Abstract
Background
Significant evidence links white matter (WM) microstructural abnormalities to cognitive impairment in schizophrenia (SZ), but the relationship of these abnormalities with functional outcome remains unclear.
Methods
In two independent cohorts (C1, C2), patients with SZ were divided into two subgroups: patients with higher cognitive performance (SZ-HCP-C1, n = 25; SZ-HCP-C2, n = 24) and patients with lower cognitive performance (SZ-LCP-C1, n = 25; SZ-LCP-C2, n = 24). Healthy controls (HC) were included in both cohorts (HC-C1, n = 52; HC-C2, n = 27). We compared fractional anisotropy (FA) of the whole-brain WM skeleton between the three groups (SZ-LCP, SZ-HCP, HC) by a whole-brain exploratory approach and an atlas-defined WM regions-of-interest approach via tract-based spatial statistics. In addition, we explored whether FA values were associated with Global Assessment of Functioning (GAF) scores in the SZ groups.
Results
In both cohorts, mean FA values of whole-brain WM skeleton were significantly lower in the SCZ-LCP group than in the SCZ-HCP group. Whereas in C1 the FA of the frontal part of the left inferior fronto-occipital fasciculus (IFOF) was positively correlated with GAF score, in C2 the FA of the temporal part of the left IFOF was positively correlated with GAF score.
Conclusions
We provide robust evidence for WM microstructural abnormalities in SZ. These abnormalities are more prominent in patients with low cognitive performance and are associated with the level of functioning.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00406-021-01363-8.
Keywords: Cognitive deficits, Fractional anisotropy, Global functioning, Schizophrenia, Replication, Confirmation
Introduction
Schizophrenia (SZ) is a severe neuropsychiatric disorder that is associated with poor social [1–3] and occupational functioning [3, 4]. This poor functioning is related to neurocognitive impairment [5–8]. Neurocognitive impairments can be present from the at-risk period to the chronic stages of SZ [9], but not all patients are affected by cognitive deficits. The fifth edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-V) describes a broad range of severities of cognitive impairment in SZ, ranging from intact to severe [10]. However, the question remains unanswered whether the different levels of impairment correlate with different neurobiological characteristics. Understanding the relationship between the degree of cognitive impairment and the underlying neurobiology is key in developing innovative neural targets to improve functional outcomes in SZ.
The disconnection hypothesis in SZ has been put forward in the form of various disconnection theories, such as disconnection of fronto-temporal regions [11, 12], formation of cortico-thalamo-cerebellar loops [13], and crossing of interhemispheric fibers in the corpus callosum [14]. White matter (WM) fibers connect brain regions structurally and functionally [15, 16], including the cortices associated with various cognitive domains [17, 18]. WM microstructural abnormalities can be revealed by diffusion tensor imaging (DTI) because the DTI signal is sensitive to the movement of water molecules and WM microstructures were shown to represent directional information of water molecules [19–21] in postmortem animal studies [22–24] and human brain dissections [25–27]. Recently, the ENIGMA Schizophrenia DTI Working Group conducted the largest international multicenter study to date; it included 4322 patients with SZ from 29 independent international cohorts and highlighted a disturbed WM integrity in widespread regions [28]. Previous studies have supported the importance of WM integrity decline for cognitive impairment [29–31] and functioning in SZ [3, 32]. However, to our knowledge, the relationship between WM integrity, cognitive function, and global functioning in SZ has not yet been investigated with the same analytical methods and replicated in two independent samples with different MRI parameters.
Therefore, we performed a replication study that applied a whole-brain exploratory approach and atlas-defined WM regions-of-interest approach via tract-based spatial statistics (TBSS) in two different cohorts. In the present study, we aimed to robustly identify microstructural differences in WM in patients with SZ subdivided into groups with good and poor cognitive performance. Moreover, we aimed to investigate the relationship between regions-of-interest (ROIs) selected on the basis of exploratory findings and functional outcomes in SZ. We hypothesized that brain WM microstructure in SZ is more severely disturbed in patients with poor cognitive performance and poor functioning.
Methods
Participants
Participants in cohort (1) were recruited from the University Hospital, LMU Munich, Germany. This study was approved by the local ethics committee of the University Hospital, LMU Munich (project number: 17–13), and written informed consent was obtained from all participants. The patients were diagnosed by two independent, experienced psychiatrists using the criteria of the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) [33]. Individuals with no current or past mental illness (according to the MINI Plus Interview [34]) were recruited into the HC group. Most patients were treated with antipsychotic medication, daily dosage of antipsychotic was calculated using the chlorpromazine equivalent method [35] and additional treatment with antidepressants and/or a benzodiazepines was assessed. In all patients, symptom severity was determined with the Positive and Negative Syndrome Scale (PANSS) [36] and level of functioning with the Global Assessment of Functioning (GAF) [37]. GAF scores were demeaned (subtracting the group mean from the individual scores, thus, the new mean is zero) to avoid overestimated associations. Parts of cohort (1) individuals were included in previous studies [38–40]. Participants from cohort (2) were recruited at the Department of Psychiatry and Psychotherapy of the University Medical Center Göttingen, and were assessed with the baseline in a previous longitudinal study [41, 42].
The demographic and clinical characteristics of the study participants in cohorts (1) and (2) are shown in Table 1. Each cohort consisted of patients with SZ and HC. In each cohort, the participants with SZ were evenly divided into a group with higher cognitive performance (HCP) and one with lower cognitive performance (LCP) on the basis of the cognitive composite score, as described in below. Cohort (1) comprised 52 HC and 50 patients with SZ, evenly subdivided into an HCP group (SZ-HCP-C1 group, n = 25) and LCP group (SZ-LCP-C1 group; n = 25), and cohort (2) comprised 27 HC and 48 patients with SZ subdivided into an HCP group (SZ-HCP-C2 group, n = 24) and LCP group (SZ-LCP-C2 group, n = 24).
Table 1.
Demographic and clinical characteristics in cohort (1) and (2)
| Cohort (1) | Post hoc comparisons (HC vs SZ-HCP-C1) (HC vs SZ-LCP-C1) (SZ-HCP-C1 vs SZ-LCP-C1) |
Cohort (2) | Post hoc comparisons (HC vs SZ-HCP-C2) (HC vs SZ-LCP-C2) (SZ-HCP-C2 vs SZ-LCP-C2) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC (n = 52) | SZ (n = 50) | p | SZ-HCP-C1 (n = 25) | SZ-LCP-C1 (n = 25) | p | HC (n = 27) | SZ (n = 48) | p | SZ-HCP-C2 (n = 24) | SZ-LCP-C2 (n = 24) | p | |||||||||||
| Age, y, mean (sd) | 32.07 | 10.95 | 35.16 | 11.26 | 0.164a | 32.56 | 9.197 | 37.76 | 12.66 | 0.097b | 36.62 | 10.91 | 35.2 | 12.4 | 0.642a | 31.91 | 10.65 | 38.66 | 13.30 | 0.127b | ||
| Sex, n, male/female | 39/13 | 42/8 | 0.261c | 20/5 | 22/3 | 0.416c | 18/9 | 33/15 | 0.852c | 16/8 | 17/7 | 0.307c | ||||||||||
| Hand preference, n, right/left | 46/6 | 46/4 | 0.547c | 24/1 | 22/3 | 0.531c | 21/6 | 42/6 | 0.270c | 21/3 | 21/3 | 0.544c | ||||||||||
| Duration of school education, y, mean (sd) | 12.27 | 1.269 | 11.52 | 2.187 | 0.036*a | 11.76 | 1.942 | 11.28 | 2.424 | 0.068b |
(0.702) (0.070) (1.000) |
11.96 | 1.372 | 11.66 | 0.425a | 12.58 | 1.442 | 10.75 | 1.648 | < 0.001b |
(0.424) (0.015*) (< 0.001***) |
|
| Duration of illness, y, mean (sd) | 8.920 | 9.023 | 7.600 | 7.852 | 10.24 | 10.04 | 0.306a | 9.916 | 8.867 | 7.791 | 8.005 | 12.04 | 9.336 | 0.097a | ||||||||
| PANSS positive score, mean (sd) | 13.96 | 5.409 | 13.92 | 5.015 | 14.00 | 5.880 | 0.959a | 14.33 | 6.302 | 13.75 | 5.712 | 14.91 | 6.915 | 0.527a | ||||||||
| PANSS negative score, mean (sd) | 16.98 | 5.235 | 15.00 | 4.627 | 18.96 | 5.135 | 0.006**a | 20.16 | 9.198 | 19.33 | 8.297 | 21.00 | 10.12 | 0.536a | ||||||||
| PANSS general score, mean (sd) | 30.44 | 8.435 | 19.32 | 7.695 | 31.56 | 9.133 | 0.353a | 37.33 | 16.76 | 33.04 | 14.49 | 41.62 | 18.04 | 0.076a | ||||||||
| PANSS total score, mean (sd) | 61.38 | 16.67 | 58.24 | 15.46 | 64.52 | 17.55 | 0.186a | 71.83 | 29.98 | 66.12 | 25.8 | 77.54 | 33.21 | 0.190a | ||||||||
| Demean GAF, mean (sd) | 55.77 | 9.66 | 58.38 | 9.588 | 53.16 | 9.186 | 0.055a | 59.93 | 11.93 | 61.75 | 12.42 | 58.12 | 11.38 | 0.298a | ||||||||
| STM score, z scores, mean (sd) | 0.516 | 0.915 | − 0.537 | 0.794 | < 0.001a | − 0.067 | 0.691 | − 1.007 | 0.592 | < 0.001b |
(0.010**) (< 0.001***) (< 0.001***) |
0.276 | 0.873 | − 0.155 | 1.051 | 0.074a | 0.440 | 0.942 | − 0.751 | 0.792 | < 0.001b |
(1.000) (< 0.001***) (< 0.001***) |
| LMT score, z scores, mean (sd) | 0.543 | 0.667 | − 0.564 | 0.989 | < 0.001a | 0.142 | 0.604 | − 1.271 | 0.727 | < 0.001b |
(0.052) (< 0.001***) (< 0.001***) |
0.234 | 0.857 | − 0.132 | 1.067 | 0.131a | 0.485 | 0.746 | − 0.749 | 0.988 | < 0.001b |
(0.924) (< 0.001***) (< 0.001***) |
| TMT-A, time (s), z scores, mean (sd) | − 0.467 | 0.858 | 0.488 | 0.914 | < 0.001a | 0.013 | 0.791 | 0.963 | 0.781 | < 0.001b |
(0.054) (< 0.001***) (< 0.001***) |
− 0.267 | 0.632 | 0.150 | 1.144 | 0.045*a | − 0.578 | 0.739 | 0.879 | 1.011 | < 0.001b |
(0.518) (< 0.001***) (< 0.001***) |
| TMT-B, time (s), z scores, mean (sd) | − 0.420 | 0.498 | 0.437 | 1.199 | < 0.001a | − 0.244 | 0.487 | 1.119 | 1.315 | < 0.001b |
(1.000) (< 0.001***) (< 0.001***) |
− 0.293 | 0.965 | 0.165 | 1.001 | 0.058a | − 0.466 | 0.503 | 0.797 | 0.981 | < 0.001b |
(1.000) (< 0.001***) (< 0.001***) |
| Cognitive composite score, z scores, mean (sd) | 0.487 | 0.548 | − 0.507 | 0.760 | < 0.001a | 0.076 | 0.375 | − 1.090 | 0.574 | < 0.001b |
(0.005**) (< 0.001***) (< 0.001***) |
0.268 | 0.565 | − 0.150 | 0.826 | 0.022*a | 0.492 | 0.471 | − 0.794 | 0.557 | < 0.001b |
(0.417) (< 0.001***) (< 0.001***) |
| Daily dose of antipsychotics, CPZ equivalents, mean (sd) | 472.1 | 325.6 | 437.4 | 265.2 | 506.8 | 379.0 | 0.456a | 548.9 | 545.4 | 600.0 | 646.3 | 497.8 | 429.9 | 0.522a | ||||||||
| Antidepressant, n, with/without | 8/42 | 3/22 | 5/20 | 0.440c | 11/37 | 5/19 | 6/18 | 0.731c | ||||||||||||||
| Benzodiazepine, n, with/without | 7/43 | 2/23 | 5/20 | 0.221c | 6/41 | 1/23 | 5/19 | 0.080c | ||||||||||||||
Significant differences among the three groups are marked. Values marked with ***, ** and * are significant at p < 0.001, p < 0.01, and p < 0.05, respectively
HC healthy controls, SZ schizophrenia, HCP high cognitive performer, LCP low cognitive performer, SD standard deviation, PANSS Positive and Negative Syndrome Scale, GAF Global Assessment of Functioning, CPZ equivalents chlorpromazine equivalents
aIndependent samples t test
bAnalysis of variance and Bonferroni’s post hoc test
cChi-square test
Neuropsychological measurements in cohorts (1) and (2)
We used neurocognitive instruments that are related to functional deficits in SZ [7, 30, 43]. Neurocognitive function was assessed by experienced psychologists with the short-term memory (STM) and long-term memory (LTM), which were obtained from previous factor analyses of the Verbal Learning Memory Test [44], and the Trail Making Test parts A (TMT-A) and B (TMT-B) [45]. Z scores, that consisted of number of remembered words (STM, LTM, high score = good performance), and seconds to complete task (TMT-A, TMT-B, high score = poor performance), were used for the neuropsychological tests. Within each cohort, a cognitive composite score was calculated with the following equation: {(STM z scores) + (LTM z scores) + (− 1) × (TMT-A z scores) + (− 1) × (TMT-B z scores)}/4.
Magnetic resonance imaging data acquisition and DTI parameters in cohort (1)
All magnetic resonance imaging (MRI) examinations were performed with a 3.0 T MR scanner (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany) with a standard 20-channel phased-array head coil. DTI was performed with 64 non-collinear diffusion-encoding directions and the following parameters: repetition time, 9600 ms; echo time, 95 ms; field of view, 244 mm; voxel size, 2.0 × 2.0 × 2.0 mm; slice thickness, 2.0 mm; 65 slices; and multiple diffusion weighting b values (b = 1000 s/mm2 and b = 0).
MRI data acquisition and DTI parameter in cohort (2)
All MRI examinations were performed with a 3.0 T MR scanner (Magnetom TIM Trio, Siemens Healthcare, Erlangen, Germany) with a standard 8-channel phased-array head coil. DTI was performed with 12 non-collinear diffusion-encoding directions and the following parameters: repetition time, 6500 ms; echo time, 96 ms; field of view, 256 mm; voxel size, 2.0 × 2.0 × 2.0 mm; slice thickness, 2.0 mm; 49 slices; and multiple diffusion weighting b values (b = 1000 s/mm2 and b = 0).
Imaging analysis in cohorts (1) and (2)
DTI data were processed with TBSS programs [46] in the FMRIB software Library (FSL), version 6.0.0. [47]. The Brain Extraction Tool was used to create a binary mask from the non-diffusion-weighted data, and the diffusion tensor and associated parameters such as fractional anisotropy (FA) maps were calculated with the DTIFIT program implemented in the FSL. Nonlinear transformation and affine registration were performed to normalize all FA data into a standard space with the nonlinear registration tool FNIRT [48]. Normalized FA images were averaged to create a mean FA image, and a mean FA skeleton was created by taking the centers of all tracts common to all participants. The voxel values of each participant’s FA map were projected onto the skeleton by searching the local maxima along the perpendicular direction from the skeleton. The resulting data were fed into the voxel-wise statistical analysis described in Sect. 2.5.
Statistical analyses in cohorts (1) and (2)
In each cohort, differences in demographic and clinical characteristics between the HC and SZ groups were analyzed by independent samples t tests for continuous variables (age, duration of school education, the z score of four neurocognitive tests and the cognitive composite score) and chi-square tests for categorical variables (sex distribution and hand preference), with a significance level of α < 0.05. Differences in demographic and clinical characteristics between the SZ-HCP and SZ-LCP groups in each cohort were analyzed with independent samples t tests for continuous variables (duration of illness, PANSS subscores, GAF scores, chlorpromazine daily dose equivalents) and chi-square tests for the number of patients taking medication, with a significance level of α < 0.05. Differences in demographic and clinical characteristics between the HC, SZ-HCP, and SZ-LCP groups in each cohort were analyzed by analysis of variance and Bonferroni’s post hoc test for continuous variables (age, duration of school education, and the z scores of each neurocognitive test) and chi-square tests for categorical variables (sex distribution and hand preference), with a significance level of α < 0.05. Group differences in demographic and clinical characteristics between the SZ groups in cohorts (1) and (2) were analyzed by independent samples t tests for continuous variables (age and duration of school education) and chi-square tests for categorical variables (sex distribution and hand preference), with a significance level of α < 0.05. All statistical analyses were performed with IBM SPSS statistics 20.
Whole-brain exploratory approach
Voxel-wise statistics of the skeletonized FA data were applied using randomize in FSL, version 6.0.0. The HC and SZ groups were compared by an analysis of covariance design, with age and sex as nuisance covariates. We randomly performed permutation-based testing with 5000 permutations and inference by threshold-free cluster enhancement (TFCE) with a threshold of less than 0.05. The mean FA values of the whole skeleton in the HC, SZ-HCP, and SZ-LCP groups were examined for differences by analysis of variance and Bonferroni’s post hoc test, with age and sex as covariates, with a significance level of α < 0.05.
Atlas-defined WM regions-of-interest approach
For atlas-based segmentation, all extracted skeletons were overlaid with the Johns Hopkins University DTI-based WM Atlas in FSL [49, 50]. Differences of the mean FA values in 20 ROIs in the HC and SZ groups were examined by independent samples t tests, with age and sex as covariates, with significance set at p < 0.00125. (= 0.05/40 WM tracts because 20 WM tracts were examined in each cohort). The mean FA values of 20 ROIs in the HC, SZ-HCP, and SZ-LCP groups were examined for differences by analysis of variance and Bonferroni’s post hoc test, with age and sex as covariates and significance set at p < 0.00125 (= 0.05/40 WM tracts because 20 WM tracts were examined in each cohort). In the voxels with a statistical difference in the mean FA values of 20 ROIs, voxel-wise multiple regression analyses were performed with TBSS to examine the relationship between FA values and demean GAF scores. We used 5000 permutations to calculate FA values using age and sex as covariates. Spearman's rank correlation test was carried out between the demeaned GAF scores and mean FA values of the voxels that were statistically significant in the voxel-wise multiple regression analysis.
Results
Demographic and clinical characteristics in cohorts (1) and (2)
Table 1 illustrates demographic and clinical characteristics of both cohorts. In cohort (1), no differences were observed in age, sex, or hand preference between the HC and SZ groups, however, duration of school education was significantly lower in the SZ group than in the HC group. Age, sex, hand preference, and duration of school education were not different between the HC, SZ-HCP, and SZ-LCP groups. Furthermore, duration of illness, PANSS positive, general, and total scores, GAF scores, chlorpromazine daily dose equivalents, and the number of patients taking an antidepressant or benzodiazepine were not different between the SZ-HCP and SZ-LCP groups. However, the SZ-LCP group had significantly higher PANSS negative scores than the SZ-HCP group (p < 0.01).
In cohort (2), no differences in age, sex, hand preference, or duration of school education were observed between the HC and SZ groups. Age, sex, and hand preference were not different between the HC, SZ-HCP, and SZ-LCP groups, but duration of school education was significantly lower in the SZ-LCP group than in the SZ-HCP group (p < 0.001). No significant differences were found between the SZ-HCP and SZ-LCP groups with regard to the duration of illness, PANSS subscales, GAF scores, chlorpromazine daily dose equivalents, and the number of patients taking an antidepressant or benzodiazepine.
Further analyses of demographic and clinical characteristics between the SZ groups in cohorts (1) and (2) are shown in Supplementary Table 1. The SZ group in cohort (2) had significantly higher PANSS negative, general, and total scores than the SZ group in cohort (1) (p < 0.05).
Group comparison of FA in cohorts (1) and (2)
Whole-brain exploratory approach
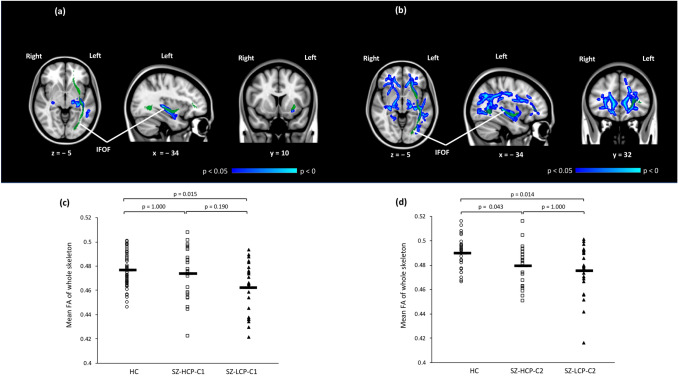
In cohort (1), the FA values in the SZ group were significantly lower than those in the HC group in the left temporal basal areas (p < 0.05, Fig. 1a). The mean FA of the whole-brain WM skeleton was significantly lower in the SZ-LCP group than in the HC group (p < 0.05, Fig. 1c).
Fig. 1.
a Difference in fractional anisotropy (FA) values between the healthy controls (HC) and schizophrenia (SZ) groups in cohort (1). Blue to light blue voxels indicate regions where the FA values were significantly lower in the SZ group than in the HC group (p < 0.05). b Difference in FA values between the HC and SZ groups in cohort (2). Blue to light blue voxels indicate regions where the FA values were significantly lower in the SZ group than in the HC group (p < 0.05). c Differences in mean fractional anisotropy (FA) values of whole-brain white matter (WM) skeleton in healthy controls (HC) and in patients with schizophrenia with higher cognitive performance (SZ-HCP-C1) and lower cognitive performance (SZ-LCP-C1) in cohort (1). The circles represent mean FA values of the whole skeleton in the HC group; the squares represent mean FA values of the whole skeleton in the SZ-HCP-C1 group; and the triangles represent mean FA values of the whole skeleton in the SZ-LCP-C1 group. d Differences in mean FA values of whole-brain WM skeleton in the HC, SZ-HCP-C2, and SZ-LCP-C2 groups in cohort (2). The circles represent mean FA values of the whole skeleton in the HC group; the squares represent mean FA values of the whole skeleton in the SZ-HCP-C2 group; and the triangles represent mean FA values of the whole skeleton in the SZ-LCP-C2 group. FA fractional anisotropy, HC healthy controls, SZ schizophrenia, SZ-HCP-C1 patients with schizophrenia and higher cognitive performance in cohort (1), SZ-HCP-C2 patients with schizophrenia and higher cognitive performance in cohort (2), SZ-LCP-C1 patients with schizophrenia with lower cognitive performance in cohort (1), SZ-LCP-C2 patients with schizophrenia with lower cognitive performance in cohort (2)
In cohort (2), the FA values in the SZ group were significantly lower than those in the HC group in widespread regions (p < 0.05, Fig. 1b). The mean FA of the whole-brain WM skeleton was significantly lower in the SZ-HCP and SZ-LCP groups than in the HC group (p < 0.05, Fig. 1d).
Atlas-defined WM regions-of-interest approach
The results of our analysis of the significant differences in WM skeleton mean FA in patients and HC in the major WM tracts as defined by the Johns Hopkins University WM Atlas are shown in Table 2. In both cohorts, the WM skeleton mean FA values in the SZ and SZ-LCP groups were significantly lower than those in the HC group in the left IFOF (Fig. 1, Table 2), i.e., significant cognition-related FA reductions in SZ were found in the left IFOF.
Table 2.
Differences in fractional anisotropy in the Johns Hopkins University White-Matter Tractography Atlas-based tract
| Cohort (1) | Post hoc comparison (HC vs SZ-HCP-C1) (HC vs SZ-LCP-C1) (SZ-HCP-C1 vs SZ-LCP-C1) |
Cohort (2) | Post hoc comparisons (HC vs SZ-HCP-C2) (HC vs SZ-LCP-C2) (SZ-HCP-C2 vs SZ-LCP-C2) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | SZ | p | SZ-HCP-C1 | SZ-LCP-C1 | p | HC | SZ | p | SZ2-HCP-C2 | SZ-LCP-C2 | p | |||||||||||
| Left anterior thalamic radiation, mean (sd) | 0.482 | 0.015 | 0.472 | 0.019 | 0.005* | 0.475 | 0.020 | 0.486 | 0.017 | 0.013* |
(0.154) (0.013*) (1.000) |
0.449 | 0.014 | 0.442 | 0.025 | 0.083 | 0.449 | 0.025 | 0.435 | 0.025 | 0.013* |
(1.000) (0.015*) (0.054) |
| Right anterior thalamic radiation, mean (sd) | 0.471 | 0.018 | 0.461 | 0.018 | 0.031* | 0.463 | 0.019 | 0.459 | 0.018 | 0.072 | 0.437 | 0.015 | 0.431 | 0.024 | 0.155 | 0.436 | 0.026 | 0.427 | 0.023 | 0.117 | ||
| Left cingulum cingulate gyrus, mean (sd) | 0.634 | 0.031 | 0.618 | 0.032 | 0.046* | 0.628 | 0.028 | 0.609 | 0.034 | 0.020* |
(1.000) (0.019*) (0.147) |
0.609 | 0.029 | 0.603 | 0.041 | 0.394 | 0.612 | 0.039 | 0.594 | 0.042 | 0.143 | |
| Right cingulum cingulate gyrus, mean (sd) | 0.592 | 0.032 | 0.574 | 0.044 | 0.067 | 0.579 | 0.040 | 0.570 | 0.048 | 0.168 | 0.566 | 0.037 | 0.561 | 0.043 | 0.438 | 0.566 | 0.041 | 0.555 | 0.046 | 0.517 | ||
| Left cingulum hippocampus, mean (sd) | 0.559 | 0.044 | 0.549 | 0.063 | 0.554 | 0.561 | 0.061 | 0.537 | 0.064 | 0.087 | 0.509 | 0.049 | 0.489 | 0.049 | 0.020* | 0.482 | 0.057 | 0.496 | 0.039 | 0.043* |
(0.043*) (0.551) (1.000) |
|
| Right cingulum hippocampus, mean (sd) | 0.562 | 0.062 | 0.554 | 0.063 | 0.735 | 0.555 | 0.079 | 0.554 | 0.061 | 0.670 | 0.502 | 0.050 | 0.493 | 0.049 | 0.178 | 0.492 | 0.052 | 0.493 | 0.047 | 0.400 | ||
| Left corticospinal tract, mean (sd) | 0.637 | 0.020 | 0.630 | 0.020 | 0.100 | 0.631 | 0.022 | 0.628 | 0.019 | 0.253 | 0.585 | 0.025 | 0.582 | 0.028 | 0.790 | 0.590 | 0.026 | 0.574 | 0.028 | 0.195 | ||
| Right corticospinal tract, mean (sd) | 0.637 | 0.021 | 0.632 | 0.025 | 0.369 | 0.632 | 0.029 | 0.633 | 0.022 | 0.575 | 0.602 | 0.026 | 0.593 | 0.031 | 0.309 | 0.598 | 0.029 | 0.588 | 0.032 | 0.481 | ||
| Forceps major, mean (sd) | 0.706 | 0.019 | 0.694 | 0.031 | 0.094 | 0.694 | 0.031 | 0.693 | 0.032 | 0.247 | 0.692 | 0.022 | 0.679 | 0.027 | 0.034* | 0.685 | 0.028 | 0.674 | 0.025 | 0.057 | ||
| Forceps minor, mean (sd) | 0.584 | 0.023 | 0.567 | 0.027 | < 0.001*** | 0.574 | 0.028 | 0.56 | 0.025 | 0.002** |
(0.033*) (0.003**) (1.000) |
0.577 | 0.029 | 0.566 | 0.028 | 0.120 | 0.577 | 0.027 | 0.554 | 0.025 | 0.017* |
(1.000) (0.021*) (0.049*) |
| Left inferior fronto-occipital fascicles, mean (sd) | 0.560 | 0.023 | 0.534 | 0.023 | < 0.001*** | 0.537 | 0.021 | 0.531 | 0.025 | < 0.001*** |
(0.002**) (< 0.001***) (1.000) |
0.530 | 0.021 | 0.517 | 0.025 | 0.014* | 0.528 | 0.025 | 0.507 | 0.019 | < 0.001*** |
(1.000) (< 0.001***) (0.007**) |
| Right inferior fronto-occipital fascicles, mean (sd) | 0.551 | 0.023 | 0.540 | 0.023 | 0.055 | 0.545 | 0.018 | 0.536 | 0.027 | 0.102 | 0.525 | 0.021 | 0.516 | 0.028 | 0.112 | 0.528 | 0.027 | 0.503 | 0.022 | < 0.001*** |
(1.000) (0.002**) (0.002**) |
|
| Left inferior longitudinal fascicles, mean (sd) | 0.555 | 0.023 | 0.533 | 0.027 | < 0.001*** | 0.536 | 0.024 | 0.531 | 0.030 | 0.004** |
(0.020*) (0.008**) (1.000) |
0.515 | 0.025 | 0.504 | 0.024 | 0.053 | 0.512 | 0.024 | 0.495 | 0.02 | 0.020* |
(1.000) (0.017**) (0.130) |
| Right inferior longitudinal fascicles, mean (sd) | 0.573 | 0.023 | 0.563 | 0.029 | 0.115 | 0.566 | 0.026 | 0.560 | 0.033 | 0.274 | 0.524 | 0.024 | 0.515 | 0.026 | 0.183 | 0.527 | 0.024 | 0.503 | 0.024 | 0.006** |
(1.000) (0.014*) (0.011*) |
|
| Left superior longitudinal fascicles, mean (sd) | 0.522 | 0.023 | 0.506 | 0.024 | 0.006* | 0.507 | 0.026 | 0.505 | 0.024 | 0.023* |
(0.044*) (0.076) (1.000) |
0.497 | 0.025 | 0.487 | 0.025 | 0.097 | 0.490 | 0.026 | 0.484 | 0.024 | 0.245 | |
| Right superior longitudinal fascicles, mean (sd) | 0.522 | 0.025 | 0.511 | 0.025 | 0.100 | 0.507 | 0.027 | 0.516 | 0.023 | 0.102 | 0.512 | 0.027 | 0.503 | 0.028 | 0.158 | 0.509 | 0.028 | 0.497 | 0.027 | 0.183 | ||
| Left superior longitudinal fascicles, temporal part, mean (sd) | 0.568 | 0.027 | 0.553 | 0.027 | 0.023 | 0.552 | 0.027 | 0.553 | 0.027 | 0.068 | 0.536 | 0.029 | 0.527 | 0.029 | 0.211 | 0.527 | 0.032 | 0.527 | 0.026 | 0.420 | ||
| Right superior longitudinal fascicles, temporal part, mean (sd) | 0.560 | 0.031 | 0.553 | 0.028 | 0.290 | 0.549 | 0.027 | 0.558 | 0.028 | 0.256 | 0.541 | 0.031 | 0.534 | 0.028 | 0.305 | 0.538 | 0.031 | 0.529 | 0.025 | 0.445 | ||
| Left uncinate fascicles, mean (sd) | 0.549 | 0.036 | 0.521 | 0.032 | < 0.001*** | 0.526 | 0.033 | 0.516 | 0.029 | 0.003** |
(0.038*) (0.004**) (1.000) |
0.487 | 0.028 | 0.481 | 0.035 | 0.239 | 0.487 | 0.034 | 0.476 | 0.035 | 0.209 | |
| Right uncinate fascicles, mean (sd) | 0.582 | 0.033 | 0.562 | 0.038 | 0.027 | 0.562 | 0.038 | 0.561 | 0.039 | 0.084 | 0.524 | 0.035 | 0.513 | 0.039 | 0.125 | 0.515 | 0.042 | 0.511 | 0.037 | 0.286 | ||
One-way ANOVA and Bonferroni’s post hoc test with age and sex as covariates. Significant differences among the three groups are marked. Values marked with ***, ** and * are significant at p < 0.05/40 = 0.00125, p < 0.01 and p < 0.05, respectively
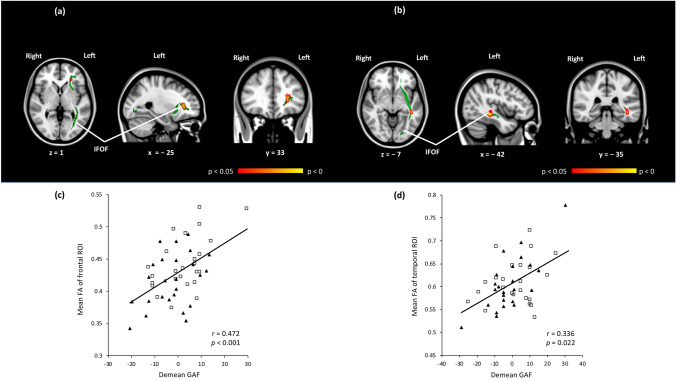
Finally, multiple regression analysis revealed that in cohort (1), FA values in the frontal part of the left IFOF were significantly associated with the demean GAF scores (r = 0.472, p < 0.001, Fig. 2a,c); and in cohort (2), FA values in the temporal part of the left IFOF were significantly associated with the demean GAF scores (r = 0.336, p = 0.022, Fig. 2b,d), i.e., significant positive associations were found between the cognitive-related FA and the demean GAF scores in the fronto-temporal part of the left IFOF in SZ.
Fig. 2.
a Red-yellow voxels indicate a significant positive relation between the fractional anisotropy (FA) values in the left inferior fronto-occipital fasciculus (IFOF) and the demean Global Assessment of Functioning (GAF) scores in the schizophrenia (SZ) group in cohort (1). b Red-yellow voxels indicate a significant positive relation between the FA values in the left IFOF and the demean GAF scores in the SZ group in cohort (2). c Scatter plot showing the relation of the demean Global Assessment of Functioning (GAF) scores and mean fractional anisotropy (FA) values of the voxels that showed a statistically significant correlation in voxel-wise multiple regression analysis in a. The squares represent patients with schizophrenia and higher cognitive performance in cohort (1), and the triangles represent the group of patients with schizophrenia and lower cognitive performance in cohort (1). d Scatter plot showing the relation of the demean GAF scores and mean FA values of the voxels that reached a statistically significant correlation in voxel-wise multiple regression analysis in b. The squares represent patients with schizophrenia and higher cognitive performance in cohort (2), and the triangles represent patients with schizophrenia and lower cognitive performance in cohort (2). FA fractional anisotropy, GAF global assessment of functioning, IFOF inferior front-occipital fascicles, SZ schizophrenia, ROI region of interest
Discussion
Our results provide robust evidence of WM microstructural abnormalities in patients with SZ, especially in those patients with lower cognitive performance (SZ-LCP). Second, in the SZ group we found a significant positive relationship between cognition-related FA values in the fronto-temporal part of the left IFOF and the GAF score.
Our results are consistent with previous findings which revealed that WM volumes were significantly smaller in patients with SZ with cognitive impairment than in healthy individuals but WM volumes in patients without cognitive impairment were not [51]. Moreover, Pérez-Iglesias et al. [52] reported that patients with SZ with cognitive impairment showed a significantly greater decrease in FA values than patients without cognitive impairment.
In this study, we could replicate in two independent cohorts of patients with SZ, that especially patients with poor cognitive performance were affected by WM microstructural abnormalities. Moreover, cognition-related WM abnormalities, which were mainly found in fronto-temporal parts of the left IFOF, were related to general, social, and occupational functioning.
It is worth emphasizing here that in our study FA was measured with different MRI scan parameters in two independent cohorts. A clinical application of structural MRI in clinical trials requires a certain robustness of readouts across different scanners and protocols, despite all biological and technical variability.
The advantage of the current study is the replication of our findings in two independent cohorts and thus increases generalizability. Therefore, our results provide robust evidence that emphasizes the relevance of large reductions in FA as indicating a neurobiological mechanism of SZ in patients with severe cognitive impairment. Beyond the group comparison, the future goal is to find a framework for individual patients [53] to allow MRI images from individuals to be directly compared with a reference. In particular, the increase in the use of MRI-based outcome measurements in clinical trials [54] requires a more precise definition and standardization, especially considering assessments of individuals.
In its atlas-based analysis, the current study found a significant positive relationship in both cohorts between cognition-related FA, mainly in the fronto-temporal part of the left IFOF, and demean GAF scores in patients. The IFOF is the longest associative bundle and connects the occipital cortex, superior parietal lobule, and temporal basal areas to the frontal lobe [55, 56]. Moreover, the fronto-temporal part of the left IFOF is the region of greater interest in SZ: As early as the last century, Wernicke [57] and Kraepelin [58] suggested the importance of the fronto-temporal network in the neuropathology of SZ, and later brain investigations with various neuroimaging methods reinforced this idea [12, 59, 60]. Previous DTI findings implicated prefrontal and temporal lobes [61–63] and the fiber tracts connecting these regions [64] in SZ. Our current finding of an association of the anisotropic reductions in the left IFOF with the cognitive composite and reduced GAF scores may be seen as confirming some previous reports from other DTI studies in SZ that used other cognitive test batteries [31, 65]. In summary, this replication study in a cohort from our previous studies [41, 42] and a new cohort extends previous work [31, 65]. We demonstrated that the origin of neurocognitive deficits and poor functional outcomes in patients with SZ may be related to neurobiological abnormalities in the left IFOF of brain networks in the temporal and frontal cortex.
An interesting finding from our analysis of the whole skeleton is that the FA values in widespread regions were significantly lower in the SZ group than in the HC group in cohort (2) but not in cohort (1). Another interesting finding from our atlas-based analysis is that we found a significant positive relation between the FA values and the demean GAF scores in the temporal part of the left IFOF in cohort (2) but in the frontal part of the left IFOF in cohort (1), i.e., the local regions in the left IFOF could not be replicated in both cohorts. This discrepancy between the independent cohorts might be explained, at least in part, by some factors affecting FA, such as differences in symptom severity for SZ [28], the use of different channel head coils in MRI research [66] and other dimensions (e.g. aggression or impulsivity[67]) that were not included in the present study.
Some limitations of this study must be noted. First, the patients were taking a variety of pharmacological agents not only at the time of scanning, but prior to the scanning (i.e., lifetime use of medication). Second, they were evenly divided with regard to the cognitive composite score and could not be classified according to unique score criteria (e.g., > 1 SD below the normative mean), though previous findings show that approximately one quarter of schizophrenia have similar cognitive performance as healthy [51]. Future studies are necessary using a greater number of patients, who are classified according to unique score criteria, with limited medication exposure and matched lifetime use of medication to confirm the results of our study.
The main strength of this study is the replication approach that used independent samples with different MRI parameters. The results provide a foundation for developing the neurobiological basis of cognitive function, which could serve as a functional proxy in SZ and other psychiatric disorders. Of particular importance will be the design of an approach to transform individual changes of the structural and functional connectome towards a clinical application. Here, cognition and functionality for psychiatric disorders will also play an important role across diagnoses.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Jacquie Klesing, BMedSci (Hons), Board-certified Editor in the Life Sciences (ELS), for editing assistance with the manuscript. The authors thank the clinical staff who helped to conduct the study and all participants, who enabled this study. Parts of the data were/are included in the PhD thesis from IP and FJR.
Abbreviations
- DTI
Diffusion tensor imaging
- FA
Fractional anisotropy
- FSL
FMRIB software library
- HC
Healthy controls
- IFOF
Inferior fronto-occipital fasciculus
- GAF
Global assessment of functioning
- PANSS
Positive and negative syndrome Scale
- STM
Short-term memory
- SZ
Schizophrenia
- SZ-HCP
Schizophrenia with higher cognitive performance
- SZ-LCP
Schizophrenia with lower cognitive performance
- TBSS
Tract-based spatial statistics
- TMT-A
Trail Making Test part A
- TMT-B
Trail Making Test part B
- LTM
Long-term memory
- WM
White matter
Author contributions
Conceptualization, SY, ST, FJR and DK; methodology, SY, ST, FJR and DK; investigation, SY, ST, BM, IP, SS, BEW, BP, UK, TW, KKV, AH, PF, FJR and DK; formal analysis, SY and ST; visualization, SY, ST, FJR and DK; resources, BM, SS, BEW, AH, PF and DK; funding acquisition, SS and PF; writing—first draft, SS, ST, EW, FJR and DK; writing—review and editing, SS, ST, BM, IP, SS, BEW, BP, UK, TW, KKV, AH, PF, EW, FJR and DK; supervision, FJR and DK.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was not supported by any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors. FR was supported by the Else Kröner-Fresenius Foundation for the Residency/PhD track of the International Max Planck Research School for Translational Psychiatry (IMPRS-TP) and by the Munich Clinician Scientist Program (MCSP) of the Faculty of Medicine at LMU Munich.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Shinichi Yamada, Florian J. Raabe and Daniel Keeser contributed equally.
Contributor Information
Florian J. Raabe, Email: florian.raabe@med.uni-muenchen.de
Daniel Keeser, Email: daniel.keeser@med.uni-muenchen.de.
References
- 1.Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr Scand. 2007;116:403–418. doi: 10.1111/j.1600-0447.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 2.Morrison RL, Bellack AS. Social functioning of schizophrenic patients: clinical and research issues. Schizophr Bull. 1987;13:715–725. doi: 10.1093/schbul/13.4.715. [DOI] [PubMed] [Google Scholar]
- 3.Wojtalik JA, Smith MJ, Keshavan MS, Eack SM. A systematic and meta-analytic review of neural correlates of functional outcome in schizophrenia. Schizophr Bull. 2017;43:1329–1347. doi: 10.1093/schbul/sbx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang HWH, Leung AY, Chung RCK, et al. Review on vocational predictors: a systematic review of predictors of vocational outcomes among individuals with schizophrenia: an update since 1998. Aust N Z J Psychiatry. 2010;44:495–504. doi: 10.3109/00048671003785716. [DOI] [PubMed] [Google Scholar]
- 5.Fett A-KJ, Viechtbauer W, Dominguez M-G, et al. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Green MF. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J Clin Psychiatry. 2016;77:20. doi: 10.4088/JCP.14074su1c.02. [DOI] [PubMed] [Google Scholar]
- 7.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 8.Zaragoza Domingo S, Bobes J, García-Portilla M-P, et al. Cognitive performance associated to functional outcomes in stable outpatients with schizophrenia. Schizophr Res Cogn. 2015;2:146–158. doi: 10.1016/j.scog.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkai P, Schmitt A. The need to develop personalized interventions to improve cognition in schizophrenia. World Psychiatry. 2019;18:170. doi: 10.1002/wps.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Association AP, Association AP. Diagnostic and statistical manual of mental disorders: DSM-5. 5. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 11.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand. 1999;99:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 12.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome. Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 13.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “Fragmented Phrene” as Schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 14.Crow TJ, Paez P, Chance SA. Callosal misconnectivity and the sex difference in psychosis. Int Rev Psychiatry. 2007;19:449–457. doi: 10.1080/09540260701486282. [DOI] [PubMed] [Google Scholar]
- 15.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler AL, Voineskos AN. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front Hum Neurosci. 2014;8:653. doi: 10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baars BJ, Franklin S, Ramsøy TZ. Global workspace dynamics: cortical “binding and propagation” enables conscious contents. Front Psychol. 2013 doi: 10.3389/fpsyg.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/S0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 19.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bihan D, Breton E. Imagerie de diffusion in vivo par résonance magnétique nucléaire. Comptes rendus de l’Académie des sciences Série 2. Mécanique Phys Chim Sci Univ Sci Terre. 1985;301:1109–1112. [Google Scholar]
- 21.Moseley ME, Cohen Y, Kucharczyk J, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- 22.Dauguet J, Peled S, Berezovskii V, et al. Comparison of fiber tracts derived from in-vivo DTI tractography with 3D histological neural tract tracer reconstruction on a macaque brain. Neuroimage. 2007;37:530–538. doi: 10.1016/j.neuroimage.2007.04.067. [DOI] [PubMed] [Google Scholar]
- 23.Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, et al. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- 24.Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48:82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::AID-MRM17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Catani M, Dell’Acqua F, Vergani F, et al. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Dell’Acqua F, Catani M. Structural human brain networks: hot topics in diffusion tractography. Curr Opin Neurol. 2012;25:375–383. doi: 10.1097/WCO.0b013e328355d544. [DOI] [PubMed] [Google Scholar]
- 28.Kelly S, Jahanshad N, Zalesky A, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23:1261–1269. doi: 10.1038/mp.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro-de-Araujo LFS, Allin M, Picchioni MM, et al. Schizophrenia moderates the relationship between white matter integrity and cognition. Schizophr Res. 2018;199:250–256. doi: 10.1016/j.schres.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochunov P, Coyle TR, Rowland LM, et al. Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatr. 2017;74:958–966. doi: 10.1001/jamapsychiatry.2017.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roalf DR, Ruparel K, Verma R, et al. White matter organization and neurocognitive performance variability in schizophrenia. Schizophr Res. 2013;143:172–178. doi: 10.1016/j.schres.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitelman SA, Torosjan Y, Newmark RE, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res. 2007;92:211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 33.ICD-10 Version:2010. https://icd.who.int/browse10/2010/en. Accessed 16 July 2021
- 34.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 35.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 36.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 37.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 38.Beller E, Keeser D, Wehn A, et al. T1-MPRAGE and T2-FLAIR segmentation of cortical and subcortical brain regions—an MRI evaluation study. Neuroradiology. 2019;61:129–136. doi: 10.1007/s00234-018-2121-2. [DOI] [PubMed] [Google Scholar]
- 39.Trossbach SV, Hecher L, Schafflick D, et al. Dysregulation of a specific immune-related network of genes biologically defines a subset of schizophrenia. Transl Psychiatry. 2019;9:1–16. doi: 10.1038/s41398-019-0486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papazova I (2021) Predicting cognition in schizophrenia applying machine learning to structural MRI data. PhD Thesis, Ludwig-Maximilians-Universität München
- 41.Malchow B, Keller K, Hasan A, et al. Effects of endurance training combined with cognitive remediation on everyday functioning, symptoms, and cognition in multiepisode schizophrenia patients. Schizophr Bull. 2015;41:847–858. doi: 10.1093/schbul/sbv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malchow B, Keeser D, Keller K, et al. Effects of endurance training on brain structures in chronic schizophrenia patients and healthy controls. Schizophr Res. 2016;173:182–191. doi: 10.1016/j.schres.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Harvey PD, Green MF, Keefe RS, Velligan DI. Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry. 2004;65:361–372. doi: 10.4088/JCP.v65n0312. [DOI] [PubMed] [Google Scholar]
- 44.Müller H, Hasse-Sander I, Horn R, et al. Rey auditory-verbal learning test: structure of a modified german version. J Clin Psychol. 1997;53:663–671. doi: 10.1002/(sici)1097-4679(199711)53:7<663::aid-jclp4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Reitan R, Wolfson D (1993) The Halstead-Reitan neuropsychology battery: theory and clinical interpretation. Hardcover Neuropsychology
- 46.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 48.Andersson JL, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. FMRIB Anal Group Univ Oxf. 2007;2:e21. [Google Scholar]
- 49.Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI atlas of human white matter. Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- 50.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wexler BE, Zhu H, Bell MD, et al. Neuropsychological near normality and brain structure abnormality in schizophrenia. Am J Psychiatry. 2009;166:189–195. doi: 10.1176/appi.ajp.2008.08020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pérez-Iglesias R, Tordesillas-Gutiérrez D, McGuire PK, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167:451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- 53.Dubois J, Adolphs R. Building a science of individual differences from fMRI. Trends Cogn Sci. 2016;20:425–443. doi: 10.1016/j.tics.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadraee A, Paulus M, Ekhtiari H. fMRI as an outcome measure in clinical trials: A systematic review in clinicaltrials.gov. Brain Behav. 2021;11:02089. doi: 10.1002/brb3.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martino J, Brogna C, Robles SG, et al. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Sarubbo S, De Benedictis A, Maldonado IL, et al. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 2013;218:21–37. doi: 10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- 57.Wernicke C. Grundriss der Psychiatrie in klinischen Vorlesungen. Thieme; 1906. [Google Scholar]
- 58.Kraepelin E. Dementia praecox and paraphrenia. Livingstone; 1919. [Google Scholar]
- 59.McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia. Psychol Med. 1996;26:663–667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- 60.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 61.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Kubicki M, McCarley R, Westin C-F, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pettersson-Yeo W, Allen P, Benetti S, et al. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Zalesky A, Fornito A, Seal ML, et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, Lai Y, Wang X, et al. Reduced white matter integrity and cognitive deficit in never-medicated chronic schizophrenia: a diffusion tensor study using TBSS. Behav Brain Res. 2013;252:157–163. doi: 10.1016/j.bbr.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 66.Panman JL, To YY, van der Ende EL, et al. Bias introduced by multiple head coils in MRI research: an 8 channel and 32 channel coil comparison. Front Neurosci. 2019;13:729. doi: 10.3389/fnins.2019.00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tesli N, Westlye LT, Storvestre GB, et al. White matter microstructure in schizophrenia patients with a history of violence. Eur Arch Psychiatry Clin Neurosci. 2021;271:623–634. doi: 10.1007/s00406-019-00988-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.