Abstract
The development of an effective vaccine against HIV is desperately needed. The successive failures of HIV vaccine efficacy trials in recent decades have shown the difficulty of inducing an appropriate protective immune response to fight HIV. Different correlates of antibody parameters associated with a decreased risk of HIV-1 acquisition have been identified. However, these parameters are difficult to reproduce and improve, possibly because they have an intricate and combined action. Here, we describe the numerous antibody (Ab) functions associated with HIV-1 protection and report the interrelated parameters regulating their complex functions. Indeed, besides neutralizing and Fc-mediated activity, additional factors such as Ab type, concentration and kinetics of induction, and Fc-receptor expression and binding capacity also influence the protective effect conferred by Abs. As these parameters were described to be associated with ethnicity, age and sex, these additional factors must be considered for the development of an effective immune response. Therefore, future vaccine designs need to consider these multifaceted Ab functions together with the demographic attributes of the patient populations.
Subject terms: HIV infections, Genetic predisposition to disease, Infection
Introduction
According to World Health Organization (WHO) data from 2020, 37.7 million people are living with HIV-1/AIDS and 68% of them are Africans [1]. In contrast to western Europe and America, where subtype B is predominant, subtype A is largely distributed in Eastern Europe and Central Asia and subtype C in East Asia. Africa shows the highest HIV-1 diversity with subtypes A and D in eastern Africa, C in southern Africa, A, G, CRF02_AG, and CRF06_cpx in western Africa, and B and CRF02_AG in northern Africa [2–4]. To fight against and end the HIV-1 pandemic, an efficient protective vaccine is needed. However, due to the high diversity of HIV-1 subtypes, vaccines need to induce antibodies (Abs) with broad inhibitory activity, i.e., antibodies able to inhibit numerous HIV-1 variants. This requirement is considered as one of the main limitations for the development of an efficient HIV vaccine [5, 6].
Over more than three decades, several HIV-1 vaccine trials have been conducted all over the world [7]. However, in HIV-1 vaccine history, only the RV144 phase III trial performed in Thailand showed a statistically significant decreased risk for HIV-1 acquisition at 42 months (31.2%) [8]. Interestingly, analysis of immune correlates for risk showed that Abs binding to the V1V2 region of gp120 correlated with a decreased risk for infection [9]. The IgG1 and IgG3 subclasses mediating antibody-dependent cell-mediated cytotoxicity (ADCC) seem to play a predominant role in protection against HIV-1 acquisition [10]. Moreover, the concentration of plasma envelope (Env)-specific IgA Abs was found to be directly correlated with a higher risk for HIV acquisition [10, 11]. These correlates of risk highlight the predominant role of isotypes and Fc-mediated functions in addition to the previously known protective role of neutralizing antibodies (NAbs). Knowledge of these new factors opens windows of opportunities for innovations in inducing a broad inhibitory humoral immune response to fight HIV and introduces new parameters to be considered, such as Fc domain/Fc receptor (FcR) interactions [12–17].
Antibodies and the pleiotropic function of the humoral response
Induction of HIV-specific Abs of various isotypes
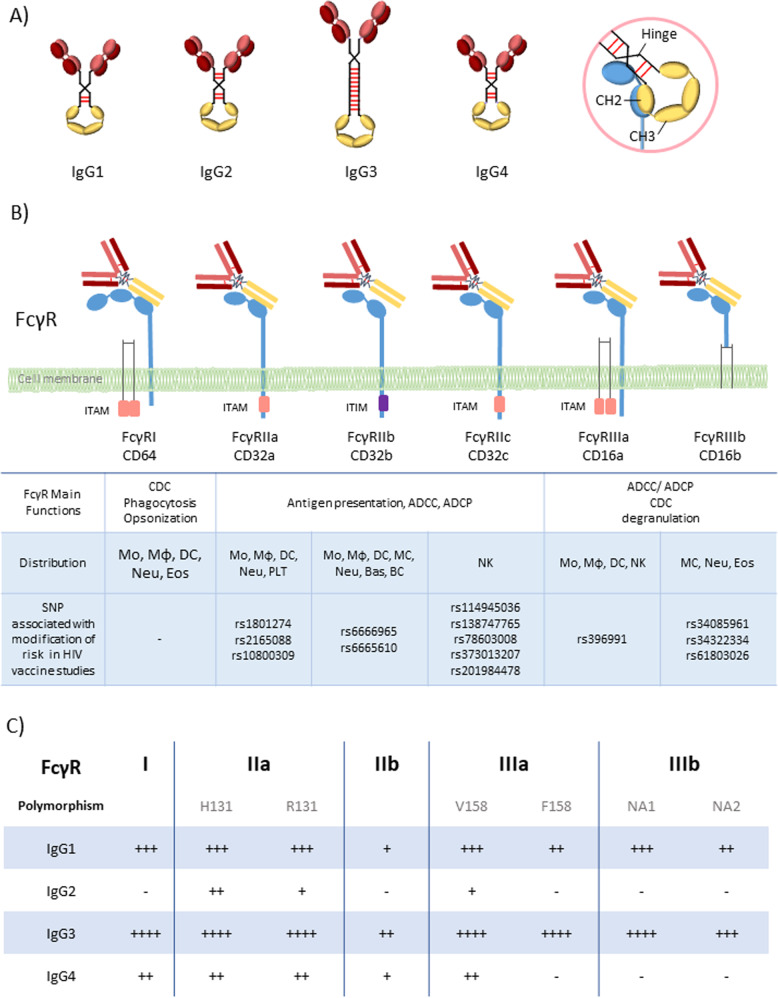
The B cells of the immune system produce Abs that are classified into five major immunoglobulin (Ig) classes or isotypes: IgM, IgG, IgA, IgD, and IgE [18]. IgG is further divided into four subclasses (Fig. 1A) that are diversely distributed according to ethnicity, sex and age, with IgG1, IgG2, IgG3, and IgG4 representing 60–72%, 20–31%, 5–10%, and <4% of total IgG, respectively [19]. IgG subclass prevalence has been reported to change over time following the course of disease and symptoms [20]. Following HIV-1 infection, the adaptive immune response predominantly induces IgG1, IgG3 and IgA [21]. In the RV144 vaccine trial, high levels of HIV-1-specific IgG3 and low Env-specific IgA correlated with a decreased risk of HIV-1 infection [10]. The various Ab isotypes and subclasses bind differently to Fc receptors at the surface of immune cells, including dendritic cells and mainly macrophages (Fig. 1B). As these cells are the best-in-class antigen-presenting cells, different Ab isotypes and subclasses directly affect Ab binding to antigen-presenting cells, modulating immune cell activation and consequently the quality of the humoral immune response that is induced [22]. Comprehensively interrogating the extensive biological Ig diversity in patients may provide critical insights that can guide the development of effective Ab-based vaccines and therapies.
Fig. 1. Antibodies and FcR-mediated functions.
A IgG subclasses. B Fc gamma receptors (FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa, FcγRIIIb), their main function, polymorphisms, and distribution on immune cells. C FcγR binding affinities of IgG subclasses. CDC complement dependent cytotoxicity, ADCC antibody-dependent cellular cytotoxicity, ADCP antibody-dependent cellular phagocytosis, Mo Monocyte, Mϕ Macrophage, DC Dendritic cell, MC Mast cell, Neu Neutrophil, Bas Basophil, Eos Eosinophil, NK Natural killer cell, BC B cell, PLT Platelet.
Two main antibody functions observed in HIV-infected patients and in vaccine trials: neutralization and Fc-mediated functions
NAbs protect cells from pathogens or infectious particles by inhibiting any effect leading to infection via the binding of their Fab domain to the infectious agent (Fig. 1B) [23, 24]. Studies of the passive injection of broadly NAbs in nonhuman primate (NHP) models demonstrate their high potential for conferring protection against HIV acquisition [23, 25]. Considering these data, immunogens aiming to induce the production of these NAbs were developed [23, 26]. Many vaccines have been designed to induce Abs targeting the envelope glycoproteins of the virus, mainly gp120 or gp160 [26–28]. However, these vaccines failed to induce broadly NAbs. Indeed, the production of broadly NAbs is extremely difficult to induce due to the need for an extensive maturation process [29, 30].
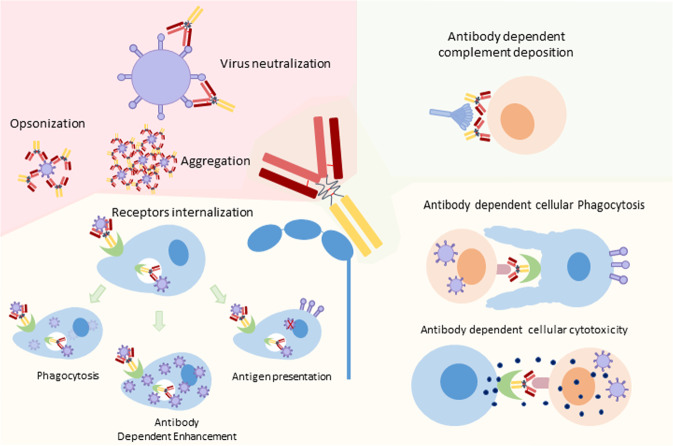
The success of the RV144 vaccine trial supported the development of new vaccine designs for the induction of Abs with additional functions, mainly Fc-mediated Ab functions [31, 32]. It has been proposed that several Fc-mediated mechanisms, including ADCC, antibody-dependent cellular phagocytosis (ADCP), antibody-dependent complement deposition (ADCD), aggregation and immune activation, participate in HIV inhibition (Figs. 1B, 2) [14, 33–37]. In addition, viruses can be directly opsonized by phagocytosis via Ab and FcR binding. The virus is then destroyed, and digested peptides can be retrieved by antigen-presenting cells for T cell activation (Fig. 2) [17, 34, 38, 39]. If the virus escapes this lysis process, opsonized virus entry may also lead to increased infection by a process called antibody-dependent enhancement (ADE) [40]. This ADE function should of course be avoided [41–43]. All these different Fc-mediated mechanisms involve the binding of the Fc domain of the Ab to the Fc receptor present on immune cells. The Fc-mediated functions of Abs are therefore also directly interconnected with FcR expression at the surface of immune cells [44, 45].
Fig. 2. HIV antibody functions.
The functions are dependent on different Ab domains: The Fab domain is involved in virus neutralization, opsonization and aggregation; the Fc domain of Ab induces the activation of the complement system; dual binding of Ab via Fab and Fc domains leads to Fc-mediated antibody function: antibody-dependent cellular phagocytosis and antibody-dependent cellular cytotoxicity; FcR internalization may lead to phagocytosis, antigen presentation or antibody-dependent enhancement.
Modulating FcR expression at the surface of immune cells
FcRs are cell surface glycoproteins that bind to the Fc domain of Abs. This binding varies according to the isotype and subclass of the Ab but also according to the type of FcR (Fig. 1B, C) [44–46]. These FcRs are differentially expressed on most immune cells, including natural killer (NK) cells, monocytes, macrophages, eosinophils, dendritic cells, B cells and even some T cells [17, 46]. There are three family classes of FcRs (I, II, and III), each of which comprises a different number of proteins: FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa and FcγRIIIb (Fig. 1B) [18]. All human FcγRs except FcγRIIB signal through an immunoreceptor tyrosine-based activating motif (ITAM), whereas FcγRIIB delivers inhibitory signals through an immunoreceptor tyrosine-based inhibitory motif (ITIM) [4, 46]. The diversity of human FcγRII and III is further increased by single nucleotide polymorphisms (SNPs) in their extracellular domains, the most studied of which are H131R in FcγR gene FCGR2A, 126C>T in FCGR2C, F158V in FCGR3A, and NA1/2 in FCGR3B (Fig. 1C). FcγRIIC has an unusual structure and is generated by an unequal crossover between FcγRIIA and FcγRIIB. FCGR2C signals through the ITAM similarly to FCGR2A. FcγRIIC (126C>T), rs114945036 presumably lead to an open reading frame with an atypical FcR protein sequence.
Importantly, the different FcR polymorphisms of the host need to be considered when analyzing FcR-mediated functions of Abs. FcγR SNPs will impact both on the the binding to the complementary Fc portion of the Abs and on the expression or activation state of the cells [46] (Fig. 1B). Increasing evidence suggests that FcγR SNPs impair receptor expression on DCs, which in turn influences the risk for HIV infection and vaccine efficacy [15, 16, 47]. Interestingly, a combination of polymorphisms may also influence FcR expression, such as the combination of rs1801274 and rs10800309 in the FcγRII coding gene FCGR2A, which affects the expression level of FcR on immature dendritic cells [48]. FcγRIIIA polymorphism appears to modify NK cell activation and, as a consequence, ADCC activity [49]. Specific polymorphisms at the FCGR2A (encoding Arg or His at position 131) and FCGR3A (encoding Phe or Val at position 158) gene loci have been associated with an HIV vaccine benefit [50]. The rs396991 SNP leads to an increased binding capacity of Abs for FcγRIIIA, which is the main receptor involved in ADCC, suggesting that the vaccine efficacy may be related to an increased efficacy of this function. More recently, Li et al. described that a tag SNP (rs114945036) in FCGR2C (126C>T, presumably leading to a stop codon or an open reading frame) was significantly associated with protection against infection with a subtype AE HIV-1 strain in the RV144 vaccine clinical trial [51]. The direct effect of this SNP is not well documented. Authors propose that it may lead to an alternative splicing, bypassing the FCGR2C-Stop codon to encode a product with an atypical FcR protein sequence, thereby modifying FcR expression or accessibility on cells [51].
Overall, the interplay between IgG subclasses, multiple FcRs and polymorphisms thereof contribute to the complexity of the Fc-mediated response [15, 46]. As a consequence, numerous studies have analyzed the association between FcR genes or their polymorphisms and the evolution of HIV disease or vaccine protection (Table 1) [50–55].
Table 1.
HIV vaccine trials.
| Vaccine trial | Year | Location | Target population | Vaccine | Ab function | Fc receptor | Vaccine efficacy | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutralization | Fc mediated | FcR | Polymorphism | Association with risk of infection | |||||||
| VaxSyn | 1987 | Canada | 72 adults | Recombinant envelope glycoprotein subunit (rgp160) of HIV | Low Tier 1 | NFD | – | – | – | No | [66, 67] |
| HIVAC-1e | 1988 | USA | 35 male adults | Recombinant vaccinia virus designed to express HIV gp160 | N/F | ADE | – | – | – | No | [68, 69] |
| Vax004 | 1998–2002 | North America | 5417 MSM and 300 women | AIDSVAX B/B gp120 with alum | Tier 1 | ADCC ADCP | FCGR2A | rs1801274 | ↓ | No | [37, 50, 52, 77, 84] |
| FCGR3A | rs397991 | ↑ | |||||||||
| Vax003 | 1999–2003 | Thailand | 2545 mem and women IDUs | AIDSVAX B/E gp120 with alum | Tier 1 | ADCC | – | – | – | No | [77, 83, 84] |
| STEP/HVTN502 | 2004–2007 | North America, Caribbean South America, and Australia | 3000 MSM and heterosexual men and women | MRKAd5 HIV-1 gag/pol/nef trivalent vaccine | Low Tier 1 | NFD | – | – | – | 1.4 increased risk of infection | [70, 71, 74, 75] |
| Phambili/HVTN503 | 2003–2007 | South Africa | 801 adults | rAd5 (gag/pol/nef) | Low Tier 1 | NFD | – | – | – | 1.7 increased risk of infection | [72, 73] |
| RV144 | 2003–2009 | Thailand | 16,402 community-risk men and women | ALVAC-HIV (vCP1521) and AIDSVAX B/E vaccine | Low Tier 1 | ADCC ADCP | FCGR2C |
rs114945036 rs138747765 rs78603008 |
↓ | 31.2% decreased risk of infection | [8, 10, 51, 54, 82, 83, 85, 86] |
| HVTN505 | 2009–2013 | USA | 2504 men or transgender women who have sex with men | Three vaccinations with DNA encoding HIV clade B gag, pol and nef as well as env from HIV clades A, B and C followed by an Ad5 vector-based vaccine encoding clade B gag and pol as well as env from clades A, B and C | Low Tier 1 | ADCC ADCP | FCGR2A | rs2165088 | ↓ | No | [54, 81, 87] |
| FCGR2C |
rs138747765 rs78603008 rs373013207 rs201984478 |
↑ | |||||||||
| FcGR3B |
rs34085961 rs34322334 rs61803026 |
↑ | |||||||||
| FCGR2B |
rs6666965 rs6665610 |
↓ | |||||||||
| HVTN305 | 2012–2017 | Thailand | 162 women and men | ALVAC-HIV and AIDSVAX B/E vaccine | Low Tier 1 | ADCC ADCP | – | – | – | No | [86, 88] |
| HVTN306 | 2013–2020 | Thailand | 360 men and women aged 20–40 years old | ALVAC-HIV and AIDSVAX B/E vaccine | Low Tier 1 | ADCC ADCP | – | – | – | No | [89, 90] |
| HVTN097 | 2012–2013 | South Africa | 100 black Africans (men and women) aged 18–40 years old | ALVAC-HIV (vCP1521) and AIDSVAX B/E vaccine | Low Tier 1 | ADCC ADCP | – | – | – | No | [91] |
| HVTN100 | 2015–2018 | South Africa | 252 men and women | ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 | Low Tier 1 | ADCC ADCP | – | – | – | No | [92–94] |
| HVTN705/Imbokodo | 2017–2021 | Sub-Saharan Africa | 2637 women ages 18 to 35 years | Ad26.Mos4.HIV, adjuvanted clade C and Mosaic gp140 HIV bivalent vaccine | – | – | – | – | – | Comparing with RV144, unable to improve the efficacy on Sub-Saharan Africa women | [31, 76, 78–80] |
Illustration of completed and documented or on-going major phase 1b to phase 3 HIV trials that analyzed the Ab and/or Fc Receptor functions.
NFD no Fc-mediated function detected, – no related publications found.
Effect of ethnicity, sex, and age on Fc-mediated Ab response to HIV
Several studies have shown that serum Ig concentrations vary according to ethnicity, sex, and age. Total IgG and IgA levels increase with age and reach the adult concentration at ~10 years of age. Thereafter, the levels of serum IgG were found to be significantly reduced with age, and the level of IgA was found to be maintained. Total IgG and IgA concentrations are higher in Black populations than in White populations [19, 56, 57]. A similar result of higher total IgG levels in HIV-infected Africans than in Caucasians and Hispanics was also found [57–60]. Notably, all these studies comparing Ab profiles according to ethnicity were performed in individuals living in the same country. The difference in Ab responses in Africans living in Africa and Caucasians living in Europe or the USA needs to be investigated to integrate the effect of geographic origin in these studies.
In addition, age-related differences in clonal expansion with decreased IgA levels and skew toward IgG2 were observed after influenza vaccination [61, 62].
These results illustrate the importance of Ab classes in vaccine studies. This difference in Ab isotypes and concentrations according to ethnicity, age and sex may directly impact FcR functions and influence the efficacy of Ab induction in HIV-vaccinated individuals.
The demonstration of the role of Fc-mediated function also brings into question the importance of FcR features. The frequencies of SNPs of FcR genes differ significantly between ethnic groups [63–65]. These differences may strongly modify the association found between FcR polymorphisms and HIV-1 protection or disease outcome. In Kawasaki disease for example, the association with the FCGR2C-ORF haplotype becomes evident only when Asians, in whom FCGR2C-ORF is a nearly absent haplotype, are excluded from the cohort [64].
Overall, analyzing Fc-mediated Ab functions without considering ethnicity, sex, and age is hazardous. These factors need to be considered for genotype/phenotype association studies, as well as for the analysis of FcR involvement in HIV vaccine trials.
FcR and Ab functions in vaccine trials
During the past three decades, several HIV-1 vaccine trials have been performed all over the world. The first vaccine trial tested the recombinant envelope glycoprotein subunit (rgp160) in 72 adults. This vaccine showed induction of NAbs but not Fc-mediated Ab responses [66, 67]. The second HIV-1 trial (HIVAC-1e) used recombinant vaccinia virus that expressed HIV-1 gp160, and its administration resulted in no induction of neutralizing Ab or Fc-mediated Ab responses, even though ADE was detected [68, 69]. Whether this lack of detectable Ab function was due to technical issues needs to be further assessed. Thereafter the following vaccine trials using envelop antigens succeeded in inducing both neutralizing and Fc-medicated Ab responses (Table 1). Of note, the CD4+ T cell-driven HIV immunogens used in the HVTN502 and HVTN503 vaccine trials did not contain envelop antigens, and led to an increased risk of infection [70–75]. FcR variants and their potential association with a decreased risk for infection were further investigated in three vaccine trials: Vax004, HVTN505 and RV144 (Fig. 1B). Although the Vax004 and HVTN505 vaccine strategies did not show efficacy, distinct FCGR polymorphisms have been associated with either an increased or decreased risk for HIV-1 acquisition (Table 1). For the RV144 vaccine trial conducted in Thailand, an association between the FCGR2C rs114940536, rs138747765, rs78603008 polymorphisms and a decreased risk for HIV acquisition was shown [51]. While focusing on fighting the HIV-1 pandemic in Africa, a similar strategy to that used in the RV144 trial was initiated in the South African area [76–79]. This trial, called HVTN702, did not reach the efficacy requirement of RV144 and was therefore stopped prematurely [80]. This failure could be explained by the fact that Black South Africans do not possess the FCGR2C haplotype that was associated with increased vaccine efficacy in the RV144 trial [63]. Collectively, the differences in FCGR2C polymorphisms in South Africa versus Thailand highlight the need for further mechanistic investigations to define the functional relevance of FcR polymorphisms in HIV-1 protection, especially in the context of vaccination. Interestingly, HVTN505 conducted in the USA showed different FcγR SNPs associated with a different hazard ratio of HIV-1 acquisition from that of RV144. In the HVTN505 trial, patients receiving the vaccine had significantly higher incidences of HIV acquisition than those receiving placebo among participants carrying the FCGR2C-TATA haplotype or the FCGR3B-AGA haplotype. Moreover, an FCGR2A SNP (rs2165088) and two FCGR2B SNPs (rs6666965 and rs666561) influenced the correlation of anti-gp140 antibody-dependent cellular phagocytosis with HIV risk [81]. Of note, the HVTN505 and RV144 trials differed in a number of points, i.e., canarypox prime/protein boost in a general low-risk Thai population in RV144 versus DNA prime/rAd5 boost in a high-risk U.S. population of men who have sex with men in HVTN505.
These results indicate that the functional impact of a given FcγR polymorphism on the risk for HIV-1 acquisition is highly context specific, depending on the specific vaccine regimen but also on other factors, such as demographics, virus quasi-species, and genetic background [53, 81, 82].
Discussion of future aspects
RV144 was the sole HIV-1 vaccine trial that showed a limited but statistically significant decreased infection risk [8, 10, 82]. As this protection was not associated with neutralization but with specific Ab types and Fc-mediated function, increased efforts were made to obtain a more in-depth characterization of the induced HIV-specific Ab response [10, 54, 82]. Indeed, in addition to HIV-specific Ab response and neutralizing activity, the specificity of the recognized epitope and Fc-mediated functions were investigated (Table 1). In addition, the FcR polymorphisms associated with infection outcome were explored [50–52, 54, 55, 81, 82]. However, taken individually, none of these factors could be associated with protection. For example, attempts to associate FcR genotypes with HIV outcome resulted in variable, sometime contradictory, results (Table 1). These results largely suggest that multiple Ab factors, including Ab class and subclass, structures, Fc domain interactions with Fc receptors, FcR locus copy number and FcR polymorphisms, may impact vaccine efficacy with synergistic or sometimes antagonistic effects [83]. Moreover, as Ab concentrations and FcR polymorphism frequencies vary according to ethnicities, analysis of correlates of infection risk need to take these additional parameters into consideration [63–65]. These results shed light on the complexity of the humoral response that may be correlated with a decreased risk of HIV-1 acquisition. Future vaccine strategies need to address humoral Ab induction as a whole challenging the different characteristics of the Abs and FcRs required to obtain the most promising combination of humoral responses associated with protection.
Acknowledgements
We would like to thank the ANRS (Agence Nationale de Recherches sur le SIDA et les hépatites virales), the Investissements d’Avenir program managed by the ANR under reference ANR-10-LABX-77 and EHVA (N°681032, Horizon 2020). Work was further supported by by the Strasbourg’s Interdisciplinary Thematic Institute (ITI) for Precision Medicine, TRANSPLANTEX NG, as part of the ITI 2021-2028 program of the University of Strasbourg, CNRS and INSERM, funded by IdEx Unistra [ANR-10-IDEX-0002] and SFRI-STRAT’US [ANR-20-SFRI-0012], the European regional development fund (European Union) INTERREG V program (project Personalis), MSD-Avenir grant AUTOGEN, the High-Level Public Health Specialized Talents Project of Beijing Municipal Health Commission (2022-2-018), and Beijing Key Laboratory for HIV/AIDS Research (BZ0089) for their financial supports.
Author contributions
CM and LYL contributed to the writing. RC and BS corrected, amended and edited the paper. All authors provided feedback on the report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Number of people (all ages) living with HIV—estimates by country. 2020. https://apps.who.int/gho/data/node.main.620?lang=en.
- 2.Hemelaar J, Gouws E, Ghys PD, Osmanov S, WHO-UNAIDS Network for HIV Isolation and Characterisation. Global trends in molecular epidemiology of HIV-1 during 2000-2007. AIDS Lond Engl. 2011;25:679–89. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lihana RW, Ssemwanga D, Abimiku A, Ndembi N. Update on HIV-1 diversity in Africa: a decade in review. AIDS Rev. 2012;14:83–100. [PubMed] [Google Scholar]
- 4.Bbosa N, Kaleebu P, Ssemwanga D. HIV subtype diversity worldwide. Curr Opin HIV AIDS. 2019;14:153–60. doi: 10.1097/COH.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 5.Rambaut A, Posada D, Crandall KA, Holmes EC. The causes and consequences of HIV evolution. Nat Rev Genet. 2004;5:52–61. doi: 10.1038/nrg1246. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert PB, McKeague IW, Eisen G, Mullins C, Guéye-NDiaye A, Mboup S, et al. Comparison of HIV-1 and HIV-2 infectivity from a prospective cohort study in Senegal. Stat Med. 2003;22:573–93. doi: 10.1002/sim.1342. [DOI] [PubMed] [Google Scholar]
- 7.Ng’uni T, Chasara C, Ndhlovu ZM. Major scientific hurdles in HIV vaccine development: historical perspective and future directions. Front Immunol. 2020;11:2761. doi: 10.3389/fimmu.2020.590780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 9.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6:228ra39. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischinger S, Dolatshahi S, Jennewein MF, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. IgG3 collaborates with IgG1 and IgA to recruit effector function in RV144 vaccinees. JCI Insight. 2020;5:e140925. doi: 10.1172/jci.insight.140925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci. 2013;110:9019–24. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boesch AW, Brown EP, Ackerman ME. The role of Fc receptors in HIV prevention and therapy. Immunol Rev. 2015;268:296–310. doi: 10.1111/imr.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 14.Wines BD, Billings H, Mclean MR, Kent SJ, Hogarth PM. Antibody functional assays as measures of Fc receptor-mediated immunity to HIV-new technologies and their impact on the HIV vaccine field. Curr HIV Res. 2017;15:202–15. doi: 10.2174/1570162X15666170320112247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su B, Dispinseri S, Iannone V, Zhang T, Wu H, Carapito R, et al. Update on Fc-mediated antibody functions against HIV-1 beyond neutralization. Front Immunol. 2019;10:2968. doi: 10.3389/fimmu.2019.02968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayr LM, Su B, Moog C. Non-neutralizing antibodies directed against HIV and their functions. Front Immunol. 2017;8:1590. doi: 10.3389/fimmu.2017.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bournazos S, Wang TT, Ravetch JV. The role and function of Fcγ receptors on myeloid cells. Microbiol Spectr. 2016;4:4–6. doi: 10.1128/microbiolspec.MCHD-0045-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maddison SE, Stewart CC, Farshy CE, Reimer CB. The relationship of race, sex, and age to concentrations of serum immunoglobulins expressed in international units in healthy adults in the USA. Bull World Health Organ. 1975;52:179–85. [PMC free article] [PubMed] [Google Scholar]
- 20.Yates NL, Lucas JT, Nolen TL, Vandergrift NA, Soderberg KA, Seaton KE, et al. Multiple HIV-1-specific IgG3 responses decline during acute HIV-1: implications for detection of incident HIV infection. AIDS. 2011;25:2089. doi: 10.1097/QAD.0b013e32834b348e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moir S, Fauci AS. B-cell responses to HIV infection. Immunol Rev. 2017;275:33–48. doi: 10.1111/imr.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alter G, Dowell KG, Brown EP, Suscovich TJ, Mikhailova A, Mahan AE, et al. High‐resolution definition of humoral immune response correlates of effective immunity against HIV. Mol Syst Biol. 2018;14:e7881. doi: 10.15252/msb.20177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hessell AJ, Haigwood NL. Neutralizing antibodies and control of HIV: moves and countermoves. Curr HIV/AIDS Rep. 2012;9:64–72. doi: 10.1007/s11904-011-0105-5. [DOI] [PubMed] [Google Scholar]
- 24.Klasse PJ. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol. 2014;2014:e157895. doi: 10.1155/2014/157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders KO, Pegu A, Georgiev IS, Zeng M, Joyce MG, Yang ZY, et al. Sustained delivery of a broadly neutralizing antibody in nonhuman primates confers long-term protection against Simian/human immunodeficiency virus infection. J Virol. 2015;89:5895–903. doi: 10.1128/JVI.00210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccin Immunol. 2010;17:1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusche JR, Lynn DL, Robert-Guroff M, Langlois AJ, Lyerly HK, Carson H, et al. Humoral immune response to the entire human immunodeficiency virus envelope glycoprotein made in insect cells. Proc Natl Acad Sci. 1987;84:6924–8. doi: 10.1073/pnas.84.19.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esparza J. A brief history of the global effort to develop a preventive HIV vaccine. Vaccine. 2013;31:3502–18. doi: 10.1016/j.vaccine.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Moore PL, Williamson C, Morris L. Virological features associated with the development of broadly neutralizing antibodies to HIV-1. Trends Microbiol. 2015;23:204–11. doi: 10.1016/j.tim.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol. 2011;85:4828–40. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rerks-Ngarm S, Pitisuttithum P, Excler JL, Nitayaphan S, Kaewkungwal J, Premsri N, et al. Randomized, double-blind evaluation of late boost strategies for HIV-uninfected vaccine recipients in the RV144 HIV vaccine efficacy trial. J Infect Dis. 2017;215:1255–63. doi: 10.1093/infdis/jix099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson SI, Moore PL. Targeting Fc effector function in vaccine design. Expert Opin Ther Targets. 2021;25:467–77. doi: 10.1080/14728222.2021.1907343. [DOI] [PubMed] [Google Scholar]
- 33.Dugast AS, Tonelli A, Berger CT, Ackerman ME, Sciaranghella G, Liu Q, et al. Decreased Fc receptor expression on innate immune cells is associated with impaired antibody-mediated cellular phagocytic activity in chronically HIV-1 infected individuals. Virology. 2011;415:160–7. doi: 10.1016/j.virol.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holl V, Peressin M, Decoville T, Schmidt S, Zolla-Pazner S, Aubertin AM, et al. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J Virol. 2006;80:6177–81. doi: 10.1128/JVI.02625-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossignol ED, Dugast AS, Compere H, Cottrell CA, Copps J, Lin S, et al. Mining HIV controllers for broad and functional antibodies to recognize and eliminate HIV-infected cells. Cell Rep. 2021;35:109167. doi: 10.1016/j.celrep.2021.109167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambotte O, Pollara J, Boufassa F, Moog C, Venet A, Haynes BF, et al. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLOS ONE. 2013;8:e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.rgp120 HIV Vaccine Study Group. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto G, Wright PF, Karzon DT. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis. 1983;148:785–94. doi: 10.1093/infdis/148.5.785. [DOI] [PubMed] [Google Scholar]
- 39.Bournazos S, Corti D, Virgin HW, Ravetch JV. Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection. Nature. 2020;588:485–90. doi: 10.1038/s41586-020-2838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russo GL, Moro M, Sommariva M, Cancila V, Boeri M, Centonze G, et al. Antibody–Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non–small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res. 2019;25:989–99. doi: 10.1158/1078-0432.CCR-18-1390. [DOI] [PubMed] [Google Scholar]
- 41.Tirado SMC, Yoon KJ. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 42.Füst G. Enhancing antibodies in HIV infection. Parasitology. 1997;115:127–40. doi: 10.1017/s0031182097001819. [DOI] [PubMed] [Google Scholar]
- 43.Kozlowski PA, Black KP, Shen L, Jackson S. High prevalence of serum IgA HIV-1 infection-enhancing antibodies in HIV-infected persons. Masking by IgG. J Immunol. 1995;154:6163–73. [PubMed] [Google Scholar]
- 44.Bournazos S, Ravetch JV. Fcγ receptor function and the design of vaccination strategies. Immunity. 2017;47:224–33. doi: 10.1016/j.immuni.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamin R, Jones AT, Hoffmann HH, Schäfer A, Kao KS, Francis RL, et al. Fc-engineered antibody therapeutics with improved anti-SARS-CoV-2 efficacy. Nature. 2021;599:465–70. doi: 10.1038/s41586-021-04017-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bournazos S, Ravetch JV. Diversification of IgG effector functions. Int Immunol. 2017;29:303–10. doi: 10.1093/intimm/dxx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carapito R, Mayr L, Molitor A, Verniquet M, Schmidt S, Tahar O, et al. A FcɣRIIa polymorphism has a HLA-B57 and HLA-B27 independent effect on HIV disease outcome. Genes Immun. 2020;21:263–8. doi: 10.1038/s41435-020-0106-8. [DOI] [PubMed] [Google Scholar]
- 48.Roederer M, Quaye L, Mangino M, Beddall MH, Mahnke Y, Chattopadhyay P, et al. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell. 2015;161:387–403. doi: 10.1016/j.cell.2015.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178:6596–603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 51.Li SS, Gilbert PB, Tomaras GD, Kijak G, Ferrari G, Thomas R, et al. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. J Clin Investig. 2014;124:3879–90. doi: 10.1172/JCI75539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forthal DN, Gabriel EE, Wang A, Landucci G, Phan TB. Association of Fcγ receptor IIIa genotype with the rate of HIV infection after gp120 vaccination. Blood. 2012;120:2836–42. doi: 10.1182/blood-2012-05-431361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LassauniŠre R, Tiemessen CT. FcγR genetic variation and HIV-1 vaccine efficacy: context and considerations. Front Immunol. 2021;12:788203. doi: 10.3389/fimmu.2021.788203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neidich SD, Fong Y, Li SS, Geraghty DE, Williamson BD, Young WC, et al. Antibody Fc effector functions and IgG3 associate with decreased HIV-1 risk. J Clin Investig. 2019;129:4838–49. doi: 10.1172/JCI126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamptey H, Bonney EY, Adu B, Kyei GB. Are Fc gamma receptor polymorphisms important in HIV-1 infection outcomes and latent reservoir size? Front Immunol. 2021;12:1356. doi: 10.3389/fimmu.2021.656894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shackelford PG, Granoff DM, Nahm MH, Scott MG, Suarez B, Pandey JP, et al. Relation of age, race, and allotype to immunoglobulin subclass concentrations. Pediatr Res. 1985;19:846–9. doi: 10.1203/00006450-198508000-00014. [DOI] [PubMed] [Google Scholar]
- 57.McGowan JP, Shah SS, Small CB, Klein RS, Schnipper SM, Chang CJ, et al. Relationship of serum immunoglobulin and IgG subclass levels to race, ethnicity and behavioral characteristics in HIV infection. Med Sci Monit Int Med J Exp Clin Res. 2006;12:CR11–6. [PubMed] [Google Scholar]
- 58.Lucey DR, Hendrix CW, Andrzejewski C, Melcher GP, Butzin CA, Henry R, et al. Comparison by race of total serum IgG, IgA, and IgM with CD4+ T-cell counts in North American persons infected with the human immunodeficiency virus type 1. J Acquir Immune Defic Syndr. 1992;5:325–32. [PubMed] [Google Scholar]
- 59.Chaisson RE, Fuchs E, Stanton DL, Quinn TC, Hendricksen C, Bartlett JG, et al. Racial heterogeneity of HIV antigenemia in people with HIV infection. AIDS Lond Engl. 1991;5:177–80. doi: 10.1097/00002030-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Gallerano D, Ndlovu P, Makupe I, Focke-Tejkl M, Fauland K, Wollmann E, et al. Comparison of the specificities of IgG, IgG-subclass, IgA and IgM reactivities in African and European HIV-infected individuals with an HIV-1 clade C proteome-based array. PLoS ONE. 2015;10:e0117204. doi: 10.1371/journal.pone.0117204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Madhun AS, Cox RJ, Haaheim LR. The effect of age and natural priming on the IgG and IgA subclass responses after parenteral influenza vaccination. J Infect Dis. 1999;180:1356–60. doi: 10.1086/315003. [DOI] [PubMed] [Google Scholar]
- 62.Powers DC. Effect of age on serum immunoglobulin G subclass antibody responses to inactivated influenza virus vaccine. J Med Virol. 1994;43:57–61. doi: 10.1002/jmv.1890430111. [DOI] [PubMed] [Google Scholar]
- 63.Lassaunière R, Tiemessen CT. Variability at the FCGR locus: characterization in Black South Africans and evidence for ethnic variation in and out of Africa. Genes Immun. 2016;17:93–104. doi: 10.1038/gene.2015.60. [DOI] [PubMed] [Google Scholar]
- 64.Nagelkerke SQ, Tacke CE, Breunis WB, Tanck MWT, Geissler J, Png E, et al. Extensive ethnic variation and linkage disequilibrium at the FCGR2/3 locus: different genetic associations revealed in Kawasaki disease. Front Immunol. 2019;10:185. doi: 10.3389/fimmu.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niederer HA, Willcocks LC, Rayner TF, Yang W, Lau YL, Williams TN, et al. Copy number, linkage disequilibrium and disease association in the FCGR locus. Hum Mol Genet. 2010;19:3282–94. doi: 10.1093/hmg/ddq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox JH, Garner RP, Redfield RR, Aronson NE, Davis C, Ruiz N, et al. Antibody-dependent cellular cytotoxicity in HIV type 1-infected patients receiving VaxSyn, a recombinant gp160 envelope vaccine. AIDS Res Hum Retroviruses. 1999;15:847–54. doi: 10.1089/088922299310755. [DOI] [PubMed] [Google Scholar]
- 67.Dolin R, Graham BS, Greenberg SB, Tacket CO, Belshe RB, Midthun K, et al. The safety and immunogenicity of a human immunodeficiency virus type 1 (HIV-1) recombinant gp160 candidate vaccine in humans. Ann Intern Med. 1991;114:119–27. doi: 10.7326/0003-4819-114-2-119. [DOI] [PubMed] [Google Scholar]
- 68.Cooney EL, Collier AC, Greenberg PD, Coombs RW, Zarling J, Arditti DE, et al. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991;337:567–72. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- 69.Montefiori DC, Graham BS, Kliks S, Wright PF. Serum antibodies to HIV-1 in recombinant vaccinia virus recipients boosted with purified recombinant gp160. NIAID AIDS Vaccine Clinical Trials Network. J Clin Immunol. 1992;12:429–39. doi: 10.1007/BF00918855. [DOI] [PubMed] [Google Scholar]
- 70.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet Lond Engl. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, del Rio C, et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012;206:258–66. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11:507–15. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gray GE, Moodie Z, Metch B, Gilbert PB, Bekker LG, Churchyard G, et al. The phase 2b HVTN 503/Phambili study test-of-concept HIV vaccine study, investigating a recombinant adenovirus type 5 HIV gag/pol/nef vaccine in South Africa: unblinded, long-term follow-up. Lancet Infect Dis. 2014;14:388–96. doi: 10.1016/S1473-3099(14)70020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li F, Finnefrock AC, Dubey SA, Korber BTM, Szinger J, Cole S, et al. Mapping HIV-1 vaccine induced T-cell responses: bias towards less-conserved regions and potential impact on vaccine efficacy in the step study. PLoS ONE. 2011;6:e20479. doi: 10.1371/journal.pone.0020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janes H, Frahm N, DeCamp A, Rolland M, Gabriel E, Wolfson J, et al. MRKAd5 HIV-1 Gag/Pol/Nef vaccine-induced T-cell responses inadequately predict distance of breakthrough HIV-1 sequences to the vaccine or viral load. PloS ONE. 2012;7:e43396. doi: 10.1371/journal.pone.0043396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bekker LG, Tatoud R, Dabis F, Feinberg M, Kaleebu P, Marovich M, et al. The complex challenges of HIV vaccine development require renewed and expanded global commitment. Lancet. 2020;395:384–8. doi: 10.1016/S0140-6736(19)32682-0. [DOI] [PubMed] [Google Scholar]
- 77.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double‐blind, placebo‐controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV‐1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 78.Janssen Vaccines & Prevention BV. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase 2b Efficacy Study of a Heterologous Prime/Boost Vaccine Regimen of Ad26.Mos4.HIV and Aluminum Phosphate-Adjuvanted Clade C Gp140 in Preventing HIV-1 Infection in Adult Women in Sub-Saharan Africa. clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT03060629.
- 79.IAVI statement on results from Phase IIb Imbokodo HIV vaccine clinical trial. IAVI. https://www.iavi.org/news-resources/features/iavi-statement-on-results-from-phase-iib-imbokodo-hiv-vaccine-clinical-trial.
- 80.Another HIV vaccine strategy fails in large-scale study. https://www.science.org/content/article/another-hiv-vaccine-strategy-fails-large-scale-study.
- 81.Li SS, Gilbert PB, Carpp LN, Pyo CW, Janes H, Fong Y, et al. Fc gamma receptor polymorphisms modulated the vaccine effect on HIV-1 risk in the HVTN 505 HIV vaccine trial. J Virol. 2019;93:e02041–18. doi: 10.1128/JVI.02041-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lassaunière R, Paximadis M, Ebrahim O, Chaisson RE, Martinson NA, Tiemessen CT. The FCGR2C allele that modulated the risk of HIV-1 infection in the Thai RV144 vaccine trial is implicated in HIV-1 disease progression. Genes Immun. 2019;20:651–9. doi: 10.1038/s41435-018-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, et al. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell. 2015;163:988–98. doi: 10.1016/j.cell.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balasubramanian P, Williams C, Shapiro MB, Sinangil F, Higgins K, Nádas A, et al. Functional antibody response against V1V2 and V3 of HIV gp120 in the VAX003 and VAX004 vaccine trials. Sci Rep. 2018;8:542. doi: 10.1038/s41598-017-18863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kijak GH, Tovanabutra S, Rerks-Ngarm S, Nitayaphan S, Eamsila C, Kunasol P, et al. Molecular evolution of the HIV-1 Thai epidemic between the time of RV144 immunogen selection to the execution of the vaccine efficacy trial. J Virol. 2013;87:7265–81. doi: 10.1128/JVI.03070-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Easterhoff D, Pollara J, Luo K, Tolbert WD, Young B, Mielke D, et al. Boosting with AIDSVAX B/E enhances Env constant region 1 and 2 antibody-dependent cellular cytotoxicity breadth and potency. J Virol. 2020;94:e01120–19. doi: 10.1128/JVI.01120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369:2083–92. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fischinger S, Shin S, Boudreau CM, Ackerman M, Rerks-Ngarm S, Pitisuttithum P, et al. Protein-based, but not viral vector alone, HIV vaccine boosting drives an IgG1-biased polyfunctional humoral immune response. JCI insight. 2020;5:e135057. doi: 10.1172/jci.insight.135057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pitisuttithum P, Nitayaphan S, Chariyalertsak S, Kaewkungwal J, Dawson P, Dhitavat J, et al. Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial. Lancet HIV. 2020;7:e238–48. doi: 10.1016/S2352-3018(19)30406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shangguan S, Ehrenberg PK, Geretz A, Yum L, Kundu G, May K, et al. Monocyte-derived transcriptome signature indicates antibody-dependent cellular phagocytosis as a potential mechanism of vaccine-induced protection against HIV-1. eLife. 2021;10:e69577. doi: 10.7554/eLife.69577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gray GE, Huang Y, Grunenberg N, Laher F, Roux S, Andersen-Nissen E, et al. Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa. Sci Transl Med. 2019;11:eaax1880. doi: 10.1126/scitranslmed.aax1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laher F, Moodie Z, Cohen KW, Grunenberg N, Bekker LG, Allen M, et al. Safety and immune responses after a 12-month booster in healthy HIV-uninfected adults in HVTN 100 in South Africa: a randomized double-blind placebo-controlled trial of ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 vaccines. PLOS Med. 2020;17:e1003038. doi: 10.1371/journal.pmed.1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bekker LG, Moodie Z, Grunenberg N, Laher F, Tomaras GD, Cohen KW, et al. Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: a phase 1/2 trial. Lancet HIV. 2018;5:e366–78. doi: 10.1016/S2352-3018(18)30071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fisher L, Zinter M, Stanfield-Oakley S, Carpp LN, Edwards RW, Denny T, et al. Vaccine-induced antibodies mediate higher antibody-dependent cellular cytotoxicity after interleukin-15 pretreatment of natural killer effector cells. Front Immunol. 2019;10:2741. doi: 10.3389/fimmu.2019.02741. [DOI] [PMC free article] [PubMed] [Google Scholar]