Abstract
Oncogenic RET fusions occur in diverse cancers. Pralsetinib is a potent, selective inhibitor of RET receptor tyrosine kinase. ARROW (NCT03037385, ongoing) was designed to evaluate pralsetinib efficacy and safety in patients with advanced RET-altered solid tumors. Twenty-nine patients with 12 different RET fusion–positive solid tumor types, excluding non-small-cell lung cancer and thyroid cancer, who had previously received or were not candidates for standard therapies, were enrolled. The most common RET fusion partners in 23 efficacy-evaluable patients were CCDC6 (26%), KIF5B (26%) and NCOA4 (13%). Overall response rate, the primary endpoint, was 57% (95% confidence interval, 35–77) among these patients. Responses were observed regardless of tumor type or RET fusion partner. Median duration of response, progression-free survival and overall survival were 12 months, 7 months and 14 months, respectively. The most common grade ≥3 treatment-related adverse events were neutropenia (31%) and anemia (14%). These data validate RET as a tissue-agnostic target with sensitivity to RET inhibition, indicating pralsetinib’s potential as a well-tolerated treatment option with rapid, robust and durable anti-tumor activity in patients with diverse RET fusion–positive solid tumors.
Subject terms: Target validation, Prognostic markers, Pharmacodynamics
Results from the precision oncology ARROW trial identify the RET receptor tyrosine kinase as a tissue-agnostic target and the drug pralsetinib’s potential as a well-tolerated treatment option with rapid, robust and durable anti-tumor activity in patients with diverse RET fusion–positive solid tumors.
Main
The RET proto-oncogene (RET) encodes a transmembrane receptor tyrosine kinase (proto-oncogene tyrosine protein kinase receptor RET) that has a physiological role in the embryonic development of the nervous system and the kidneys1,2. RET fusions and mutations induce oncogenic transformation, leading to the aberrant activation of RET receptor tyrosine kinase3. RET fusions can be found in 1–2% of non-small-cell lung cancers (NSCLCs), approximately 20% of papillary thyroid cancers and <1% of many other solid tumors, including ovarian, pancreatic, salivary and colorectal cancers4–8.
Pralsetinib (formerly known as BLU-667, Blueprint Medicines Corporation) is a selective RET inhibitor that potently targets RET kinases, including RET fusion proteins. The recommended phase 2 dose of 400 mg once daily (QD) orally administered pralsetinib was determined in phase 1 of the ARROW study9. Pralsetinib has low affinity for off-target kinases. In a biochemical assay, pralsetinib was 88-fold more selective for RET than for vascular endothelial growth factor receptor 2 (VEGFR2), a tyrosine kinase receptor that is targeted by multi-kinase inhibitors such as cabozantinib and vandetanib2. Based on the results from the registrational phase 1/2 ARROW study (NCT03037385)10,11, pralsetinib was approved in several countries globally, including the United States, for treatment of metastatic RET fusion–positive NSCLC, advanced or metastatic RET-mutant medullary thyroid cancer and RET fusion–positive thyroid cancer12, as well as in the European Union for treatment of advanced RET fusion–positive NSCLC13.
Pre-clinical and early clinical evidence suggests that RET fusions lead to oncogene addiction across tumor types and have the potential to be targeted by selective RET inhibition. Recent tumor-agnostic drug approvals have demonstrated that patients can benefit from select molecularly targeted therapies irrespective of tumor histology14–18. These landmark approvals have heralded the era of precision oncology for tissue-agnostic targets. Since the approvals of pralsetinib and selpercatinib in NSCLC and thyroid cancer12,19, biomarker testing for RET alterations is recommended in treatment guidelines for patients with these tumor types20–22. However, this is not standard of care across all disease indications where RET alterations are recognized as oncogenic drivers23,24.
ARROW is a phase 1/2 study of the highly selective RET inhibitor pralsetinib in patients with medullary thyroid cancer, RET-altered NSCLC and other RET-altered solid tumors. Efficacy and safety of pralsetinib in patients with RET-altered NSCLC and thyroid cancer from the ARROW study were previously reported10,11. After recent approvals of pralsetinib in patients with RET-altered NSCLC and thyroid cancers and respective publications of these data, here we present interim data on the efficacy and safety of pralsetinib in prospectively identified patients with diverse RET fusion–positive tumors.
Results
Patients
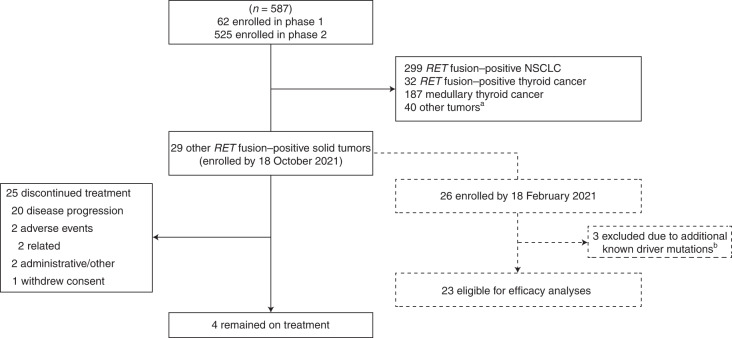
Between 17 March 2017 and the data cutoff date of 18 October 2021, 587 patients were enrolled across all groups (Fig. 1 and Extended Data Fig. 1). Of these, 29 patients had RET fusion–positive solid tumors, excluding RET fusion–positive NSCLC or thyroid cancer, and were included in the safety population presented here. In total, 28 patients (96%) received a starting dose of pralsetinib 400 mg QD, and one patient (4%) received a starting dose of pralsetinib 200 mg/100 mg twice daily but transitioned to 400 mg QD after 3.4 months; the latter patient was the only patient included from the dose-escalation phase of the ARROW trial. At the data cutoff date, four patients (14%) remained on treatment, and 25 patients (86%) had discontinued treatment for the following reasons: disease progression (20 patients (69%)); administrative/other (two patients (7%)); adverse events (AEs) (two patients (7%), of which both were treatment-related—grade 3 thrombocytopenia and grade 3 neutropenia); and withdrew consent (one patient (3%)).
Fig. 1. Patient disposition.
A flowchart that illustrates enrollment of patients with RET fusion–positive solid tumors in the safety (n = 29) and efficacy-evaluable (n = 23) populations in the context of the overall study population of 587 patients, as well as the status of these patients at the data cutoff. aOther RET-mutant tumors (n = 15), no or unknown RET status (n = 2) and prior treatment with a RET inhibitor (n = 23). bThree patients (two with colon cancer and one with cholangiocarcinoma) had additional driver mutations (KRAS, PIK3CB and BRAF).
Extended Data Fig. 1. Prior therapies at baseline.
All Ns are target enrollment. Safety and efficacy analyses presented herein were based on the shaded group (5).
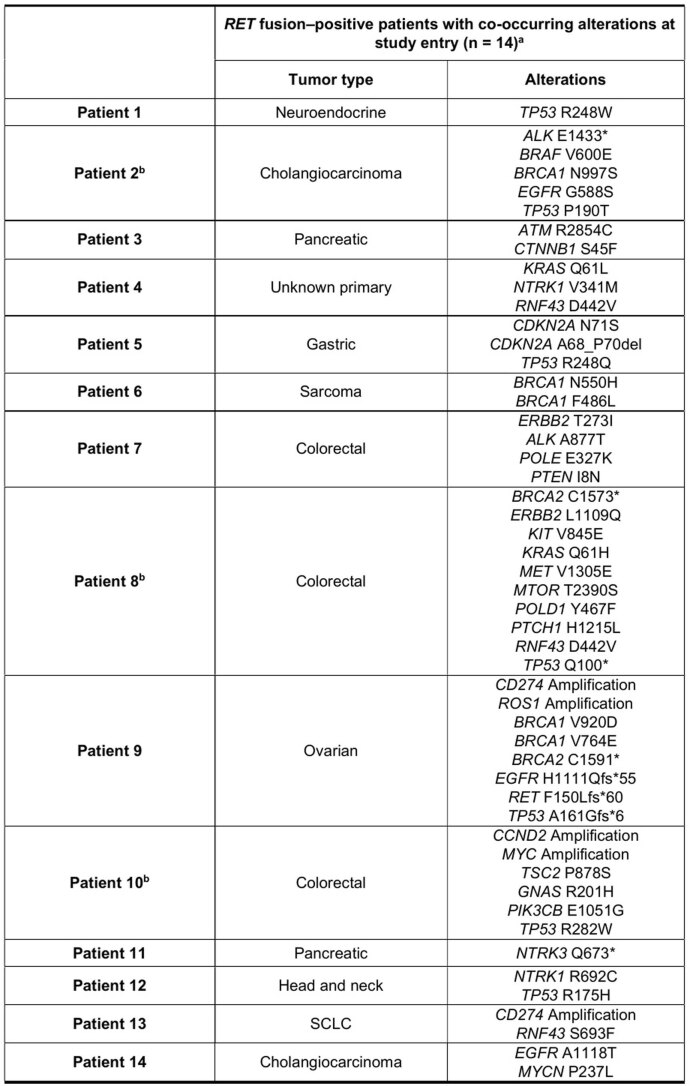
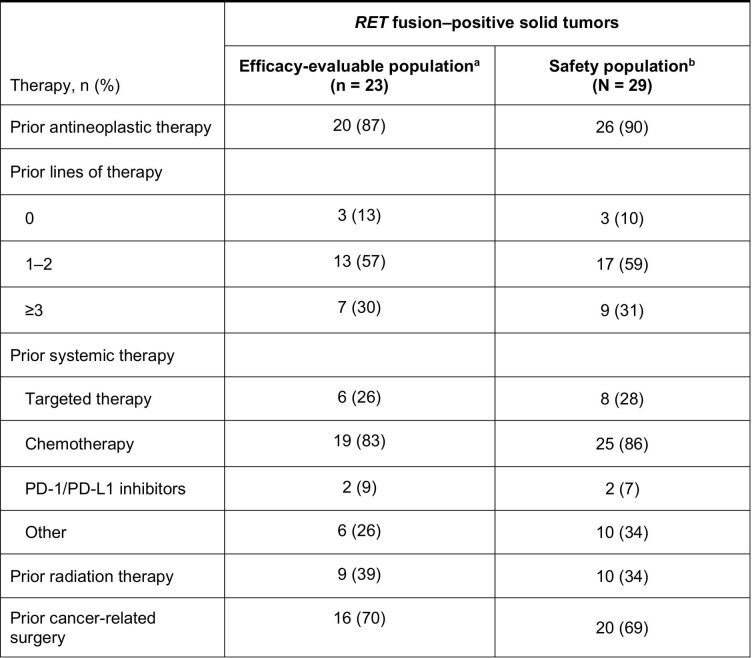
Twenty-six of the 29 patients in the safety population enrolled by the 18 October 2021 data cutoff, with RET fusion–positive solid tumors excluding RET fusion–positive NSCLC or thyroid cancer, were enrolled by the efficacy enrollment cutoff date of 18 February 2021. Fourteen of 26 patients were confirmed to have co-occurring alterations at study entry (Extended Data Fig. 2). RET fusions were identified as the only oncogenic driver in 23 patients (three patients had other oncogenic drivers in addition to RET and were excluded from the efficacy-evaluable population due to this pre-specified criterion). Among these 23 patients evaluable for efficacy, the median age was 53 years (range, 31–71); 14 patients (61%) were female; 20 (87%) patients had metastatic disease; and 20 (87%) patients had received prior therapies at baseline (Table 1 and Extended Data Fig. 3). The most common cancer diagnoses in the efficacy-evaluable population were pancreatic cancer (four patients (17%)), cholangiocarcinoma (three patients (13%)), neuroendocrine cancer (three patients (13%)), sarcoma (three patients (13%): malignant mesenchymal tumor (one patient (4%)), mixed sarcoma and adenocarcinoma (one patient (4%)), malignant isolated fibroma (one patient (4%))), head and neck cancer (two patients (9%): sweat gland cancer (one patient (4%)), salivary duct cancer (one patient (4%))), colorectal cancer (two patients (9%)) and small-cell lung cancer (SCLC) (two patients (9%)). Three patients had stage 3 disease, including one patient with stage 3 ovarian cancer who had received nine prior lines of therapy and one patient each with stage 3B gastric cancer or sarcoma who had both received one prior line of therapy. Two patients had not received prior systemic therapy, both of whom had stage 4 head and neck cancer.
Extended Data Fig. 2. Co-occurring alterations in patients with RET fusions in the ARROW trial at study entry.
a12 patients from the efficacy population did not have any genomics carried out except CRF before the first treatment and were therefore not included. bTwo patients with colorectal cancer and one patient with cholangiocarcinoma were excluded from efficacy analyses due to additional co-occurring driver alterations of PIK3CB E1051G, KRAS Q61H and BRAF V600E, respectively. After enrollment, although the patients were allowed to continue on-study, considering concurrent activating events the sponsor later excluded these patients from the efficacy cohort as the concurrent drivers were adjudicated centrally.
Table 1.
Patient demographics and baseline characteristics
| Demographic/characteristic | RET fusion–positive solid tumors | |
|---|---|---|
| Efficacy-evaluable populationa (n = 23) | Safety populationb (n = 29) | |
| Median age (range), years | 53 (31–71) | 55 (25–75) |
| Sex, n (%) | ||
| Female | 14 (61) | 18 (62) |
| Male | 9 (39) | 11 (38) |
| Race, n (%) | ||
| White | 15 (65) | 20 (69) |
| Asian | 7 (30) | 8 (28) |
| Black | 1 (4) | 1 (3) |
| ECOG performance status, n (%) | ||
| 0 | 7 (30) | 11 (38) |
| 1 | 16 (70) | 18 (62) |
| Tumor type, n (%) | ||
| Pancreatic | 4 (17) | 5 (17) |
| Cholangiocarcinoma | 3 (13) | 4 (14) |
| Neuroendocrine | 3 (13) | 3 (10) |
| Sarcoma | 3 (13) | 3 (10) |
| Head and neck | 2 (9) | 2 (7) |
| Colorectal | 2 (9) | 5 (17) |
| SCLC | 2 (9) | 2 (7) |
| Unknown primary | 1 (4) | 1 (3) |
| Gastric | 1 (4) | 1 (3) |
| Ovarian | 1 (4) | 1 (3) |
| Thymic | 1 (4) | 1 (3) |
| CNS | 0 | 1 (3) |
| History of CNS metastases, n (%) | 6 (26) | 7 (24) |
| TNM stage, n (%) | ||
| III | 3 (13) | 4 (14) |
| IV | 20 (87) | 24 (83) |
| Unknown | 0 | 1 (3) |
| RET fusion partner, n (%) | ||
| CCDC6 | 6 (26) | 9 (31) |
| KIF5B | 6 (26) | 6 (21) |
| NCOA4 | 3 (13) | 4 (14) |
| Otherc | 5 (22) | 6 (21) |
| Unknown | 3 (13) | 4 (14) |
| Median prior lines of therapy, n (range) | 2 (1–9) | 2 (1–9) |
Baseline demographic and clinical characteristics of patients in the safety population (n = 29) and efficacy-evaluable population (n = 23) with RET fusion–positive solid tumors. Three patients in the safety population were not enrolled by the efficacy enrollment date of 18 February 2021, and an additional three patients had oncogenic drivers in addition to RET and were excluded from the efficacy-evaluable population. aEnrollment as of 18 February 2021 and data cutoff date 18 October 2021. bEnrollment and data cutoff date as of 18 October 2021. cIncludes ANKRD26, MYH10, NUP93, SATB1, PRKG1 and TRIM24, and TRIM33 and JMJD1C. CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; TNM, tumor, node, metastases.
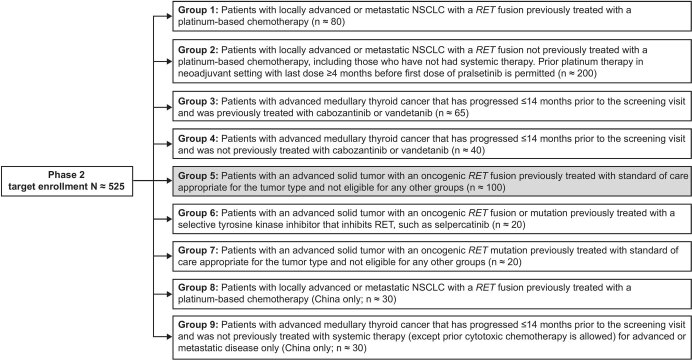
Extended Data Fig. 3. Phase 2 study design.
aEnrollment as of 18 February 2021 and data cutoff date 18 October 2021. bEnrollment and data cutoff date as of 18 October 2021. PD-1, programmed death-1; PD-L1, programmed death ligand-1.
RET fusions were identified by next-generation sequencing (NGS) in 16 patients (70%), by fluorescence in situ hybridization (FISH) in five patients (22%) and by GeneTrails Solid Tumor Fusion Panel and local NGS each in one patient (4%). Central circulating tumor DNA (ctDNA) analysis was also performed in patients for whom FISH was used, with the aim of identifying the RET fusion partners. The most common RET fusion partners were CCDC6 (six patients (26%)), KIF5B (six patients (26%)) and NCOA4 (three patients (13%)) (Table 1). None of the tumors in the patients with pancreatic cancer was identified to harbor KRAS mutations.
Efficacy
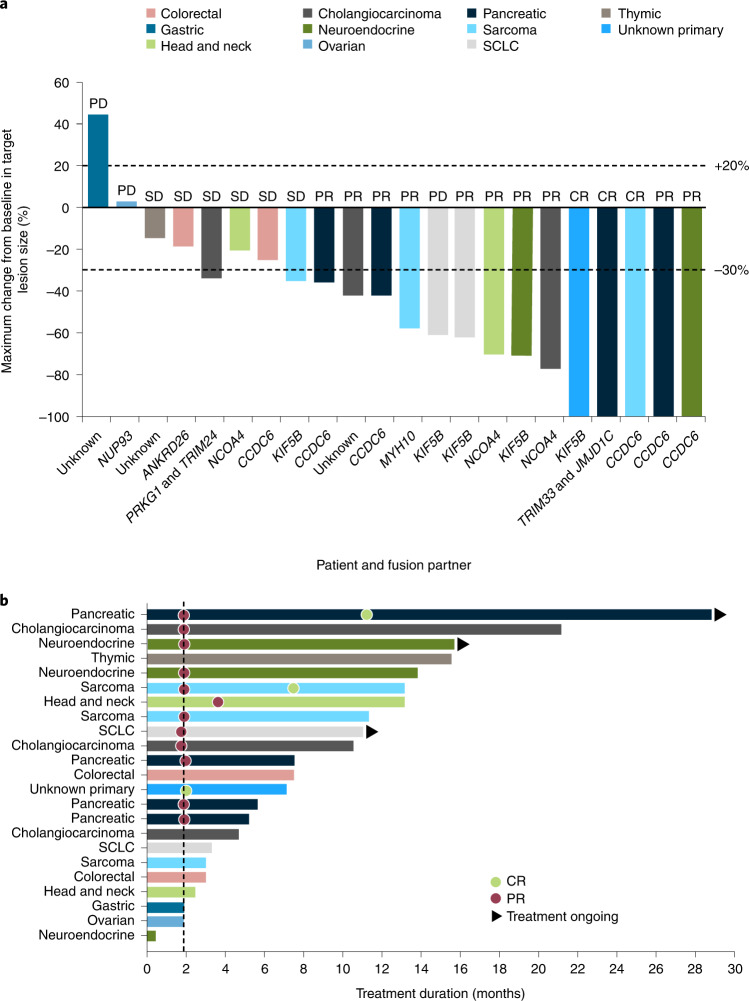
Overall response rate (ORR) was the primary endpoint of phase 2 of this study. In the 23 patients eligible for efficacy analyses, the ORR was 57% (95% confidence interval (CI), 35–77); three (13%) had a confirmed complete response (CR); and ten (43%) had a confirmed partial response (PR) (Table 2). Target tumor shrinkage per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was seen in 91% of patients with post-baseline tumor assessments (Fig. 2a); one patient with progression based on a new site of disease did not have post-baseline assessment of RECIST target lesions.
Table 2.
Summary of tumor response
| Response, n (%) | RET fusion–positive solid tumors (n = 23)a |
|---|---|
| ORRb (95% CI) | 13 (57) (35–77) |
| CR | 3 (13) |
| PR | 10 (43) |
| SD | 6 (26) |
| PD | 4 (17) |
| CBRc (95% CI) | 16 (70) (47–87) |
| DCRd (95% CI) | 19 (83) (61–95) |
| Median DOR, months (95% CI)e | 11.7 (5.5–19.0) |
Response rates and the number of patients with each individual response per RECIST version 1.1 in the efficacy-evaluable population (n = 23). Two-sided 95% CIs were based on exact binomial distributions using the Clopper–Pearson method. aEnrollment as of 18 February 2021 and data cutoff date 18 October 2021. Excludes three patients (two with colon cancer and one with cholangiocarcinoma) with additional driver mutations (KRAS, PIK3CB and BRAF). bConfirmed CR or PR. cConfirmed CR, PR or SD ≥16 weeks. dConfirmed CR, PR or SD. eKaplan–Meier estimated.
Fig. 2. Individual tumor response and treatment duration waterfall and swimlane plots for the efficacy-evaluable population.
In 23 patients eligible for efficacy analyses: a, tumor response by BICR and maximum change from baseline in target lesion size, showing each patient’s tumor type and RET fusion partner; b, treatment duration, indicating the corresponding tumor type and the timeline for response, where the dotted line represents median time to response (1.9 months). One patient with progression based on a new site of disease did not have post-baseline assessment of RECIST target lesions and so is not shown in a.
Confirmed tumor responses were observed in all four patients with pancreatic cancer (including one CR), two of three patients with cholangiocarcinoma, two of three patients with sarcoma (including one CR), two of three patients with neuroendocrine cancer and single patients with head and neck cancer and unknown primary tumor (CR). The other patient with cholangiocarcinoma had a single timepoint response before discontinuing treatment due to an AE.
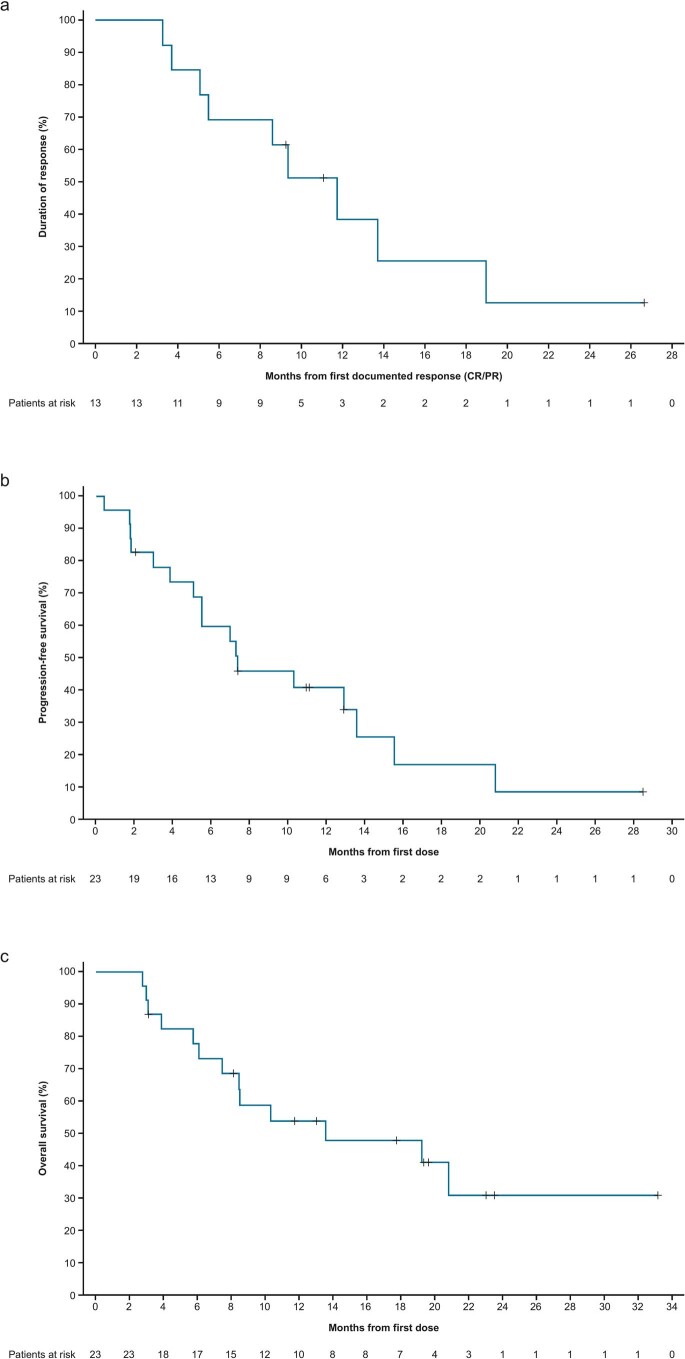
The clinical benefit rate (CBR) was 70% (16/23 patients), and the disease control rate (DCR) was 83% (19/23 patients) (Table 2). Median duration of response (DOR) was 11.7 months (95% CI, 5.5–19.0) with a median follow-up of 26.7 months (95% CI, 9.3–26.7) (Extended Data Fig. 4a). DOR rates were 69% (95% CI, 44–94) at 6 months and 39% (95% CI, 8–69) at 12 months. Of the 13 patients with CR or PR, DOR was ≥6 months for nine (69%) (Fig. 2b); two (15%) had response durations of ≥18 months, one (8%) of whom had a response duration of ≥24 months. Median time to response was 1.9 months (range, 1.7–3.6); at data cutoff, 31% (4/13) of patients had ongoing responses.
Extended Data Fig. 4.
a, Duration of response in 13 patients with RET fusion–positive tumors in the efficacy-evaluable population with a confirmed CR or PR. b, Progression-free survival in 23 patients with RET fusion–positive tumors in the efficacy-evaluable population. c, Overall survival in 23 patients with RET fusion–positive tumors in the efficacy-evaluable population.
Although individual results may vary, among the patients with tumor response, three cases were particularly notable. A man in his early 30s had pancreatic cancer (2.5-cm pancreatic head mass) with multiple hepatic metastases (largest 2.3 cm and 2.4 cm) and multiple peripancreatic lymph nodes at treatment initiation, and his tumor harbored RET–TRIM33 and RET–JMJD1C fusions; other genomic alterations detected on liver biopsy were FGFR4 p.R493Q, which was a variant of unknown significance, and PTCH1 p.1287_1303del and PTEN copy number loss, which were not considered actionable drivers. Baseline cancer antigen (CA) 19-9 was 12.6 U ml−1, and baseline carcinoembryonic antigen was 2.5 ng ml−1. This patient, who had previously experienced progressive disease (PD) and treatment-limiting toxicity on one prior line of chemotherapy (PD and toxicity on capecitabine), had a CR with pralsetinib (100% decrease in the sum of lesion diameters (SLD)). This patient continued treatment with an ongoing CR at a treatment duration of 33.1 months as of the 18 October 2021 data cutoff.
A woman in her early 50s had an intrahepatic cholangiocarcinoma with a RET–NCOA4 fusion and metastases to liver and bone on diagnosis. Diagnostic imaging revealed a large mass with at least a 13 × 8 cm diameter and 20 satellites of different diameters (most ~15 mm) (Fig. 3a,b). The patient had a PR with pralsetinib after experiencing a best response of PD on all three prior lines of therapy (received for ≤3 months: gemcitabine/cisplatin/abraxane, erlotinib/bevacizumab and osimertinib). Other genomic alterations identified in this patient were EGFR A1118T, which was not an actionable driver, and CDKN2A/B loss, which was a variant of unknown significance; microsatellite status was stable, and tumor mutation burden was low (three mutations per megabase). Liver immunohistochemistry was positive for CK7 and CDX-2 and negative for CK20. Throughout treatment with pralsetinib, CA 19-9 reduced from 1 × 106 U ml−1 to 82 U ml−1, and CA-125 reduced from 1,591 U ml−1 to 16.4 U ml−1, with rapid and near-complete clearance of RET–NCOA4 fusion ctDNA. This patient received treatment with continued tumor shrinkage (to a maximum of 77% reduction in measurable disease) for 20.7 months before ultimately succumbing to PD, leading to treatment discontinuation and death before the data cutoff.
Fig. 3. Time-dependent disease evaluations in two patients after pralsetinib treatment.
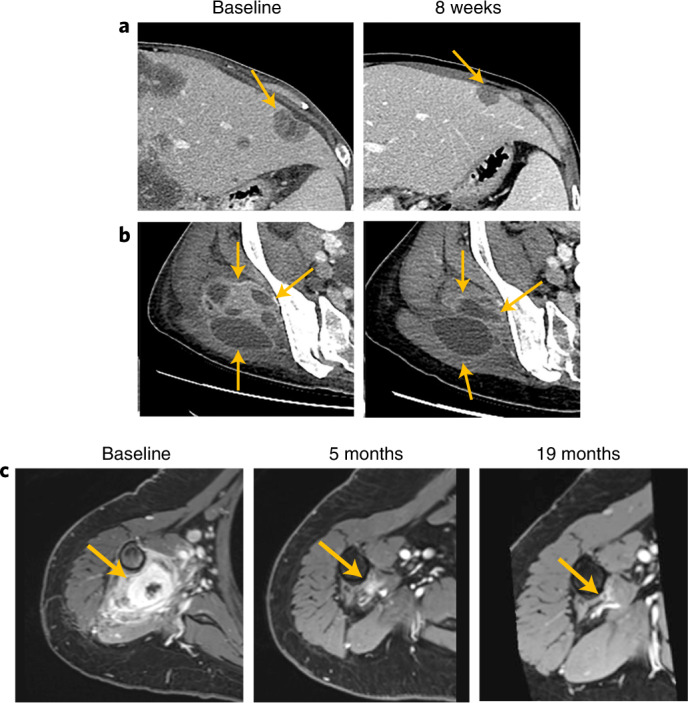
Baseline and 8-week disease evaluation in a 51-year-old woman with RET–NCOA4 fusion–positive cholangiocarcinoma: a, at first disease evaluation after 8 weeks receiving pralsetinib, a left hepatic lobe lesion measuring 2 × 3 cm at baseline had reduced to 1.2 × 1.9 cm; b, a prior heterogeneously enhancing soft tissue mass in the right gluteal muscles had decreased in size and enhancement and showed increased cystic and necrotic components compared to baseline scans. c, Baseline, 5-month and 19-month disease evaluation in a 60-year-old woman with a RET–CCDC6 fusion–positive sarcoma presenting as two muscular masses in the right upper arm.
A woman in her early 60s with a sarcoma (malignant mesenchymal tumor) with a RET–CCDC6 fusion and no metastases had a PR after 1.9 months of pralsetinib treatment that had evolved to a CR at the time of the data cutoff (100% decrease in SLD), with treatment duration of 19.4 months (Fig. 3c). She had two muscular masses on her right upper arm, and the initial pathologic diagnosis after resection was pigmented villonodular synovitis (PVNS). Given this diagnosis, the patient received imatinib as first-line therapy but progressed, with locoregional recurrence with at least four macroscopic tumor nodules in the right arm that were considered inoperable. In parallel to RNA sequencing analysis that confirmed a RET–CCDC6 fusion, transcriptomic analysis suggested that the diagnosis was not PVNS but, rather, an undifferentiated histiocytic tumor. Other mutations in the whole-exome RNA sequence included an in-frame fusion of type FN1-PRG4 as well as the reciprocal transcript (also in-frame) and mutations in exon 43 of ATM (p.R2105G) and exon 5 of PDGFRB (p.R251H). These three mutations were variants of unknown significance. At the time of treatment initiation, a total of seven nodules (8–50 mm) were found on pre-treatment magnetic resonance imaging (MRI) in the soft tissue of the right arm as well as two right axillary lymph nodes (10 mm and 12 mm). As of October 2021, the CR was ongoing after 19.6 months of follow-up.
Among all 23 patients, median progression-free survival (PFS) was 7.4 months (95% CI, 5.1–13.6) at a median follow-up of 28.5 months (95% CI, 10.9–28.5) (Extended Data Fig. 4b), with a PFS rate of 60% (95% CI, 39–80) at 6 months and 41% (95% CI, 20–62) at 12 months. Median overall survival (OS) was 13.6 months (95% CI, 7.5–not reached) with median follow-up of 23.5 months (95% CI, 19.8–23.9) (Extended Data Fig. 4c), with an OS rate of 78% (95% CI, 61–95) at 6 months and 54% (95% CI, 33–75) at 12 months.
Safety
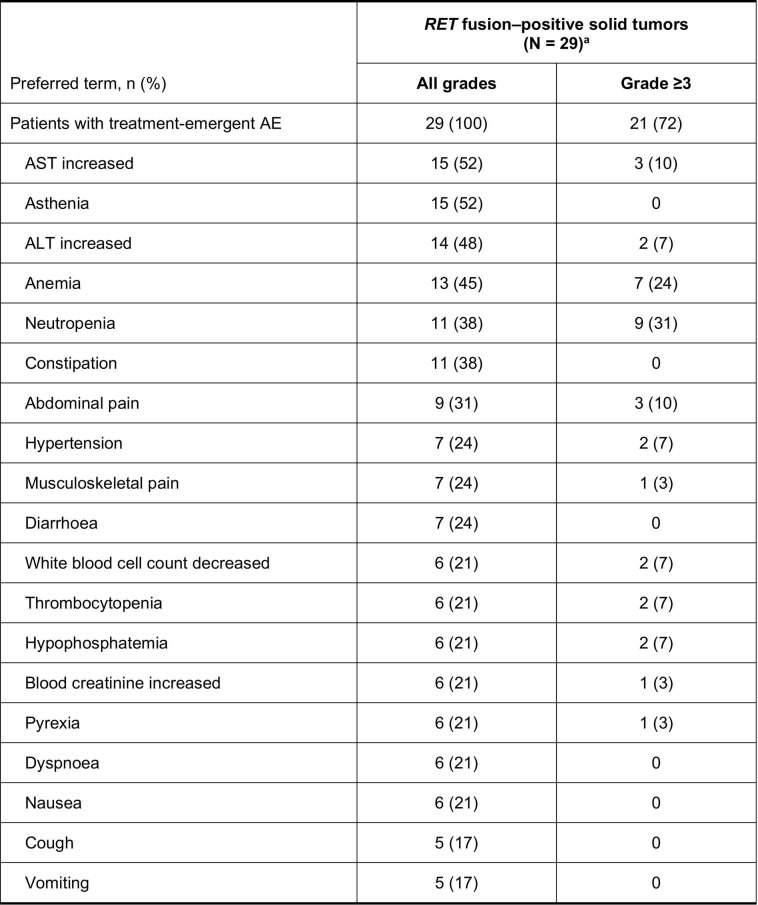
For the 29 patients in the safety population, median relative dose intensity was 86% (range, 51–124), with a median daily dose of 397 mg (range, 212–400), and median time on treatment was 7.0 months (range, 0.4–33.1). Dose intensity was calculated based on starting dose; as the patient who initiated at 200 mg/100 mg twice daily dosing subsequently received 400 mg QD, this patient had a dose intensity >100%. All patients experienced treatment-emergent AEs, of whom 21 (72%) experienced grade ≥3 events (Extended Data Fig. 5). Treatment-related adverse events (TRAEs) occurred in 25 patients (86%), of whom 20 (69%) experienced a grade ≥3 TRAE (Table 3). The most common any-grade TRAEs were increased aspartate transaminase (AST; 11 patients (38%)), increased alanine transaminase (ALT; ten patients (34%)) and neutropenia (ten patients (34%)). Grade 4 events were experienced by two patients (7%); one patient experienced thrombocytopenia, and one patient experienced thrombocytopenia, pancytopenia and acute kidney injury. One death occurred in which the cause was unknown; this patient had multiple possible causes of death, including disease progression, pulmonary infection, respiratory failure, cardiac insufficiency and hypertensive heart disease. The death was recorded as treatment related because the cause could not be unequivocally excluded.
Extended Data Fig. 5. Treatment-emergent adverse events occurring in ≥15% of patients.
aEnrollment and data cutoff date as of 18 October 2021.
Table 3.
Treatment-related adverse events (TRAEs)
| Preferred term, n (%) | RET fusion–positive solid tumors (n = 29)a | |
|---|---|---|
| All grades | Grade ≥3 | |
| Patients with TRAEs | 25 (86) | 20 (69) |
| Increased AST | 11 (38) | 3 (10) |
| Increased ALT | 10 (34) | 2 (7) |
| Neutropenia | 10 (34) | 9 (31) |
| Anemia | 9 (31) | 4 (14) |
| Constipation | 7 (24) | 0 |
| Decreased white blood cell count | 6 (21) | 2 (7) |
| Thrombocytopenia | 5 (17) | 2 (7) |
| Hypertension | 5 (17) | 2 (7) |
| Asthenia | 5 (17) | 0 |
TRAEs that occurred in ≥15% of patients in the safety population (n = 29), which were graded according to the Common Terminology Criteria for Adverse Events version 4.03, with terms pooled. aEnrollment and data cutoff date as of 18 October 2021.
In total, 17 patients (59%) had transient dose interruptions due to TRAEs, and 13 patients (45%) had permanent dose reductions due to TRAEs. The most common TRAEs leading to dose interruption were neutropenia (eight patients (28%)), anemia and increased AST (each three patients (10%)) and thrombocytopenia and increased ALT (each two patients (7%)). The most common TRAEs leading to dose reduction were neutropenia (eight patients (28%)), anemia, increased AST and increased ALT (each two patients (7%)).
Discussion
In this phase 1/2 study of pralsetinib in patients with advanced or metastatic RET fusion–positive solid tumors, almost all of whom were previously treated with systemic therapy, pralsetinib showed robust and durable anti-tumor activity regardless of tumor type or RET fusion partner. RET fusions have been identified as oncogenic drivers in multiple tumor types4–8,25, and, generally, standard therapies that are effective in tumors without oncogenic drivers are less effective than targeted therapies26–28. Precision oncology paradigms that comprise identification of oncogenic alterations through clinical NGS and subsequent application of genomically targeted therapies are applicable to multiple malignancies. Herein, RET fusions defined a unique subset of alterations across multiple tumor types (>15 including NSCLC and multiple subtypes of thyroid cancer) targeted by pralsetinib, validating RET as a tissue-agnostic target.
In this patient group, whose disease was resistant to prior treatments where available, treatment with pralsetinib resulted in an ORR of 57% across seven tumor types, and clinical benefit was reported in 70% of patients by blinded independent central review (BICR). This compares to an ORR of 61% and 70% in patients with RET fusion–positive NSCLC who received prior platinum therapy and no prior systemic treatment, respectively, and an ORR of 89% for patients with RET fusion–positive thyroid cancer in previously published data on the ARROW study10,11. Despite the small number of patients, responses were seen in all four patients with pancreatic cancer (including an ongoing CR with treatment duration of 33.1 months) as well as in two of the three patients with cholangiocarcinoma (including a patient who received treatment for over 20 months after a best response of PD on all three prior lines of therapy). These are encouraging findings because these tumor types are difficult to treat, and the unmet need for better treatments to improve clinical benefit is high. Indeed, response rates for standard-of-care therapies are 26% in biliary cancers (cisplatin plus gemcitabine29) and 23–32% (first-line oxaliplatin-based combination chemotherapy and first-line gemcitabine plus nab-paclitaxel) in pancreatic cancer30,31. In ARROW, responses were seen in treatment-naive patients who were not candidates for standard therapies and in patients who had received several prior lines of therapy, highlighting the need for targeted therapies across a range of tumor types for patients who currently have no standard of care and for those who have exhausted all other options. The strategy of treating patients with RET fusion–positive solid tumors with targeted therapies is also supported by results with selpercatinib: in an analysis that included adult patients with locally advanced or metastatic RET fusion–positive non-lung/non-thyroid solid tumors who received selpercatinib twice daily, the ORR was 47%32. The efficacy-evaluable population comprised patients who were enrolled long enough to allow a 6-month follow-up from their first dose.
The safety profile reported in this analysis is consistent with previously reported results in patients with RET fusion–positive NSCLC and thyroid cancer from the ARROW study10,11, with no new safety signals identified, and no effect of pralsetinib on QT interval was observed11. The most common TRAEs were increased ALT/AST and neutropenia. Common TRAEs seen with selpercatinib include increased ALT/AST, dry mouth, diarrhea and fatigue1,32. For patients with other solid tumors who received selpercatinib in the LIBRETTO-001 study, a grade ≥3 TRAE of QT interval prolongation was reported in 4% of patients32.
ARROW is a single-arm study with no comparator group. The safety population for the cohort analyzed here included a small heterogeneous number of patients (n = 29); despite this, all but two patients included in the efficacy-evaluable population (n = 23), a subset of the safety population, experienced a tumor shrinkage. In combination with the robust activity seen in patients with NSCLC and thyroid cancer in the ARROW study10,11, these data further support the potential of pralsetinib to address the unmet medical need across a broad range of RET-altered tumor types with differing histology.
Overall, these data highlight the need for broad RET testing, preferably by NGS, to identify candidates who may benefit from treatment with pralsetinib. Enrollment of patients with other RET fusion–positive solid tumors in ARROW is ongoing.
Methods
Study design and patient population
ARROW (NCT03037385) is an open-label, international, phase 1/2 study evaluating the efficacy and safety of pralsetinib across various RET-altered solid tumors conducted at 84 sites across 13 countries. The phase 1 dose-escalation portion identified the maximum tolerated dose and recommended phase 2 dose of pralsetinib as 400 mg QD10. Adults with unresectable, locally advanced or metastatic solid tumors were enrolled into nine phase 2 groups as defined by disease type and prior therapy status. This current analysis reports results for the subgroup of patients with RET fusion–positive solid tumor types, excluding NSCLC and thyroid cancer, who were enrolled in the phase 1 study portion and in the phase 2 expansion group 5. In accordance with study eligibility requirements, these patients had previously received or were not candidates for appropriate standard-of-care therapy. Additional eligibility criteria were as previously reported10.
This study was conducted in accordance with the ethical principles of Good Clinical Practice and the Declaration of Helsinki, and was based on the International Council for Harmonisation E6 requirements. The full protocol was approved by the institutional review board or independent ethics committee of each participating site, and all patients provided signed informed consent. The name of each participating institute, organization or site whose ethical committee approved the protocol is provided in the Supplementary Information.
Outcomes
Phase 2 primary endpoints were ORR (defined as the proportion of patients who had confirmed CR or PR per RECIST version 1.1) and safety. Key secondary endpoints included CBR (defined as the proportion of patients who had confirmed CR, PR or SD lasting ≥16 weeks); DCR (defined as the proportion of patients who had confirmed CR, PR or SD); DOR (defined as time from first documented tumor response (CR/PR) until first documented disease progression or death); PFS (defined as time from first dose of pralsetinib to first documented disease progression or death due to any cause); and OS (defined as time from first dose of pralsetinib to death due to any cause).
Assessments
Tumor response per RECIST version 1.1 was assessed by BICR. Computed tomography or MRI of all known disease sites was performed at screening and approximately every 8 weeks during treatment. For the purpose of study eligibility, RET fusions were identified by local testing using NGS, FISH or GeneTrails Solid Tumor Fusion Panel, which used DNA and RNA, with RNA used to identify RET fusions. In accordance with the statistical analysis plan, patients were confirmed as RET fusion positive if any one of these methods returned a positive fusion result. The presence of concurrent non-RET fusion oncogenic drivers was determined prospectively based on local testing and/or by retrospective central analysis if necessary. As per the study protocol, concurrent drivers were defined as known primary driver alterations consistent with the scientific literature for different tumor types, and the final decision was made by the sponsor. AEs were graded according to the Common Terminology Criteria for Adverse Events version 4.03, and terms were pooled.
Statistical analysis
All patients with RET fusion–positive solid tumors, excluding NSCLC and thyroid cancer, who were enrolled by the analysis cutoff date (18 October 2021) were included in the safety analyses. Of these patients, those who began treatment by the enrollment cutoff date (18 February 2021), who had baseline measurable disease per BICR, who had at least one evaluable post-baseline disease response assessment and who were without other known oncogenic mutations were included in efficacy analyses. The enrollment cutoff for efficacy analyses was employed to provide adequate follow-up time for responses to pralsetinib. Two-sided 95% CIs were based on exact binomial distributions using the Clopper–Pearson method. DOR, PFS and OS were analyzed using the Kaplan–Meier method. Estimates of follow-up duration for DOR, PFS and OS were based on the inverse Kaplan–Meier method, with 95% CIs based on the Greenwood formula.
For group 5, which excluded patients with RET fusion–positive NSCLC but included patients with RET fusion–positive thyroid cancer, a total sample size of 100 patients with solid tumors harboring a RET fusion was intended to allow >90% power at the two-sided significance level of 0.05 for testing the assumption of null hypothesis of ORR = 0.1 versus the alternative ORR = 0.3. As results for 20 patients with RET fusion–positive thyroid cancer were reported previously11, patients with these cancers were excluded from this interim analysis. All statistical analyses were performed with SAS version 9.4 software.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-022-01931-y.
Supplementary information
Protocol and list of study sites whose ethics committees approved the protocol
Acknowledgements
The authors would like to thank the patients, their families and all investigators involved in this study. V.S. is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. V.S. acknowledges support of the Jacquelyn A. Brady Fund. V.S. is supported by US National Institutes of Health grants R01CA242845 and R01CA273168. MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (1U01 CA180964), a National Center for Advancing Translational Sciences grant (UL1 TR000371) and the MD Anderson Cancer Center Support grant (P30 CA016672). Medical writing support, including assisting authors with the development of the outline as well as initial draft and incorporation of comments, was provided by N. Tracey and W. Wheddon; editorial support, including submission, was provided by E. Sims and T. Taylor, all of Paragon (Knutsford, United Kingdom), supported by Blueprint Medicines, according to Good Publication Practice guidelines. The sponsor was involved in the study design and collection, analysis and interpretation of data, as well as data checking of information provided in the article. However, ultimate responsibility for opinions, conclusions and data interpretation lies with the authors. E.G. is supported by the Caixa Research Advanced Oncology Research Program (supported by Fundació La Caixa, LCF/PR/CE07/50610001). E.N. is supported by the Carlos III National Health Institute grant (PI21/00789) and Horizon 2020 (H2020-SC1-2019-Single-Stage-RTD). M. Schuler is supported by the Oncology Center of Excellence Grant/German Cancer Aid (70112273) and the German Cancer Consortium, partner site: University Hospital Essen (BMBF 613-71043-1). G.C. is supported by an OPTIMA (optimal treatment for patients with solid tumors in Europe through artificial intelligence) grant (101034347). The ARROW study (NCT03037385) was supported by Blueprint Medicines and F. Hoffmann-La Roche.
Extended data
Author contributions
All authors were involved in data interpretation and in the writing, revision and critical review of the article. All authors have approved the submitted version and are accountable for their contributions and the integrity of the work.
Peer review
Peer review information
Nature Medicine thanks Alison Schram, Shun Lu, Wei Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary editors: Javier Carmona and Joao Monteiro, in collaboration with the Nature Medicine team.
Data availability
The anonymized derived data from the registrational ARROW study (NCT03037385) that underlie the results reported in this article may be made available after Roche and/or Blueprint Medicines have received regulatory approval for pralsetinib in the United States and the European Union in the tumor-agnostic setting described herein or upon terminating its clinical development in this setting. Qualified researchers can then request access to individual patient-level clinical data through a data request platform. At the time of writing, this platform is Vivli (https://vivli.org/ourmember/roche/). As RET fusions are rare alterations, the anonymization of patient-level data in patient subgroups or trial cohorts of fewer than 50 patients may be difficult to achieve. As a result, Roche will assess the feasibility of anonymization and, therefore, data release as part of the review of inquiries. For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing.
Competing interests
V.S. reports research funding/grant support for clinical trials from AbbVie, Agensys, Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicines, Boston Biomedical, Boston Pharmaceuticals, Celgene, D3 Bio, Dragonfly Therapeutics, Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Incyte, Inhibrx, Loxo Oncology, MedImmune, MultiVir, NanoCarrier, National Comprehensive Cancer Network, NCI-CTEP, Northwest Biotherapeutics, Novartis, PharmaMar, Pfizer, Relay Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, MD Anderson Cancer Center and Vegenics; travel support from ASCO, ESMO, Helsinn Healthcare, Incyte, Novartis and PharmaMar; consultancy/advisory board participation for Helsinn Healthcare, Incyte, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi-Sankyo, and R-Pharm US; and other relationship with Medscape. P.A.C. reports receiving honoraria from Amgen, Blueprint Medicines, Merck Serono, Merck Sharp & Dohme, Novartis, and Roche/Genentech; travel, accommodations and expenses from Amgen, Bristol Myers Squibb, Merck Sharp & Dohme, NETRIS Pharma, Novartis and Roche; non-financial support from Merck Sharpe & Dohme, Novartis and Plexxikon; research funding from Novartis and Merck Sharpe & Dohme; and research funding to institution from AbbVie, AstraZeneca, Bayer, Blueprint Medicines, Bristol Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Innate Pharma, Janssen Pharmaceuticals, Loxo Oncology, Merck Serono, Merck Sharp & Dohme, Novartis, Plexxikon, Roche/Genentech, Taiho Pharmaceutical, Toray Industries and Transgene. S.S. is an advisory board member for Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Checkmab, Daiichi-Sankyo, Merck, Roche/Genentech and Seattle Genetics. E.G. reports personal fees from Alkermes, Bristol Myers Squibb, Boehringer Ingelheim, Ellipses Pharma, Genentech, F. Hoffmann-La Roche, Janssen Pharmaceuticals, Merck Sharpe & Dohme, Neomed Therapeutics, Seattle Genetics, TFS Health Science and Thermo Fisher Scientific; research funding from Novartis, Roche and Thermo Fisher Scientific; and travel grants from Glycotope and Menarini. L.P.A. reports personal fees from Amgen, AstraZeneca, Bayer, Blueprint Medicines, Bristol Myers Squibb, Eli Lilly, Merck, Merck Sharpe & Dohme, Ipsen, Mirati Therapeutics, Novartis, Pfizer, PharmaMar, Roche, Sanofi and Servier; grants from AstraZeneca, Bristol Myers Squibb, Merck Sharpe & Dohme and Pfizer; and other funding from Altum Sequencing and Genomica. P.G. reports a consulting or advisory role for AstraZeneca, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Guardant Health, Janssen Pharmaceuticals, Novartis, Merck Sharpe & Dohme, Pfizer, Roche and Takeda; speakers bureau for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Gilead Sciences, MSD Oncology, Novartis, Pfizer, ROVI, Roche and Takeda; travel, accommodations and expenses support from AstraZeneca, Bristol Myers Squibb and Roche; and research funding from Guardant Health and Sysmex. E.N. reports consulting or advisory role and lectures for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Merck-Serono, MSD Oncology, Pfizer, Roche and Takeda; and research funding from Bristol Myers Squibb, Merck-Serono, Pfizer and Roche. J.V. reports having consulted and/or having advisory roles for AstraZeneca, Bristol Myers Squibb and Seattle Genetics/Astellas; and institutional research funding from Agendia, Arvinas, Astellas Pharma, AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, Celldex, Clovis Oncology, Deciphera, Eisai, Exelixis, Fortis, Roche/Genetech, Ignyta, Incyte, Innocrin Pharma, Eli Lilly, Loxo, Merck, Novartis, Pfizer, Polyphor and Rgenix. G.L. reports having consulted and/or having advisory roles for AstraZeneca and Pfizer; received travel, accommodations and expenses from Boehringer Ingelheim, E.R. Squibb & Sons, Janssen Pharmaceuticals and Pfizer; received honoraria from Boehringer Ingelheim; received research funding from AstraZeneca; and received research funding to their institution from AbbVie, Adaptimmune Therapeutics, AstraZeneca, Bavarian Nordic, Blueprint Medicines, Bristol Myers Squibb, Eli Lilly, EMD Serono, G1 Therapeutics, GlaxoSmithKline, Janssen Pharmaceuticals, Merck Sharpe & Dohme, Novartis, Pfizer, Rgenix, Roche/Genentech and Tesaro. G.P.K. received research funding/grant support for clinical trials from AbbVie, Blueprint Medicines, Daiichi, Merck, Cullinan and Takeda. D.W.B. declares no conflicts of interest. M. Seetharam. reports having consulted and/or having an advisory role for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Janssen, MorphoSys, Novartis, Roche and Takeda; and received honoraria from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharpe & Dohme and Novartis; and institutional research funding from AstraZeneca and Bristol Myers Squibb. J.C. declares no conflicts of interest. H.Z. is an employee and shareholder of Blueprint Medicines. J.G. reports having a consulting or advisory role with Arvinas, Blueprint Medicines, Genocea Biosciences and Tesaro. A.Z. is an employee and shareholder of Blueprint Medicines. M. Schuler. reports having a consulting or advisory role for Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Roche, Sanofi and Takeda; having received honoraria from Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Merck Serono, Novartis and Roche; having received research funding to his institution from AstraZeneca and Bristol Myers Squibb; and his institution has patents, royalties and other intellectual property of a highly sensitive method for mutation detection by PCR. Y.F. received honoraria as a speaker from AstraZeneca, Bristol Myers Squibb, CStone, Hengrui, Innovent Biologics, Lilly China, Roche, Merck Sharpe & Dohme, Novartis and Pfizer. G.C. reports having a consulting or advisory role for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Eli Lilly, Foundation Medicine, GlaxoSmithKline, Novartis, Pfizer, Roche/Genentech, Samsung, Exact Sciences, Merck and Seagent; having served on speakers bureau for Daiichi-Sankyo, Eli Lilly, Foundation Medicine, Novartis, Pfizer, Roche/Genentech and Samsung; having received travel, accommodations and expenses support from Roche/Genentech, Daichii-Sankyo and Pfizer; having received honoraria from Ellipses Pharma; and having received research funding from Merck.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41591-022-01931-y.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-022-01931-y.
References
- 1.Drilon A, et al. Efficacy of selpercatinib in RET fusion-positive non-small cell lung cancer. N. Engl. J. Med. 2020;383:813–824. doi: 10.1056/NEJMoa2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subbiah V, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8:836–849. doi: 10.1158/2159-8290.CD-18-0338. [DOI] [PubMed] [Google Scholar]
- 3.Subbiah V, Cote GJ. Advances in targeting RET-dependent cancers. Cancer Discov. 2020;10:498–505. doi: 10.1158/2159-8290.CD-19-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato S, et al. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin. Cancer Res. 2017;23:1988–1997. doi: 10.1158/1078-0432.CCR-16-1679. [DOI] [PubMed] [Google Scholar]
- 5.Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology. 2007;148:936–941. doi: 10.1210/en.2006-0921. [DOI] [PubMed] [Google Scholar]
- 6.Kohno T, et al. KIF5B–RET fusions in lung adenocarcinoma. Nat. Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipson D, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat. Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Rolle AF, et al. Identification and characterization of RET fusions in advanced colorectal cancer. Oncotarget. 2015;6:28929–28937. doi: 10.18632/oncotarget.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbiah, V. et al. Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in ARROW, a phase 1 stidy of advanced, RET-altered solid tumors. Cancer Res.78, CTO43 https://aacrjournals.org/cancerres/article/78/13_Supplement/CT043/631013/Abstract-CT043-Highly-potent-and-selective-RET (2018).
- 10.Gainor JF, et al. Pralsetinib for RET fusion–positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22:959–969. doi: 10.1016/S1470-2045(21)00247-3. [DOI] [PubMed] [Google Scholar]
- 11.Subbiah V, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021;9:491–501. doi: 10.1016/S2213-8587(21)00120-0. [DOI] [PubMed] [Google Scholar]
- 12.Blueprint Medicines. GAVRETO (pralsetinib). Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214701s000lbl.pdf (2020).
- 13.Roche Registration. GAVRETO (pralsetinib). Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/gavreto-epar-product-information_en.pdf (2021).
- 14.Looney AM, Nawaz K, Webster RM. Tumour-agnostic therapies. Nat. Rev. Drug Discov. 2020;19:383–384. doi: 10.1038/d41573-020-00015-1. [DOI] [PubMed] [Google Scholar]
- 15.Genentech USA. ROZLYTREK (entrectinib) capsules. Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf (2019).
- 16.Bayer Healthcare Pharmaceuticals. VITRAKVI (larotrectinib). Prescribing information. http://labeling.bayerhealthcare.com/html/products/pi/vitrakvi_PI.pdf (2020).
- 17.Merck Sharpe & Dohme. KEYTRUDA (pembrolizumab) injection. Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf (2019).
- 18.GlaxoSmithKline. JEMPERLI (dostarlimab-gxly). Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761223s000lbl.pdf (2021).
- 19.Eli Lilly USA. RETEVMO (selpercatinib) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213246s000lbl.pdf (2020).
- 20.National Comprehensive Cancer Network (NCCN). Non-Small Cell Lung Cancer (version 7). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (2021).
- 21.National Comprehensive Cancer Network (NCCN). Thyroid Carcinoma (version 1.2021). https://www.nccn.org/professionals/physician_gls/PDF/thyroid.pdf (2021).
- 22.Filetti S, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019;30:1856–1883. doi: 10.1093/annonc/mdz400. [DOI] [PubMed] [Google Scholar]
- 23.European Society for Medical Oncology. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-15SEPT2020.pdf (2020). [DOI] [PubMed]
- 24.Planchard D, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 25.Paratala BS, et al. RET rearrangements are actionable alterations in breast cancer. Nat. Commun. 2018;9:4821. doi: 10.1038/s41467-018-07341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maemondo M, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 27.Peters S, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 28.Soria J-C, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 29.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 30.Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 31.Von Hoff DD, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subbiah, V. et al. Efficacy and safety of selpercatinib in RET fusion–positive cancers other than lung or thyroid cancers. Annual Meeting of the American Association for Cancer Research. https://ecancer.org/en/video/9679-efficacy-and-safety-of-selpercatinib-in-ret-fusion-positive-cancers-other-than-lung-or-thyroid-cancers (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol and list of study sites whose ethics committees approved the protocol
Data Availability Statement
The anonymized derived data from the registrational ARROW study (NCT03037385) that underlie the results reported in this article may be made available after Roche and/or Blueprint Medicines have received regulatory approval for pralsetinib in the United States and the European Union in the tumor-agnostic setting described herein or upon terminating its clinical development in this setting. Qualified researchers can then request access to individual patient-level clinical data through a data request platform. At the time of writing, this platform is Vivli (https://vivli.org/ourmember/roche/). As RET fusions are rare alterations, the anonymization of patient-level data in patient subgroups or trial cohorts of fewer than 50 patients may be difficult to achieve. As a result, Roche will assess the feasibility of anonymization and, therefore, data release as part of the review of inquiries. For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing.