Significance
γδ T cells are an abundant T cell population that is critical in mucosal tissue homeostasis and immunoregulation, while its detailed developmental mechanisms remain poorly understood. Previous studies have demonstrated N6-methyladenosine (m6A) plays important roles in controlling the homeostasis and differentiation of CD4+ αβ T cells. However, the roles of m6A RNA modification in the development and function of γδ T cells remain unclear. Here, we report that ALKBH5-mediated m6A RNA demethylation regulates the early development of γδ T cell progenitors by specifically fine-tuning the messenger RNA (mRNA) expression of NOTCH signaling genes.
Keywords: RNA m6A modification, ALKBH5, γδ T cell development, Jagged1/Notch2 signaling, developmental checkpoint
Abstract
γδ T cells are an abundant T cell population at the mucosa and are important in providing immune surveillance as well as maintaining tissue homeostasis. However, despite γδ T cells’ origin in the thymus, detailed mechanisms regulating γδ T cell development remain poorly understood. N6-methyladenosine (m6A) represents one of the most common posttranscriptional modifications of messenger RNA (mRNA) in mammalian cells, but whether it plays a role in γδ T cell biology is still unclear. Here, we show that depletion of the m6A demethylase ALKBH5 in lymphocytes specifically induces an expansion of γδ T cells, which confers enhanced protection against gastrointestinal Salmonella typhimurium infection. Mechanistically, loss of ALKBH5 favors the development of γδ T cell precursors by increasing the abundance of m6A RNA modification in thymocytes, which further reduces the expression of several target genes including Notch signaling components Jagged1 and Notch2. As a result, impairment of Jagged1/Notch2 signaling contributes to enhanced proliferation and differentiation of γδ T cell precursors, leading to an expanded mature γδ T cell repertoire. Taken together, our results indicate a checkpoint role of ALKBH5 and m6A modification in the regulation of γδ T cell early development.
Lymphocytes in the thymus are subdivided into two major populations based on their surface markers of αβ and γδ T cell antigen receptors (TCRs) (1). Besides innate immune biology, γδ T cells also form adaptive immune subsets and represent a link between innate and adaptive immunity (2). Similar to conventional αβ T cells, γδ T lymphocytes can produce different chemokines and various cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-4 (IL-4), IL-17, IL-21, IL-22, and so on, and differentiate into different effector profiles based on their ability to produce either IFN-γ (γδT1), IL-17 (γδT17), or both IL-4 and IFN-γ (γδNKT) (3, 4). In addition, some γδ T cells also produce particular cytokines, such as keratinocyte growth factor, connective tissue growth factor, and macrophage colony-stimulating factor, or antimicrobial peptides (5, 6). Accordingly, γδ T cells show great promise in the development of novel immunotherapies for mucocutaneous infections, autoimmune diseases, and malignancies (6–8).
It has been shown that αβ T and γδ T cells originate from a common thymic progenitor, which is a CD4/CD8 double-negative (DN) lymphocyte (9). The decision between αβ T and γδ T cells occurs at the DN stage, when precommitment selection and signal strength models can determine αβ/γδ lineage commitment (10). Different from αβ T lymphocytes, γδ T cells are abundant during the embryonic stage, and the functions of some γδ T cells are programmed during thymic development (3, 11). The development and function of γδ T cells do not only depend on the antigen recognition mode, and they have the inherent ability to produce cytokines such as IFN-γ and IL-17. It is well-identified that development of these cytokine-producing γδ T subsets is largely preprogrammed in the thymus. During the development of both the αβ T and γδ T lineages, the αβ/γδ selection is mainly dependent on Zap70/Syk–mediated signaling, which is regulated by protein tyrosine kinase p56 (Lck) (12–15). TCR-γδ signaling strength exerts a critical role in thymic acquisition of γδ T cell effector fate (16, 17). Strong TCR-γδ signaling induces a general IFN-γ–producing γδ T phenotype (17–19), while weak or no TCR-γδ signaling promotes the development of the γδT17 subset (19–22). Additionally, Notch signaling pathways play an important role in T cell lineage commitment and function (23). It is now clear that thymocyte progenitors that receive a strong Notch1 signal preferentially enter the αβ T cell lineage at the expense of γδ T cells (24); however, which stage Notch signaling start to act, and the underlying developmental checkpoints that regulate murine γδ T cell lineage commitment events in the thymus, are still unresolved.
N6-methyladenosine (m6A) is the most prevalent and abundant mammalian RNA modification. m6A can modulate almost every aspect of messenger RNA (mRNA) metabolism, and its comprehensive roles are mediated by specific RNA-binding protein complexes including “writers” (mainly METTL3 and METTL14), “erasers” (ALKBH5 and FTO), and “readers” (25, 26). m6A RNA methylation is involved in a variety of physiological and pathological processes, such as maintenance of pluripotency in embryonic stem cells (27, 28), sex determination (29), energy metabolism (30), antiviral immunity (31), DNA damage response (32), colon homeostasis (33), tumorigenesis (34, 35), and antitumor immunity (36). Our previous work demonstrated that m6A RNA methylation controls αβ T cell homeostasis and differentiation (37), sustains regulatory T cell suppressive functions (38), and regulates macrophage activation and polarization (39). However, γδ T cells as another important T cell subset are also critical in maintaining T cell homeostasis and immunoregulation, and whether m6A RNA modification governs the development and function of γδ T cells is unclear.
Lck kinase is expressed in the earliest thymic immigrants and in all T cell subsets, but not in B and natural killer (NK) cells (40, 41). Lck-Cre transgenic mice have been widely used to study the involvement of different genes in T cell development and function (42, 43). Here, using Alkbh5f/f Lck-Cre and Alkbh5f/f CD4-Cre conditional knockout (KO) mice, we identified that loss of ALKBH5 specifically expands the γδ T cell population in Lck-Cre mice, which confers enhanced immune protection against Salmonella typhimurium infection but has little impact on the αβ T cell compartment in either of the two strains. We observed that loss of ALKBH5 is a specific driver for the development of γδ T cell progenitors, and is responsible for a significant expansion of γδ T cells starting at the embryonic stage. Furthermore, we discovered that ALKBH5 deficiency-mediated m6A RNA hypermethylation induces the suppression of the Jagged1/Notch2 signaling environment in which γδ T precursors can acquire a strengthened capacity to proliferate and differentiate. This study demonstrates that m6A RNA modification plays a vital role in regulating the early development of murine γδ T cells, serving as a potential developmental checkpoint in the decision between αβ T and γδ T cells.
Results
ALKBH5 Deficiency Leads to an Expanded γδ T Cell Population and Enhanced Protection against S. typhimurium Infection.
Dynamic removal of the m6A modification on mRNA is a likely mode of regulation of T cell development but its contribution is unknown. Further, ALKBH5 is highly expressed across different subsets of T lymphocytes (SI Appendix, Fig. S1A), implying it plays important roles in regulating T cell biology. We therefore crossed Alkbh5f/f mice with Lck-Cre transgenic mice to assess the function of ALKBH5 and m6A RNA modification in developing T cell populations (SI Appendix, Fig. S1 B–D). Interestingly, loss of ALKBH5 resulted in a significant expansion of γδ T cells in both the thymus and peripheral tissues including spleen, peripheral lymph nodes (pLNs), mesenteric lymph nodes (mLNs), intestinal epithelial lymphocytes (IELs), and lamina propria lymphocytes (LPLs) of colons (Fig. 1 A and B and SI Appendix, Fig. S2). On the other hand, αβ T cells were unchanged between Alkbh5f/f wild-type (WT) and Alkbh5f/f Lck+ mice. To further confirm this observation in αβ T cells, we examined the frequencies and counts of different αβ T cell subsets in peripheral lymphoid tissues. Although we observed a slight reduction of CD8 T cells and a slightly skewed naïve–memory balance in the spleen and pLNs of Alkbh5f/f Lck+ mice (SI Appendix, Fig. S3), the total populations were comparable (SI Appendix, Fig. S4). We further crossed Alkbh5f/f mice with CD4-Cre transgenic mice to assess and exclude the effects of ALKBH5 on αβ T cells, and confirmed that ALKBH5 deletion did not affect the proportion and balance of αβ T cell subsets in Alkbh5f/f, CD4-Cre mice at steady state (SI Appendix, Fig. S5 A–C). γδ T cells have previously been categorized into three subpopulations based on CD8 expression: CD8αβ+, CD8αα+, and CD8− cells (44). Of note, loss of ALKBH5 did not alter the frequencies of these three subpopulations (SI Appendix, Fig. S6 A and B), but the numbers of all subsets were markedly increased due to expansion of total γδ T cells in Alkbh5f/f Lck+ mice (SI Appendix, Fig. S6C). These results suggest that loss of ALKBH5 selectively expands γδ T cells.
Fig. 1.
ALKBH5 deficiency leads to an expanded γδ T cell population and enhanced protection against S. typhimurium infection. (A) Representative dot plots showing the balance of αβ/γδ T cells isolated from the indicated organs of Alkbh5f/f Lck+ and Alkbh5f/f mice. (B) Statistical analysis of cell numbers in A is reported (WT, n = 7; KO, n = 6). Unpaired t test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). (C) Weight loss of Alkbh5f/f (n = 8) or Alkbh5f/f Lck+ (n = 9) mice infected with S. typhimurium. Unpaired t test was used for statistical analysis. Data represent one out of three independent experiments (mean ± SEM). (D) Survival curve for S. typhimurium–infected Alkbh5f/f (n = 12) or Alkbh5f/f Lck+ (n = 8) mice. Log-rank test was used for analysis. Data represent one out of three independent experiments. (E and F) S. typhimurium colony-forming units (CFUs) per gram of feces (E) and cecum, spleen, and liver (F) from Alkbh5f/f (n = 7) or Alkbh5f/f Lck+ (n = 7) mice, 4 d postinfection. Mann–Whitney U test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). (G) Weight loss of Alkbh5f/f TCRδ−/− (n = 11) or Alkbh5f/f TCRδ−/− Lck+ (n = 9) mice infected with S. typhimurium. Unpaired t test was used for statistical analysis. Data represent one out of three independent experiments (mean ± SEM). (H) Survival curve for S. typhimurium–infected Alkbh5f/f TCRδ−/− (n = 11) or Alkbh5f/f TCRδ−/− Lck+ (n = 9) mice. Log-rank test was used for analysis. Data represent one out of three independent experiments. (I) S. typhimurium CFU/g of feces from Alkbh5f/f TCRδ−/− (n = 11) or Alkbh5f/f TCRδ−/− Lck+ (n = 9) mice, 4 d postinfection. Mann–Whitney U test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s., not significant.
It has been documented that γδ T cells have important roles in controlling mucocutaneous infections (6, 7). We next performed an S. typhimurium model of colonic infection to determine the functional consequences of the expanded γδ T cells in Alkbh5f/f Lck+ mice. Strikingly, Alkbh5f/f Lck+ mice were better protected from infection than their WT littermates, manifested in significantly reduced weight loss and delayed fatality (Fig. 1 C and D). Moreover, Alkbh5f/f Lck+ mice had a lower bacterial burden in their feces, cecum, spleen, and liver than WT littermate mice (Fig. 1 E and F). To exclude the possibility that the protective phenotype observed in Alkbh5f/f Lck+ mice during infection might be mediated by CD4+ T lymphocytes, we performed S. typhimurium infection with Alkbh5f/f CD4-Cre mice. We found that ALKBH5 deletion in αβ T cells did not contribute better protection to Alkbh5f/f CD4-Cre than their littermate controls (SI Appendix, Fig. S5 E–G). To further confirm that the protective phenotype observed in Alkbh5f/f Lck+ mice during infection was mediated by γδ T cells rather than other lymphocyte populations, we crossed Alkbh5f/f and Alkbh5f/f Lck+ mice onto TCRδ-deficient (TCRδ−/−) mice for targeted deletion of γδ T cells. Of note, Alkbh5f/f TCRδ−/− Lck+ mice showed similar pathological outcomes compared with Alkbh5f/f TCRδ−/− mice upon S. typhimurium infection, including comparable weight loss, survival rate, and fecal bacterial burden (Fig. 1 G–I). Taken together, these results indicate that expanded γδ T cells in Alkbh5f/f Lck+ mice lead to a more efficient immune response against S. typhimurium.
Loss of ALKBH5 Has No Impact on IFN-γ and IL-17 Production, Proliferation, and Apoptosis of Mature γδ T Cells.
Similar to αβ T cells, γδ T cells can exert their immune functions by secreting proinflammatory cytokines such as IFN-γ, IL-17A, and IL-4 (3, 4, 17–19). To further address the phenotype of S. typhimurium infection as to whether it was only attributable to an increased number of γδ T cells or also to their enhanced function, we next assayed the cytokine changes secreted by γδ T cells in WT and Alkbh5f/f Lck+ mice. γδ T cell effector fate is largely dependent on TCR-γδ signaling strength in the thymus (16, 17). We confirm no big changes in the percentage of IFN-γ– or IL-17–producing γδ T cells between Alkbh5f/f and Alkbh5f/f Lck+ mice in the thymus and peripheral immune tissues (SI Appendix, Figs. S7 A and B and S8 C and D), suggesting that the quality of γδ T cells and the strength of TCR-γδ signaling are not affected by ALKBH5 deficiency. Furthermore, we also found that loss of ALKBH5 did not alter the proportion of IFN-γ–, IL-17–, or IL-4–producing αβ T cells in both Lck-Cre and CD4-Cre mice compared with their littermate control WT mice (SI Appendix, Figs. S5D and S8 A and B). Thus, we conclude that the quality of γδ T cells and the strength of TCR-γδ signaling are not affected by ALKBH5 deficiency.
To better understand the quantitative increase of γδ T cells in Alkbh5f/f Lck+ mice, we tested whether proliferation and apoptosis of mature γδ T cells had changed in those mice. Surprisingly, we did not observe enhanced proliferation or impaired apoptosis in mature γδ T cells isolated from the thymus of Alkbh5f/f Lck+ mice (SI Appendix, Fig. S7 C–F). Together, these findings imply that loss of ALKBH5 neither changes the cytokine profile nor alters proliferation or apoptosis of mature γδ T cells.
Depletion of ALKBH5 in Thymocytes Promotes the Expansion of γδ T Cell Precursors.
To further explore mechanistically how ALKBH5 deficiency facilitates the expansion of γδ T cells, we next investigated whether loss of ALKBH5 affected the proportion and number of γδ T progenitors at early developmental stages. Since αβ T cells and γδ T cells are both derived from thymic DN cells, we first evaluated these cells isolated from Alkbh5f/f Lck+ mice and WT littermates. Interestingly, Alkbh5f/f Lck+ mice had significantly more DN lymphocytes than WT mice in the thymus (Fig. 2 A–C). It is well-known that the DN population can be further subdivided into four subsets according to their expression of surface markers CD44 and CD25: CD44+CD25− (DN1) cells, CD44+CD25+ (DN2) cells, CD44–CD25+ (DN3) cells, and CD44–CD25− (DN4) cells (45). In Lck-Cre transgenic mice, the ALKBH5 deletion is initiated at the DN2 stage and completed by the DN3 stage (42, 46). In accordance with this, we observed that the frequency of DN3 cells and the numbers of DN2 and DN3 cells were markedly increased in Alkbh5f/f Lck+ mice (Fig. 2 D–F), suggesting that ALKBH5 is involved in the development of early γδ T cell precursors. To exclude the effect of Lck-Cre toxicity, we used Lck-Cre–positive littermate controls to further detect positive data in γδ T cells, and we also found markedly increased numbers and frequencies of γδ T, DN, DN2, and DN3 cells in the Alkbh5f/f Lck+ group compared with the Lck+ WT group (SI Appendix, Fig. S9).
Fig. 2.
Depletion of ALKBH5 in thymocytes promotes the expansion of γδ T cell precursors. (A) Representative dot plots showing CD4+, CD8+, double-positive (DP), and DN populations isolated from the thymus of Alkbh5f/f Lck+ and Alkbh5f/f mice. (B) Statistical analysis of frequencies for each population in A is reported (WT, n = 7; KO, n = 6). Unpaired t test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). (C) Statistical analysis of cell numbers for DN populations in A is reported (WT, n = 7; KO, n = 6). Unpaired t test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). (D) Representative dot plots showing thymic lymphocytes at different DN stages. Cells were isolated from the thymus of either Alkbh5f/f Lck+ or Alkbh5f/f mice. (E) Statistical analysis of frequencies for each DN subpopulation in D is reported (WT, n = 7; KO, n = 6). Unpaired t test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). (F) Statistical analysis of cell number for cells at DN2 and DN3 stages in D is reported (WT, n = 7; KO, n = 6). Unpaired t test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). (G) Gating strategies for flow cytometric analysis of different subsets of γδ T cell precursors in the thymus. Basically, immature TCRvγ1.1+ γδ T cells were identified as CD45+, CD4–, CD8–, TCRγδ+, and TCRvγ1.1+ cells. TCRvγ1.1+ γδ T precursor cells were further identified as CD73+, TCRvγ1.1+ γδ T cells. NKTCRvγ1.1 precursor cells were further identified as CD73+, CD24–, NK1.1+, TCRvγ1.1+ γδ T cells. Similarly, immature TCRvγ2+ γδ T cells (CD45+, CD4–, CD8–, TCRγδ+, TCRvγ2+), TCRvγ2+ γδ T precursor cells (CD73+, TCRvγ2+ γδ T cells), and NKTCRvγ2 precursor cells (CD73+, CD24–, NK1.1+, TCRvγ2+ γδ T cells) were identified. (H and I) Statistical analysis of cell numbers for immature TCRvγ1.1+ (H) and immature TCRvγ2+ (I) γδ T cells at the DN stage, as well as their precursor subsets, is reported (WT, n = 6; KO, n = 6). Unpaired t test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s., not significant.
Until now, few surface markers have been identified on developing γδ T cells, and most studies have used CD73+ TCRγδ+ DN lymphocytes as γδ T progenitors that are committed to the γδ lineage (18, 47). Human γδ T cells are usually characterized based on the features of their TCRδ variable region (Vδ), while mouse γδ T cell subsets are distinguished by the Vγ chain they bear, most of which are either Vγ1.1 or Vγ2 (48–50). Therefore, we next analyzed whether loss of ALKBH5 affects CD73-expressing TCRδ+ Vγ1.1+ and TCRδ+ Vγ2+ progenitors (Fig. 2G). We observed that ALKBH5 deletion slightly affected the frequency of TCRδ+ Vγ1.1+ and TCRδ+ Vγ2+ cells at the DN stage, as well as their progenitor subsets in thymus (SI Appendix, Fig. S10). Of note, compared with Alkbh5f/f mice, Alkbh5f/f Lck+ mice had more immature TCRδ+ Vγ1.1+ cells, TCRδ+ Vγ1.1+ precursor cells, and γδNKT Vγ1.1+ progenitors at the DN stage (Fig. 2H). Similarly, increased numbers of immature TCRδ+ Vγ2+ cells, TCRδ+ Vγ2+ precursor cells, and γδNKT Vγ2+ progenitors at the DN stage (Fig. 2I) were found in Alkbh5f/f Lck+ mice. Finally, we further determined the number of mature γδ T cell subsets in the thymus, and found highly increased counts in all these subpopulations in Alkbh5f/f Lck+ mice compared with control Alkbh5f/f mice (SI Appendix, Fig. S11), indicating that the increased number of mature γδ T cells observed above is due to the expansion of γδ T cell precursors.
Absence of ALKBH5 Promotes the Proliferation of γδ T Cell Precursors and Expands the γδ T Cell Repertoire during the Embryonic Stage.
These above findings prompted us to hypothesize that ALKBH5 deficiency might increase the proliferation rate of γδ T cell progenitors. Therefore, we labeled the cells in vivo with bromodeoxyuridine (BrdU), a thymidine analog used to identify proliferating cells. As expected, the frequency of BrdU-positive γδ T cell progenitors was significantly higher in Alkbh5f/f Lck+ mice compared with WT littermates (Fig. 3 A and B). On the other hand, no obvious changes were observed regarding the apoptotic rate of these γδ T cell progenitors (Fig. 3 C and D), suggesting that γδ T cell precursors in Alkbh5f/f Lck+ mice were expanded because of increased proliferation rather than reduced cell death.
Fig. 3.
Absence of ALKBH5 promotes the proliferation of γδ T cell precursors and expands the γδ T cell repertoire during the embryonic stage. (A) Flow cytometric analysis of thymic γδ T cell precursors after in vivo BrdU labeling from Alkbh5f/f Lck+ and Alkbh5f/f mice. (B) Frequency of BrdU+ γδ T cell precursors in A is reported (WT, n = 6; KO, n = 7). Unpaired t test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). (C) Flow cytometric analysis of thymic γδ T cell precursors undergoing apoptosis from Alkbh5f/f Lck+ and Alkbh5f/f mice. (D) Frequency of apoptotic cells (Annexin V+) in C is reported (WT, n = 6; KO, n = 8). Unpaired t test was used for statistical analysis. Each dot represents one mouse. Data represent one out of three independent experiments (mean ± SD). (E) Flow cytometric analysis of thymic γδ T cells at different developmental stages from Alkbh5f/f Lck+ and Alkbh5f/f mice. (F) Statistical analysis of the frequencies for thymic γδ T cells in E. Unpaired t test was used for statistical analysis. Each dot represents one mouse (WT, n = 5; KO, n = 7 for E13.5, E14.5, and E15.5; WT, n = 3; KO, n = 4 for E18.5). Data represent two independent experiments combined (mean ± SD). ***P < 0.001, ****P < 0.0001; n.s., not significant.
From a developmental point of view, it has been shown that murine γδ T cells begin to appear during the middle and late period of embryonic development (embryonic day ∼13.5; ∼E13.5) (17). We then analyzed the quantity of γδ T cells in the thymus of Alkbh5f/f Lck+ mice at different fetal stages. Surprisingly, the increased frequency of γδ T cells was observed in Alkbh5f/f Lck+ mice as early as day E14.5 (Fig. 3 E and F), suggesting that ALKBH5 affected the γδ T cell population during midterm fetal development. Taken together, these data indicate that loss of ALKBH5 promotes the proliferation of γδ T cell precursors and leads to an expanded γδ T cell repertoire during embryonic development.
Loss of ALKBH5 Results in an Altered Jagged1/Notch2 Signaling Pathway in γδ T Cell Precursors.
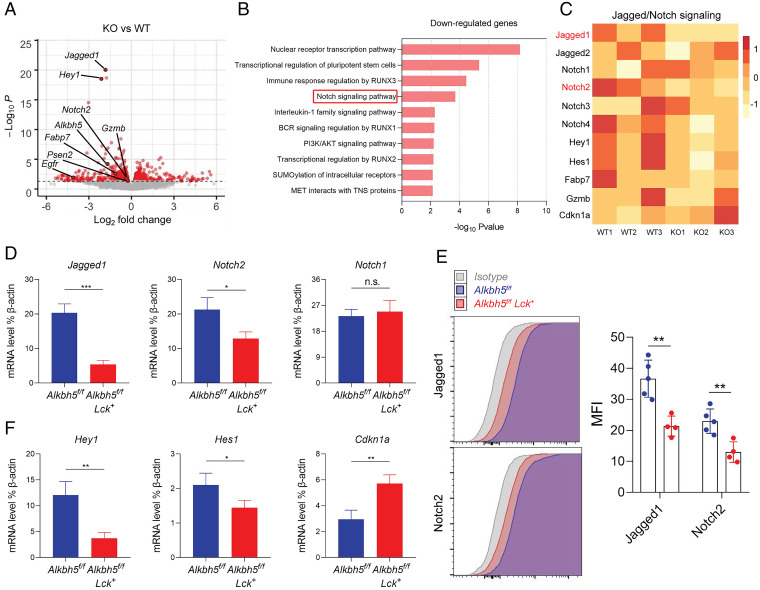
To explore the molecular mechanisms underlying the expanded γδ T cell progenitors in the absence of ALKBH5, we performed RNA sequencing (RNA-seq) on γδ T cell precursors isolated from the thymus in Alkbh5f/f and Alkbh5f/f Lck+ cohoused independent littermates. Overall, about 698 genes (P < 0.05) were differentially expressed in ALKBH5-deficient γδ T cell progenitors, including 339 down-regulated genes and 359 up-regulated genes (SI Appendix, Fig. S12A). Since m6A RNA modification is mainly involved in mRNA decay, we reasoned that removing its eraser ALKBH5 likely would promote the degradation of certain mRNA transcripts with increased m6A levels. Among those differentially expressed genes identified from Alkbh5f/f Lck+ mice, Jagged1 was the most markedly down-regulated gene (Fig. 4A). Jagged1 is a key ligand for Notch, a pathway which is both necessary and sufficient for T cell lineage commitment (23). Pathway analysis revealed that Notch signaling was one of the most substantially reduced signaling cascades in the γδ T cell progenitors isolated from Alkbh5f/f Lck+ mice (Fig. 4B). In-depth analysis further showed that Jagged/Notch signaling genes and their target genes, such as Jagged1, Notch2, Hey1, Hes1, Fabp7, and Gzmb, were indeed down-regulated, suggesting that the Jagged1/Notch2 signaling pathway was functionally suppressed in ALKBH5-deficient γδ T cell precursors (Fig. 4 A and C and SI Appendix, Table S1). In addition, pathway analysis implied that most of the genes up-regulated in the absence of ALKBH5 are involved in regulating the cell cycle (SI Appendix, Fig. S12B and Table S2), which is also consistent with our flow cytometric analysis of γδ T cell progenitors mentioned above (Fig. 3 A and B). Next, we validated by qPCR and flow analysis that the expression of Jagged1 and Notch2 was down-regulated at both mRNA and protein levels in the γδ T cell progenitors of Alkbh5f/f Lck+ mice (Fig. 4 D and E). Besides, downstream components of Notch signaling such as its effector genes Hey1 and Hes1 (23, 51) were also confirmed to be markedly decreased, while the cell-cycle factor Cdkn1a (52) was significantly increased in the absence of ALKBH5 (Fig. 4 C and F). Taken together, this evidence suggests that ALKBH5 regulates the development of γδ T cells through Jagged1/Notch2 signaling, thereby regulating the proliferation of γδ T cell precursors.
Fig. 4.
Loss of ALKBH5 results in an altered Jagged1/Notch2 signaling pathway in γδ T cell precursors. (A) Volcano plot of the differentially expressed genes between γδ T cell precursors isolated from Alkbh5f/f Lck+ mice and Alkbh5f/f mice. The down-regulated genes, such as Jagged1, Hey1, Notch2, Fabp7, Gzmb, Egfr, and Psen2, are highlighted. Data represent three independent experiments combined. (B) Gene Ontology enrichment analysis of biological processes for down-regulated genes in ALKBH5-deficient γδ T cell precursors. (C) Heatmap showing Jagged/Notch signaling-related genes across different samples. Data represent three independent experiments combined. Paired t test was used for statistical analysis (WT1/KO1, WT2/KO2, and WT3/KO3). (D) qPCR analysis of Jagged1, Notch1, and Notch2 mRNA expression in γδ T cell precursors isolated from Alkbh5f/f Lck+ (n = 3) and Alkbh5f/f (n = 3) mice. Unpaired t test was used for statistical analysis. Data represent one out of three independent experiments (mean ± SD). (E, Left) Flow cytometric analysis of Jagged1 and Notch2 protein levels in γδ T cell precursors isolated from Alkbh5f/f Lck+ (n = 4) and Alkbh5f/f (n = 5) mice. (E, Right) Statistical analysis of mean fluorescence intensity (MFI) of Jagged1 and Notch2 proteins. Unpaired t test was used for statistical analysis. Data represent one out of three independent experiments. (F) qPCR analysis of Notch signaling downstream genes Hey1, Hes1, and Cdkn1a in γδ T cell precursors isolated from Alkbh5f/f Lck+ (n = 3) and Alkbh5f/f (n = 3) mice. Unpaired t test was used for statistical analysis. Data represent one out of three independent experiments (mean ± SD). *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant.
We next sought to understand whether impaired Jagged1/Notch2 signaling in the absence of ALKBH5 occurs only in γδ T cell progenitors but not in mature γδ T cells. Therefore, we performed RNA-seq on mature γδ T cells isolated from three independent Alkbh5f/f Lck+ mice as well as WT littermates. We found many fewer up- and down-regulated genes in ALKBH5 KO mature γδ T cells compared with precursor T cells. Surprisingly, the dysregulation of Jagged1/Notch2 signaling and related gene expression observed in γδ T cell progenitor cells was absent in γδ T mature cells in Alkbh5f/f Lck+ mice (SI Appendix, Fig. S13 B and D–F and Table S3). Of note, the level of Jagged1 and Notch2 mRNAs markedly decreased in WT mature γδ T cells compared with WT γδ T progenitors (SI Appendix, Fig. S13C and Tables S1 and S3), indicating that Jagged1 expression in γδ T cells may have temporospatial characteristics. Taken together, this evidence indicates that ALKBH5 regulates the development of γδ T cells by influencing the expression of Jagged1 and Notch2 mRNAs, and through these other Notch-related genes in γδ T cell progenitors.
ALKBH5 Deficiency Enhances m6A RNA Modification on Jagged1 and Notch2 mRNAs to Decrease Their Stability and Expression.
To address mechanistically how ALKBH5 affects Jagged1 and Notch2 mRNA levels, we first confirmed by dot blot and mass spectrometry that the m6A levels in total mRNA of thymus lymphocytes increased in Alkbh5f/f Lck+ mice compared with Alkbh5f/f mice (Fig. 5 A and B). Based on our previous m6A RNA immunoprecipitation (RIP)–seq data from T cells (37, 53), we found highly enriched and specific m6A peaks on Jagged1 and Notch2 mRNAs (Fig. 5C). m6A RIP combined with qPCR revealed that Jagged1 and Notch2 m6A enrichment was markedly increased in Alkbh5f/f Lck+ mice compared with WT littermates (Fig. 5D). RNA m6A methylation is understood to mainly affect RNA stability. To further prove that increased m6A led to more rapid degradation of Jagged1 and Notch2 mRNAs, we performed RNA decay analysis and identified that Jagged1 transcription was more rapidly degraded in Alkbh5f/f Lck+ mice than in WT littermates (Fig. 5E). Although the degradation of Notch2 transcription was slightly accelerated in the absence of ALKBH5 (Fig. 5F), it could be enough to reduce the expression level of Notch2 mRNA over time compared with WT littermates. These data suggest that ALKBH5 deletion enhances m6A modification on Jagged1 and Notch2 mRNAs to decrease their stability and expression in γδ T cells.
Fig. 5.
ALKBH5 deficiency enhances m6A RNA modification on Jagged1 and Notch2 mRNAs to decrease their stability and expression. (A) Western blot of ALKBH5 protein (Top) and dot blot of m6A levels (200 or 400 ng total RNA; Bottom) in the thymocytes isolated from Alkbh5f/f Lck+ and Alkbh5f/f mice (n = 3 or 4 per group). Data represent one out of three independent experiments. (B) Liquid Chromatography/Mass Spectrometry (LC-MC) analysis of m6A RNA modification in the thymocytes isolated from Alkbh5f/f Lck+ and Alkbh5f/f mice (n = 6 or 7 per group). Unpaired t test was used for statistical analysis. Data represent one out of three independent experiments (mean ± SD). (C) m6A RIP–seq analysis of Jagged1 and Notch2 mRNA in CD4+ T lymphocytes. The primers of Jagged1 and Notch2 genes used in D targeting the transcripts are highlighted. (D) m6A RIP–qPCR analysis showing m6A enrichment of Jagged1 and Notch2 mRNAs in total thymic γδ T cells from Alkbh5f/f Lck+ and Alkbh5f/f cohoused three independent littermates. Results are presented relative to those obtained with immunoglobulin G (IgG). β-actin, m6A negative control; Myc peak, m6A positive control. Paired t test was used for statistical analysis. n = 3 to 5 mice in each group for a one-time independent experiment. Data represent three independent experiments combined (mean ± SD). (E) RNA decay analysis showing Jagged1 mRNA degradation in total thymic γδ T cells treated with actinomycin D for 2 and 4 h from Alkbh5f/f Lck+ and Alkbh5f/f cohoused three independent littermates. The residual RNAs (mRNA remaining) were normalized to 0 h. Paired t test was used for statistical analysis. n = 3 to 5 mice in each group for a one-time independent experiment. Data represent three independent experiments combined (mean ± SD). (F) RNA decay analysis showing Notch2 mRNA degradation in total thymic γδ T cells treated with actinomycin D for 2 and 4 h from Alkbh5f/f Lck+ and Alkbh5f/f cohoused three independent littermates. The residual RNAs (mRNA remaining) were normalized to 0 h. Paired t test was used for statistical analysis. n = 3 to 5 mice in each group for a one-time independent experiment. Data represent three independent experiments combined (mean ± SD). *P < 0.05, **P < 0.01; n.s., not significant.
To further confirm that defective Jagged1 signaling contributed to the observed specific γδ T cell expansion in Alkbh5f/f Lck-Cre mice, we crossed Jagged1f/f mice with Lck-Cre transgenic mice to assess the function of Jagged1 in developing T cell populations. Although Jagged1 KO in T lymphocytes did not affect the early development of T cells (SI Appendix, Fig. S14 A and B), the frequency of γδ T cells was significantly increased in the thymus and spleen from Jagged1f/f Lck+ mice compared with the control littermates (SI Appendix, Fig. S14 C and D), suggesting the inactivation of Jagged1 also contributes to the expansion of the γδ T cell pool which phenocopies Alkbh5f/f Lck-Cre mice. Collectively, loss of ALKBH5 promotes the development of γδ T cell progenitors through targeted suppression of Jagged1/Notch2 signaling.
Discussion
In this study, we discovered that ALKBH5 in thymic lymphocytes plays a critical role in the cell-fate decision between αβ T and γδ T cell lineages by targeting the Jagged1/Notch2 signaling pathway. We observed that loss of ALKBH5 results in a marked expansion of γδ T cells, with a minor effect on αβ T cells in both the thymus and periphery. It is well-demonstrated that γδ T cells are required to combat invasive bacterial infection, and may have great promise for the development of novel immunotherapies (6, 7). Surprisingly, expanded γδ T cells led to a more efficient immune response against S. typhimurium infection, while the depletion of γδ T cells eliminated this protective role in Alkbh5f/f Lck+ mice, together suggesting that loss of ALKBH5 specifically affects the expansion of γδ T cells. ALKBH5 deletion had no impact on cytokine profiles, and affected neither proliferation nor apoptosis of mature γδ T cells; it did, however, expand the pool of developing γδ T cell progenitors and enhanced their proliferative capacity during embryonic development. Previously, the Notch signaling pathway was shown to be necessary and sufficient for T cell lineage commitment (23). However, whether specific Notch receptor–ligand interactions influence the later cell-fate decision by early thymocyte progenitors to become γδ T cells in mice is still unknown (54, 55). Here, our data demonstrate that loss of ALKBH5-mediated RNA m6A levels promotes the proliferation and differentiation of γδ T precursors through targeting the Notch signaling genes, and show which specific Notch receptor–ligand interaction controls the development of murine γδ T cell progenitors in the thymus.
Our findings raise the intriguing question of how ALKBH5 affects the proportion and number of γδ T cells in the thymus. Although αβ and γδ T cells originate from a common thymic lymphocyte progenitor (9), γδ T cell precursors are believed to branch off from the αβ T cell development pathway before or at the time of TCR-α rearrangement at the DN2 stage (42). There is evidence that DN progenitors have the potential to change the timing of the divergence of αβ and γδ T cell lineages to the late DN2 to DN3 developmental stages (56). Our previous work shows that m6A writer METTL3 deletion has much greater impact and not opposite roles over T cell function compared with that of m6A eraser ALKBH5 deletion or FTO deletion (37, 57), possibly due to unknown m6A erasers and a much more complicated regulatory network of m6A function. In the present study, we observed that the frequency of DN3 cells and the number of DN2 and DN3 cells are significantly increased in the thymus in the absence of ALKBH5, suggesting that ALKBH5 deletion in thymic lymphocytes enhances the γδ T precursor pool at the DN2 to DN3 stages. Furthermore, our data also show that ALKBH5 deficiency results in a significant expansion of γδ T cell progenitors in the thymus, including TCRδ+ Vγ1.1+, TCRδ+ Vγ2+, and γδNKT subsets. However, the generation of γδ T cell subpopulations is developmentally regulated during ontogeny, such that Vγ5 cells develop during the fetal period, Vγ6 cells around birth, Vγ4 cells in the neonatal period, and Vγ1 and Vγ7 cells at the adult stage (41, 58). After their egress from the thymus, several γδ T cell subsets naturally establish residency in predetermined mucosal and epithelial locations, as exemplified by the restricted location of the murine Vγ5+ subset to the intestinal epithelium and epidermis, and loss of Vγ5+Vδ1+ cells due to a failure of thymic selection (59–61). Vγ5+Vδ1+ cells comprise 90% of mouse epidermal γδ T cells, and the intraepithelial lymphocyte network of TCRVγ5+ plays a critical role in the regulation of cutaneous inflammation (60, 61). Therefore, we do not rule out other subsets of γδ T cell precursors that may have changed during ontogeny in the absence of ALKBH5. Further studies will address which populations of other γδ T cell precursors are differentially induced by the loss of ALKBH5 during ontogeny.
Notch signaling is involved in the regulation of lymphocyte development and function (23). In mammals, this pathway is composed of four Notch receptors (Notch1 to Notch4) and five ligands: Jagged1, Jagged2, Delta1, Delta3, and Delta4. Recent evidence indicates that Notch receptor–ligand interactions have been implicated in governing cell-fate decisions during T lymphocyte differentiation (24, 54, 62, 63). By RNA-seq and m6A epigenomic analysis, we confirm that loss of ALKBH5-mediated m6A RNA modification directly suppresses Jagged1/Notch2 signaling in γδ T cell progenitors, which in turn promotes the development of immature γδ T cells. However, we readily admit that there might be other signaling pathways contributing to the observed γδ T cell progenitor defects upon ALKBH5 deletion. In humans, Jagged1 induces mainly αβ lineage differentiation, whereas Jagged2-mediated Notch3 activation primarily results in γδ T cell development and represses αβ lineage differentiation by inhibiting TCR-β formation (54). Interestingly, murine bone marrow–derived stem cells do not respond to Jagged1 signals, while Jagged1 signals can influence the differentiation of T cell progenitors along γδ T cell lineages during a brief window of their development between the DN1 and DN3 stages of thymic development (55). These results suggest the different mechanisms of γδ T cell development in mice and humans. Out of curiosity, we compared the expression of Jagged1 and Notch2 in mature and immature γδ T cells, and were surprised to find that the level of Jagged1 and Notch2 expression in mature γδ T cells is very low and significantly decreased compared with γδ T cell precursors (SI Appendix, Fig. S13C), pointing to a potential role of Jagged1/Notch2 in γδ T cell development. Recent studies have shown critical roles of RNA m6A eraser ALKBH5 in acute myeloid leukemia stem cells by specifically regulating a limited number of genes in leukemia cells (64, 65). Here, we observe that loss of ALKBH5 only markedly reduces the expression of Jagged1 and Notch2, without detectable changes in any other receptors or ligands. Furthermore, Notch signaling downstream effectors, such as Hey1 and Hes1, are significantly decreased in the absence of ALKBH5. Taken together, these data indicate that RNA m6A modification may serve as one checkpoint in the development of murine γδ T cells, by at least controlling Notch signaling strength.
Remarkably, Runx3 signaling is another top down-regulated pathway in the ALKBH5 KO group (Fig. 5B), which reportedly promotes the development of DN TCRγδ+ thymocytes (66, 67). In addition, IL-1 signaling is important in the development and activation of certain subgroups of γδ T cells, such as dermal Vγ4 and Vγ6T17 cells (68–70). Further analysis showed only Jagged1, Spp1, and Rorc genes are differentially expressed in Runx3 signaling and no significant difference in IL-1 signaling based on our RNA-seq data, indicating Jagged1/Notch2 signaling plays the dominant role in γδ T cell development in our study. Collectively, we show that the RNA m6A modification eraser ALKBH5 regulates the development of murine γδ T cell precursors in the thymus mainly through a specific interaction between Jagged1 and Notch2. Further investigation is needed to characterize the regulation of the m6A checkpoint and identify more downstream “responders” that control the development of γδ T cell precursors.
Materials and Methods
Alkbh5 conditional KO mice (Alkbh5f/f) were generated as previously described with CRISPR-Cas9 technology by insertion of two loxp sites into Alkbh5 genome loci (57). The guide RNA (gRNA) and donor oligonucleotides used in this study are listed as described previously (71).
Briefly, gRNA and single-stranded DNA Ultramer (IDT) donor oligonucleotides were synthesized and injected into C57BL/6N embryos. Alkbh5f/f mouse generation and conditionally deleted Alkbh5 in thymic lymphocytes by generating Alkbh5f/f Lck-Cre mice. We further crossed Alkbh5f/f Lck-Cre with TCRδ−/− mice to get Alkbh5 and TCRδ double-KO mice. Lck-Cre (B6.Cg strain; stock no. 003802), CD4-Cre (B6.Cg strain; stock no. 022071), TCRδ−/− (B6.129P2 strain; stock no. 002120), and Jagged1f/f (B6.129S-Jag1tm2Grid/J; stock no. 010618) mice were obtained from The Jackson Laboratory, and have been backcrossed to C57BL/6N mice (Charles River Laboratories) for more than 10 generations. With the Alkbh5f/f mouse generation, we conditionally deleted Alkbh5 in CD4+ lymphocytes to obtain Alkbh5f/f CD4-Cre mice.
We used Alkbh5f/f without Lck-Cre or Lck-Cre–positive littermates as WT controls for Alkbh5f/f Lck-Cre mice, Alkbh5f/f without CD4-Cre as WT controls for Alkbh5f/f CD4-Cre mice, and Jagged1f/f without Lck-Cre as WT controls for Jagged1f/f Lck-Cre mice. All WT and KO mice used in most of the experiments described here were sex- and age- (6- to 10-wk-old) matched littermates and were cohoused after weaning. Unless they came with special instructions, mice were randomly assigned to different experimental groups and each cage contained animals of all different experimental groups. Both male and female mice were used in experiments. All mice were kept under specific pathogen-free conditions, on a 12-h/12-h on/off light cycle, maintained at 72 (±2) °F with 70% humidity in the animal facility at Yale University. Animal procedures were approved by the Institutional Animal Care and Use Committee of Yale University. Mice were infected with S. typhimurium, and purified mature γδ T cells and γδ T progenitors were sorted at steady state and then sequenced (SI Appendix, Materials and Methods).
Supplementary Material
Acknowledgments
We thank L. Yang for support with m6A RIP–qPCR analysis. We also thank all members of the H.-B.L. laboratory and R.A.F. laboratory for technical and administrative support. This work was supported by the National Natural Science Foundation of China (82030042/32070917/82111540277 to H.-B.L.), Chongqing International Institute for Immunology (2021YJC01 to H.-B.L.), Ministry of Science and Technology of China (2021YFA1100800 to H.-B.L.), Shanghai Science and Technology Commission (20JC1417400/201409005500/20JC1410100 to H.-B.L.), Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20212501 to H.-B.L.), China Postdoctoral Science Foundation (2021M702160 to C.D.), and Howard Hughes Medical Institute (R.A.F.).
Footnotes
Reviewers: P.J.B., National Cancer Institute; and C.J., University of Pennsylvania.
Competing interest statement: R.A.F. is an advisor to GlaxoSmithKline, Zai Lab, and Ventus Therapeutics.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2203318119/-/DCSupplemental.
Data Availability
Raw FASTQ files for the RNA-seq libraries are deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (BioProject accession no. PRJNA681681 (72); https://www.ncbi.nlm.nih.gov/bioproject/PRJNA681681).
All study data are included in the article and/or SI Appendix.
References
- 1.Miller J. F., The golden anniversary of the thymus. Nat. Rev. Immunol. 11, 489–495 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Davey M. S., et al. , Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 8, 14760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonneville M., O’Brien R. L., Born W. K., Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10, 467–478 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Sutton C. E., et al. , Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Mamedov M. R., et al. , A macrophage colony-stimulating-factor-producing γδ T cell subset prevents malarial parasitemic recurrence. Immunity 48, 350–363.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y. L., et al. , γδ T cells and their potential for immunotherapy. Int. J. Biol. Sci. 10, 119–135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho J. S., et al. , IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva-Santos B., Serre K., Norell H., γδ T cells in cancer. Nat. Rev. Immunol. 15, 683–691 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Kreslavsky T., Gleimer M., von Boehmer H., Alphabeta versus gammadelta lineage choice at the first TCR-controlled checkpoint. Curr. Opin. Immunol. 22, 185–192 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennington D. J., Silva-Santos B., Hayday A. C., Gammadelta T cell development—Having the strength to get there. Curr. Opin. Immunol. 17, 108–115 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Shiromizu C. M., Jancic C. C., γδ T lymphocytes: An effector cell in autoimmunity and infection. Front. Immunol. 9, 2389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Oers N. S., Lowin-Kropf B., Finlay D., Connolly K., Weiss A., α β T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity 5, 429–436 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Negishi I., et al. , Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature 376, 435–438 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Prinz I., et al. , Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat. Immunol. 7, 995–1003 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Muro R., et al. , γδTCR recruits the Syk/PI3K axis to drive proinflammatory differentiation program. J. Clin. Invest. 128, 415–426 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muñoz-Ruiz M., et al. , TCR signal strength controls thymic differentiation of discrete proinflammatory γδ T cell subsets. Nat. Immunol. 17, 721–727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumaria N., Martin S., Pennington D. J., Developmental origins of murine γδ T-cell subsets. Immunology 156, 299–304 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buus T. B., Ødum N., Geisler C., Lauritsen J. P. H., Three distinct developmental pathways for adaptive and two IFN-γ-producing γδ T subsets in adult thymus. Nat. Commun. 8, 1911 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turchinovich G., Hayday A. C., Skint-1 identifies a common molecular mechanism for the development of interferon-γ-secreting versus interleukin-17-secreting γδ T cells. Immunity 35, 59–68 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Haas J. D., et al. , Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity 37, 48–59 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Sumaria N., Grandjean C. L., Silva-Santos B., Pennington D. J., Strong TCRγδ signaling prohibits thymic development of IL-17A-secreting γδ T cells. Cell Rep. 19, 2469–2476 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spidale N. A., et al. , Interleukin-17-producing γδ T cells originate from SOX13+ progenitors that are independent of γδTCR signaling. Immunity 49, 857–872.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radtke F., Wilson A., Mancini S. J., MacDonald H. R., Notch regulation of lymphocyte development and function. Nat. Immunol. 5, 247–253 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Washburn T., et al. , Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell 88, 833–843 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Frye M., Harada B. T., Behm M., He C., RNA modifications modulate gene expression during development. Science 361, 1346–1349 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y., Hsu P. J., Chen Y. S., Yang Y. G., Dynamic transcriptomic m6A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 28, 616–624 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geula S., et al. , Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 347, 1002–1006 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Batista P. J., et al. , m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15, 707–719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haussmann I. U., et al. , m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature 540, 301–304 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Fischer J., et al. , Inactivation of the Fto gene protects from obesity. Nature 458, 894–898 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., et al. , N6-methyladenosine RNA modification-mediated cellular metabolism rewiring inhibits viral replication. Science 365, 1171–1176 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Xiang Y., et al. , RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature 543, 573–576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T., et al. , m6A mRNA modification maintains colonic epithelial cell homeostasis via NF-κB-mediated antiapoptotic pathway. Sci. Adv. 8, eabl5723 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S., et al. , m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 31, 591–606.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z., et al. , FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine RNA demethylase. Cancer Cell 31, 127–141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han D., et al. , Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H. B., et al. , m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548, 338–342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong J., et al. , m6A mRNA methylation sustains Treg suppressive functions. Cell Res. 28, 253–256 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong J., et al. , Pooled CRISPR screening identifies m6A as a positive regulator of macrophage activation. Sci. Adv. 7, eabd4742 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu C., et al. , Progression of T cell lineage restriction in the earliest subpopulation of murine adult thymus visualized by the expression of lck proximal promoter activity. Int. Immunol. 13, 105–117 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Muro R., Takayanagi H., Nitta T., T cell receptor signaling for γδT cell development. Inflamm. Regen. 39, 6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanigaki K., et al. , Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity 20, 611–622 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Carow B., Gao Y., Coquet J., Reilly M., Rottenberg M. E., lck-driven Cre expression alters T cell development in the thymus and the frequencies and functions of peripheral T cell subsets. J. Immunol. 197, 2261–2268 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Kadivar M., Petersson J., Svensson L., Marsal J., CD8αβ+ γδ T cells: A novel T cell subset with a potential role in inflammatory bowel disease. J. Immunol. 197, 4584–4592 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Rodewald H. R., Fehling H. J., Molecular and cellular events in early thymocyte development. Adv. Immunol. 69, 1–112 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Wolfer A., et al. , Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat. Immunol. 2, 235–241 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Coffey F., et al. , The TCR ligand-inducible expression of CD73 marks γδ lineage commitment and a metastable intermediate in effector specification. J. Exp. Med. 211, 329–343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams E. J., Gu S., Luoma A. M., Human gamma delta T cells: Evolution and ligand recognition. Cell. Immunol. 296, 31–40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carding S. R., Egan P. J., Gammadelta T cells: Functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Garman R. D., Doherty P. J., Raulet D. H., Diversity, rearrangement, and expression of murine T cell gamma genes. Cell 45, 733–742 (1986). [DOI] [PubMed] [Google Scholar]
- 51.Iso T., Kedes L., Hamamori Y., HES and HERP families: Multiple effectors of the Notch signaling pathway. J. Cell. Physiol. 194, 237–255 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Rangarajan A., et al. , Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20, 3427–3436 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Y., et al. , m6A modification prevents formation of endogenous double-stranded RNAs and deleterious innate immune responses during hematopoietic development. Immunity 52, 1007–1021.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van de Walle I., et al. , Specific Notch receptor-ligand interactions control human TCR-αβ/γδ development by inducing differential Notch signal strength. J. Exp. Med. 210, 683–697 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lehar S. M., Dooley J., Farr A. G., Bevan M. J., Notch ligands Delta1 and Jagged1 transmit distinct signals to T-cell precursors. Blood 105, 1440–1447 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ciofani M., Knowles G. C., Wiest D. L., von Boehmer H., Zúñiga-Pflücker J. C., Stage-specific and differential Notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity 25, 105–116 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Zhou J., et al. , m6A demethylase ALKBH5 controls CD4+ T cell pathogenicity and promotes autoimmunity. Sci. Adv. 7, eabg0470 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vantourout P., Hayday A., Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khairallah C., Chu T. H., Sheridan B. S., Tissue adaptations of memory and tissue-resident gamma delta T cells. Front. Immunol. 9, 2636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyden L. M., et al. , Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat. Genet. 40, 656–662 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Girardi M., et al. , Resident skin-specific gammadelta T cells provide local, nonredundant regulation of cutaneous inflammation. J. Exp. Med. 195, 855–867 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hozumi K., et al. , Delta-like 4 is indispensable in thymic environment specific for T cell development. J. Exp. Med. 205, 2507–2513 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koch U., et al. , Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J. Exp. Med. 205, 2515–2523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen C., et al. , RNA demethylase ALKBH5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell Stem Cell 27, 64–80.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J., et al. , Leukemogenic chromatin alterations promote AML leukemia stem cells via a KDM4C-ALKBH5-AXL signaling axis. Cell Stem Cell 27, 81–97.e8 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Woolf E., Brenner O., Goldenberg D., Levanon D., Groner Y., Runx3 regulates dendritic epidermal T cell development. Dev. Biol. 303, 703–714 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Plužarić V., et al. , Differential skewing of circulating MR1-restricted and γδ T cells in human psoriasis vulgaris. Front. Immunol. 11, 572924 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai Y., et al. , Differential developmental requirement and peripheral regulation for dermal Vγ4 and Vγ6T17 cells in health and inflammation. Nat. Commun. 5, 3986 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee P., et al. , Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of γδT-cells. eLife 6, e28875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai Y., et al. , Differential roles of the mTOR-STAT3 signaling in dermal γδ T cell effector function in skin inflammation. Cell Rep. 27, 3034–3048.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henao-Mejia J., et al. , Generation of genetically modified mice using the CRISPR-Cas9 genome-editing system. Cold Spring Harb. Protoc. 2016, pdb.prot090704 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yale University, Exploring the role of m6A RNA modification in gd T cell development. Mus musculus (ID 681681). NCBI BioProject. https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA681681. Deposited 30 November 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw FASTQ files for the RNA-seq libraries are deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (BioProject accession no. PRJNA681681 (72); https://www.ncbi.nlm.nih.gov/bioproject/PRJNA681681).
All study data are included in the article and/or SI Appendix.