Abstract
Aim
To estimate neutralizing antibody (NAb) immunity against SARS-CoV-2 in 739 healthcare personnel (HCP) vaccinated with three doses of BNT162b2 mRNA vaccine.
Methods
Serum samples were collected at 3, 6, and 9 months after the second vaccine dose and at 7–55 days after the third dose. Samples were tested for NAbs against SARS-CoV-2 receptor binding domain.
Results
The mean inhibition rates at 3, 6, and 9 months after the second dose were 86.33%, 73.38%, and 61.18%, and increased to 95.57% after the booster dose. Younger HCP and HCP with past SARS-CoV-2 infection had higher inhibition rates while there was an inverse correlation between NAb levels and comorbidities or tobacco use (p-values < 0.001). Increased NAb titers were also noticed in women (p-value = 0.033), especially at the end of the 9-month study period.
Conclusion
NAb levels increased considerably after a booster mRNA vaccine dose. Host factors and past SARS-CoV-2 infection influence NAb titers.
Keywords: SARS-CoV-2, COVID-19, mRNA vaccine, Neutralizing antibodies, Healthcare personnel
1. Introduction
Within less than one year after the declaration of the coronavirus disease 2019 (COVID-19) pandemic, the first mRNA COVID-19 vaccines were authorized for emergency use as a key control measure [1], [2]. Yet, humankind continues to experience serious morbidity and mortality [3], [4]. According to the World Health Organization, as of 3 July 2022, over 546 million confirmed cases and over 6.2 million deaths have been reported globally [5]. Although it is almost certain that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will become endemic the next years, there are uncertainties regarding the characteristics of transmission to endemicity in light of the emergence of new virus variants, the duration of immunity after natural infection or vaccination, and the suboptimal COVID-19 vaccination rates almost globally [6], [7], [8], [9].
Neutralization assays have been widely used to study humoral immunity against SARS-CoV-2 [10], [11], [12]. Neutralizing antibody (NAb) immunity is elicited post-natural infection or is vaccine-induced and confers cell protection from virus intrusion which is mediated through binding to angiotensin-converting enzyme 2 (ACE2) receptor [10]. There are published data on NAb immunity after three doses of mRNA vaccines [13], [14], [15], [16], [17]. We studied NAb responses after three doses of mRNA BNT162b2 vaccine in healthcare personnel (HCP) in Greece. The association of NAb immunity with the characteristics of HCP and a laboratory-confirmed past SARS-CoV-2 infection was also investigated.
2. Methods
2.1. COVID-19 vaccination campaign
In Greece vaccination of HCP started on January 4, 2021. Two doses of Pfizer-BioNTech BNT162b2 mRNA vaccine are administered three weeks apart. From September 2021 a third (booster) dose was given to HCP.
2.2. Study design, data collection, and sera sampling
The study was conducted from April to December 2021 at Red Cross General Hospital, a COVID-19 referral hospital in Athens. HCP who had received three doses of BNT162b2 mRNA vaccine were eligible to participate. The following data were collected using one questionnaire per participant: age, gender, comorbidities, use of immunosuppressive drugs, body mass index (BMI), tobacco smoking, regular exercise, and mild post-vaccination side-effects. The dates of COVID-19 vaccinations and past SARS-CoV-2 infections (if any) were retrieved from the National COVID-19 Vaccination Registry and the National Registry of SARS-CoV-2 Infections, respectively. Participants were invited for serum sampling at 3, 6, and 9 months after the second vaccine dose and at 7–55 days after the third dose. The survey and sera sampling were conducted concomitantly and prospectively.
2.3. Detection of total neutralizing antibodies against SARS-CoV-2
Serum samples were tested for NAbs against SARS-CoV-2 receptor binding domain (RBD) with GenScript NAb detection kit (GenScript, Piscataway, USA). The GenScript Nab detection kit is a FDA-approved commercial kit with high sensitivity and specificity, and perfectly correlating results with gold standard methods Virus Neutralization Tests (VNT; conventional or pseudovirus-based) and Plaque Reduction Neutralization Tests (PRNT) [18]. Negative neutralization ability was defined as a neutralization rate of < 30% [18]. The inhibition rate of a sample was calculated as follows: [1-optical density (OD) of sample/OD of negative control] × 100. OD was measured at 450 nm. Residual serum samples collected before 2018 were used as negative controls.
2.4. Diagnosis of SARS-CoV-2 infection
RNA was extracted from nasopharyngeal swabs using the STARMag 96 Kit (Seegene Technologies), followed by real-time PCR using the Allplex SARS-CoV-2 Assay. PCR amplification was run on a CFX96 real-time thermal cycler (Bio-Rad Laboratories) and data were analyzed with the SARS-CoV-2 Viewer (Seegene).
2.5. Detection of SARS-CoV-2 variants of concern (VOC) and of interest (VOI)
The following SARS-CoV-2 mutations were detected with Allplex™ SARS-CoV-2 Variants I and II Assay according to manufacturer instructions: E484K, HV69/70 deletion, N501Y (S gene), and mutations K417N, K417T, L452R, W152C (S gene), respectively.
2.6. Flow cytometry analysis
Samples of EDTA-anticoagulated peripheral blood were collected from patients and tested within 6 h. CD3 +/ CD4 +/ CD8 + T cells, CD19 + B cells, CD3 -/ CD16 +/ CD56 + NK cells and TCRγδ Τ cells (cells/μl) were measured via multiple-color cytometry analysis Beckman Coulter.
2.7. Statistical analysis
Frequencies and percentages were used for categorical variables. Comparisons were performed by using the two-tailed t-test for continuous variables and the chi-square test for categorical variables. P-values < 0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics 21 software.
2.8. Ethical approval
The study protocol was approved by the Scientific Committee of the hospital. Informed consent was obtained from participants. The study was performed in compliance with the national and European regulations.
3. Results
A total of 739 HCP who had received three doses of BNT162b2 mRNA vaccine were studied. Table 1 shows their characteristics.
Table 1.
Characteristics of tested HCP
|
Characteristic |
N (%) |
|
|---|---|---|
| (n = 739) | ||
| Age, years (n = 714) | ||
| < 40 | 207 (29.0%) | |
| 40–59 | 435 (60.9%) | |
| > 60 | 72 (10.1%) | |
| Gender (n = 739) | ||
| male | 238 (32.2%) | |
| female | 501 (67.8%) | |
| Comorbidities1 (n = 498) | 118 (23.7%) | |
| Immunosupressive drugs (n = 511) | 19 (3.7%) | |
| BMI (n = 467) | ||
| < 20 | 32 (6.9%) | |
| 20–25 | 188 (40.2%) | |
| > 25–30 | 167 (35.8%) | |
| > 30 | 80 (17.1%) | |
| Smoking (n = 510) | ||
| yes | 167 (32.7%) | |
| no | 343 (67.3%) | |
| Regular physical exercise (n = 513) | ||
| yes | 111 (21.6%) | |
| no | 402 (78.4%) | |
| SARS-CoV-2 infection2 (n = 739) | 72 (9.7 %) | |
| before vaccination | 37 | |
| before 1st sampling3 | 1 3 | |
| before 2nd sampling4 | 3 | |
| before 3rd sampling5 | 19 | |
| Mild side effects post-vaccination (n = 510) | 269 (52.7%) | |
n: the number of participants who answered this question; HCP: healthcare personnel; BMI: body mass index; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; RT-PCR: real-time polymerase chain reaction.
Co-morbidities included diabetes mellitus (26 HCP), autoimmune disorder (44 HCP), and other (57 HCP).
confirmed by RT-PCR.
after 2nd vaccine dose.
after first sampling and before third vaccine dose.
after second sampling and before third vaccine dose.
A total of 486 HCP (66.3%) provided a first serum sample at a mean of 95.3 (range: 52–135) days after the second vaccine dose. Of them, 426 HCP (84.0%) had a neutralization level of > 75%, indicating a high protective level, while only 4 (0.8%) had negative neutralization ability. Overall, the mean inhibition rate at first sampling was 86.33%. A second serum sample was collected in 473 HCP at a mean of 184.5 (range: 136–225) days after the second dose. The mean inhibition rate of the second sample was 73.38%, while 14 HCP (3.0%) had no neutralization ability (Fig. 1 A). A third serum sample was available in 212 HCP at a mean of 262.9 (range: 226–322) days after the second vaccine dose. The mean inhibition rate in the third measurement was 61.18%, while 22 HCP (10.4%) had no neutralization activity (Fig. 1A).
Fig. 1.
(A) Mean inhibition rates in HCP by sampling period, (B) inhibition rates in selected individuals that had negative neutralization ability before booster dose Error Bars 95% CI.
Sampling 1: samples collected approximately 3 months after the second vaccine dose (mean: 95.3 days, range: 52–135 days). Sampling 2: samples collected approximately 6 months after the second vaccine dose (mean: 184.6 days, range: 136–225 days). Sampling 3: samples collected approximately 9 months after the second vaccine dose (mean: 262.9 days, range: 226–322 days). The effect of booster dose was estimated approximately 3 weeks after the booster dose (mean 21.5 days, range: 7–55 days) HCP: healthcare personnel; CI: confidence interval
In order to study the impact of a booster dose on NAb levels, serum samples were collected from 102 HCP at a mean of 21.5 (range: 7–55) days after the third vaccine dose. The NAb levels increased dramatically to 95.58% (Fig. 1A). The neutralization level in the control group did not exceed the limit of 30% (average 12.24 %, maximum 24.02%). Subsequently, we focused on individuals who had no neutralization ability (<30% inhibition rates) before the booster dose, In almost all these cases the inhibition rate exceeded 90%, with the exception of one case, that was further studied (Fig. 1B). At this particular case (54 year old woman with no comorbidities), lymphocyte subpopulations were determined by flow cytometry. A low percentage (16%) and absolute number (255 cells/μl) of CD3 +/ CD4 + T cells and a high percentage (20.6%) of TCRγδΤ lymphocytes were found.
Table 2 shows the mean inhibition rates according to HCP characteristics. HCP with no history of past SARS-CoV-2 infection noted significant reduction of mean inhibition rates during the 9-month study period. In contrast, there was no significant reduction of the mean inhibition rate during the 9-month period after the second vaccine dose in HCP with past SARS-CoV-2 infection (mean inhibition rates: 95.41%, 92.29%, and 93.62%, respectively) (Table 2, Fig. 2 A).
Table 2.
Mean inhibition rates per time sampling1 and characteristic of HCP
| First sampling | Second sampling | Third sampling | |||||
|---|---|---|---|---|---|---|---|
| n | mean inhibition (%) | n | mean inhibition (%) | n | mean inhibition (%) | p-value | |
| SARS-CoV-2 infection2 | |||||||
| no | 507 | 86.33 (13.96) | 473 | 73.38 (19.27) | 212 | 61.18 (21.62) | < 0.001 |
| yes | 37 | 95.41 (6.50) | 37 | 92.29 (10.98) | 18 | 93.62 (13.04) | |
| Gender | |||||||
| male | 163 | 83.56 (16.30) | 139 | 72.08 (20.17) | 55 | 55.91(22.98) | 0.033 |
| female | 344 | 87.64 (12.51) | 334 | 73.92 (18.88) | 157 | 63.02 (20.88) | |
| Age (years) | |||||||
| <40 | 133 | 91.16 (10.96) | 115 | 78.60 (18.29) | 39 | 70.02 (21.54) | < 0.001 |
| 40–59 | 306 | 85.16 (13.53) | 297 | 73.31 (18.63) | 149 | 60.83 (21.01) | |
| ≥60 | 55 | 76.93 (17.20) | 53 | 63.37 (20.80) | 21 | 48.49 (19.99) | |
| Mild adverse events3 | |||||||
| yes | 163 | 88.56 (12.75) | 208 | 75.16 (18.40) | 91 | 63.18 (21.59) | 0.023 |
| no | 175 | 85.30 (14.80) | 179 | 71.43 (19.63) | 89 | 59.27 (21.54) | |
| Comorbidities | |||||||
| yes | 76 | 81.52 (17.55) | 89 | 69.81 (19.30) | 44 | 56.57 (22.11) | < 0.001 |
| no | 255 | 88.30 (12.41) | 291 | 74.43 (18.94) | 134 | 63.0 (21.17) | |
| Immunosuppressive drugs | |||||||
| yes | 10 | 60.61 (25.79) | 13 | 50.81 (22.41) | 7 | 42.56 (20.23) | < 0.001 |
| no | 328 | 87.68 (12.64) | 374 | 73.87 (18.80) | 174 | 62.07 (21.13) | |
| BMI | |||||||
| < 20 | 18 | 87.29 (12.60) | 22 | 73.46 (18.74) | 13 | 60.43 (22.21) | 0.780 |
| 20–25 | 122 | 86.84 (12.96) | 139 | 73.39 (18.35) | 75 | 60.99 (20.48) | |
| 25,1–30 | 118 | 86.44 (14.95) | 128 | 75.32 (17.97) | 51 | 59.14 (23.54) | |
| > 30 | 52 | 87.39 (15.49) | 65 | 70.22 (20.70) | 22 | 63.18 (21.92) | |
| Smoking | |||||||
| yes | 114 | 80.93 (17.32) | 123 | 65.82 (21.04) | 63 | 51.06 (21.80) | < 0.001 |
| no | 224 | 89.84 (10.64) | 264 | 76.96 (16.95) | 117 | 66.67 (19.43) | |
| Regular physical excersice | |||||||
| yes | 74 | 86.89 (13.12) | 75 | 72.86 (16.71) | 47 | 60.28 (20.13) | 0.458 |
| no | 266 | 86.90 (14.12) | 312 | 73.57 (19.58) | 134 | 61.69 (22.10) | |
n: number of samples; HCP: healthcare personnel; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; BMI: body mass index.
after the 2nd vaccine dose
92 samples collected from 72 HCP with SARS-CoV-2 infection
after vaccination
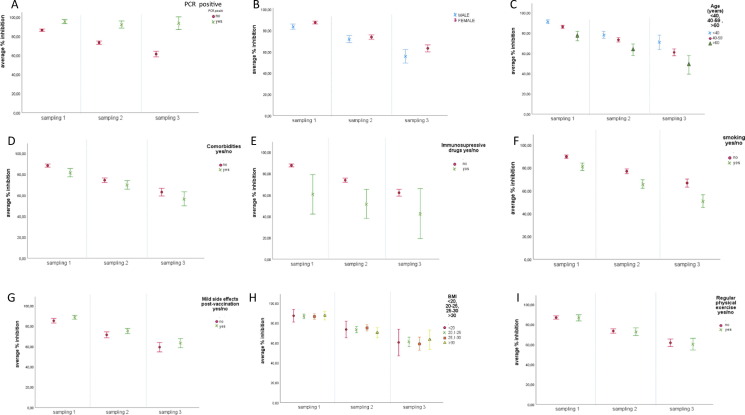
Fig. 2.
Mean inhibition rates per time sampling1 and: (A) SARS-CoV2 infection: no past SARS-CoV-2 infection and
no past SARS-CoV-2 infection and past SARS-CoV-2 infection;(B) gender:
past SARS-CoV-2 infection;(B) gender: male and
male and female;(C) age:
female;(C) age: < 40 years old,
< 40 years old, 40-59 years old and
40-59 years old and > 60 years old;(D) comorbidities:
> 60 years old;(D) comorbidities: no,
no, yes;(E) use of immunosuppressive drugs:
yes;(E) use of immunosuppressive drugs: no,
no, yes;(F) tobacco use:
yes;(F) tobacco use: no,
no, yes;(G) mild adverse events post vaccination:
yes;(G) mild adverse events post vaccination: no,
no, yes;(H) BMI:
yes;(H) BMI: < 20,
< 20, 20.1–25,
20.1–25, 25.1–30 and
25.1–30 and > 30;(I) regular physical exercise:
> 30;(I) regular physical exercise: no,
no, yes.
yes.
Sampling 1: samples collected approximately 3 months after the second vaccine dose (mean: 95.3 days, range: 52–135 days). Sampling 2: samples collected approximately 6 months after the second vaccine dose (mean: 184.6 days, range: 136–225 days).Sampling 3: samples collected approximately 9 months after the second vaccine dose (mean: 262.9 days, range: 226–322 days). Error Bars 95% CI SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; BMI: body mass index; CI: confidence interval
We further investigated the association between NAbs levels at 3, 6, and 9 months after the second vaccine dose and baseline characteristics of HCP (Table 2). Women had significantly higher mean inhibition rates than males in all three measurements (p-value = 0.033), mainly in the third measurement (p-value < 0.001) (Fig. 2B). Stratification of NAb levels by age showed a negative correlation with age. In particular, HCP < 40 years and HCP 40–59 years old had significantly higher inhibition rates at 3, 6, and 9 months after the second vaccine dose compared to HCP ≥ 60 years old (p-value < 0.001) (Fig. 2C). On the other hand, there was a significant negative correlation between NAb levels and the presence of comorbidities, immunosuppressive drugs or regular tobacco smoking (p-value < 0.001 for all comparisons) (Fig. 2D, 2F). Furthermore, HCP with mild adverse events post-vaccination had significantly higher mean inhibition rates compared with HCP without mild adverse events (p-value = 0.023) (Fig. 2G). There was no significant association between regular physical exercise or BMI and NAb levels.
Among 72 HCP with a history of past SARS-CoV-2 infection, 35 were infected after COVID-19 vaccination, including 10 within 90 days post-vaccination, 3 within 91–180 days post-vaccination, and 22 > 180 days post- vaccination. All tested SARS-CoV-2 samples were identified as B.1.1.7 or B.1.617 variants. According to our results, high levels of NAb did not prevent SARS-CoV-2 infection, which is in accordance with the fact that very high levels of NAb were detected few days before SARS-CoV-2 infection in several cases. For example, there was one HCP with SARS-CoV-2 infection 14 days after NAb measurement estimated at 91.7%, and another case of infection 12 days after NAb measurement estimated at 93.5%. None of these cases required hospitalization.
4. Discussion
In order to study the humoral immunogenicity of the mRNA BNT162b2 COVID-19 vaccine, we estimated the NAb levels in serum samples of 739 HCP collected at 3, 6, and 9 months after the second vaccine dose and at 7–55 days after the booster vaccine dose. Breakthrough infections in HCP after full COVID-19 vaccination may jeopardize healthcare services [19]. Although there are published data on NAb levels after a third mRNA vaccine dose [13], [14], [15], [16], [17], further studies are needed to define the magnitude and duration of immunity among HCP after a booster dose.
According to others [8], [9], we found that four out of five vaccinated HCP had strong evidence of a high protective level 3 months after the second mRNA vaccine dose (86.33% inhibition). Nonetheless, in our series, NAb titers gradually waned and ended to 73.38% and 61.18% 6 and 9 months post-vaccination, respectively. A study which was conducted to assess the impact of several SARS-CoV-2 variants on antibodies elicited by mRNA vaccine showed that immune responses waned but were still detectable 6 months post-vaccination in most individuals, along with functional B cells and induction of long-term humoral and cellular immunity [20].
In our series, NAb levels increased almost uniformly to > 90% inhibition rate after a booster dose. Similarly, a mean inhibition rate of 95.9% was detected one month after a third (booster) dose administered to HCP nine months post-full vaccination with the mRNA BNT162b2 vaccine [14]. Another recently published study among 328 HCP showed that a BNT162b2 booster dose increased antibody levels 4-fold compared to the second dose, inducing cross-protective neutralizing antibodies against several SARS-CoV-2 variants [16]. Robust humoral responses were also noted after a BNT162b2 third dose, superior to those recorded after the second dose, among immunocompetent and immunocompromised individuals [15], as well as among persons > 60 years old [17].
Another finding of our study is the significant association between host factors and NAb response after vaccination. In our series, women and younger HCP had significantly higher inhibitory rates throughout the 9-month study period after the second vaccine dose. Recent evidence indicates an association between age, gender, host factors, and post-vaccination immune responses after COVID-19 vaccination [21]. In this latter study, significantly greater antibody titers after a single dose of mRNA vaccine have been detected in younger and normal-weight individuals [21]. Similarly, weaker immune responses have been induced after a booster dose in older HCP (55–65 years old) compared to 35–54 years age-group [16]. In our series we found no significant association between NAb levels and BMI. However, there was a significant inverse association between NAb levels, comorbidities, and tobacco smoking. Studies show evidence of host-associated variations in immune responses to other vaccines, including influenza and measles-mumps-rubella vaccines [22], [23]. It is well established that vaccine-induced immunity against influenza gradually wanes over time and this may particularly affect immunocompromised individuals and the elderly [21].
We also investigated the role of past SARS-CoV-2 infection and found that a history of laboratory-confirmed infection was associated with sustained high NAb immune responses in all three measurements after the second vaccine dose. High levels of anti-RBD IgG and NAb titers have been also detected after only one dose of mRNA vaccine among most vaccinated persons recovered from COVID-19 [12].
A limitation of the current study is the fact that four serum samples were not available in all participants. Another limitation is that there were no samples available beyond 55 days after the booster dose. The role of repeated, thought undiagnosed, natural exposure to SARS-CoV-2 which may boost HCP’ immunity should also be considered. A clear strength is the prospective and concomitant collection of serum samples and data from a large number of HCP, which gave us the opportunity to explore the role of host characteristics. Finally, data about vaccinations and past SARS-CoV-2 infections were retrieved from the national registries.
In conclusion, our study offers insight on NAb immune responses after three doses of mRNA vaccine. In addition, our study provides information to understand the association between host factors, past SARS-CoV-2 infection, and immunogenicity after mRNA vaccination. Higher NAb titers after two doses of mRNA vaccine were detected over a 9-month period in women, younger individuals, no smokers, and individuals with past SARS-CoV-2 infection. A booster vaccine dose was associated with high NAb levels. Further studies are needed to explore the duration of immune responses after a third vaccine dose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We are thankful to the participating healthcare personnel. The opinions presented in this article are those of the authors and do not necessarily represent those of their institutions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Lythgoe M.P., Middleton P. Comparison of COVID-19 vaccine approvals at the US food and drug administration, european medicines agency, and health canada. JAMA Network Open. 2021;4(6):e2114531. doi: 10.1001/jamanetworkopen.2021.14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puthumana J., Egilman A.C., Zhang A.D., Schwartz J.L., Ross J.S. Speed, evidence, and safety characteristics of vaccine approvals by the US Food and Drug Administration. JAMA Intern Med. 2021;181(4):559. doi: 10.1001/jamainternmed.2020.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garber A.M. Learning from excess pandemic deaths. JAMA. 2021;325(17):1729. doi: 10.1001/jama.2021.5120. [DOI] [PubMed] [Google Scholar]
- 4.Faust J.S., Chen A.J., Nguemeni Tiako M.J., Du C., Li S.-X., Krumholz H.M., et al. Leading causes of death among adults aged 25 to 44 years by race and ethnicity in Texas during the COVID-19 pandemic, March to December 2020. JAMA Intern Med. 2022;182(1):87. doi: 10.1001/jamainternmed.2021.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weekly epidemiological update on COVID-19 – 11 January 2022. World Health Organization, available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---6-july-2022 (accessed July 12 2022).
- 6.Lavine J.S., Bjornstad O.N., Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371(6530):741–745. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sah P., Moghadas S.M., Vilches T.N., Shoukat A., Singer B.H., Hotez P.J., et al. Implications of suboptimal COVID-19 vaccination coverage in Florida and Texas. Lancet Infect Dis. 2021;21(11):1493–1494. doi: 10.1016/S1473-3099(21)00620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doria-Rose N., Suthar M.S., Makowski M., O’Connell S., McDermott A.B., Flach B., et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y., Wang J., Li Q., Hu H., Lu J., Chen Z. Advances in neutralization assays for SARS-CoV-2. Scand J Immunol. 2021;94(3) doi: 10.1111/sji.v94.310.1111/sji.13088. [DOI] [Google Scholar]
- 11.Mazzini L., Martinuzzi D., Hyseni I., Benincasa L., Molesti E., Casa E., et al. Comparative analyses of SARS-CoV-2 binding (IgG, IgM, IgA) and neutralizing antibodies from human serum samples. J Immunol Methods. 2021;489:112937. doi: 10.1016/j.jim.2020.112937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demonbreun A.R., Sancilio A., Velez M.P., Ryan D.T., Saber R., Vaught L.A., et al. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. EClinicalMedicine. 2021;38:101018. doi: 10.1016/j.eclinm.2021.101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall V.G., Ferreira V.H., Ku T., Ierullo M., Majchrzak-Kita B., Chaparro C., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terpos E., Karalis V., Sklirou A.D., Apostolakou F., Ntanasis-Stathopoulos I., Bagratuni T., et al. Third dose of the BNT162b2 vaccine results in very high levels of neutralizing antibodies against SARS-CoV-2; results of a prospective study in 150 health professionals in Greece. Am J Hematol. 2022;97:E147–E150. doi: 10.1002/ajh.26468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro Ben David S., Mizrahi B., Rahamim-Cohen D., Supino-Rosin L., Shahar A., Hermoni-Alon S., et al. Robust antibody response after a third BNT162b2 vaccine compared to the second among immunocompromised and healthy individuals, a prospective longitudinal cohort study. Vaccine. 2022;40(30):4038–4045. doi: 10.1016/j.vaccine.2022.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belik M., Jalkanen P., Lundberg R., Reinholm A., Laine L., Väisänen E., et al. Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat Commun. 2022;13(1) doi: 10.1038/s41467-022-30162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilboa M, Mandelboim M, Indenbaum V, Lustig Y, Cohen C, Rahav G, et al. Early immunogenicity and safety of the third dose of BNT162b2 messenger RNA coronavirus disease 2019 vaccine among adults older than 60 years: real-world experience. J Infect Dis 2022;225:785-92. [DOI] [PubMed]
- 18.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.-C., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 19.Ledda C., Costantino C., Motta G., Cunsolo R., Stracquadanio P., Liberti G., et al. SARS-CoV-2 mRNA vaccine breakthrough infections in fully vaccinated healthcare personnel: a systemic review. Trop Med Infect Dis. 2022;7:9. doi: 10.3390/tropicalmed7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374(6572) doi: 10.1126/science:abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellini R., Venuti A., Pimpinelli F., Abril E., Blandino G., Campo F., et al. Early onset of SARS-COV-2 antibodies after first dose of BNT162b2: correlation with age, gender and BMI. Vaccines. 2021;9(7):685. doi: 10.3390/vaccines9070685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poland G.A. Influenza vaccine failure: failure to protect or failure to understand? Expert Rev Vaccines. 2018;17(6):495–502. doi: 10.1080/14760584.2018.1484284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riggenbach M.M., Haralambieva I.H., Ovsyannikova I.G., Schaid D.J., Poland G.A., Kennedy R.B. Mumps virus-specific immune response outcomes and sex-based differences in a cohort of healthy adolescents. Clin Immunol. 2022;234:108912. doi: 10.1016/j.clim.2021.108912. [DOI] [PMC free article] [PubMed] [Google Scholar]