Significance
Nav1.7 has been targeted for pain management for its well-established role in pain sensation. Hundreds of mutations of Nav1.7 have been found in patients with pain disorders. Structures of Nav1.7 captured in different conformations will reveal its working mechanism and facilitate drug discovery. Here we present the rational design of a Nav1.7 variant, Nav1.7-M11, that may be trapped in the closed-state inactivation conformation at 0 mV. Cryoelectron microscopy analysis of Nav1.7-M11 reveals voltage-sensing domain in the first repeat (VSDI) in the completely down conformation, VSDII at an intermediate state, and the pore domain tightly closed. Structural comparison of Nav1.7-M11 with the WT channel provides unprecedented insight into the electromechanical coupling details and affords mechanistic interpretation for a number of pain-related mutations.
Keywords: Nav1.7, cryo-EM structure, electromechanical coupling, resting VSD, pain
Abstract
Voltage-gated sodium (Nav) channel Nav1.7 has been targeted for the development of nonaddictive pain killers. Structures of Nav1.7 in distinct functional states will offer an advanced mechanistic understanding and aid drug discovery. Here we report the cryoelectron microscopy analysis of a human Nav1.7 variant that, with 11 rationally introduced point mutations, has a markedly right-shifted activation voltage curve with V1/2 reaching 69 mV. The voltage-sensing domain in the first repeat (VSDI) in a 2.7-Å resolution structure displays a completely down (deactivated) conformation. Compared to the structure of WT Nav1.7, three gating charge (GC) residues in VSDI are transferred to the cytosolic side through a combination of helix unwinding and spiral sliding of S4I and ∼20° domain rotation. A conserved WNФФD motif on the cytoplasmic end of S3I stabilizes the down conformation of VSDI. One GC residue is transferred in VSDII mainly through helix sliding. Accompanying GC transfer in VSDI and VSDII, rearrangement and contraction of the intracellular gate is achieved through concerted movements of adjacent segments, including S4-5I, S4-5II, S5II, and all S6 segments. Our studies provide important insight into the electromechanical coupling mechanism of the single-chain voltage-gated ion channels and afford molecular interpretations for a number of pain-associated mutations whose pathogenic mechanism cannot be revealed from previously reported Nav structures.
The voltage-gated sodium (Nav) channel Nav1.7, encoded by SCN9A, is highly expressed in the pain-sensing neurons at the dorsal root ganglia and amplifies small membrane depolarizations for action potential firing. Loss of Nav1.7 function can abolish pain sensation, while potentiation of its channel activity is associated with extreme pain disorders. Nav1.7 has thus been targeted for developing next-generation pain killers (1–7). It is critical to capture the structures of Nav1.7 in multiple functional states to facilitate drug discovery.
Unlike the homotetrameric Kv and bacterial Nav channels, eukaryotic Nav channels, as well as Cav channels, are made of one single-polypeptide chain that comprises four homologous transmembrane repeats (8, 9) (SI Appendix, Fig. S1). Similar to all other voltage-gated ion channels, Nav channels contain two basic functional modules, the central ion-conducting pore domain (PD) and four flanking voltage-sensing domains (VSDs). Each VSD consists of four transmembrane segments (S1 to S4), among which S4 is responsible for detecting membrane potentials (9, 10). Four to six Arg or Lys residues, known as the gating charges (GC), occur at three-residue intervals on S4 (11). These positively charged residues face the cytosolic side at resting membrane potential, defined as the “down” or deactivated state. Upon membrane depolarization, GC residues move toward the extracellular side to reach an “up” or activated state (12).
Two to three GCs are transferred in each VSD during Nav channel activation (13). The conformational shifts of the VSDs are allosterically transmitted to the PD to control pore gating, a process known as the electromechanical coupling (EMC) (14). The primary steps in an EMC cycle of Nav channels include transition from the resting to the activated state in response to membrane depolarization (activation), closure of the channel in millisecond-scale after activation (fast inactivation), and return to the resting state upon hyperpolarization (deinactivation) (15).
Prior to structural elucidation, decades of electrophysiological, biophysical, and pharmacological characterizations have predicted the following conformational features for the major states (15, 16). At the resting state, the VSDs are “down” and the PD is closed. The activated state has opposite conformations, with “up” VSDs and conductive PD. Nav channels can inactivate through both fast and slow mechanisms. An inactivated channel is nonconductive and the VSDs are at least partially up because of the depolarized membrane potential. Whereas fast inactivation is executed by the Ile/Phe/Met (IFM) motif, which is positioned on the short linker between repeats III and IV (the III-IV linker) (17), the determinants and mechanism for slow inactivation remains elusive.
As the resting state of WT Nav channels is held by a strong electric field across the membrane and the activated state is only transient, the inactivated conformation may represent the most stable state in the absence of transmembrane voltages or gating modifiers. Indeed, the majority of the resolved structures of unmodified eukaryotic Nav channels shares a similar conformation that is featured with loosely closed PD and up VSDs, consistent with the inactivated state. Of note, the IFM motif wedges into a receptor site that was first discovered in the structure of EeNav1.4 (Nav1.4 homolog from electric eel) and then observed in all human and rat Nav channel structures (18–26). Based on the structures and reported functional characterizations, we proposed a “door wedge” allosteric blocking mechanism for fast inactivation, wherein insertion of the IFM motif in the accommodation site that is at the corner outside S6III and S6IV drives the contraction of the S6 helical bundle to close the intracellular gate (18).
The outlier in the Nav structure gallery is NavPaS, the first eukaryotic Nav channel whose structure was resolved (27). The GC residues in VSDs I, II, and IV of NavPaS are 1 to 1.5 helical turns lower than the corresponding ones in the vertebrate Nav structures; the PD is tightly closed without fenestrations; and the S4-5 constriction ring is more contracted. The most distinctive feature occurs in the III-IV linker, which cofolds with a globular carboxy terminal domain, keeping the IFM-corresponding residues far from the binding site. Unfortunately, the functional state of the NavPaS structure cannot be defined due to the difficulty of recording NavPaS.
To dissect the EMC of Nav channels, various strategies have been employed to lock the channels in different states. A chimera was generated by grafting VSDIV and eight residues on the adjacent S5I from Nav1.7 to the scaffold of NavPaS. The S4 segment in the grafted VSDIV is one helical turn higher than that in the original NavPaS-VSDIV. Upon binding to AaH2, an α-scorpion toxin, two GC residues are transferred toward the cytosolic side (28). Similarly, the S4IV segment of rat Nav1.5 also moves down by two GC residues in the presence of another scorpion toxin LqhIII (29). Despite the conformational changes of VSDIV in these channels, the PD remains nearly unchanged, preventing mechanistic dissection of the EMC.
Here we report structure-based engineering of a functional Nav1.7 variant that exhibits substantially right-shifted voltage dependence for activation. Structural comparison of this variant, in which VSDI is in a completely down state, with WT Nav1.7 (26) reveals multimodal conformational changes of VSDI and VSDII that are propagated to intracellular gating through cross-talk between adjacent segments. A conserved WNФФD motif (Ф for hydrophobic residue) on the S3I segment is discovered to stabilize the down conformation of VSDI.
Results
Structure-Guided Engineering of Right-Shifting Nav1.7 Variants.
Toward the goal of capturing Nav1.7 in a resting state, we set out to introduce point mutations that might lead to the right shift of activation and steady-state inactivation threshold beyond 0 mV. Mutations were selected based on the following rationales.
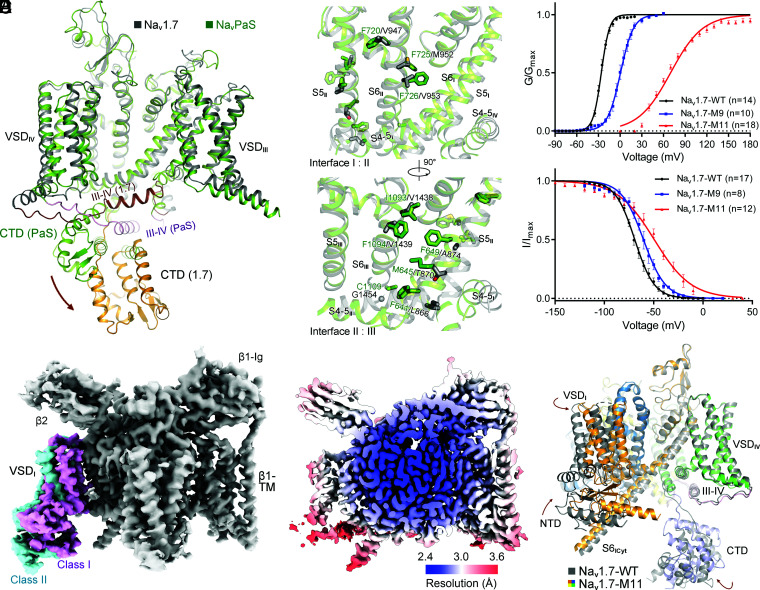
First, the lack of fenestration on the PD of NavPaS (30) suggested that more energy, equivalent of more depolarized voltage, may be required to disrupt the interactions of the pore-forming segments for channel opening. Indeed, sequence comparison of NavPaS and human Nav channels reveals that the former contains larger number of bulky residues on S5 and S6 in repeats II and III. We thereby replaced the small fenestration-constituting residues in Nav1.7 with the corresponding larger ones from NavPaS (Fig. 1A and B and SI Appendix, Fig. S1). A combination of nine point mutations—L866F, T870M, and A874F on S5II; V947F, M952F, and V953F on S6II; and V1438I, V1439F, and G1454C on S6III (the resulting variant named as Nav1.7-M9)—shifted the V1/2 of the activation curve of Nav1.7 from −26.00 ± 0.26 mV to −1.59 ± 0.33 mV and the inactivation curve from −69.21 ± 0.37 mV to −60.40 ± 0.56 mV (Fig. 1B and C and SI Appendix, Fig. S2A and Table S1).
Fig. 1.
A rationally designed Nav1.7 variant with right-shifted voltage-dependence for activation and inactivation displays marked conformational shifts from the WT channel. (A) Distinct conformations of WT human Nav1.7 and NavPaS. Shown here is a side view of the superimposed α subunit of Nav1.7 and NavPaS (PDB ID codes: 7W9K and 6A95, respectively). The brown arrow indicates the swing of the carboxy terminal domain (CTD) from NavPaS to Nav1.7. (B) Structure-guided introduction of mutations that may strengthen the interactions between adjacent repeats in the PD of Nav1.7. Nine small residues on S5II, S6II, and S6III in Nav1.7 are replaced by the corresponding ones in NavPaS, which contains more bulky residues on the interface of adjacent repeats. The Nav1.7 variant containing L866F, T870M, A874F, V947F, M952F, V953F, V1438I, V1439F, and G1454C is designated Nav1.7-M9. (C) Right shift of both activation and inactivation curves of Nav1.7 variants. V1/2 of the activation curve for Nav1.7-M9 is shifted from −26.02 ± 0.24 mV to 0.55 ± 0.43 mV, and that of the inactivation curve changes from −69.21 ± 0.37 mV to −60.40 ± 0.56 mV. Two additional mutations, E156K on S2I and G779R on S2II, led to a pronounced right shift of the activation threshold with the V1/2 reaching 69.37 ± 0.80 mV. This variant, designated Nav1.7-M11, was applied for cryo-EM structural analysis. Data points are presented as mean ± SEM and n is the number of recorded cells. Statistical significance was assessed using one-way ANOVA analysis. (D) Two distinct conformations of VSDI were observed in the 3D EM reconstructions of Nav1.7-M11. For simplicity, the label Nav1.7-M11 or M11 refers to class I hereafter, unless otherwise indicated. (E) Heat map for resolution distribution of M11. Local resolutions were calculated in RELION 3.0. (F) Overall structural shifts between M11 and WT. Shown here is a side view of the superimposed structures. Structures of the β1 and β2 subunits are omitted in all structure figures. The brown arrows indicate the conformational shifts from WT (gray) to M11 (domain colored). Movies S1–S4 illustrate the conformational changes presented in different figures. Maps were prepared in Chimera (48) and structure figures were prepared in PyMol (49).
Second, numerous disease-related mutations have been characterized to cause positive shift of the activation voltage. Inspired by our structural analysis of Nav1.5 disease mutations (24, 25), two right-shifting point mutations, E156K on S2I and G779R on S2II (31, 32), were introduced to Nav1.7-M9, and the resulting variant Nav1.7-M11 exhibited a markedly right-shifted and less steep activation curve with the V1/2 reaching 69.37 ± 0.80 mV. No current could be recorded below 30 mV for Nav1.7-M11. The inactivation curve is also right-shifted with a reduced slope, V1/2 reaching −49.44 ± 0.95 mV (Fig. 1C and SI Appendix, Fig. S2A and Table S1).
When the preholding potential is set at −120 mV, Nav1.7 completely activates at 0 mV, followed by fast inactivation (Fig. 1C and SI Appendix, Fig. S2A and Table S1). But when held for prolonged time at more depolarized potentials, such as −70 mV or −50 mV, the WT channel can enter closed-state inactivation (CSI) (SI Appendix, Fig. S2B) (33, 34). Our electrophysiological characterizations suggest that while Nav1.7-WT and Nav1.7-M9 likely display conformations of open-state inactivation (OSI) after protein extraction and purification in the absence of any electric field, Nav1.7-M11 may be trapped in the CSI state. To examine potential conformational differences between the two states, we set out to solve the structure of Nav1.7-M11.
Two Distinct Conformations of VSDI in Nav1.7-M11.
Following our established protocols (26), a three-dimensional (3D) electron microscopy (EM) reconstruction for Nav1.7-M11 in the presence of the β1 and β2 subunits were obtained at an averaged resolution of 2.8 Å of 912,322 selected particles. Unlike Nav1.7-WT, VSDI was only resolved to 8 to 10 Å at this stage. Combining maximum-likelihood–based classification and local refinement, two classes of 3D reconstructions were obtained with overall resolutions of 2.7 Å (class I) and 2.8 Å (class II) (Fig. 1D and SI Appendix, Figs. S3 and S4 and Table S2). The two classes only diverge in VSDI, which rotates by ∼20° around the PD (Fig. 1D and SI Appendix, Fig. S5). Structural comparison shows that class II is more similar to WT (SI Appendix, Fig. S5). We will mainly focus on class I, whose overall resolution reaches 2.7 Å (Fig. 1E), for analysis. For simplicity, Nav1.7-M11 or M11 hereafter refers to the class I structure if not otherwise indicated.
When the structures of M11 and WT are superimposed, the extracellular loops, the extracellular half of the PD, VSDIII and VSDIV remain identical, while all other segments, exemplified by VSDI, undergo conformational changes to different degrees (Fig. 1F and Movie S1). In the following, we will illustrate the major structural rearrangements from WT to M11 and present an analysis of the coupling mechanism. Movements of structural segments toward the extracellular and intracellular side will be described as upward and downward, respectively, and motions toward the center and periphery of the channel in the membrane-parallel plane will be described as inward and outward, respectively.
Completely Deactivated (Down) VSDI.
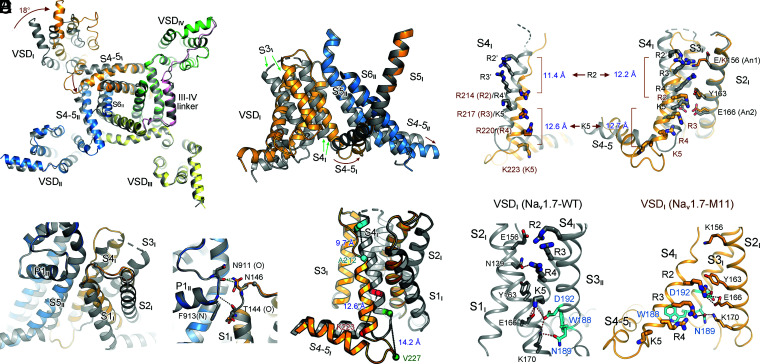
VSDI contains four GC residues: Arg214 (R2), Arg217 (R3), Arg220 (R4), and Lys223 (K5). In the context of the overall structural comparison of the α-subunit from WT and M11, VSDI pivots around the extracellular contact point between S1I and the PD (Fig. 2A and B). In addition to a ∼20° domain-wise rotation of VSDI, the movement of S4I combines several modes of structural transitions, resulting in the transfer of three GC residues, R2 to R4, across the occluding residue, Tyr163 on S2I, to the intracellular side in M11 (Fig. 2C and D and Movies S1–S3). It is noted that rotation of VSDI is also observed in the cryo-EM structures of Nav1.8, in which multiple conformations for VSDI were resolved. Despite substantial structural rearrangements, all the VSDI conformers in Nav1.8 remain in the up states (35).
Fig. 2.
Multimodal conformational changes of VSDI between its up and down states. (A) Large-degree conformational changes between M11 and WT mainly occur in repeats I and II. (Left) An intracellular view of the superimposed α subunits from M11 (domain colored) and WT (dark gray). Intracellular segments are omitted for visual clarity. (Right) A side view of the rotation of VSDI and the ensuing S4-5I segment. Brown arrows indicate the shift of the corresponding segments from WT to M11. (B) The pivot for VSDI rotation. VSDI swings around the contact between the extracellular tips of S1I and P1II. (Right) The interactions between the carbonyl oxygen of Thr144 and the side chain of Asn146 in VSDI with main-chain groups of the starting turn of P1II helix are preserved in M11 and WT, representing the pivoting point for VSDI rotation. (C) Dissection of conformational changes of VSDI between the up (WT) and down (M11) states. An enlarged view of the superimposed VSDI and the S4-5I linker from A is shown. Structural shifts of S4I combine helical unwinding, tilted sliding, and axial rotation. Cα atoms of the residues that mark the termini of S4I helices in the two structures are shown as spheres. The linear displacement of the Cα atoms of these residues in the context of overall structural comparison are indicated. S1–S3I segments rotate around S4I. (D) Downward transfer of three GC residues in VSDI from WT to M11. The linear displacement of the Cα atoms of R2 and K5 residues, which mark the two ends, in the context of overall structural comparison (Left) and individual VSDI comparison (Right) are indicated. The WT residues are labeled with apostrophe. (Right) When the structures of VSDI are individually compared, S1–S3 can be completely overlaid. (E) A conserved WNФФD motif on the cytoplasmic terminus of S3I stabilizes the GC residues in the down conformation of VSDI. Ф stands for hydrophobic residues. (Left) Coordination of GC residues in the up VSDI in WT. S1I is omitted for visual clarity. (Right) R2–R4 are stabilized by the WNФФD motif in the down VSDI in M11. The top GC residue R2 forms cation–π interaction with the occluding Tyr163 on S2I and electrostatic interaction with Asp192 in the WNФФD motif. Red and black dashes indicate distances of 2.8 to 3.5 Å and 4.5 to 5.5 Å, respectively.
S4I undergoes a spiral and tilted sliding toward the cytosol from WT to M11 with the backbone traversing the membrane by 11 to 14 Å (Fig. 2C and D). Unexpectedly, the last one-and-half helical turns of S4I in WT, consisting of residues 222LKTISV227, unwind to become an extended linker that connects S4I and S4-5I. The extracellular end of S4I is also unwound by half a helical turn. Together, S4I in WT comprises two more helical turns than that in M11 (Fig. 2C and D).
When individual VSDI is compared between WT and M11, there is no internal movement among S1–S3I segments. They rotate as a rigid body accompanying the gating charge transfer (Fig. 2D). The driving force for the rotation of S1–S3I appears to be the sliding motion and the secondary structural shift of S4I on the extracellular side. When the extracellular segment of S4I is unwound from WT to M11, S3I is dragged toward the PD. When S4I moves upward, the restored helical turns, which requires more space, pushes S3I away (Fig. 2C and D and Movie S2).
Due to the unwinding of the cytosolic helical turns of S4I, the ensuing S4-5I linker, which rotates toward S4-5II, remains at similar height in these two structures despite the pronounced downward translocation of S4I. Therefore, the different directions of motions, vertical for S4I and horizontal for S4-5I, are reconciled through secondary structural transformation of S4I (Fig. 2A and C and Movie S2).
The structure of VSDI in Nav1.7-M11 represents a VSD in the completely deactivated or resting state, as all its GC residues face the cytosolic side. Compared to the up state in the WT, GC residues are coordinated by a different set of polar residues (Fig. 2E). In the down conformation, the top R2 is most stably anchored, through cation–π interaction with the occluding Tyr163 on S2I and polar interaction with the invariant Asp192 on S3I. R3 is out of reach for direct hydrogen bonds (H-bonds) with any surrounding residues, but it may be coordinated by Glu166 on S2I and Asn189/Asp192 on S3I through water-mediated H-bonds. R4 may also form water-mediated H-bonds with Asp186 on the S2-3I linker and Asn189 on S3I, in addition to cation–π interaction with Trp188 (Fig. 2E, Right).
Among the GC-coordinating residues in the down state, Tyr163, Glu166, and Asp192 constitute the conserved charge transfer center (36). Of particular note, Asp192 and its preceding sequence on the cytosolic end of S3I, 186DPWNWL191D192, are invariant in all VSDI of human Nav channels (SI Appendix, Fig. S1). Sequence analysis shows that the WNФФD (Ф representing hydrophobic residues) motif is conserved in all VSDs except VSDIII, where Asn is replaced by Cys (SI Appendix, Fig. S1). Supporting the functional significance of this motif, Nav1.1 variants that each contain a single point substitution—W190R, N191K, N191Y, D194G, and D194N—are all associated with epileptic encephalopathy, early infantile, 6 (EIEE6), one of the most severe generalized epilepsies (37–40). Structure of Nav1.7-VSDI in the down conformation thus reveals the molecular basis for the functional significance of this conserved motif.
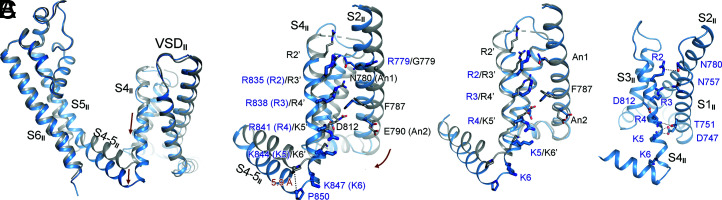
One GC Transfer in VSDII.
The structures of VSDII are nearly identical in the two classes of Nav1.7-M11, except for minor rotation of side chains (SI Appendix, Fig. S5E). One GC residue moves down from that in WT, placing both K5 and K6 below the occluding Phe787 (Fig. 3A). The GC transfer is also accompanied with a subtle but discernible overall domain rotation (Fig. 3A and Movie S2). The transfer of one GC is achieved through helix sliding of S4II by about 5-Å displacement of the backbone (Fig. 3B and Movie S2). There is no secondary structural transition of S4II. In contrast to the relatively minor shift of S4II, the S4-5II segment undergoes a marked dislocation. The entire helix drops down almost parallelly to the cytoplasm by ∼6 Å (Fig. 3A).
Fig. 3.
Structural changes of VSDII between Nav1.7-M11 and Nav1.7-WT. (A) Conformational changes of S1–S6 in repeat II between M11 and WT. (Left) The two structures are superimposed as in Fig. 2A. Shown here is a side view of the superimposed repeat II. Although the shift of VSDII is to a smaller degree than that of VSDI, the S4-5II, S5II, and S6II segments display pronounced changes. (Right) VSDII undergoes a minor rotation around the PD accompanying the transfer of one GC residue. In the WT channel (dark gray), R2′–R4’ of VSDII are above the occluding residue Phe787. In M11 (blue), only R2 and R3 remain above. (B) GC transfer in VSDII is mainly achieved through helix sliding of S4II. S1II is omitted for visual clarity. (C) Coordination of the GC residues in M11-VSDII by polar residues. Potential direct and water-mediated hydrogen bonds are indicated by black dashed lines.
Coordination of the GC residues in VSDII in this conformation mainly involves water-mediated electrostatic interactions between R2-K5 and several polar residues on S1II (Fig. 3C). The top R2 interacts with Asn780 on S2II. R3 is out of reach of any surrounding residues for H-bond formation, but it may be coordinated by Asn757 on S1II through water-mediated H-bonds. R4 may form direct or water-mediated H-bonds with Thr751 on S1II and Asp812 on S3II, respectively. K5 is stabilized by Asp747 on S1II.
A Tightly Closed PD in Nav1.7-M11.
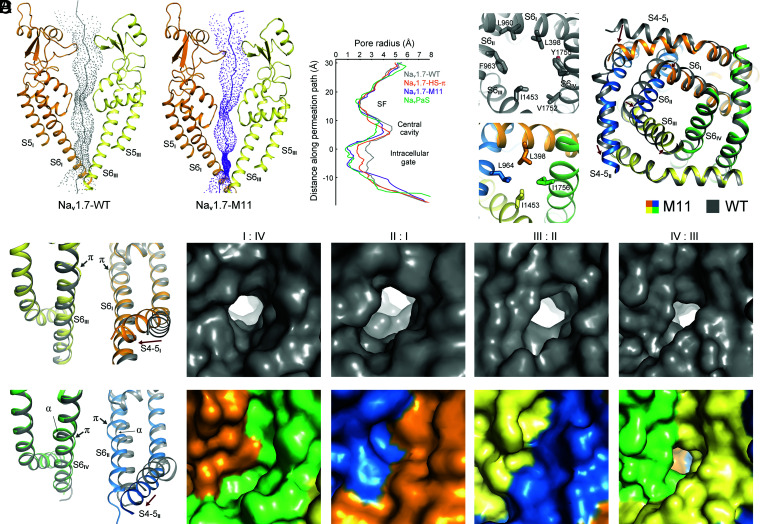
In addition to the conformational shifts of VSDI and VSDII, M11 lacks the detergent molecule that penetrates the intracellular gate, a shared feature in all structures of ligand-free WT human Nav channels. Indeed, calculation of the channel permeation path shows that the radius of the constriction point of the intracellular gate is only ∼0.9 Å in M11, shortened by ∼1.5 Å from that of the WT channel. It is also narrower than that of Nav1.7(E406K) in the presence of Huwentoxin-IV (designated Nav1.7-HS) (26). The overall contour of the permeation path of M11 is largely similar to that of NavPaS, although their PD segments still deviate considerably (Fig. 4A and SI Appendix, Fig. S6).
Fig. 4.
The PD of Nav1.7-M11 is substantially contracted compared to the WT channel. (A) The radius of the intracellular gate of M11 is reduced by over 1.5 Å compared to WT. The permeation paths of WT and M11 were calculated in HOLE (50). The corresponding pore radii are compared (Right). HS–π refers to the structure of HWTX-IV and saxitoxin-bound Nav1.7 (PDB ID code 7W9P). NavPaS refers to the structure in the presence of Dc1a and tetrodotoxin (PDB code ID 6A95). HS–π and NavPaS are included to indicate the level of PD contraction from WT to M11. (B) Contraction of the pore-forming segments of M11. (Left) Comparison of the intracellular gate of WT (Upper) and M11 (Lower). The gating residues are shown as sticks. (Right) An intracellular view of the superimposed PD of M11 and WT. Shifts of the corresponding segments from WT to M11 are indicated by red arrows. Contraction of the gate is mainly caused by rotation and inward motion of S6II. (C) All the S6 segments in M11 contain π helical turns. Side views of the PD from diagonal repeats of superimposed WT and M11 are presented. In WT channel, only S6I and S6III possess π-helical turns. (D) Only one small fenestration remains on the interface of repeats III and IV in the structure of M11. Corresponding side views of the surface presentation of the four side walls of the PD in WT (Upper) and M11 (Lower) are shown.
The composition of gating residues from the S6 tetrahelical bundle is different between WT and M11. In WT, the contour of the intracellular gate is of an oval shape, constituted by six residues: Leu398, Leu960, Phe963, Ile1453, Val1752, and Tyr1755. In contrast, four residues—Leu398, Leu964, Ile1453, and Ile1756—interact closely to seal the intracellular gate of M11 (Fig. 4B, Left, and Movie S3). Closure of the intracellular gate is a result of swing motion of S6II and S6III as well as α→π transition of S6II and S6IV (Fig. 4B, Right, Fig. 4C, and Movie S3). In M11, all four S6 segments contain one π helical turn in the middle (Fig. 4C). Accompanying these rearrangements, the central cavity shrinks (Fig. 4A) and the side walls of the PD other than the III and IV interface have no fenestrations (Fig. 4D).
In the following session, we will analyze the allosteric coupling of pore gating with the structural shifts of VSDI and VSDII through cross-talk of adjacent segments in all repeats.
EMC through Concerted Motions of Adjacent Segments.
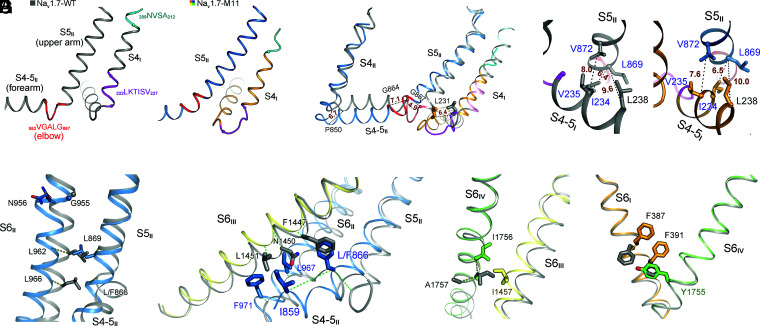
As VSDI undergoes the most dramatic conformational transition, we attempt to delineate the route of EMC from VSDI to the intracellular gate. Three coupling nodes are found, one between S4-5I and the joint connecting S4-5II and S5II (Fig. 5A–C), next between S5II and S6II (Fig. 5D), and finally the cross-talk among all S6 segments (Fig. 5E–G). Movement of S4II may also contribute to the shift of S4-5II and S5II.
Fig. 5.
Electromechanical coupling of GC transfer to intracellular gating through concerted motions of adjacent segments. (A) Prominent secondary structure transition of the adjacent S4I and S4-5II segments. The corresponding sequences that undergo helical winding/unwinding between WT and M11 are highlighted with the same color in the two structures. Of note, the unwound sharp turn (elbow, colored red) between S4-5II and S5II in WT is transformed to a curved helical turn in M11. (B) Concerted structural shifts of S4-5I segments with the adjacent S4-5II and S5II segments. Structures of WT (gray) and M11 (domain colored) are superimposed as in Fig. 2A. Displacement of the Cα atoms of terminal residues on S4I, S4-5I, S4-5II, and S5II are indicated with dashed lines and labeled in ångstroms. (C) A hydrophobic cluster on the interface of S4-5I and S5II couples the concerted motions of these two segments. The distances between the indicated Cα atoms are labeled in ångstroms. (D) Concerted motions of S6II and S5II. The Cα atoms of the corresponding residues in the superimposed M11 and WT structures are connected by dashed lines to indicate their displacements. Leu962 and L966 on S6II would respectively clash with Leu869 and Leu866 (mutated to Phe in M11) on S5II had S6II not moved concertedly. (E) S6III is pushed inwardly by S4-5II and S6II. Displacements of the Cα atoms of the corresponding residues in M11 and WT are indicated by dashed lines. Representative residues in WT and M11 are shown to indict potential clashes should S6III not move accordingly. (F) Concerted motions of S6III and S6IV. S6IV undergoes an α→π transition that avoids clash between Ile1457 and Ile1756. (G) The subtle shift of S6IV leads to local change of S6I. Rotation of the aromatic rings of Phe387 and Phe391 seals the fenestration on the interface of repeats I and IV (Fig. 4D). It is noted that the conformational changes from WT to M11 depicted in F and G are nearly identical to those of Nav1.7 upon HWTX-IV binding to VSDII (26).
In all previously reported structures of eukaryotic Nav channels, NavPaS included, S4-5II (forearm) and S5II (upper arm) are connected by a sharp turn (elbow). In M11, the “arm” extends forward and the elbow becomes a smoothly curved helix (Fig. 5A). This structural transition appears to be mainly driven by the shift of S4-5I, whose N terminus moves toward the elbow by a displacement of over 6 Å (Fig. 5B). The distance between the N terminus of S4-5I and the cytoplasmic end of S5II remains nearly unchanged despite the marked conformational difference between WT and M11, demonstrating coupled motions.
The coupling is mediated by a conserved hydrophobic cluster composed of Ile234, Val235, and Leu238 on S4-5I and Leu869 and Val872 on S5II (Fig. 5C, SI Appendix, Fig. S1, and Movie S2). While S4-5I mainly pushes the elbow and S5II, the downward sliding of S4II may drag the overall S4-5II helix to sink toward the cytoplasmic side (Fig. 3A). As there is only one GC transfer in VSDII, the physiological coupling between S4II and S4-5II awaits further examination.
Tilting of S5II directly drives S6II movement (Fig. 5D and Movie S3). If S6II had not shifted concertedly, there would be steric clashes between residues on S6II and S5II (Fig. 5D). Motions of these repeat II segments further propagate to S6III; the latter has to tilt toward S6IV to avoid clash with residues on S4-5II, S5II, and S6II (Fig. 5E). The middle turn of S6IV undergoes an α→π transition, replacing Ile1757 with a smaller Ala1757 to avoid collision with the shifted Ile1457 on S6III (Fig. 5F). Finally, to avoid colliding with Tyr1755 on S6IV, S6I slightly moves inward and the aromatic rings of Phe387 and Phe391 rotate up, eliminating the fenestration between repeats I and IV (Fig. 5G). It is noted that the coupled conformational changes from S6III to S6I described in Fig. 5F and G are identical with those observed in Nav1.7 upon toxin binding to VSDII (26).
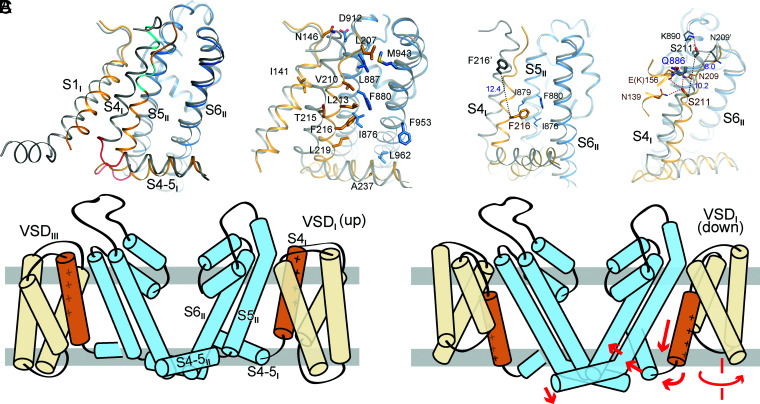
Substantial Rearrangement of the Interface between VSDI and the PD.
The motions of VSDI and VSDII relative to the PD lead to the rearrangement of their respective interface with the neighboring pore segments (SI Appendix, Fig. S7). As S5III remains unchanged and S4II only undergoes sliding by one helical turn, their interface is mediated by a similar set of residues except for the shift of their relative positions (Movie S2). In contrast, the interface of VSDI and the PD undergoes substantial rearrangements. The contact between VSDI and S5II is more extensive in M11 than in WT, with a dozen additional interacting residues (Fig. 6A and SI Appendix, Fig. S7).
Fig. 6.
Structural interpretation of representative Nav1.7 mutations related to pain disorders. (A) Rearrangement of the interface between VSDI and the PD. (Right) More residues are engaged in the interactions of the down VSDI with S5II and S6II. Shown here are the additional residues that mediate the VSDI–PD interface in Nav1.7-M11. Please refer to SI Appendix, Fig. S7 for detailed comparison of the VSDI–PD interface in WT and M11. (B) Mechanistic interpretation for representative primary erythermalgia mutations that map to the interface of S4I and S5II. (Left) F216S may destabilize the interaction between the down VSDI and S5II. (Right) S211P and Q886E may both alter the interactions between the down VSDI and the PD. Residues in WT are labeled with apostrophe. (C) A schematic summary of the propagation of conformational changes for EMC when VSDI moves down. The WT and M11 structures likely represent the conformations of OSI and CSI, respectively.
Discussion
Structural Insight into Mutations in Pain Disorders.
Structural comparison of WT and M11 affords important mechanistic insight into a number of disease-causing mutations that could not be interpreted from previous structures (SI Appendix, Table S3 and Movie S4). Instead of enumerating all concerned residues, we select several representative ones that are associated with primary erythermalgia (PERYTHM).
The PERYTHM mutation F216S causes left shift of the activation V1/2 by 11 mV (41). The functional role of Phe216, which is positioned on the second helical turn of S4I and points to the lipid bilayer in previous structures, was unclear. In the present deactivated VSDI, Phe216, which undergoes a marked spiral translocation through a linear displacement by more than 12 Å from the previous structures, is surrounded by three hydrophobic residues on S5II, Ile876, Ile879, and Phe880 (Fig. 6B, Left). Substitution of Phe with Ser may weaken the interaction of S4I with these hydrophobic residues and thus lower the energy barrier, equivalent of a less-depolarized voltage, for the upward movement of S4I upon depolarization.
Another PERYTHM-related mutation that led to left shift of the activation curve is S211P (42). In the previous structures, Ser is on the extracellular tip of S4I and close to Lys890, a residue on the extended helix of S5II. Such conformation cannot explain the left shift of the activation curve by the pathogenic mutation, as Ser→Pro at this position appears to disfavor the up state of VSDI (Fig. 6B, Right). In the structure of M11, Ser211, whose Cα atom moves down by ∼10 Å, points to the hydrophilic interior of VSDI (Fig. 6B). S211P may thus facilitate activation by destabilizing the hydrophilic cluster in the down state of VSDI. It is noted that the Ser211-coordinating residue Asn209 is close to Gln886 on S5II. Q886E is also a PERYTHM-related mutation, although its main disease-causing mechanism may be attributed to its increased stabilizing power of Arg214 (R2) in the up state (Fig. 6B).
In addition to the interface between VSDI and the PD, four of the five hydrophobic residues that mediate the coupling between S4-5I and S5II are susceptible to disease-related mutations (Fig. 5C). Nav1.7 variants, I234T, L869F, and L869H, are found in patients with PERYTHM (43, 44). In addition to pain-related mutations in Nav1.7, a Nav1.5 variant, V240M (equivalent of V235M in Nav1.7), has been identified in patients with Brugada syndrome or type 3 long QT syndrome (23, 24, 45). Substitutions of Nav1.1-Val896 (equivalent of Nav1.7-Val872) with bulkier residues, Phe, Ile, or Leu, are associated with different forms of epileptic seizures (23). These pathogenic mutations support the structurally-revealed key role of this conserved hydrophobic cluster in EMC.
Closed-State Inactivation.
Nav1.7-M11 activates only when the membrane voltage is above 30 mV (Fig. 1C). No current was ever recorded when the preholding potential was set to 0 mV. Therefore, the structure of Nav1.7-M11 should represent the CSI conformation. The structure is largely consistent with previous reports of activated VSDIII and VSDIV, deactivated VSDI and VSDII, and a closed pore for CSI of Nav channels. Although VSDII is not completely deactivated, it can be related to voltage-dependent conformations associated with CSI (34).
Our recently reported structure of human Cav2.2 also has VSDII down and the other VSDs up (46). While we were performing more comprehensive experiments before assigning a functional state for that structure, Dong et al. (47) defined it as the CSI state. Indeed, the structures of Cav2.2 and Nav1.7-M11 share common features of one deactivated VSD and tightly closed PD. Other Nav and Cav channels structures that are featured with all up VSDs and loosely closed PD likely correspond to the OSI conformation.
Our original goal was to capture the structure of Nav1.7 in the resting state; M11 represents a milestone toward this goal. Structural comparison of WT and M11 affords unprecedented insight into the EMC mechanism (Fig. 6C). Our present study exemplifies the importance of structural resolution of Nav channels in different states, as the functional significance of many residues may not be clear in one conformation. Only in the structure of M11 did we discover the conserved WNФФD motif (Fig. 2D) and the coupling cluster between S4-5I and S5II (Fig. 5C), and establish the structure–function relationship of a number of pathogenic mutations (Fig. 6B). These characterizations lay the foundation to dissect the delicate EMC process of the asymmetric, single-chain Nav as well as Cav channels and shed light on drug development targeting these channels.
Supplementary Material
Acknowledgments
We thank Xiaomin Li, Fan Yang, and Jianlin Lei for technical support during electron microscopy image acquisition; The Tsinghua University Branch of the China National Center for Protein Sciences (Beijing) for supporting the cryoelectron microscopy facility; and the computational facility support from Tsinghua University Branch of China National Center for Protein Sciences (Beijing). This work was funded by Beijing Nova Program (Z191100001119127) from Beijing Municipal Science and Technology Commission. N.Y. has been supported by the Shirley M. Tilghman endowed professorship from Princeton University since 2017.
Footnotes
Reviewers: L.F., Stanford University; and V.Y.-Y., University of California, Davis.
The authors declare no competing interest.
See Profile, e2210772119, in vol. 119, issue 33.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2209164119/-/DCSupplemental.
Data Availability
Cryo-EM maps and structural model data have been deposited in the Electron Microscopy Database, https://www.ebi.ac.uk/emdb/ (EMDB codes EMD-33484 and EMD-33485) (51, 52) and the Protein Data Bank, http://www.wwpdb.org (PDB ID codes 7XVE and 7XVF) (53, 54).
References
- 1.Klugbauer N., Lacinova L., Flockerzi V., Hofmann F., Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J. 14, 1084–1090 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y., et al. , Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J. Med. Genet. 41, 171–174 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nassar M. A., et al. , Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. U.S.A. 101, 12706–12711 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummins T. R., Dib-Hajj S. D., Waxman S. G., Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J. Neurosci. 24, 8232–8236 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox J. J., et al. , An SCN9A channelopathy causes congenital inability to experience pain. Nature 444, 894–898 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett D. L., Woods C. G., Painful and painless channelopathies. Lancet Neurol. 13, 587–599 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Alsaloum M., Higerd G. P., Effraim P. R., Waxman S. G., Status of peripheral sodium channel blockers for non-addictive pain treatment. Nat. Rev. Neurol. 16, 689–705 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Ren D., et al. , A prokaryotic voltage-gated sodium channel. Science 294, 2372–2375 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Yu F. H., Yarov-Yarovoy V., Gutman G. A., Catterall W. A., Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol. Rev. 57, 387–395 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Long S. B., Campbell E. B., Mackinnon R., Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Bezanilla F., Gating currents. J. Gen. Physiol. 150, 911–932 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong C. M., Bezanilla F., Charge movement associated with the opening and closing of the activation gates of the Na channels. J. Gen. Physiol. 63, 533–552 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang N., George A. L. Jr., Horn R., Molecular basis of charge movement in voltage-gated sodium channels. Neuron 16, 113–122 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Long S. B., Campbell E. B., Mackinnon R., Voltage sensor of Kv1.2: Structural basis of electromechanical coupling. Science 309, 903–908 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Ahern C. A., Payandeh J., Bosmans F., Chanda B., The hitchhiker’s guide to the voltage-gated sodium channel galaxy. J. Gen. Physiol. 147, 1–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarov-Yarovoy V., et al. , Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc. Natl. Acad. Sci. U.S.A. 109, E93–E102 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West J. W., et al. , A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc. Natl. Acad. Sci. U.S.A. 89, 10910–10914 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Z., et al. , Structure of the Nav1.4-β1 complex from electric eel. Cell 170, 470–482.e11 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Pan X., et al. , Structure of the human voltage-gated sodium channel Nav1.4 in complex with beta1. Science 362, eaau2486 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Pan X., et al. , Molecular basis for pore blockade of human Na(+) channel Nav1.2 by the mu-conotoxin KIIIA. Science 363, 1309–1313 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Shen H., Liu D., Wu K., Lei J., Yan N., Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science 363, 1303–1308 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Jiang D., et al. , Structure of the cardiac sodium channel. Cell 180, 122–134.e10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan X., et al. , Comparative structural analysis of human Nav1.1 and Nav1.5 reveals mutational hotspots for sodium channelopathies. Proc. Natl. Acad. Sci. U.S.A.. 118, e2100066118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., et al. , Structure of human Nav1.5 reveals the fast inactivation-related segments as a mutational hotspot for the long QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 118, e2100069118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z., et al. , Structural basis for pore blockade of the human cardiac sodium channel Nav1.5 by the antiarrhythmic drug quinidine. Angew. Chem. Int. Ed. Engl. (2021). [DOI] [PubMed] [Google Scholar]
- 26.Huang G., et al. , High-resolution structures of human Nav1.7 reveal gating modulation through α-π helical transition of S6IV. Cell Rep. 39, 110735 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Shen H., et al. , Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 355, eaal4326 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Clairfeuille T., et al. , Structural basis of α-scorpion toxin action on Nav channels. Science 363, eaav8573 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Jiang D., et al. , Structural basis for voltage-sensor trapping of the cardiac sodium channel by a deathstalker scorpion toxin. Nat. Commun. 12, 128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen H., et al. , Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science 362, eaau2596 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Smits J. P., et al. , A mutation in the human cardiac sodium channel (E161K) contributes to sick sinus syndrome, conduction disease and Brugada syndrome in two families. J. Mol. Cell. Cardiol. 38, 969–981 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Potet F., et al. , Novel Brugada SCN5A mutation leading to ST segment elevation in the inferior or the right precordial leads. J. Cardiovasc. Electrophysiol. 14, 200–203 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Bean B. P., Sodium channel inactivation in the crayfish giant axon. Must channels open before inactivating? Biophys. J. 35, 595–614 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angsutararux P., Kang P. W., Zhu W., Silva J. R., Conformations of voltage-sensing domain III differentially define NaV channel closed- and open-state inactivation. J. Gen. Physiol. 153, e202112891 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang X., et al. , Structural basis for high-voltage activation and subtype-specific inhibition of human Nav1.8. Proc. Natl. Acad. Sci. U.S.A. (2022), 10.1073/pnas.2208211119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao X., Lee A., Limapichat W., Dougherty D. A., MacKinnon R., A gating charge transfer center in voltage sensors. Science 328, 67–73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J. W., et al. ; Epilepsy Genetic Study Group Japan, Prevalence of SCN1A mutations in children with suspected Dravet syndrome and intractable childhood epilepsy. Epilepsy Res. 102, 195–200 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Depienne C., et al. , Spectrum of SCN1A gene mutations associated with Dravet syndrome: Analysis of 333 patients. J. Med. Genet. 46, 183–191 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Mancardi M. M., et al. , Familial occurrence of febrile seizures and epilepsy in severe myoclonic epilepsy of infancy (SMEI) patients with SCN1A mutations. Epilepsia 47, 1629–1635 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Heron S. E., et al. , De novo SCN1A mutations in Dravet syndrome and related epileptic encephalopathies are largely of paternal origin. J. Med. Genet. 47, 137–141 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Choi J. S., Dib-Hajj S. D., Waxman S. G., Inherited erythermalgia: Limb pain from an S4 charge-neutral Na channelopathy. Neurology 67, 1563–1567 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Estacion M., et al. , Can robots patch-clamp as well as humans? Characterization of a novel sodium channel mutation. J. Physiol. 588, 1915–1927 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn H. S., et al. , A new Nav1.7 sodium channel mutation I234T in a child with severe pain. Eur. J. Pain 14, 944–950 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Huang W., Liu M., Yan S. F., Yan N., Structure-based assessment of disease-related mutations in human voltage-gated sodium channels. Protein Cell 8, 401–438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fatima A., et al. , The disease-specific phenotype in cardiomyocytes derived from induced pluripotent stem cells of two long QT syndrome type 3 patients. PLoS One 8, e83005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao S., Yao X., Yan N., Structure of human Cav2.2 channel blocked by the painkiller ziconotide. Nature 596, 143–147 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong Y., et al. , Closed-state inactivation and pore-blocker modulation mechanisms of human CaV2.2. Cell Rep. 37, 109931 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Pettersen E. F., et al. , UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 49.DeLano W. L., The PyMOL Molecular Graphics System (Delano Scientific, San Carlos, CA, 2002). PyMOL 2.5.2. http://pymol.org/2/. Accessed 16 March 2022. [Google Scholar]
- 50.Smart O. S., Neduvelil J. G., Wang X., Wallace B. A., Sansom M. S., HOLE: A program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360, 376 (1996). [DOI] [PubMed] [Google Scholar]
- 51.Huang G., et al., Data from “Unwinding and spiral sliding of S4 and domain rotation of VSD during the electromechanical coupling in Nav1.7.” Electron Microscopy Database. http://www.ebi.ac.uk/pdbe-srv/emsearch/atlas/33484_summary.html. Deposited 22 May 2022. [DOI] [PMC free article] [PubMed]
- 52.Huang G., et al., Data from “Unwinding and spiral sliding of S4 and domain rotation of VSD during the electromechanical coupling in Nav1.7.” Electron Microscopy Database. http://www.ebi.ac.uk/pdbe-srv/emsearch/atlas/33485_summary.html. Deposited 22 May 2022. [DOI] [PMC free article] [PubMed]
- 53.Huang G., et al., Data from “Unwinding and spiral sliding of S4 and domain rotation of VSD during the electromechanical coupling in Nav1.7.” Protein Data Bank. http://www.rcsb.org/pdb/explore/explore.do?structureId=7XVE. Deposited 22 May 2022. [DOI] [PMC free article] [PubMed]
- 54.Huang G., et al., Data from “Unwinding and spiral sliding of S4 and domain rotation of VSD during the electromechanical coupling in Nav1.7.” Protein Data Bank. http://www.rcsb.org/pdb/explore/explore.do?structureId=7XVF. Deposited 22 May 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cryo-EM maps and structural model data have been deposited in the Electron Microscopy Database, https://www.ebi.ac.uk/emdb/ (EMDB codes EMD-33484 and EMD-33485) (51, 52) and the Protein Data Bank, http://www.wwpdb.org (PDB ID codes 7XVE and 7XVF) (53, 54).