Abstract
Haemophilia A (HA) and B (HB) are X-linked hereditary bleeding disorders caused by lack of activity of coagulation factors VIII (FVIII) or IX (FIX), respectively. Besides conventional products, modern replacement therapies include FVIII or FIX concentrates with an extended half-life (EHL-FVIII/FIX). Two main strategies for measuring plasma FVIII or FIX activity are applied: the one-stage clotting assay (OSCA) and the chromogenic substrate assay (CSA), both calibrated against plasma (FVIII/FIX) standards. Due to the structural modifications of EHL-FVIII/FIX, reagent-dependent assay discrepancies have been described when measuring the activity of these molecules. Assay discrepancies have also been observed in FVIII/FIX gene therapy approaches. On the other hand, nonfactor replacement by the bispecific antibody emicizumab, a FVIIIa-mimicking molecule, artificially shortens activated partial thromboplastin time–based clotting times, making standard OSCAs inapplicable for analysis of samples from patients treated with this drug. In this review, we aim to give an overview on both, the currently applied and future therapies in HA and HB with or without inhibitors and corresponding test systems suitable for accompanying diagnostics.
Keywords: haemophilia A/B, factor concentrates, extended half-life products, emicizumab, gene therapy
Zusammenfassung
Bei der Hämophilie A (HA) und B (HB) handelt es sich um X-chromosomal vererbte Blutungsstörungen, die durch einen Mangel an Aktivität der Gerinnungsfaktoren VIII (FVIII) bzw. IX (FIX) verursacht werden. Im Rahmen entsprechender Substitutionstherapien kommen heute auch FVIII- oder FIX-Konzentrate mit einer verlängerten Halbwertszeit (EHL-FVIII/FIX) zum Einsatz. Zur Bestimmung der Aktivität von FVIII- bzw. FIX werden zwei grundlegend verschiedene Testprinzipien angewandt, Einstufen-Gerinnungstests (One-Stage Clotting Assay [OSCA]) und chromogene Testverfahren (CS-Assays [CSA]), welche gegen plasmatische Faktoren kalibriert werden. Aufgrund der strukturellen Modifikationen von EHL-FVIII/FIX sind testabhängige Diskrepanzen bei der Bestimmung der Aktivität dieser Moleküle beschrieben worden. Solche Diskrepanzen wurden auch im Rahmen der FVIII-/FIX-Gentherapie beobachtet. Unter Emicizumab, einem bispezifischen, monoklonalen Antikörper zur Behandlung der HA, sind die Gerinnungszeiten der aktivierten partiellen Thromboplastinzeit (aPTT) artifiziell verkürzt. Dadurch sind etablierte, aPTT-basierte Einstufen-Gerinnungstests für die Analyze von Proben von Patienten unter Emicizumab nicht anwendbar. Mit dieser Übersichtsarbeit soll ein Überblick sowohl über die Prinzipien der derzeit angewandten und zukünftigen Therapieoptionen als auch über entsprechende Testverfahren in der HA und HB gegeben werden.
Schlüsselwörter: Hämophilie A/B, Faktorenkonzentrate, verlängerte Halbwertszeit, Emicizumab, Gentherapie
Introduction
Haemophilia A (HA) and B (HB) are X-linked hereditary bleeding disorders caused by lack of activity of coagulation factors VIII (FVIII) or IX (FIX). 1 To date, treatment of HA and HB is dominated by replacement therapies using plasma-derived or recombinant FVIII/FIX concentrates, including those with an extended half-life (EHL-FVIII/FIX). 2 3 Especially in HA, a serious side effect of factor replacement remains the development of inhibitors. Approximately 30% of treated patients are affected, and require treatment with FVIII bypassing agents, such as recombinant activated FVII (rFVIIa) or plasma-derived activated prothrombin complex concentrate (aPCC). 4 Besides these established bypassing drugs, recombinant porcine FVIII has become available for the treatment of HA inhibitor patients. 5
In addition to conventional intravenous factor replacement or bypassing schemes, emicizumab, a subcutaneously administered, bispecific monoclonal antibody that mimics the cofactor functions of activated FVIII (FVIIIa), has recently been approved for the treatment of HA patients with or without inhibitors. 6 7 Furthermore, 20 years after the first attempts, gene therapy is becoming a real treatment option in both HA and HB. 8
For diagnosis and therapy monitoring, two main strategies for measuring plasma FVIII or FIX activity are applied in laboratories worldwide: the activated partial thromboplastin time (aPTT)-based one-stage clotting assay (OSCA) and the chromogenic substrate assay (CSA); both usually calibrated against plasma (FVIII/FIX) standard preparations. 9 Nevertheless, owing to different underlying assay principles, gene mutation-dependent assay discrepancies have been described, requiring the application of both assay types for initial diagnosis and staging of disease severity. 10 Regarding post-infusion monitoring, recombinant FVIII/FIX concentrates also show discrepant results, on the one hand between OSCA and CSA, and, on the other hand, between different types of OSCA. 11 Due to deviant characteristics of expressed molecules, assay discrepancies have also been observed in FVIII/FIX gene therapy. Moreover, emicizumab excessively shortens aPTT-clotting times, making this assay or aPTT-based standard OSC assays inapplicable for the analysis of samples obtained from patients treated with this FVIIIa-mimetic drug. 12
The aforementioned issues illustrate that monitoring of modern HA/HB treatment options, including EHL concentrates, gene therapy and nonfactor replacement (e.g., emicizumab), require correspondingly adapted and/or qualified assays to ensure accurate and meaningful results. In the present review, we aim to give an overview on the molecules and mechanisms behind these therapies and to describe suitable test systems.
Basic Principles of Functional Assays in Haemophilia Testing
FVIII/FIX Activity Assays
Two major strategies for measuring plasma FVIII or FIX activity are applied, the aPTT-based OSCA and the CSA. 9 The basic principles of these different types of assays are illustrated in Fig. 1 . In the OSCA, the patient sample is diluted either using FVIII- or FIX-deficient plasma, followed by an aPTT measurement. Under such conditions, the aPTT solely depends on the FVIII or FIX activity in the original sample. Although the aPTT is a two-step process (addition of the aPTT reagent and incubation, followed by addition of CaCl 2 as the starting reagent), aPTT-based determination of factor activity is designated as an OSCA. Hereby, formation of the tenase-complex (FIXa + FVIIIa) after recalcification and subsequent formation of prothrombinase, thrombin and fibrin proceeds without interruption ( Fig. 1A ). In contrast, the CSA is designed and denoted as a two-stage assay. During the first stage, the patient sample is strongly diluted and a specific reagent added ( Fig. 1B : FVIII CSA; Fig. 1C : FIX CSA) assembling the tenase complex. After incubation, during the second stage of the CSA, the amount of generated FXa, which reflects the FVIII or FIX activity in the original sample, is measured by addition of a corresponding chromogenic peptide substrate. Both the OSCA and the CSA are usually calibrated by the use of plasma standards calibrated against the World Health Organization (WHO) International Standard (IS) for factor VIII (and von Willebrand factor [vWF]) in plasma (available from the ‘National Institute for Biological Standards and Control’ [NIBSC], code: 07/316) or the WHO IS for blood coagulation factors II, VII, IX, X in plasma (NIBSC code: 09/172) by the manufacturer. 13 14
Fig. 1.
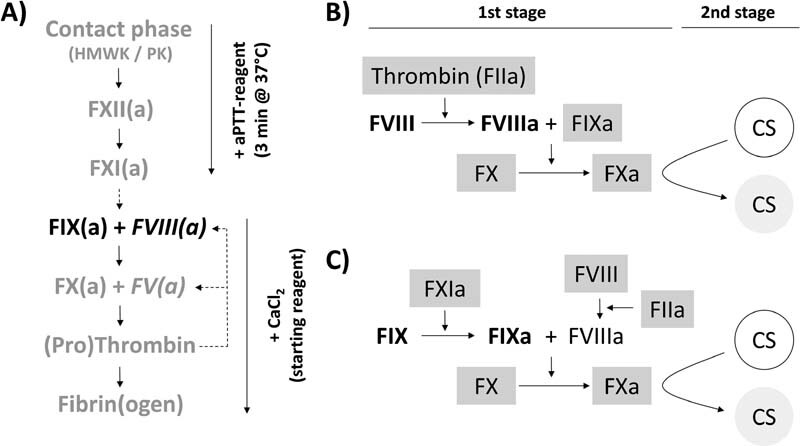
Schematic overview on the principles of FVIII/FIX one-stage clotting (OSC) and (two-stage) chromogenic substrate (CS) assays . ( A ) OSC assays. Due to dilution of the patient sample in FVIII- or FIX-deficient plasma (containing defined amounts of the remaining coagulation factors [grey characters]), either FVIII or FIX stemming from the patient sample (black characters) becomes the only variable that influences the activated partial thromboplastin time (aPTT) of the mixture: in brief, addition of the aPTT reagent activates the intrinsic, non-Ca 2+ -dependent pathway of the mixture (down to FXI[a]) during initial incubation. After recalcification, the clotting time, that, inter alia, depends on the thrombin-based feedback activation (dotted lines) of cofactors FV and FVIII (italic characters), is measured and FVIII or FIX activities calculated from corresponding plasma-based standard curves. Although the aPTT is a two-step process, aPTT-based determination of factor activity is designated as one-stage clotting assay since the activity of the forming tenase-complex (FIXa + FVIIIa) is reflected in one go by the clotting time measured after recalcification. ( B and C ) (Two-stage) CS assays for the determination of FVIII or FIX activity, respectively. During the first stage, plasma samples are diluted and reagents (factors) that allow for activation of plasma FVIII or FIX (bold characters), assembly of the tenase complex as well as activation of FX are added (characters with grey background). After incubation, the amount of generated FXa is quantified during the second stage of the procedure by addition of a chromogenic peptide substrate (CS). Underlying original plasma FVIII or FIX activities are again calculated from corresponding plasma calibrators.
Besides the general differences between OSCA and CSA, there are also significant methodological variances within these classes of assays. Regarding OSCAs, a multitude of differently composed aPTT reagents based on different contact phase activators (including ellagic acid, polyphenols, different types of silica, and kaolin) are available from different manufacturers and are used in laboratories worldwide. Within the class of CSAs, differences between assays mainly comprise the use of either human or bovine factors and differing factor compositions and concentrations. 15
Detection of FVIII/FIX Inhibitors ([Nijmegen]-Bethesda Assay)
The detection and quantification of antibodies that inhibit (neutralise) the activity of FVIII or FIX is usually done using the Bethesda protocol. 16 17 In case of FVIII inhibitor testing, the Nijmegen-modification (Nijmegen-Bethesda Assay [NBA]) is regularly applied. 18 The basic principle of the NBA is illustrated in Fig. 2 . In brief, (heat inactivated) patient sample and, if necessary, dilutions thereof are mixed (1 + 1) with (buffered) pooled plasma ([B]PP). After incubation, the remaining factor activities are determined. In parallel, a control reaction consisting of BPP and factor-deficient plasma or buffer is tested. The relative activity (RA) is calculated as shown in Fig. 2A . By definition, an inhibitor titre of one Bethesda unit (BU) per mL inhibits the factor activity by 50%, a titre of 2 BU/mL by 75% ( Fig. 2B ). To exclude any determinations based on borderline or low (poorly reproducible) factor activities, the range of valid RAs is limited from 75% (defining the detection limit of 0.42 BU/mL) down to 25%. Accordingly, samples with titres of greater than 2 BU/mL need further testing with increasing dilutions ( Fig. 2B, C ).
Fig. 2.
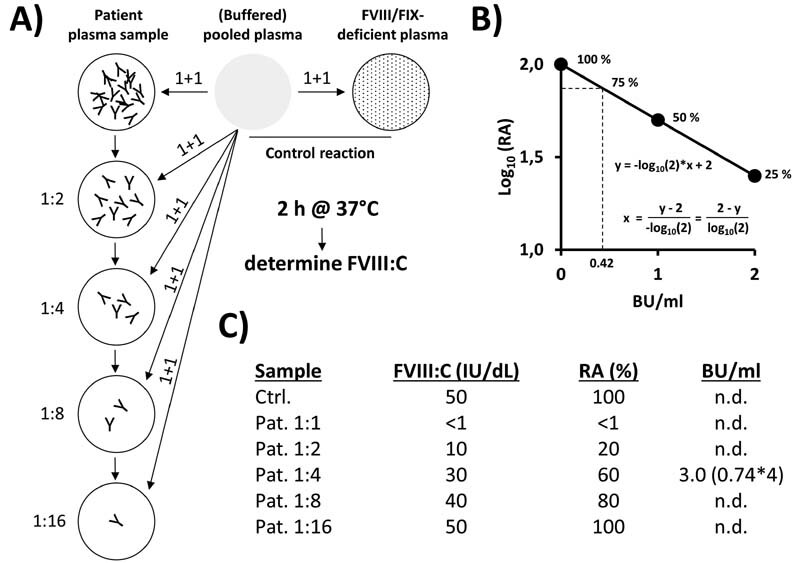
Principle of the (Nijmegen)-Bethesda Assay for quantitative determination of inhibitory antibodies against FVIII or FIX. (A) In an exemplary procedure, a heat-inactivated patient plasma sample with an inhibitor titre of 3 Bethesda units (BU)/mL is serially diluted in the respective factor-deficient plasma (or buffer) and the single dilutions are mixed with equal amounts (1 + 1) of (buffered) pooled plasma ([B]PP). As a control, the (B)PP is mixed 1 + 1 with factor-deficient plasma. After incubation (e.g., 2 hours at 37 °C for FVIII inhibitor testing, no such long incubation required for FIX inhibitor testing), (residual) factor activities are measured and calculated relative (%) to that of the control reaction (relative activities [RA], panel C ). ( B ) Inhibitor titres are usually given as BU/mL according to the definition that one BU/mL inhibits the FVIII/FIX activity by 50% (50% RA) and two BE/mL by further 50% (25% RA). The corresponding ideal interpolation function (including 0 BU/mL = 100% RA) that allows the calculation of BU/mL from determined RA values is shown in the graph (RA [ y -axis, log 10 ] versus BU/mL [ x -axis]). The defined range of valid RAs is limited from 75% down to 25%, necessitating the testing of sample dilutions in case of inhibitor titres of > 2 BU/mL. ( C ) Measured factor activities and calculated RA values for the exemplary inhibitor-positive plasma sample (dilutions). Only the 1:4 dilution shows a RA value (60%) within the defined acceptable range of 25 to 75%. Based on the formula shown in panel B , a BU/mL titre of 0.74 is calculated, corresponding to 3 BU/mL in the original sample after correction for the initial dilution.
Heat inactivation of patient plasma samples (e.g., 56 °C for 30 minutes) is considered as an important pre-analytical step to eliminate remaining factor activities and to liberate anti-FVIII antibodies bound to FVIII or cross-reacting material. 19 20 In principle, the kind of assay used for the measurement of remaining factor activities (OSC- or CS-assay) is of minor importance. However, the use of CSA formats may generally lower the potential interference of non-target inhibitory substances such as lupus anticoagulants and may therefore increase assay specificity. 21 22
Commonly Used OSC and CS Assay Reagents
A recent survey initiated by the section ‘haemophilia’ of the German (D), Austrian (A), and Swiss (CH) Society of Thrombosis and Haemostasis Research (GTH) among haemophilia centres in the DACH countries revealed that the majority of centres primarily apply the OSCA format for both plasma FVIII and FIX activity measurements. Nevertheless, more than 80% of centres also have a FVIII CSA available, while the application of a FIX CSA is less common. Manufacturers of the used coagulation analysers are Siemens Healthineers ([Siemens], Erlangen, Germany), IL Werfen (Barcelona, Spain), and Stago (Asnières-sur-Seine, France). Accordingly, the most often applied aPTT reagents comprise the following: Actin, Actin FS, Actin FSL, and Pathromtin SL (Siemens); APTT-SP, SynthAFax, and SynthASil (IL Werfen); STA-PTT Automate (A), (STA-)Cephascreen, and (STA-)C.K. Prest (Stago). The most common FVIII CSA include the following: FVIII Chromogenic Assay (Siemens), Electrachrome FVIII (IL Werfen), TriniCHROM FVIII:C (Stago), COAMATIC FACTOR VIII (Chromogenix/IL), BIOPHEN FVIII:C (Hyphen-Biomed, Neuville-sur-Oise, France), and TECHNOCHROM FVIII:C (Technoclone, Vienna, Austria). Regarding chromogenic determination of FIX activity, only two CE/IVD-marked FIX CS assays are currently available on the European market: the BIOPHEN FIX (Hyphen-Biomed) and the Rox Factor IX assays (Rossix, Mölndal, Sweden). In this article, with respect to potential specific assay discrepancies, we focus on the aforementioned reagents and kits.
Assay Discrepancies in the Diagnosis of HA and HB
Discrepancies between the two major test principles (OSCA and CSA) have been observed in patients with mild and moderate forms of HA. 23 24 25 26 Several gene mutations have been shown to be associated with discrepant results. 27 28 29 In a recently published study on nonsevere HA, however, even discrepancies between different OSC assays have been observed, calling for a larger cohort study to systematically address such inter-reagent variations. 30
Also with respect to HB it has been reported that FIX CSA appear to correlate better with the clinical picture at least in some cases of nonsevere HB. The authors concluded that both assays are required for a correct diagnosis and classification. 31
Accordingly, the recent World Federation of Hemophilia (WFH) guidelines for the management of haemophilia recommends the use of more than one type of factor assay for the detection and classification of all forms of HA or HB. 10
Drugs for and Monitoring of Factor Replacement Therapies in HA and HB
Conventional Plasma-Derived and Recombinant FVIII and FIX Concentrates
The treatment of HA and HB has developed from transfusion of whole blood, plasma, and cryoprecipitate to more targeted replacement therapies under use of plasma-derived (pd) or recombinant (r) human FVIII or FIX concentrates. 32
When compared with CSA, lower OSCA-based FVIII activities have been observed for highly purified plasma-derived as well as recombinant full-length (rFL)- or B-domain-deleted (BDD)-FVIII concentrates. 9 It is noteworthy that according to specifications of the European Pharmacopoeia, potency labeling of FVIII concentrates in Europe is usually based on results from CSAs against the WHO IS for factor VIII concentrate (NIBSC code: 07/350). 33 Thus, depending on the applied FVIII concentrate, significantly lower FVIII activity levels may be observed when using OSCAs rather than CSAs for the analysis of post-infusion samples. Such discrepancies, which are mainly attributable to assay-specific differences in phospholipid composition, usually do not exceed 20% for highly purified pd- or rFL-FVIII concentrates and, therefore, are considered to be clinically irrelevant. 9 34
Higher differences have been observed for certain recombinant BDD (rBDD)-FVIII concentrates which lack the FVIII B-domain. 9 35 Moroctocog alfa (ReFacto® [AF]) has up to 50% lower activity when measured by OSCAs, with strong dependency on the aPTT reagent. 36 To address this particular issue, a ReFacto AF Laboratory Standard for testing of plasma samples from patients receiving ReFacto AF has been made available. 37
In contrast to FVIII concentrates, potency labeling of FIX concentrates released to the European market is usually done using an OSCA in combination with the WHO IS for FIX concentrate (NIBSC code: 07/182). 38 The IS for FIX as well as FVIII concentrate are plasma-derived preparations. It was demonstrated that rFIX concentrates yielded up to 30% lower activity levels in an OSCA calibrated against the IS for FIX concentrate when compared with a correspondingly calibrated CSA. Furthermore, also reagent-related discrepancies between applied OSCAs were found, while such effects were not observed when testing pd-FIX concentrates. 39 40
The aforementioned findings demonstrate that, due to structural and/or matrix-dependent differences between plasma-based assay calibrators and FVIII or FIX drug molecules, the introduction of recombinant factor concentrates led to assay discrepancies of varying degrees during both potency labeling and analyses of post-infusion samples. With the advent of modified recombinant EHL-FVIII/FIX concentrates, such discrepancies, as described below, have become more frequent.
Extended Half-Life (EHL) FVIII and FIX Concentrates
At present, a total of five EHL-FVIII concentrates (Damoctocog alfa pegol [Jivi®], Efmoroctocog alfa [Elocta®], Lonoctocog alfa [Afstyla®], Rurioctocog alfa pegol [Adynovi®], and Turoctocog alfa pegol [Esperoct®]) and three EHL-FIX concentrates (Albutrepenonacog alfa [Idelvion®], Eftrenonacog alfa [Alprolix®], and Nonacog Beta Pegol [Refixia®]) are available in Europe. Compared with conventional plasma-derived or recombinant factor concentrates, these EHL products have a 1.2- to 1.5-fold (FVIII, corresponding to the half-life of vWF) or 5-fold (FIX) prolonged half-life in the circulation. 3 The modifications, introduced to achieve these characteristics (fusion with Fc fragment, albumin fusion or PEGylation) have various effects on the activation and/or activity pattern of these FVIII/FIX drug molecules. Consequently, divergent assessments of factor activity compared with plasma-derived FVIII or FIX used for assay calibration are observed. 11 Potential assay discrepancies between commonly used test systems are described below for each factor concentrate. A synopsis is presented in Table 1 .
Table 1. Summary of EHL/modified FVIII/FIX concentrates and applicable test systems.
| EHL-FVIII concentrates | Porcine rFVIII | EHL-FIX concentrates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adynovi | Afstyla | Elocta | Esperoct | Jivi | Obizur | Alprolix | Idelvion | Refixia | |
| OSC assays (aPTT reagents) | |||||||||
| Activator: ellagic acid/polyphenols | |||||||||
| Actin (Siemens) | ? | *2 | ? | √ | √ | ? | ? | √ | x ↓ |
| Actin FS (Siemens) | (√) | *2 | √ | √ | o ↑ | √ | o ↑ | x ↓ | x ↓ |
| Actin FSL (Siemens) | (√) | *2 | √ | √ | √ | √ | o ↑ | x ↓ | x ↓ |
| SynthAFax (IL Werfen) | ? | *2 | ? | ? | √ | ? | x ↑ | x ↑ | √ |
| Cephascreen (Stago) | (√) | *2 | √ | ? | √ | √ | o ↑ | ? | √ |
| Activator: Silica | |||||||||
| Pathromtin SL (Siemens) | ? | *2 | √ | √ | o ↑ | ? | √ | √ | x ↑ |
| APTT SP (IL Werfen) | (√) | *2 | ? | x ↓ | x ↓ | √ | ? | √ | x ↑ |
| SynthASil (IL Werfen) | (√) | *2 | √ | ? | o ↑ | √ | √ | √ | x ↓ |
| STA PPT A (Stago) | (√) | *2 | √ | x ↓ | x ↓ | √ | √ | √ | x ↑ |
| Activator: Kaolin | |||||||||
| C.K. Prest (Stago) | (√) | *2 | √ | √ | o ↑ | √ | x ↓ | x ↓ | x ↓ |
| FVIII CS assays | |||||||||
| Factor VIII chromogen (Siemens) | (√) | √ | √ ↑ | √ ↑ | o ↓ | x ↓ | |||
| COAMATIC FVIII (Chromogenix/IL) Werfen) | (√) | √ | √ ↑ | √ ↑ | √ | x ↓ | |||
| Biophen FVIII (Hyphen-BioMed) | ? | √ | √ ↑ | √ ↑ | √ | ? | |||
| Electrachrome FVIII (IL Werfen) | (√) | ? | √ ↑ | √ ↑ | √ | x ↓ | |||
| Technochrom Factor VIII:C (Technoclone) | ? | √ | √ ↑ | √ ↑ | ? | ? | |||
| FIX CS assays | |||||||||
| BIOPHEN Factor IX (Hyphen) | (√) | x ↓ | √ | ||||||
| ROX Factor IX (Rossix) | (√) | x ↓ | √ | ||||||
Abbreviations: aPTT, activated partial thromboplastin time; EHL, extended half-life.
√ Acceptable recoveries described in the literature, may be applied for analysis of post-infusion samples.
(√) Acceptable recoveries found in field study but contradictory information available.
o Moderate and/or system-dependent discrepancies described.
x Not recommended for analysis of post-infusion samples due to described significant discrepancies.
? No individual or only insufficient data available.
*2 manufacturers' recommendation to apply a conversion factor of 2 (see text for potential restrictions).
↑ Overestimation observed.
↓ Underestimation observed.
Efmoroctocog Alfa (Elocta)
Efmoroctocog alfa (rFVIIIFc, Elocta) is a fusion protein combining a B-domain-deleted FVIII molecule and the Fc fragment of human immunoglobulin G1 (IgG1), to extend the circulatory half-life. 41
In an initial international field study based on FVIII deficiency plasma spiked with rFVIIIFc at different target concentrations, results obtained with various OSCAs showed good recovery and were comparable to those of correspondingly prepared rFL-FVIII preparations (Advate) analysed in parallel. 42 During a more recent UK National External Quality Assessment Scheme for Blood Coagulation [NEQAS BC]), based on spiked as well as post-infusion samples, the initial findings could be confirmed, again for a broad spectrum of applied OSCAs/aPTT reagents. 43
In contrast to the OSCA results, higher results for rFVIIIFc are obtained when samples are analysed by CSAs. 35 42 43 44 It was assumed that these discrepancies stem from differences between the WHO IS for FVIII concentrate or plasma. 42 However, it is generally concluded that both the OSCA and the CSA may be used to safely monitor rFVIIIFc-based factor FVIII replacement therapies. However, overestimation of Elocta by CSAs should be considered ( Table 1 ).
Damoctocog Alfa Pegol (Jivi)
Damoctocog alfa pegol (Jivi) is a rBDD FVIII molecule with extended half-life due to site-specific PEGylation. 45
During an international field study, a variety of FVIII OSCAs and CSAs were investigated using FVIII-deficient plasma spiked with various Jivi target concentrations. Overall, good recovery and agreement of results was stated for the applied CSAs (Siemens FVIII Chromogenic Assay, Electrachrome FVIII, and COAMATIC FACTOR VIII) within this study design. 46 While this was also true for most of the investigated OSCAs, exceptions were identified. For example, moderate overestimation especially at low-to-medium Jivi input concentrations was observed when the aPTT reagents (Actin FS, Pathromtin SL, SynthASil or CK Prest) were used, although results were not consistent between participating laboratories. 46 In contrast, two silica-based aPTT reagents, APTT-SP and STA-PTT A, consistently showed pronounced underestimation of Jivi, an effect that had already been observed before. 47
However, the earlier-described performance of OSCAs and CSAs could only be partially confirmed during more recent studies based on spiked plasma samples. 48 49 While OSCAs based on the aPTT reagents (Pathromtin SL, SynthASil, and CK Prest) showed more acceptable results, evaluation of different CSAs yielded more inconsistent findings. For example, when using the TriniCHROM FVIII:C assay, Jivi was underestimated by approximately 40% at all tested input concentrations. 48 Underestimation was also observed with the Siemens FVIII Chromogenic Assay. 48 49 In contrast, the COAMATIC FACTOR VIII and BIOPHEN FVIII:C (Hyphen-Biomed) CSA showed acceptable results. 48
Based on these findings, it is concluded that APTT-SP and STA-PTT A should not be used for OSC-based FVIII activity measurements in samples from patients treated with Jivi. The same is true for the TriniCHROM FVIII:C CSA. Furthermore, it appears recommendable that local laboratories evaluate the use of Actin FS, Pathromtin SL-, SynthASil-, or CK Prest-based OSCAs as well as the Siemens FVIII Chromogenic Assay with respect to the accuracy of Jivi activity measurements.
Lonoctocog Alfa (Afstyla)
Lonoctocog alfa (rFVIII-SC, Afstyla) is a B-domain-truncated single-chain FVIII molecule with improved binding to vWF, resulting in a higher stability and a prolonged half-life (approximately 1.2-fold) of the molecule in the circulation. 50 51
Initially, an international field study based on FVIII-deficient plasma spiked with rFVIII-SC at four different input concentrations was conducted. Overall, while the applied CSAs showed good recovery and agreement of results, analysis of samples by OSCAs revealed underestimation of the activity of rFVIII-SC by approximately 45%; the finding was independent of the aPTT reagent used. 52 Although it was shown that the interlaboratory variability of results was increased at low target concentrations, approval of Afstyla came with the stipulation that the results of activity measurements obtained with OSCAs shall be multiplied by 2 ( Table 1 ). 52
However, during a further study also based on spiked plasma samples, it was shown that, especially at low concentrations and depending on the aPTT reagent used, overestimation of Afstyla may occur when using the conversion factor as described earlier. In this regard, the aPTT reagents SynthAFax and Actin FS were particularly conspicuous. 53
Based on these findings, we conclude that the application of a CSA is the first choice for reliable determination of Afstyla activity in post-infusion samples. When using an OSCA in conjunction with the proposed conversion factor of 2, possible overestimation, especially at low Afstyla plasma levels, must be taken into account. While local evaluation of applied OSCAs is generally advisable, verification of SynthAFax- or Actin FS-based OSC assays is especially recommended.
Rurioctocog Alfa Pegol (Adynovi)
Rurioctocog alfa pegol (PEG-rFVIII, BAX 855, Adynovi) is a rFL-FVIII molecule conjugated with a polyethylene glycol (PEG) reagent (approximately 60% attached to the B-domain) for half-life extension. 54
In an initial international field study based on congenital FVIII-deficient plasma spiked with PEG-rFVIII at different input concentrations, obtained results showed concentration-dependent overestimation of PEG-rFVIII. However, comparable results were obtained with analogous rFL-FVIII preparations (Octocog alfa [Advate®]) tested in parallel. Especially for OSCAs that utilise aPTT reagents based on ellagic acid or polyphenol, overestimation (25–40%) was observed at lower PEG-rFVIII or Advate input concentrations. When silica- or kaolin-based aPTT reagents were used, target activity values were found. In contrast, overestimation (25–30%) of both FVIII preparations by CSAs was observed at higher PEG-rFVIII input concentrations. Overall, however, there appeared to be good comparability of measurement results between PEG-rFVIII and the conventional FVIII product Advate using a wide range of measurement systems. 55
In contrast to the results described earlier, another field study showed a more significant (concentration-dependent) overestimation of Adynovi when using both OSCAs and CSAs. Interestingly, this effect was also seen using product-specific calibrators. 56 It is noted by the authors that, unlike the initial study described earlier, no congenital FVIII-deficient plasma was used for sample preparations. Furthermore, also a narrower repertory of reagents was compared within that study. 56
Nevertheless, since the currently available literature contains inconsistent information regarding the reliability of test systems for the determination of Adynovi plasma activity, individual evaluation of local assays may be considered ( Table 1 ). 57
Turoctocog Alfa Pegol (Esperoct)
Turoctocog alfa pegol (N8-GP, Esperoct) is a rFVIII molecule with extended half-life due to site-specific (glyco)PEGylation within the truncated B-domain of the construct. 58
Previously performed studies revealed overestimation of Esperoct by CSA, albeit on a clinically acceptable level (<30%). 59 60 Also during a more recent study, measuring of patients' samples taken during the pathfinder clinical trials program showed comparable results between an applied CSA (Chromogenix Coatest SP FVIII) and an OSCA based on Actin FS. 61
Regarding analysis by different OSCAs, inconsistent results have been described, especially for the aPTT reagents SynthASiL and SynthAFax (IL Werfen). Significant underestimation was found when APTT-SP (IL Werfen), TriniCLOT (Stago), or STA PTT-Automate (Stago) was used. 60 62 Accordingly, these aPTT reagents should not be applied for the analysis of Esperoct by OSCAs ( Table 1 ). As being true for Elocta, overestimation of Esperoct by CSAs should be considered ( Table 1 ).
Albutrepenonacog Alfa (Idelvion)
Albutrepenonacog Alfa (rIX-FP, Idelvion) is a recombinant fusion protein combining a FIX molecule with albumin for extended half-life. 63
In several studies, Pathromtin SL, which is also used for potency labeling of Idelvion, has been shown to be well suited as aPTT reagent in FIX OSCAs for monitoring of post-infusion samples. 64 65 Although inconsistent results have been observed, most of the other applied aPTT reagents showed acceptable results, with the exception of Actin FS and CK Prest, that showed significant underestimation of Idelvion, while, in contrast, significant overestimation was found for SynthAFax as well as for the available FIX CSAs. 66 67 68 Interestingly, it has been recently proposed that the latter is due to impaired activation of rFIX-FP during Pathromtin SL (and colloidal silica)-based OSC measurements. 69
On the basis of the data published to date, an OSCA for the measurement of Idelvion appears to be preferable; however, the above-described aPTT reagents should not be applied ( Table 1 ).
Eftrenonacog Alfa (Alprolix)
Eftrenonacog alfa (Alprolix) is a recombinant fusion protein with extended half-life that combines a FIX molecule with the Fc fragment of human IgG1. 70
While potency labeling of Alprolix is done using an OSCA based on Actin, overestimation of Alprolix especially at lower concentrations was found when using OSCAs that apply ellagic acid–based aPTT reagents. While this was especially and reproducibly true for SynthAFax, a high interlaboratory variability of results was found for other aPTT reagents tested. 71 72 In contrast to the ellagic acid–based aPTT reagents, silica-based reagents showed more acceptable results, while the kaolin-based CK Prest demonstrated significant underestimation. When using a CSA for sample analysis, acceptable recoveries were found. 72 However, a recent single-centre study revealed analyser-dependent differences of assay results, as well as underestimation of Alprolix by CSAs at lower input concentrations. 73 Furthermore, another field study also demonstrated interlaboratory differences during analysis of Alprolix, which, however, were not different from that of pFIX and rFIX processed in parallel. 74
In summary, one may conclude that, due to over- or underestimation of Alprolix, by SynthAFax or CK Prest, respectively, these two aPTT reagents should not be used in OSCAs applied for the determination of Alprolix activity. Furthermore, local evaluation of (other) ellagic acid–based aPTT reagents should be performed ( Table 1 ).
Nonacog Beta Pegol (Refixia)
Nonacog Beta Pegol (Refixia) is a recombinant FIX molecule with site-specific (glyco)PEGylation for half-life extension. 75
Laboratory studies showed that the application of FIX CSAs yields acceptable results when measuring Refixia in post-infusion samples. 76 In contrast, a high variability of results was found when applying a series of different OSCAs. When using polyphenol- or ellagic acid–based aPTT reagents, acceptable results (STA-Cephascreen, SynthAFax, or DG Synth), but also significant underestimation of Refixia (e.g., using Actin FS and Actin FSL), were found. Underestimation was also found when using kaolin-based CK Prest. Regarding the silica-based aPTT reagents, however, SynthASil (colloidal silica) led to an underestimation of more than 50%, while, as also true for most of the other silica-based reagents, Pathromtin SL (silicon dioxide particles) led to severe overestimation of Refixia. 77 As a cause for the latter effect, it could be demonstrated that the presence of silica leads to an activation of Refixia by FXIa but also kallikrein already during the incubation phase of the aPTT reagent in the absence of Ca 2+ ions. 78
Thus, as outlined above, and also summarised in Table 1 , CSAs and only selected OSCAs (aPTT reagents) appear to be suitable for reliable determination of Refixia activity in post-infusion samples. 79 80
Laboratory Diagnostics during Treatment of HA with Emicizumab (Hemlibra)
Emicizumab (Hemlibra®) is a subcutaneously administered, humanised, monoclonal, bi-specific IgG4 antibody that binds FIX(a) and FX(a), thereby mimicking the cofactor function of FVIIIa. 81 In contrast to FVIIIa, emicizumab circulates at high (target) concentrations (30–80 µg/mL [200–530 nM] as measured in plasma) and binds only with moderate affinity (KD = 1–2 µM) and non-discriminatorily to the human (pro)enzyme variants of FIX(a) or FX(a). Simultaneous binding of emicizumab to FIXa and FX, both presenting properly aligned and at high local concentrations on a phospholipid surface at the site of the injury, is prerequisite for its cofactor activity during FIXa-mediated generation of FXa. 82 83
Interference of Emicizumab with Established Test Systems
In contrast to FVIII, emicizumab does not require activation and is not subject to regulation. The former characteristic leads to significant shortening of aPTT-based clotting times even at subtherapeutic plasma concentrations of emicizumab. The reason is that emicizumab requires no thrombin-mediated feedback activation but is ‘active’ as it is. Accordingly, in the presence of emicizumab, aPTT-based OSCAs configured and calibrated for quantitative detection of plasmatic coagulation factor activity exhibit clotting times outside the dynamic range of these test systems. 84 85
In contrast, emicizumab at therapeutic concentrations is dynamically detected by FVIII CSAs based on human FIXa and FX (cf. Fig. 1B ). However, it is important to note that any determined ‘FVIII activities’ must not be translated to FVIII-equivalent effects of emicizumab in vivo, but are merely derived from assay calibration against FVIII. A different picture emerges when using FVIII CSAs based on bovine factors. Such assays are not able to detect emicizumab, as it only binds to human FIX(a) and FX(a), and are therefore specific tools to measure (endogenous or exogenous) FVIII in the presence of emicizumab. 12 84 85 However, assays to monitor the combined effect of FVIII and emicizumab are currently not available.
Determination of Plasma Levels of Emicizumab
To enable the determination of emicizumab in plasma, a modified FVIII OSCA has been developed. Using a higher pre-diluted plasma sample and appropriate calibrators and controls, it determines plasma concentrations (activity) of emicizumab between 10 and 100 μg/mL, adequately covering the therapeutic range of the drug. 6 7
Using emicizumab calibrators, quantitative determination of emicizumab also appears possible using human factor-based FVIII CSAs. 86 It must be noted that any additionally present FVIII may distort measurement results especially of this assay type. Should it be necessary to determine the plasma level of emicizumab in the presence of FVIII, heat inactivation of the plasma sample may be considered. 85
Laboratory Diagnostics in HA or HB with Inhibitors
Neutralising anti-FVIII (drug) antibodies, so-called inhibitors, occur in up to 30% of treated patients with severe HA. In contrast, the formation of FIX inhibitors in severe HB is much less common. 4 87
In case of HA or HB with inhibitors, bypassing agents such as recombinant activated FVII (rFVIIa) or aPCCs may be applied. 88 In HA with inhibitors, emicizumab has become a treatment option. 6 89 In addition, recombinant porcine FVIII (rpFVIII, Susoctocog alfa [Obizur®]) has been approved for the treatment of bleeding episodes in adults with acquired HA (AHA). 5
To eliminate inhibitors formed in treated HA or HB patients, immune tolerance induction (ITI) therapy is currently the therapy of choice. Hereby, FVIII or FIX concentrates are administered at high frequencies and doses. While up to 70% of HA patients with inhibitors are in remission after ITI therapy, this strategy is less successful in patients suffering from HB with inhibitors. 87 88 90
Functional detection and quantification of FVIII or FIX inhibitors is usually done using the (Nijmegen)-Bethesda Assay ( Fig. 2 ). Furthermore, enzyme-linked immunosorbent assay (ELISA)-based analysis may be used to assess total (neutralising and non-neutralising) anti-drug antibody (ADA) levels. 91 With the introduction of modified (EHL-)FVIII/FIX concentrates and emicizumab to the clinic, however, traditional testing schemes need to be adapted and/or extended. 11
Inhibitor Testing in HA Patients Treated with Emicizumab
As outlined earlier, emicizumab is immediately active and does not require thrombin-mediated feedback activation. In contrast to FVIII present in a sample after substitution, emicizumab cannot be inactivated by heat. 11 A straightforward solution to this problem comes with the fact that emicizumab is not recognised by FVIII CSAs based on bovine factors. Accordingly, the application of such an assay for the determination of (residual) FVIII activities during NBA leads to reliable results, independent of the presence of emicizumab in the sample to be analysed. 92
Inhibitor Testing in HA and HB Patients Treated with EHL-FVIII/FIX Concentrates
The formation of anti-PEG has been observed in previously conventionally treated HA patients substituted with pegylated EHL-FVIII concentrates. 93 94 95 96 While in the majority of cases, these antibodies did not affect FVIII function, also loss of treatment efficacy was observed. 96 In these cases, the antibodies were directed against the PEG moiety, specific for the EHL-FVIII molecule and did not cross-react with unmodified FVIII. These circumstances allowed for successful further treatment of patients with conventional FVIII concentrates after observed loss of efficacy of therapy with pegylated EHL-FVIII. 96
In general, to specifically detect and quantify any inhibitory ADAs, EHL-specific (N)BAs may be performed. The EHL factor to be addressed is diluted to (approx.) 1 IU/mL in (imidazole-buffered) FVIII- or FIX-deficient plasma to serve as the substrate during the assay. While this may be done using (remainders of) the reconstituted drug, the availability of corresponding laboratory standards would be favorable.
The risk of FVIII or FIX inhibitor development in previously untreated patients (PUPs) under therapy with EHL factor concentrates might be reduced but remains significant. 97 98 Consequently, with respect to functional inhibitor testing by the (N)BA, this raises the question whether or not established standard conditions for heat inactivation of patient samples to eliminate any remaining factor activities ( Fig. 2 ) also apply for EHL-FVIII or FIX molecules. Indeed, corresponding analysis confirmed that the activity of all currently approved EHL factor concentrates can be efficiently inactivated when incubated at 56 °C for 30 minutes. 99 100
Monitoring of and Inhibitor Testing in the Treatment of AHA with Susoctocog Alfa (Obizur)
Susoctocog alfa (Obizur) is a rpFVIII concentrate approved for the treatment of acute bleedings in AHA. In contrast to human (EHL-)FVIII concentrates, however, potency labeling of Obizur uses a FVIII OSCA rather than a CSA. 55 In an international field study, based on congenital FVIII-deficient plasma spiked with Obizur at different input concentrations, OSCAs with a variety of aPTT reagents showed acceptable results. In contrast, applied CSAs underestimated the activity of Obizur by approximately 50%. 55 Accordingly, a FVIII OSCA should be used for both, monitoring of Obizur plasma levels during therapy as well as determination of (residual) FVIII activities in Obizur-specific NBAs ( Table 1 ).
Indeed, while Obizur has been developed with the intention to bypass neutralising antibodies against human FVIII, cross-reactivity of such inhibitors with the underlying rpFVIII molecule has been described. 101 Thus, it may become necessary to perform specific testing for inhibitors against Obizur. For doing so, a corresponding laboratory standard is available from the manufacturer (Takeda). When diluted to 1 U/mL in (imidazole-buffered) FVIII-deficient plasma, this mixture, instead of FVIII in buffered pooled plasma ( Fig. 2A ), serves as substrate during the NBA.
Outlook to Future Treatment Options
In addition to currently available treatment options for HA and HB, a variety of novel molecules and innovative therapy strategies is currently investigated in a series of clinical trials. Among others, these comprise FVIII/FIX gene therapy, factor concentrates with further improved properties and/or the possibility for subcutaneous application as well as strategies for rebalancing pro- and anticoagulant mechanisms. 102 103 104 105 As also true for the currently established therapies, also these novel treatment regimens do and will prompt questions about the general need for treatment monitoring but also about their impact on assay results as well as the applicability of established test systems for patient sample analysis. 11 106
Currently, five (three HA and two HB) gene therapy approaches are within phase 3 clinical trials, making a licensed product possible within the near future. 8
In gene constructs used for HA, the factor VIII cassette is B-domain deleted, consistently resulting in OSCA results which were approximately 1.5 times higher than those obtained with chromogenic assays. 107 Interestingly, this contrasts with the factor level discrepancies found in patients treated with BDD FVIII concentrates, where OSCAs generally yields lower results than CSAs. 36 65
In gene therapy for HB, a full-size FIX gene with higher specific activity than the native FIX gene (Padua variant) is most often used. FIX OSCAs yield approximately 1.5 times higher results than chromogenic assays, while aPTT-reagent-dependent differences have been observed. 108
Further research is needed to determine the relative accuracy of the different assay principles in patients with gene therapy in relation to clinical response and also in relation to the results of patients treated with different factor concentrates. Recently, it has been recommended to apply both assay principles to quantify FVIII and FIX activity in gene therapy trials and that the possibly more accurate lower values measured by the chromogenic test be relied upon. 10
Summary and Conclusion
High inter-laboratory variation in the measurement specifically of EHL-factors in post-infusion samples has become apparent. To some extent, this variation is considered to be within the range of ‘traditional’ (but previously less robustly examined) laboratory result variation, as observed for the measurement of conventional recombinant factor molecules. However, it was our aim to highlight that there are inappropriate test systems for certain factors, or inappropriate factors for certain test systems. Such combinations must be avoided to maintain patient safety. The treating physician and the laboratory need to harmonise their toolboxes to ensure that factor activities are assessed within acceptable ranges for all factor concentrates prescribed in the respective centre. As a minimum requirement, significant overestimation of factor activity must be excluded to ensure appropriate laboratory-based controls for prophylactic and therapeutic factor administration. While product-specific calibrators may be helpful to correct for potential assay discrepancies, this approach appears to be impractical in clinical routine due to the variety of available concentrates. However, OSCAs will still meet most requirements. Emicizumab poses considerable additional challenges, especially for small- and medium-size coagulation laboratories, because the use of CSAs becomes mandatory when examining samples from HA patients treated with this antibody. In the end, coagulation laboratories processing samples for HA and HB patients under factor replacement or alternative therapy will have to establish two different assays for FVIII and FIX, respectively: one OSCA and one CSA—at least. With new therapeutic options lying ahead, this will become more pressing during the next years.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Bolton-Maggs P H, Pasi K J.Haemophilias A and B Lancet 2003361(9371)1801–1809. [DOI] [PubMed] [Google Scholar]
- 2.Aledort L, Mannucci P M, Schramm W, Tarantino M. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019;17(06):479–486. doi: 10.2450/2019.0211-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdary P. Extended half-life recombinant products in haemophilia clinical practice - expectations, opportunities and challenges. Thromb Res. 2020;196:609–617. doi: 10.1016/j.thromres.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Cormier M, Batty P, Tarrant J, Lillicrap D. Advances in knowledge of inhibitor formation in severe haemophilia A. Br J Haematol. 2020;189(01):39–53. doi: 10.1111/bjh.16377. [DOI] [PubMed] [Google Scholar]
- 5.Burness C B, Scott L J. Susoctocog alfa: a review in acquired haemophilia A. Drugs. 2016;76(07):815–821. doi: 10.1007/s40265-016-0576-1. [DOI] [PubMed] [Google Scholar]
- 6.Oldenburg J, Mahlangu J N, Kim B. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(09):809–818. doi: 10.1056/NEJMoa1703068. [DOI] [PubMed] [Google Scholar]
- 7.Mahlangu J, Oldenburg J, Paz-Priel I. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(09):811–822. doi: 10.1056/NEJMoa1803550. [DOI] [PubMed] [Google Scholar]
- 8.Batty P, Lillicrap D. Hemophilia gene therapy: approaching the first licensed product. HemaSphere. 2021;5(03):e540. doi: 10.1097/HS9.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peyvandi F, Oldenburg J, Friedman K D. A critical appraisal of one-stage and chromogenic assays of factor VIII activity. J Thromb Haemost. 2016;14(02):248–261. doi: 10.1111/jth.13215. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava A, Santagostino E, Dougall A. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26 06:1–158. doi: 10.1111/hae.14046. [DOI] [PubMed] [Google Scholar]
- 11.Peyvandi F, Kenet G, Pekrul I, Pruthi R K, Ramge P, Spannagl M. Laboratory testing in hemophilia: impact of factor and non-factor replacement therapy on coagulation assays. J Thromb Haemost. 2020;18(06):1242–1255. doi: 10.1111/jth.14784. [DOI] [PubMed] [Google Scholar]
- 12.Bowyer A E, Lowe A E, Tiefenbacher S. Laboratory issues in gene therapy and emicizumab. Haemophilia. 2021;27 03:142–147. doi: 10.1111/hae.13976. [DOI] [PubMed] [Google Scholar]
- 13.SSC Sub-Committees on Factor VIII/Factor IX and von Willebrand factor of ISTH . Hubbard A R, Hamill M, Beeharry M, Bevan S A, Heath A B. Value assignment of the WHO 6th International Standard for blood coagulation factor VIII and von Willebrand factor in plasma (07/316) J Thromb Haemost. 2011;9(10):2100–2102. doi: 10.1111/j.1538-7836.2011.04471.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilmot H V, Rakowski K, Gray E. The traceability of commercial plasma calibrators to the plasma international standards for factor VIII and factor IX. Int J Lab Hematol. 2020;42(06):810–818. doi: 10.1111/ijlh.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marlar R A, Strandberg K, Shima M, Adcock D M. Clinical utility and impact of the use of the chromogenic vs one-stage factor activity assays in haemophilia A and B. Eur J Haematol. 2020;104(01):3–14. doi: 10.1111/ejh.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasper C K, Aledort L, Aronson D. Proceedings: a more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975;34(02):612. [PubMed] [Google Scholar]
- 17.Gadarowski J J, Jr, Czapek E E, Ontiveros J D, Pedraza J L. Modification of the Bethesda assay for factor VIII or IX inhibitors to improve efficiency. Acta Haematol. 1988;80(03):134–138. doi: 10.1159/000205619. [DOI] [PubMed] [Google Scholar]
- 18.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73(02):247–251. [PubMed] [Google Scholar]
- 19.Boylan B, Miller C H. Effects of pre-analytical heat treatment in factor VIII (FVIII) inhibitor assays on FVIII antibody levels. Haemophilia. 2018;24(03):487–491. doi: 10.1111/hae.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemophilia Inhibitor Research Study Investigators . Miller C H, Boylan B, Shapiro A D, Lentz S R, Wicklund B M. Limit of detection and threshold for positivity of the Centers for Disease Control and Prevention assay for factor VIII inhibitors. J Thromb Haemost. 2017;15(10):1971–1976. doi: 10.1111/jth.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemophilia Inhibitor Research Study Investigators . Miller C H, Rice A S, Boylan B. Characteristics of hemophilia patients with factor VIII inhibitors detected by prospective screening. Am J Hematol. 2015;90(10):871–876. doi: 10.1002/ajh.24104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rampersad A G, Boylan B, Miller C H, Shapiro A. Distinguishing lupus anticoagulants from factor VIII inhibitors in haemophilic and non-haemophilic patients. Haemophilia. 2018;24(05):807–814. doi: 10.1111/hae.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldenburg J, Pavlova A. Discrepancy between one-stage and chromogenic factor VIII activity assay results can lead to misdiagnosis of haemophilia A phenotype. Hamostaseologie. 2010;30(04):207–211. [PubMed] [Google Scholar]
- 24.Duncan E M, Rodgers S E, McRae S J. Diagnostic testing for mild hemophilia a in patients with discrepant one-stage, two-stage, and chromogenic factor VIII:C assays. Semin Thromb Hemost. 2013;39(03):272–282. doi: 10.1055/s-0033-1334863. [DOI] [PubMed] [Google Scholar]
- 25.Bowyer A E, Van Veen J J, Goodeve A C, Kitchen S, Makris M. Specific and global coagulation assays in the diagnosis of discrepant mild hemophilia A. Haematologica. 2013;98(12):1980–1987. doi: 10.3324/haematol.2013.088088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trossaert M, Lienhart A, Nougier C. Diagnosis and management challenges in patients with mild haemophilia A and discrepant FVIII measurements. Haemophilia. 2014;20(04):550–558. doi: 10.1111/hae.12381. [DOI] [PubMed] [Google Scholar]
- 27.Bowyer A E, Goodeve A, Liesner R, Mumford A D, Kitchen S, Makris M. p.Tyr365Cys change in factor VIII: haemophilia A, but not as we know it. Br J Haematol. 2011;154(05):618–625. doi: 10.1111/j.1365-2141.2011.08688.x. [DOI] [PubMed] [Google Scholar]
- 28.Lyall H, Hill M, Westby J, Grimley C, Dolan G. Tyr346–>Cys mutation results in factor VIII:C assay discrepancy and a normal bleeding phenotype - is this mild haemophilia A? Haemophilia. 2008;14(01):78–80. doi: 10.1111/j.1365-2516.2007.01557.x. [DOI] [PubMed] [Google Scholar]
- 29.Strålfors A, Mikovic D, Schmidt D. Genetics and hemostatic potential in persons with mild to moderate hemophilia A with a discrepancy between one-stage and chromogenic FVIII assays. Thromb Haemost. 2021;121(01):27–35. doi: 10.1055/s-0040-1715443. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki A, Suzuki N, Kanematsu T. Impact of variation in reagent combinations for one-stage clotting assay on assay discrepancy in nonsevere haemophilia A. Int J Lab Hematol. 2021;43(01):131–138. doi: 10.1111/ijlh.13335. [DOI] [PubMed] [Google Scholar]
- 31.Kihlberg K, Strandberg K, Rosén S, Ljung R, Astermark J. Discrepancies between the one-stage clotting assay and the chromogenic assay in haemophilia B. Haemophilia. 2017;23(04):620–627. doi: 10.1111/hae.13219. [DOI] [PubMed] [Google Scholar]
- 32.Franchini M, Mannucci P M. The history of hemophilia. Semin Thromb Hemost. 2014;40(05):571–576. doi: 10.1055/s-0034-1381232. [DOI] [PubMed] [Google Scholar]
- 33.Dodt J, Hubbard A R, Wicks S J. Potency determination of factor VIII and factor IX for new product labelling and postinfusion testing: challenges for caregivers and regulators. Haemophilia. 2015;21(04):543–549. doi: 10.1111/hae.12634. [DOI] [PubMed] [Google Scholar]
- 34.Mikaelsson M, Oswaldsson U, Sandberg H. Influence of phospholipids on the assessment of factor VIII activity. Haemophilia. 1998;4(04):646–650. doi: 10.1046/j.1365-2516.1998.440646.x. [DOI] [PubMed] [Google Scholar]
- 35.Augustsson C, Norström E, Andersson N G, Zetterberg E, Astermark J, Strandberg K. Monitoring standard and extended half-life products in hemophilia: assay discrepancies for factor VIII and IX in pre- and postinfusion samples. Res Pract Thromb Haemost. 2020;4(07):1114–1120. doi: 10.1002/rth2.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikaelsson M, Oswaldsson U, Jankowski M A.Measurement of factor VIII activity of B-domain deleted recombinant factor VIII Semin Hematol 200138(2, Suppl 4):13–23. [DOI] [PubMed] [Google Scholar]
- 37.Pouplard C, Ternisien C, Desconclois C, Lasne D, Aillaud M F, Caron C. Discrepancies between one stage assay and chromogenic substrate assay in patients treated with recombinant or plasma-derived FVIII and usefulness of a specific standard in ReFacto AF ® -treated patients . Haemophilia. 2016;22(02):e101–e103. doi: 10.1111/hae.12867. [DOI] [PubMed] [Google Scholar]
- 38.Gray E, Pickering W, Hockley J. Collaborative study for the establishment of replacement batches for human coagulation factor IX concentrate reference standards. Pharmeur Bio. 2008;2008(01):19–30. [PubMed] [Google Scholar]
- 39.Wilmot H V, Hogwood J, Gray E. Recombinant factor IX: discrepancies between one-stage clotting and chromogenic assays. Haemophilia. 2014;20(06):891–897. doi: 10.1111/hae.12449. [DOI] [PubMed] [Google Scholar]
- 40.Wilmot H V, Gray E. Potency estimates for recombinant factor IX in the one-stage clotting assay are influenced by more than just the choice of activated partial thromboplastin time reagent. Haemophilia. 2018;24(05):e363–e368. doi: 10.1111/hae.13556. [DOI] [PubMed] [Google Scholar]
- 41.Peters R T, Toby G, Lu Q. Biochemical and functional characterization of a recombinant monomeric factor VIII-Fc fusion protein. J Thromb Haemost. 2013;11(01):132–141. doi: 10.1111/jth.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommer J M, Moore N, McGuffie-Valentine B. Comparative field study evaluating the activity of recombinant factor VIII Fc fusion protein in plasma samples at clinical haemostasis laboratories. Haemophilia. 2014;20(02):294–300. doi: 10.1111/hae.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitchen S, Jennings I, Makris M, Kitchen D P, Woods T AL, Walker I D. Clotting and chromogenic factor VIII assay variability in post-infusion and spiked samples containing full-length recombinant FVIII or recombinant factor VIII Fc fusion protein (rFVIIIFc) Int J Lab Hematol. 2019;41(02):176–183. doi: 10.1111/ijlh.12940. [DOI] [PubMed] [Google Scholar]
- 44.Owaidah T M, Alzahrani H A, Al-Numair N S, Alnosair A O, Aguilos A M, Saleh M. Assessing the performance of extended half-life coagulation factor VIII, FC fusion protein by using chromogenic and one-stage assays in Saudi hemophilia A patients. Adv Hematol. 2020;2020:8.768074E6. doi: 10.1155/2020/8768074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coyle T E, Reding M T, Lin J C, Michaels L A, Shah A, Powell J. Phase I study of BAY 94-9027, a PEGylated B-domain-deleted recombinant factor VIII with an extended half-life, in subjects with hemophilia A. J Thromb Haemost. 2014;12(04):488–496. doi: 10.1111/jth.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Church N, Leong L, Katterle Y. Factor VIII activity of BAY 94-9027 is accurately measured with most commonly used assays: results from an international laboratory study. Haemophilia. 2018;24(05):823–832. doi: 10.1111/hae.13564. [DOI] [PubMed] [Google Scholar]
- 47.Gu J M, Ramsey P, Evans V. Evaluation of the activated partial thromboplastin time assay for clinical monitoring of PEGylated recombinant factor VIII (BAY 94-9027) for haemophilia A. Haemophilia. 2014;20(04):593–600. doi: 10.1111/hae.12374. [DOI] [PubMed] [Google Scholar]
- 48.Meijer P, Marlar R A, Teare J M, Adcock D. Inter-laboratory evaluation of the recovery of Bay 94–9027 [Jivi®] with one-stage clotting and chromogenic assays. Blood. 2019;134 01:1124. [Google Scholar]
- 49.Müller J, Goldmann G, Marquardt N, Pötzsch B, Oldenburg J.Extended half-life factor VIII/factor IX products: assay discrepancies and implications for hemophilia management Hamostaseologie 202040(S 01):S15–S20. [DOI] [PubMed] [Google Scholar]
- 50.Schmidbauer S, Witzel R, Robbel L. Physicochemical characterisation of rVIII-SingleChain, a novel recombinant single-chain factor VIII. Thromb Res. 2015;136(02):388–395. doi: 10.1016/j.thromres.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Klamroth R, Simpson M, von Depka-Prondzinski M. Comparative pharmacokinetics of rVIII-SingleChain and octocog alfa (Advate(®) ) in patients with severe haemophilia A. Haemophilia. 2016;22(05):730–738. doi: 10.1111/hae.12985. [DOI] [PubMed] [Google Scholar]
- 52.St Ledger K, Feussner A, Kalina U. International comparative field study evaluating the assay performance of AFSTYLA in plasma samples at clinical hemostasis laboratories. J Thromb Haemost. 2018;16(03):555–564. doi: 10.1111/jth.13932. [DOI] [PubMed] [Google Scholar]
- 53.Bowyer A, Key N, Dalton D, Kitchen S, Makris M. The coagulation laboratory monitoring of Afstyla single-chain FVIII concentrate. Haemophilia. 2017;23(05):e469–e470. doi: 10.1111/hae.13290. [DOI] [PubMed] [Google Scholar]
- 54.Turecek P L, Bossard M J, Graninger M. BAX 855, a PEGylated rFVIII product with prolonged half-life. Development, functional and structural characterisation. Hamostaseologie. 2012;32 01:S29–S38. [PubMed] [Google Scholar]
- 55.Turecek P L, Romeder-Finger S, Apostol C. A world-wide survey and field study in clinical haemostasis laboratories to evaluate FVIII:C activity assay variability of ADYNOVATE and OBIZUR in comparison with ADVATE. Haemophilia. 2016;22(06):957–965. doi: 10.1111/hae.13001. [DOI] [PubMed] [Google Scholar]
- 56.Bulla O, Poncet A, Alberio L. Impact of a product-specific reference standard for the measurement of a PEGylated rFVIII activity: the Swiss Multicentre Field Study. Haemophilia. 2017;23(04):e335–e339. doi: 10.1111/hae.13250. [DOI] [PubMed] [Google Scholar]
- 57.Gray E, Kitchen S, Bowyer A. Laboratory measurement of factor replacement therapies in the treatment of congenital haemophilia: a United Kingdom Haemophilia Centre Doctors' Organisation guideline. Haemophilia. 2020;26(01):6–16. doi: 10.1111/hae.13907. [DOI] [PubMed] [Google Scholar]
- 58.Stennicke H R, Kjalke M, Karpf D M. A novel B-domain O-glycoPEGylated FVIII (N8-GP) demonstrates full efficacy and prolonged effect in hemophilic mice models. Blood. 2013;121(11):2108–2116. doi: 10.1182/blood-2012-01-407494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pickering W, Hansen M, Kjalke M, Ezban M. Factor VIII chromogenic assays can be used for potency labeling and postadministration monitoring of N8-GP. J Thromb Haemost. 2016;14(08):1579–1587. doi: 10.1111/jth.13375. [DOI] [PubMed] [Google Scholar]
- 60.Hillarp A, Bowyer A, Ezban M, Persson P, Kitchen S. Measuring FVIII activity of glycopegylated recombinant factor VIII, N8-GP, with commercially available one-stage clotting and chromogenic assay kits: a two-centre study. Haemophilia. 2017;23(03):458–465. doi: 10.1111/hae.13168. [DOI] [PubMed] [Google Scholar]
- 61.Møss J, Clausen W HO, Ezban M. Measuring factor VIII activity in samples from patients treated with N8-GP (Esperoct ® ; turoctocog alfa pegol) during the pathfinder clinical trials programme . Haemophilia. 2021;27(03):e389–e392. doi: 10.1111/hae.14173. [DOI] [PubMed] [Google Scholar]
- 62.Ezban M, Hansen M, Kjalke M. An overview of turoctocog alfa pegol (N8-GP; ESPEROCT ® ) assay performance: implications for postadministration monitoring . Haemophilia. 2020;26(01):156–163. doi: 10.1111/hae.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Metzner H J, Weimer T, Kronthaler U, Lang W, Schulte S. Genetic fusion to albumin improves the pharmacokinetic properties of factor IX. Thromb Haemost. 2009;102(04):634–644. doi: 10.1160/TH09-04-0255. [DOI] [PubMed] [Google Scholar]
- 64.Santagostino E, Negrier C, Klamroth R. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in hemophilia B patients. Blood. 2012;120(12):2405–2411. doi: 10.1182/blood-2012-05-429688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitchen S, Tiefenbacher S, Gosselin R. Factor activity assays for monitoring extended half-life FVIII and factor IX replacement therapies. Semin Thromb Hemost. 2017;43(03):331–337. doi: 10.1055/s-0037-1598058. [DOI] [PubMed] [Google Scholar]
- 66.Horn C, Négrier C, Kalina U, Seifert W, Friedman K D. Performance of a recombinant fusion protein linking coagulation factor IX with recombinant albumin in one-stage clotting assays. J Thromb Haemost. 2019;17(01):138–148. doi: 10.1111/jth.14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.French Study Group on Laboratory Management of Bleeding Disorders (BIMHO Group) . Pouplard C, Galinat H, Ternisien C. Multicentre evaluation of CK Prest® for assaying plasma levels of factor IX fused with albumin (Idelvion®) Haemophilia. 2019;25(05):e327–e330. doi: 10.1111/hae.13812. [DOI] [PubMed] [Google Scholar]
- 68.Bowyer A, Sampson B, Shepherd F, Jones R, Kitchen S, Makris M.The FIX C assessment of extended half-life recombinant factor IX products in clinical practice Haemophilia 2018240156(Abstract P053) [Google Scholar]
- 69.Rosén S, Bryngelhed P. FIX potency of rFIX-albumin fusion protein is underestimated by one-stage methods using silica-based APTT reagents. Haemophilia. 2020;26(02):340–345. doi: 10.1111/hae.13915. [DOI] [PubMed] [Google Scholar]
- 70.Peters R T, Low S C, Kamphaus G D. Prolonged activity of factor IX as a monomeric Fc fusion protein. Blood. 2010;115(10):2057–2064. doi: 10.1182/blood-2009-08-239665. [DOI] [PubMed] [Google Scholar]
- 71.Sommer J M, Buyue Y, Bardan S. Comparative field study: impact of laboratory assay variability on the assessment of recombinant factor IX Fc fusion protein (rFIXFc) activity. Thromb Haemost. 2014;112(05):932–940. doi: 10.1160/th13-11-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sommer J M, Sadeghi-Khomami A, Barnowski C, Wikén M, Willemze A J. Real-world assay variability between laboratories in monitoring of recombinant factor IX Fc fusion protein activity in plasma samples. Int J Lab Hematol. 2020;42(03):350–358. doi: 10.1111/ijlh.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sinegre T, Trayaud A, Tardieu M. Measurements of eftrenonacog alfa by 19 different combinations reagents/instrument: a single-centre study. Haemophilia. 2020;26(03):543–552. doi: 10.1111/hae.14003. [DOI] [PubMed] [Google Scholar]
- 74.Fukutake K, Kobayashi T, Sommer J M, Hirakata T, Recombinant F IX. Recombinant FIX Fc fusion protein activity assessment with the one-stage clotting assay: a multicenter, assessor-blinded, prospective study in Japan (J-Field Study) Int J Lab Hematol. 2020;42(02):162–169. doi: 10.1111/ijlh.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Østergaard H, Bjelke J R, Hansen L. Prolonged half-life and preserved enzymatic properties of factor IX selectively PEGylated on native N-glycans in the activation peptide. Blood. 2011;118(08):2333–2341. doi: 10.1182/blood-2011-02-336172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young G, Ezban M, Clausen W HO, Negrier C, Oldenburg J, Shima M. Chromogenic analysis of FIX activity in haemophilia B patients treated with nonacog beta pegol. Haemophilia. 2017;23(06):e528–e530. doi: 10.1111/hae.13348. [DOI] [PubMed] [Google Scholar]
- 77.Bowyer A E, Hillarp A, Ezban M, Persson P, Kitchen S. Measuring factor IX activity of nonacog beta pegol with commercially available one-stage clotting and chromogenic assay kits: a two-center study. J Thromb Haemost. 2016;14(07):1428–1435. doi: 10.1111/jth.13348. [DOI] [PubMed] [Google Scholar]
- 78.Rosén P, Rosén S, Ezban M, Persson E. Overestimation of N-glycoPEGylated factor IX activity in a one-stage factor IX clotting assay owing to silica-mediated premature conversion to activated factor IX. J Thromb Haemost. 2016;14(07):1420–1427. doi: 10.1111/jth.13359. [DOI] [PubMed] [Google Scholar]
- 79.Tiefenbacher S, Bohra R, Amiral J. Qualification of a select one-stage activated partial thromboplastin time-based clotting assay and two chromogenic assays for the post-administration monitoring of nonacog beta pegol. J Thromb Haemost. 2017;15(10):1901–1912. doi: 10.1111/jth.13787. [DOI] [PubMed] [Google Scholar]
- 80.Ezban M, Hermit M B, Persson E. FIXing postinfusion monitoring: assay experiences with N9-GP (nonacog beta pegol; Refixia ® ; Rebinyn ® ) . Haemophilia. 2019;25(01):154–161. doi: 10.1111/hae.13671. [DOI] [PubMed] [Google Scholar]
- 81.Sampei Z, Igawa T, Soeda T. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PLoS One. 2013;8(02):e57479. doi: 10.1371/journal.pone.0057479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitazawa T, Igawa T, Sampei Z. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18(10):1570–1574. doi: 10.1038/nm.2942. [DOI] [PubMed] [Google Scholar]
- 83.Lenting P J, Denis C V, Christophe O D. Emicizumab, a bispecific antibody recognizing coagulation factors IX and X: how does it actually compare to factor VIII? Blood. 2017;130(23):2463–2468. doi: 10.1182/blood-2017-08-801662. [DOI] [PubMed] [Google Scholar]
- 84.Adamkewicz J I, Chen D C, Paz-Priel I. Effects and interferences of emicizumab, a humanised bispecific antibody mimicking activated factor VIII cofactor function, on coagulation assays. Thromb Haemost. 2019;119(07):1084–1093. doi: 10.1055/s-0039-1688687. [DOI] [PubMed] [Google Scholar]
- 85.Müller J, Pekrul I, Pötzsch B, Berning B, Oldenburg J, Spannagl M. Laboratory monitoring in emicizumab-treated persons with hemophilia A. Thromb Haemost. 2019;119(09):1384–1393. doi: 10.1055/s-0039-1692427. [DOI] [PubMed] [Google Scholar]
- 86.Amiral J, Seghatchian J. Usefulness of chromogenic assays for potency assignment and recovery of plasma-derived FVIII and FIX concentrates or their recombinant long acting therapeutic equivalents with potential application in treated pediatric hemophiliac patients. Transfus Apheresis Sci. 2018;57(03):363–369. doi: 10.1016/j.transci.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 87.Santoro C, Quintavalle G, Castaman G. Inhibitors in hemophilia B. Semin Thromb Hemost. 2018;44(06):578–589. doi: 10.1055/s-0038-1660817. [DOI] [PubMed] [Google Scholar]
- 88.Lusher J M. Inhibitor antibodies to factor VIII and factor IX: management. Semin Thromb Hemost. 2000;26(02):179–188. doi: 10.1055/s-2000-9821. [DOI] [PubMed] [Google Scholar]
- 89.‘Ständige Kommission Hämophilie’ (Haemophilia board) of the German, Swiss Austrian Society for Thrombosis Haemostasis Research (GTH) . Holstein K, Albisetti M, Bidlingmaier C. Practical guidance of the GTH Haemophilia Board on the use of emicizumab in patients with haemophilia A. Hamostaseologie. 2020;40(05):561–571. doi: 10.1055/a-1127-6476. [DOI] [PubMed] [Google Scholar]
- 90.Warrier I, Ewenstein B M, Koerper M A. Factor IX inhibitors and anaphylaxis in hemophilia B. J Pediatr Hematol Oncol. 1997;19(01):23–27. doi: 10.1097/00043426-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 91.Abdi A, Bordbar M R, Hassan S. Prevalence and incidence of non-neutralizing antibodies in congenital hemophilia A - a systematic review and meta-analysis. Front Immunol. 2020;11:563. doi: 10.3389/fimmu.2020.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miller C H, Boylan B, Payne A B, Driggers J, Bean C J. Validation of the chromogenic Bethesda assay for factor VIII inhibitors in hemophilia a patients receiving Emicizumab. Int J Lab Hematol. 2021;43(02):e84–e86. doi: 10.1111/ijlh.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Konkle B A, Stasyshyn O, Chowdary P. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(09):1078–1085. doi: 10.1182/blood-2015-03-630897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mullins E S, Stasyshyn O, Alvarez-Román M T. Extended half-life pegylated, full-length recombinant factor VIII for prophylaxis in children with severe haemophilia A. Haemophilia. 2017;23(02):238–246. doi: 10.1111/hae.13119. [DOI] [PubMed] [Google Scholar]
- 95.Meunier S, Alamelu J, Ehrenforth S. Safety and efficacy of a glycoPEGylated rFVIII (turoctocog alpha pegol, N8-GP) in paediatric patients with severe haemophilia A. Thromb Haemost. 2017;117(09):1705–1713. doi: 10.1160/TH17-03-0166. [DOI] [PubMed] [Google Scholar]
- 96.Santagostino E, Kenet G, Fischer K, Biss T, Ahuja S, Steele M. PROTECT VIII Kids: BAY 94-9027 (PEGylated Recombinant Factor VIII) safety and efficacy in previously treated children with severe haemophilia A. Haemophilia. 2020;26(03):e55–e65. doi: 10.1111/hae.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lieuw K. Many factor VIII products available in the treatment of hemophilia A: an embarrassment of riches? J Blood Med. 2017;8:67–73. doi: 10.2147/JBM.S103796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schiavoni M, Napolitano M, Giuffrida G. Status of recombinant factor VIII concentrate treatment for hemophilia A in Italy: characteristics and clinical benefits. Front Med (Lausanne) 2019;6:261. doi: 10.3389/fmed.2019.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fylling K A, Tange J I, Chen D, Pruthi R K. Heat inactivation of extended half-life factor VIII concentrates. Haemophilia. 2019;25(02):e130–e131. doi: 10.1111/hae.13700. [DOI] [PubMed] [Google Scholar]
- 100.Payne A B, Ellingsen D, Driggers J, Bean C J, Miller C H. Evaluation of pre-analytic heat treatment protocol used in the CDC Nijmegen-Bethesda assay for heat inactivation of extended half-life haemophilia treatment products. Haemophilia. 2020;26(01):e28–e30. doi: 10.1111/hae.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Türkantoz H, Königs C, Knöbl P. Cross-reacting inhibitors against recombinant porcine factor VIII in acquired hemophilia A: data from the GTH-AH 01/2010 study. J Thromb Haemost. 2020;18(01):36–43. doi: 10.1111/jth.14618. [DOI] [PubMed] [Google Scholar]
- 102.Mancuso M E, Mahlangu J N, Pipe S W.The changing treatment landscape in haemophilia: from standard half-life clotting factor concentrates to gene editing Lancet 2021397(10274):630–640. [DOI] [PubMed] [Google Scholar]
- 103.Croteau S E, Wang M, Wheeler A P. 2021 clinical trials update: innovations in hemophilia therapy. Am J Hematol. 2021;96(01):128–144. doi: 10.1002/ajh.26018. [DOI] [PubMed] [Google Scholar]
- 104.Miesbach W, Eladly F. Current and future options of haemophilia A treatments. Expert Opin Biol Ther. 2021;21(11):1395–1402. doi: 10.1080/14712598.2021.1908993. [DOI] [PubMed] [Google Scholar]
- 105.Trinchero A, Sholzberg M, Matino D. The evolution of hemophilia care: clinical and laboratory advances, opportunities, and challenges. Hamostaseologie. 2020;40(03):311–321. doi: 10.1055/a-1175-6530. [DOI] [PubMed] [Google Scholar]
- 106.Tripodi A, Chantarangkul V, Novembrino C, Peyvandi F. Advances in the treatment of hemophilia: implications for laboratory testing. Clin Chem. 2019;65(02):254–262. doi: 10.1373/clinchem.2017.284356. [DOI] [PubMed] [Google Scholar]
- 107.Rangarajan S, Walsh L, Lester W. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377(26):2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 108.Robinson M M, George L A, Carr M E. Factor IX assay discrepancies in the setting of liver gene therapy using a hyperfunctional variant factor IX-Padua. J Thromb Haemost. 2021;19(05):1212–1218. doi: 10.1111/jth.15281. [DOI] [PMC free article] [PubMed] [Google Scholar]


