Abstract
Hemorrhagic shock causes vascular endothelial glycocalyx (EGCX) damage and systemic inflammation. Dexmedetomidine (DEX) has anti-inflammatory and EGCX-protective effects, but its effect on hemorrhagic shock has not been investigated. Therefore, we investigated whether DEX reduces inflammation and protects EGCX during hemorrhagic shock. Anesthetized Sprague-Dawley rats were randomly assigned to five groups (n=7 per group): no shock (SHAM), hemorrhagic shock (HS), hemorrhagic shock with DEX (HS+DEX), hemorrhagic shock with DEX and the α7 nicotinic type acetylcholine receptor antagonist methyllycaconitine citrate (HS+DEX/MLA), and hemorrhagic shock with MLA (HS+MLA). HS was induced by shedding blood to a mean blood pressure of 25–30 mmHg, which was maintained for 30 min, after which rats were resuscitated with Ringer’s lactate solution at three times the bleeding volume. The survival rate was assessed up to 3 h after the start of fluid resuscitation. Serum tumor necrosis factor-alpha (TNF-α) and syndecan-1 concentrations, and wet-to-dry ratio of the heart were measured 90 min after the start of fluid resuscitation. The survival rate after 3 h was significantly higher in the HS+DEX group than in the HS group. Serum TNF-α and syndecan-1 concentrations, and the wet-to-dry ratio of heart were elevated by HS, but significantly decreased by DEX. These effects were antagonized by MLA. DEX suppressed the inflammatory response and serum syndecan-1 elevation, and prolonged survival in rats with HS.
Keywords: cholinergic pathway, dexmedetomidine, endothelial glycocalyx, hemorrhagic shock
Introduction
Hemorrhagic shock (HS) is the leading cause of unexpected perioperative death in Japan [1]. This is a major challenge for anesthesiologists who play a central role in perioperative medicine. Even if temporary resuscitation is achieved with appropriate fluid or blood transfusion, multiple organ failure can subsequently occur, resulting in a poor prognosis [2]. Systemic inflammatory responses are known to be secondary to HS and might make a major contribution to multiple organ failure after HS [2, 3]. During systemic inflammation, impaired vascular endothelial glycocalyx (EGCX) increases vascular permeability [4]. Hyperpermeability promotes edema, impairs tissue microcirculation, and causes organ damage [5]. Intravital microscopy demonstrated the shedding of EGCX during HS [6], and the degradation of EGCX was reported to lead to poor survival [7, 8]. Therefore, the control of inflammation and protection of EGCX may help prevent the progression of multiple organ failure and reduce mortality [9]. Although several drugs were previously reported to protect EGCX [7, 8, 10], clinically sufficient therapeutics have yet to be established.
Dexmedetomidine (DEX) is a sedative that acts on the α2 adrenergic receptor and is commonly used in intensive care units [11]. Recent studies reported that DEX has anti-inflammatory effects mediated via cholinergic anti-inflammatory pathways [12,13,14]. In addition, we reported that DEX protected glycocalyx in a rat heat stroke model [15]. However, the effect of DEX on EGCX in HS is still unknown.
We hypothesized that DEX protects against EGCX injury after HS and examined whether its action was related to the control of inflammation via the cholinergic anti-inflammatory pathway.
Materials and Methods
Animal preparation
This study was approved by the Ethical Committee for Animal Experiments and the Laboratory Animal Facility of Hamamatsu University School of Medicine (2018039). Overall, 70 male Sprague-Dawley rats (aged 13–15 weeks old, weighing 380–430 g) were obtained from Japan SLC, Inc. (Shizuoka, Japan). All rats were acclimated to a 12/12-h light/dark cycle at a temperature of 24°C and were provided free access to standard feed and water at all times. After the induction of anesthesia with isoflurane (Mylan, Tokyo, Japan), the rats underwent a tracheotomy and were intubated with a 19-gauge fluororesin catheter (Hakko Medical Device Division, Nagano, Japan). The core body temperature of the rats was measured using a rectal probe (BWT-100A; Bio Research Center, Nagoya, Japan) and maintained at 37°C with a heating lamp. A 24-gauge catheter (B Braun, Melsungen, Germany) was inserted into the descending aorta from the left femoral artery to measure the arterial pressure and heart rate. A catheter (SP31; Natsume Seisakusho, Tokyo, Japan) was placed into the right femoral vein for drug administration. A 20-gauge catheter (B Braun, Melsungen, Germany) was placed into the right femoral artery for sampling, blood withdrawal, and fluid resuscitation. After all catheters had been placed, pentobarbital sodium (Nacalai Tesque, Inc., Kyoto, Japan) and remifentanil (Daiichi Sankyo, Tokyo, Japan) were administered continuously at 20 mg/kg/h and 2 µg/kg/min, respectively, to maintain anesthesia instead of isoflurane. The rats were artificially ventilated (rate, 60/min; tidal volume, 0.8 ml/100 g; FiO2, 21%; Shinano Seisakusho, Tokyo, Japan) during the experiments. Mean arterial pressure and heart rate were continuously recorded. Data were recorded on a data acquisition system (PowerLab 8/30, Chart v5.5.6; ADInstruments, New Zealand).
Hemorrhagic shock model
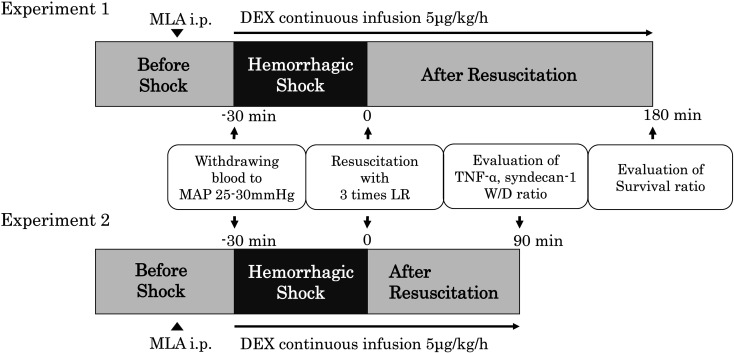
After stabilization, the rats were randomly divided into five groups: no shock (SHAM), hemorrhagic shock (HS), hemorrhagic shock with dexmedetomidine (HS+DEX), hemorrhagic shock with dexmedetomidine and the α7nAChR antagonist methyllycaconitine citrate (HS+DEX/MLA), and hemorrhagic shock with methyllycaconitine (HS+MLA). HS was induced using the fixed-pressure model as previously described with minor modifications [16]. Blood was withdrawn over 5 min until the mean arterial pressure (MAP) decreased to 25–30 mmHg and maintained at this level by further blood withdrawal and reinfusion of shed blood for 30 min after reaching the target pressure. After the shock phase, rats were resuscitated by the administration of Ringer’s lactate solution at three volumes of the shed blood at 2.5 ml/min. Additionally, DEX was administered at 5 µg/kg/h from the start of blood withdrawal in the HS+DEX group, whereas MLA (5 mg/kg for experiment 1; 10 mg/kg for experiment 2) was administered intraperitoneally 15 min prior to blood withdrawal in the HS+DEX/MLA group. In the HS+MLA group, the treatment was identical to that in the HS group, except that MLA was administered intraperitoneally 15 min prior to blood withdrawal. Rats in the SHAM group underwent all of the surgical procedures that the other groups underwent. The experimental protocols are shown in Fig. 1. All surviving rats were sacrificed by exsanguination under anesthesia.
Fig. 1.
Experimental protocol of hemorrhagic shock and fluid resuscitation. DEX, dexmedetomidine; MLA, methyllycaconitine; MAP, mean arterial pressure; LR, Ringer’s lactate solution; TNF-α, tumor necrosis factor-alpha; W/D, wet-to-dry ratio.
Experiment 1: Survival time analysis (n=7 per group)
The end of the experiment was defined when the MAP decreased to <10 mmHg. The time from the beginning of fluid resuscitation to the end of the experiment and survival rates for 180 min from the beginning of fluid resuscitation were measured. An arterial blood sample was collected for the measurement of PaO2, PaCO2, and hemoglobin (Hb) concentration at the start of blood withdrawal (ABL90 FLEX; Radiometer Medical ApS, Brønshøj, Denmark), and the shed blood volume was recorded.
Experiment 2: Blood analysis and wet-to-dry ratio of hearts (n=7 per group)
Ninety minutes after the beginning of fluid resuscitation, an arterial blood sample was collected for the measurement of PaO2, PaCO2, and hemoglobin concentration, and then thoracotomy for blood sampling from the right atrium was performed. Plasma concentrations of syndecan-1 (Cloud-Clone Corp., Katy, TX, USA) and TNF-α (R&D Systems, Inc., Minneapolis, MN, USA) were measured with an ELISA kit, in accordance with the manufacturer’s instruction. Next, PBS was administered from the left ventricle for 2 min, and the heart was excised and immediately weighed. It was then dried in a 50°C oven for 72 h and reweighed, and the wet-to-dry ratio was calculated. We chose the heart for evaluating organ edema because it is sensitive to edema and has a greater influence on functional impairment [17].
Statistical analysis
Data are expressed as the mean ± SD. The means of each group were compared by one-way analysis of variance (ANOVA). If there was a significant difference in the overall comparison of the groups, comparisons between two groups were performed by the Holm test. Survival rates were evaluated by Kaplan-Meier analysis and log rank test. P-values <0.05 were considered statistically significant. All statistical analyses were performed using the R statistical package (version 4.0.2, R Foundation for Statistical Computing).
Results
Experiment 1
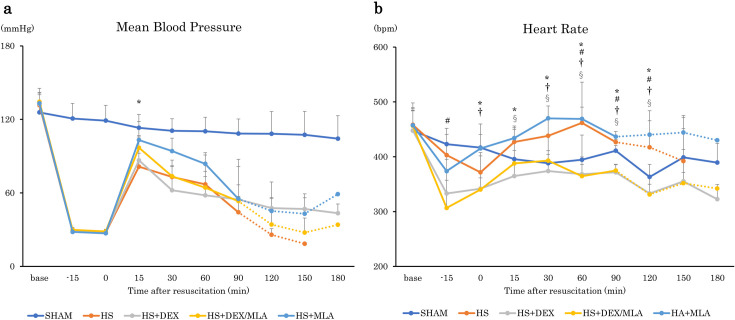
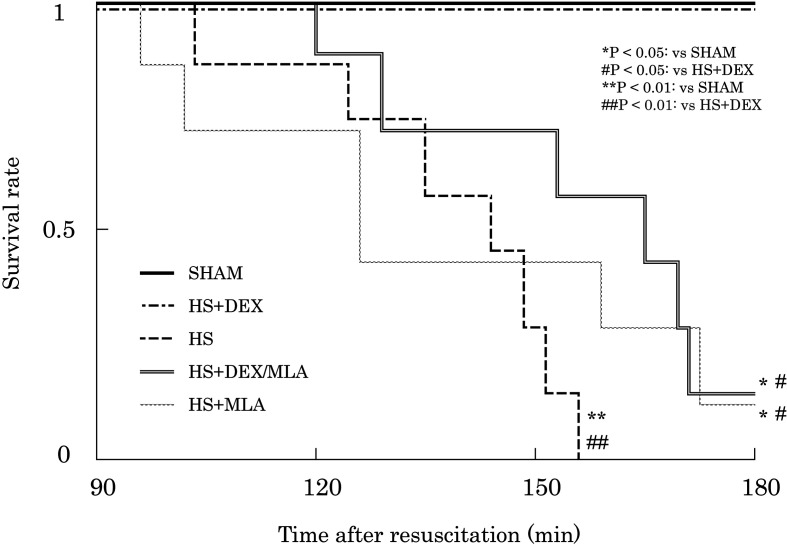
There were no significant differences in body weight, baseline PaO2, PaCO2, Hb concentration, or shed blood volume among the experimental groups. In addition, no significant difference in MAP was found among the groups that underwent HS, except at 30 min after resuscitation (HS+DEX vs. HS+MLA, P=0.002) (Fig. 2a). The heart rate of the rats in some groups administered DEX was lower than that in groups not administered it at several timepoints (Fig. 2b). A higher 180-min survival rate was observed in the SHAM and HS+DEX groups (100%) than in the HS group (0%, P=0.0014), the HS+DEX/MLA group (14.3%, P=0.0133), or the HS+MLA group (14.3%, P=0.0137) (Fig. 3). There were no differences in survival among the HS, HS+DEX/MLA, and HS+MLA groups.
Fig. 2.
Time course of hemodynamic parameters after resuscitation. a Mean arterial pressure. b Heart rate. The error bars represent the standard deviation of the mean. SHAM, no shock; HS, hemorrhagic shock; HS+DEX, hemorrhagic shock with dexmedetomidine; HS+DEX/MLA, hemorrhagic shock with dexmedetomidine and methyllycaconitine citrate; HS+MLA, hemorrhagic shock with methyllycaconitine citrate. *P<0.05: HS+MLA vs. HS+DEX. #P<0.05: HS vs. HS+DEX/MLA. †P<0.05: HS+MLA vs. HS+DEX/MLA. §P<0.05: HS vs. HS+DEX.
Fig. 3.
Kaplan–Meier survival curves. SHAM, no shock; HS, hemorrhagic shock; HS+DEX, hemorrhagic shock with dexmedetomidine; HS+DEX/MLA, hemorrhagic shock with dexmedetomidine and methyllycaconitine citrate; HS+MLA, hemorrhagic shock with methyllycaconitine citrate.
Experiment 2
There were no significant differences in the PaO2 and PaCO2 90 min after fluid resuscitation, but the Hb concentration was significantly lower in the HS+DEX group (8.5 ± 0.9 g/dL) than in the HS (10.6 ± 0.9, P=0.005), HS+DEX/MLA (10.6 ± 1.6, P=0.023), and HS+MLA (10.8 ± 0.9, P=0.004) groups (Table 1). The shed blood volumes were similar among all experimental groups.
Table 1 . Results of blood gas analysis and wet to dry ratio 90 minutes after fluid resuscitation, and amount of bleeding.
| Item | SHAM | HS | HS+DEX | HS+DEX/MLA | HS+MLA | P |
|---|---|---|---|---|---|---|
| Body weight (g) | 407 ± 12 | 414 ± 17 | 409 ± 15 | 406 ± 15 | 412 ± 14 | 0.977 |
| Shed blood volume (ml) | 9.5 ± 0.3 | 9.2 ± 0.5 | 9.4 ± 0.5 | 9.5 ± 0.6 | 0.958 | |
| PaO2 (mmHg) | 89.4 ± 7.2 | 88.9 ± 9.5 | 96.7 ± 5.6 | 90.5 ± 10.2 | 92.3 ± 7.2 | 0.259 |
| PaCO2 (mmHg) | 37.3 ± 4.0 | 34.5 ± 3.5 | 30.4 ± 2.3 | 31.9 ± 5.6 | 34.5 ± 3.7 | 0.333 |
| Hemoglobin (g/dL) | 13.4 ± 0.3 | 10.8 ± 0.9a,b | 8.8 ± 0.7a | 10.4 ± 1.6a,c | 10.8 ± 0.8a,c | <0.001 |
| wet to dry ratio | 4.7 ± 0.2 | 5.4 ± 0.4a,b | 4.7 ± 0.3 | 5.2 ± 0.2c | 5.3 ± 0.3a,c | <0.001 |
Data are expressed as mean±standard deviation. P values were calculated from ANOVA followed by the Holm post hoc test. SHAM no shock, HS hemorrhagic shock, HS+DEX hemorrhagic shock with dexmedetomidine, HS+DEX/MLA hemorrhagic shock with dexmedetomidine and methyllycaconitine citrate, HS+MLA hemorrhagic shock with methyllycaconitine citrate. aP<0.01 vs. SHAM. bP<0.01 vs. HS+DEX. cP<0.05 vs. HS+DEX.
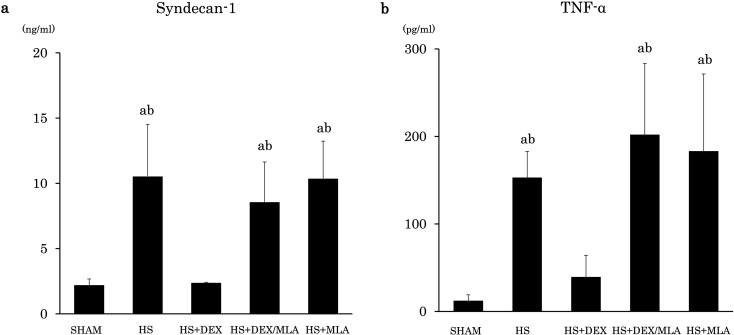
For biomarkers, the serum syndecan-1 level was 2.2 ± 0.5 ng/ml in the SHAM group, 10.5 ± 4.0 ng/ml in the HS group, 2.4 ± 0.1 ng/ml in the HS+DEX group, 8.5 ± 3.1 ng/ml in the HS+DEX/MLA group, and 10.3 ± 2.9 ng/ml in the HS+MLA group. The HS group showed higher syndecan-1 levels than the SHAM group (P<0.001) and the HS+DEX group (P<0.001). The HS+DEX/MLA group also showed higher syndecan-1 levels than the SHAM group (P=0.001) and the HS+DEX group (P=0.002). There were no differences in the serum syndecan-1 levels among the HS, HS+DEX/MLA, and HS+MLA groups, or between the SHAM and HS+DEX groups (Fig. 4a).
Fig. 4.
a Serum concentration of syndecan-1. b Serum concentration of TNFα. SHAM, no shock; HS, hemorrhagic shock; HS+DEX, hemorrhagic shock with dexmedetomidine; HS+DEX/MLA, hemorrhagic shock with dexmedetomidine and methyllycaconitine citrate; HS+MLA, hemorrhagic shock with methyllycaconitine citrate; TNF-α, tumor necrosis factor-alpha. aP<0.01 vs. SHAM. bP<0.01 vs. HS+DEX.
Similarly, the serum TNF-α level was 12.3 ± 6.7 pg/ml in the SHAM group, 153.0 ± 30.0 pg/ml in the HS group, 39.3 ± 24.8 pg/ml in the HS+DEX group, 202.0 ± 81.2 pg/ml in the HS+DEX/MLA group, and 183.1 ± 88.3 in the HS+MLA group. The HS group showed higher TNF-α levels than the SHAM group (P<0.001) and the HS+DEX group (P=0.007). The HS+DEX/MLA group also showed higher TNF-α levels than the SHAM group (P<0.001) and the HS+DEX group (P<0.001). There were no differences in the serum TNF-α levels among the HS, HS+DEX/MLA, and HS+MLA groups, or between the SHAM and HS+DEX groups (Fig. 4b).
The wet-to-dry ratios of hearts in the HS, HS+DEX/MLA, and HS+MLA groups were significantly higher than those in the SHAM and HS+DEX groups, indicating increased cardiac muscle edema (Table 1).
Discussion
This study showed that DEX suppressed the elevation of serum TNF-α and syndecan-1 levels in a rat HS model. In addition, myocardial edema was decreased, and survival time was prolonged. These protective effects were antagonized by administration of the α7 nicotinic type acetylcholine receptor antagonist methyllycaconitine. To the best of our knowledge, this is the first study to show that DEX suppresses serum syndecan-1 elevation in HS.
A DEX dose of 5 µg/kg/h used in rats is equivalent to a human dose of 0.8 µg/kg/h based on body surface area [18], which is similar to the daily clinical dose. It is thus expected that similar results of DEX for HS will be obtained in clinical practice.
EGCX on the luminal surface of vascular endothelial cells was reported to contribute to the permeability barrier formed by the vessel wall [4, 19]. Syndecan-1 released into the bloodstream during EGCX shedding is a major component of EGCX and a sensitive marker of vascular endothelial damage [9]. It was reported that an increase in syndecan-1 was associated with increased vascular permeability in trauma patients [20], and endothelial dysfunction and vascular permeability have been associated with increased morbidity and mortality [21]. EGCX is injured by inflammatory cytokines, especially TNF-α, which rapidly activates heparinase and damages EGCX [9, 22].
In our experiments, the HS group had higher TNFα, syndecan-1, and Hb levels than the SHAM group. This suggests that the infused fluid likely extravasated to the interstitium making it difficult to maintain the blood volume because of damage to EGCX associated with the inflammatory response. The water content of the heart increased after HS and fluid resuscitation in our experiments, which is considered to have caused myocardial edema related to increased vascular permeability associated with EGCX injury. Indeed, a microscopy study demonstrated that the experimental removal of EGCX by treatment with hyaluronidase increased the permeability of myocardial microvessels leading to an increased interstitial space in the myocardium [23]. The heart is highly sensitive to increases in microvascular permeability and the accumulation of myocardial edema. A slight increase in interstitial fluid volume can significantly impair cardiac function [17]. Moreover, because the cardiac function deteriorated by edema is not easily improved [17], vascular hyperpermeability in the heart has great pathological significance. To summarize, syndecan-1 elevation associated with EGCX injury might increase vascular permeability, reduce circulating blood volume, and decrease cardiac function related to myocardial edema, leading to a poor survival rate.
However, the increases in serum TNF-α and syndecan-1 levels and the wet-to-dry ratio of hearts were suppressed in the DEX group. This suggests that the suppression of excessive inflammation retained EGCX, reduced vascular hyperpermeability, and led to better survival. Previously, we reported that DEX protected EGCX and improved survival in a rat heat stroke model [15]. A similar effect was observed in the current study, using a rat HS model, and we showed that this was related to an anti-inflammatory mechanism.
Previous studies revealed that DEX suppresses inflammatory cytokines [24, 25], and that the cholinergic anti-inflammatory pathway mediated by efferent vagal nerve activation plays a critical role in the regulation of inflammation [12,13,14]. Vagal nerve activation stimulates the α7 nicotinic acetylcholine receptor on spleen macrophages via the splenic nerve, and suppresses the release of the pro-inflammatory cytokine TNF-α [26]. However, excessive inflammation suppressed vagal nerve activity [27] and significantly reduced cholinergic anti-inflammatory effects [28]. DEX increased the activity of the cervical vagal nerve and improved survival in a mouse sepsis model by inhibiting the release of inflammatory cytokines [12]. In the current study, pretreatment with MLA, a specific antagonist of the α7 nicotinic acetylcholine receptor, attenuated the anti-inflammatory effect, suppressed serum syndecan-1 elevation, and reduced edema, which suggests that these effects were mediated via the cholinergic anti-inflammatory pathway, supporting the results of previous studies.
Our study had some limitations. First, we administered DEX at the same time as starting blood removal in this experimental protocol. However, DEX has hemodynamic effects including bradycardia and hypotension, even at the clinical dose used [29]. The administration of DEX in situations of ongoing bleeding may promote hypotension. However, in studies in which DEX was administered before and after the onset of ischemia–reperfusion, it was effective before the onset but not after it [30,31,32]. Further examination of the timing of DEX administration is required to achieve optimal efficacy and safety. Second, we used the serum concentration of syndecan-1 as a marker of glycocalyx damage, and did not directly observe the degradation of EGCX. Previous reports showed that the degree of EGCX damage measured by electron microscopy correlated with the serum syndecan-1 concentration [7, 33]. We assume that syndecan-1 level is one of the markers suitable for reflecting glycocalyx damage.
In conclusion, DEX suppressed the inflammatory response and serum syndecan-1 level in a rat HS model. DEX also reduced vascular hyperpermeability, prevented myocardial edema, and prolonged survival. These effects were antagonized by MLA, suggesting that the cholinergic anti-inflammatory pathway is involved in the protective effects of DEX.
Acknowledgments
The authors thank I. Ohta, Y. Kumakiri, and S. Mikawa (Hamamatsu University School of Medicine) for their technical assistance. The authors also thank J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. This work was supported by JSPS KAKENHI (Grant Numbers JP19K09371, JP19K09391, and JP21K09042).
References
- 1.Kawashima Y, Irita K, Morita K, Tuzaki K, Sawa T. Preoperative hemorrhagic shock and intraoperative bleeding: two main causes of surgical deaths in Japan. Nihon Yuketsu Gakkai Zasshi. 2005; 51: 23–31. [Google Scholar]
- 2.Liu H, Xiao X, Sun C, Sun D, Li Y, Yang M. Systemic inflammation and multiple organ injury in traumatic hemorrhagic shock. Front Biosci. 2015; 20: 927–933. doi: 10.2741/4347 [DOI] [PubMed] [Google Scholar]
- 3.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995; 332: 1351–1362. doi: 10.1056/NEJM199505183322008 [DOI] [PubMed] [Google Scholar]
- 4.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010; 363: 689–691. doi: 10.1056/NEJMcibr1007320 [DOI] [PubMed] [Google Scholar]
- 5.Plante GE, Chakir M, Lehoux S, Lortie M. Disorders of body fluid balance: a new look into the mechanisms of disease. Can J Cardiol. 1995; 11: 788–802. [PubMed] [Google Scholar]
- 6.Torres Filho I, Torres LN, Sondeen JL, Polykratis IA, Dubick MA. In vivo evaluation of venular glycocalyx during hemorrhagic shock in rats using intravital microscopy. Microvasc Res. 2013; 85: 128–133. doi: 10.1016/j.mvr.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 7.Sato T, Mimuro S, Katoh T, Kurita T, Truong SK, Kobayashi K, et al. 1.2% Hydrogen gas inhalation protects the endothelial glycocalyx during hemorrhagic shock: a prospective laboratory study in rats. J Anesth. 2020; 34: 268–275. doi: 10.1007/s00540-020-02737-3 [DOI] [PubMed] [Google Scholar]
- 8.Tamura T, Sano M, Matsuoka T, Yoshizawa J, Yamamoto R, Katsumata Y, et al. Hydrogen Gas Inhalation Attenuates Endothelial Glycocalyx Damage and Stabilizes Hemodynamics in a Rat Hemorrhagic Shock Model. Shock. 2020; 54: 377–385. doi: 10.1097/SHK.0000000000001459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao RN, Tang L, Xia ZY, Xia R. Endothelial glycocalyx as a potential theriapeutic target in organ injuries. Chin Med J (Engl). 2019; 132: 963–975. doi: 10.1097/CM9.0000000000000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashandy GM. Implications of recent accumulating knowledge about endothelial glycocalyx on anesthetic management. J Anesth. 2015; 29: 269–278. doi: 10.1007/s00540-014-1887-6 [DOI] [PubMed] [Google Scholar]
- 11.Sano H, Doi M, Mimuro S, Yu S, Kurita T, Sato S. Evaluation of the hypnotic and hemodynamic effects of dexmedetomidine on propofol-sedated swine. Exp Anim. 2010; 59: 199–205. doi: 10.1538/expanim.59.199 [DOI] [PubMed] [Google Scholar]
- 12.Xiang H, Hu B, Li Z, Li J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014; 37: 1763–1770. doi: 10.1007/s10753-014-9906-1 [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Wang Y, Ning Q, Gong C, Zhang Y, Zhang L, et al. The role of spleen in the treatment of experimental lipopolysaccharide-induced sepsis with dexmedetomidine. Springerplus. 2015; 4: 800. doi: 10.1186/s40064-015-1598-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Wang Y, Wang Y, Ning Q, Zhang Y, Gong C, et al. Dexmedetomidine attenuates inflammatory reaction in the lung tissues of septic mice by activating cholinergic anti-inflammatory pathway. Int Immunopharmacol. 2016; 35: 210–216. doi: 10.1016/j.intimp.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K, Mimuro S, Sato T, Kobayashi A, Kawashima S, Makino H, et al. Dexmedetomidine preserves the endothelial glycocalyx and improves survival in a rat heatstroke model. J Anesth. 2018; 32: 880–885. doi: 10.1007/s00540-018-2568-7 [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka T, Suzuki M, Sano M, Hayashida K, Tamura T, Homma K, et al. Hydrogen gas inhalation inhibits progression to the “irreversible” stage of shock after severe hemorrhage in rats. J Trauma Acute Care Surg. 2017; 83: 469–475. doi: 10.1097/TA.0000000000001620 [DOI] [PubMed] [Google Scholar]
- 17.Dongaonkar RM, Stewart RH, Geissler HJ, Laine GA. Myocardial microvascular permeability, interstitial oedema, and compromised cardiac function. Cardiovasc Res. 2010; 87: 331–339. doi: 10.1093/cvr/cvq145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22: 659–661. doi: 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- 19.Okada H, Takemura G, Suzuki K, Oda K, Takada C, Hotta Y, et al. Three-dimensional ultrastructure of capillary endothelial glycocalyx under normal and experimental endotoxemic conditions. Crit Care. 2017; 21: 261. doi: 10.1186/s13054-017-1841-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahbar E, Cardenas JC, Baimukanova G, Usadi B, Bruhn R, Pati S, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015; 13: 117. doi: 10.1186/s12967-015-0481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011; 6: e23530. doi: 10.1371/journal.pone.0023530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012; 18: 1217–1223. doi: 10.1038/nm.2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003; 92: 592–594. doi: 10.1161/01.RES.0000065917.53950.75 [DOI] [PubMed] [Google Scholar]
- 24.Tan F, Chen Y, Yuan D, Gong C, Li X, Zhou S. Dexmedetomidine protects against acute kidney injury through downregulating inflammatory reactions in endotoxemia rats. Biomed Rep. 2015; 3: 365–370. doi: 10.3892/br.2015.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004; 32: 1322–1326. doi: 10.1097/01.CCM.0000128579.84228.2A [DOI] [PubMed] [Google Scholar]
- 26.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009; 265: 663–679. doi: 10.1111/j.1365-2796.2009.02098.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnaby D, Ferrick K, Kaplan DT, Shah S, Bijur P, Gallagher EJ. Heart rate variability in emergency department patients with sepsis. Acad Emerg Med. 2002; 9: 661–670. doi: 10.1197/aemj.9.7.661 [DOI] [PubMed] [Google Scholar]
- 28.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011; 269: 45–53. doi: 10.1111/j.1365-2796.2010.02321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Constantin JM, Momon A, Mantz J, Payen JF, De Jonghe B, Perbet S, et al. Efficacy and safety of sedation with dexmedetomidine in critical care patients: a meta-analysis of randomized controlled trials. Anaesth Crit Care Pain Med. 2016; 35: 7–15. doi: 10.1016/j.accpm.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 30.Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao X, et al. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology. 2012; 116: 1035–1046. doi: 10.1097/ALN.0b013e3182503964 [DOI] [PubMed] [Google Scholar]
- 31.Mimuro S, Katoh T, Suzuki A, Yu S, Adachi YU, Uraoka M, et al. Deterioration of myocardial injury due to dexmedetomidine administration after myocardial ischaemia. Resuscitation. 2010; 81: 1714–1717. doi: 10.1016/j.resuscitation.2010.07.021 [DOI] [PubMed] [Google Scholar]
- 32.Okada H, Kurita T, Mochizuki T, Morita K, Sato S. The cardioprotective effect of dexmedetomidine on global ischaemia in isolated rat hearts. Resuscitation. 2007; 74: 538–545. doi: 10.1016/j.resuscitation.2007.01.032 [DOI] [PubMed] [Google Scholar]
- 33.Truong SK, Katoh T, Mimuro S, Sato T, Kobayashi K, Nakajima Y. Inhalation of 2% Hydrogen Improves Survival Rate and Attenuates Shedding of Vascular Endothelial Glycocalyx in Rats with Heat Stroke. Shock. 2021; 56: 593–600. doi: 10.1097/SHK.0000000000001797 [DOI] [PubMed] [Google Scholar]