Abstract
INTRODUCTION:
The mitochondrial genome has small open reading frames (sORF) which produce measurable mitochondrial-derived peptides (MDPs), including humanin, SHLP2, and MOTS-c. Previously, among men undergoing prostate biopsy, we found higher serum SHLP2 was linked with lower PC risk in European American men (EAM), while null associations were found in African American men (AAM). Here, in different patients undergoing prostate biopsy, we tested the link between SHLP2, humanin and MOTS-c and prostate cancer (PC) risk by race.
METHODS:
Plasma SHLP2, humanin, and MOTS-c were measured in 198 men (50/49 EAM/AAM cases; 50/49 EAM/AAM controls) undergoing biopsy. Logistic and multinomial regression models tested associations between each MDP and PC diagnosis, low-grade (grade group, GG1) and high-grade (GG2–5). Models were adjusted for age, body mass index, digital rectal examination, and PSA. We tested interactions between MDPs and race.
RESULTS:
Among controls, humanin was similar by race (p=0.60), but both SHLP2 (p=0.007) and MOTS-c (p=0.026) were lower in AAM controls versus EAM controls. Among EAM, higher MDP values were associated with lower PC risk (all p≤0.001), with null associations in AAM (all p-interactions≤0.01). Similarly, higher MDP expression was associated with decreased risk of low- and high-grade PC in EAM (all p≤0.005) with null associations in AAM.
CONCLUSIONS:
Higher MDP levels were associated with lower PC risk in EAM but not AAM. Generally, AAM controls had lower MDP levels. These data support MDPs and mitochondrial dysfunction in PC, suggesting greater dysfunction in AAM may contribute to excess PC risk. Future larger studies are needed to confirm these results.
Keywords: prostate cancer, mitochondrial genome, mitochondrial-derived peptides (MDPs), mitochondrial dysfunction, disparities
INTRODUCTION
Prostate cancer (PC) is the most common cancer in men and second leading cause of cancer death in men in the United States (1). Current screening for PC is highly reliant on PSA testing. However, given the high false-positive rate of PSA, most men who undergo a biopsy for concerns about PC turn out either not to have cancer or are diagnosed with clinically insignificant cancer (2). To overcome this, newer biomarkers are needed.
One relatively unexplored, but potentially promising source of PC biomarkers is the mitochondria, as reviewed in Sita-Lumsden et al. (3) One study among 130 Chinese men with PC, found that mitochondrial DNA (mtDNA) deletions were associated with aggressive PC (4). In addition, the two mitochondrial ribosomal RNAs, the 12S and 16S rRNAs regions of the mtDNA contain genetic variations. As reviewed by Kalsbeek et al. (5), only a few small studies with PC patients from different ethnic and cultural backgrounds reported association between PC and alterations within the 12S and 16S rRNAs region of the mtDNA (5, 6). However, results have not been validated and these mtDNA genetic markers are not currently used clinically in PC.
Beyond variations in the mtDNA, Nishimoto and colleagues identified the first peptide encoded within the 16S rRNA (MT-RNR2) in a small open reading frame (sORF), named humanin (7). Further examination of the 16S rRNA region, led by our group, identified 6 additional small humanin-like peptides (SHLPs) 1–6 (8), and MOTS-c, the only MDP located within the 12S rRNA region also known as the MT-RNR1 gene (9). Determining the exact function of these MDPs is still an on-going effort, but data to date suggest these MDPs may act as hormones (10), as all the currently discovered MDPs are detectable in the circulation and have bioactive properties (8). For example, humanin is a 24-amino acid peptide reported to exert anti-inflammatory effects (11) as well as inhibiting mitochondrial-generated reactive oxygen species (ROS) (12, 13) and reducing the levels of free-circulating insulin-like growth factor-I (IGF-I) (14). Among the SHLPs 1–6, SHLP2 and SHLP3 had the most profound effects on cellular biology, specifically, increasing mitochondrial oxygen consumption rate and ATP while reducing elevated levels of ROS (8), which might help disruption of ROS-mediated signaling pathways that activate pro-oncogenic signaling. Lastly, MOTS-c is known to be involved in mitochondrial regulation of energy metabolism, insulin sensitivity and folate cycle pathways (9, 15, 16). Given the MDPs properties on metabolic pathways related to cancer, including insulin-resistance, folate and ROS pathways, it raises the possibility that MDPs may have a role in PC risk (17–20).
To date only one small prior study from our group assessed the association between MDPs and PC risk. Among 100 Veteran African American men (AAM) and 100 Veteran European American men (EAM), we found low SHLP2 levels were strongly correlated with increased PC risk, but only in EAM (14). While a less clear link was seen in AAM, a SHLP2 level <350 pg/ml was strongly linked with increased PC risk in both races. Herein, we sought to validate the results from our prior study but using plasma instead of serum as done in our prior study. We also tested 2 additional MDPs for their association with PC risk in AAM and EAM undergoing prostate biopsy at the Durham VA Health Care System (DVAHCS) using the identical study design, but a different set of men from our earlier study (14). Consistent with our earlier data on SHLP2, we hypothesized that lower expression of all MDPs would be associated with increased PC risk and the results would be stronger among EAM.
MATERIAL AND METHODS
Study population and data collection
Patients undergoing prostate biopsy at DVAHCS for suspicion of PC (elevated PSA or suspicious digital rectal examination [DRE]) were prospectively recruited between 2007 and 2018. Patients provided written informed consent, and the study was approved by the Institutional Review Board. From all subjects, blood was drawn on the date of biopsy prior to the procedure. Cases were defined as patients diagnosed with PC at biopsy, and controls were patients with a negative biopsy. Of 378 patients enrolled since 2016 (beginning of plasma sample collection), we randomly selected 199 patients (50 EAM cases, 50 EAM controls, 50 AAM cases, and 49 AAM controls), all who had plasma available and complete data on PSA, age, and BMI.
Sample preparation
Prior to assay, humanin, SHLP2 and MOTS-c in plasma were extracted in 90% acetonitrile and 10% 1N HCl. Briefly, 200 μl of extraction reagent was added to 100 μl of plasma, gently mixed and incubated at room temperature for 30 min. The mixture was centrifuged, and the supernatant removed and dried by Speedvac. The dried extract was reconstituted with 250 μl of phosphate buffer (50 mM sodium phosphate, 150 mM sodium chloride, 0.5% Tween-20, pH 7.6) and then centrifuged at 13,000rpm for 10 minutes. The supernatants were then transferred to a new tube for enzyme-linked immunosorbent assay (ELISA) assays.
Humanin, SHLP-2 and MOTS-c ELISA
Circulating levels of MDPs including humanin, SHLP2 and MOTS-c were measured by in-house sandwich ELISA (8, 9, 21). In brief, a rabbit anti-human analogue for humanin, HNG, and SHLP2 polyclonal anti-sera was produced by Harlan Laboratories, respectively (Indianapolis, IN) and a rabbit-anti MOTS-c anti-sera was produced by Yenzym Antibodies, LLC (South San Francisco, CA). IgG subclasses purified with a protein A column chromatography (Pierce, Rockfold, IL) were used as capture antibody. IgG was further purified with a peptide-conjugated ligand affinity column and labeled with biotin. This biotinylated ligand affinity purified IgG was used as detection antibody. To measure endogenous humanin, SHLP2, and MOTS-c levels, synthetic humanin, SHLP2 and MOTS-c were used as standard within range 0.05 ng/ml to 10 ng/ml. Ninety-six-well microtiter plates were coated with capture antibody at 0.5 ug/well in 200 μl of 50 mM sodium bicarbonate buffer, pH 9.5, incubated 3–4 hours at room temperature on a shaker then washed with wash buffer followed by 2 washes with Superblock buffer (Pierce Chemicals, Rockford, IL). Standards, controls or extracted samples and pre-tittered detection antibody were added to the appropriate wells and incubated overnight. After wash, streptavidin-HRP conjugate was added to well and further incubated for 30 min at room temperature. After 4 washes with wash buffer, 200 μl/well of 1-step ultra TMB were added and incubated for 10–20 min. The reaction was stopped by the addition of 50 μl/well 2N H2SO4 and absorbance was measured on a plate spectrophotometer (Molecular Designs, Sunnyvale, CA) at 490 nm. All analyses were conducted blinded to knowledge of case-control status and all plates had a relatively equal mixture of cases and controls as well as equal mixture by race.
Statistical analysis
Patient characteristics were summarized and stratified by case-control status and by race. Continuous variables were compared using Wilcoxon rank-sum tests and categorical variables were compared using chi-squared tests. The difference in distribution of MDPs between cases and controls, stratified by race, was tested using Wilcoxon rank-sum tests.
Univariable and multivariable logistic regression models were used to assess the association between each MDP (SHLP2, humanin, and MOTS-c) and case-control status. The MDPs were modeled continuously per 10 pg/ml. All models were stratified by race, and the multivariable models were adjusted for age at biopsy (continuous), body mass index (BMI) (continuous), DRE findings (normal vs. abnormal), and PSA before biopsy (continuous). Similarly, univariable and multivariable multinomial logistic regression were used to test the association between each MDP and high-grade PC (vs. no cancer) and low-grade PC (vs. no cancer), stratified by race. Low-grade PC was defined as grade group (GG) 1 and high-grade PC was defined as GG 2–5. The interaction between each MDP and race in predicting overall PC and PC grade was tested using a Wald test. Area under the receiver operating characteristic curve (AUC) was calculated for each outcome (overall PC and high-grade PC) for models including MDP alone, base model (age at biopsy, BMI, DRE, and PSA at biopsy), and base model plus each MDP. Given statistically significant interactions between MDP and race for both overall PC and PC grade (all p-interaction<0.05), all analyses were stratified by race.
Given these MDPs are thought to be metabolic markers, which may in turn relate to BMI and or diabetes, we performed a secondary analysis testing the interaction between each MDP and BMI as well as diabetes for overall PC and PC grade. All statistical tests were performed with SAS 9.4 and p<0.05 was considered statistically significant.
Data Availability Statement:
The data analyzed in this study are available from the Durham VA Medical Center. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors upon reasonable request with the permission of the VA.
RESULTS
Patient characteristics
Baseline characteristics of the patients are shown in Table 1. Cases had higher PSA (median 7.2 vs. 5.9 ng/ml, p<0.001) than controls, as expected. Age at biopsy, BMI, DRE findings, and history of diabetes were similar between cases and controls. Of the cases, there were 36 low-grade (grade group, GG, 1) and 63 high-grade (GG 2–5). AAM were younger at biopsy compared to EAM (median age 66 vs. 68 years, p=0.004). Otherwise, BMI, PSA, DRE and history of diabetes, and cancer grade were similar between races.
Table 1:
Baseline patient characteristics by cancer status and race
| Cancer status | Race | |||||
|---|---|---|---|---|---|---|
| Cases (N=100) | Controls(N=99) | AAM (N=99) | EAM (N=100) | p value | ||
|
| ||||||
| Age at biopsy | 0.6461 | 0.0041 | ||||
| Median | 67 | 67 | 66 | 68 | ||
| Q1, Q3 | 63, 70 | 63, 69 | 61, 69 | 64, 70 | ||
| Race | 1.0002 | - | ||||
| White | 50 (51%) | 50 (51%) | ||||
| Black | 49 (49%) | 49 (49%) | ||||
| BMI (kg/m2) | 0.9061 | 0.2101 | ||||
| Median | 29.9 | 29.5 | 29.5 | 29.8 | ||
| Q1, Q3 | 26.2, 33.6 | 26.8, 32.5 | 26.3, 32.6 | 27.2, 33.6 | ||
| PSA at biopsy (ng/mL) | <0.0011 | 0.3481 | ||||
| Median | 7.2 | 5.9 | 6.4 | 6.5 | ||
| Q1, Q3 | 5.5, 10.5 | 4.3, 7.8 | 5.2, 9.9 | 4.9, 8.2 | ||
| Digital rectal exam | 0.0742 | 0.2922 | ||||
| Not suspicious | 68 (69%) | 79 (80%) | 76 (78%) | 71 (71%) | ||
| Suspicious | 31 (31%) | 20 (20%) | 22 (22%) | 29 (29%) | ||
| History of diabetes? | 0.0862 | 0.9902 | ||||
| Missing | 33 | 36 | 37 | 32 | ||
| No | 51 (77%) | 40 (63%) | 43 (70%) | 48 (71%) | ||
| Yes | 15 (23%) | 23 (37%) | 18 (30%) | 20 (29%) | ||
| Biopsy grade group | - | 0.6402 | ||||
| No cancer | 0 | 99 | 49 | 50 | ||
| 1 | 36 (36%) | 0 (0%) | 17 (35%) | 19 (38%) | ||
| 2–3 | 40 (40%) | 0 (0%) | 22 (45%) | 18 (36%) | ||
| 4–5 | 23 (23%) | 0 (0%) | 10 (20%) | 13 (26%) | ||
Wilcoxon
Chi-Square
When only comparing controls, humanin levels were similar by race (p=0.60), but both SHLP2 (p=0.007) and MOTS-c (p=0.026) were lower in AAM vs. EAM (Fig 1B).
Figure 1B:
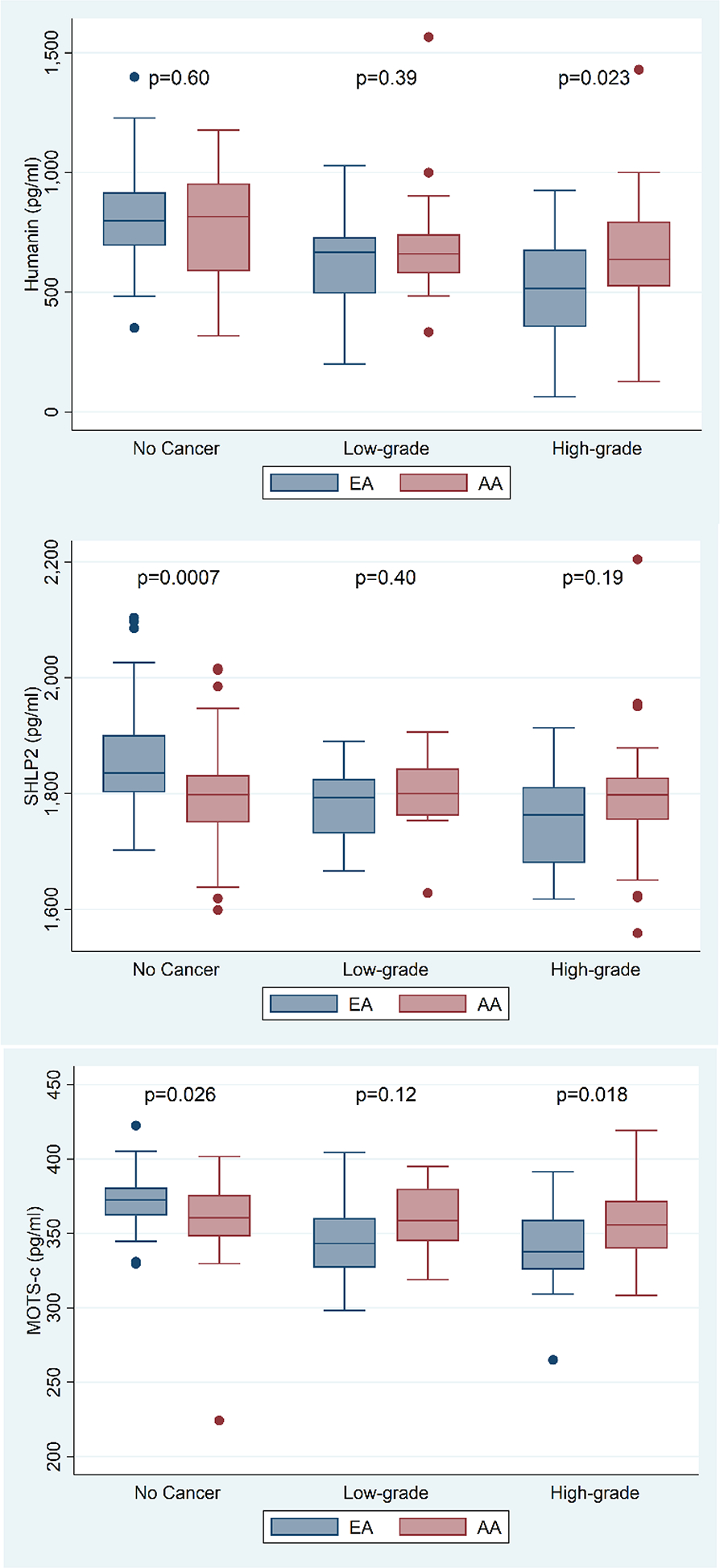
Distribution of humanin, SHLP2 and MOTS-c levels categorized by no cancer, low- and high-grade PC per race.
Plasma levels of MDPs in AAM
Among AAM, humanin values were lower in cases vs. controls (p=0.015, Table 2). When stratified by controls vs. low-grade vs. high-grade, levels were similarly lower in both low-grade and high-grade groups vs. controls (p=0.046, Figure 1A). By contrast, SHLP2 and MOTS-c were unrelated to overall PC risk (all p≥0.39, Table 2) or PC grade (all p≥0.66, Figure 1A).
Table 2:
Plasma levels of humanin, SHLP2 and MOTS-c levels by race and case/control status
| AAM cases (N=50) | AAM controls(N=49) | p value | EAM Cases (N=50) | EAM controls (N=50) | p va1ue | |
|---|---|---|---|---|---|---|
|
| ||||||
| Humanin (pg/ml) | 0.0151 | <0.0011 | ||||
| Median | 654.7 | 815.9 | 546.6 | 798.7 | ||
| Q1, Q3 | 527.2, 793.6 | 587.0, 954.9 | 392.4, 702.0 | 694.0, 917.4 | ||
| SHLP2 (pg/ml) | 0.7741 | <0.0011 | ||||
| Median | 1800.2 | 1798.5 | 1786.2 | 1835.5 | ||
| Q1, Q3 | 1757.6, 1832.2 | 1750.0, 1832.4 | 1722.5, 1814.0 | 1802.3, 1901.0 | ||
| MOTS-c (pg/ml) | 0.3921 | <0.0011 | ||||
| Median | 356.8 | 360.4 | 338.9 | 372.5 | ||
| Q1, Q3 | 340.8, 379.4 | 348.0, 375.9 | 325.8, 359.1 | 362.1, 380.9 | ||
Wilcoxon rank sum
Figure 1A:
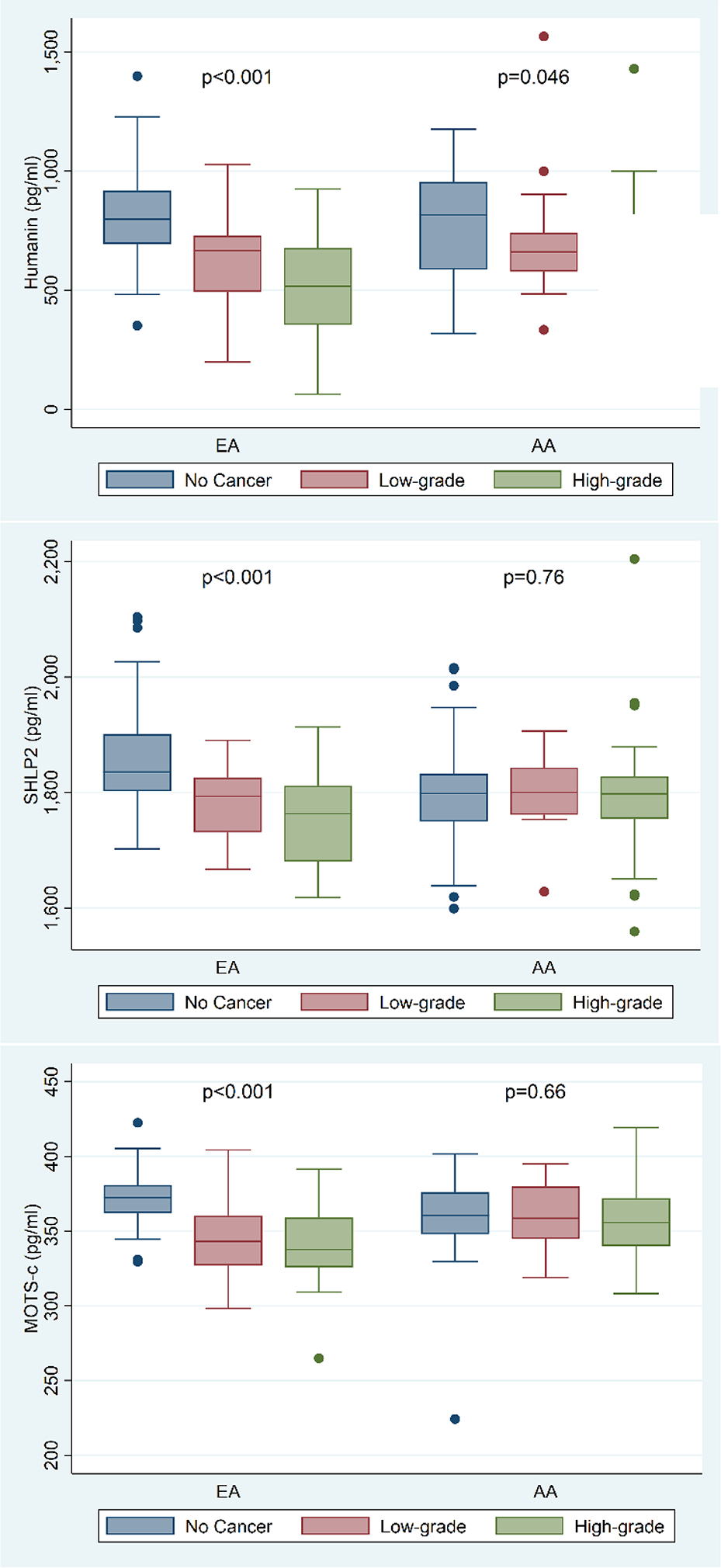
Distribution of humanin, SHLP2 and MOTS-c by race stratified by control vs. low-grade vs. high-grade with highgrade tumors.
On both univariable and multivariable analysis, there remained no association between SHLP2 or MOTS-c and PC diagnosis (all p≥0.57; Table 3). Similar null results were seen when examined by tumor grade. In contrast, for humanin, higher levels were associated with lower odds of overall PC (p=0.043) and high-grade PC (p=0.029) on univariable analysis. While the results became non-significant on adjusted analysis, the odds ratio still favored an inverse association between humanin and overall PC (OR=0.98, p=0.054) and high-grade PC (OR=0.98, p=0.065) (Table 3).
Table 3:
Association between humanin, SHLP2 and MOTS-c and overall risk of PC and risk of PC grade, stratified by race
| EAM | AAM | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | p** | |
| Humanin * | |||||
| Unadjusted | |||||
| Overall PC (vs. none) | 0.94 (0.92–0.97) | <.0001 | 0.98 (0.97–0.999) | 0.043 | |
| No PC | Ref. | Ref. | |||
| Low-grade PC | 0.96 (0.93–0.98) | 0.003 | 0.99 (0.97–1.01) | 0.37 | |
| High-grade PC | 0.93 (0.90–0.96) | <.0001 | 0.98 (0.96–0.998) | 0.029 | |
| Adjusted | |||||
| Overall PC (vs. none) | 0.95 (0.92–0.97) | <.0001 | 0.98 (0.97–1.00) | 0.054 | 0.014 |
| No PC | Ref. | Ref. | 0.048 | ||
| Low-grade PC | 0.96 (0.93–0.99) | 0.004 | 0.99 (0.96–1.01) | 0.21 | |
| High-grade PC | 0.94 (0.91–0.97) | <.0001 | 0.98 (0.96–1.00) | 0.065 | |
| SHLP2 * | |||||
| Unadjusted | |||||
| Overall PC (vs. none) | 0.86 (0.80–0.92) | <.0001 | 1.00 (0.96–1.05) | 0.94 | |
| No PC | Ref. | Ref. | |||
| Low-grade PC | 0.89 (0.81–0.96) | 0.005 | 1.01 (0.95–1.07) | 0.76 | |
| High-grade PC | 0.84 (0.77–0.91) | <.0001 | 1.00 (0.95–1.05) | 0.92 | |
| Adjusted | |||||
| Overall PC (vs. none) | 0.85 (0.78–0.93) | 0.0002 | 1.01 (0.96–1.06) | 0.75 | 0.0004 |
| No PC | Ref. | Ref. | 0.001 | ||
| Low-grade PC | 0.87 (0.79–0.96) | 0.005 | 1.01 (0.95–1.07) | 0.75 | |
| High-grade PC | 0.83 (0.75–0.91) | 0.0002 | 1.01 (0.95–1.07) | 0.79 | |
| MOTS-c * | |||||
| Unadjusted | |||||
| Overall PC (vs. none) | 0.54 (0.42–0.70) | <.0001 | 0.97 (0.84–1.13) | 0.72 | |
| No PC | Ref. | Ref. | |||
| Low-grade PC | 0.58 (0.43–0.77) | 0.0002 | 0.97 (0.79–1.19) | 0.80 | |
| High-grade PC | 0.52 (0.40–0.69) | <.0001 | 0.97 (0.83–1.15) | 0.75 | |
| Adjusted | |||||
| Overall PC (vs. none) | 0.57 (0.44–0.73) | <.0001 | 0.97 (0.83–1.14) | 0.73 | 0.0003 |
| No PC | Ref. | Ref. | 0.001 | ||
| Low-grade PC | 0.58 (0.43–0.77) | 0.0002 | 0.94 (0.77–1.15) | 0.57 | |
| High-grade PC | 0.55 (0.41–0.73) | <.0001 | 1.00 (0.82–1.21) | 0.99 | |
Multivariable models are adjusted for age at biopsy, BMI, DRE, and PSA at biopsy.
Biomarker modeled per 10 pg/ml.
p** = p-value for biomarker and race interaction.
Among AAM, data for overall PC shows that AUC for the base model (0.65, Table 4) was higher than the AUC for each MDP (0.52–0.64). Further, adding the MDPs to the base model resulted in minimal to no improvement in AUC (0.65–0.69) (Table 4). Likewise, AUCs were minimally improved when adding different combinations of MDPs into the model (0.66–0.69, Table 5).
Table 4:
Area under the curve (AUC) for humanin, SHLP2 and MOTS-c models of single MDPs for PC and high-grade PC diagnosis
| PC vs. no PC | High-grade PC vs. low-grade or no PC | |||||
|---|---|---|---|---|---|---|
| MDP alone | Base model | Base model + MDP | MDP alone | Base model | Base model + MDP | |
| EAM | ||||||
| Humanin | 0.80 | 0.65 | 0.82 | 0.78 | 0.77 | 0.86 |
| SHLP2 | 0.77 | 0.65 | 0.81 | 0.74 | 0.77 | 0.84 |
| MOTS-c | 0.83 | 0.65 | 0.84 | 0.76 | 0.77 | 0.82 |
| AAM | ||||||
| Humanin | 0.64 | 0.65 | 0.69 | 0.63 | 0.72 | 0.73 |
| SHLP2 | 0.52 | 0.65 | 0.65 | 0.52 | 0.72 | 0.72 |
| MOTS-c | 0.55 | 0.65 | 0.66 | 0.55 | 0.72 | 0.72 |
Table 5:
Area under the curve (AUC) for humanin, SHLP2 and MOTS-c combination models for PC and high-grade PC diagnosis
| PC vs. no PC | High-grade PC vs. low-grade or no PC | |||||
|---|---|---|---|---|---|---|
| MDPs alone | Base model | Base model + MDPs | MDPs alone | Base model | Base model + MDPs | |
| EAM | ||||||
| Humanin + SHLP2 | 0.83 | 0.65 | 0.85 | 0.80 | 0.77 | 0.86 |
| Humanin + MOTS-c | 0.84 | 0.65 | 0.85 | 0.80 | 0.77 | 0.86 |
| SHLP2 + MOTS-c | 0.84 | 0.65 | 0.86 | 0.79 | 0.77 | 0.85 |
| Humanin + SHLP2 + MOTS-c | 0.85 | 0.65 | 0.86 | 0.80 | 0.77 | 0.86 |
| AAM | ||||||
| Humanin + SHLP2 | 0.65 | 0.65 | 0.68 | 0.63 | 0.72 | 0.73 |
| Humanin + MOTS-c | 0.65 | 0.65 | 0.69 | 0.64 | 0.72 | 0.73 |
| SHLP2 + MOTS-c | 0.55 | 0.65 | 0.66 | 0.53 | 0.72 | 0.72 |
| Humanin + SHLP2 + MOTS-c | 0.65 | 0.65 | 0.68 | 0.64 | 0.72 | 0.73 |
Similar to data for overall PC, among AAM, each MDP had a low AUC (0.52–0.63) for high-grade PC especially compared to the base model alone (0.72). Adding the MDPs to the base model resulted in minimal to no improvement in AUC over the base model alone (0.72–0.73) (Table 4). Likewise, adding different combinations of MDPs to the base model for high-grade PC, resulted in no improvement to the AUC relative to the baseline model alone (Table 5).
Plasma levels of MDPs in EAM
Among EAM, all MDP values were lower in cases vs. controls (all p<0.001; Table 2). Results remained significant when stratified by controls vs. low-grade vs. high-grade with high-grade tumors having slightly lower levels than low-grade tumors (all p<0.001; Figure 1A). On both univariable and multivariable analyses, higher MDP values remained significantly associated with lower PC risk (all p≤0.0002; Table 3). When examined by grade, higher levels of all MDPs were associated with lower odds of both low-grade and high-grade PC, with the association being slightly stronger for high-grade disease (all p≤0.005; Table 3).
In EAM, the base model of clinical and demographic features for predicting overall PC had an AUC of 0.65 (Table 4). The AUCs for each MDP alone predicting PC ranged from 0.77–0.83. When individual MDPs were added to the base model, the AUCs increased to 0.81–0.84. When different combinations of MDPs were added to the base model, AUCs were slightly further improved to 0.85–0.86 (Table 5).
For predicting high-grade disease, similar patterns were seen. In EAM, the AUC for the base model of clinical and demographic features for high grade PC was 0.77 (Table 4). The AUCs for each MDP alone predicting high grade ranged from 0.74–0.78. When individual MDPs were added to the base model, the AUCs increased to 0.82–0.86 (Table 4). While various combinations of MDPs had slightly better AUCs than each MDP alone (range 0.79–0.80), combining this to the base model resulted in an AUC range of 0.85–0.86, which was not meaningfully better than the base model with single MDPs (0.82–0.86, Table 5).
Sensitivity analysis
In analyses stratified by race testing for interactions between MDPs and BMI (stratified as <30 vs. ≥30 kg/m2) for predicting both overall PC and by grade, results were largely null except the interaction between MOTS-c and PC grade among AAM (p=0.025, Supplementary Table 1). However, as these analyses involved 12 different statistical tests, after Bonferroni correction, these results were no longer statistically significant.
In analyses stratified by race testing for interactions between MDPs and diabetes at the time of biopsy for predicting both overall PC and by grade, we found no significant interactions (all p-interaction ≥0.14, Supplementary Table 2)
DISCUSSION
Mitochondrial dysfunction is an important cancer-driving event (22, 23) and mitochondrial impairment appears to be involved in PC pathophysiology (24). As such, it stands to reason that circulating mitochondria-derived circulating markers, such as MDPs (8), may be associated with PC risk. Indeed, we previously found that low serum levels of one MDP, SHLP2, were strongly correlated with PC risk in EAM (25), while a less clear link was seen in AAM, suggesting an race-specific regulation. In the present study we sought to validate those results and further measured two other MDPs, humanin and MOTS-c, in a different cohort of 199 EAM and AAM undergoing prostate biopsy. We found that overall, cases had lower levels of MDPs, including SHLP2, humanin and MOTS-c compared to controls. Furthermore, after adjusting for confounders, higher MDP levels were associated with lower PC risk, including low- and high-grade PC, but similar to our prior results, significant associations were only seen in EAM. While no associations between MDPs and PC risk were found in AAM, AAM controls had significantly lower levels of two MDPs (SHLP2 and MOTS-c) compared to EAM controls. These data validate our previous results and support the importance of MDPs and mitochondrial dysfunction in PC, suggesting greater dysfunction in AAM (as evidenced by lower levels in controls), which may contribute to excess PC risk in this group. Moreover, these data support further evaluation of MDPs as novel PC biomarkers.
Our findings are important and point to biological factors in PC racial disparities. Previous studies showed higher genetic PC risk scores among men with African ancestry than in men with European ancestry (26). In this regard, we confirmed the genetic ancestry of these self-reported individuals via haplogroup assignment (data not shown). Beyond the fact that overall, MDPs’ levels were lower in PC patients, we found that higher levels of MDPs were statistically significantly associated with lower PC risk only in EAM. This may reflect racial/ethnic differences in mitochondrial dysfunction and metabolic dysregulation. This phenomenon could contribute to the well-known increased PC risk in AAM compared to EAM (27). Intriguingly, as high SHLP2 and MOTS-c levels were equally strongly linked with the risk of low- and high-grade cancer in EAM, it suggests these are markers of cancer risk, not specifically aggressive cancer. As such, the fact that AAM, even controls, had lower levels than EAM, is further consistent with the increased risk of PC among AAM.
Several mechanistic pathways may link MDPs and PC risk. Humanin, SHLP2 and MOTS-c are reportedly regulators of insulin action. Treatment with exogenous humanin, SHLP2 and MOTS-c peptides in several in vivo models improved insulin sensitivity, mainly by suppressing hepatic glucose production and enhancement of peripheral glucose uptake (8, 9, 28). Hyperinsulinemia appears to be associated with a higher risk of PC (17, 29). Interestingly, MOTS-c is also implicated in obesity and insulin resistance pathogenesis and known to reduce circulating IL-6 and TNFα levels and indirectly activate the metabolic regulator AMPK (9). Other roles of these MDPs relate to their ability to inhibit PC cell growth (30), induce apoptosis and inhibit migration and invasion (31). These preclinical findings support our observations that higher levels are associated with lower PC risk (at least among EAM). As such, these markers are not traditional cancer biomarkers, such as PSA, which are released by the tumor, but may rather reflect a metabolic environment, in which a cancer is more likely to develop. Future studies should focus on human specimens to find a correlation between the insulin/inflammation pathways, MDPs and PC. Additionally, SHLP2 is known to enhance mitochondrial metabolism and suppress ROS production (8). Thus, the decreased levels reported in our study may reflect a dent on MDP’s cytoprotective role, which may help PC development.
While these results certainly suggest a novel biological link between MDPs and PC, they also show potential clinical implications for PC diagnosis. The addition of single MDPs to the base model (age at biopsy, BMI, DRE, and PSA at biopsy), show dramatic improvements in AUC among EAM, for PC vs. no PC (0.84 vs. 0.65) as well as for low-grade and high-grade PC (0.86 vs. 0.77). These data on MDPs predictive values are on par with commercially available screening tools, such as the serum based 4K score and the urine-based SelectMDX and ExoDx™ tests. For example, a recent study of 457 prostate biopsies showed similar AUC when using ExoDx, 4K score and Select MDx (0.849, 0.835 and 0.699, respectively) (32). Furthermore, in another study, a combined model (MRI and biomarkers) had better predictive ability for detecting high-grade PC than MRI alone (33). Thus, their data suggested that MRI alone was not sensitive enough to detect all high-grade PC and that 4K or ExoDx testing alone could be sufficient when deciding to proceed with biopsy (33). As such, if our results are validated in future larger studies, the MDPs studied herein may be clinically useful biomarkers to identify which patients with an elevated PSA require a biopsy and which can be safely followed given a very low risk of high-grade PC.
Although the current study has several strengths including the clinical data of a racially diverse cohort of men undergoing prostate biopsy, there are also limitations, including the retrospective design of the study, and small sample size. Furthermore, lack of detailed metabolic parameters such as insulin and glucose levels could have provided more mechanistic insights into the link between MDPs and PC risk. Another limitation was none of the men underwent a pre-biopsy MRI, which is increasingly part of the standard of care for diagnosing PC. The last limitation encountered is the need for robust studies in the scientific community that unequivocally establish MDPs as potential biomarkers. Despite these limitations, our study adds important evidence on racial differences in the associations between MDPs and PC risk.
In summary, in an equal-access setting of men undergoing prostate biopsy, three MDPs, SHLP2, humanin and MOTS-c, were associated with PC risk in EAM but not in AAM. Importantly, among controls, for 2 of the 3 MDPs, AAM had significantly lower levels suggesting baseline mitochondrial dysfunction that may underscore the increased risk of PC among AAM. Future larger studies are needed to confirm these results as well as to better understand the biological link between MDPs and PC risk.
Supplementary Material
Acknowledgments
This research was supported by grants from DoD Award Number W81XWH-18-1-0575 # PC171069.
Sources of funding:
Supported in part by DODW81XWH-17-1-0612 and U54CA233465
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest.
References
- 1.Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity - United States, 2001–2017. MMWR Morb Mortal Wkly Rep. 2020;69(41):1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaytoun OM, Stephenson AJ, Fareed K, El-Shafei A, Gao T, Levy D, et al. When serial prostate biopsy is recommended: most cancers detected are clinically insignificant. BJU Int. 2012;110(7):987–92. [DOI] [PubMed] [Google Scholar]
- 3.Sita-Lumsden A, Fletcher CE, Dart DA, Brooke GN, Waxman J, Bevan CL. Circulating nucleic acids as biomarkers of prostate cancer. Biomark Med. 2013;7(6):867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu JJ, Yan T. Effect of mtDNA mutation on tumor malignant degree in patients with prostate cancer. Aging Male. 2010;13(3):159–65. [DOI] [PubMed] [Google Scholar]
- 5.Kalsbeek AMF, Chan EKF, Corcoran NM, Hovens CM, Hayes VM. Mitochondrial genome variation and prostate cancer: a review of the mutational landscape and application to clinical management. Oncotarget. 2017;8(41):71342–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Zaera M, Abril J, Gonzalez L, Aguilo F, Condom E, Nadal M, et al. Identification of somatic and germline mitochondrial DNA sequence variants in prostate cancer patients. Mutat Res. 2006;595(1–2):42–51. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, et al. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer’s disease-relevant insults. J Neurosci. 2001;21(23):9235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY). 2016;8(4):796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21(3):443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarse K, Ristow M. A mitochondrially encoded hormone ameliorates obesity and insulin resistance. Cell Metab. 2015;21(3):355–6. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol Biol Cell. 2009;20(12):2864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, et al. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88(2):360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blasco N, Bea A, Bares G, Giron C, Navaridas R, Irazoki A, et al. Involvement of the mitochondrial nuclease EndoG in the regulation of cell proliferation through the control of reactive oxygen species. Redox Biol. 2020;37:101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao J, Kim SJ, Cohen P, Yen K. Humanin: Functional Interfaces with IGF-I. Growth Horm IGF Res. 2016;29:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SJ, Miller B, Kumagai H, Yen K, Cohen P. MOTS-c: an equal opportunity insulin sensitizer. J Mol Med (Berl). 2019;97(4):487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albanes D, Weinstein SJ, Wright ME, Mannisto S, Limburg PJ, Snyder K, et al. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst. 2009;101(18):1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandeya DR, Mittal A, Sathian B, Bhatta B. Role of hyperinsulinemia in increased risk of prostate cancer: a case control study from Kathmandu Valley. Asian Pac J Cancer Prev. 2014;15(2):1031–3. [DOI] [PubMed] [Google Scholar]
- 19.Prabhat P, Tewari R, Natu SM, Dalela D, Goel A, Tandon P, et al. Is central obesity, hyperinsulinemia and dyslipidemia associated with high-grade prostate cancer? A descriptive cross-sectional study. Indian J Urol. 2010;26(4):502–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shukla S, Srivastava JK, Shankar E, Kanwal R, Nawab A, Sharma H, et al. Oxidative Stress and Antioxidant Status in High-Risk Prostate Cancer Subjects. Diagnostics (Basel). 2020;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin YP, Keni J, Wan J, Mehta H, Anene F, Jia Y, et al. Pharmacokinetics and tissue distribution of humanin and its analogues in male rodents. Endocrinology. 2013;154(10):3739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12(10):685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28(3):265–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parr R, Harbottle A, Jakupciak JP, Singh G. Mitochondria and cancer. Biomed Res Int. 2013;2013:763703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao J, Howard L, Wan J, Wiggins E, Vidal A, Cohen P, et al. Low circulating levels of the mitochondrial-peptide hormone SHLP2: novel biomarker for prostate cancer risk. Oncotarget. 2017;8(55):94900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conti DV, Darst BF, Moss LC, Saunders EJ, Sheng X, Chou A, et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet. 2021;53(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaines AR, Turner EL, Moorman PG, Freedland SJ, Keto CJ, McPhail ME, et al. The association between race and prostate cancer risk on initial biopsy in an equal access, multiethnic cohort. Cancer Causes Control. 2014;25(8):1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4(7):e6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammarsten J, Hogstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39(2):151–8. [DOI] [PubMed] [Google Scholar]
- 30.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321(1):161–7. [DOI] [PubMed] [Google Scholar]
- 31.Kim SJ, Miller B, Mehta HH, Xiao J, Wan J, Arpawong TE, et al. The mitochondrial-derived peptide MOTS-c is a regulator of plasma metabolites and enhances insulin sensitivity. Physiol Rep. 2019;7(13):e14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claire de la Calle JC, Herlemann Annika, Chu Carissa, Gadzinski Adam, Au Yeung Reuben, Cooperberg Matthew, Shinohara Katsuto, Carroll Peter, Nguyen Hao, Fasulo Vittorio, Saita Alberto, editor Evaluation of Biomarkers 4K Score, SelectMDx and ExoDx™, PSAD, TRUS and MRI for the Detection of High-Grade Prostate Cancer. 20th Annual Meeting of the Society of Urologic Oncology; 2019; Renaissance Washington DC Downtown Hotel, Washington, DC. [Google Scholar]
- 33.Fasulo V, Cowan JE, Maggi M, Washington SL 3rd, Nguyen HG, Shinohara K, et al. Characteristics of Cancer Progression on Serial Biopsy in Men on Active Surveillance for Early-stage Prostate Cancer: Implications for Focal Therapy. Eur Urol Oncol. 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study are available from the Durham VA Medical Center. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors upon reasonable request with the permission of the VA.


