Significance
Dopamine receptors in the brain are important targets for the therapeutic treatment of psychiatric disorders. Dopamine receptors are generally thought to be activated by dopamine. In the present study, however, we reveal that noradrenaline can also robustly activate dopamine D1 receptors in the mouse hippocampus. Noradrenaline-activated D1 receptor signaling was highly sensitive to the neuronal activity and experience of mice. Chronic stress and voluntary exercise synergistically augmented noradrenaline–D1 receptor signaling. This augmented noradrenaline–D1 receptor signaling promoted the induction of hippocampal neuronal plasticity by an antidepressant drug acting on the noradrenergic system. Our results suggest that noradrenaline–D1 receptor signaling increases the efficacy of antidepressant treatment and therefore can be a unique therapeutic target for augmenting antidepressant medication.
Keywords: monoamine, dentate gyrus, mossy fiber, stress, exercise
Abstract
Dopamine D1 receptors (D1Rs) in the hippocampal dentate gyrus (DG) are essential for antidepressant effects. However, the midbrain dopaminergic neurons, the major source of dopamine in the brain, only sparsely project to DG, suggesting possible activation of DG D1Rs by endogenous substances other than dopamine. We have examined this possibility using electrophysiological and biochemical techniques and found robust activation of D1Rs in mouse DG neurons by noradrenaline. Noradrenaline at the micromolar range potentiated synaptic transmission at the DG output and increased the phosphorylation of protein kinase A substrates in DG via activation of D1Rs and β adrenergic receptors. Neuronal excitation preferentially enhanced noradrenaline-induced synaptic potentiation mediated by D1Rs with minor effects on β-receptor–dependent potentiation. Increased voluntary exercise by wheel running also enhanced noradrenaline-induced, D1R-mediated synaptic potentiation, suggesting a distinct functional role of the noradrenaline–D1R signaling. We then examined the role of this signaling in antidepressant effects using mice exposed to chronic restraint stress. In the stressed mice, an antidepressant acting on the noradrenergic system induced a mature-to-immature change in the DG neuron phenotype, a previously proposed cellular substrate for antidepressant action. This effect was evident only in mice subjected to wheel running and blocked by a D1R antagonist. These results suggest a critical role of noradrenaline-induced activation of D1Rs in antidepressant effects in DG. Experience-dependent regulation of noradrenaline–D1R signaling may determine responsiveness to antidepressant drugs in depressive disorders.
Hippocampal dopamine D1 receptors (D1Rs) have been implicated in regulation of learning and memory (1, 2). Accumulating evidence also suggests a critical involvement of hippocampal D1Rs, especially those expressed in the dentate gyrus (DG) neurons, in antidepressant action (3–7). D1R activation induces robust synaptic potentiation at the synapse formed by the mossy fiber (MF) axons of the dentate granule cells (GCs) (8). Pharmacological and physical antidepressant treatments augment this D1R-dependent synaptic potentiation (3, 4) and increase D1R expression in the DG (4, 5). Furthermore, D1R activation contributes to antidepressant-induced behavioral changes and neuronal plasticity in the DG (5). Midbrain dopaminergic neurons are the major source of brain dopamine and have been thought to be responsible for activation of dopamine receptors in the DG (9). However, projection of these dopaminergic neurons to the hippocampus including DG is relatively sparse (10–13). In addition, the dopamine content in the hippocampus is much lower than other monoamines (14–16). Therefore, dopamine may mediate only a part of dopamine-receptor–dependent hippocampal functions, and endogenous substances other than dopamine may also be involved.
Noradrenergic fibers from the locus coeruleus abundantly project to the hippocampus (13, 17). The noradrenaline content in the hippocampus is 10- to 100-fold higher than dopamine (14–16). Previous studies have shown that optogenetic manipulation of the noradrenergic projection to the hippocampus modulated performance of learning tasks in a D1R-dependent manner (13, 18, 19). It is likely that noradrenaline can activate D1Rs as an endogenous agonist, thereby regulating hippocampal functions. However, none of the previous studies focused on this possibility, because it is generally believed that noradrenaline activates D1Rs at tens of micromolar concentrations (20), a range that may exceed physiological levels.
In the present study, we examined whether noradrenaline could modulate hippocampal neuronal signaling by activating D1Rs using electrophysiological and biochemical techniques, with a particular focus on the dentate GCs and their MF synaptic output. We found that noradrenaline at the micromolar range causes robust changes in neuronal transmission in all hippocampal subregions examined and increases protein phosphorylation in the DG via D1R activation. Notably, noradrenaline–D1R signaling at the MF synapse was activated by submicromolar noradrenaline and substantially enhanced in an activity- or experience-dependent manner. A possible involvement of this noradrenaline–D1R signaling in antidepressant effects was further examined. Antidepressant treatments can change the phenotype of mature dentate GCs into an immature-like state, a process characterized as “dematuration” of GCs (5, 21–24). We found that experience-dependent augmentation of noradrenaline–D1R signaling promotes the induction of dematuration by a noradrenergic antidepressant. Our results suggest that D1R signaling activated by noradrenaline plays a critical role in regulating the antidepressant efficacy.
Results
Noradrenaline Activates Hippocampal Dopamine D1 Receptors at Micromolar Concentrations.
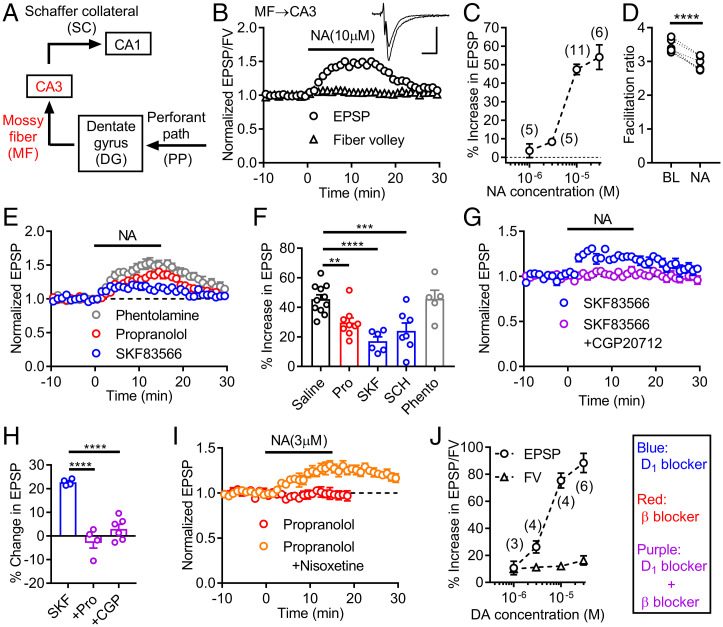
Dopamine D1Rs regulate synaptic transmission and neuronal excitability in the somatodendritic region and at the axonal output of the dentate GCs (8, 25). We first examined the effects of noradrenaline at the MF axon to CA3 pyramidal cell synapse (Fig. 1A). Dopamine potentiates MF synaptic transmission by activating presynaptic D1Rs (4, 8). Previous studies have shown that noradrenaline had little effect on basal MF synaptic transmission in rat hippocampal slices (26) or inhibited synaptic transmission via α adrenergic receptors in hippocampal slice cultures (27). We found that noradrenaline (10 μM) potentiated excitatory postsynaptic potentials (EPSPs) at the MF synapse in mouse hippocampal slices, with only minor effects on presynaptic fiber volleys (Fig. 1 B and C). This synaptic potentiation was accompanied by a decrease in a presynaptic form of synaptic facilitation induced by repetitive stimulation (Fig. 1D), suggesting that noradrenaline potentiated MF synaptic transmission via presynaptic mechanisms. Noradrenaline-induced synaptic potentiation was partially blocked by an antagonist of β adrenergic receptors, but not of α adrenergic receptors (Fig. 1 E and F). Antagonists of dopamine D1Rs also partially blocked noradrenaline-induced synaptic potentiation (Fig. 1 E and F). The D1-antagonist–resistant potentiation was suppressed by the broad β receptor antagonist propranolol or the β1-receptor–specific antagonist CGP20712 (Fig. 1 G and H), indicating that noradrenaline-induced potentiation is mediated by dopamine D1 and β1 adrenergic receptors. Noradrenaline had minimal effects at low micromolar concentrations (Fig. 1C). However, this is due to uptake of noradrenaline into noradrenergic nerve terminals in the hippocampus, because addition of the noradrenaline transporter inhibitor nisoxetine unveiled robust D1R-dependent potentiation by low micromolar noradrenaline (Fig. 1I). These results revealed that noradrenaline can activate dopamine D1Rs at the MF output of the GCs at the micromolar concentration range. As compared with noradrenaline, dopamine had larger potentiating effects on both EPSPs and fiber volleys (Fig. 1J). Since dopamine-induced potentiation at the MF synapse is almost exclusively mediated by D1Rs (4, 8), dopamine is more effective in activating D1Rs than noradrenaline (see also Fig. 3F).
Fig. 1.
Activation of D1 receptors by noradrenaline potentiates mossy fiber synaptic transmission in the hippocampus. (A) Schematic diagram of hippocampal excitatory circuit highlighting mossy fiber (MF)–CA3 connection. (B) Effects of noradrenaline (NA) applied at the bar on presynaptic fiber volley (FV) and EPSPs at MF–CA3 synapse. Sample traces show averaged field potentials before and during NA application (scale bars: 10 ms, 0.2 mV). (C) Dependence of synaptic potentiation on NA concentrations. (D) Decrease in triple-pulse facilitation at 200-ms intervals by NA. BL: baseline. Paired t test (t5 = 11.77, ****P < 0.0001). (E) NA-induced synaptic potentiation in D1 antagonist SKF83566 (SKF) (200 nM), β antagonist propranolol (Pro) (10 μM) or α antagonist phentolamine (Phento) (20 μM). (F) Summary of antagonist effects on NA-induced synaptic potentiation. SCH: SCH23390 (D1 antagonist, 50 nM). One-way ANOVA (F4,33 = 10.2, P < 0.0001) followed by Dunnett's test (**P = 0.0085, ***P = 0.0009, ****P = 0.0001). (G) Block of SKF-resistant potentiation by β1 antagonist CGP20712 (CGP) (100 nM). (H) Summary of β antagonist effects on SKF-resistant potentiation. One-way ANOVA (F2,11 = 35.66, P < 0.0001) followed by Dunnett's test (****P = 0.0001). (I) D1-receptor–dependent synaptic potentiation by low micromolar NA in presence (n = 6) and absence (n = 3) of nisoxetine (1 μM). (J) Dependence of FV and EPSP potentiation on dopamine (DA) concentrations. The number of data is shown in the graph in C and J. Data are presented as means ± SEM in all figures with or without individual values.
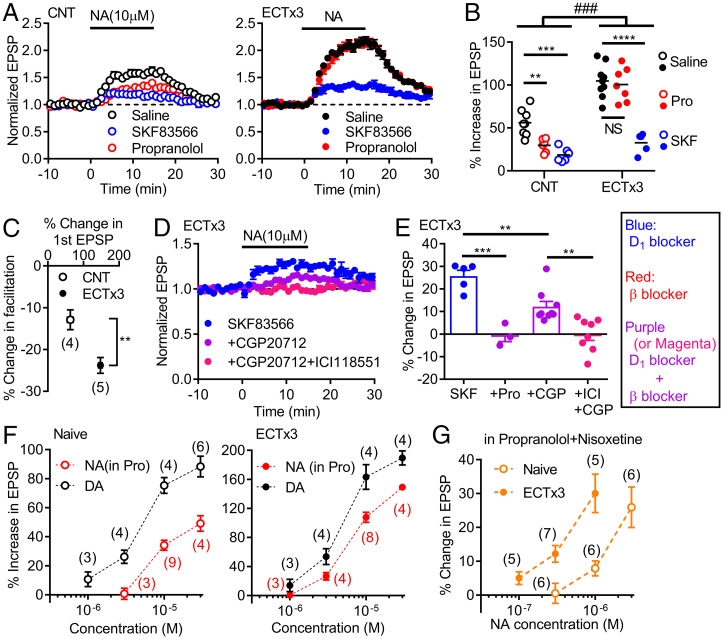
Fig. 3.
Activity-dependent enhancement of noradrenaline–D1 receptor signaling. (A) Effects of NA in control mice (CNT) and mice treated with three times of ECT (ECTx3) in normal saline or in antagonists. (B) Summary of data shown in A. Two-way ANOVA (ECT effect, F1,35 = 83.22, P < 0.0001; drug effect, F2,35 = 42.98, P < 0.0001; interaction, F2,35 = 10.13, ###P = 0.0003) followed by Tukey's test (NS: not significant, **P = 0.0083, ***P = 0.0002, ****P < 0.0001). (C) Effects of ECTx3 on reduction of triple-pulse facilitation by NA (t7 = 3.644, **P = 0.082). (D) Effects of β receptor antagonists on SKF-resistant component of NA-induced potentiation in ECT-treated mice. (E) Summary of β antagonist effects on SKF-resistant component of NA-induced potentiation. CGP (100–200 nM), ICI: ICI118551 (β2 antagonist, 100–200 nM). One-way ANOVA (F3,21 = 17.37, P < 0.0001) followed by Bonferroni's test (**P < 0.01, ***P = 0.0001). (F) Dependence of D1-mediated synaptic potentiation on NA and DA concentrations in naive and ECT-treated mice. The DA data in naive mice are the same as those in Fig. 1J. (G) Dependence of D1-mediated potentiation on NA concentrations in nisoxetine. The number of data is shown in the graph in C, F, and G.
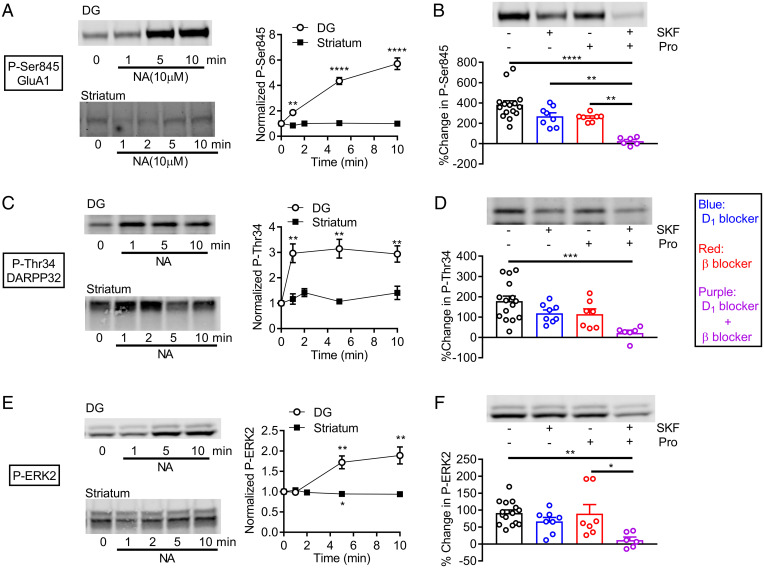
We next examined the effects of noradrenaline at the somatodendritic region of the GCs. Population spikes (PSs) were evoked by stimulating the perforant path input to the DG (SI Appendix, Fig. S1A). Noradrenaline at 10 μM strongly potentiated PSs and had only small potentiating effects on EPSPs (SI Appendix, Fig. S1B). The β and α1 antagonist labetalol suppressed potentiation of EPSPs and reduced PS potentiation (SI Appendix, Fig. S1 C–F). Propranolol had similar effects (SI Appendix, Fig. S1 D and F), confirming an involvement of β receptors. The D1 antagonist SKF83566 applied alone had no significant effects (SI Appendix, Fig. S1 C–F). In the presence of labetalol, however, SKF83566 suppressed potentiation of PSs without affecting EPSPs (SI Appendix, Fig. S1 C–F). In the presence of SKF83566 and labetalol, noradrenaline caused inhibition of PSs that was suppressed by the α receptor antagonist phentolamine, and block of α and β receptors isolated D1R-mediated potentiation of PSs that was abolished by D1R antagonists (SI Appendix, Fig. S1 C, D, G, and H). The D1R-mediated potentiation of PSs was observed along the longitudinal axis of the hippocampus with no clear differences between dorsal and ventral regions (SI Appendix, Fig. S1I). These results indicate that activation of D1Rs by noradrenaline potentiates PSs in the GCs but has no detectable effects on EPSPs. In the CA1 region, noradrenaline potentiated PSs without affecting EPSPs (SI Appendix, Fig. S2 A and B). This PS potentiation was largely suppressed by the β antagonist (SI Appendix, Fig. S2 C and D). As in the DG, SKF83566 significantly suppressed potentiation of PSs only in the presence of the β antagonist, revealing α-receptor–dependent inhibition of PSs (SI Appendix, Fig. S2D). Isolated D1R-dependent potentiation was detected in the presence of α and β receptor antagonists (SI Appendix, Fig. S2 C and D). Taken together, these results indicate that spike generation in both DG GCs and CA1 pyramidal cells is facilitated by noradrenaline via activation of D1 and β receptors and inhibited via activation of α receptors. The intact β receptor functioning masked the effect of the D1R antagonist on noradrenaline-induced potentiation of PSs, suggesting an overlap or redundancy in signaling pathways between D1 and β receptors (see Discussion). In addition to the electrophysiological studies, we conducted a biochemical assay of D1R activation by noradrenaline. Noradrenaline strongly increased the phosphorylation of downstream substrates of protein kinase A signaling, GluA1, DARPP32, and ERK2, in slices of the hippocampal DG, but not of the striatum (Fig. 2 A, C, and E). The increased phosphorylation in the DG was blocked by addition of propranolol and SKF83566 (Fig. 2 B, D, and F). However, neither propranolol nor SKF83566 alone had significant effects, again suggesting the overlap in the signaling pathway. Taken together, these results indicate that noradrenaline at the micromolar range activates dopamine D1 receptors expressed in the hippocampus, but not in the striatum.
Fig. 2.
D1-receptor–dependent protein phosphorylation by noradrenaline in the dentate gyrus. (A) Effects of NA on GluA1 phosphorylation in dentate gyrus (DG) (n = 7 for each point) and striatum (n = 4). Typical immunoblots for detection of phospho-Ser845 (left) and quantified data (right). One-sample t test (**P = 0.0019, ****P < 0.0001, compared with 1). (B) Effects of SKF83566 (500 nM) and propranolol on NA-induced GluA1 phosphorylation. Typical immunoblots (top) and quantified data (bottom). Two-way ANOVA (SKF effect, F1,32 = 19.28, P = 0.0001; Pro effect, F1,32 = 22.07, P < 0.0001) followed by Tukey's test (**P < 0.005, ****P < 0.0001). (C) Effects of noradrenaline on DARPP32 phosphorylation (phospho-Thr34) in slices of DG and striatum. One-sample t test (**P < 0.005). DG: n = 6, striatum: n = 4. (D) Antagonist effects on NA-induced phosphorylation of DARPP32 in DG. Two-way ANOVA (SKF effect, F1,32 = 8.549, P = 0.0063; Pro effect, F1,32 = 9.497, P = 0.0042) followed by Tukey's test (***P = 0.0006). (E) Effects of noradrenaline on ERK2 phosphorylation in slices of DG and striatum. One-sample t test (*P < 0.05, **P < 0.01). DG: n = 7, striatum: n = 4. (F) Antagonist effects on NA-induced phosphorylation of ERK2 in DG. Two-way ANOVA (SKF effect, F1,32 = 11.65, P = 0.0018) followed by Tukey's test (*P = 0.0123, **P = 0.0025). Phospho-proteins were normalized to total proteins and then data were normalized to values obtained with time 0 (A, C, and E) or control without noradrenaline (B, D, and F).
Activity-Dependent Augmentation of Noradrenaline–D1R Signaling.
A unique feature of D1R signaling at the MF synapse is marked augmentation by neuronal excitation (4). To further characterize the D1R signaling activated by noradrenaline, we next examined the effect of electroconvulsive treatment (ECT) that causes massive neuronal excitation and consistently augments dopamine-induced synaptic potentiation at the MF synapse (Fig. 3F) (4). In mice treated with ECT, noradrenaline-induced potentiation at the MF synapse was significantly augmented (Fig. 3 A and B). The reduction of synaptic facilitation by noradrenaline was also augmented by ECT, suggesting presynaptic mechanisms in the effect of ECT (Fig. 3C). Unexpectedly, ECT also changed the sensitivity to the antagonists. While the D1R antagonist SKF83566 strongly reduced noradrenaline-induced potentiation in both control and ECT-treated mice, the β antagonist propranolol applied alone reduced the synaptic potentiation only in control mice (Fig. 3 A and B). In ECT-treated mice, however, propranolol suppressed the SKF83566-resistant potentiation (Fig. 3E), suggesting that the effect of propranolol in the absence of SKF83566 was masked by intact D1R functioning. These results suggest that the β receptor signaling overlaps with a part of the D1R signaling at the MF synapse only after ECT. In addition, the overlapping β receptor signaling was partly mediated by β2 receptors (Fig. 3 D and E). Therefore, ECT reorganized D1- and β-receptor–dependent signaling at the MF synapse. Since synthetic β receptor agonists potentiated the MF synaptic transmission via β2 receptors in naive mice (SI Appendix, Fig. S3), ECT appeared to increase the sensitivity of β2 receptors to noradrenaline. A comparison of the effects of noradrenaline and dopamine revealed that noradrenaline was more effective in activating D1Rs after ECT, and there was an apparent increase in potency of noradrenaline (Fig. 3F). In the presence of the noradrenaline uptake inhibitor, even submicromolar noradrenaline was able to activate D1Rs after ECT (Fig. 3G), excluding a possibility that altered noradrenaline uptake caused the apparent increase in potency. These results indicate that ECT facilitated noradrenaline–D1R signaling at the MF synapse, possibly via the reorganization of the D1 and β receptor signaling, rather than simply augmenting D1R-dependent synaptic modulation. In the DG, ECT did not significantly increase D1R-dependent potentiation of PSs, although it changed the time course of potentiation (SI Appendix, Fig. S4A). In CA1, ECT tended to reduce D1R-dependent potentiation of PSs (SI Appendix, Fig. S4B). Therefore, the activity-dependent facilitation of the noradrenaline–D1R signaling is specific to the MF system.
Interaction between D1 and β Receptors.
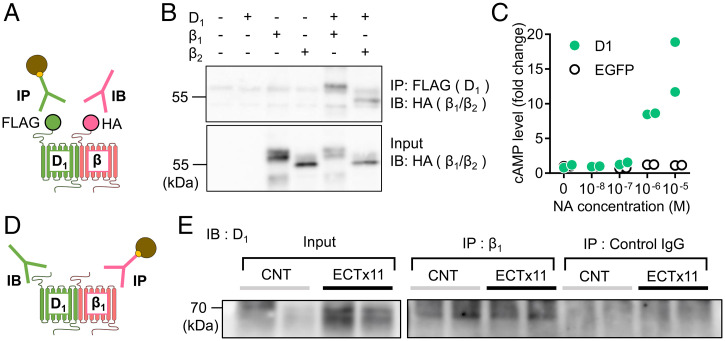
Above results showed the significant overlap between D1 and β receptor signaling. G-protein–coupled receptors, including dopamine receptors, can form a heteromeric complex (28, 29). Heteromeric D1 and β receptors may be a molecular basis for such overlap in signaling. To examine formation of D1–β heteromers, we performed a coimmunoprecipitation assay (Fig. 4A). FLAG-tagged D1Rs were coexpressed with HA-tagged β1 or β2 receptors in HEK293T cells. After immunoprecipitation with a FLAG antibody, blotting with an HA antibody detected β1 and β2 receptors coprecipitated with D1Rs (Fig. 4B). Furthermore, interaction between native D1 and β receptors was examined using whole hippocampal tissue lysates (Fig. 4D). After immunoprecipitation with a β1 receptor antibody, a D1R antibody detected coprecipitated D1R in control and ECT-treated mice (Fig. 4E and SI Appendix, Fig. S5B). There was no clear difference in the D1–β1 coprecipitation between control and ECT-treated mice, while ECT increased D1R expression levels (SI Appendix, Fig. S5A). These results suggest that D1 and β receptors can form the heteromeric receptor complex. In HEK293T cells expressing D1Rs alone, noradrenaline increased cAMP production at the micromolar range (Fig. 4C). Therefore, the complex formation is not required for noradrenaline responsiveness and may regulate efficacy and/or potency of D1R activation by noradrenaline (see Discussion).
Fig. 4.
Interaction between D1 and β receptors. (A) Schematic diagram showing experimental design of coimmunoprecipitation assay using tagged receptors. IP: immunoprecipitation, IB: immunoblot. (B) Coimmunoprecipitation of FLAG-tagged D1 and HA-tagged β1 or β2 receptors expressed in HEK293T cells. (C) NA-induced cAMP production in HEK293T cells expressing EGFP or D1 receptors (n = 2 each). (D) Experimental design of coimmunoprecipitation assay of native receptors. (E) Coimmunoprecipitation of hippocampal D1 and β1 receptors in control and mice treated with 11 times of ECT (ECTx11). IgG: immunoglobulin G. Experiments were repeated three times with similar results (SI Appendix, Fig. S5B).
Experience-Dependent Augmentation of Noradrenaline–D1R Signaling Facilitates Antidepressant Effects.
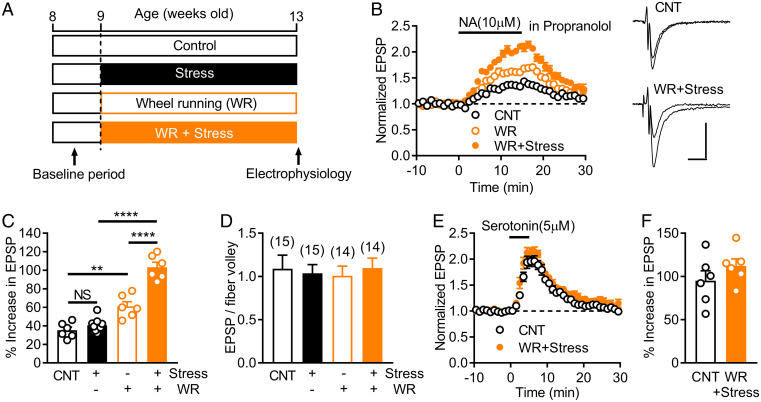
The noradrenergic system has been implicated in stress-induced alterations of the central nervous system and antidepressant effects (30), and D1Rs in the dentate GCs contribute to antidepressant effects (5, 7). Finally, we examined an involvement of the noradrenaline–D1R signaling in antidepressant effects, focusing on possible influence of stress on this signaling. Mice were subjected to 4 wk of restraint stress (Fig. 5A). Noradrenaline-induced MF synaptic potentiation mediated by D1Rs was recorded in the presence of propranolol. Chronic restraint stress had no significant effect on this noradrenaline–D1R signaling at the MF synapse (Fig. 5C). On the other hand, increased voluntary exercise by wheel running (Fig. 5A), which often causes neuronal changes opposite to those induced by stress and has antidepressant-like effects (31), significantly increased the noradrenaline–D1R signaling (Fig. 5 B and C). Low micromolar noradrenaline was able to induce significant D1R-dependent synaptic potentiation in mice subjected to wheel running (SI Appendix, Fig. S6A). Unexpectedly, chronic stress enhanced the effect of wheel running on the noradrenaline–D1R signaling (Fig. 5 B and C). Chronic stress combined with wheel running enhanced the D1R-antagonist–sensitive component of noradrenaline-induced synaptic potentiation (SI Appendix, Fig. S7), confirming the enhancing effect on the noradrenaline–D1R signaling. The basal synaptic efficacy was not affected by chronic stress or wheel running (Fig. 5D). In addition, chronic stress combined with wheel running had no significant effect on serotonin 5-HT4-receptor–dependent synaptic modulation that shares the intracellular signaling pathway with the D1R-dependent modulation (32) (Fig. 5 E and F). These results indicate that chronic stress and voluntary exercise synergistically and specifically enhance the noradrenaline–D1R signaling.
Fig. 5.
Synergistic augmentation of noradrenaline–D1 receptor signaling by chronic stress and exercise. (A) Timeline of chronic restraint stress and increased voluntary exercise by wheel running (WR). (B) Effects of chronic restraint stress and WR on NA-induced MF synaptic potentiation mediated by D1 receptors. Recordings were made in the presence of propranolol. Sample traces show averaged field potentials before and during NA application (scale bars: 10 ms, 0.2 mV). (C) Summary of effects of stress and WR on D1-mediated potentiation. Two-way ANOVA (stress effect, F1,23 = 35.29, P < 0.0001; WR effect, F1,23 = 122.9, P < 0.0001; interaction, F1,23 = 21.36, P = 0.0001) followed by Tukey's test (**P = 0.0012, ****P < 0.0001). (D) Lack of effects of stress and WR on the basal MF synaptic efficacy assessed by EPSP to fiber volley ratios. The number of data is shown in the graph. (E) Effects of stress and WR on serotonin-induced MF synaptic potentiation. (F) Summary of serotonin-induced potentiation.
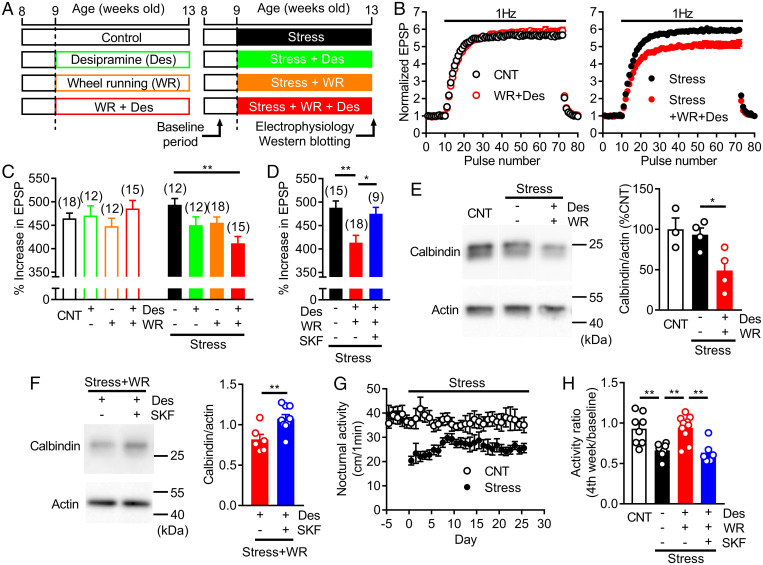
We then examined a contribution of this synergistic enhancement of the noradrenaline–D1R signaling to dematuration of GCs, a proposed cellular substrate for antidepressant action (5, 21–24). Dematuration is characterized by altered expression of maturation stage markers and the appearance of immature-like functional properties in GCs. Prominent frequency facilitation at the MF synapse represents the functional maturity of GCs and is attenuated by serotonergic antidepressants (21, 22). We used the noradrenergic antidepressant desipramine, which acts as a highly potent noradrenaline reuptake inhibitor (33). Acute desipramine significantly augmented D1R-dependent synaptic potentiation induced by low micromolar noradrenaline (SI Appendix, Fig. S6B). Chronic treatment with desipramine (Fig. 6A) did not change frequency facilitation when applied alone or in combination with wheel running (Fig. 6 B and C). In mice exposed to chronic restraint stress, desipramine alone had no significant effect. However, desipramine combined with wheel running significantly reduced frequency facilitation in stressed mice (Fig. 6 B and C). The reduction of facilitation was blocked by SKF83566 (Fig. 6D), suggesting an involvement of D1Rs. Furthermore, we found that the expression level of the mature GC marker calbindin was significantly reduced by desipramine combined with wheel running in stressed mice (Fig. 6E). In mice coadministered with SKF83566, the calbindin expression level was higher than that in mice treated with desipramine alone (Fig. 6F). These results suggest that the synergistic enhancement of the noradrenaline–D1R signaling in stressed mice subjected to wheel running facilitated the induction of GC dematuration by the noradrenergic antidepressant. We also examined behavioral effects of desipramine in stressed mice. While chronic stress did not change anxiety- or depression-related behavior in commonly used behavioral paradigms (SI Appendix, Fig. S8), it significantly reduced voluntary activity in home cages (Fig. 6G). Desipramine combined with wheel running reversed the reduced activity in stressed mice, and SKF83566 blocked this effect (Fig. 6H), suggesting a beneficial effect of the desipramine treatment combined with wheel running on stress-induced behavioral impairment.
Fig. 6.
Experience-dependent augmentation of noradrenaline–D1 receptor signaling facilitates effects of noradrenergic antidepressant. (A) Timeline of restraint stress, WR, and desipramine (Des) treatment. (B) Effects of chronic Des (30 mg/kg/day for 4 wk) and WR on 1-Hz frequency facilitation at MF synapse in nonstressed (left) and stressed (right) mice. (C) Summary of effects of Des and WR on frequency facilitation in nonstressed and stressed mice. Two-way ANOVA (stress effect, F1,106 = 1.757, P = 0.1879; treatment effect, F3,106 = 1.657, P = 0.1809; interaction, F3,106 = 4.424, P = 0.0057) followed by Dunnett's test (**P = 0.0011). (D) Effects of SKF (1 mg/kg/day) on reduction of frequency facilitation caused by Des and WR in stressed mice. One-way ANOVA (F2,39 = 7.508, P = 0.0017) followed by Dunnett's test (*P = 0.0247, **P = 0.0015). (E) Effects of Des and WR on calbindin expression in DG. Typical immunoblots (left) and quantified data (right). One-way ANOVA (F2,8 = 5.809, P = 0.0276) followed by Dunnett's test (*P = 0.0399). (F) Effects of SKF on calbindin expression in DG of Des-treated mice (t13 = 3.164, **P = 0.0075). (G) Effects of chronic stress on nocturnal home cage activity. (H) D1-receptor–dependent reversal of stress-induced decrease in home cage activity by Des and WR. One-way ANOVA (F3,28 = 9.789, P = 0.0001) followed by Bonferroni's test (**P < 0.01). The number of data is shown in the graph in C and D.
Discussion
We have revealed that noradrenaline can activate dopamine D1Rs in the mouse hippocampus, but not in the striatum, at the submicromolar to micromolar concentration range. This noradrenaline–D1R signaling was strongly augmented by neuronal excitation, a process that may include reorganization of D1- and β-receptor–dependent signaling. Chronic stress and voluntary exercise synergistically enhanced the noradrenaline–D1R signaling, thereby facilitating antidepressant-induced dematuration of DG neurons. Our results suggest that experience-dependent plasticity of the noradrenaline–D1R signaling determines the responsiveness to antidepressant drugs.
Previous in vivo microdialysis studies have estimated that the basal extracellular noradrenaline concentration in the rodent hippocampus is 14–35 nM (34, 35). In general, transmitter concentrations near the release sites are much higher than the basal extracellular levels. While microdialysis studies estimated that the basal glutamate concentration in the hippocampus was 1–3 μM (36, 37), an electrophysiological study suggested that the peak glutamate concentration outside synapses could reach up to about 200 μM (38). Therefore, noradrenaline concentrations may reach micromolar levels around noradrenergic terminals. We showed that micromolar noradrenaline can activate D1R in all hippocampus subregions examined. In the presence of intact noradrenaline uptake activity, low micromolar noradrenaline activated D1Rs at the MF synapse in mice subjected to wheel running (SI Appendix, Fig. S6A), but not in naive mice (Fig. 1I), suggesting a critical influence of experience on the sensitivity of D1Rs to noradrenaline. Our finding is consistent with the previous observations that the noradrenergic projection regulated performance of hippocampus-dependent learning tasks via D1Rs (13, 18, 19). However, these studies favored an alternative interpretation that dopamine coreleased from the noradrenergic fibers activated D1Rs. On the basis of the biosynthetic pathway of catecholamines, high-frequency firing of noradrenergic fibers is predicted to increase the vesicular content of dopamine relative to noradrenaline (19). The amount of dopamine released from optogenetically activated noradrenergic fibers projecting to the hippocampus is about 10% of noradrenaline after prolonged high-frequency firing (18). Therefore, assuming micromolar levels of noradrenaline as estimated above, hippocampal D1Rs near noradrenergic terminals may be primarily activated by noradrenaline, although our results do not exclude the possibility of D1R activation by coreleased dopamine (also see below).
The overlap or redundancy in D1 and β receptor signaling was suggested by pharmacological experiments. We observed that the D1R antagonist failed to cause significant effects in the absence of the β receptor antagonist or vice versa. Especially, at the MF synapse in ECT-treated mice, the effect of the β antagonist propranolol was detectable only after blocking D1Rs (Fig. 3), suggesting that D1R activation completely occluded the effect of β receptor activation. Both D1 and β receptors are coupled with Gs–cAMP signaling pathways. It has been suggested that the cAMP signaling can be compartmentalized (39, 40). If D1 and β receptors are localized close to each other, activation of either of them may saturate the local cAMP signaling, which could explain our results showing that blocking D1 or β receptors alone had insignificant effects. Such colocalization can be best exemplified by the formation of heteromeric D1 and β receptor complex. Indeed, our coimmunoprecipitation studies suggested the formation of the D1–β receptor complex. As discussed below, we speculate that the formation of the heteromeric receptor complex is essential for regulation of the noradrenaline–D1R signaling. At the MF synapse, the overlap of D1 and β receptor signaling was seen in ECT-treated mice, but not in control mice (Fig. 3). ECT causes a marked increase in D1R expression levels (4), which may be required to induce colocalization of D1 and β receptors.
The sensitivity of D1Rs to noradrenaline was different between brain regions. Micromolar noradrenaline caused robust activation of D1Rs in the hippocampus, but not in the striatum. A bioluminescence resonance energy transfer assay showed that the ligand affinity of recombinant human D1Rs depends on the subtype of Gα subunit. The D1R coupled to Gαolf has a lower affinity for noradrenaline than that coupled to Gαs (41). Gαolf is the predominant subtype of Gα coupled with D1Rs in the striatum (42), which may explain the failure in activation of striatal D1Rs by micromolar noradrenaline. At the MF synapse, the D1R sensitive to submicromolar noradrenaline was induced by ECT. ECT also produced the overlap between D1 and β signaling and increased the sensitivity of β2 receptors to noradrenaline. Heteromeric β1–β2 receptors show a higher affinity to synthetic agonists than homomeric β1 receptors (29). Since we found that D1Rs can interact with β1 and β2 receptors, a heteromeric complex including D1 and β2 or β1–β2 receptors is a candidate for the D1R with the higher affinity for noradrenaline.
ECT augmented the noradrenaline-induced D1R-dependent synaptic potentiation by about 200% at the maximal concentration of 30 μM, while dopamine-induced synaptic potentiation was increased by about 100% (Fig. 3F). Since D1Rs solely mediate dopamine-induced potentiation at the MF synapse even after ECT (4), it is conceivable that ECT increased the efficacy of D1R activation by noradrenaline. While ECT strongly up-regulates D1R expression (SI Appendix, Fig. S5A) (4), an increase in the number of D1Rs would equally enhance noradrenaline- and dopamine-induced potentiation. Therefore, we hypothesize that the reorganization of D1 and β signaling, most probably the formation of the heteromeric D1–β receptor complexes, underlies the increase in the efficacy as well as potency of D1R activation by noradrenaline.
We observed coimmunoprecipitation of D1 and β receptors in the hippocampal tissue lysates from both control and ECT-treated mice. Coimmunoprecipitation of these receptors in control mice supports the idea that the heteromeric receptor complex is the molecular basis for the overlap in the D1 and β receptor signaling observed by electrophysiological and biochemical studies in naive mice (SI Appendix, Fig. S1 and Fig. 2). Although we hypothesized that ECT caused the reorganization of D1 and β signaling at the MF synapse via formation of the heteromeric receptor complex, there was no obvious difference in the coimmunoprecipitation between control and ECT-treated mice. Since ECT did not significantly increase noradrenaline-induced potentiation of PSs mediated by D1Rs in the DG and CA1 (SI Appendix, Fig. S4), the reorganization of D1 and β signaling may be specifically induced at the MF system. An increase in heteromeric receptor complexes specific to the MF synapse, if any, may not be detected by coimmunoprecipitation in whole hippocampal tissue lysates.
Many lines of evidence suggest a critical role of the hippocampal DG in depression and antidepressant medication (7, 43, 44). We have previously demonstrated that antidepressant drugs and ECT induce immature-like phenotypes in the dentate GCs and at their output MF synapse and proposed this dematuration as the common neuronal mechanism underlying pharmacological and physical antidepressant treatments (5, 21–24). Although serotonergic antidepressants consistently induce GC dematuration in nonstressed mice (5, 21, 23, 45), the noradrenergic antidepressant desipramine failed to induce dematuration even when combined with exercise in nonstressed mice. This is probably related to lower functional levels of endogenous catecholamines at the MF system as compared with serotonin (46). Stress facilitated the induction of dematuration by desipramine, most likely via augmentation of the noradrenaline–D1R signaling. Since desipramine inhibits uptake of dopamine as well as noradrenaline, D1R activation by dopamine coreleased from noradrenergic fibers may also contribute to the induction of dematuration by desipramine. As described above, the amount of dopamine that can be released from noradrenergic fibers in the hippocampus is predicted to be at most 10% of noradrenaline. In the presence of desipramine, noradrenaline at 3 μM, a concentration that may be reached around noradrenergic terminals as estimated above, caused strong D1R-dependent synaptic potentiation in mice subjected to stress and wheel running (SI Appendix, Fig. S6B). In the same condition, a 10-fold lower concentration of dopamine also induced robust potentiation, although it was smaller in magnitude than that by noradrenaline (SI Appendix, Fig. S6B). Therefore, while the noradrenaline–D1R signaling is likely to play a major role in the antidepressant effect of desipramine, dopamine could also make a significant contribution, depending on its vesicular content. Since the dopamine transporter is expressed, if any, at a very low level in the hippocampus (47, 48), the noradrenaline transporter may be essential for clearance of dopamine released from the dopaminergic fibers in addition to the noradrenergic fibers. Indeed, systemic administration of the noradrenaline transporter inhibitor can raise extracellular dopamine levels in the hippocampus (49). However, the dopamine level is much less sensitive to the noradrenaline transporter inhibitor as compared with the extracellular noradrenaline level (50). Given sparse dopaminergic innervation to the DG and CA3 region (11–13), it is unlikely that dopamine solely mediates D1R receptor activation involved in the effects of desipramine, although we cannot exclude this possibility. Dematuration by the serotonergic antidepressant fluoxetine requires 5-HT4 receptors (21, 23), which are abundantly expressed in the GCs and along the MF tract (23, 51). Since both 5-HT4 and D1Rs are coupled with Gs, cAMP-dependent signaling is likely to be a common downstream pathway for the induction of dematuration. The noradrenaline–D1R signaling was observed at the somatodendritic and axonal regions of GCs. At present, it is unknown whether somatodendritic or axonal D1Rs are required for the induction of dematuration. Experience- or activity-dependent augmentation of noradrenaline–D1R signaling was prominent at the MF synapse. The extent of fluoxetine-induced dematuration was significantly correlated with the magnitude of 5-HT4-receptor–mediated potentiation at the MF synapse (21). Therefore, it is possible that axonal cAMP signaling plays a critical role in GC dematuration.
Although chronic restraint stress combined with wheel running enhanced the noradrenaline–D1R signaling at the MF synapse, it did not significantly change 5-HT4-receptor–dependent synaptic potentiation (Fig. 5). The exact mechanism underlying this selective enhancement is unknown. The dentate GCs show an acute increase in the c-fos expression, a measure of neuronal activity, after voluntary exercise (52, 53). The D1R expression in the DG is highly sensitive to neuronal activity. While single ECT strongly up-regulates D1R expression levels (4), repeated ECT does not significantly change 5-HT4 receptor expression levels (46). This differential sensitivity to neuronal activity can explain the selective enhancement of the noradrenaline–D1R signaling. In addition to up-regulation of the D1R expression, neuronal activity could increase the efficacy of D1R activation by noradrenaline as demonstrated in ECT-treated mice. Furthermore, chronic restraint stress may boost the activity-dependent up-regulation of the D1R signaling via corticosterone (22), resulting in the marked enhancement of the noradrenaline–D1R signaling by restraint stress combined with wheel running.
The central noradrenergic system has long been thought to be an important target of antidepressant drugs, although it is not precisely understood how the noradrenergic system is involved in the pathology of depression and antidepressant effects. Antidepressant drugs have been assumed to reverse dysfunction of monoaminergic systems caused by stress and/or other risk factors for depression (30, 54). However, our finding implies that antidepressant drugs make use of stress-induced adaptive changes in the noradrenaline–D1R signaling to induce dematuration of GCs. Stress and antidepressant treatment can activate shared cellular responses that mediate resilience to stress (55, 56). The noradrenaline–D1R signaling may play a role as stress resilience that facilitates the antidepressant efficacy in depressive disorders. Activation of D1Rs contributes to the effects of the serotonergic antidepressant fluoxetine in the DG (5), including GC dematuration and increased adult neurogenesis, another candidate cellular substrate for antidepressant action (44). Therefore, the noradrenaline–D1R signaling may be commonly involved in the effects of different classes of antidepressant drugs. Chronic corticosterone treatment that mimics chronic stress exposure augments D1R signaling at the MF synapse and concomitantly facilitates the induction of dematuration by fluoxetine (22). Chronic corticosterone also facilitates the fluoxetine-induced increase of adult neurogenesis in the DG (57), and antidepressant drugs more consistently increase adult neurogenesis in stressed animals than in naive animals (58). These lines of evidence, including the present study, suggest that antidepressant drugs activate the process of adaptive neuronal plasticity that is initiated by chronic stress rather than simply reversing stress-induced alterations in the brain. Our present finding suggests that the enhancement of the noradrenaline–D1R signaling plays a pivotal role in linking stress with antidepressant-induced plasticity. The enhancement of the noradrenaline–D1R signaling by stress was conditional, requiring increased voluntary activity. This synergistic experience-dependent regulation of the noradrenaline–D1R signaling, possibly influenced by other unknown factors, may determine the antidepressant responsiveness, which suggests a unique target for therapeutic treatments of depressive disorders refractory to antidepressant medication.
Materials and Methods
Adult male C57BL/6J mice were used in all experiments. Animal use and procedures were approved by the Animal Care and Use Committee of Nippon Medical School, Tokyo University of Science, and Kurume University. Electrophysiological recordings were made using acute hippocampal slices (8, 46). In protein phosphorylation analyses, the regions of the dentate gyrus or the striatum were dissected from coronal brain slices (5, 59). Bilateral electroconvulsive treatment was administered to mice anesthetized with isoflurane (24). HEK293T cells transfected with plasmids carrying FLAG-tagged D1 receptors (60) and/or HA-tagged β1/β2 receptors were used for cAMP measurements and receptor coimmunoprecipitation analyses. Mice were treated with oral desipramine, exposed to chronic restraint stress or wheel running, or subjected to combination of these treatments. These mice were used for electrophysiological experiments, home cage activity monitoring (61), and western blotting. For detailed methods, see SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Tomoyuki Furuyashiki for providing a plasmid containing D1–FLAG and for valuable discussion on the present study. We thank Dr. Takahide Shuto and Yasunori Mikahara for technical assistance. This work was supported by Japan Society for the Promotion of Science KAKENHI Grant No. 19K06961 (to K.K.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2117903119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
References
- 1.da Silva W. C., Köhler C. C., Radiske A., Cammarota M., D1/D5 dopamine receptors modulate spatial memory formation. Neurobiol. Learn. Mem. 97, 271–275 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Hansen N., Manahan-Vaughan D., Dopamine D1/D5 receptors mediate informational saliency that promotes persistent hippocampal long-term plasticity. Cereb. Cortex 24, 845–858 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi K., Haneda E., Higuchi M., Suhara T., Suzuki H., Chronic fluoxetine selectively upregulates dopamine D1-like receptors in the hippocampus. Neuropsychopharmacology 37, 1500–1508 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi K., et al. , Rapid and lasting enhancement of dopaminergic modulation at the hippocampal mossy fiber synapse by electroconvulsive treatment. J. Neurophysiol. 117, 284–289 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shuto T., et al. , Obligatory roles of dopamine D1 receptors in the dentate gyrus in antidepressant actions of a selective serotonin reuptake inhibitor, fluoxetine. Mol. Psychiatry 25, 1229–1244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra A., Singh S., Tiwari V., Parul S., Shukla S., Dopamine D1 receptor activation improves adult hippocampal neurogenesis and exerts anxiolytic and antidepressant-like effect via activation of Wnt/β-catenin pathways in rat model of Parkinson’s disease. Neurochem. Int. 122, 170–186 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Umschweif G., Greengard P., Sagi Y., The dentate gyrus in depression. Eur. J. Neurosci. 53, 39–64 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi K., Suzuki H., Dopamine selectively potentiates hippocampal mossy fiber to CA3 synaptic transmission. Neuropharmacology 52, 552–561 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Du H., et al. , Dopaminergic inputs in the dentate gyrus direct the choice of memory encoding. Proc. Natl. Acad. Sci. U.S.A. 113, E5501–E5510 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson L. W., The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 9, 321–353 (1982). [DOI] [PubMed] [Google Scholar]
- 11.Gasbarri A., Packard M. G., Campana E., Pacitti C., Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res. Bull. 33, 445–452 (1994). [DOI] [PubMed] [Google Scholar]
- 12.McNamara C. G., Tejero-Cantero Á., Trouche S., Campo-Urriza N., Dupret D., Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat. Neurosci. 17, 1658–1660 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi T., et al. , Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt R. H., Bhatnagar R. K., Assessment of the effects of neonatal subcutaneous 6-hydroxydopamine on noradrenergic and dopaminergic innervation of the cerebral cortex. Brain Res. 166, 309–319 (1979). [DOI] [PubMed] [Google Scholar]
- 15.Koshimizu H., et al. , Comprehensive behavioral analysis and quantification of brain free amino acids of C57BL/6J congenic mice carrying the 1473G allele in tryptophan hydroxylase-2. Neuropsychopharmacol. Rep. 39, 56–60 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Y., Su Y., Jiang Y., Effect of the warming and tonifying kidney-yang recipe on monoamine neurotransmitters and pathological morphology of hippocampus tissue in depression model rats. Technol. Health Care 28 (S1), 237–244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loy R., Koziell D. A., Lindsey J. D., Moore R. Y., Noradrenergic innervation of the adult rat hippocampal formation. J. Comp. Neurol. 189, 699–710 (1980). [DOI] [PubMed] [Google Scholar]
- 18.Kempadoo K. A., Mosharov E. V., Choi S. J., Sulzer D., Kandel E. R., Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. U.S.A. 113, 14835–14840 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagatsuma A., et al. , Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc. Natl. Acad. Sci. U.S.A. 115, E310–E316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunahara R. K., et al. , Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350, 614–619 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K., et al. , Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc. Natl. Acad. Sci. U.S.A. 107, 8434–8439 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi K., et al. , Corticosterone facilitates fluoxetine-induced neuronal plasticity in the hippocampus. PLoS One 8, e63662 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imoto Y., et al. , Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus. Mol. Brain 8, 29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imoto Y., Segi-Nishida E., Suzuki H., Kobayashi K., Rapid and stable changes in maturation-related phenotypes of the adult hippocampal neurons by electroconvulsive treatment. Mol. Brain 10, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton T. J., et al. , Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proc. Natl. Acad. Sci. U.S.A. 107, 18185–18190 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopkins W. F., Johnston D., Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J. Neurophysiol. 59, 667–687 (1988). [DOI] [PubMed] [Google Scholar]
- 27.Scanziani M., Gähwiler B. H., Thompson S. M., Presynaptic inhibition of excitatory synaptic transmission mediated by alpha adrenergic receptors in area CA3 of the rat hippocampus in vitro. J. Neurosci. 13, 5393–5401 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misganaw D., Heteromerization of dopaminergic receptors in the brain: Pharmacological implications. Pharmacol. Res. 170, 105600 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Zhu W.-Z., et al. , Heterodimerization of β1- and β2-adrenergic receptor subtypes optimizes β-adrenergic modulation of cardiac contractility. Circ. Res. 97, 244–251 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Seki K., Yoshida S., Jaiswal M. K., Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural Regen. Res. 13, 1159–1169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yau S.-Y., Lau B. W., So K.-F., Adult hippocampal neurogenesis: A possible way how physical exercise counteracts stress. Cell Transplant. 20, 99–111 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi K., Ikeda Y., Haneda E., Suzuki H., Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J. Neurosci. 28, 6272–6280 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatsumi M., Groshan K., Blakely R. D., Richelson E., Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 340, 249–258 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Abercrombie E. D., Keller R. W. Jr., Zigmond M. J., Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: Pharmacological and behavioral studies. Neuroscience 27, 897–904 (1988). [DOI] [PubMed] [Google Scholar]
- 35.Harley C. W., Lalies M. D., Nutt D. J., Estimating the synaptic concentration of norepinephrine in dentate gyrus which produces beta-receptor mediated long-lasting potentiation in vivo using microdialysis and intracerebroventricular norepinephrine. Brain Res. 710, 293–298 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Lerma J., Herranz A. S., Herreras O., Abraira V., Martín del Río R., In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 384, 145–155 (1986). [DOI] [PubMed] [Google Scholar]
- 37.Baker D. A., Xi Z.-X., Shen H., Swanson C. J., Kalivas P. W., The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci. 22, 9134–9141 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzubay J. A., Jahr C. E., The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft. J. Neurosci. 19, 5265–5274 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lefkimmiatis K., Zaccolo M., cAMP signaling in subcellular compartments. Pharmacol. Ther. 143, 295–304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghigo A., Mika D., cAMP/PKA signaling compartmentalization in cardiomyocytes: Lessons from FRET-based biosensors. J. Mol. Cell. Cardiol. 131, 112–121 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Yano H., et al. , Gs- versus Golf-dependent functional selectivity mediated by the dopamine D1 receptor. Nat. Commun. 9, 486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuang X., Belluscio L., Hen R., G(olf)α mediates dopamine D1 receptor signaling. J. Neurosci. 20, RC91 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi K., Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol. Neurobiol. 39, 24–36 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Castrén E., Hen R., Neuronal plasticity and antidepressant actions. Trends Neurosci. 36, 259–267 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohira K., Miyakawa T., Chronic treatment with fluoxetine for more than 6 weeks decreases neurogenesis in the subventricular zone of adult mice. Mol. Brain 4, 10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi K., et al. , Predominant role of serotonin at the hippocampal mossy fiber synapse with redundant monoaminergic modulation. iScience 23, 101025 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mennicken F., Savasta M., Peretti-Renucci R., Feuerstein C., Autoradiographic localization of dopamine uptake sites in the rat brain with 3H-GBR 12935. J. Neural Transm. (Vienna) 87, 1–14 (1992). [DOI] [PubMed] [Google Scholar]
- 48.Kwon O. B., et al. , Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc. Natl. Acad. Sci. U.S.A. 105, 15587–15592 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borgkvist A., Malmlöf T., Feltmann K., Lindskog M., Schilström B., Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int. J. Neuropsychopharmacol. 15, 531–540 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Montezinho L. P., et al. , The effects of acute treatment with escitalopram on the different stages of contextual fear conditioning are reversed by atomoxetine. Psychopharmacology (Berl.) 212, 131–143 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Vilaró M. T., Cortés R., Mengod G., Serotonin 5-HT4 receptors and their mRNAs in rat and guinea pig brain: Distribution and effects of neurotoxic lesions. J. Comp. Neurol. 484, 418–439 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Clark P. J., Brzezinska W. J., Puchalski E. K., Krone D. A., Rhodes J. S., Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus 19, 937–950 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark P. J., et al. , Adult hippocampal neurogenesis and c-Fos induction during escalation of voluntary wheel running in C57BL/6J mice. Behav. Brain Res. 213, 246–252 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillhouse T. M., Porter J. H., A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp. Clin. Psychopharmacol. 23, 1–21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vialou V., et al. , DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat. Neurosci. 13, 745–752 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedman A. K., et al. , KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat. Commun. 7, 11671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.David D. J., et al. , Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62, 479–493 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller B. R., Hen R., The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 30, 51–58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishi A., et al. , Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J. Neurosci. 28, 10460–10471 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ehrlich A. T., Furuyashiki T., Kitaoka S., Kakizuka A., Narumiya S., Prostaglandin E receptor EP1 forms a complex with dopamine D1 receptor and directs D1-induced cAMP production to adenylyl cyclase 7 through mobilizing G(βγ) subunits in human embryonic kidney 293T cells. Mol. Pharmacol. 84, 476–486 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi K., Ikeda Y., Suzuki H., Behavioral destabilization induced by the selective serotonin reuptake inhibitor fluoxetine. Mol. Brain 4, 12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.