Significance
Cancer cell dissemination is the seed for metastasis and adversely linked to patients’ benefit. Critical for hematogenous dissemination is the entrance of the cancer cell into the circulation, which is regulated by vascular permeability within the primary tumor. Here, we describe pathophysiological communication between endothelial cells, tumor infiltrating neutrophils, and the complement system, with implications for vascular barrier opening and melanoma cell dissemination. Experiments in complement-deficient animals indicate that interference with complement-mediated activation of neutrophils stabilizes blood vessel integrity and abolishes the systemic spread of melanoma cells.
Keywords: membrane attack complex, neutrophil, NETs, endothelial cell, melanoma
Abstract
The microenvironment of malignant melanomas defines the properties of tumor blood vessels and regulates infiltration and vascular dissemination of immune and cancer cells, respectively. Previous research in other cancer entities suggested the complement system as an essential part of the tumor microenvironment. Here, we confirm activation of the complement system in samples of melanoma patients and murine melanomas. We identified the tumor endothelium as the starting point of the complement cascade. Generation of complement-derived C5a promoted the recruitment of neutrophils. Upon contact with the vascular endothelium, neutrophils were further activated by complement membrane attack complexes (MACs). MAC-activated neutrophils release neutrophil extracellular traps (NETs). Close to the blood vessel wall, NETs opened the endothelial barrier as indicated by an enhanced vascular leakage. This facilitated the entrance of melanoma cells into the circulation and their systemic spread. Depletion of neutrophils or lack of MAC formation in complement component 6 (C6)–deficient animals protected the vascular endothelium and prevented vascular intravasation of melanoma cells. Our data suggest that inhibition of MAC-mediated neutrophil activation is a potent strategy to abolish hematogenous dissemination in melanoma.
The complement system, a cascade of consecutive proteases, belongs to innate immunity and is thus part of the fundamental pathogen defense (1). Formation of the membrane attack complex (MAC), a multiprotein complex composed of the complement factors C5b, C6, C7, C8, and C9, is the endpoint of the complement cascade. Upstream of MAC formation, proteolytic cleavage of the complement factors C3 and C5 results in the generation of the anaphylatoxins C3a and C5a. Both cleavage products are efficient chemotactic molecules attracting immune cells such as neutrophils into the inflamed tissue (1). In addition, tissue recruitment of neutrophils is supported by the vascular deposition of C3b and iC3b, additional biologically active cleavage products of C3 (2). The complement cascade is commonly initiated by either the classical, lectin, or alternative pathway. Although all three pathways have the same terminal route leading to MAC formation, the molecular triggers of the complement cascade are diverse and comprise, e.g., immune complexes (classical pathway), carbohydrate structures (lectin pathway), or the spontaneous hydrolysis of C3 (alternative pathway). A growing body of evidence indicates that the complement cascade is also activated in tumors (3–6). Although the complement system has the potential to eliminate cancer cells and to suppress tumor progression, it may also shift the tumor microenvironment toward a tumor-supportive milieu (3, 7, 8). The actual impact of the complement system is complex and its explicit role during tumor progression depends on the tumor entity, stage, and therapy (4, 9–11).
In melanoma, the role of the complement is largely unknown (4). However, melanoma progression is strongly directed by the tumor microenvironment, suggesting a relevant contribution of the complement system. Neutrophils may bridge the tumor microenvironment and tumor-associated complement activation. C5a can attract and activate neutrophils, whereas neutrophils can further amplify complement activation through the release of factor P (12). Recent advances in melanoma therapy through the introduction of immune checkpoint inhibitors highlight the essential role of the tumor microenvironment. Although the infiltration of cytotoxic T lymphocytes for therapy response and patients’ outcome is evident, the role of neutrophils within the tumor microenvironment and their impact on melanoma progression is still controversial. Systemic depletion of neutrophils was shown to reduce the hematogenous spread of melanoma cells (13), suggesting a prometastatic role of neutrophils. Hematogenous spread and metastasis of cancer cells can be divided into distinct steps. First, cancer cells cross the endothelial barrier within the primary tumor and intravasate into the circulation (termed “intravasation”). Blood flowing cancer cells travel to distant organs where they attach to the vascular endothelium and extravasate into the adjacent tissue (termed “extravasation”). Once arrived in the target organ, the disseminated cancer cell is either eliminated by the immune system, stays dormant, or proliferates to form a metastasis. Recent investigations of neutrophil homeostasis in different organs suggest that neutrophils, patrolling at the luminal site of blood vessels, eliminate disseminating melanoma cells during extravasation (14). However, neutrophils may also support the extravasation process through the release of neutrophil extracellular traps (NETs) (15). NETs are composed of chromosomal DNA decorated with various cytotoxic proteins such as neutrophil elastase, myeloid peroxidase, and histones (16). The molecular impact of NETs is versatile and their contribution to tumor cell adhesion has previously been proposed (16–18). In melanoma, the role of NETs has remained enigmatic. Experiments in mice suggest that the exogenous induction of NETosis by granulocyte colony–stimulating factor enhance tumor growth (19). More recent data indicate that NETs can directly eliminate melanoma cells, suggesting a tumor-suppressive effect (20). Recent studies investigating the impact of NETs on breast cancer cell dissemination indicate that NET release (also termed “NETosis”) is associated with trapping and extravasation of blood circulating cancer cells (21). It was also reported that NETs are able to enhance the motility of breast cancer cells, promoting their recruitment into premetastatic livers (22). Of note, by using intravenous tumor cell injection models, most reports investigated the impact of NETs on cancer cell extravasation and the escape of circulating tumor cells from the blood stream. However, the role of neutrophils and NETs on cancer cell intravasation, that is, the entrance of cancer cells into the circulation at the site of the primary tumor, remains largely unexplored. In the present study, we investigated whether the complement system is activated in primary melanomas, involved in the activation and recruitment of neutrophils, and contribute to melanoma cell intravasation.
Results
The Complement System Is Activated in Human Melanoma Patients.
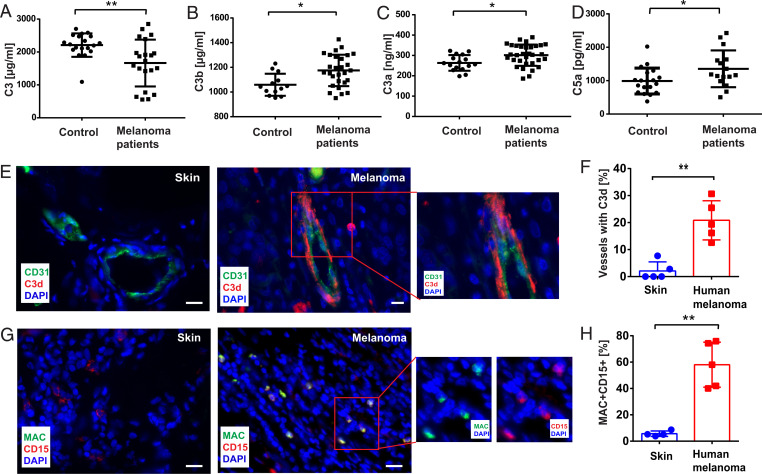
To analyze the impact of the complement system in melanoma, we investigated the plasma levels of the complement factors C3, C3a, C3b, and C5a in stage IV melanoma patients (Fig. 1 A–D). In healthy controls, the mean plasma level of C3 was 2210 ± 81.75 μg/mL (n = 19) (Fig. 1A). In cancer patients, C3 was significantly decreased to 1,665 ± 155.3 μg/mL (n = 21), suggesting the consumption of C3 due to complement activation. We analyzed plasma levels of the C3-derived fragments C3b and C3a (Fig. 1 B and C). The concentrations of both C3 cleavage products were significantly increased in plasma of cancer patients, indicating melanoma-related complement activation. C5a is the downstream product of the C3b-catalyzed cleavage of C5. To further confirm the course of the complement cascade, we measured the plasma levels of C5a (Fig. 1D). In line with the supposed activation of the complement system, C5a levels were significantly elevated in the blood of stage IV melanoma patients. As shown in SI Appendix, Fig. S1A, tumor ulceration had no impact on C5a plasma levels. In contrast to epithelial-derived cancers such as breast cancer, squamous cell, or renal cell carcinomas, melanoma cells produce only negligible amounts of complement factors (23, 24), which is also indicated by low abundance of related mRNA transcripts within tumor tissues and different melanoma cell lines (SI Appendix, Fig. S1 B and C). To understand the site of complement activation in melanoma, we evaluated the presence of C3 cleavage fragments C3d, C3b, and iC3b in cryosections of primary melanomas obtained from stage IV patients. Representative images of hematoxylin and eosin–stained tissues are shown in SI Appendix, Fig. S1D, and corresponding Breslow thicknesses are listed. None of the analyzed primary melanomas were ulcerated. Healthy skin was used as control tissue. Immunofluorescence analysis revealed that C3d/C3b/iC3b depositions were found at the endothelium of tumor blood vessels, suggesting that the complement cascade was triggered at the vascular wall (Fig. 1 E and F). In line with the results of Bulla et al. (23), we found no complement activation on the surface of melanoma cells, which can be explained by the low expression of C3 in melanoma cells. We further analyzed the presence and localization of MACs in tumor tissues, using the specific antibody AE11 (25). We found that about 58% of all tumor-related neutrophils were MAC-positive (Fig. 1 G and H). Next, we compared complement activation in melanomas of different stages (SI Appendix, Fig. S1E) and less malignant skin tumor entities (SI Appendix, Fig. S1 F and G). The complement was activated (vascular deposition of C3d) in stages IV and III melanoma patients, but not in stage II patients. Only very low levels of complement activation (vascular deposition of C3b and MAC formation on neutrophils) were detected in basal cell carcinomas (BCCs), keratoacanthoma (KA) and nevocytic nevi (NCN). Taken together, our data suggest activation of the complement system in melanoma. Moreover, elevated levels of MAC-positive neutrophils in the cancer tissue indicated a cross-talk between the humoral complement system and cell-mediated neutrophil innate immunity.
Fig. 1.
Complement activation in human melanoma patients. Systemic complement effector levels in plasma samples from control donors and stage IV melanoma patients (A–D) were analyzed by enzyme-linked immunosorbent assay (ELISA). C3 levels in blood samples of melanoma patients were decreased compared with healthy control donors (A). In contrast, C3 activation fragments C3b and anaphylatoxins C3a and C5a were increased in the plasma of melanoma patients (B–D). Immunofluorescence analysis of C3d/C3b/iC3b (E) or CD15 and MAC (G) in cryosections (Scale bars, 20 µm) of human melanoma tissues (stage IV, n = 5) and healthy skin was performed; CD31 was used as a blood vascular endothelial cell marker. Nuclei were stained with DAPI. Representative images of melanoma tissue show blood vessel–associated deposition of C3d/C3b/iC3b, whereas analysis of healthy skin indicated the absence of C3 fragments (E). Quantification revealed significantly increased numbers of vessels with C3d/C3b/iC3b deposition in tumors compared with healthy skin (F). MAC was associated with neutrophils in tumor tissue, whereas no MACs were formed on healthy skin neutrophils (G). Neutrophils with or without MAC were quantified and the number of MAC-positive neutrophils in tumor tissues was significantly increased compared with healthy skin (H). Bars indicate the mean ± SD, *P < 0.05, **P < 0.005.
Classical and Lectin Pathways of the Complement Are Activated in Melanoma.
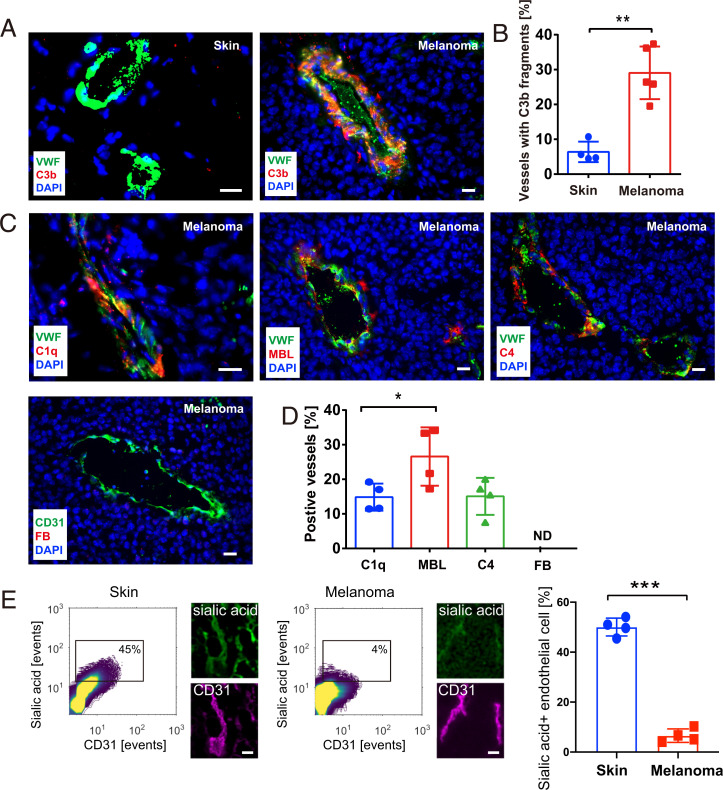
To further investigate the impact of the complement system on melanoma progression and the potential connection to neutrophil activation, we checked the levels of different complement factors by immunofluorescence microscopy of primary murine melanoma tissue sections. Tumors were generated by the intradermal injection of ret transgenic murine melanoma cells into the dorsal skin of mice. In the first week after injection, tumors grew locally and represented early-stage melanomas. Four weeks postinjection, advanced tumors had been developed. In line with previous findings (23), we detected no complement factor deposition and complement activation along the tumor blood vessels in 1-wk-old melanomas (SI Appendix, Fig. S2A). In more advanced melanomas and in agreement with our patient tissue analysis, C3 cleavage products (C3b/iC3b/C3c) were extensively deposited along the tumor blood vessel walls (∼30% vessels). Deposition of C3 fragments was virtually absent in control skin samples (Fig. 2 A and B). Moreover, we did not detect an enhanced C3b/iC3b/C3c deposition in the vasculature of kidneys, lungs, and livers (SI Appendix, Fig. S2B). Together, the data indicate local and tumor-specific complement activation. In about 25% of the tumor blood vessels, we detected depositions of mannose-binding lectin (MBL). MBL initiates the lectin pathway, suggesting lectin pathway–mediated complement activation in melanoma (Fig. 2 C and D). Significantly less abundant was the vascular deposition of C1q, a marker for the classical complement pathway. Factor B was not detectable in any of the analyzed blood vessels, suggesting no alternative pathway activation. Specificity of the applied factor B antibody was validated in sections of liver tissues (SI Appendix, Fig. S2C). Consistent with the supposed activation of the classical and especially the lectin pathway we found vascular depositions of C4. MBL recognizes glycan structures containing mannose, fucose, N-acetyl-glucosamine, or glucose. MBL binding requires the sugar residue to be at a terminal nonreducing position (26). In healthy tissue, glycans are mostly terminated by sialic acid preventing the binding of MBL, which avoids the erroneous recognition of self-tissues (27). Lack of terminal sialic acid favors MBL binding and complement activation through the lectin pathway (28). To check whether the composition of vascular glycosylation was changed by the presence of the tumor, we compared the glycosylation of skin and tumor endothelial cells. To this end, we profiled related tissues using specific lectins to detect sialic acid, fucose, mannose, N-acetyl-glucosamine, and galactose. The correlation of the spatial overlap between lectin and endothelial cell staining in skin and tumor tissues was quantified. Representative contour plots showing the correlation between sialic acid exposure and the blood vessel marker CD31 are presented in Fig. 2E. In comparison to skin endothelial cells, we detected significantly less sialic acid on the tumor-associated endothelium. Loss of sialic acid was less prominent in less advanced, 1-wk-old murine melanomas (SI Appendix, Fig. S2D). In contrast to sialic acid, the levels of fucose, mannose, galactose, and N-acetyl-glucosamine were not significantly changed (SI Appendix, Fig. S2E). Sialic acid is enzymatically removed from the terminal site of glycans by sialidases. Surveys of public transcriptome data (29, 30) revealed that increased expression of sialidase-3 (NEU3) in melanoma is significantly linked to decreased patient survival (SI Appendix, Fig. S2F). Additionally NEU3 expression was significantly higher in metastatic foci when compared to primary melanoma tissues (SI Appendix, Fig. S2G). Thus, our data suggest tumor endothelium as a platform for complement activation upstream of MAC formation.
Fig. 2.
Complement activation at the tumor endothelium. Cryosections (n = 4 to 5, 4 wk) (Scale bars, 20 µm) of primary murine melanomas were stained for VWF (green) and C3 cleavage products (C3b/iC3b/C3c, red) (A). Nuclei were stained with DAPI (blue). Representative images show that C3 cleavage products were deposited along tumor blood vessels. In healthy mouse control skin, no C3 fragments were detected, indicating a quiescent complement milieu (A). Quantitative analysis demonstrated an increased number of tumor blood vessels with C3 fragment deposition, as compared with healthy skin (B). Immunofluorescence images of early complement components C1q, MBL, C4, and FB (factor B) (C). (Scale bars, 20 µm.) Quantification indicates a significantly high abundance of MBL-positive vessels. Blood vessel wall deposition of factor B was not detectable (D). Contour plots correlating the fluorescence signal of sialic acid recognizing lectins with the fluorescence of the endothelial cell marker CD31. Lines of the contour plot are color coded. High abundance of correlating fluorescence signals are shown in yellow, low abundance in purple. Small microscopic Insets show representative fields of view. Gates within the contour plot were used for quantitative comparison of tissues shown as bar diagram (E). (Scale bars, 50 µm.) Bars indicate the mean ± SD, *P < 0.05, **P < 0.005, ***P < 0.0005, ND = not detectable.
MAC Associates with Neutrophils in Murine Tumors.
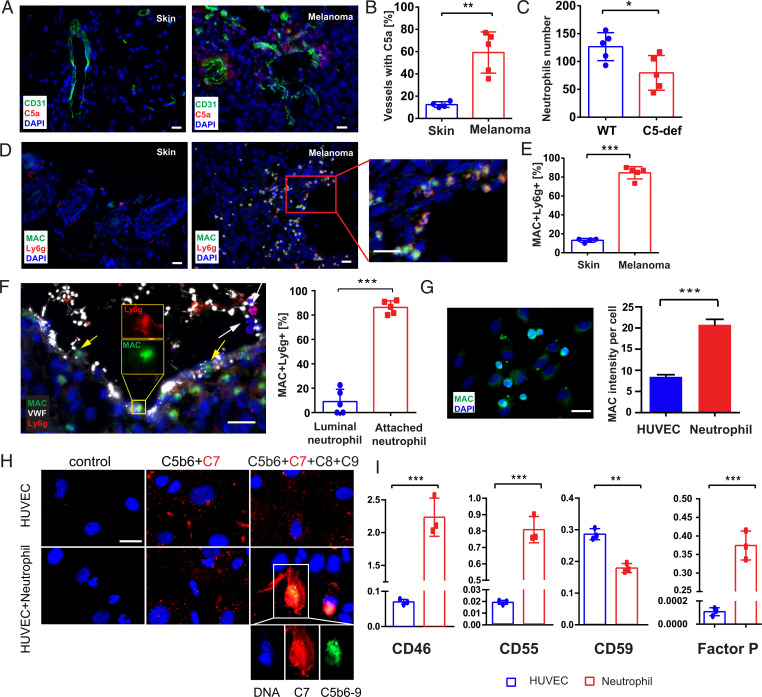
We found elevated C5a levels in the proximity of tumor blood vessels when compared to blood vessels of healthy skin (Fig. 3 A and B). C5a colocalized with C3b fragments, suggesting that C5a was generated in the course of the complement reaction (SI Appendix, Fig. S3A). In line with increased C5a levels, we also found recruitment of neutrophils to C5a-rich areas (SI Appendix, Fig. S3B). To further prove the mechanistic connection between C5a and neutrophil recruitment, we analyzed experimental melanoma growth in C5-deficient (C5-def) mice. While the growth of the primary tumor was not affected by the lack of C5 (SI Appendix, Fig. S3C), the number of tumor-associated neutrophils was significantly reduced (Fig. 3C).
Fig. 3.
Occurrence of MAC-positive neutrophils in primary melanomas. Cryosections (n = 4 to 5) (Scale bars, 20 µm) of primary tumors were stained for CD31 (green) and C5a (red) (A). Nuclei were stained with DAPI (blue). In healthy mouse control skin, no C5a was detected but a punctuate C5a staining was found in the proximity of mice melanoma blood vessels (A). Quantification revealed a significantly increased number of vessels with C5a accumulation (B). Neutrophil recruitment to tumors of C5-def mice was decreased in comparison to wild-type animals (C). Cryosections (n = 4 to 5) (Scale bars, 20 µm) of primary tumors were stained for Ly6G (red) and MAC (green). In healthy mouse control skin, no MACs were detected, whereas in the melanoma tissue MAC was associated with neutrophils (D). Quantification revealed a significantly increased number of MAC-positive neutrophils (E). Representative image of luminal MAC-negative neutrophils distant to the blood vessel wall (F, white arrow) (Scale bars, 20 µm) and MAC-positive neutrophils upon contact with the endothelium (F, yellow arrows, dotted line reflecting endothelial–luminal interface). Quantitative analysis indicated that the majority of blood vessel wall–attached neutrophils were MAC-positive (F). Representative image of neutrophils cocultured with CD31 antibody sensitized HUVECs in 10% NHS (G). MAC was detected by immunofluorescence (G). (Scale bar, 20 µm.) HUVECs and neutrophils were distinguished by the shape of their nuclei (HUVEC: oval nucleus; neutrophils: polymorphous nucleus). Quantification of fluorescence intensities revealed that neutrophils are prone to accumulate MAC (G). HUVECs were treated with fluorescently labeled C5b-C7 (MAC precursor). After removing the nonbound C5b-C7, neutrophils were added to the HUVECs. Addition of recombinant C8 and C9 completed MAC formation on neutrophils, suggesting a bystander effect (H). Gene expression of complement regulatory proteins (CD46, CD55, CD59, and factor P) in HUVECs and neutrophils suggest that neutrophils are more susceptible to MAC accumulation (I). Bars indicate the mean ± SD, *P < 0.05, **P < 0.005, ***P < 0.0005.
Because we found MAC-positive neutrophils in human melanomas, we analyzed the occurrence of MAC also in murine tissue samples. In analogy to our human tissue analysis, MAC and tumor-infiltrating neutrophils colocalized (Fig. 3 D and E). MAC was neither associated with tumor-infiltrating macrophages, dendritic cells, T cells (SI Appendix, Fig. S3D), nor with neutrophils in peripheral organs such as lung, liver, and kidney (SI Appendix, Fig. S3E). We validated our immunofluorescence-based tissue analyses by flow cytometry. In line with our microscopic results we identified MAC-positive neutrophils in tumors but not in other tissues (SI Appendix, Fig. S3F). Our findings suggest that the complement cascade upstream of MAC is initiated on the tumor endothelium, whereas the terminal MAC formation occurs on adjacent neutrophils. This suggests a close cross-talk between tumor microenvironment-primed endothelial cells and infiltrating neutrophils. Indeed, we found that neutrophils become MAC-positive at the interface of the blood vessels (Fig. 3F and SI Appendix, Fig. S3G, yellow arrows), while neutrophils without physical contact to the endothelium remained MAC-negative (Fig. 3F and SI Appendix, Fig. S3G, white arrow). This result suggests the occurrence of a process, previously referred to as bystander effect (31). The bystander effect describes the transfer of the MAC precursor C5b-7 from a complement-activating surface to a naïve, adjacent bystander cell (32). Requirement for the bystander mechanism is the close physical contact between the donor and acceptor ensuring the transfer of the lipophilic C5b-7 complex to the accepting plasma membrane (33, 34). While the bystander effect has been described in different diseases such malaria or Alzheimer’s disease (35, 36), the molecular transfer of MAC or its precursor from endothelial cells to neutrophils has not been reported. Therefore, to further investigate the cellular and molecular cross-talk between endothelial cells, the complement system and neutrophils we developed an in vitro model. Complement activation was initiated on human umbilical vein endothelial cell (HUVEC) surfaces followed by the addition of freshly isolated human neutrophils. Normal human serum (NHS) was used as a complement source. To promote the adhesion of neutrophils, we stimulated the endothelium with human melanoma cell culture supernatant (37) (SI Appendix, Fig. S3H). MAC formation was analyzed by immunofluorescence microscopy and flow cytometry. In agreement with our melanoma tissue analysis, we detected MAC not on the endothelium but exclusively in association with neutrophils (Fig. 3G and SI Appendix, Fig. S3I). In further experiments, we incubated HUVECs with purified C5b6 in complex with fluorescently labeled C7 (C7-CL555). After addition of naïve neutrophils, we detected spots of C5b-7–CL555 on HUVECs but also on neutrophils, suggesting the transfer of the MAC precursor. Further addition of purified C8 and C9 promoted the formation of MAC (C5b-9) on neutrophils but not on HUVECs (Fig. 3H).
Previously, the acceptance of MAC by the bystander cells was assumed to be facilitated by a reduced expression of complement-protecting proteins at the cellular surface (32, 38). To understand the susceptibility of neutrophils for MAC accumulation but protection of the endothelium, we analyzed the expression of the complement regulatory proteins, CD46, CD55, CD59, and properdin in HUVECs and neutrophils (Fig. 3I). CD46 serves as a cofactor for factor I–mediated proteolysis of C3b/C4b and CD55 accelerates the decay of the C3 convertase (1). CD59 prevents recruitment of C9 to the C5b-8 complex and inhibits the final step of MAC formation (1). In agreement with our assumption that neutrophils are prone to accumulate MAC, HUVECs were found to express higher levels of CD59 than neutrophils. Therefore, HUVECs appear to be able to prevent MAC deposition on their plasma membrane, whereas low levels of CD46 and CD55 suggest vulnerability to the deposition of early complement factors C3b and C3 convertase. In contrast, neutrophils showed high levels of CD46 and CD55, but comparable low levels of CD59, suggesting an insufficient protection against MAC. Moreover and in contrast to HUVECs, neutrophils expressed high levels of properdin, the only known positive regulator of the complement system. This is also in agreement with the high expression of properdin by neutrophils in murine melanomas, which we found in close proximity to the tumor blood vessel wall (SI Appendix, Fig. S3J).
MAC-Positive Neutrophils Produce Reactive Oxygen Species and Release NETs.
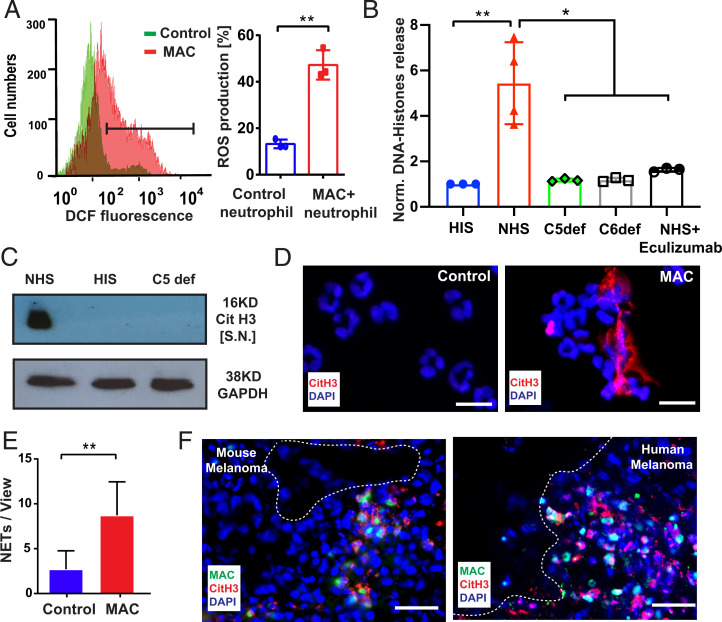
We analyzed the interplay of MAC and neutrophils in more detail. The induction of MACs on neutrophils was performed as described by B. P. Morgan (39) using CD15-directed sensitizing IgM. Neutrophils were treated with either NHS as a complement source or heat inactive serum (HIS). We confirmed MAC formation on neutrophils in the NHS group by flow cytometry and cellular accumulation of C9 by Western blot (SI Appendix, Fig. S4 A and B); HIS-treated neutrophils remained MAC negative. To prove the potential activation of neutrophils, we measured the release of reactive oxygen species (ROS). Neutrophils treated with NHS produced elevated intracellular ROS levels as indicated by the increased oxidation of the intracellular fluorescent probe 2′,7′-dichlorofluorescin diacetate (DCFHDA) (Fig. 4A and SI Appendix, Fig. S4C). We verified these results by a luminol-dependent chemiluminescence assay (SI Appendix, Fig. S4C). Neutrophils treated in the presence of HIS were unable to produce ROS, indicating that addition of the neutrophil-sensitizing antibodies in the absence of active complement factors was not sufficient for activation. C5a can induce the swelling of neutrophils but not ROS production (SI Appendix, Fig. S4 D and E). To exclude that ROS production was mainly triggered by C3a receptor (C3aR) or C5aR signaling, we used specific receptor antagonists (SI Appendix, Fig. S4F). Moreover, potential signaling via the C3b receptor CD11b/CD18 can be excluded, because ROS production was reduced to basal levels in C5- and C6-deficient sera and in the presence of the C5 neutralizing antibody eculizumab (SI Appendix, Fig. S4G). ROS formation by neutrophils is a hallmark of NETosis. We therefore analyzed whether MAC can induce the release of DNA–histone fragments. Neutrophils were treated with NHS, HIS, eculizumab/NHS, C5-, or C6-depleted serum for 1 h. DNA–histone fragments were largely increased in supernatants of NHS-treated neutrophils, but not in neutrophils incubated either in HIS, eculizumab/NHS, C5-, or C6-depleted serum (Fig. 4B). Moreover, we found that C5a stimulation did not induce release of DNA–histone fragments (SI Appendix, Fig. S4H). Addition of C3aR and C5aR antagonists was unable to block release of DNA–histone fragments, excluding essential contribution of C3aR or C5aR receptor signaling (SI Appendix, Fig. S4I). To confirm that MAC-mediated release of DNA–histone fragments is related to NETosis, we measured the citrullination of histone 3 (CitH3) as a common NET marker. A robust Western blot signal of CitH3 was detected in MAC-positive neutrophil supernatants (Fig. 4C). In contrast, no CitH3 was found in HIS and C5-depleted serum (Fig. 4C). Immunofluorescence images of NHS-treated neutrophils showed that externalized DNA structures colocalized with NET-associated CitH3. Neither DNA release nor a positive CitH3 staining was detectable in the HIS-treated control group (Fig. 4 D and E). Next, we examined the release of CitH3 from MAC-positive neutrophils in murine and human tumor tissues. Fig. 4F shows that CitH3 and MAC were formed in close proximity to each other, suggesting MAC-induced NETosis in human and murine melanomas. Of note, NETosis formation was not prevented in tumor-bearing mice treatment with the C5aR antagonist PMX-53 (SI Appendix, Fig. S4J).
Fig. 4.
MAC promotes ROS production and NET formation. Sensitized neutrophils were treated with 10% NHS for 30 min to induce MAC formation on neutrophils; 10% HIS was used as a control. Flow cytometric analysis of MAC-mediated intracellular ROS production (A). Sensitized neutrophils were treated with 10% NHS for 1 h to induce NETosis. HIS- or C5-depleted serum were used as control. ELISA was used to analyze the release of DNA–histone fragments in the supernatants. NHS-treated neutrophils released significantly more DNA–histone fragments, compared with other control groups (B). Citrullinated histone 3 (CitH3) was detected in NHS-treated neutrophil supernatant by Western blot (C). NHS-treated neutrophils and HIS-treated control neutrophils were stained with an anti-CitH3 antibody (red) and DAPI (blue). Fluorescence microscopy revealed NETs in colocalization with CitH3 of MAC-positive neutrophils. CitH3 staining was negative on control neutrophils (D). (Scale bar, 20 µm.) For quantification, NETs were counted on the whole slide and displayed as NETs per field of view (E). Representative immunofluorescence staining of MAC (green) and CitH3 (red) in the cryosections (n = 5) (Scale bars, 20 µm.) of a murine melanoma tumor and a human MM tissue showing the close association between CitH3 and MAC (F, dotted line reflecting endothelial-luminal interface). Bars indicate the mean ± SD, *P < 0.05, **P < 0.005.
Complement Inhibition Blocks Neutrophil Activation In Vitro and In Vivo.
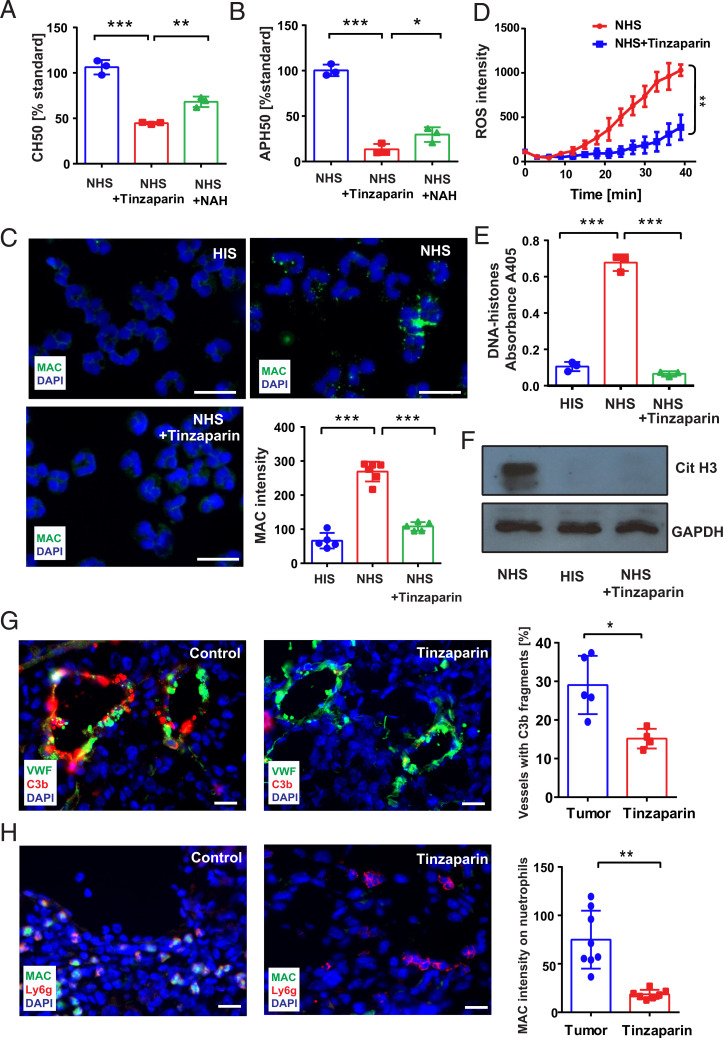
The finding of complement-mediated neutrophil activation prompted us to assess whether blockage of the complement system can affect neutrophil activation. Low-molecular-weight heparin (LMWH) has previously been reported as broad complement inhibitor (40, 41). We compared the complement inhibitory activity of the two LMWHs, tinzaparin, and N-acetylheparin (NAH). Both LMWHs blocked complement activation in CH50 and APH50 assays; however tinzaparin was significantly more efficient than NAH (Fig. 5 A and B). As expected, tinzaparin prevented MAC formation on neutrophils (Fig. 5C) and abolished NETosis as indicated by a reduced release of DNA–histone fragments and CitH3 into cell supernatants (Fig. 5 E and F). In comparison to NAH, tinzaparin was again more efficient in blocking NETosis (SI Appendix, Fig. S5 A and B) and ROS release from neutrophils (Fig. 5D). Daily intradermal administration of tinzaparin to melanoma cell–engrafted mice prevented the deposition of C3b/iC3b at tumor blood vessel walls (Fig. 5G). In agreement with our in vitro experiments, tinzaparin decreased the number of MAC-positive neutrophils in tumors (Fig. 5H) and prevented NETosis (SI Appendix, Fig. S5C).
Fig. 5.
Tinzaparin inhibits complement activation in vitro and in vivo. Hemolytic complement function of the classical pathway (CH50) and the alternative pathway (APH50) was significantly reduced by the LMWH tinzaparin and NAH. Tinzaparin was more efficient than NAH (A and B). Representative immunofluorescence images of sensitized neutrophils treated with 10% NHS or with 10% NHS supplemented with tinzaparin (100 IU/mL). The speckled MAC staining on NHS-treated neutrophils was significantly abolished by tinzaparin (C). (Scale bar, 20 µm.) Luminol chemiluminescence assay showed a reduction of MAC-induced ROS release in the presence of tinzaparin (D). Tinzaparin blocked NETosis in NHS-treated neutrophils as indicated by the significantly reduced release of DNA–histone fragments (E) and CitH3 (F). Cryosections of primary tumors were analyzed by immunofluorescence staining (n = 5). (Scale bar, 20 µm.) Tinzaparin treatment reduced the deposition of C3 fragments along the blood vessel wall compared with control tumors (G). Quantification revealed a significant reduction of C3b/iC3b deposition after tinzaparin treatment (G). MAC-positive neutrophils were present in tumors of control mice (H). In contrast, treatment with tinzaparin resulted in a decreased MAC formation on neutrophils. Quantification showed a significant reduction in colocalization of MAC and neutrophils after tinzaparin treatment (H). Bars indicate the mean ± SD, *P < 0.05, **P < 0.005, ***P < 0.0005.
Perivascular-Activated Neutrophils Contribute to Endothelial Barrier Disruption.
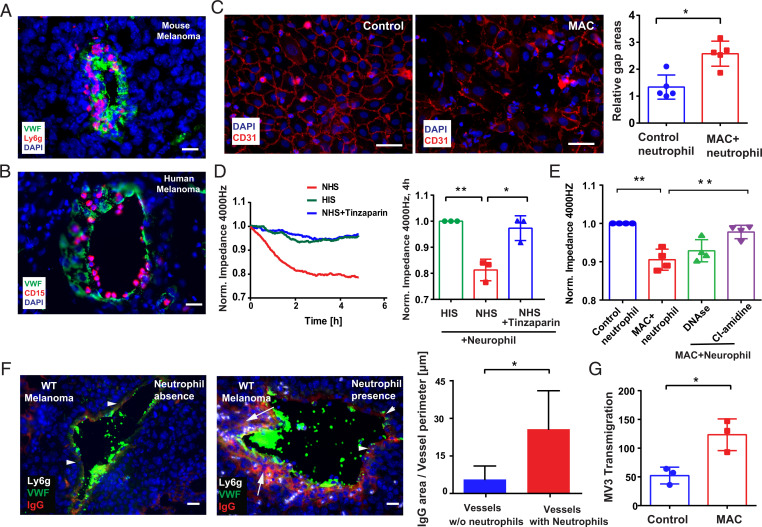
The presence of C5a around the tumor vasculature (Fig. 3 A and B) correlated with an enhanced recruitment of neutrophils to the vessel wall (Fig. 6 A and B and SI Appendix, Fig. S6). Our data further suggest that complement-activated neutrophils produce ROS and release NETs (Fig. 4). Therefore, we postulated that, in close contact to endothelial cells, MAC-activated neutrophils could increase vascular permeability. To study the neutrophil-endothelial cell cross-talk, we cocultured HUVECs with NHS-pretreated neutrophils in vitro. Cell–cell junctions were analyzed by staining of CD31, a common marker for endothelial cell layer integrity (42). As shown in Fig. 6C, MAC-positive neutrophils induced gap formation in a confluent endothelial cell monolayer. Data from electric cell-substrate impedance sensing (ECIS) further confirmed that coculture with MAC-positive neutrophils decreased the transendothelial electrical resistance (TEER) of an endothelial monolayer, indicating opening of the endothelial barrier (Fig. 6D). Inhibition of MAC formation by tinzaparin abolished TEER decrease (Fig. 6D). Also blockage of NETosis by the protein arginine deiminase 4 (PAD4) inhibitor CI-amidine prevented the decrease of TEER (Fig. 6E). Treatment with DNase counteracted the endothelial destructive effect of NETs only slightly (Fig. 6E). We also measured permeability of the endothelial barrier in our tumor tissues. For this purpose, we quantified the leakage of plasma proteins (IgG was used as marker) from the blood vessel into the adjacent tissue (Fig. 6F). In the absence of perivascular neutrophils, IgG located only along the blood vessel wall (Fig. 6F, arrowheads); however, IgG was found in the tissue beyond the blood vessels in neutrophil-rich regions (Fig. 6F, arrows). Quantitative analysis revealed significantly more IgG leakage from blood vessels upon neutrophil accumulation. Of note, IgG accumulation upon vascular leakage may also fuel further complement activation via the classical pathway. As shown in Fig. 2C, we detected classical pathway activation in about 15% of the tumoral blood vessels. To prove whether an impaired endothelial barrier may promote tumor cell intravasation, we measured the transmigration of human melanoma cells (MV3) by Transwell assays. In line with an increased vascular permeability, the number of transmigrated melanoma cells through an endothelial monolayer was almost twofold increased in the presence of MAC-positive neutrophils (Fig. 6G). MAC-positive neutrophils were washed before addition to endothelial cells to avoid contamination with complement components such as C3a or C5a that may induce vascular leakage independently of activated neutrophils. Taken together, our data indicate that perivascular MAC-positive neutrophils increase the endothelial barrier permeability, promoting tumor cell transmigration.
Fig. 6.
Activated neutrophils opened endothelial barrier. Numerous neutrophils marginated along or penetrate into the mouse and human tumor blood vessel wall (A and B, n = 5). (Scale bar, 20 µm.) Integrity of a HUVEC layer after 6 h of treatment with MAC-positive neutrophils or control neutrophils (C). (Scale bar, 50 µm.) Gap areas in the HUVEC monolayer were increased after coculture with MAC-positive neutrophils (C). Permeability of HUVEC was determined using ECIS. Coculture with MAC-activated neutrophils decreased the impedance of the endothelial monolayer, indicating increased vascular permeability (D). Supplementation of NHS with tinzaparin (100 IU/mL) counteracted the complement-induced endothelial dysfunction (D). The PAD4 inhibitor CI-amidine (50 µM) prevented the neutrophil-induced breakdown of the endothelial resistance. Treatment with DNase (100 U/mL) was less effective (E). In the absence of perivascular neutrophils, plasma protein leakage (IgG was used as marker) located only along the blood vessel wall (arrowheads, F). (Scale bar, 20 µm.) In neutrophil-rich regions, IgG leaked out into the tissue and was detected beyond the blood vessel border (arrows, F). The ratio between the IgG leakage areas and the vessel perimeter was quantified (F). Transmigration of the human melanoma cells MV3 through confluent HUVEC layers cocultured for 6 h prior to the tumor cell challenge with MAC-positive neutrophils or control neutrophils (G). Bars indicate the mean ± SD, *P < 0.05, **P < 0.005.
MAC-Activated Neutrophils Promoted Hematogenous Dissemination of Melanoma Cells.
Although our experiments with tinzaparin indicated that complement inhibition prevents complement-mediated neutrophil activation (Fig. 5) and endothelial barrier disruption (Fig. 6D), LMWHs are not complement specific but act also as inhibitors of the coagulation cascade. To underpin the explicit function of the complement system, we performed further experiments in C6-def mice. C6 is a central part of MAC and in contrast to C5/C5a it is not involved in neutrophil recruitment. Therefore, C6 deficiency prevents MAC formation, whereas recruitment of neutrophils remained unaffected (SI Appendix, Fig. S7 A and B).
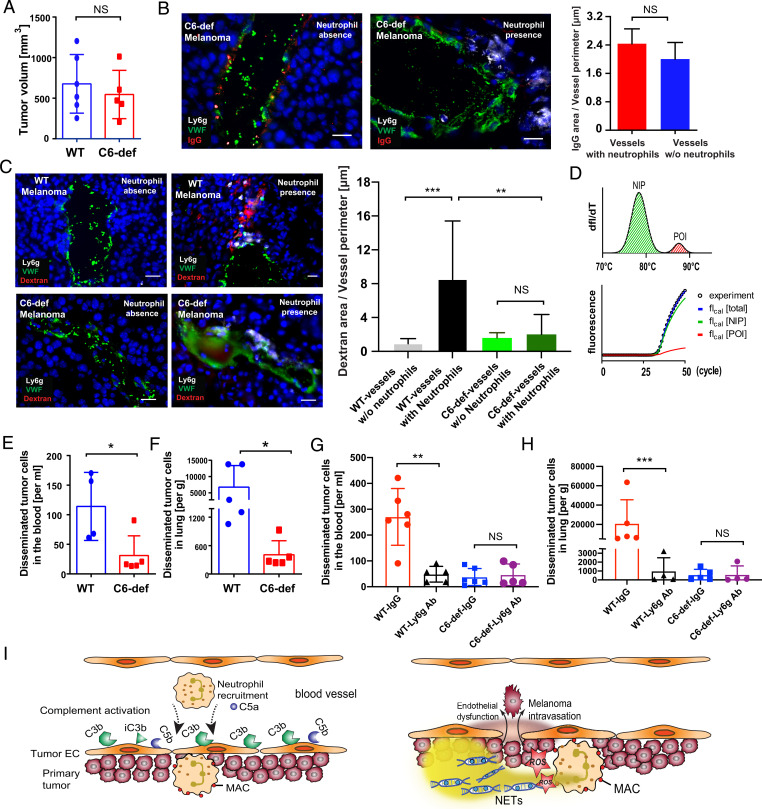
In the next set of experiments, we aimed to prove our hypothesis that MAC-mediated neutrophil activation increases vascular permeability and promotes intravasation of melanoma cells from the primary tumor into the circulation. To study the intravasation process, we inoculated melanoma cells overexpressing luciferase 2 into the dorsal skin of wild-type (WT) and C6-def mice. Four weeks after implanting the melanoma cells, we drew blood from the animals and dissected the primary tumors and the lungs. Notably, the volume of the primary tumor was not affected by lack of C6 (Fig. 7A). As expected, C6 deficiency abolished MAC formation (SI Appendix, Fig. S7A) and blood vessel permeability was not increased in neutrophil-rich regions (Fig. 7B). We obtained similar results in C5-def mice and animals treated with the NETosis blocker CI-amidine (SI Appendix, Fig. S7 C and D). To further confirm the increased vascular permeability in vascular regions rich in MAC-positive neutrophils, we measured the vascular leakage of intravenously injected tetramethylrhodamine (TRITC)-labeled dextran in WT and C6-def mice. Compared with melanomas of WT animals, we detected significantly less dextran leakage in C6-def mice (Fig. 7C). To measure the numbers of blood-circulating and organ-disseminated melanoma cells, we applied quantitative PCR (qPCR) as this is currently the most sensitive detection method to measure systemic cancer cell spread (43, 44). To this end, we isolated genomic DNA from collected blood samples and probed the luciferase 2 gene locus by a modified highly sensitive PCR approach further referred to as melting curve–assisted quantitative PCR (MC-qPCR). This technique enables the detection of fewer than 10 circulating tumor cells per milliliter of blood (Fig. 7D). Details on the accuracy and validity of our MC-qPCR method are provided in the method section. As shown in Fig. 7E, we detected low numbers (31 ± 29.9) of circulating melanoma cells per milliliter of blood in C6-def animals. In comparison, in WT animal blood, melanoma cell count was more than threefold elevated (114 ± 49.8 cells per milliliter of blood) (Fig. 7E). This indicates a significantly protected endothelium in C6-def animals and increased melanoma cell intravasation and tumor blood vessel permeability in WT mice. Next, we aimed to investigate whether the number of melanoma cells disseminated into the lungs was also reduced in mice lacking C6. Because the short lifetime of melanoma-bearing mice prevents the formation of macroscopic metastasis, we used our MC-qPCR method to detect disseminated melanoma cells within dissected lung tissues. As indicated in Fig. 7F and in line with the number of blood-circulating cells, we found significantly fewer disseminated melanoma cells in lungs of C6-def animals when compared to WT mice. In additional experiments, we depleted neutrophils in WT and C6-def animals to clarify their impact on melanoma cell intravasation and dissemination. Immunofluorescence staining of tumor tissues and flow cytometric analysis of blood samples were used to confirm the in vivo depletion of neutrophils (SI Appendix, Fig. S8). Fig. 7 G and H show that depletion of neutrophils in WT mice reduced the number of melanoma cells in the blood and the lungs of tumor-bearing animals. Neutrophil depletion in C6-def mice did not further decrease melanoma cell dissemination. In summary, our findings indicate that the complement system and especially the activation of tumor-infiltrating neutrophils by MAC, promotes the vascular leakage within the primary tumor. As a consequence, the entrance of melanoma cells into the circulation is increased and leads to enhanced systemic dissemination.
Fig. 7.
MAC-activated neutrophils promoted hematogenous intravasation of melanoma cells. WT mice (n = 5) and C6-def mice (n = 5) received an intradermal injection of 7.5 × 105 B16F10-luc2 melanoma cells. After 28 d, mice were killed. C6 deficiency did not affect the primary tumor growth (A). The permeability of endothelial barrier was determined by measuring IgG leakage. No IgG leakage was detected in blood vessels of C6-def animals (B, n = 5). (Scale bars, 20 µm.) The vascular leakage of intravenously injected TRITC-labeled dextran in WT and C6-def mice was measured by fluorescence microscopy. Compared with melanomas of WT animals, we detected significantly less dextran leakage in C6-def mice (C). Blood and lung samples were analyzed by MC-qPCR. MC-qPCR was based on the separation of the amplification curve into signals of nonintended products (NIP) and products of interest (POI) as defined by the melting curve (D). Comparison of blood samples received from WT and C6-def mice indicated significantly less blood circulating melanoma cells when C6 was deficient (E). The number of melanoma cells disseminated into the lung was reduced in C6-def mice when compared to WT mice (F). Depletion of neutrophils in WT mice reduces the number of disseminating melanoma cells in blood and lung tissues. Depletion of neutrophils was without significant effect in C6-def animals (G and H). Bars indicate the mean ± SD, *P < 0.05, **P < 0.005, ***P < 0.0005, NS = not significant. The schematic overview shows the cross-talk between the complement system, neutrophils, and endothelial cells in the tumor microenvironment (I). Complement activation at the endothelial–blood interface leads to the C5a-mediated recruitment of neutrophils. Upon contact with the endothelium, MAC is formed on neutrophils, which mediates neutrophil activation characterized by ROS release and NETosis. Complement-activated neutrophils increase the permeability of the endothelium allowing the hematogenous intravasation of melanoma cells.
Discussion
The aim of our study was to investigate the involvement of and cross-talk between the complement, neutrophils, and the tumor endothelium in melanoma. Although prior studies indicated that complement activation in the tumor microenvironment can enhance tumor growth (3, 45, 46) and that neutrophils may play an offensive role in tumor cell extravasation (47–50), our data provide first molecular insights that MAC-activated neutrophils increase endothelial permeability and melanoma cell intravasation (Fig. 7I).
A growing body of data indicates that an imbalanced complement activation in the tumor microenvironment participates in chronic inflammation and suppressive immune responses (7). Clinical data demonstrate complement activation in lung, ovarian, and colorectal cancer patients (46). However, knowledge of complement activation in human melanoma is scarce (4). Here, we show that in stage IV melanoma patients the levels of complement activation products C3b, C3a, and C5a, were elevated in blood samples. In agreement with patients’ data and previous studies (24), we found that in comparison to other tumor entities, melanoma cells express only low amounts of complement components, suggesting other complement factor sources. Further inspection of late-stage melanoma tissues indicated that complement activation was initiated at the tumor endothelium, suggesting a complement supporting milieu. That primary melanomas exhibit a microenvironment that favors complement activation was further supported by comparative analysis of distinct skin tumor types (melanoma, BCC, KA, and NCN). Our data indicate that the role of complement-mediated tumor inflammation differs in different cancer types and stages and that the tumor microenvironment as a trigger of the complement cascade also needs to be considered in future research.
Previous clinical studies analyzing colorectal (51), pancreatic ductal adenocarcinoma (6), and ovarian cancer (52) suggested the involvement of the lectin pathway. However, molecular activators of the complement cascade remain to be identified. In melanoma, our data indicate the involvement of the lectin pathway and its onset on the tumor endothelium as indicated by the vascular deposition of the MBL. Recognition of self-tissue by MBL is prevented under healthy conditions. Cellular stress or cancer-related glycosylation patterns have been linked to MBL binding and complement activation (53). Recently, lectin pathway activation was found to be trigged by endothelial cells with an aberrant glycosylation (53). To understand whether the binding of MBL to melanoma-educated endothelial cells was also related to an aberrant glycosylation, we compared the glycosylation of skin and tumor endothelial cells. We found significantly reduced levels of sialic acid on the tumor endothelium. Sialic acid is known to mask potential MBL ligands in healthy tissues (26). Therefore, lack of sialic acid appears to be a reasonable explanation for lectin pathway activation melanoma. Interestingly, we also found that melanoma patient survival was adversely connected to increased expression levels of sialidases within the tumor tissues, highlighting the biological relevance of sialic acid turnover in melanoma. However, the glycosylation of mammalian cells is very complex, comprising a large diversity of sugar residues assembled to different glycans with varying patterns and biological properties (54–56). For this reason, future research is required to fully understand complement-regulating mechanisms of cellular glycosylations.
In accordance with the notion of tumor blood vessel–restricted complement activation, we also detected high levels of C5a in close proximity to the tumor vasculature. C5a is a potent chemoattractant for proinflammatory leukocytes and recruits neutrophils to areas of inflammation (1). In addition, the work by Marks et al. (2) reported that enforced deposition of complement factors at endothelial cells is a potent and rapid stimulus for neutrophil adhesion. Thus, the enrichment of biologically active complement C3 fragments and C5a along the vasculature is a sound explanation for the observed recruitment of neutrophils around tumor blood vessels and is in line with reduced neutrophil numbers in tumors of C5-def mice. Next to their attraction, C3a and C5a can also promote neutrophil activation (57, 58). C5a can induce the swelling of neutrophils, which might represent the reorganization of the cytoskeleton necessary for chemotaxis (59). More recently, neutrophil expansion was shown to take place shortly prior to NETosis, also suggesting C5a as potential cotrigger for NETosis (60). We tested the production of ROS and the release of NETs in the presence of C3a and C5a receptor antagonists. In our experimental settings, neutrophil activation was not reduced by the receptor antagonists. Moreover we were not able to induce NETosis by recombinant C5a in the absence of a costimulatory agent. This is also in line with previous literature (61, 62) and the recent work by Fattahi et al. postulating MAC to trigger inflammasome activation and histone release from neutrophils (63).
In the present work, we were able to identify MAC as a potent trigger for NETosis. Our data suggest that neutrophils gain MAC upon direct physical contact with the tumor endothelium. This implies a close association between complement activation at the endothelium and tissue-infiltrating neutrophils. Our findings point to an accumulation of MAC on neutrophils through the direct transfer of C5b-7 complexes (precursor of MAC) from endothelial cells also known as the bystander effect. C5b-7 is not covalently bound to cell surfaces but a lipophilic and metastable complex that dynamically forms either protein micelles in the fluid phase or is incorporated into plasma membranes (33). Further complex formation of C5b-7 with C8 and C9 at lipid bilayers finally leads to its firm integration into plasma membranes. Requirement for the bystander transfer of the MAC precursor C5b-7 is the direct physical contact between the donor and acceptor. Postulated already in the last century, a growing body of evidence suggests the occurrence of bystander mechanisms in different cellular setups and diseases such as neuromyelitis optica, malaria, or Alzheimer’s disease (35, 36, 38) and was, e.g., shown to induce NLRP3 inflammasome activation in macrophages (64). Our experiments indicate the transfer of C5b-7 from endothelial cells to neutrophils shedding light on the endothelial–neutrophil cross-talk in the course of disease.
Nucleated cells have several protection mechanisms to counteract complement-mediated cellular lysis. For example, CD59 efficiently prevents MAC assembly on cellular surfaces (65). We found that in comparison to endothelial cells, neutrophils express lower CD59 levels, suggesting that MAC formation at neutrophil surfaces is more favored than on endothelial cells. Susceptibility to MAC has previously been linked to a low expression of CD59 (32, 38). However, further molecular parameters such as the lipid composition or deformation of the plasma membrane during adhesion and vascular transmigration, high local concentrations of C5b-7 or other costimulatory molecules may contribute to MAC binding by neutrophils. Once formed at their surface, MAC can either form a pore within the neutrophil membrane or is endocytosed to promote neutrophil activation (66). Among several different pathways, MAC permits Ca2+ influx and interacts with other signaling molecules involving G protein–coupled receptors and NF-κB (67–69). MAC was also shown to alter cell proliferation, to induce or inhibit apoptosis and activate inflammasomes (67–69). Here we report MAC-mediated neutrophil activation in tumors. Moreover, we identified MAC accumulation as a pathophysiological trigger for NETs in the tumor microenvironment.
Our data show that NETting neutrophils remained in close proximity to the tumor endothelium to open the endothelial barrier. NETs expose histones and proteases such as matrix metalloprotease-9 or elastase known to destabilize the vascular endothelium (70–72). To confirm that the increased vascular permeability promoted the intravasation of melanoma cells into the blood circulation, we conducted experiments in C6-def mice (73). In agreement with our hypothesis, we found in comparison to WT mice significantly less blood circulating and lung-disseminated melanoma cells in C6-def animals.
Recent research (55, 74, 75) and ongoing clinical trials (NCT03665129) indicate that complement inhibition especially in combination with immune checkpoint therapy may prolong the life of cancer patients. However, even more recent evaluations of large sets of patient data revealed that under immune checkpoint therapy the risk of suffering from severe thrombotic events is considerably increased (76, 77). The coagulation and the complement systems are evolutionary close relatives with several molecular overlaps. For instance, thrombin, a key protease of the coagulation system, is able to convert C5 into C5a and C5b in parallel with the complement-related C5 convertase (78). Therefore, simultaneous attenuation of the complement system and the coagulation cascade appears to be a reasonable therapeutic approach in cancer patients. In the present work, we showed that the anticoagulant tinzaparin was a potent inhibitor of MAC-mediated neutrophil activation. Therefore, coadministration of immune checkpoint inhibitors and LMWHs to block cancer-associated coagulation and complement activation may improve current cancer therapies. In future, the therapeutic application of C6- or C9-neutralizing antibodies or peptides that inhibit MAC assembly might represent a more specific possibility to prevent MAC-mediated neutrophil activation in melanoma.
Taken together, we provide a mechanism of the cross-talk between complement, neutrophils, and the tumor endothelium in melanoma. The results reported here extend the role of the complement system as a component of tumor-promoting inflammation and indicate that MAC-activated neutrophils in close proximity to the vasculature are associated with an increased endothelial permeability and melanoma cell intravasation.
Materials and Methods
Please see SI Appendix, Materials and Methods for a detailed description of materials and methods.
Supplementary Material
Acknowledgments
We thank Anita Ignatius (Universitätsklinikum Ulm) and John D. Lambris (University of Pennsylvania) for providing C6-def mice. Portions of the paper were developed from the doctoral thesis of X.L. This study was supported by the Heike und Wolfgang Mühlbauer Stiftung, the European Union’s Seventh Framework Program for Research, Technological Development and Demonstration under grant agreement 613931, and the Deutsche Forschungsgemeinschaft within the RTG 2099, the IRTG 1549, SFB/Transregio 23, and SHENC-Unit FOR 1543. The C5-def mice experiment was supported by a grant to W.H. from the National Natural Science Foundation of China (81572827). The PhD position of X.L. and Y.W. was supported by the program of China Scholarship Council. The PhD position of Y.W. was supported by the Hamburg Pro Exzellenzia Scholarship program.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2122716119/-/DCSupplemental.
Data, Materials, and Software Availability
The applied codes for lectin staining analysis and the applied gating strategies are freely available (https://github.com/gorzelac/tissue-lectin.git) (79). All other study data are included in the article and/or SI Appendix.
References
- 1.Merle N. S., Church S. E., Fremeaux-Bacchi V., Roumenina L. T., Complement system. Part I—Molecular mechanisms of activation and regulation. Front. Immunol. 6, 262 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks R. M., R. F. Todd, 3rd, Ward P. A., Rapid induction of neutrophil-endothelial adhesion by endothelial complement fixation. Nature 339, 314–317 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Reis E. S., Mastellos D. C., Ricklin D., Mantovani A., Lambris J. D., Complement in cancer: Untangling an intricate relationship. Nat. Rev. Immunol. 18, 5–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roumenina L. T., Daugan M. V., Petitprez F., Sautès-Fridman C., Fridman W. H., Context-dependent roles of complement in cancer. Nat. Rev. Cancer 19, 698–715 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Medler T. R., et al. , Complement C5a fosters squamous carcinogenesis and limits T cell response to chemotherapy. Cancer Cell 34, 561–578.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aykut B., et al. , The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markiewski M. M., et al. , Modulation of the antitumor immune response by complement. Nat. Immunol. 9, 1225–1235 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwak J. W., et al. , Complement activation via a C3a receptor pathway alters CD4+ T lymphocytes and mediates lung cancer progression. Cancer Res. 78, 143–156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daugan M. V., et al. , Intracellular factor H drives tumor progression independently of the complement cascade. Cancer Immunol. Res. 9, 909–925 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Daugan M. V., et al. , Complement C1s and C4d as prognostic biomarkers in renal cancer: Emergence of noncanonical functions of C1s. Cancer Immunol. Res. 9, 891–908 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Fishelson Z., Kirschfink M., Complement C5b-9 and cancer: Mechanisms of cell damage, cancer counteractions, and approaches for intervention. Front. Immunol. 10, 752 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camous L., et al. , Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood 117, 1340–1349 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Bald T., Landsberg J., Jansen P., Gaffal E., Tüting T., Phorbol ester-induced neutrophilic inflammatory responses selectively promote metastatic spread of melanoma in a TLR4-dependent manner. OncoImmunology 5, e1078964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casanova-Acebes M., et al. , Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 215, 2778–2795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Z., et al. , Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat. Commun. 12, 6202 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demers M., et al. , Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. U.S.A. 109, 13076–13081 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demers M., Wagner D. D., NETosis: A new factor in tumor progression and cancer-associated thrombosis. Semin. Thromb. Hemost. 40, 277–283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinod K., et al. , Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl. Acad. Sci. U.S.A. 110, 8674–8679 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demers M., et al. , Priming of neutrophils toward NETosis promotes tumor growth. OncoImmunology 5, e1134073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schedel F., et al. , Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigment Cell Melanoma Res. 33, 63–73 (2020). [DOI] [PubMed] [Google Scholar]
- 21.McDowell S. A. C., et al. , Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat. Can. 2, 545–562 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Yang L., et al. , DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583, 133–138 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Bulla R., et al. , C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation. Nat. Commun. 7, 10346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roumenina L. T., et al. , Tumor cells hijack macrophage-produced complement C1q to promote tumor growth. Cancer Immunol. Res. 7, 1091–1105 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Mollnes T. E., Lea T., Harboe M., Tschopp J., Monoclonal antibodies recognizing a neoantigen of poly(C9) detect the human terminal complement complex in tissue and plasma. Scand. J. Immunol. 22, 183–195 (1985). [DOI] [PubMed] [Google Scholar]
- 26.Holmskov U., Thiel S., Jensenius J. C., Collections and ficolins: Humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21, 547–578 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Ip W. K., Takahashi K., Ezekowitz R. A., Stuart L. M., Mannose-binding lectin and innate immunity. Immunol. Rev. 230, 9–21 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki N., et al. , Highly fucosylated N-glycan ligands for mannan-binding protein expressed specifically on CD26 (DPPVI) isolated from a human colorectal carcinoma cell line, SW1116. Glycobiology 19, 437–450 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Cerami E., et al. , The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J., et al. , Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lachmann P. J., Thompson R. A., Reactive lysis: The complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J. Exp. Med. 131, 643–657 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan T., Smith A. J., Verkman A. S., Complement-dependent bystander injury to neurons in AQP4-IgG seropositive neuromyelitis optica. J. Neuroinflammation 15, 294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preissner K. T., Podack E. R., Müller-Eberhard H. J., The membrane attack complex of complement: Relation of C7 to the metastable membrane binding site of the intermediate complex C5b-7. J. Immunol. (Baltimore, MD: 1950) 135, 445–451 (1985). [PubMed] [Google Scholar]
- 34.Doorduijn D. J., et al. , Bacterial killing by complement requires direct anchoring of membrane attack complex precursor C5b-7. PLoS Pathog. 16, e1008606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee M., Guo J. P., Schwab C., McGeer E. G., McGeer P. L., Selective inhibition of the membrane attack complex of complement by low molecular weight components of the aurin tricarboxylic acid synthetic complex. Neurobiol. Aging 33, 2237–2246 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Dasari P., et al. , Malarial anemia: Digestive vacuole of Plasmodium falciparum mediates complement deposition on bystander cells to provoke hemophagocytosis. Med. Microbiol. Immunol. (Berl.) 203, 383–393 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Strozyk E. A., et al. , Melanoma-derived IL-1 converts vascular endothelium to a proinflammatory and procoagulatory phenotype via NFκB activation. Exp. Dermatol. 23, 670–676 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Tradtrantip L., Yao X., Su T., Smith A. J., Verkman A. S., Bystander mechanism for complement-initiated early oligodendrocyte injury in neuromyelitis optica. Acta Neuropathol. 134, 35–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan B. P., Non-lethal complement-membrane attack on human neutrophils: Transient cell swelling and metabolic depletion. Immunology 63, 71–77 (1988). [PMC free article] [PubMed] [Google Scholar]
- 40.Zaferani A., et al. , Heparin/heparan sulphate interactions with complement—A possible target for reduction of renal function loss? Nephrol. Dial. Transplant. 29, 515–522 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Girardi G., Redecha P., Salmon J. E., Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat. Med. 10, 1222–1226 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Privratsky J. R., Newman P. J., PECAM-1: Regulator of endothelial junctional integrity. Cell Tissue Res. 355, 607–619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eyles J., et al. , Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 120, 2030–2039 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arenberger P., Arenbergerova M., Vohradnikova O., Kremen J., Early detection of melanoma progression by quantitative real-time RT-PCR analysis for multiple melanoma markers. Keio J. Med. 57, 57–64 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Cho M. S., et al. , Autocrine effects of tumor-derived complement. Cell Rep. 6, 1085–1095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pio R., Ajona D., Lambris J. D., Complement inhibition in cancer therapy. Semin. Immunol. 25, 54–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coffelt S. B., Wellenstein M. D., de Visser K. E., Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 16, 431–446 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Chen M. B., et al. , Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc. Natl. Acad. Sci. U.S.A. 115, 7022–7027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Rayes T., et al. , Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl. Acad. Sci. U.S.A. 112, 16000–16005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang W., Li Q., Ferrara N., Metastatic growth instructed by neutrophil-derived transferrin. Proc. Natl. Acad. Sci. U.S.A. 115, 11060–11065 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ytting H., Christensen I. J., Jensenius J. C., Thiel S., Nielsen H. J., Preoperative mannan-binding lectin pathway and prognosis in colorectal cancer. Cancer Immunol. Immunother. 54, 265–272 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swierzko A. S., et al. , Mannose-Binding Lectin (MBL) and MBL-associated serine protease-2 (MASP-2) in women with malignant and benign ovarian tumours. Cancer Immunol. Immunother. 63, 1129–1140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talsma D. T., et al. , Endothelial heparan sulfate deficiency reduces inflammation and fibrosis in murine diabetic nephropathy. Lab. Invest. 98, 427–438 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalagara T., et al. , The endothelial glycocalyx anchors von Willebrand factor fibers to the vascular endothelium. Blood Adv. 2, 2347–2357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magrini E., et al. , Complement activation promoted by the lectin pathway mediates C3aR-dependent sarcoma progression and immunosuppression. Nat. Can. 2, 218–232 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jedlicka J., Becker B. F., Chappell D., Endothelial glycocalyx. Crit. Care Clin. 36, 217–232 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Fattahi F., et al. , Organ distribution of histones after intravenous infusion of FITC histones or after sepsis. Immunol. Res. 61, 177–186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X., et al. , Reduced neutrophil extracellular trap formation during ischemia reperfusion injury in C3 KO mice: C3 requirement for NETs release. Front. Immunol. 13, 781273 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denk S., et al. , Complement C5a-induced changes in neutrophil morphology during inflammation. Scand. J. Immunol. 86, 143–155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neubert E., et al. , Chromatin swelling drives neutrophil extracellular trap release. Nat. Commun. 9, 3767 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schreiber A., et al. , C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 20, 289–298 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khameneh H. J., et al. , C5a regulates IL-1β production and leukocyte recruitment in a murine model of monosodium urate crystal-induced peritonitis. Front. Pharmacol. 8, 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fattahi F., et al. , Requirement of complement C6 for intact innate immune responses in mice. J. Immunol. (Baltimore, MD: 1950) 205, 251–260 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Suresh R., Chandrasekaran P., Sutterwala F. S., Mosser D. M., Complement-mediated ‘bystander’ damage initiates host NLRP3 inflammasome activation. J. Cell Sci. 129, 1928–1939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brodsky R. A., Paroxysmal nocturnal hemoglobinuria. Blood 124, 2804–2811 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morgan B. P., Dankert J. R., Esser A. F., Recovery of human neutrophils from complement attack: Removal of the membrane attack complex by endocytosis and exocytosis. J. Immunol. (Baltimore, MD: 1950) 138, 246–253 (1987). [PubMed] [Google Scholar]
- 67.Morgan B. P., The membrane attack complex as an inflammatory trigger. Immunobiology 221, 747–751 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Cole D. S., Morgan B. P., Beyond lysis: How complement influences cell fate. Clin. Sci. (London, England: 1979) 104, 455–466 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Jane-wit D., et al. , Complement membrane attack complexes activate noncanonical NF-κB by forming an Akt+ NIK+ signalosome on Rab5+ endosomes. Proc. Natl. Acad. Sci. U.S.A. 112, 9686–9691 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pieterse E., et al. , Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler. Thromb. Vasc. Biol. 37, 1371–1379 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Saffarzadeh M., et al. , Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS One 7, e32366 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carmona-Rivera C., Zhao W., Yalavarthi S., Kaplan M. J., Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 74, 1417–1424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mödinger Y., et al. , Reduced terminal complement complex formation in mice manifests in low bone mass and impaired fracture healing. Am. J. Pathol. 189, 147–161 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Zha H., et al. , Intracellular activation of complement C3 leads to PD-L1 antibody treatment resistance by modulating tumor-associated macrophages. Cancer Immunol. Res. 7, 193–207 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Ajona D., et al. , A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. 7, 694–703 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Moik F., et al. , Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood 137, 1669–1678 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bauer A. T., Gorzelanny C., Gebhardt C., Pantel K., Schneider S. W., Interplay between coagulation and inflammation in cancer: Limitations and therapeutic opportunities. Cancer Treat. Rev. 102, 102322 (2022). [DOI] [PubMed] [Google Scholar]
- 78.Krisinger M. J., et al. , Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood 120, 1717–1725 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Gorzelanny C., Tissue-lectin. GitHub. https://github.com/gorzelac/tissue-lectin. Deposited 22 April 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The applied codes for lectin staining analysis and the applied gating strategies are freely available (https://github.com/gorzelac/tissue-lectin.git) (79). All other study data are included in the article and/or SI Appendix.