | 2 (1.5) | 2 (1.5) | 1.000 |
| Overall, n (%) | 25 (18.7) | 31 (23.3) | .351 |
3.4. Salvage therapy and medical costs
BQT was recommended as a salvage regimen for 22 patients in whom the eradication of H. pylori infection failed after completing the empirical CoT or TaT. Four of the 22 patients (3 in the CoT group and 1 in the TaT group) refused to receive BQT; therefore, only 18 patients received the second-line BQT for 14 days. Levofloxacin-based triple therapy (14 days) was recommended to 1 patient after the first-line BQT failed. All patients for whom the eradication of H. pylori infection failed after CoT or TaT achieved successful eradication with the salvage regimes, except for 2 patients (one in each group) who were lost to follow-up.
The medical cost for each patient treated with CoT was $413, which included physician consultant fee, gastroscopy with sedation, rapid urease test, eradication medication, and urease breath test. In the TaT group, the medical costs per patient for triple therapy and BQT were $463 and $470, respectively. The medical costs for the patients who failed the eradication after CoT, triple therapy, and BQT were $539, $589, and $561, respectively. The average cost per patient for the successful eradication of H. pylori was $424 for CoT and $468 for TaT (Table 3).
Table 3.
Medical costs involved in the 2 therapies.
| Concomitant therapy (n = 145) | Tailored therapy (n = 145) | |||
|---|---|---|---|---|
| Success | Fail | Success | Fail | |
| Consultation fee | 24 | 36 | 24 | 36 |
| Endoscopy with sedation | 254 | 254 | 254 | 254 |
| Rapid urease test | 23 | 23 | ||
| DPO-PCR test | 78 | 78 | ||
| Concomitant therapy | 79 | 79 | ||
| Triple therapy | 74 | 74 | ||
| Bismuth quadruple therapy | 81 | 81 | ||
| Triple therapy with levofloxacin | 46 | |||
| Urea breath test | 33 | 66 | 33 | 66 |
| Average cost | 334 | 379 | 389 | 434 |
| Cost per 1 successful treatment* | 424 | 468 | ||
All values are expressed in US dollars.
Cost included the first-line therapy and rescue therapy after failure of first-line treatment.
DPO-PCR = dual-priming oligonucleotide polymerase chain reaction.
4. Discussion
In this study, the eradication rates of H. pylori infection were not significantly different between empirical CoT and DPO-PCR-based TaT according to ITT (82.8% and 85.5%, respectively, P = .520) and PP (88.6% and 94.6%, respectively, P = .084) analyses. However, the TaT group showed higher eradication rates for H. pylori harboring A2142G and/or A2143G point mutations than the empirical CoT group (ITT 87.5% and 69.8%, respectively, P = .050; PP 97.1% and 76.9%, respectively, P = .011). A recent Korean guideline, revised in 2020, recommends PAM or BQT for patients who are infected with H. pylori harboring A2142G and/or A2143G point mutations.[5,6] Although PAM was considered as the first-line treatment to overcome clarithromycin resistance of H. pylori, it did not achieve an acceptable eradication success rate (70–74%).[18,22] Our findings indicate that BQT should be considered as the first-line treatment regimen for patients infected with H. pylori exhibiting clarithromycin resistance. Moreover, PAM and CoT should be discontinued as the first-line treatments for infections caused by clarithromycin-resistant H. pylori.
The clarithromycin-resistance rate of H. pylori has increased from 21.2% to 45.9% in Korea over the last 15 years.[9] Several guidelines recommend testing for clarithromycin resistance before considering triple therapy as the first-line treatment in areas with high clarithromycin-resistance rate.[4–6] Maastricht V/Florence Consensus Report recommends the use of CoT and BQT in areas with high clarithromycin resistance rate (>15%) and low-to-intermediate metronidazole resistance rate (<40%).[1] A recent culture-based study of the antibiotic resistance profile of H. pylori in Korea showed that clarithromycin, metronidazole, and dual resistance rates were 17.8%, 16.2%, and 7.1%, respectively.[23] A recent Korean nationwide randomized controlled trial reported that the eradication rates of CoT and sequential therapy were 90.6% and 85.0%, respectively, as per PP analyses.[24] Therefore, CoT and sequential therapy were included as the first-line empirical regimens in the revised Korean guidelines for the treatment of H. pylori infection.[6] However, the metronidazole-resistance rate of H. pylori is also currently increasing >40% in Korea.[9] Maastricht V guideline recommends BQT as the first-line therapy in areas with high dual clarithromycin-metronidazole resistance rate (>15%).[1] The effectiveness of H. pylori eradication regimens is graded as excellent (>95%), good (91–95%), borderline (85–89%), and unacceptable (<85%) based on PP analysis.[25] In the present study, the eradication rate of CoT was suboptimal (<90%) overall[26] and unacceptable (76.9%) in patients infected with H. pylori harboring A2142G and/or A2143G point mutations.
In addition to the suboptimal eradication rates, in this study, CoT included multiple antibiotics, namely clarithromycin, metronidazole, and amoxicillin, which can lead to antibiotic overuse.[27] Antibiotic overuse can accelerate the spread of antibiotic-resistant bacteria, not limited to H. pylori. Eradication regimens based on antimicrobial susceptibility tests can reach the highest eradication rates.[28] However, culture-based antimicrobial susceptibility tests are costly, time consuming, and require trained technologists. Therefore, H. pylori tissue culture is only available in specialized centers. DPO-PCR test is cost effective and less time consuming compared with culture-based test, and it is widely used to identify mutations in the 23S rRNA gene related to clarithromycin resistance in Korea. Although the average cost of successful eradication in 1 patient was higher for TaT than that for CoT, TaT can increase the eradication rate of clarithromycin-resistant H. pylori and reduce the overuse of antibiotics.
Empirical BQT, as the first-line treatment, can be an option to overcome the dual clarithromycin-metronidazole resistance. A recent study comparing the empirical BQT and TaT based on DPO-PCR test showed that a 10-day empirical BQT was more effective and less expensive than TaT.[29] Another study showed that the eradication rates of 7-day empirical BQT were similar to those of TaT based on DPO-PCR test and that the former was more cost effective.[30] However, there are only few salvage regimens after BQT failure, and the 4 times daily dosing can decrease the adherence to medication.[31] To reduce this disadvantage, twice daily administration with the same dose of standard BQT was tested.[32] Twice daily BQT for 1 week showed an eradication rate similar to that of 4 times daily BQT for 1 week (93.9% and 92.9%, respectively). Twice daily modified BQT (amoxicillin instead of tetracycline) for 14 days was also evaluated, and it exhibited a good eradication rate (93.5% in PP analysis).[33] Further studies to determine the salvage therapy after BQT failure are needed before considering BQT as a preferred first-line empirical therapy.
In addition to empirical BQT, an empirical treatment consisting of vonoprazan, a potassium competitive acid blocker (P-CAB), can overcome the dual clarithromycin-metronidazole resistance. Japanese studies showed that vonoprazan-based dual or triple therapy had significantly higher eradication rate than that of PPI in patients infected with clarithromycin-resistant H. pylori.[34,35] Stronger and longer-lasting inhibition of gastric acid is considered the reason for the high eradication rate. Vonoprazan is a promising regimen to treat multi-drug resistant bacterial infections, but unfortunately, it is not available in Korea. The eradication rate of triple therapy with tegoprazan, another kind of P-CAB available in Korea, was compared with standard triple therapy with PPI. Tegoprazan 50 mg combined with amoxicillin and clarithromycin did not achieve an acceptable eradication rate.[36] Authors explained that tegoprazan 50 mg (twice the dose used for gastroesophageal reflux disease) appears insufficient in suppressing the gastric acid and in achieving an acceptable eradication rate. In triple therapy with vonoprazan, the 20 mg dose is 4 times the dose used for gastroesophageal reflux disease.[35] Further studies using higher doses of tegoprazan or fexuprazan, a new P-CAB drug, are required to evaluate the role of P-CAB in the eradication of clarithromycin-resistant H. pylori.[37]
Our study has several limitations. First, H. pylori culture and antimicrobial susceptibility tests were not performed. Nevertheless, in a previous study, the accuracy of DPO-PCR test was determined to be approximately 98% based on comparison of results with those of the culture-based antibiotic resistance test.[38,39] Second, Second, we did not investigate virulence factors (vacA and cagA). A meta-analysis reported that infection with vacA s1, cagA positive strains is more conducive to eradication therapy than vacA s2, cagA-negative.[40] Although it would be a limitation, previous report demonstrated that most H. pylori strain of Korean patients carry vacA s1 and cagA.[41] Third, he 6 tertiary hospitals that enrolled patients in this randomized controlled study were located in Busan and Kyungsangnam-do, Korea. Nationwide multicenter studies should be performed to overcome this limitation.
In summary, although CoT and TaT showed similar overall eradication rates for H. pylori, the eradication rate of CoT was suboptimal, especially for patients infected with 23S rRNA gene point mutation-positive H. pylori. Therefore, BQT is a recommend regimen to treat infections with H. pylori harboring 23S rRNA point mutations.
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by SJ Kim, SR Jee, MI Park, K Jung, GH Kim, MW Lee, J Lee, JS Jang, and M Koh. Data analysis were performed by SJ Kim. SJ Kim wrote the first draft of the manuscript. SR Jee reviewed edited this manuscript. All authors read and approved the final manuscript
Abbreviations:
- BQT =
- bismuth quadruple therapy
- CoT =
- concomitant therapy
- DPO-PCR =
- dual-priming oligonucleotide-based polymerase chain reaction
- H. pylori =
- Helicobacter pylori
- ITT =
- intention-to-treat
- P-CAB =
- potassium competitive acid blocker
- PAM =
- PPI, amoxicillin metronidazole
- PP =
- per-protocol
- PPI =
- proton-pump inhibitor
- rRNA =
- ribosomal RNA
- TaT =
- tailored therapy
The authors have no conflicts of interest to disclose.
This work was supported by the Korean College of Helicobacter and Upper Gastrointestinal Research Foundation Grant (KCHUGR-201902506).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Kim SJ, Jee SR, Park MI, Jung K, Kim GH, Lee MW, Lee J, Jang JS, Koh M. A randomized controlled trial to compare Helicobacter pylori eradication rates between the empirical concomitant therapy and tailored therapy based on 23S rRNA point mutations. Medicine 2022;101:33(e30069).
Contributor Information
Sam Ryong Jee, Email: tokimom@nate.com.
Moo In Park, Email: gimipark2003@naver.com.
Kyoungwon Jung, Email: forjkw@gmail.com.
Gwang Ha Kim, Email: doc0224@pusan.ac.kr.
Moon Won Lee, Email: injemed76@naver.com.
Jin Lee, Email: injemed76@naver.com.
Jin Seok Jang, Email: jsjang@dau.ac.kr.
Myeongseok Koh, Email: kohdolchu@dau.ac.kr.
References
- [1].Malfertheiner P, Megraud F, O’Morain CA, et al. Management of helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- [2].Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–39. [DOI] [PubMed] [Google Scholar]
- [3].Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69.e14. [DOI] [PubMed] [Google Scholar]
- [4].Mahachai V, Vilaichone RK, Pittayanon R, et al. Helicobacter pylori management in ASEAN: the Bangkok consensus report. J Gastroenterol Hepatol. 2018;33:37–56. [DOI] [PubMed] [Google Scholar]
- [5].Kato M, Ota H, Okuda M, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter. 2019;24:e12597. [DOI] [PubMed] [Google Scholar]
- [6].Jung HK, Kang SJ, Lee YC, et al. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver. 2021;15:168–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013;62:3–26. [DOI] [PubMed] [Google Scholar]
- [8].Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1–20. [DOI] [PubMed] [Google Scholar]
- [9].Lee JY, Kim N, Nam RH, et al. Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 2019;24:e12660. [DOI] [PubMed] [Google Scholar]
- [10].Kim BJ, Yang CH, Song HJ, et al. Online registry for nationwide database of Helicobacter pylori eradication in Korea: correlation of antibiotic use density with eradication success. Helicobacter. 2019;24:e12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Houben MH, van de Beek D, Hensen EF, et al. A systematic review of Helicobacter pylori eradication therapy--the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047–55. [DOI] [PubMed] [Google Scholar]
- [12].Fischbach LA, van Zanten S, Dickason J. Meta-analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004;20:1071–82. [DOI] [PubMed] [Google Scholar]
- [13].Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–57. [DOI] [PubMed] [Google Scholar]
- [14].Huang YK, Wu MC, Wang SS, et al. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. J Dig Dis. 2012;13:232–8. [DOI] [PubMed] [Google Scholar]
- [15].Georgopoulos SD. “Concomitant” or “sequential” eradication of Helicobacter pylori: which regimen comes first? Ann Gastroenterol. 2014;27:280–1. [PMC free article] [PubMed] [Google Scholar]
- [16].Smith SM, O’Morain C, McNamara D. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol. 2014;20:9912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerrits MM, van Vliet AH, Kuipers EJ, et al. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis. 2006;6:699–709. [DOI] [PubMed] [Google Scholar]
- [18].Ong S, Kim SE, Kim JH, et al. Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: a multicenter randomized controlled trial. Helicobacter. 2019;24:e12654. [DOI] [PubMed] [Google Scholar]
- [19].Choi YI, Chung JW, Park DK, et al. Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: a comparative, open trial. World J Gastroenterol. 2019;25:6743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kwon YH, Kim N, Lee JY, et al. The diagnostic validity of citric acid-free, high dose (13)C-Urea breath test after Helicobacter pylori eradication in Korea. Helicobacter. 2015;20:159–68. [DOI] [PubMed] [Google Scholar]
- [21].Jung YS, Park CH, Park JH, et al. Efficacy of Helicobacter pylori eradication therapies in Korea: a systematic review and network meta-analysis. Helicobacter. 2017;22:e12389. [DOI] [PubMed] [Google Scholar]
- [22].Gweon TG, Kim JS, Kim BW. An economic modeling study of Helicobacter pylori eradication: comparison of dual priming oligonucleotide-based multiplex polymerase chain reaction and empirical treatment. Gut Liver. 2018;12:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee JH, Ahn JY, Choi KD, et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: a prospective multicenter study. Helicobacter. 2019;24:e12592. [DOI] [PubMed] [Google Scholar]
- [24].Kim BJ, Lee H, Lee YC, et al. Ten-day concomitant, 10-day sequential, and 7-day triple therapy as first-line treatment for Helicobacter pylori infection: a nationwide randomized trial in Korea. Gut Liver. 2019;13:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–86.e3; Discussion e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–8. [DOI] [PubMed] [Google Scholar]
- [27].Shiotani A, Lu H, Dore MP, et al. Treating Helicobacter pylori effectively while minimizing misuse of antibiotics. Cleve Clin J Med. 2017;84:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee JW, Kim N, Nam RH, et al. Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter. 2019;24:e12561. [DOI] [PubMed] [Google Scholar]
- [29].Chang YW, Shin GY, Kim JW, et al. Cost-effectiveness of empirical bismuth-based quadruple therapy and tailored therapy after clarithromycin resistance tests for Helicobacter pylori eradication. Dig Dis Sci. 2021;67:1222–1230. [DOI] [PubMed] [Google Scholar]
- [30].Cha B, Bang BW, Shin JB, et al. Bismuth containing quadruple therapy versus tailored therapy as first-line treatments for Helicobacter pylori infection in a high clarithromycin resistance area. Scand J Gastroenterol. 2021;56:1017–22. [DOI] [PubMed] [Google Scholar]
- [31].Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. [DOI] [PubMed] [Google Scholar]
- [32].Kim J, Gong EJ, Seo M, et al. Efficacy of twice a day bismuth quadruple therapy for second-line treatment of Helicobacter pylori infection. J Pers Med. 2022;12. doi: 10.3390/jpm12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cho JH, Jin SY, Park S. Comparison of tailored Helicobacter pylori eradication versus modified bismuth quadruple therapy in Korea: a randomized controlled trial. Expert Rev Anti Infect Ther. 2021;1:7. [DOI] [PubMed] [Google Scholar]
- [34].Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69:1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim JY, Lee SY, Kim H, et al. Efficacy of seven-day potassium-competitive acid blocker-based first-line Helicobacter pylori eradication therapy administered with bismuth. Yonsei Med J. 2021;62:708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jeong YS, Kim MS, Lee N, et al. Development of physiologically based pharmacokinetic model for orally administered fexuprazan in humans. Pharmaceutics. 2021;13:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kwon YH, Kim N, Lee JY, et al. Comparison of the efficacy of culture-based tailored therapy for Helicobacter pylori eradication with that of the traditional second-line rescue therapy in Korean patients: a prospective single tertiary center study. Scand J Gastroenterol. 2016;51:270–6. [DOI] [PubMed] [Google Scholar]
- [39].Kwon YH, Jeon SW, Nam SY, et al. Efficacy of tailored therapy for Helicobacter pylori eradication based on clarithromycin resistance and survey of previous antibiotic exposure: a single-center prospective pilot study. Helicobacter. 2019;24:e12585. [DOI] [PubMed] [Google Scholar]
- [40].Wang D, Li Q, Gong Y, et al. The association between vacA or cagA status and eradication outcome of Helicobacter pylori infection: a meta-analysis. PLoS One. 2017;12:e0177455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim SY, Woo CW, Lee YM, et al. Genotyping CagA, VacA subtype, IceA1, and BabA of Helicobacter pylori isolates from Korean patients, and their association with gastroduodenal diseases. J Korean Med Sci. 2001;16:579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]