Abstract
The alternative sigma factor ςB of Bacillus subtilis is required for the induction of approximately 100 genes after the imposition of a whole range of stresses and energy limitation. In this study, we investigated the impact of a null mutation in sigB on the stress and starvation survival of B. subtilis. sigB mutants which failed to induce the regulon following stress displayed an at least 50- to 100-fold decrease in survival of severe heat (54°C) or ethanol (9%) shock, salt (10%) stress, and acid (pH 4.3) stress, as well as freezing and desiccation, compared to the wild type. Preloading cells with ςB-dependent general stress proteins prior to growth-inhibiting stress conferred considerable protection against heat and salt. Exhaustion of glucose or phosphate induced the ςB response, but surprisingly, ςB did not seem to be required for starvation survival. Starved wild-type cells exhibited about 10-fold greater resistance to salt stress than exponentially growing cells. The data argue that the expression of ςB-dependent genes provides nonsporulated B. subtilis cells with a nonspecific multiple stress resistance that may be relevant for stress survival in the natural ecosystem.
One of the earliest responses of a Bacillus subtilis cell population to different stressful environmental conditions is the striking and immediate induction of a large number of general stress proteins. Heat, acid, ethanol, and salt stress or starvation for glucose, oxygen, or phosphate induces probably more than 100 stress genes that form the ςB-dependent general stress regulon (4, 11, 14, 45). The mechanism of this induction has been elucidated: the sigB operon itself is induced by all of the stress and starvation signals (9, 10, 13, 25, 28, 45). The complex signal transduction network conveying the different stress and starvation stimuli to sigB has been elucidated by the laboratories of Price, Haldenwang, and Losick: all of these stimuli enhance the activity of ςB, thereby inducing the sigB operon itself and consequently the entire ςB regulon (1–3, 8, 9, 15, 19, 29, 47, 48, 51).
Contrary to the wealth of information on the regulation of the activity of ςB, the physiological function of ςB remained obscure despite the increasing list of newly described genes subject to ςB control (24). Based on the induction profile, we suggested in 1986 that these proteins may have a rather nonspecific but nevertheless essential protective function under stress regardless of the specific growth-limiting factor (38). Later we found that the translational capacity used for the production of general stress proteins increases from about 1% in growing cells to 20 or even 30% in starved or stressed cells, suggesting a crucial physiological role of the proteins in stress adaptation (11). Elucidation of the structure and function of this stress and starvation regulon seemed to be essential for understanding the physiology of growth-restricted bacterial cells in general.
Two principal approaches should help to elucidate the function of the general stress regulon: (i) identification of the function of stress proteins belonging to the ςB regulon may allow predictions on the function of the entire regulon, and (ii) comparison of the stress tolerance of wild-type cells with that of strains carrying mutations in sigB itself or in ςB-dependent general stress genes will provide experimental evidence for the function of the entire regulon.
Some of the general stress proteins identified so far seem to be involved in the nonspecific protection from oxidative stress, from osmotic and water stress, or from heat stress. Therefore, we recently concluded that the products of ςB-dependent stress genes most likely provide B. subtilis cells in transition from a growing to a nongrowing state with a comprehensive multiple stress resistance machine in anticipation of future stress during long-term survival as nongrowing vegetative cells. Furthermore, we suggested that this stress-resistant state of growth-restricted cells could be an essential part of a survival strategy alternative to sporulation (for a review, see reference 26).
However, the phenotypic studies of sigB mutants available up to 1995 did not indicate any essential role in stress adaptation. ςB mutants were impaired in neither growth nor sporulation (12, 20). Furthermore, ςB seemed to be nonessential for the survival of B. subtilis following heat shock, since a sigB null mutant was as temperature resistant as the wild type (10). First hints for a role of ςB-dependent genes emerged when an insertional inactivation of the ςB-dependent ctc gene resulted in a sporulation frequency at 48°C lower than that in the wild type (43).
Considering the discrepancies between the growing list of newly described ςB-dependent genes on the one hand and the lack of a distinct stress-sensitive phenotype of sigB mutants on the other, a systematic reinvestigation of the function of the ςB-dependent stress response became necessary. Recently, a sigB mutant was found to be extremely sensitive to the radical-producing agent cumene hydroperoxide both during growth and in stationary phase (5). Furthermore, the sigB mutant was impaired in the development of the resistance against hydrogen peroxide nonspecifically acquired in glucose-starved cells (21) as well as against acid or alkaline stress (22). In this report, we present the results of a systematic study on the stress resistance of sigB mutants compared to the wild type, underlining the suggestion that ςB is responsible for the development of multiple stress resistance in nongrowing B. subtilis cells.
MATERIALS AND METHODS
Bacterial strains.
The B. subtilis wild-type strain 168 (trpC2), its isogenic sigB mutant ML6 (trpC2 sigB::ΔHindIII-EcoRV::cat) (27), and BSA115 were grown either in LB or in a synthetic medium described previously (42). In BSA115 (trpC2 rsbU::kan PBΔ28::Pspac rsbW313 pTET-I SPβ ctc::lacZ) (44), rsbW is inactivated by a frameshift mutation and the downstream half of the sigB operon is placed under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter Pspac. Inclusion of IPTG triggers the production of active ςB and hyperinduction of the ςB regulon in this strain.
Growth conditions and viability assay.
The cultures were inoculated into prewarmed LB or synthetic medium; during exponential growth, the cultures (6 × 107 to 8 × 107 cells per ml) were exposed to the stresses according to the following scheme: for heat shock, the culture was transferred to 54°C; for ethanol stress, ethanol was added to a final concentration of 9% (vol/vol); for salt stress, solid NaCl was added to a final concentration of 10% (wt/vol); for acid shock, 1 N HCl was added to shift the pH of the culture from 7.5 to 4.3; for freezing, aliquots of the culture were rapidly frozen at −20°C, thawed, and plated at the time indicated. Cells were preadapted if indicated by incubation for 30 min either in the presence of 4% NaCl, at 48°C, or at pH 5.2. Thereafter, the stress strength was raised to the level described above. The influence of preloading cells with ςB-dependent stress proteins was investigated by dividing an exponentially growing culture of BSA115 60 min (LB) or 120 min (synthetic medium) prior to stress and including 1 mM IPTG (final concentration) in one half of the culture. Both parts were exposed to the stresses as described above. The osmoprotectant glycine betaine was added in some experiments to a final concentration of 1 mM.
At the time points indicated, aliquots of the culture were sampled and diluted in 0.9% (wt/vol) NaCl solution, and appropriate dilutions were plated on LB agar plates to determine cell viability. All experiments were performed at least in triplicate, and the results of typical experiments are displayed in the figures.
RESULTS
Role of ςB in heat and ethanol stress resistance.
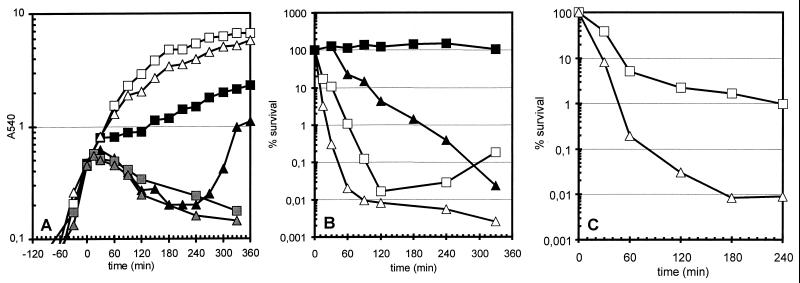
Transferring B. subtilis from 37 to 48°C is commonly applied to induce the expression of the ςB regulon (10, 45), but the sigB mutant displayed no difference in either growth or survival compared with the wild type (data not shown). However, assuming that ςB-dependent gene expression serves to prepare the cell for more severe stress conditions, then the effect of the lack of ςB could be more pronounced at harsher conditions. Therefore, the heat shock was repeated by shifting bacteria from 37 to 52°C. Whereas wild-type bacteria continued to grow, albeit at a reduced rate, the sigB mutant strain ceased growth and started to lyse (Fig. 1A). Since this reduction in optical density of the sigB mutant was accompanied by a slight decrease in survival only (data not shown), the heat shock was extended to 54°C, which also prevented growth of the wild type (Fig. 1A). At this critical point when even the wild-type strain was severely impaired, viability declined dramatically within 60 min for both strains, but striking differences in survival between the wild type and the sigB mutant became visible (Fig. 1B). A mild heat preadaptation period at 48°C prevented the decrease in viability in the wild type but not in the sigB mutant. As a result, the differences in survival between the two strains became much more pronounced: after 5 h, a more than 1,000-fold difference in heat stress survival between the wild type and the sigB mutant was found. Nevertheless, we observed a protective effect by mild heat stress also in the sigB mutant, most likely because of the induction of the ςB-independent chaperone-encoding genes (Fig. 1B).
FIG. 1.
Growth and survival of B. subtilis strains during heat shock and ethanol stress. The wild-type strain 168 (squares) and its isogenic sigB mutant ML6 (triangles) were grown in LB and exposed to either heat shock (A and B) or ethanol stress (C). (A) The growth of cultures was monitored by measuring the optical density at 540 nm. The strains were cultivated at 37°C (open symbols) or transferred at time zero to 52°C (black symbols) or 54°C (gray symbols). (B) Survival was determined by plating appropriate dilutions of samples taken at the time indicated. The number of cells of each strain present at the time zero immediately before treatment was set at 100%. Open symbols, cultures shifted to 54°C at time zero; filled symbols, cultures incubated for 30 min at 48°C prior to transfer to the final temperature of 54°C. (C) Viability was assayed immediately before and at different time points after the addition of ethanol to a final concentration of 9% (vol/vol).
Ethanol treatment seems to induce cell damage similar to that caused by heat shock (36), and ethanol-treated cells of a sigB mutant displayed a lower growth yield than the corresponding wild-type strain (22). Therefore, results similar to those observed for heat shock were expected for ethanol. The sigB mutant showed indeed an approximately 100-fold greater drop in cell survival in response to treatment with 9% ethanol than its wild-type counterpart (Fig. 1C).
Role of ςB in resistance to salt stress.
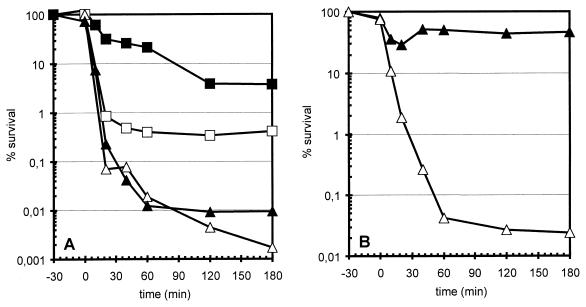
Comparison of the growth patterns of sigB mutant cells and wild-type bacteria after exposure to NaCl in the range from 4 to 16% (final concentration) revealed no difference between the two strains (data not shown). However, the strains displayed clear differences in survival of such harsh conditions. The survival rate of the wild-type strain was approximately 50-fold higher than that of the sigB mutant (Fig. 2A). An attempt to protect these cells by a mild (4% NaCl) salt preadaptation for 30 min increased the viability of the wild type 10-fold but had no effect on the sigB mutant (Fig. 2A). In contrast to heat shock, the pretreatment did not completely protect the wild-type bacteria.
FIG. 2.
Survival of B. subtilis strains during salt stress. The wild-type strain 168 (squares) and its isogenic sigB mutant ML6 (triangles) were grown in a synthetic medium and exposed to salt stress. Survival was determined as described in the legend to Fig. 1. (A) Open symbols represent cultures exposed to 10% (wt/vol) sodium chloride at time zero; filled symbols represent cultures pretreated with a mild salt stress of 4% NaCl for 30 min before the sodium chloride concentration was raised to 10% (wt/vol) at time zero. (B) The sigB mutant ML6 was grown in synthetic medium with (closed triangles) or without (open triangles) 1 mM glycine betaine; 30 min before the sodium chloride concentration was increased to 10% (wt/vol), the bacteria were exposed to a mild salt stress of 4% NaCl.
Glycine betaine is a very effective osmoprotectant in B. subtilis (30). We wanted to determine whether glycine betaine can compensate for the missing induction of the ςB response in the sigB mutant. Addition of 1 mM glycine betaine to growing sigB mutant cells before the imposition of salt stress triggered an almost complete protection against the otherwise lethal salt stress (Fig. 2B). This result indicates that induction of ςB-dependent general stress genes and accumulation of osmoprotective substances constitute alternative mechanisms for protection against the deleterious effects of growth-inhibiting salt concentrations. Inclusion of 1 mM glycine betaine in the culture medium did not protect heat-damaged cells (data not shown), indicating that heat and salt seem to cause distinctively different types of damage.
Artificial induction of the ςB response and cross-adaptation produce stress resistance.
To separate the effects of the ςB response from protection triggered by induction of specific stress proteins, we analyzed the effects of cross-adaptation and preloading cells with ςB-dependent general stress proteins.
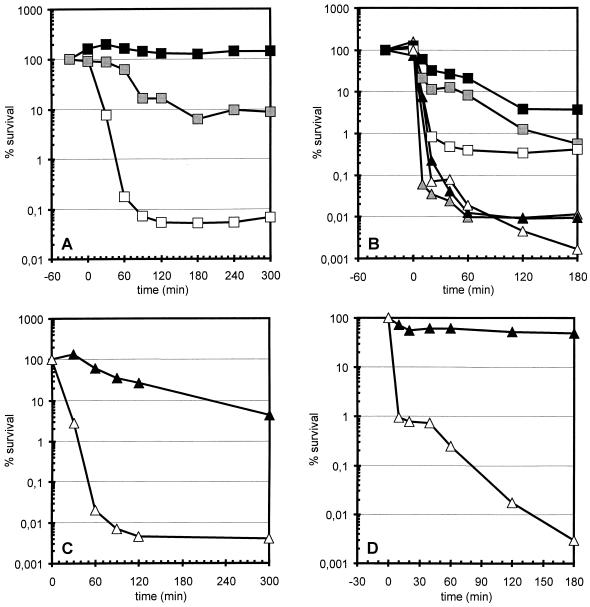
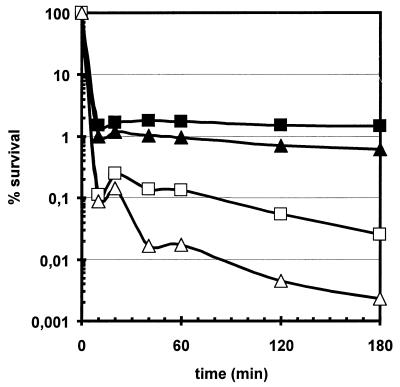
A mild heat preadaptation which also induced the ςB-dependent stress genes was almost as effective as a mild salt pretreatment in protection against severe salt stress at least within 60 min after imposition of the salt stress (Fig. 3B). As expected, this cross-protection occurred in the wild type but not in the sigB mutant. Preincubating wild-type cells with 4% NaCl for 30 min prior to a heat treatment at 54°C similarly provided a significant but incomplete protection (Fig. 3A).
FIG. 3.
Influence of cross-adaptation (A and B) and preloading bacteria with general stress proteins (C and D) on survival of heat shock (A and C) or salt stress (B and D). Bacteria were grown in a synthetic medium, and during exponential growth the cultures were exposed to heat shock or salt stress. Survival was determined as described in the legend to Fig. 1. (A) The wild-type strain 168 was pretreated for 30 min with either a mild heat shock of 48°C (black squares) or a mild salt stress of 4% NaCl (gray squares) prior to the shift to 54°C or directly exposed to 54°C (open squares) at time zero. (B) The wild-type strain 168 (squares) or its isogenic sigB counterpart ML6 (triangles) were exposed to 10% (wt/vol) sodium chloride at time zero. Cells were immediately exposed to the severe salt stress (open symbols) or pretreated for 30 min with 4% NaCl (black symbols) or a mild heat shock of 48°C (gray symbols). (C and D) B. subtilis BSA115 was grown in synthetic medium. During exponential growth, the culture was divided and IPTG was added to one half to a final concentration of 1 mM; 120 min after the addition of IPTG (time zero), both cultures were exposed to either 54°C (C) or 10% (wt/vol) NaCl (D). Open triangles, no IPTG; closed triangles, 1 mM IPTG added.
In another set of experiments, the level of ςB-dependent stress proteins was varied without exposing bacteria to stress. In B. subtilis BSA115, a sigB operon with a null mutation in the primary negative regulator rsbW is placed under the control of the IPTG-inducible promoter Pspac. Addition of IPTG prior to heat shock triggered the accumulation of large amounts of general stress proteins in the absence of stress and partially prevented the sharp decline in cell survival at 54°C observed in the absence of IPTG (Fig. 3C). In the case of severe salt stress, the addition of IPTG almost completely restored cell viability, indicating that the ςB-dependent stress response seems to be mainly responsible for the resistance against severe salt stress (Fig. 3D).
ςB and acid stress resistance.
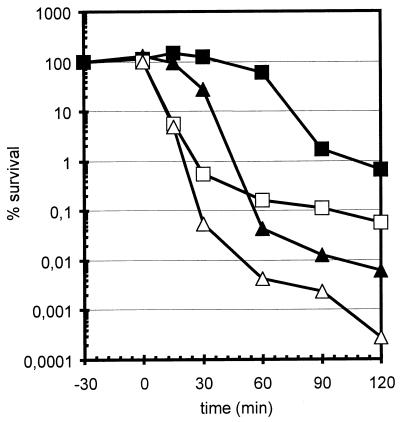
Exponentially growing cells treated at pH 4.3 showed a dramatic drop in cell survival within 60 min, but the sigB mutant was about 50- to 100-fold more sensitive than its wild-type counterpart (Fig. 4). Both strains, however, exhibited an about 10-fold increase in cell viability after a 30-min preexposure to mild acid stress (pH 5.2), indicating that this protective acid-adaptive response does not depend solely on ςB.
FIG. 4.
Influence of ςB on survival of acid shock. During exponential growth in LB, cultures of the wild-type strain 168 (squares) and the sigB mutant ML6 (triangles) were exposed to acid shock. Survival was determined as described in the legend to Fig. 1. Acid shock was imposed either directly at time zero by shifting the pH of the culture medium from 7.5 to 4.3 (open symbols) or after a preadaptation for 30 min with a intermediate acid shock at pH 5.2 (filled symbols).
Influence of ςB on survival of freezing and thawing.
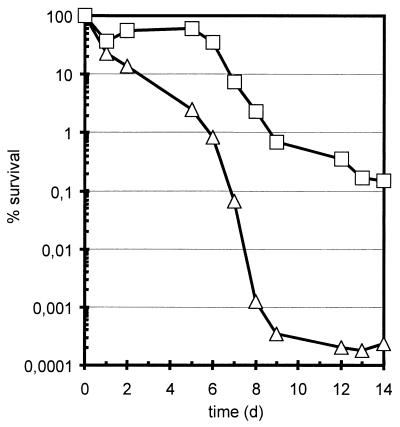
The effect on survival of B. subtilis of changing the water activity inside the cell during freezing and thawing was tested. Samples of the B. subtilis wild-type strain 168 and its isogenic sigB mutant strain ML6 were taken 1 h after the cultures had entered the stationary phase as a result of the exhaustion of glucose from a synthetic medium and immediately frozen at −20°C without the addition of a cryoprotective substance. Over a period of 14 days, we thawed aliquots of the samples each day at room temperature and assayed viability by plating on LB agar plates. The results shown in Fig. 5 indicate that the wild-type cells survived the treatment 50- to 100-fold better than the sigB mutant. These findings were supported by desiccation experiments: the wild-type strain survived lyophilization at least 10-fold better than the sigB strain (data not shown).
FIG. 5.
Influence of ςB on survival of freezing and thawing. One hour after cultures of the wild-type strain (squares) and the sigB mutant (triangles) had entered the stationary phase as a result of the exhaustion of glucose, samples were removed and immediately frozen at −20°C. Each day, aliquots were thawed at room temperature and appropriate dilutions were plated on LB agar plates. The number of cells present during the sampling was set at 100%.
Role of ςB in starvation survival.
ςB does not seem to be essential for starvation survival under glucose or phosphate limitation in a minimal medium. Similar starvation survival kinetics were measured for the wild type and the sigB mutant (data not shown). It is well known that glucose-starved cells become resistant to oxidative stress (for a review, see reference 18). This nonspecifically acquired stress resistance depends on ςB because glucose-starved cells of a sigB mutant were much more sensitive to oxidative stress than starved wild-type cells (21). Because glucose starvation activates the ςB response, we wondered if the resistance to other stresses in glucose-starved cells is also enhanced under these conditions. Surprisingly, glucose-starved cells were as sensitive to heat stress as exponentially growing cells (not shown). In the case of salt stress resistance, the difference between growing and glucose-starved cells was at least 10-fold (Fig. 6). However, glucose-starved wild-type and sigB mutant cells differed only two- to threefold in salt stress resistance, because of the development of a ςB-independent salt resistance in both strains during glucose starvation (Fig. 6).
FIG. 6.
Influence of glucose starvation on survival of salt stress. The wild-type strain 168 (squares) and its isogenic sigB mutant ML6 (triangles) were grown in a synthetic medium and exposed to salt stress (12% [wt/vol], final concentration) either during exponential growth (open symbols) or 1 h after the cultures had entered the stationary phase as a result of the exhaustion of glucose (filled symbols). Survival was determined as described in the legend to Fig. 1. Salt was added at time zero.
DISCUSSION
ςB was identified as the first bacterial alternative sigma factor almost 20 years ago, but interest in ςB declined after it was determined that this sigma factor is not involved in sporulation (12, 20, 27, 28). The discovery that ςB, whose activity itself is tightly regulated by a set of different environmental stimuli, controls a large general stress and starvation regulon activated in nongrowing cells may have been responsible for the revival of attention to ςB (10, 13, 14, 45). Several laboratories started to investigate not only the role of ςB in the adaptation to growth-restricting conditions but also the pathogenicity of gram-positive bacteria such as Staphylococcus aureus, Listeria monocytogenes, and mycobacteria (7, 17, 32, 33, 50).
The discrepancy between a constantly increasing list of newly described ςB-dependent stress genes (26) and the lack of any altered phenotype of sigB mutants (12, 20) called for a careful reinvestigation of the physiological role of ςB. It was tempting to speculate that this large stress regulon might fulfill a crucial adaptive function under stress, but experimental evidence was lacking. Recently, new data revealed that ςB is necessary for the adaptation to oxidative stress (21) as well as to acid or alkaline stress (22). It is interesting that the ςB regulon is clearly involved in adaptation to oxidative and alkaline stress, but neither induces this ςB regulon. On the other hand, no evidence has been presented for any role of ςB in protection against heat and salt stress, which are excellent inducers of the more than 100 genes of the ςB regulon. The results presented in this report suggest that general stress proteins are required during growth inhibition resulting from severe heat or salt stress. Conditions which reduce but still permit growth do not require ςB-dependent stress proteins for the development of resistance. Instead, specific stress proteins such as the systems used for the uptake and synthesis of osmoprotective substances sufficiently protect the cells during, for example, less severe osmotic stress (49; for a review, see reference (30).
Schulz et al. (40) and Li and Wong (34) found that dnaK or groESL mutants of B. subtilis were impaired in thermotolerance. Our data clearly demonstrate that besides chaperones, ςB-dependent stress proteins are also involved in heat stress resistance in B. subtilis. The increase in the survival rates at 54°C of sigB mutant cells after pretreatment at 48°C (Fig. 1) might be explained by the induction of the DnaK and GroEL and also of the Clp machinery in the sigB mutant (11, 31). The much stronger, almost complete protective effect of the same mild heat shock pretreatment of the wild type argues for a major role of general stress proteins in this heat stress resistance (Fig. 1). Such a conclusion gains support from the protection from severe heat shock by cross-adaptation with a mild salt stress or preloading cells of BSA115 with ςB-dependent stress proteins in the absence of stress (Fig. 3). Both variants yield only partial protection since they failed to induce the heat-specific chaperones, corroborating the essential function of chaperones in this adaptation (34, 40, 46).
A great difference in survival of wild-type and sigB mutant cells was also found when both strains were challenged with a severe, growth-inhibiting salt stress. The difference was enhanced after a 30-min preadaptation period with a mild salt stress (4% sodium chloride [Fig. 2]). This pretreatment increased the viability of the wild type more than 10-fold but did not substantially stimulate the survival rate of sigB mutant cells. This result suggests that contrary to the heat stress response where heat specific (chaperones) and general stress proteins contributed to the stress resistance, general stress proteins (and not salt stress-specific proteins) seem to play the major role in this acquired resistance against severe salt stress. This suggestion is supported by the fact that an artificial increase of the ςB level by IPTG addition in strain BSA115 provided almost complete protection against the toxic salt stress but only partial protection against the lethal heat challenge (Fig. 3). Because the induction of general stress proteins seemed to be crucial for the acquired salt stress resistance, it is reasonable that a mild heat shock would be nearly as effective as a mild salt pretreatment (Fig. 2 and 3). Altogether, the results presented in this and related reports clearly show that ςB-dependent stress proteins provide the stressed or starved cells with multiple stress resistance (5, 6, 21, 22). This stress resistance includes heat, ethanol, or salt stress resistance and resistance against acid or alkaline stress as found by Gaidenko and Price (22) or against oxidative stress. The multiple stress resistance also involves resistance to freezing and thawing. However, in the latter case it cannot be distinguished whether the killing is caused by water stress or by oxidative stress, which was shown to occur in yeast during aerobic freezing and thawing (37).
A similar multiple stress resistance in nutrient-starved Escherichia coli cells depends on RpoS, confirming the suggestion that the two regulons may fulfill similar adaptive functions in nongrowing cells (25). However, ςB does not contribute to the starvation survival potential as RpoS does for E. coli (35) either in B. subtilis (this study and reference 22) or in S. aureus (16). This conclusion is supported by a recent study of Schweder et al., who found that the B. subtilis wild-type strain has no growth advantage compared with a sigB mutant grown in a glucose-limited chemostat in the absence of additional stresses (41). These results indicate that ςB-dependent stress proteins do not contribute to the starvation survival potential of glucose-starved cells; rather, the main function of ςB is to protect the starved cell against future stress that may emerge during long-term survival. Glucose-starved cells develop an oxidative stress resistance that depends on ςB (21). This nonspecific and multiple stress resistance of glucose-starved cells also includes salt stress resistance that increased more than 10-fold during starvation. Surprisingly, the salt resistance acquired during glucose starvation is only partially ςB dependent (Fig. 6). Thermal resistance, however, does not significantly increase after glucose exhaustion compared to exponentially growth despite the fact that the ςB regulon is fully active. Failure of development of a full heat stress resistance might at least partially be accounted for by the lack of induction of chaperones in glucose-starved cells (11).
Therefore, the main function of ςB during energy depletion should be to equip glucose-starved cells with nonspecific resistance to oxidative stress, including resistance to freezing and thawing or to UV light damage (not shown here). In addition to this function, ςB is essential for the interaction with a severe heat, ethanol, acid, or salt challenge, cross-protection between these stimuli included. Oxidative stress, changing osmotic or salt stress conditions triggered by desiccation and rain, and also acid or heat stress may be typical growth-restricting conditions in the upper layers of soil, the natural habitat of B. subtilis. The ςB-dependent stress response could be particularly relevant for cell survival in soil under conditions that do not allow efficient sporulation such as high osmolarity or low cell density (23, 39). Therefore, it is tempting to speculate that sigB mutants in soil have a survival disadvantage compared to the wild type, but experimental evidence is still lacking.
The function of the sigB regulon in multiple and prospective stress resistance is in good accordance with recent data on the function of ςB-dependent genes (for a review, see reference 26). These genes form a complex regulon that may comprise more than 100 genes. The identification of their function was one of our main strategies to uncover the function of the regulon. It is an attractive goal for future research to assign the single general stress proteins to the different components of the multiple stress resistance network typical of stressed or starving B. subtilis cells.
ACKNOWLEDGMENTS
We thank Anita Harang for excellent technical assistance throughout this investigation. We also thank E. Bremer for stimulating discussion.
This work was supported by grants from the Deutsche Forschungsgemeinschaft to M.H. and U.V. and by a grant from the Fonds der Chemischen Industrie to M.H.
REFERENCES
- 1.Akbar S, Kang C M, Gaidenko T A, Price C W. Modulator protein RsbR regulates environmental signalling in the general stress pathway of Bacillus subtilis. Mol Microbiol. 1997;24:567–578. doi: 10.1046/j.1365-2958.1997.3631732.x. [DOI] [PubMed] [Google Scholar]
- 2.Alper S, Dufour A, Garsin D A, Duncan L, Losick R. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J Mol Biol. 1996;260:165–177. doi: 10.1006/jmbi.1996.0390. [DOI] [PubMed] [Google Scholar]
- 3.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 4.Antelmann H, Bernhardt J, Schmid R, Mach H, Völker U, Hecker M. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis. 1997;18:1451–1463. doi: 10.1002/elps.1150180820. [DOI] [PubMed] [Google Scholar]
- 5.Antelmann H, Engelmann S, Schmid R, Hecker M. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J Bacteriol. 1996;178:6571–6578. doi: 10.1128/jb.178.22.6571-6578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antelmann H, Engelmann S, Schmid R, Sorokin A, Lapidus A, Hecker M. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor ςB in Bacillus subtilis. J Bacteriol. 1997;179:7251–7256. doi: 10.1128/jb.179.23.7251-7256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker L A, Cetin M S, Hutkins R W, Benson A K. Identification of the gene encoding the alternative sigma factor ςB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;180:4547–4554. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson A K, Haldenwang W G. Bacillus subtilis ςB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc Natl Acad Sci USA. 1993;90:2330–2334. doi: 10.1073/pnas.90.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benson A K, Haldenwang W G. Characterization of a regulatory network that controls ςB expression in Bacillus subtilis. J Bacteriol. 1992;174:749–757. doi: 10.1128/jb.174.3.749-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson A K, Haldenwang W G. The ςB-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J Bacteriol. 1993;175:1929–1935. doi: 10.1128/jb.175.7.1929-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernhardt J, Völker U, Völker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two-dimensional protein electrophoresis study. Microbiology. 1997;143:999–1017. doi: 10.1099/00221287-143-3-999. [DOI] [PubMed] [Google Scholar]
- 12.Binnie C, Lampe M, Losick R. Gene encoding the sigma 37 species of RNA polymerase sigma factor from Bacillus subtilis. Proc Natl Acad Sci USA. 1986;83:5943–5947. doi: 10.1073/pnas.83.16.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boylan S A, Redfield A R, Brody M S, Price C W. Stress-induced activation of the ςB transcription factor of Bacillus subtilis. J Bacteriol. 1993;175:7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boylan S A, Redfield A R, Price C W. Transcription factor ςB of Bacillus subtilis controls a large stationary-phase regulon. J Bacteriol. 1993;175:3957–3963. doi: 10.1128/jb.175.13.3957-3963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boylan S A, Rutherford A, Thomas S M, Price C W. Activation of Bacillus subtilis transcription factor ςB by a regulatory pathway responsive to stationary-phase signals. J Bacteriol. 1992;174:3695–3706. doi: 10.1128/jb.174.11.3695-3706.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan P F, Foster S J, Ingham E, Clements M O. The Staphylococcus aureus alternative sigma factor ςB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMaio J, Zhang Y, Ko C, Bshai W. Mycobacterium tuberculosis sigF is part of a gene cluster with similarities to the Bacillus subtilis sigF and sigB operons. Tuberc Lung Dis. 1997;78:3–12. doi: 10.1016/s0962-8479(97)90010-1. [DOI] [PubMed] [Google Scholar]
- 18.Dowds B C A. The oxidative stress response in Bacillus subtilis. FEMS Microbiol Lett. 1994;124:255–263. doi: 10.1111/j.1574-6968.1994.tb07294.x. [DOI] [PubMed] [Google Scholar]
- 19.Dufour A, Haldenwang W G. Interactions between a Bacillus subtilis anti-sigma factor (RsbW) and its antagonist (RsbV) J Bacteriol. 1994;176:1813–1820. doi: 10.1128/jb.176.7.1813-1820.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan M L, Kalman S S, Thomas S M, Price C W. Gene encoding the 37,000-dalton minor sigma factor of Bacillus subtilis RNA polymerase: isolation, nucleotide sequence, chromosomal locus, and cryptic function. J Bacteriol. 1987;169:771–778. doi: 10.1128/jb.169.2.771-778.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelmann S, Hecker M. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol Lett. 1996;145:63–69. doi: 10.1111/j.1574-6968.1996.tb08557.x. [DOI] [PubMed] [Google Scholar]
- 22.Gaidenko T A, Price C W. General stress transcription factor-ςB and sporulation transcription factor-ςH each contribute to survival of Bacillus subtilis under extreme growth conditions. J Bacteriol. 1998;180:3730–3733. doi: 10.1128/jb.180.14.3730-3733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman A, Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci USA. 1988;85:4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 26.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the ςB regulon. Mol Microbiol. 1998;29:1129–1136. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 27.Igo M, Lampe M, Ray C, Schafer W, Moran C P, Jr, Losick R. Genetic studies of a secondary RNA polymerase sigma factor in Bacillus subtilis. J Bacteriol. 1987;169:3464–3469. doi: 10.1128/jb.169.8.3464-3469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalman S, Duncan M L, Thomas S M, Price C W. Similar organization of the sigB and spoIIA operons encoding alternate sigma factors of Bacillus subtilis RNA polymerase. J Bacteriol. 1990;172:5575–5585. doi: 10.1128/jb.172.10.5575-5585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang C M, Vijay K, Price C W. Serine kinase activity of a Bacillus subtilis switch protein is required to transduce environmental stress signals but not to activate its target PP2C phosphatase. Mol Microbiol. 1998;30:189–196. doi: 10.1046/j.1365-2958.1998.01052.x. [DOI] [PubMed] [Google Scholar]
- 30.Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolarity environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 31.Krüger E, Msadek T, Hecker M. Alternate promoters direct stress-induced transcription of the Bacillus subtilis clpC operon. Mol Microbiol. 1996;20:713–723. doi: 10.1111/j.1365-2958.1996.tb02511.x. [DOI] [PubMed] [Google Scholar]
- 32.Kullik I, Giachino P. The alternative sigma factor ςB in Staphylococcus aureus—regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 33.Kullik I, Giachino P, Fuchs T. Deletion of the alternative sigma factor ςB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Wong S. Cloning and characterization of the groESL operon from Bacillus subtilis. J Bacteriol. 1992;174:3981–3992. doi: 10.1128/jb.174.12.3981-3992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matin A, Auger E A, Blum P H, Schultz J E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- 36.Mogk A, Völker A, Engelmann S, Hecker M, Schumann W, Völker U. Nonnative proteins signal induction of the Bacillus subtilis CIRCE regulon. J Bacteriol. 1998;180:2895–2900. doi: 10.1128/jb.180.11.2895-2900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J, Grant C, Davies M, Dawes I. The cytoplasmic Cu,Zn superoxide dismutase of Saccharomyces cerevisiae is required for resistance to freeze-thaw stress—generation of free radicals during freezing and thawing. J Biol Chem. 1998;273:22921–22928. doi: 10.1074/jbc.273.36.22921. [DOI] [PubMed] [Google Scholar]
- 38.Richter A, Hecker M. Heat-shock proteins in Bacillus subtilis: a two-dimensional gel electrophoresis study. FEMS Microbiol Lett. 1986;36:69–71. [Google Scholar]
- 39.Ruzal S M, Lopez C, Rivas E, Sanchez-Rivas C. Osmotic strength blocks sporulation at stage II by impeding activation of early sigma factors in Bacillus subtilis. Curr Microbiol. 1998;36:75–79. doi: 10.1007/s002849900282. [DOI] [PubMed] [Google Scholar]
- 40.Schulz A, Tzschaschel B, Schumann W. Isolation and analysis of mutants of the dnaK operon of Bacillus subtilis. Mol Microbiol. 1995;15:421–429. doi: 10.1111/j.1365-2958.1995.tb02256.x. [DOI] [PubMed] [Google Scholar]
- 41.Schweder, T., A. Kolyschkow, U. Völker, and M. Hecker. Analysis of the expression and function of the ςB-dependent general stress regulon of Bacillus subtilis during slow growth. Arch. Microbiol., in press. [DOI] [PubMed]
- 42.Stülke J, Hanschke R, Hecker M. Temporal activation of beta-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J Gen Microbiol. 1993;139:2041–2045. doi: 10.1099/00221287-139-9-2041. [DOI] [PubMed] [Google Scholar]
- 43.Truitt C L, Weaver E A, Haldenwang W G. Effects on growth and sporulation of inactivation of a Bacillus subtilis gene (ctc) transcribed in vitro by minor vegetative cell RNA polymerases (E-ς37, E-ς32) Mol Gen Genet. 1988;212:166–171. doi: 10.1007/BF00322460. [DOI] [PubMed] [Google Scholar]
- 44.Völker U, Dufour A, Haldenwang W G. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of ςB. J Bacteriol. 1995;177:114–122. doi: 10.1128/jb.177.1.114-122.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 46.Völker U, Mach H, Schmid R, Hecker H. Stress proteins and cross-protection by heat shock and salt stress in Bacillus subtilis. J Gen Microbiol. 1992;138:2125–2135. doi: 10.1099/00221287-138-10-2125. [DOI] [PubMed] [Google Scholar]
- 47.Völker U, Völker A, Haldenwang W G. Reactivation of the Bacillus subtilis anti-ςB antagonist, RsbV, by stress- or starvation-induced phosphatase activities. J Bacteriol. 1996;178:5456–5463. doi: 10.1128/jb.178.18.5456-5463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Völker U, Völker A, Maul B, Hecker M, Dufour A, Haldenwang W G. Separate mechanisms activate ςB of Bacillus subtilis in response to environmental and metabolic stresses. J Bacteriol. 1995;177:3771–3780. doi: 10.1128/jb.177.13.3771-3780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VonBlohn C, Kempf B, Kappes R M, Bremer E. Osmostress response in Bacillus subtilis—characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol Microbiol. 1997;25:175–187. doi: 10.1046/j.1365-2958.1997.4441809.x. [DOI] [PubMed] [Google Scholar]
- 50.Wu S W, Delencastre H, Tomasz A. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase—molecular cloning and DNA sequencing. J Bacteriol. 1996;178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X F, Kang C M, Brody M S, Price C W. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 1996;10:2265–2275. doi: 10.1101/gad.10.18.2265. [DOI] [PubMed] [Google Scholar]