Abstract
Purpose
Breast cancer (BC) patients’ (pts) management was affected by a global reorganization after Coronavirus disease 2019 (COVID-19). Our multicenter study aimed to assess the impact of COVID-19 on access to diagnosis, staging and treatment for BC pts compared to pre-pandemic.
Methods
Medical records of all consecutive newly diagnosed BC pts referred to 6 Italian Institutions between March and December 2020 were assessed. Monthly access rate and temporal intervals between date of symptoms onset, radiological, cytohistological diagnosis and treatment start were analyzed and compared with 2019.
Results
A reduction (25%) in newly diagnosed BC was observed compared to 2019 (666 vs 890). New BC pts in 2020 were less likely to be diagnosed with early stage BC (77% vs 83%, p < 0.01), had a worse performance status according to the Eastern Cooperative Oncology Group (ECOG PS) (19.8% had PS > 0 in 2020 vs 16.5% in 2019, p < 0.01) and fewer pts were asymptomatic at diagnosis in 2020 (54% vs 71%,p < 0.01). COVID-19 did not negatively impact in terms of access to diagnosis, staging and treatment. Time intervals between symptom onset and radiological diagnosis, symptom onset and cytohistological diagnosis, cytohistological diagnosis and treatment start were maintained or improved. However, less cases were discussed in multidisciplinary tumor meetings during 2020 (60% vs 73%, p < 0.01).
Conclusions
Our data proved an alarming reduction of early stage BC associated with the COVID-19 crisis in 2020. Despite the upheaval generated by the pandemic, our study shed light on the effective performance delivered by Italian Oncology Departments to guarantee diagnostic-therapeutic pathways.
Keywords: Breast cancer, COVID-19, Diagnostic delay, Therapeutic delay, Multidisciplinary discussions
Highlights
-
•
COVID-19 impacted on breast cancer (BC) diagnoses with a reduction of 25% in 2020.
-
•
Fewer early-stage BC and more symptomatic patients were diagnosed during 2020.
-
•
Timing of access to BC diagnosis, staging and treatment has not been affected by COVID-19.
-
•
Less BC cases were reviewed in multidisciplinary tumor meetings during 2020.
1. Introduction
In March 2020, the Coronavirus Disease 2019 (COVID-19) was declared a global pandemic by the World Health Organization, reporting 175 million infections and over 3 million deaths to date. Italy was the first European country and one of the most affected, especially during the first pandemic wave [1]. Consequently, there was a complete reorganization of the National Health System, including reallocation of crucial human and economic health resources. This inevitably impacted on hospital's admissions for non-communicable diseases hampering both inpatients and outpatients care. In particular, all elective activities were paused or postponed in order to preserve the health care system capacity for COVID-19 patients [2]. The Italian Government introduced emergency social restrictions to reduce the human-to-human viral transmission and protect the most vulnerable individuals, such as cancer patients at risk of deferring, suspending or never starting anticancer treatment due to COVID-19 infection [3,4]. In this setting, Oncology Departments have made many efforts in order to guarantee access to care and high-quality standards for diagnostic-therapeutic pathways [[5], [6], [7]] according to international guidelines [8]. However, screening programs have been temporarily halted or delayed between March and May 2020, with a potential unfavorable impact on cancer patients' prognosis [9,10]. Female breast cancer (BC) represents the most commonly diagnosed tumor worldwide with 2.26 million cases in 2020 [11]. In Italy, approximately 55 000 women receive a BC diagnosis every year [12]. The majority of these diagnoses, especially in the early stage, results from screening programs, with an estimated prognosis improvement of 45% in western countries during the past 10–20 years [13]. Recent studies suggest that pausing BC screening programs during lockdown produced delayed cancer diagnoses associated with significant repercussions on cancer mortality and health economic losses [14,15]. Gathering data from 6 Italian Institutions, the aim of our multicenter study was to investigate COVID-19 impact on new BC diagnoses in terms of access to diagnosis, staging and treatment after March 2020 compared to pre-pandemic period.
2. Materials and methods
2.1. Study design and population
Patient data were retrieved from the COVID-DELAY study (“Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of cancer patients in Italy”). The study included patients with lung, colorectal and breast cancer diagnosis. Hereby we report data on the BC cohort. Primary objective was to assess whether the COVID-19 outbreak impacted timing of diagnosis and access to treatment of BC patients in 2020. To investigate this objective, we assessed the total number of new diagnoses, access rate (number of patients/month) and temporal intervals between date of symptoms onset, diagnosis, first oncological appointment, treatment start and first radiological reassessment. We compared the obtained data with those of the same months of 2019 (“pre-pandemic” period). As a secondary objective, we also assessed the impact of COVID outbreak on the stage of disease at diagnosis in 2020 compared to 2019.
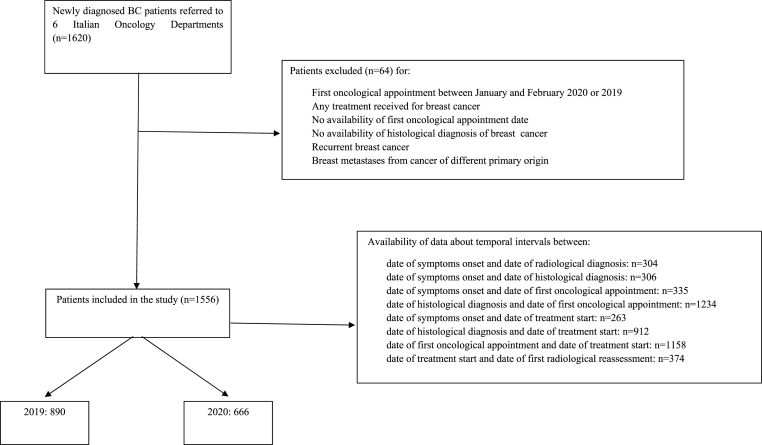
In total, 6 Italian Institutions (mostly secondary care centers, one university hospital and one tertiary referral hospital) provided data about BC patients. Clinical records of all consecutive patients with histologically or cytologically proven diagnosis of BC referred to those Institutions between March and December 2020 or 2019, who received at least one type of oncological treatment (either surgery, radiotherapy, or systemic therapy including endocrine therapy) after diagnosis and had available data about their diagnostic-therapeutic pathways (date of symptom onset, radiological diagnosis, cytohistological diagnosis, first oncological appointment, treatment start, and first radiological reassessment), were reviewed (Supplementary Table 1). Patients with relapsed BC or breast metastases from cancer of a different organ were excluded. Baseline (at diagnosis) data about patient, tumor and treatment characteristics were also retrieved and differences between the two years were computed. To avoid negative values, patients who had a BC diagnosis following the first oncological appointment (as per standard practice of some referral hospitals) were not included in the calculation of these specific temporal intervals. Time intervals between symptom onset and date of diagnosis/first oncological appointment/treatment start was only computed for patients who were symptomatic at diagnosis (Fig. 1).
Fig. 1.
STROBE diagram. Identification and selection of study population according to inclusion and exclusion criteria.
Approval to conduct this study was granted by the respective local ethical committees on human experimentation of each participating center, after previous approval by the coordinating center (“Comitato Etico Regionale delle Marche - C.E.R.M.”, Reference Number 2021 139). As subgroup analyses, we also investigated whether the COVID-19 outbreak impacted differently BC patients according to the lockdown period, the infection rate of the provinces where BC patients were diagnosed (high-vs medium/low-infected provinces) [16], and the hospital volume (high volume: ≥200 new BC diagnoses in the 2-year timeframe vs low/medium volume: <200 diagnoses). As a conventional time interval of about 1 month between diagnosis and first oncological appointment was expected, April 1, 2020–June 30, 2020 was considered as a reference time period for the lockdown, instead of March 8, 2020–May 4, 2020 actually imposed by the Italian Government.
2.2. Statistical analysis
A 20% reduction of newly diagnosed BC cases in the pandemic year (2020) compared to 2019 was postulated. Therefore, assuming a 95% confidence interval (95%CI) range of 10% (±5%), a sample size of at least 250 newly diagnosed BC patients in 2019, corresponding to 200 new diagnoses in 2020 was required to test the null hypothesis. Baseline patient, disease and treatment characteristics were presented using count and percentage for categorical variables, median, and range for continuous variables. Pearson chi-square or Fisher's exact tests (for categorical variables) and paired Student t-test, or the Mann-Whitney U test (for continuous variables) were used for analysis of differences between 2020 and 2019. All statistical analyses were performed using R 3.6.0 (R Foundation for Statistical Computing) with a two-tailed level of significance of p < 0.05.
3. Results
3.1. Patients characteristics
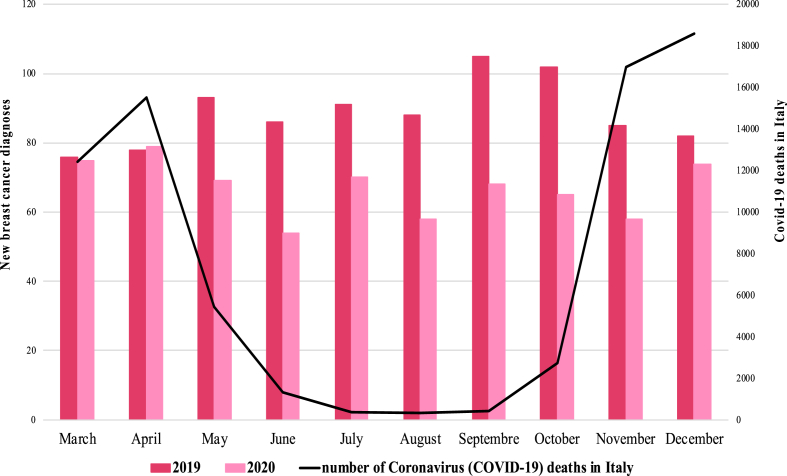
A total of 1556 patients were included in the study (Fig. 1). A notable reduction (−25%) in newly diagnosed BC was seen in 2020 (n = 666) when compared with 2019 (n = 890). The mean monthly access rate was significantly reduced in 2020 compared to pre-pandemic year (66.6 vs 89.0, p < 0.01) (Table 1, Fig. 2).
Table 1.
Monthly differences of new breast cancer diagnoses between 2019 and 2020. April, May, and June 2020 (in bold type) were considered as the lockdown timeframe.
| Month | 2019 | 2020 | Absolute difference | % |
|---|---|---|---|---|
| March | 76 | 75 | −1 | −1.3% |
| April | 78 | 79 | 1 | 1.3% |
| May | 93 | 69 | −24 | −25.8% |
| June | 86 | 54 | −32 | −37.2% |
| July | 91 | 70 | −21 | −23.1% |
| August | 88 | 58 | −30 | −34.1% |
| September | 105 | 68 | −37 | −35.2% |
| October | 102 | 65 | −37 | −36.3% |
| November | 85 | 58 | −27 | −31.7% |
| December | 82 | 74 | −8 | −9.8% |
Fig. 2.
New breast cancer diagnosis between 2019 and 2020 for each month.
3.2. Impact on patient and tumor characteristics at diagnosis
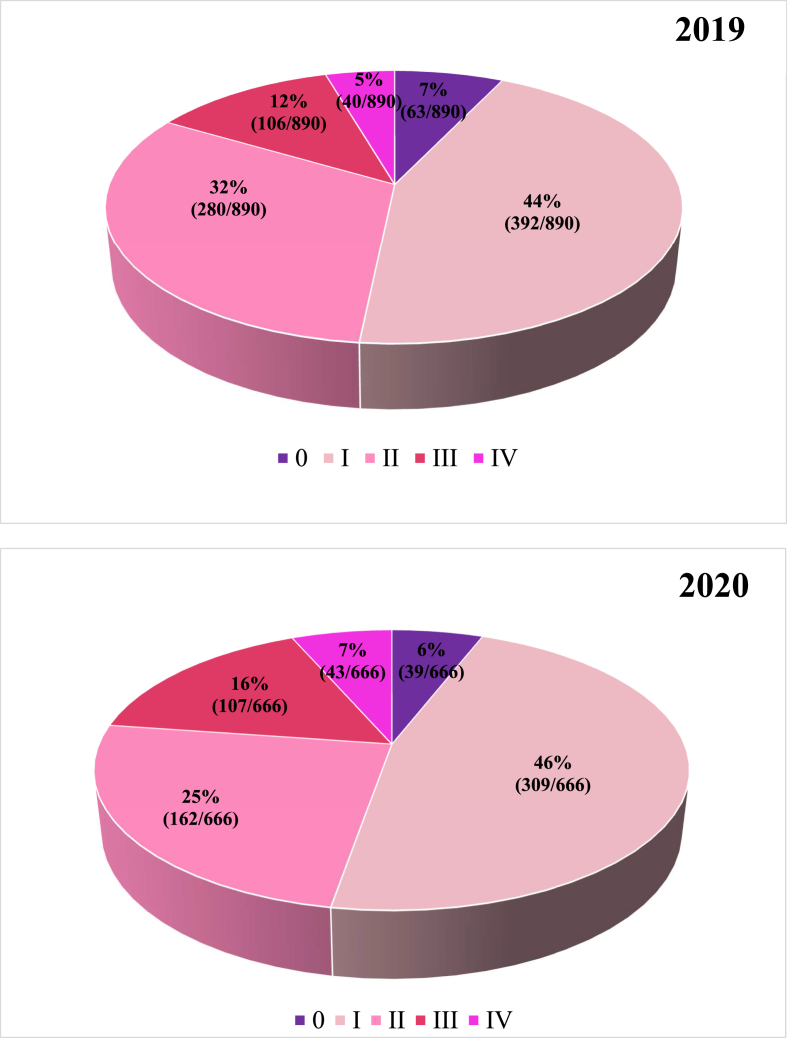
Median age was similar in the two groups (61 years in 2020 vs 62 years in 2019, p = 0.62). Male patients represented a small portion in both cohorts with no significant difference between years (0.3% in 2020 vs 0.7% in 2019, p = 0.50) and only 3% of patients had a bilateral disease in both cohorts (p = 1.00). Histotype and “molecular subtype” were similar regardless of the year (p = 0.51 and p = 0.79, respectively) with ductal histotype accounting for 78% and “luminal A″ subtype for 42% of the cases in both years. The clinical stage at diagnosis was different between the two cohorts. Specifically, in 2020 new BC patients were less likely to be diagnosed with early stage disease (stage 0-I-II) compared to previous year (77% vs 83%, p < 0.01) (Fig. 3). Looking at symptom onset, a significantly lower number of asymptomatic patients was diagnosed in 2020 compared to 2019 (54% vs 71%, p < 0.01). The ECOG Performance Status (PS) at the start of treatment was significantly different between the two years, with 19.8% of patients with PS > 0 in 2020 vs 16.5% in 2019 (p < 0.01). This made the pair with the more advanced stage at diagnosis, as stage III-IV patients were more likely to be symptomatic (p < 0.01) and have higher ECOG PS (p < 0.01).
Fig. 3.
Difference in breast cancer stages at diagnosis between 2019 and 2020.
3.3. Impact on treatment
Regarding the treatment setting, a lower percentage of BC patients received adjuvant therapy in 2020 compared to 2019 (66% vs 70%, p = 0.01). However, no significant difference emerged between the two years looking at the number of patients treated with radiotherapy (64% in 2020 vs 66% in 2019, p = 0.32). The proportion of patients treated in clinical trials were similar in both cohorts (0.3% in 2020 vs 0.5% in 2019, p = 0.70). The multidisciplinary management of patients was significantly impacted by the pandemic: 60% of new BC cases were reviewed in 2020 in multidisciplinary team meetings compared to 73% in 2019 (p < 0.01).
Demographic, clinicopathological and treatment characteristics by year of treatment are summarized in Table 2.
Table 2.
Patients’ characteristics by year of diagnosis.
| Characteristic | 2019 (%) | 2020 (%) | p value | |
|---|---|---|---|---|
| Patients | 890 | 666 | ||
| Monthly access rate | 89.0 | 66.6 | <0.01 | |
| Median age (IQR) | 62 (29–96) | 61 (27–94) | 0.62 | |
| Male gender | 6 (0.7) | 2 (0.3) | 0.50 | |
| Asymptomatic disease onset * | 371 (71) | 219 (54) | <0.01 | |
| Bilateral Disease | 27 (3) | 20 (3) | 1.00 | |
| Histological subtype * | 0.51 | |||
| Ductal | 695 (78) | 523 (78) | ||
| Lobular | 96 (11) | 62 (9) | ||
| Mixed | 59 (7) | 55 (8) | ||
| Other | 37 (4) | 26 (5) | ||
| Molecular subtype * | 0.79 | |||
| Luminal A | 351 (42) | 260 (42) | ||
| Luminal B HER2- | 276 (33) | 221 (35) | ||
| Luminal B HER2+ | 44 (5) | 32 (5) | ||
| HER2+ | 83 (10) | 63 (10) | ||
| TN | 85 (10) | 53 (8) | ||
| Stage at diagnosis * | <0.01 | |||
| Stage 0-I-II | 735 (83) | 510 (77) | ||
| Stage III-IV | 146 (17) | 150 (23) | ||
| Grading * | 0.17 | |||
| 1 | 136 (16) | 82 (13) | ||
| 2 | 440 (52) | 345 (53) | ||
| 3 | 273 (32) | 222 (34) | ||
| Treatment * | 0.01 | |||
| Adjuvant | 618 (70) | 440 (66) | ||
| Neoadjuvant | 96 (10) | 104 (16) | ||
| Metastatic | 41 (5) | 40 (6) | ||
| Follow up | 132 (15) | 81 (12) | ||
| Multidisciplinary discussion * | <0.01 | |||
| Yes | 653 (73) | 399 (60) | ||
| No | 236 (27) | 266 (40) | ||
| Treatment within clinical trials * | 0.70 | |||
| Yes | 4 (0.5) | 2 (0.3) | ||
| No | 751 (99.5) | 580 (99.7) | ||
| Radiotherapy * | 0.32 | |||
| Yes | 565 (66) | 388 (64) | ||
| No | 288 (34) | 222 (36) | ||
| ECOG PS at treatment initiation * | <0.01 | |||
| 0 | 592 (83.5) | 446 (80.2) | ||
| 1 | 107 (15.1) | 85 (15.3) | ||
| 2 | 10 (1.4) | 24 (4.3) | ||
| 3 | 0 (0) | 1 (0.2) |
Abbreviations: IQR, interquartile range.
* % has been calculated excluding NA (not available) values.
3.4. Impact on timing of diagnosis and treatment
Looking at BC patients’ management, COVID-19 did not seem to negatively impact 2020 in terms of access to diagnosis, staging and treatment (Table 3 and Supplementary Fig. S1).
Table 3.
Temporal intervals between date of symptoms onset, radiological diagnosis, cytohistological diagnosis, first oncological appointment, treatment start, and first radiological reassessment by year of diagnosis.
| Time interval | 2019 Median, days (range) | 2020 Median, days (range) | P valuea |
|---|---|---|---|
| Symptom onset/radiological diagnosis | 24.5 (0–1492) | 20 (0–346) | 0.08 |
| Symptom onset/cytohistological diagnosis | 38 (1–1521) | 32 (0–361) | 0.08 |
| Symptom onset/first oncological appointment | 96 (4–1537) | 81 (7–557) | 0.03 |
| Cytohistological diagnosis/first oncological appointment | 63 (-5–500) | 49 (0–458) | <0.01b |
| Symptom onset/treatment start | 113 (29–453) | 94.5 (17–453) | <0.01b |
| Cytohistological diagnosis/treatment start | 75 (0–413) | 61 (0–412) | <0.01b |
| First oncological appointment/treatment start | 9.5 (0–390) | 8 (0–368) | 0.12 |
| Treatment start/first radiological evaluation | 104 (6–455) | 71.5 (2–385) | <0.01b |
Mann-Whitney U test comparing time intervals between 2019 and 2020. P values were calculated excluding patients with unknown values. Data of patients who had their breast cancer diagnosis after first oncological appointment (as per standard practice of referral Hospitals) were also excluded in the calculation of these specific temporal intervals.
Statistically significant (P < 00.05).
In particular, time intervals between symptoms onset and radiological diagnosis (median 20 days in 2020 vs 24.5 in 2019, p = 0.08) and symptoms onset and cytohistological diagnosis (median 32 vs 38 days, p = 0.08) were similar between the two years. The intervals between symptoms onset and first oncological visit (median 81 vs 96 days, p = 0.03) and cytohistological diagnosis and first oncological visit (median 49 vs 63 days, p < 0.01) appeared even shorter in 2020. Moreover, focusing on access to treatment, time intervals between symptoms onset and treatment start (median 94.5 vs 113 days, p < 0.01) and cytohistological diagnosis and treatment start (median 61 vs 75 days, p < 0.01) were also improved in 2020 compared to 2019. Temporal intervals between first oncological appointment and treatment start were similar in 2020 compared to 2019 (median 8 vs 9.5 days, p = 0.12); conversely, time interval between treatment start and first radiological re-evaluation was significantly reduced in 2020 (median 71.5 vs 104 days, p < 0.01). Temporal intervals by year are shown in Table 3.
3.5. Exploratory analyses
Considering that COVID-19 might have impacted differently on BC diagnoses according to time of the year, hospital volume and provincial infection rate, sensitivity analyses were performed in some subgroups of patients. No significant difference appeared in terms of new BC diagnoses between the lockdown period and the rest of 2020 (−21% vs −26%, p = 0.64). June 2020 was the month with the greatest percentage drop in terms of new BC diagnoses (−37%) (Fig. 2). Regarding the percentage of patients referring to high-volume hospitals vs low/medium-volume hospitals, no significant difference emerged during the pandemic compared to 2019. The percentage was 87% in both years (p = 0.83). Moreover, no difference was described in terms of BC patients referred to hospitals in high-infected vs low/medium-infected provinces during the pandemic (58% in 2020 vs 61% in 2019, p = 0.23).
4. Discussion
4.1. COVID-19 impact on cancer care and study rationale
Following the direct consequences of COVID-19 on a close to collapse global health care system, the 2020 leaves the cancer care setting in complete awe of future prospects. With our country in the eye of the pandemic storm, Italian medical oncologists strived to navigate the uncharted waters of COVID-19 [5,17]. When desperate times called for drastic measures, diagnostic procedures and screening programs were deprioritized, routine clinical practices (such as follow up visits and multidisciplinary tumor meetings) reoriented to virtual care to reduce risk exposure and ease the pressure on hospital facilities [6,18]. Cancer patients, as a highly vulnerable population to better manage at a safe distance [19,20], remotely experienced this sudden disaster response coping with the fear of being left orphan of specialist care [21,22]. Considering the downside of this forced historic transition to telemedicine, the post-pandemic oncological scenario will hardly look the same in the forthcoming years [23]. Held hostage by such a global health crisis, alarming predictions have shortly warned the scientific community against neglecting the enduring cancer pandemic by diverting the attention on COVID-19 outbreak [24]. With a great research interest now growing on the repercussions of COVID-19 on cancer incidence and mortality rates, we aimed to shed light on the effectiveness of the measures adopted to deliver new tailored standards of Oncology care for BC patients in Italy after March 2020.
4.2. Key findings within the pandemic context and future implications
Our data indicate a steep reduction (−25%) in BC new diagnoses in 2020 (n = 666) compared to pre-pandemic time (n = 890), reflecting previous concerning findings worldwide [[25], [26], [27], [28]]. Such drop shows consistency with former observations demonstrating the greater diagnostic backlog after the shutdown of screening programs [26].
Specifically, according to Kaufmann et al. BC diagnosis experienced the sharpest decrease, among several malignancies, in their first pandemic wave's report of United States (US) weekly cancer incidence [25]. Firstly however, our expanded analysis throughout 2020 revealed that this decline hit its highest point (26%) after the flattening of the first epidemic curve and the resumption of cancer screenings (June 2020). This finding also suggests that the composite outcome of diagnostic delays might gain more consideration as the follow up period extends due to the long-tail effect of shutting down the screening programs [26]. As the most common diagnosed tumor globally, mainly benefitting in the early stage disease from screening detection [29], it is of utmost importance to consider the potential effect of BC delayed diagnoses on patients' outcome, ultimately resulting in divergent therapeutic intents (curative vs palliative) [30]. In this regard, our study has strikingly proved that new incidence BC in 2020 were detected at a more advanced stage (23% vs 17%, p < 0.01) and more frequently with a symptomatic onset compared to pre-pandemic time. As a matter of fact, patients may have avoided seeking immediate consultation with their general practitioner/specialist due to the pandemic.
The notable setback in early stage (stage 0-I-II) diagnoses (77% vs 83%, p < 0.01) demonstrated after March 2020 parallels the dramatic decline in cancer screenings reported by the Italian National Screening Network (−34.5% of mammography invitations in the first 9 months of 2020) [31] and by London et al. in their extensive US networks assessment (mammography dropping by 89.2% during lockdown) [32]. As additional evidence of the role played by the screening's interruption, our analysis also highlighted a reduction in asymptomatic breast cancer diagnoses (−17% in 2020 vs 2019) that mainly result from mammography screenings. Although it is too soon to accurately predict how this much-feared upstaging effect might bear upon cancer survival, early findings from a large United Kingdom (UK) modeling study have suggested for BC mortality rate an increase of 9.6% in the 5 years following diagnosis [33,34].
In addition, we cannot afford to overlook the economic impact on a pandemic-exhausted health system of the projected excess in cancer deaths resulting from delayed diagnosis. It might outdo on a per capita basis, as estimated by Gheorghe et al., the productivity loss accountable to COVID-19 [14].
Differing from former US and European investigations regarding continuum of care [35,36], our results proves that Italian Oncology Departments were up to the challenge of unwaveringly keeping the cancer care ship afloat despite the unparalleled times. In particular, no gap between years occurred from our analysis in the management system of BC patients in terms of temporal intervals at any step of the Oncology care pathway. This unexpectedly improved performance given during 2020 in the context of diagnostic - therapeutic pathway might be partly explained with the decline in the number of early-stage BC cases more likely amenable to surgery, thus hastening the referral of late stage BC to medical oncologists. However, our study observed a 13% drop in post-pandemic multidisciplinary BC cases discussions (60% vs 73%, p < 0.01). In this regard, Schroeder et al. have formerly investigated the challenges posed by COVID-19 on tumor board members' interactions, hampered by social distancing along with technical and organizational issues after specialists’ reallocation to COVID-19 units [37]. With the multidisciplinary decision-making process firmly laying the ground for best Oncology practice, it may not be a stretch to speculate the magnitude of this result on cancer outcome [38].
4.3. Study's strengths and limitations
We acknowledge that our work has potential limitations as a retrospective investigation. In the present study patients with recurrent disease were excluded in order to analyze an homogeneous sample of new BC diagnoses and to avoid potential biases related to the oncological management during the follow up period for patients with previous BC. This decision could be considered a potential limitation of the work, taken into account that COVID-19 might have also and equally impacted on diagnosis and treatment of BC relapses. Since the COVID-19 vaccination campaign started at the end of December 2020 in Italy (after the observation period of our study), the effect of the plan for the prevention of SARS COV-2 infections on cancer care was not an object of the present analysis. In addition, we cannot rule out a priori other potential confounders not solely attributed to the COVID-19 pandemic that may have, even partially, influenced our findings.
Nevertheless, as a multicenter study carried out from a broad national collaboration [39], it reflects the heterogeneous response to COVID-19 across different levels, including pandemic geographic distribution and local governments’ crisis management. More significantly, previous investigations were largely conducted through network comparisons at major risk of inadequacies in reporting high-quality and real-time data from electronic records during the immediate crisis response [40,41]. By reviewing 1556 medical chart records, our real-world study owns the value of avoiding this clinical-analytic gap and potential informatic reporting biases in such turbulent times. Even though a stratified analysis by age did not show any differences among the study population, future focus might interestingly unveil social and geographical disparities on provision of care for underserved and frailer patients [42].
5. Conclusions
Ultimately from our analysis we can conclude that, while COVID-19 has left its trail on cancer care, the impact of the fewer but later-stage BC diagnoses might clearly unfold in the years to come. Pandemic's challenges considered, our study offers a valuable picture of Italian Oncology Departments' performance to ensure timely diagnosis, staging and treatment for BC patients during the first pandemic year. Setting the bar of Oncology to revised standards of care, Italian oncologists managed to prevent cancer patients from paying the highest price of such disaster unpreparedness. Moreover, as the pandemic continues, the path already covered should indicate the next steps towards the end of the COVID-19 tunnel. Further studies with a forward-looking perspective, including comparisons between European countries differently affected by the pandemic, are warranted to address how COVID-19 crisis will globally resonate in tomorrow's Oncology.
Supplementary Materials: Supplementary Fig. 1: Boxplots showing temporal intervals between date of symptoms onset, radiological diagnosis, cytohistological diagnosis, first oncological appointment, treatment start, and first radiological reassessment in 2019 and 2020; Supplementary Table 1: Institutions participating in the COVID-DELAY study.
Informed consent statement
Informed consent was obtained from all subjects involved in the study. Results presented in this article contain no personally identifiable information from the study.
Prior presentation
The preliminary results of this article have been presented at the ESMO Congress in September 2021 in the form of an e-poster by first author Prof. Rossana Berardi. The e-poster has been also selected for a post-ESMO meeting webinar supported by Eli Lilly.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Comitato Etico Regionale delle Marche - C.E.R.M., (protocol code 2021 139 approved on 15th April 2021).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Rita Chiari received fees for speaker's bureau and advisory boards participation in BMS, MSD, Roche, Pfizer, AZD, Takeda, Amgen, Boheringer, Novartis. Rossana Berardi is a consultant/advisory board member for Astra Zeneca, Boehringer Ingelheim, Novartis, MSD, Otsuka, Eli-Lilly, Roche. Nicla La Verde declare the following financial interests/personal relationships which may be considered as potential competing interests: grants from EISAI; speaker bureau, travel expenses for conference from ROCHE, GENTILI; advisory role from NOVARTIS and CELGENE; advisor role, travel expenses for conference from PFIZER; advisory board from MSD. All other authors declare that there is no conflict of interest.
Acknowledgments
The Authors thank the patients, the investigators and their teams who took part in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.08.007.
Contributor Information
Giulia Mentrasti, Email: giulia.mentrasti@ospedaliriuniti.marche.it.
Luca Cantini, Email: lucacantini.med@gmail.com.
Patrizia Vici, Email: patrizia.vici@ifo.it.
Nicola D'Ostilio, Email: nicola.dostilio@gmail.com.
Nicla La Verde, Email: nicla.laverde@asst-fbf-sacco.it.
Rita Chiari, Email: rita.chiari@aulss6.veneto.it.
Vittorio Paolucci, Email: vittorio.paolucci@sanita.marche.it.
Sonia Crocetti, Email: crocetti.sonia16@gmail.com.
Chiara De Filippis, Email: chiadefi@gmail.com.
Federica Pecci, Email: peccifede91@gmail.com.
Francesca Sofia Di Lisa, Email: fs.dilisa@libero.it.
Donatella Traisci, Email: donatella.traisci@asl2abruzzo.it.
Maria Silvia Cona, Email: cona.silvia@asst-fbf-sacco.it.
Linda Nicolardi, Email: linda.nicolardi@aulss6.veneto.it.
Laura Pizzuti, Email: laura.pizzuti@ifo.gov.it.
Simona Gildetti, Email: simona.gildetti@asl2abruzzo.it.
Simone Oldani, Email: simone.oldani1@studenti.unimi.it.
Arianna Della Mora, Email: arianna.dellamora@gmail.com.
Marco Luigi Bruno Rocchi, Email: marco.rocchi@uniurb.it.
Rossana Berardi, Email: r.berardi@staff.univpm.it.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Worldometer . 2021. COVID live update: 234,240,846 cases and 4,791,252 deaths from the Coronavirus.https://www.worldometers.info/coronavirus/ accessed. [Google Scholar]
- 2.Buonomo O.C., Materazzo M., Pellicciaro M., Caspi J., Piccione E., Vanni G. Tor vergata university-hospital in the beginning of COVID-19-era: experience and recommendation for breast cancer patients. In Vivo. 2020;34(3 Suppl):1661–1665. doi: 10.21873/invivo.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covid-19 - situazione in italia. https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?id=5351&area=nuovoCoronavirus&menu=vuoto; 2021 accessed 30 September 30 2021.
- 4.Garassino M.C., Vyas M., de Vries E.G.E., Kanesvaran R., Giuliani R., Peters S., et al. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann Oncol. 2021;32(5):579–581. doi: 10.1016/J.ANNONC.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curigliano G. How to guarantee the best of care to patients with cancer during the COVID-19 epidemic: the Italian experience. Oncol. 2020;25(6):463–467. doi: 10.1634/THEONCOLOGIST.2020-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curigliano G., Banerjee S., Cervantes A., Garassino M.C., Garrido P., Girard N., et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31(10):1320–1335. doi: 10.1016/J.ANNONC.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berardi R., Torniai M., Cona M.S., Cecere F.L., Chiari R., Guarneri V., et al. Social distress among medical oncologists and other healthcare professionals during the first wave of COVID-19 pandemic in Italy. ESMO Open. 2021;6(2) doi: 10.1016/J.ESMOOP.2021.100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ESMO. Cancer . 2021. Patient management during the COVID-19 pandemic.https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic accessed. [Google Scholar]
- 9.Vanni G., Pellicciari M., Materazzo M., Bruno V., Oldani C., Pistolese C.A., et al. Lockdown of breast cancer screening for COVID-19: possible scenario. In Vivo. 2020;34(5):3047–3053. doi: 10.21873/INVIVO.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakouny Z., Paciotti M., Schmidt A.L., Lipsitz S.R., Choueiri T.K., Trinh Q.-D. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458–460. doi: 10.1001/JAMAONCOL.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global Cancer Observatory. 2021. https://gco.iarc.fr/ accessed 30 September 30 2021. [Google Scholar]
- 12.Aiom I. 2021. Numeri del cancro in Italia.https://www.aiom.it/i-numeri-del-cancro-in-italia/ accessed. [Google Scholar]
- 13.Berry D.A., Cronin K.A., Plevritis S.K., Fryback D.G., Clarke L., Zelen M., et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 14.Gheorghe A., Maringe C., Spice J., Purushotham A., Chalkidou K., Rachet B., et al. Economic impact of avoidable cancer deaths caused by diagnostic delay during the COVID-19 pandemic: a national population-based modelling study in England, UK. Eur J Cancer. 2021;152:233–242. doi: 10.1016/j.ejca.2021.04.019. https://doi.org/0.1016/J.EJCA.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oldani C., Vanni G., Buonomo O.C. COVID-19 unintended effects on breast cancer in Italy after the great lockdown. Front Public Health. 2020;8 doi: 10.3389/FPUBH.2020.601748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riccardo F., Ajelli M., Andrianou X.D., Bella A., Del Manso M., Fabiani M., et al. Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Euro Surveill. 2020;25(49) doi: 10.2807/1560-7917.ES.2020.25.49.2000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballatore Z., Bastianelli L., Merloni F., Ranallo N., Cantini L., Marcantognini G., et al. Scientia potentia est: how the Italian world of Oncology changes in the COVID-19 pandemic. JCO Glob Oncol. 2020;6:1017–1023. doi: 10.1200/GO.20.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riera R., Bagattini Â.M., Pacheco R.L., Pachito D.V., Roitberg F., Ilbawi A. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Glob Oncol. 2021;7:311–323. doi: 10.1200/GO.20.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 21.Tsamakis K., Gavriatopoulou M., Schizas D., Stravodimou A., Mougkou A., Tsiptsios D., et al. Oncology during the COVID-19 pandemic: challenges, dilemmas and the psychosocial impact on cancer patients. Oncol Lett. 2020;20(1):441–447. doi: 10.3892/OL.2020.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballatore Z., Merloni F., Ranallo N., Bastianelli L., Vitarelli F., Cantini L., et al. Cancer patient perspective in the arena of COVID‐19 pandemic. Psycho Oncol. 2022;31(1):39–45. doi: 10.1002/pon.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharpless N.E. COVID-19 and cancer. Science. 2020;368(6497):1290. doi: 10.1126/SCIENCE.ABD3377. [DOI] [PubMed] [Google Scholar]
- 24.Horn L., Garassino M. COVID-19 in patients with cancer: managing a pandemic within a pandemic. Nat Rev Clin Oncol. 2021;18(1):1–2. doi: 10.1038/s41571-020-00441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the Coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3(8) doi: 10.1001/JAMANETWORKOPEN.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M., et al. Fewer cancer diagnoses during the COVID-19 epidemic in The Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones D., Neal R.D., Duffy S.R.G., Scott S.E., Whitaker K.L., Brain K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21(6):748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yabroff K.R., Wu X.-C., Negoita S., Stevens J., Coyle L., Zhao J., et al. JNCI J Natl Cancer Inst; 2021. Association of the COVID-19 pandemic with patterns of statewide cancer services. djab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–789. doi: 10.1002/IJC.33588. [DOI] [PubMed] [Google Scholar]
- 30.Vanni G., Pellicciaro M., Materazzo M., Palombi L., Buonomo O.C. Breast cancer diagnosis in coronavirus-era: alert from Italy. Front Oncol. 2020;10:938. doi: 10.3389/FONC.2020.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osservatorio Nazionale Screening . 2021. Rapporto sulla ripartenza degli screening - settembre 2020.https://www.osservatorionazionalescreening.it/content/rapporto-sulla-ripartenza-degli-screening-settembre-2020 accessed. [Google Scholar]
- 32.London J.W., Fazio-Eynullayeva E., Palchuk M.B., Sankey P., McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maringe C., Spicer J., Morris M., Purushotham A., Nolte E., Sullivan R., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sud A., Torr B., Jones M.E., Broggio J., Scott S., Loveday C., et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papautsky E.L., Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184(1):249–254. doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brugel M., Carlier C., Essner C., Debreuve-Theresette A., Beck M.F., Merrouche Y., et al. Dramatic changes in Oncology care pathways during the COVID-19 pandemic: the French ONCOCARE-COV study. Oncol. 2021;26(2):e338–e341. doi: 10.1002/onco.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroeder B.A., Cuevas E., Graber J.J. Multidisciplinary tumor boards present technical and financial challenges in the COVID-19 era. Ann Oncol. 2021;32(7):933. doi: 10.1016/J.ANNONC.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kesson E.M., Allardice G.M., George W.D., Burns H.J.G., Morrison D.S. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344 doi: 10.1136/BMJ.E2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantini L., Mentrasti G., Russo G.L., Signorelli D., Pasello G., Rijavec E., et al. Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of lung cancer patients in Italy (COVID-DELAY study): fewer cases and higher stages from a real-world scenario. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sudat S.E.K., Robinson S.C., Mudiganti S., Mani A., Pressman A.R. Mind the clinical-analytic gap: electronic health records and COVID-19 pandemic response. J Biomed Inf. 2021;116 doi: 10.1016/j.jbi.2021.103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulos J., Zhu L., Shah A.D. Data gaps in electronic health record (EHR) systems: an audit of problem list completeness during the COVID-19 pandemic. Int J Med Inf. 2021;150 doi: 10.1016/j.ijmedinf.2021.104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battisti N.M.L., Mislang A.R., Cooper L., O'Donovan A., Audisio R.A., Cheung K.L., et al. Adapting care for older cancer patients during the COVID-19 pandemic: recommendations from the international society of geriatric Oncology (SIOG) COVID-19 working group. J Geriatr Oncol. 2020;11(8):1190–1198. doi: 10.1016/j.jgo.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.