Abstract
This study attempted to determine the expression of p21-activated kinase 4 (PAK4) in non-small cell lung cancer (NSCLC) tissues and the normal lung tissues. The correlation between PAK4 expression and prognosis of NSCLC patients was also evaluated in the present study. The expression level of PAK4 was measured by high-performance liquid chromatography method. Chi-square test was adopted to explore the relationship of PAK4 expression and clinical features. Kaplan-Meier survival curves were plotted to delineate the overall survival rate of NSCLC patients. Cox regression analysis was performed to evaluate the prognostic significance of PAK4 expression in NSCLC. The PAK4 expression in NSCLC tissue samples was significantly higher than that in normal lung tissues (P<0.001) and shared significant correlation with Eastern Cooperative Oncology Group score, histological type, and distant metastasis (P<0.05). Survival curve revealed that NSCLC patients with high PAK4 expression had relatively higher mortality than those with low PAK4 expression (P = .001). Cox regression analysis explained that PAK4 expression was associated with the prognosis of NSCLC patients (P = .024; HR, 3.104; 95% CI, 1.164–8.278). In a word, PAK4 was highly expressed in NSCLC tissues and could act as a prognostic factor for NSCLC patients.
Keywords: HPLC, non-small cell lung cancer, PAK4, prognosis
1. Introduction
Lung cancer is one of the most frequent malignant diseases and currently is one of the leading causes of cancer-related deaths all over the world.[1–3] The incidence and mortality are increasing every year because of environmental pollution and cigarette abuse.[4] Non-small cell lung cancer (NSCLC) is the capital type of lung cancer, which accounts for more than 85% of lung cancer cases.[5–7] Most NSCLC patients are diagnosed as advanced stage, which is considered to be a crucial reason for short survival of NSCLC patients.[8,9] Treatments for NSCLC patients are mainly surgical resection, chemotherapy, and radiotherapy.[10,11] Despite advances in the treatments, there has been little improvement in the survival of NSCLC patients over the past three decades. And more than 60% patients suffer distant metastasis or local recurrence of NSCLC after treatments.[12] Thus, it is urgently needed to find novel, sensitive biomarkers for early diagnosis and prognosis of NSCLC patients.
The p21-activated kinase (PAK) family of serine/threonine kinases are key signaling proteins that play important roles in many cellular processes, including cell signaling, cytoskeletal organization, cell proliferation, migration, and survival.[13–15] Based on homology, six PAKs are identified and classified into 2 groups: group I (PAK1-3) and group II (PAK4-6).[16–18] p21-activated kinase 4 (PAK4) is the most divergent member of PAK family and locates at 19q13.2-13.3.[19,20] PAK4 is first identified as an effector protein for Rho GTPase Cdc42.[21] The overexpression of PAK4 has been identified in various cancers, such as breast cancer, gastric cancer, pancreatic cancer, and ovarian cancer.[22–25] Moreover, a relationship between the overexpression of PAK4 and poor prognosis of ovarian cancer patients has been proved.[25] Furthermore, previous studies have explored the role of PAK1 in squamous NSCLC cells and demonstrated that PAK1 was required for proliferation of squamous NSCLC cells.[26] However, until now, there is no report on the association between PAK4 and NSCLC.
In this study, we aimed to investigate the expression of PAK4 in NSCLC and estimate the role of PAK4 in the prognosis of NSCLC patients.
2. Materials and Methods
2.1. Patients and specimens
The cancer tissue samples were obtained from 75 NSCLC patients in Anqing Hospital Affiliated to Medical University of Anhui. All the patients were diagnosed with NSCLC through histologically and cytologically examinations by 2 experienced pathologists. None of the patients received any antitumor treatments before tissue collection, and they received standard treatments by the same medical team in the hospital. Normal lung tissues (n = 31) as controls were collected from patients who were treated with pulmonectomy for benign pulmonary disease. The tissue specimens used for PAK4 detection were Ct-guided/bronchoscopic-guided biopsy specimens. The cancer tissue specimens were with tumor contents more than 60%. The case and controls were matched in age and gender. The present study was approved by Ethics Committee of Anqing Hospital Affiliated to Medical University of Anhui and patients all signed the informed consents in advance.
All the patients enrolled in a 5-year follow-up investigation. The patients whose deaths were not related to NSCLC would be excluded from our study. The preoperative clinical characteristics of the NSCLC patients were collected from their medical records. There were 38 males and 37 females, and their mean age was 63.25 ± 7.63 years. Forty-two patients had smoking history. After tissue collection, 32 patients received radiotherapy or chemotherapy, whereas 43 patients were treated by surgery. According to tumor node metastasis staging, 51 patients diagnosed with stages I–II, and the rest 24 patients were confirmed with stages III–IV. Histological examinations demonstrated that 41 patients were diagnosed with adenocarcinoma, and 34 patients were diagnosed with squamous cell carcinoma. Distant metastasis was observed in 36 patients. The NSCLC patients’ general well-being and activities of daily life were quantified by Eastern Cooperative Oncology Group (ECOG) score. The scoring system runs from 0 to 5, with 0 denoting perfect health and 5 death. The patients with ECOG score no more than 2 means that they could take care themselves. Among the included NSCLC patients, 29 cases exhibited ECOG scores no more than 2, while 46 patients had ECOG score more than 2. The detailed clinical information of the NSCLC patients was summarized in Table 1.
Table 1.
Relationship between PAK4 expression and clinical characteristics.
| Characteristics | Case no. | Expression | χ2 | P value | |
|---|---|---|---|---|---|
| High | Low | ||||
| Sex | |||||
| Male | 38 | 25 | 13 | 0.455 | .500 |
| Female | 37 | 27 | 10 | ||
| Smoking | |||||
| Yes | 42 | 30 | 12 | 0.197 | .657 |
| No | 33 | 22 | 11 | ||
| Treatment | |||||
| Radio- or chemotherapy | 32 | 24 | 8 | 0.843 | .359 |
| Surgery | 43 | 28 | 15 | ||
| ECOG score | |||||
| ≦ 2 | 29 | 16 | 13 | 4.459 | .035 |
| > 2 | 46 | 36 | 10 | ||
| TNM stage | |||||
| I–II | 51 | 29 | 21 | .155 | |
| III–IV | 24 | 18 | 6 | ||
| Histological type | |||||
| Adenocarcinoma | 41 | 33 | 8 | 5.292 | .021 |
| Squamous cell carcinoma | 34 | 19 | 15 | ||
| Distant metastasis | |||||
| Yes | 36 | 29 | 7 | 4.101 | .043 |
| No | 39 | 23 | 16 | ||
ECOG = Eastern Cooperative Oncology Group, PAK4 = p21-activated kinase 4, TNM = tumor node metastasis.
2.2. High-performance liquid chromatography
The high-performance liquid chromatography method was adopted to detect the protein expression level of PAK4. Five-gram tissue sample (prier to treatments) was weighed and homogenized with the addition of 20-mL methyl cyanide. Then, the homogenate was centrifuged at 4000 r/min for 15 minutes. Supernatant was collected and then dried by a rotary evaporator, followed by resolution with 1-mL mobile phase. Kromasil C18 column (250 × 4.6 mm, 5 µm) was used, and the column temperature was maintained at 30 °C. The mobile phase consisted of methyl cyanide and water (pH = 2.5) (v:v = 83:17). The flow rate was 1 mL/min, and the detection wavelength was 280 nm. PAK4 protein of 0.05 g was weighed and dissolved in a 10-mL volumetric flask by mobile phase and prepared for standard solution. Every sample had 3 repetitions, and the final result was average value of the 3.
2.3. Statistical analysis
All data were analyzed by SPSS 18.0 (SPSS Inc, IL). The relationship between PAK4 expression and clinical characteristics was analyzed by Chi-square test. Overall survival of NSCLC patients was described by Kaplan-Meier method. The Cox regression was conducted to evaluate the relevance between PAK4 expression and prognosis of NSCLC patients. P < .05 was considered as statistically significant.
3. Results
3.1. Increased expression of PAK4 in NSCLC samples
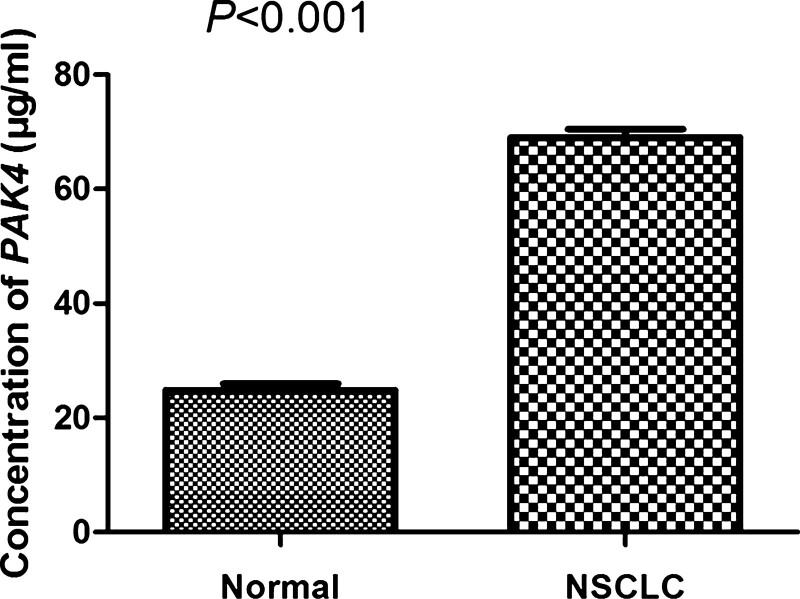
The expression of PAK4 was firstly examined by high-performance liquid chromatography in 75 NSCLC samples and 31 normal lung tissue samples. The concentration of PAK4 in NSCLC tissues was 69.03 ± 12.71, while that in the normal lung tissues was 24.84 ± 6.15. The expression level of PAK4 was significantly higher in NSCLC than that in the normal lung tissues (Fig. 1; P < .001), indicating that abnormal PAK4 expression might be associated with NSCLC pathogenesis.
Figure 1.
PAK4 expression in NSCLC tissues and the normal tissues was assayed by HPLC, respectively. The results were presented as mean ± SD. PAK4 was highly expressed in NSCLC tissues compared to the normal lung tissues (P < .001). HPLC = high-performance liquid chromatography, NSCLC = non-small cell lung cancer, PAK4 = p21-activated kinase 4, SD = XXX.
3.2. The association of PAK4 expression with clinicopathological characteristics of NSCLC patients
To further address the association between PAK4 expression and NSCLC, several clinical factors of NSCLC were analyzed. All NSCLC samples were manually divided into 2 groups: the high expression group with the PAK4 concentration of more than 76.01, and the others belonged to the low group. The statistical analysis revealed that high expression of PAK4 was tightly related to ECOG score, histological type, and distant metastasis (Table 1; P < .05). However, there was no statistical significance between PAK4 expression and sex, smoking, treatment, or tumor node metastasis stage (Table 1; P > .05).
3.3. Upregulation of PAK4-predicted unfavorable prognosis of NSCLC patients
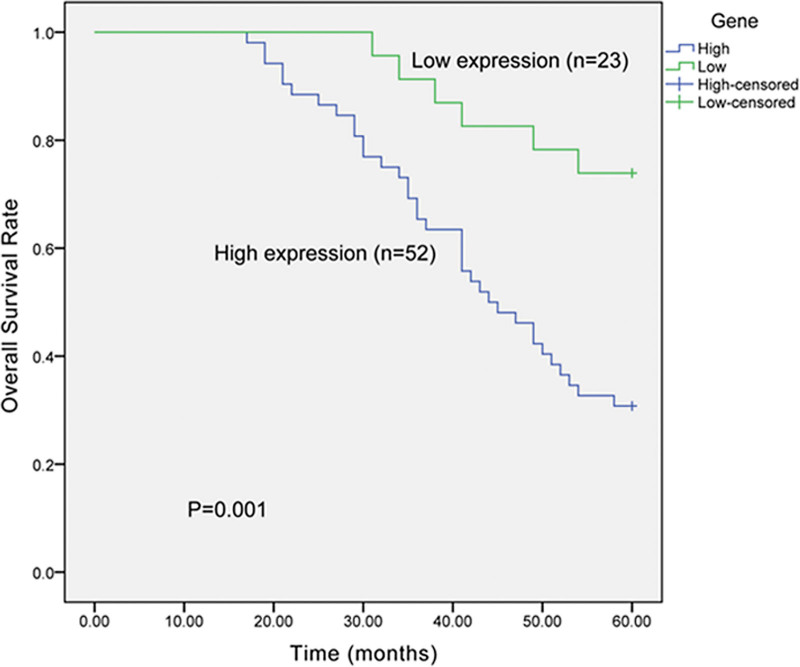
Kaplan-Meier analysis was adopted to evaluate the effects of PAK4 expression on the overall survival of NSCLC patients. The survival curves displayed that the NSCLC patients with high expression of PAK4 had shorter overall survival time than those with low PAK4 expression (Fig. 2; P = .001). The overall 5-year survival rate of patients with high PAK4 expression was 30.8% (16 out of 52), compared with 73.9% (17 out of 23) of patients with low PAK4 expression. Multivariate analysis demonstrated that PAK4 expression shared significant relevance with prognosis of NSCLC patients (Table 2, P = .024; HR, 3.104; 95% CI, 1.164–8.278), suggesting that PAK4 could be a candidate factor for prognosis of NSCLC patients.
Figure 2.
Kaplan-Meier curves showed the influence of PAK4 expression on overall survival of NSCLC patients. The overall survival rate of NSCLC patients with high PAK4 expression was significantly lower than those with low PAK4 expression (P = .001). NSCLC = non-small cell lung cancer, PAK4 = p21-activated kinase 4.
Table 2.
Multivariate analyses for clinical factors and PAK4 expression in patients with NSCLC.
| Characteristics | P value | HR (95% CI) |
|---|---|---|
| Sex | .533 | 0.810 (0.418–1.571) |
| Smoking | .396 | 0.749 (0.385–1.459) |
| Treatment | .228 | 0.662 (0.338–1.296 |
| Histological type | .647 | 1.197 (0.555–2.581) |
| Distant metastasis | .653 | 1.168 (0.593–2.302) |
| PAK4 expression | .024 | 3.104 (1.164–8.278) |
CI = XXX, HR = XXX, NSCLC = non-small cell lung cancer, PAK4 = p21-activated kinase 4.
4. Discussion
NSCLC is the most frequent lung cancer, and its most common 3 types are adenocarcinoma, squamous cell carcinoma, and large cell anaplastic carcinoma. Although there are several therapies, including surgery, radiotherapy, and chemotherapy, most NSCLC patients experience recurrence because of the poor sensitivity to those treatments. The 5-year survival rate of NSCLC patients is low, and the prognosis is dismal.[27] Therefore, identification of novel molecular markers, which could improve the diagnosis and prognosis stratification and serve as possible therapy targets, is of great importance. In addition, several clinicopathological factors have been found to relate with the risk of lung cancer, such as sex, tumor stage and vascular invasion. However, the accuracy of the markers is not sufficient, so finding a novel and specific targeted gene for therapy and prognosis of NSCLC patients is necessary.
Recently, the research on PAKs has become a hotpot in several fields, including tumor occurrence, development, and metastasis. PAK4, as an important member of PAK family, develops a variety of biological effects, such as regulation of cell cycles, cell growth, and cell apoptosis. Siu et al[25] showed that PAK4 regulated the cell proliferation in ovarian cancer. Moreover, it has been reported that PAK4 participated in signal pathways of tumor cells, functioning on proliferation, invasion, and metastasis of tumor cells. Thus, PAK4 might be a novel target for therapy and prognosis of tumors. Precious studies have demonstrated that PAK4 was overexpressed in a variety of cancer cells, and knockdown of PAK4 reduced the proliferation of various cancer cells. For instance, Kimmelman et al[24] have confirmed that PAK4 was overexpressed in pancreas cancer. Meanwhile, Wong et al[14] have suggested that knockdown of PAK4 reduced proliferation and migration of tumoral cells in breast cancer. Minden[13] proved that overexpression of PAK4 was observed in breast cancer. However, we would like to know the role of PAK4 in NSCLC.
The results of our study were in accordance with the above observations. We found that the expression of PAK4 in NSCLC tissues was higher than that in the normal lung tissues, suggesting that PAK4 could be a predictive marker for the therapy and prognosis of NSCLC. To our knowledge, this is the first time to demonstrate the prognostic role of PAK4 in NSCLC. In the present study, Chi-square test revealed that high expression of PAK4 was related to ECOG score, histological type, and distant metastasis. ECOG score is frequently used to quantify cancer patients’ general well-being and activities of daily life, runs from 1 to 5. High scores predict poor living status. Among the included NSCLC patients, 69.2% patients with high expression of PAK4 exhibited ECOG score more than 2, while only 43.5% patients with low PAK4 expression high expression had ECOG score more than 5.
High expression of PAK4 predicted poor living status of NSCLC patients. The following survival outcome proved that the overall survival rate of patients with high PAK4 expression was significantly lower than those with low PAK4 expression. Multivariate analysis delineated that overexpression of PAK4 was correlated with poor prognosis of NSCLC patients after adjusted to the potential confusing factors, indicating that PAK4 might be an independent prognostic factor for NSCLC patients.
The rapid and uncontrolled growth of cancer cells is primarily due to the development of apoptotic resistance and enhanced cell-cycle progression.[28] Studies have demonstrated that PAK4 can regulate the cell-cycle progression of cancer cells through several signal pathways. Tyagi et al[29] have illustrated that PAK4 regulated the proliferation and survival of pancreatic cancer through the NF-κB pathway. Fu et al[30] have identified that PAK4 functioned on cisplatin resistance in gastric cancer cells by PI3K/Akt- and MEK/ERK-dependent pathways. Besides, Siu et al[25] have confirmed that PAK4 induced ovarian cancer cell proliferation via Pak4/c-Src/EGFR pathway. Based on the previous reports, we can further investigate the mechanism of PAK4 on the progression of NSCLC and provide novel possible therapy for the diagnosis and treatment of NSCLC patients.
Despite the encouraging results, some limitations in current study should be stated. Firstly, the sample size was relatively small that might influence the statistical power of our results. Secondly, the molecular mechanisms of PAK4 in NSCLC had not been explored in our study. Finally, the association between PAK4 expression and other prognostic and diagnostic markers in NSCLC was not analyzed. The combined application of PAK4 with other biomarkers might improve the clinical values of these parameters. Therefore, further well-designed studies with larger sample size are required to address the above issues.
Generally speaking, PAK4 was significantly overexpressed in NSCLC, and statistical significance was found between PAK4 expression and clinicopathological features. Most importantly, PAK4 could serve as a novel independent biomarker in NSCLC patients.
Footnotes
How to cite this article: Li C, Ji D, Duan A, Jiang Q. PAK4 expression is associated with the prognosis in non-small cell lung cancer. Medicine 2022;101:33(e30050).
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Jin T, Jin J, Li X, et al. Prognostic implications of ezrin and phosphorylated ezrin expression in non-small cell lung cancer. BMC Cancer. 2014;14:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang YR, Zang SZ, Zhong CL, et al. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Path. 2014;7:6929–35. [PMC free article] [PubMed] [Google Scholar]
- [3].Nii K, Tokunaga Y, Liu D, et al. Overexpression of G protein-coupled receptor 87 correlates with poorer tumor differentiation and higher tumor proliferation in non-small-cell lung cancer. Mol Clin Oncol. 2014;2:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. [DOI] [PubMed] [Google Scholar]
- [5].Zhou H, Wu A, Fu W, et al. Significance of semaphorin-3A and MMP-14 protein expression in non-small cell lung cancer. Oncol lett. 2014;7:1395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jiang Y, Wang C, Lv B, et al. Expression level of serum human epididymis 4 and its prognostic significance in human non-small cell lung cancer. Int J Clin Exp Med. 2014;7:5568–72. [PMC free article] [PubMed] [Google Scholar]
- [7].Guo S, Yan F, Xu J, et al. Identification and validation of the methylation biomarkers of non-small cell lung cancer (NSCLC). Clin Epigenetics. 2015;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen C, Zhao Z, Liu Y, et al. microRNA-99a is downregulated and promotes proliferation, migration and invasion in non-small cell lung cancer A549 and H1299 cells. Oncol Lett. 2015;9:1128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Raungrut P, Wongkotsila A, Lirdprapamongkol K, et al. Prognostic significance of 14-3-3gamma overexpression in advanced non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:3513–8. [DOI] [PubMed] [Google Scholar]
- [10].Yan B, Zhang W, Jiang LY, et al. Reduced E-cadherin expression is a prognostic biomarker of non-small cell lung cancer: a meta-analysis based on 2395 subjects. Int J Clin Exp Med. 2014;7:4352–6. [PMC free article] [PubMed] [Google Scholar]
- [11].Liu B, Xu K, Jiang Y, et al. Aberrant expression of Per1, Per2 and Per3 and their prognostic relevance in non-small cell lung cancer. Int J Clin Exp Path. 2014;7:7863–71. [PMC free article] [PubMed] [Google Scholar]
- [12].Jassem J, Skokowski J, Dziadziuszko R, et al. Results of surgical treatment of non-small cell lung cancer: validation of the new postoperative pathologic TNM classification. J Thorac Cardiovasc Surg. 2000;119:1141–6. [DOI] [PubMed] [Google Scholar]
- [13].Minden A. The pak4 protein kinase in breast cancer. ISRN Oncol. 2012;2012:694201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wong LE, Chen N, Karantza V, et al. The Pak4 protein kinase is required for oncogenic transformation of MDA-MB-231 breast cancer cells. Oncogenesis. 2013;2:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eswaran J, Soundararajan M, Kumar R, et al. UnPAKing the class differences among p21-activated kinases. Trends Biochem Sci. 2008;33:394–403. [DOI] [PubMed] [Google Scholar]
- [16].Zhang H, Li Z, Viklund EK, et al. P21-activated kinase 4 interacts with integrin alpha v beta 5 and regulates alpha v beta 5-mediated cell migration. J Cell Biol. 2002;158:1287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tabusa H, Brooks T, Massey AJ. Knockdown of PAK4 or PAK1 inhibits the proliferation of mutant KRAS colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT signaling. Mol Cancer Res. 2013;11:109–21. [DOI] [PubMed] [Google Scholar]
- [18].Law SH, Sargent TD. Maternal pak4 expression is required for primitive myelopoiesis in zebrafish. Mech Dev. 2013;130:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang HJ, Siu MK, Yeung MC, et al. Overexpressed PAK4 promotes proliferation, migration and invasion of choriocarcinoma. Carcinogenesis. 2011;32:765–71. [DOI] [PubMed] [Google Scholar]
- [20].Lu Y, Pan ZZ, Devaux Y, et al. p21-activated protein kinase 4 (PAK4) interacts with the keratinocyte growth factor receptor and participates in keratinocyte growth factor-mediated inhibition of oxidant-induced cell death. J Biol Chem. 2003;278:10374–80. [DOI] [PubMed] [Google Scholar]
- [21].Callow MG, Clairvoyant F, Zhu S, et al. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem. 2002;277:550–8. [DOI] [PubMed] [Google Scholar]
- [22].Liu Y, Chen N, Cui X, et al. The protein kinase Pak4 disrupts mammary acinar architecture and promotes mammary tumorigenesis. Oncogene. 2010;29:5883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ahn HK, Jang J, Lee J, et al. P21-activated kinase 4 overexpression in metastatic gastric cancer patients. Transl Oncol. 2011;4:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kimmelman AC, Hezel AF, Aguirre AJ, et al. Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer. Proc Natl Acad Sci USA. 2008;105:19372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Siu MK, Chan HY, Kong DS, et al. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci USA. 2010;107:18622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ong CC, Jubb AM, Haverty PM, et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci USA. 2011;108:7177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yin QW, Sun XF, Yang GT, et al. Increased expression of microRNA-150 is associated with poor prognosis in non-small cell lung cancer. Int J Clin Exp Path. 2015;8:842–6. [PMC free article] [PubMed] [Google Scholar]
- [28].Arora S, Bhardwaj A, Srivastava SK, et al. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS One. 2011;6:e21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tyagi N, Bhardwaj A, Singh AP, et al. p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT- and ERK-dependent activation of NF-kappaB pathway. Oncotarget. 2014;5:8778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fu X, Feng J, Zeng D, et al. PAK4 confers cisplatin resistance in gastric cancer cells via PI3K/Akt- and MEK/ERK-dependent pathways. Biosci Rep. 2014;34:e00094. [DOI] [PMC free article] [PubMed] [Google Scholar]