Abstract
Diabetic retinopathy (DR) is the prevalent microvascular complication of diabetes mellitus (DM), and it may lead to permanent blindness. The previous publication has indicated that both inflammatory response and oxidative stress are critical factors involved in DR progression, however, the accurate regulatory mechanism remains to be revealed. Src homology region 2 (SH2)-containing protein tyrosine phosphatase 2 (SHP2), a member of the protein tyrosine phosphatase family, was reported to play a role in diabetic nephropathy, whereas its function in DR was unknown and required further exploration. The level of phosphorylated, not the total, SHP2 increased in the retinas of rats with streptozotocin injection-induced DM. Further, the intravitreal injection of SHP2 shRNA lentivirus alleviated retinal pathological changes, and inhibited inflammatory response and oxidative stress, which were accompanied with Yes-associated protein 1 (YAP1) deactivation in DR rats. Additional co-immunoprecipitation results confirmed the interaction of SHP2 and YAP1. Collectively, our data preliminarily show that DR amelioration-induced by SHP2 inhibition in rats may attribute to the deactivation of YAP1 pathway.
Keywords: diabetic retinopathy, Src homology region 2 (SH2)-containing protein tyrosine phosphatase 2 (SHP2), Yes-associated protein 1 (YAP1)
Introduction
Diabetic retinopathy (DR), as the common microvascular complication of diabetes mellitus (DM), remains to be the principal cause of visual loss in working-age adults during the past two decades [1, 2]. Previous statistical data prompted that the prevalence of DR at all stages has been down-regulated since 1980 on account of improved DM treatment, whereas visual impairment and loss induced by DR were increased from 1990 to 2015, especially in low and middle-income countries [3,4,5]. Furthermore, as predicted by the World Health Organization (WHO), the number of DM patients at risk of losing sight is expected to double by 2030 [6]. At present, except for tight blood glucose control, novel technologies, such as laser photocoagulation treatment and luocinolone acetonide implant, are changing the therapeutic strategy for DR [7, 8]. Although the new technologies improved the cost-effectiveness, the treatment outcome is difficult to satisfy most patients. Therefore, it is essential to explore the pathomechanism of DR to develop more effective treatments.
According to the severity, DR is classified into several grades, including mild, moderate, severe non-proliferative, and proliferative DR. The proliferative DR is defined by the emergence of neovascularization in the retinas [9]. Once the DM patients are diagnosed with mild non-proliferative DR, they will quickly (only about 2 to 3 years) develop into a more advanced stage or even proliferative DR [10]. Hence, inhibiting the development from mild non-proliferative DR to the advanced stage may be a new turning point in DR treatment. Recent research suggests that Src homology region 2 (SH2)-containing protein tyrosine phosphatase 2 (SHP2), a non-receptor phosphotyrosine phosphatase, shows ubiquitous cytoplasmic and nuclear localization in various vertebrate cells [11]. SHP2 is encoded by the PTPN11 gene, and it possesses a protein tyrosine phosphatase catalytic domain and two tandem Src homology-2 domains [12]. A previous study carried out by Shi et al. indicated that the tyrosine phosphorylation of SHP2 was enhanced in the glomeruli of DM rats, and that the protective effects of fluvastatin on diabetic nephropathy might be attributed to the de-phosphorylation of SHP2 on the tyrosine site [13]. Their following in vivo experiment confirmed that hyperglycemic condition promoted the tyrosine phosphorylation of SHP2 in glomerular mesangial cells, supporting the above hypothesis [13]. This previous study also implicated that the abnormal activation of SHP2 aggravated retinal ganglion cell injury via blocking brain derived neurotrophic factor (BDNF)/SHP2 dephosphorylates tropomyosin receptor kinase B (TrkB) pathway [14]. Inversely, SHP2 knockdown mediated by adeno-associated virus serotype 2 preserved retinal ganglion cell loss in a glaucoma animal model, indicating that SHP2 could potentially serve as a therapeutic target for retinopathy [14].
It is well accepted that the role of inflammation and oxidative stress in DR progression cannot be ignored [15,16,17,18]. It has been newly reported that the beneficial effects of aflibercept on high glucose-elicited retinal damage are achieved by inhibiting inflammation [19]. Clinically, application of Coenzyme Q10 and combined antioxidant therapy for 6 months is effective and safe for DR patients [20]. Meanwhile, a recent study has reported that the phosphorylated SHP2 level is elevated in the intestine diagnosed with inflammatory bowel disease, accompanied by decreased anti-inflammatory cytokine IL-10 secretion [21]. Zheng et al. have found that the induction of SHP2 mutation is involved in several pathogenic processes in primary mouse embryonic fibroblasts, including oxidative stress [22]. These studies imply an involvement of SHP2 in diseases associated with inflammation and oxidative stress. SHP2 deletion is known to inhibit the activity of Yes-associated protein 1 (YAP1), which can alleviate DR injury [23, 24]. These findings thus suggest that SHP2 may be one of the possible targets for treating DR.
Here, we hypothesized that SHP2 was a potential target for DR treatment. For this, we explored the expression and phosphorylation of SHP2 in the retinas of diabetic rats and further investigated its biological function in vivo and in vitro.
Materials and Methods
Animal treatment
Healthy Sprague-Dawley rats (male, 8–9 weeks, 220–250 g) were purchased from Liaoning Changsheng Biotechnology Co., Ltd. (SCXK (Liao) 2020-0001) (Shenyang, China). All rats were kept in a standard laboratory environment (temperature 22 ± 1°C, humidity 45–55%, 12 h light/dark cycle) and provided with normal rat chow and water. All the experimental procedures strictly adhere to the Guide for Care and Use of Laboratory Animals. The Ethics Committee of The Second Hospital of Jilin University approved all the experimental operations.
Establishment of animal models: Rats were intraperitoneally injected with 65 mg/kg streptozotocin (STZ, dissolved in 0.01 mol/L citric acid buffer, pH 4.5) (Aladdin, Shanghai, China) after overnight fasting to establish a diabetes model. Control rats received an equal volume of citric acid buffer. The fasting blood glucose at 72 h post STZ injection was detected (Sinocare, Changsha, China). Rats with a blood glucose over 16.7 mmol/L were subjected to following experiments.
SHP2 expression manipulation: Diabetic rats received intravitreal injection of 1 µl SHP2 shRNA lentivirus (LV-shSHP2, 1 × 108 TU/ml) or the negative control lentivirus (LV-shNC) at 24 h and week 4 after blood glucose determination. All rats were sacrificed at the end of week 8. The concentration of blood glucose was measured after rats were sacrificed. Timeline for the experimental procedure was shown in Fig. 1A.
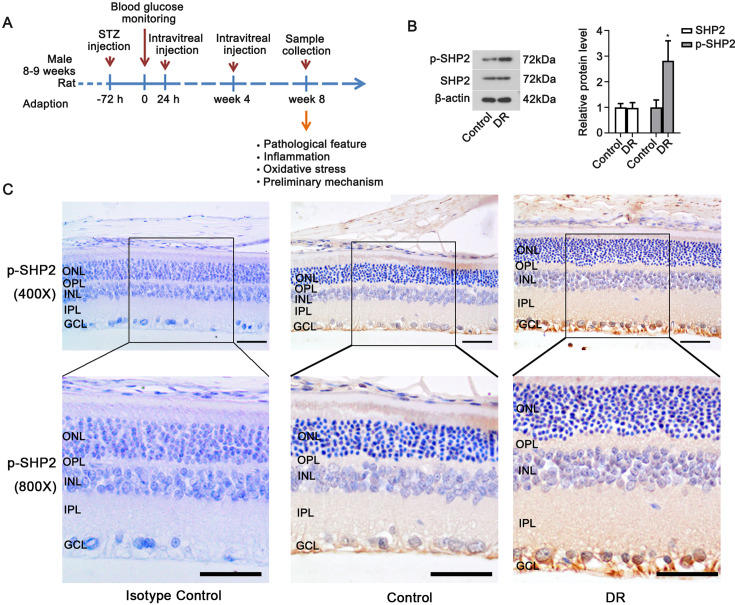
Fig. 1.
Expression of SHP2 in the retinal tissues of diabetic rats. (A) Timeline for the experimental procedure. (B) Western blot was used to detect SHP2 and p-SHP2 (Tyr580) levels in rat retinal tissues. Data were represented as mean ± SD (n=6). The data from two groups were analyzed by t-test. *P<0.05 vs. the control group. (C) Immunohistochemistry was used to confirm p-SHP2 (Tyr580) protein distribution in the retinal tissues (upper: 400× magnification, scale bar=50 µm; bottom: 800× magnification, scale bar=25 µm). DR: diabetic retinopathy group. ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer; GCL: ganglion cell layer.
Hematoxylin and eosin (HE) staining
The aforementioned retinas were paraformaldehyde-fixed, embedded, sectioned into slices of 5 µm. After being deparaffinized in xylene (Aladdin) and rehydrated with 95%, 85% and 75% ethanol in turn, the retinal sections were stained in hematoxylin solution (Solarbio, Beijing, China) for 5 min and counterstained in eosin (Sangon, Shanghai, China) for 3 min. The morphological changes of retinal tissues were observed under a light microscope (BX53, Olympus, Tokyo, Japan) at 200× magnification.
Immunohistochemistry
The rehydrated sections were incubated in 3% H2O2 for 15 min, blocked in goat serum for 15 min at room temperature, and then incubated with primary antibodies at 4°C overnight. After washing using PBS, the retinal sections were incubated in horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody at 37°C for 1 h. Eventually, the sections were stained in diaminobenzidine solution (Solarbio) and counterstained in hematoxylin solution (Solarbio). The retinal sections of control rats exposed to rabbit IgG rather than the primary antibody were served as the isotype control. Images were observed under a light microscope (BX53, Olympus) at 400× magnification. Antibodies used here were shown in Supplementary Table 1.
Immunofluorescence
Similar to the procedure of immunohistochemistry, the retinal sections were treated with primary antibodies against GFAP and CD45 overnight at 4°C, Cy3-labeled goat anti-mouse IgG secondary antibody at room temperature for 1 h, and finally 4’, 6-diamidino-2-phenylindole (DAPI). Immune-positive protein expression was observed under a fluorescence microscope (BX53). Antibodies used here were shown in Supplementary Table 1.
Dihydroethidium (DHE) staining
The sectioned retinal sample (10-µm thickness) was washed with distilled water, incubated in DHE dye (Beyotime Institute of Biotechnology, Shanghai, China) at 37°C for 1 h in dark according to the manufacturer’s instructions. The fluorescence in the retinal tissues was observed under a light microscope (BX53) at 400× magnification.
Real-time polymerase chain reaction (RT-PCR)
Retinal samples were used to isolate total RNA through using Trizol reagent (Tiangen Biotech (Beijing) Co., Ltd., China). The total RNA was synthesized into corresponding cDNA via super M-MLV reverse transcriptase (Beyotime Institute of Biotechnology). ExicyclerTM 96 System (Bioneer, Daejeon, Korea) was used to detect the fluorescence, and the relative mRNA expression of targeted genes was calculated via 2−ΔΔCT method. β-actin was regarded as the housekeeping gene. The primers obtained from GenScript (Nanjing, China) were shown in Supplementary Table 2.
Western blot
The total protein was isolated from retinal tissues using RIPA reagent (Solarbio) containing PMSF (Solarbio). Afterwards, the protein concentration was quantified by a BCA protein concentration assay kit (Solarbio), and electrically separated by 10% sodium dodecyl sulfate-polyacrylamide gel. Protein in the target region was transferred to the PVDF membranes (Millipore, Billerica, MA, USA), which were then blocked in 5% skimmed milk (Sangon) for 1 h at room temperature. The blocked PVDF membranes were incubated in primary antibodies overnight at 4°C and then in goat anti-rabbit/mouse HRP-conjugated secondary antibody 37°C for 1 h. Protein bands were visualized via chemiluminescent (ECL) (Solarbio, China) and quantified by Gel-Pro-Analyzer software. Antibodies used here were shown in Supplementary Table 1.
Co-immunoprecipitation
HEK293T cells were obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (Shanghai, China) to certify the binding between SHP2 and YAP1. In brief, HEK293T cells were transfected with SHP2-Flag vector or/and YAP1-HA vector for 48 h. Afterwards, the cells were incubated in Protein A agarose at 4°C for 2 h and separated by sodium dodecyl sulfate-polyacrylamide gel. After transferring to the PVDF membrane, the membranes were blocked in 5% skimmed milk for 1 h at room temperature. In turn, the membranes were exposed to the primary antibody (HA-tag (WB, rabbit) antibody and flag-tag (WB, rabbit) antibody, ABclonal, Wuhan, China) overnight at 4°C and goat anti-rabbit HRP-conjugated secondary antibody 37°C for 1 h. Finally, the target bands were visualized by using ECL reaction (Solarbio). Antibodies used here were shown in Supplementary Table 1.
Statistical analysis
Data were presented as the mean ± SD. All the in vivo and in vitro experiments were independently repeated for three times. Data were analyzed via one-way ANOVA test followed by Tukey’s test among multiple groups or two-tailed unpaired Student’s t-test between two groups using Graph Pad Prism 8.0 software. P<0.05 was considered statistically significant.
Results
SHP2 expression in the retinal tissues of diabetic rats
The expression and phosphorylation of SHP2 in the retina of diabetic rats were detected with Western blot assay. As shown in Fig. 1B, the retinal expression of total SHP2 in diabetic rats was similar to that in control animals, while the phosphorylated SHP2 (at Tyr580) was markedly increased in diabetic rats (P<0.05). Immunohistochemical staining results also showed that phosphorylated SHP2 was widely distributed in the retina of diabetic rats (Fig. 1C). The above data suggested that the level of phosphorylated SHP2 was elevated in the retinal tissues of diabetic rats.
Effect of SHP2 silencing on pathological changes in the retinas of diabetic rats
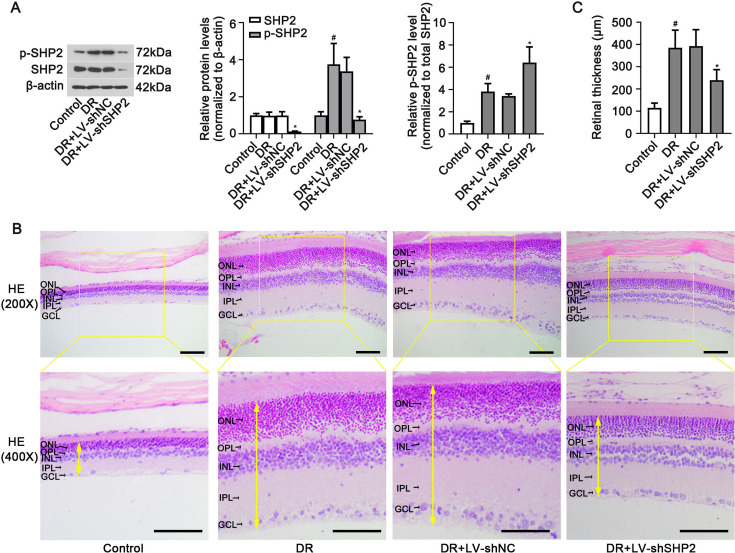
To knock p-SHP2 level down in the retina, the diabetic rats were intravitreally injected with LV-shSHP2. Knockdown of SHP2 via intravitreal injection of LV-shSHP2 did not alter blood glucose levels of diabetic rats (Supplementary Table 3). As shown in Fig. 2A, the levels of both SHP2 and p-SHP2 were decreased in the retinal tissues of diabetic rats (P<0.05). However, the phosphorylation level of p-SHP2 in LV-shSHP2 treatment, which was normalized to SHP2 protein, was increased, compared to that of LV-shNC treatment. As exhibited in Fig. 2B, intact and clear retinal structure could be found in the control group. In diabetic rats, the thickness of retina was increased (Fig. 2C, P<0.05) accompanied by loosening retinal layers. These morphological changes could be remitted by SHP2 knockdown (Fig. 2B and C, P<0.05). The above data suggested that retinal SHP2 knockdown may be beneficial for DR.
Fig. 2.
Effects of SHP2 silencing on pathological changes in the retinas of diabetic rats. LV-shRNA or LV-shSHP2 particles were delivered to rats with DR. The protein levels of phosphorylated SHP2Tyr580 (p-SHP2) and total SHP2 in retinas were determined with (A) Western blot analysis (left), and normalized to β-actin (middle). The p-SHP2/SHP2 ratio was calculated. (B) Representative retinal images of HE staining (upper: 200× magnification, scale bar=100 µm; bottom: 400× magnification, scale bar=50 µm). (C) Retinal thickness. DR: diabetic retinopathy group. Data were represented as mean ± SD (n=6). The yellow two-way arrows represent retinal thickness. The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. #P<0.05 vs. the control group and *P<0.05 vs. the DR+LV-shNC group.
Effect of SHP2 expression on inflammation in the retinas of diabetic rats
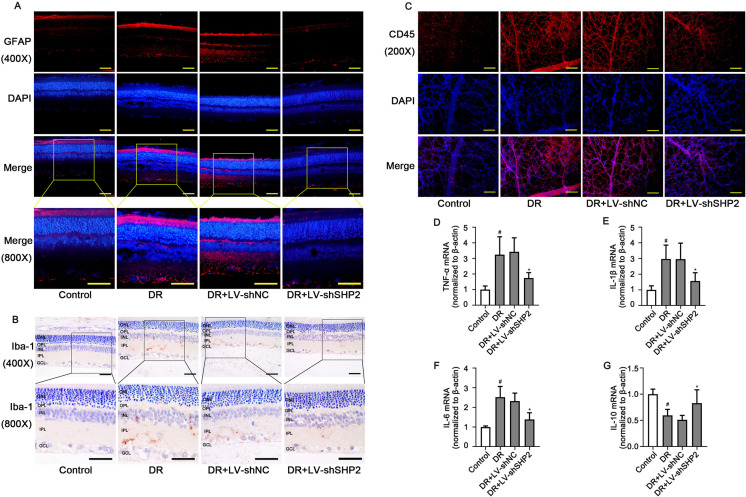
Müller cell gliosis, vascular leukocyte adhesion, microglia recruitment, as well as relative inflammatory factor levels were all detected to evaluate inflammation in the retinal tissues of diabetic rats. Müller cell gliosis labeled by GFAP protein was reduced by retinal SHP2 knockdown (Fig. 3A). As illustrated in Fig. 3B, the recruitment of Iba1-positive microglia in the retina was also decreased with SHP2 silencing. CD45 is a transmembrane protein tyrosine phosphatase that marks leukocytes [25]. Results of immunofluorescence staining using anti-CD45 antibody revealed that SHP2 knockdown markedly reduced leukocytes in the retina of diabetic rats (Fig. 3C). Moreover, the retinal mRNA levels of pro-inflammatory TNF-α, IL-1β, and IL-6 were down-regulated, while that of anti-inflammatory IL-10 was up-regulated in diabetic rats injected with LV-shSHP2 (Figs. 3D–G, P<0.05). The above data suggested that retinal SHP2 knockdown could attenuate retinal inflammation associated with DM.
Fig. 3.
Effect of SHP2 expression on inflammation in the retinas of diabetic rats. (A) Representative images of immunofluorescence probing GFAP in the retinal tissues (upper: 400× magnification, scale bar=50 µm; bottom: 800× magnification, scale bar=25 µm). (B) Representative images of immunohistochemistry targeting Iba-1 in the retinal tissues (upper: 400× magnification, scale bar=50 µm; bottom: 800× magnification, scale bar=25 µm). (C) Representative images of immunofluorescence probing CD45 in the retinal tissues (200 × magnification, scale bar=100 µm). The relative mRNA levels of (D) TNF-α, (E) IL-1β, (F) IL-6, and (G) IL-10 in the retinal tissues. DR: diabetic retinopathy group. Data were represented as mean ± SD (n=6). The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. #P<0.05 vs. the control group and *P<0.05 vs. the DR+LV-shNC group.
Effect of SHP2 expression on oxidative stress in the retinas of diabetic rats
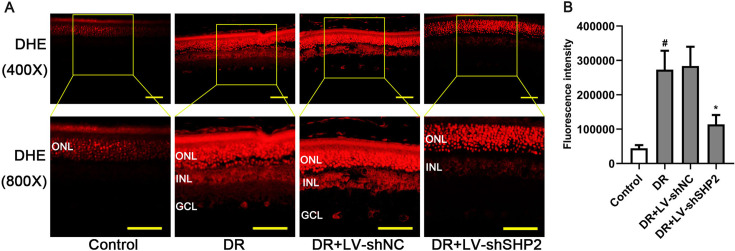
DHE staining was utilized to measure ROS generation in the retinal tissues. As indicated in Fig. 4A, oxidative stress labeled with DHE was aggravated in several retinal layers, including outer nuclear layer (ONL), inner nuclear layer (INL) and ganglion cell layer (GCL), in diabetic rats. Retinal ROS production in diabetic rats was significantly inhibited by SHP2 knockdown (Figs. 4A and B, P<0.05). The above data suggested that retinal SHP2 knockdown could alleviate retinal oxidative stress associated with DM.
Fig. 4.
Effect of SHP2 expression on oxidative stress in the retinas of diabetic rats. (A) Representative images and (B) quantification of DHE staining in the retinal tissues (upper: 400× magnification, scale bar=50 µm; bottom: 800× magnification, scale bar=25 µm). DR: diabetic retinopathy group. Data were represented as mean ± SD (n=6). The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. #P<0.05 vs. the control group and *P<0.05 vs. the DR group.
SHP2 regulates YAP1 activity in the retinas of diabetic rats
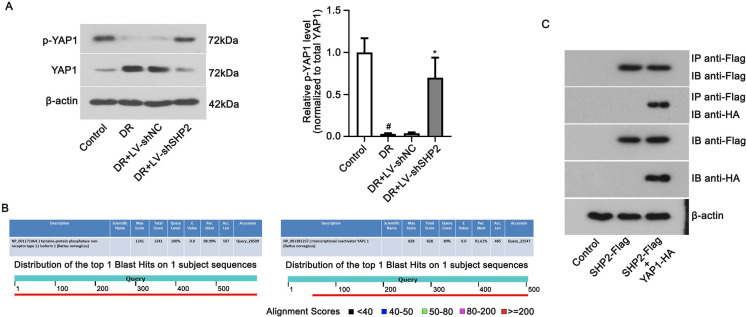
To further investigate mechanisms underlying SHP2’s role in DR progress, the expression of YAP1 was detected. As shown in Fig. 5A, the phosphorylation of YAP1 at Y357 was weakened in diabetic retinas, which was enhanced when SHP2 was silenced (P<0.05). Total expression of YAP1 altered in the opposite manner with phosphorylated YAP1 in the retina (P<0.05). The BLASTP analysis further confirmed the high degree of homology in SHP2 and YAP1 between Homo sapiens and Rattus norvegicus (Fig. 5B). The results of co-immunoprecipitation confirmed the interaction of SHP2 and YAP1 (Fig. 5C), indicating that YAP1 activity was regulated by SHP2. The above data suggested that SHP2 might be involved in the development of DR by regulating the activity of YAP1.
Fig. 5.
SHP2 regulates YAP1 activity in the retinas of diabetic rats. (A) The ratio of phosphorylated YAP1Y357 (p-YAP1) to total YAP1 were analyzed in the retinal tissues with LV-shSHP2 injection. DR: diabetic retinopathy group. LV-shSHP2: SHP2 shRNA lentivirus. Data were represented as mean ± SD (n=6). The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. #P<0.05 vs. the control group and *P<0.05 vs. the DR+LV-shNC group. (B) Homology of SHP2 and YAP1 between Homo sapiens and Rattus norvegicus by BLASTP analysis. (C) Co-immunoprecipitation was used to examine SHP2 and YAP1 interaction in HEK293T cells. SHP2-Flag and YAP1-HA plasmids were co-transfected into HEK293T cells.
Discussion
In the present study, the expression and phosphorylation of SHP2 were first determined in the retinas of rats with or without DR. Then, the biological function of SHP2 was investigated in DR via loss-of-function experiments of SHP2. Data of our study showed that the phosphorylated rather than the total SHP2 was up-regulated in the retinas of diabetic rats. SHP2 knockdown alleviated retinal pathological changes, inhibited inflammation and oxidative stress, thereby slowing down DR progression in rats. In addition, this therapeutic effect of SHP2 knockdown was likely to be achieved by inhibiting the activity of YAP1.
Currently, rigorous blood glucose control is the first choice for the prevention and treatment of DR. Meanwhile, it has been reported that the disease progression of DR is rapidly developed [8]. For most DR patients, they are likely to develop more advanced or even proliferative DR within about 2–3 years following the diagnosis of mild non-proliferative DR [8]. Therefore, inhibiting DR development at the early stage is urgent. Phosphorylation at Tyr-580 of SHP2 has been demonstrated to stimulate its tyrosine phosphatase activity by interacting with C-terminal SH2 domain [26]. In the present study, total protein levels of SHP2 were unchanged, but its phosphorylation level at Tyr-580 was significantly increased in the retina of diabetic rats, suggesting the activation of SHP2 in DR. Given this finding, we believed that knockdown of SHP2 ameliorated DR in rats probably via inhibiting its phosphorylation. The upregulation of relative phosphorylated SHP2 (p-SHP2)/SHP2 ratio induced by SHP2 shRNA did not change the fact that the absolute p-SHP2 level was downregulated. Exploring the precise role of SHP2 by applying a specific inhibitor that only targets phosphorylated SHP2 is further needed.
Generally, emerging evidence has revealed the function of SHP2 in cell survival, proliferation and migration [27,28,29]. Recent studies also demonstrate the involvement of SHP2 in the regulation of inflammation and oxidative stress. The role of SHP2 in inflammation-related diseases is still debatable. Liu et al. have expounded that SHP2 deficiency availed caspase-1 splitting, which subsequently led to pro-inflammatory IL-18 elevation in macrophages stimulated with lipopolysaccharide (LPS) plus palmitic acid (PA), and in liver tissues derived from high-fat diet-treated mice [30]. Their team also reported that SHP2 knockdown in mouse macrophages facilitates NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome hyperactivation, accelerates IL-1β and IL-18 production, thereby deteriorating murine peritonitis [31]. Phosphorylated SHP2 level was elevated in the colonic macrophages and blood monocytes collected from patients with inflammatory bowel disease (IBD) [21]. However, on the contrary, macrophage SHP2 deficiency protected mice against colitis [21]. Our study also elucidated that tyrosine phosphorylation of SHP2 was enhanced in diabetic retinas, which was consistent with earlier studies [13,14]. The role of oxidative stress in DR should not be underestimated. Previous studies have found that the therapeutic strategies targeting oxidative stress disorders improve the outcome of DR patients [18]. Our findings showing that SHP2 knockdown reduced renal ROS generation suggested SHP2 as a potential target in oxidative stress associated with DR.
Multiple signaling pathways are regulated by SHP2, we only explored the molecules both involving DR and SHP2. Bioinformatic analysis results combined with previous research data indicated that there existed an interaction between SHP2 and YAP1 [23]. Several previous studies mainly focused on the role of YAP1 in stem cell proliferation and progenitor dedifferentiation [32, 33]. Served as a major downstream molecule, YAP1 activity is negatively modulated by the tumor suppressor Hippo pathway, whose activation contributes to tumor growth restriction and apoptosis [34, 35]. The recent work of Han et al. presented that YAP1 was overexpressed in retinas of diabetic mice, and that knocking down YAP1 inhibited the proliferation, migration, and angiogenesis in retinal microvascular endothelial cells, indicating that upregulation of YAP1 contributes to DR progression [24]. Our study also demonstrated that activity of YAP1 was reduced in response to SHP2 silencing, which supported the findings from Han et al. [24].
From these results, we concluded that DR amelioration-induced by SHP2 inhibition in rats may attribute to the deactivation of YAP1 pathway. These findings might provide a new insight into the therapeutic strategy for DR.
Conflicts of Interest
The authors declared no conflict of interest.
Supplementary
Acknowledgments
Not applicable.
References
- 1.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003; 110: 1677–1682. doi: 10.1016/S0161-6420(03)00475-5 [DOI] [PubMed] [Google Scholar]
- 2.Kollias AN, Ulbig MW. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl Int. 2010; 107: 75–83, quiz 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vujosevic S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020; 8: 337–347. doi: 10.1016/S2213-8587(19)30411-5 [DOI] [PubMed] [Google Scholar]
- 4.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017; 5: e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 5.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35: 556–564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27: 1047–1053. doi: 10.2337/diacare.27.5.1047 [DOI] [PubMed] [Google Scholar]
- 7.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991; 98:(Suppl): 766–785. doi: 10.1016/S0161-6420(13)38011-7 [DOI] [PubMed] [Google Scholar]
- 8.Bressler SB, Odia I, Glassman AR, Danis RP, Grover S, Hampton GR, et al. Changes in diabetic retinopathy severity when treating diabetic macular edema with ranibizumab DRCR.NET protocol I 5-year report. Retina. 2018; 38: 1896–1904. doi: 10.1097/IAE.0000000000002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Chen H, Su SB. Neuroinflammatory responses in diabetic retinopathy. J Neuroinflammation. 2015; 12: 141. doi: 10.1186/s12974-015-0368-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez B, Peplow PV. MicroRNAs as biomarkers of diabetic retinopathy and disease progression. Neural Regen Res. 2019; 14: 1858–1869. doi: 10.4103/1673-5374.259602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Zhang F, Niu R. Functions of Shp2 in cancer. J Cell Mol Med. 2015; 19: 2075–2083. doi: 10.1111/jcmm.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Qu J, Zhao M, Xu Q, Sun Y. Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacol Res. 2020; 152: 104595. doi: 10.1016/j.phrs.2019.104595 [DOI] [PubMed] [Google Scholar]
- 13.Shi YH, Zhao S, Wang C, Li Y, Duan HJ. Fluvastatin inhibits activation of JAK and STAT proteins in diabetic rat glomeruli and mesangial cells under high glucose conditions. Acta Pharmacol Sin. 2007; 28: 1938–1946. doi: 10.1111/j.1745-7254.2007.00653.x [DOI] [PubMed] [Google Scholar]
- 14.Chitranshi N, Dheer Y, Mirzaei M, Wu Y, Salekdeh GH, Abbasi M, et al. Loss of Shp2 Rescues BDNF/TrkB Signaling and Contributes to Improved Retinal Ganglion Cell Neuroprotection. Mol Ther. 2019; 27: 424–441. doi: 10.1016/j.ymthe.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ucgun NI, Zeki-Fikret C, Yildirim Z. Inflammation and diabetic retinopathy. Mol Vis. 2020; 26: 718–721. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Lo ACY. Diabetic retinopathy: Pathophysiology and treatments. Int J Mol Sci. 2018; 19: E1816. doi: 10.3390/ijms19061816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018; 61: 29–38. doi: 10.1007/s00125-017-4435-8 [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez ML, Pérez S, Mena-Mollá S, Desco MC, Ortega ÁL. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid Med Cell Longev. 2019; 2019: 4940825. doi: 10.1155/2019/4940825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazzara F, Fidilio A, Platania CBM, Giurdanella G, Salomone S, Leggio GM, et al. Aflibercept regulates retinal inflammation elicited by high glucose via the PlGF/ERK pathway. Biochem Pharmacol. 2019; 168: 341–351. doi: 10.1016/j.bcp.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, Miller-Arrevillaga G, Pacheco-Moisés FP, Román-Pintos LM, et al. The effect of ubiquinone and combined antioxidant therapy on oxidative stress markers in non-proliferative diabetic retinopathy: A phase IIa, randomized, double-blind, and placebo-controlled study. Redox Rep. 2016; 21: 155–163. doi: 10.1179/1351000215Y.0000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao P, Zhang H, Zhang Y, Zheng M, Liu R, Zhao Y, et al. Phosphatase Shp2 exacerbates intestinal inflammation by disrupting macrophage responsiveness to interleukin-10. J Exp Med. 2019; 216: 337–349. doi: 10.1084/jem.20181198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng H, Li S, Hsu P, Qu CK. Induction of a tumor-associated activating mutation in protein tyrosine phosphatase Ptpn11 (Shp2) enhances mitochondrial metabolism, leading to oxidative stress and senescence. J Biol Chem. 2013; 288: 25727–25738. doi: 10.1074/jbc.M113.462291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Si X, Gu S, Wang M, Shen J, Li H, et al. Allosteric Inhibitors of SHP2 with Therapeutic Potential for Cancer Treatment. J Med Chem. 2017; 60: 10205–10219. doi: 10.1021/acs.jmedchem.7b01520 [DOI] [PubMed] [Google Scholar]
- 24.Han N, Tian W, Yu N, Yu L. YAP1 is required for the angiogenesis in retinal microvascular endothelial cells via the inhibition of MALAT1-mediated miR-200b-3p in high glucose-induced diabetic retinopathy. J Cell Physiol. 2020; 235: 1309–1320. doi: 10.1002/jcp.29047 [DOI] [PubMed] [Google Scholar]
- 25.Thomas ML. The leukocyte common antigen family. Annu Rev Immunol. 1989; 7: 339–369. doi: 10.1146/annurev.iy.07.040189.002011 [DOI] [PubMed] [Google Scholar]
- 26.Lu W, Gong D, Bar-Sagi D, Cole PA. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol Cell. 2001; 8: 759–769. doi: 10.1016/S1097-2765(01)00369-0 [DOI] [PubMed] [Google Scholar]
- 27.Mohi MG, Neel BG. The role of Shp2 (PTPN11) in cancer. Curr Opin Genet Dev. 2007; 17: 23–30. doi: 10.1016/j.gde.2006.12.011 [DOI] [PubMed] [Google Scholar]
- 28.Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007; 109: 862–867. doi: 10.1182/blood-2006-07-028829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matozaki T, Murata Y, Saito Y, Okazawa H, Ohnishi H. Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation. Cancer Sci. 2009; 100: 1786–1793. doi: 10.1111/j.1349-7006.2009.01257.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Yin Y, Wang M, Fan T, Zhu Y, Shen L, et al. Disrupting phosphatase SHP2 in macrophages protects mice from high-fat diet-induced hepatic steatosis and insulin resistance by elevating IL-18 levels. J Biol Chem. 2020; 295: 10842–10856. doi: 10.1074/jbc.RA119.011840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo W, Liu W, Chen Z, Gu Y, Peng S, Shen L, et al. Tyrosine phosphatase SHP2 negatively regulates NLRP3 inflammasome activation via ANT1-dependent mitochondrial homeostasis. Nat Commun. 2017; 8: 2168. doi: 10.1038/s41467-017-02351-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014; 94: 1287–1312. doi: 10.1152/physrev.00005.2014 [DOI] [PubMed] [Google Scholar]
- 33.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013; 27: 355–371. doi: 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maruyama J, Inami K, Michishita F, Jiang X, Iwasa H, Nakagawa K, et al. Novel YAP1 Activator, Identified by Transcription-Based Functional Screen, Limits Multiple Myeloma Growth. Mol Cancer Res. 2018; 16: 197–211. doi: 10.1158/1541-7786.MCR-17-0382 [DOI] [PubMed] [Google Scholar]
- 35.Xiao Y, Zhang H, Ma Q, Huang R, Lu J, Liang X, et al. YAP1-mediated pancreatic stellate cell activation inhibits pancreatic cancer cell proliferation. Cancer Lett. 2019; 462: 51–60. doi: 10.1016/j.canlet.2019.07.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.