Abstract
Background -
Ablation of VT is limited by the inability to create penetrating lesions to reach intra-myocardial origins. Intramural needle ablation using in-catheter, heated Saline Enhanced Radio Frequency (SERF) energy uses convective heating to increase heat transfer and produce deeper, controllable lesions at intramural targets. This first-in-human trial was designed to evaluate the safety and efficacy of SERF needle ablation in patients with refractory VT.
Methods -
Thirty-two subjects from 6 centers underwent needle electrode ablation. Each had recurrent drug-refractory monomorphic VT after ICD implantation and prior standard ablation. During the SERF study procedure, one or more VTs were induced and mapped. The SERF needle catheter was used to create intramural lesions at targeted VT site(s). Acute procedural success was defined as non-inducibility of the clinical VT after the procedure. Patients underwent follow-up at 30 days, and 3 and 6 months, with ICD interrogation at follow-up to determine VT recurrence.
Results -
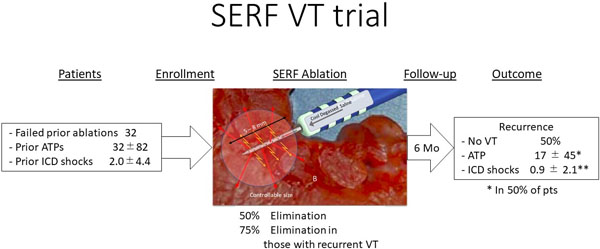
These refractory VT patients (91% male, 66±10 yrs., EF 35±11%; 56% ischemic and 44% non-ischemic) had a median of 45 device therapies (shock/ATP) for VT in the 3–6 months pre-SERF ablation. The study catheter was used to deliver an average of 10±5 lesions per case, with an average of 430±295 sec of RF time, 122±65 min of catheter use time, and a procedural duration of 4.3±1.3 hours. Acute procedural success was 97% for eliminating the clinical VT. At average follow-up of 5 months (n=32), device therapies were reduced by 89%. Complications included 2 periprocedural deaths: an embolic mesenteric infarct and cardiogenic shock, 2 mild strokes, and a pericardial effusion treated with pericardiocentesis (n=1).
Conclusions -
Intramural heated saline needle ablation showed complete acute and satisfactory mid-term control of difficult VTs failing 1–5 prior ablations and drug therapy. Further study is warranted to define safety and longer-term efficacy.
Graphical Abstract
Introduction
Ablation is an important component of the therapeutic armamentarium for treating life threatening ventricular tachycardias (VT) in patients with heart disease1–5. Over the past several decades, a variety of different techniques and technologies have been utilized to facilitate more effective ablation6–14. Although advances including changes in ablation and electroanatomic mapping techniques, and methods for defining the underlying arrhythmia substrate have improved acute and long-term success rates15, 16. outcomes with standard radiofrequency ablation continue to be disappointing3, 4, 6, 7, 10, 11, 13, 17. In multicenter trials, approximately 50 to 80% of patients treated with ablation for post infarction VT experience recurrences, and outcomes are somewhat more limited for those with nonischemic cardiomyopathy18–21.
Origin of the arrhythmia substrate deeper to the endocardium, beyond the reach of conventional RF ablation is an important cause of ablation failure22, 23. A recent human study observed that some or all of the arrhythmia substrate is intramural in the majority of scar-related VTs24. As a result, there has been intensified focus on identifying innovative approaches to ablation including the revisiting of early DC Pulse delivery25, as well as the use of photon and particle therapies26, 27–30.
Heated saline-enhanced radiofrequency (SERF) ablation has also been forwarded as a potentially more effective means of creating larger, more penetrating lesions26, 30–33. This approach incorporates a heating stage within the catheter tip so that heated saline can be injected though the needle directly into the target tissue as RF current is delivered. Heated saline injection markedly increases convective heat transport during RF application. Lesion size increases as the duration of RF application is increased and can be sufficient to create transmural left ventricular lesions from an endocardial catheter position27, 29, 34, 35.
The purpose of this study was to evaluate the technical feasibility and procedural safety of using a novel needle electrode catheter that disperses heated saline into the tissue combined with RF energy applied from the needle to treat recurrent sustained, monomorphic VTs that have been refractory to antiarrhythmic drug therapy, ICDs, and conventional catheter ablation therapy. These results were presented as a Late Breaking Clinical Trial at the Heart Rhythm Society 2020 meeting28.
Methods
The study was designed as a non-randomized, open-label, single group, interventional study, conducted to evaluate the safety and performance of the Durablate Catheter and Thermedical Ablation System (Thermedical, Waltham, MA) in subjects with VT. The studies were separately approved under US IDE and Canadian Clinical Trial Authorization and by all sites Institutional Review Boards. All patients provided informed consent. The data methods used in the analysis, and materials used to conduct the research are available for reproducing the results or replicating the procedure.
Patient Characteristics
Thirty-two patients with refractory VT due to structural heart disease, each of whom had previously undergone defibrillator (ICD) implantation and a subsequent standard catheter ablation were enrolled between January 2017 and March 2020. Inclusion and exclusion criteria are listed in Table 1. All patients had been treated with one or more antiarrhythmic agents and an ICD and had recurrence of VT despite treatment with one or more post-ICD implant standard RF ablations.
Table 1:
Inclusion and Exclusion Criteria for the SERF Needle Ablation Trial
| INCLUSION CRITERIA |
|---|
|
| EXCLUSION CRITERIA |
|
Substrate and Arrhythmia Assessment
The target site of the VT was assessed from previously obtained data including: the 12-lead ECG of the VT, ICD interrogation, mapping data from prior procedures, and cardiac imaging with echocardiography, CTA with delayed enhancement, or MRI with gadolinium in attempt to identify areas of potential scar that could contribute to the arrhythmia substrate. Patients were then taken to the clinical electrophysiology laboratory and catheters placed according to the centers’ standard protocols. Intracardiac ultrasound image analysis was undertaken in real time to identify the location of potential scar, and specific ventricular anatomy. The baseline study included programmed stimulation for induction of VT, if VT was not present spontaneously. The protocol required programmed stimulation using two drive cycle lengths from two right ventricular sites with up to three extra stimuli. LV access was via transseptal approach using a deflectable sheath (Agilis, Abbott) or retrograde aortic approach using an SL-1 sheath (Abbott), depending on the anticipated target VT location. Initial mapping during sinus rhythm and / or VT was generally performed with a conventional electrode catheter and electroanatomic mapping system (Carto, Biosense Webster, Inc.; Precision, Abbott, Inc.; Rhythmia, Boston Scientific, Inc.).
Needle Ablation Device
The Thermedical (SERF) Ablation System’s Durablate deflectable catheter (Figure 1), incorporates a 25-guage stainless steel needle inserted into the myocardium to deliver the RF energy in conjunction with injected heated saline through 30 side holes in the needle. Electrograms could be recorded from 4 distal electrodes at the tip of the catheter. The electrosurgical generator provided radiofrequency (RF) energy up to 50 Watts, simultaneously with the delivery of saline heated to 60° C at a flow rate of 10 ml/min (Figure 2)28–33.
Figure 1.
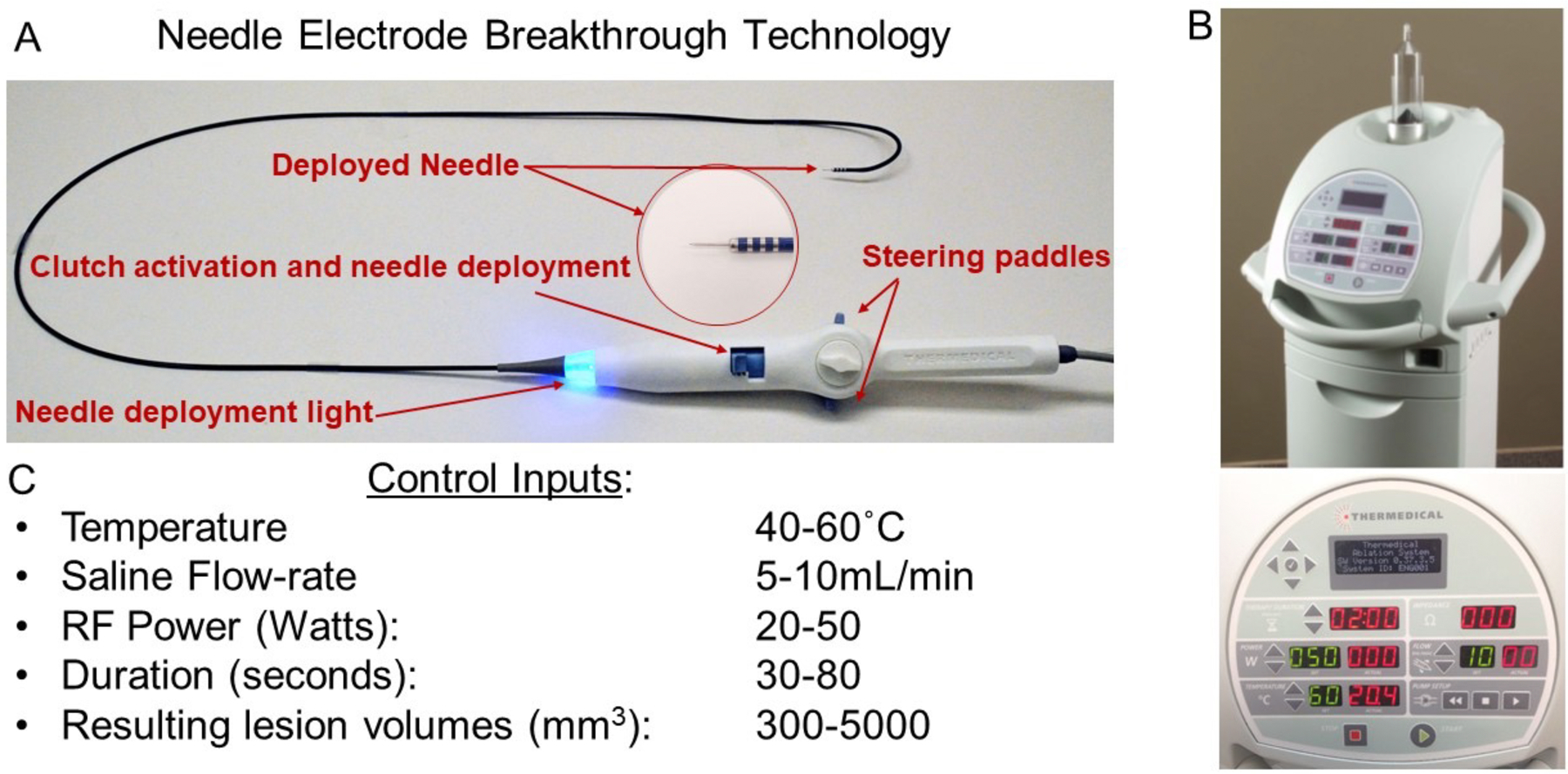
SERF Needle Electrode and Ablation Generator System. The deflectable catheter (Panel A) contains a needle that can be extended to 4 or 8 mm depths. Panel B show the ablation system console with monitoring utilities. Panel C shows the range of programmable settings for heated needle energy delivery.
Figure 2.
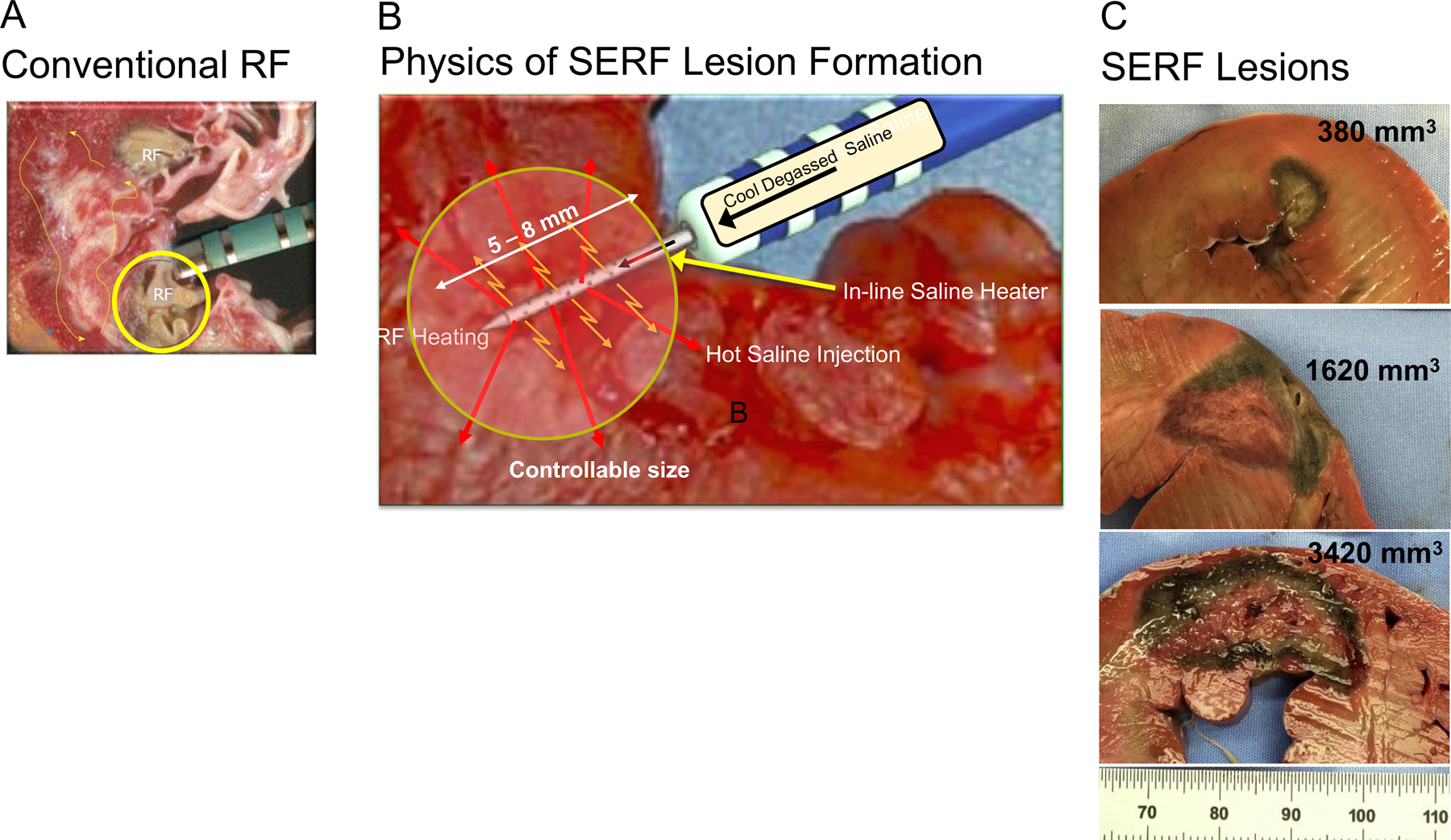
Lesion Creation with Standard RF and SERF Heated Saline Ablations. Panel A shows RF catheter tip/tissue contact and a representative endocardial surface lesion using a standard catheter design. Panel B is a rendition showing the positioning of the SERF catheter along with the needle extension into the myocardium. Heated saline is injected through side holes into the tissue, markedly increasing convective heating (orange arrows) during RF application to the needle. Panel C shows a representative spectrum of needle ablation lesions with size and depth of extension to the mid and epicardial myocardium from a previous animal model. [31, 32] [29] [33] [28–30]
Ablation Approach
Energy was delivered at selected endocardial sites felt to be in close proximity to the VT origin based on imaging, electrograms, and mapping data. A 12-lead ECG or 12-lead Holter or information from a prior ablation with matching follow-up data showing an identical VT was required. This was used where possible, as that replicating the clinical VT. Two channel morphologies from an ICD was not felt to be adequate to call a “clinical VT.”
The ablation catheter tip was positioned in a preferred perpendicular catheter tip/tissue orientation. The extendable needle was deployed into the target ablation area, at an intra-myocardial position confirmed by fluoroscopy or ICE. The needle depth was selected as 6 to 8 mm depending on the total thickness of the myocardium and any observed scar on ICE. Saline was injected through the needle into the myocardium immediately before and continuing during RF application to increase heat transfer capacity and thereby carry thermal energy deeper into the tissue. Subsequent catheter tip/tissue contact, RF deliveries and lesion development were monitored by intracardiac ultrasound and biplane fluoroscopy. Failure to completely insert the catheter needle into the myocardium produced a flurry of microbubbles as saline flowed back into the LV cavity from the needle as easily visualized on ICE. In the presence of significant (grade 2–3) microbubbles27, 29, 30, 34–37, energy delivery was terminated, and the catheter repositioned. Ablation settings were selected based on preclinical studies to achieve a desired lesion volume and depth (Table 2). The acute endpoint of the ablation procedure was the non-inducibility of the clinically relevant VT. In 14 cases, a conventional ablation catheter was also used for residual ablation targets that were not felt to require intramural ablation. An attempt to ablate all inducible VTs was not required but was left to the discretion of the primary site operator.
Table 2:
Ablation Settings
| Setting Number | Duration (s) |
Power (W) |
Flowrate (mL/min) |
Saline Temperature (°C) |
Target Lesion Volume* (cc) |
|---|---|---|---|---|---|
| 1 | 30 | 20 | 10 | 60 | 0.50 |
| 2 | 40 | 25 | 10 | 60 | 1.10 |
| 3 | 55 | 35 | 10 | 60 | 2.30 |
| 4 | 65 | 45 | 10 | 60 | 3.60 |
| 5 | 80 | 50 | 10 | 60 | 5.00 |
Study Endpoints
The trial design sought to establish both safety and efficacy information. Primary Safety Measures were defined as serious adverse events within 30 days that were definitely device related. The Primary Acute Efficacy Measure was non-inducibility of the targeted clinical VT at end of ablation procedure. Secondary Safety Measures included: 1) freedom from acute procedural complications probably or definitely related to the ablation procedure (within 24 hours of ablation) 2) 48 hour prolonged duration of hospitalization (days) following ablation; 3) Number of probably or definitely device related anticipated SAEs within 6 months following ablation; 4) Number of hospitalizations for recurrent VT up to 6 months following ablation; and 5) Percentage of subjects experiencing all-cause and cardiovascular mortality within 6 months of follow-up.
Follow Up
Study visit follow-ups occurred at 30 days, and at 3 and 6 months, and included ICD device interrogation, and assessment of incidence of recurrent VT. Specific patient well-being measures, neurologic function and adverse event occurrence at all follow-ups captured parallel information.
Statistical Methods
Continuous variables are provided as mean ± standard deviation along with the range of that variable, and significance of differences computed using paired T or Kruskal-Wallace testing. Categorical comparisons were made with Chi Square or Fischer Exact test methods. The use of parametric versus nonparametric methods was dependent on the uniformity of distribution of the individual parameter values. A p<0.05 was taken as significant.
Results
Demographics
The 32 patients enrolled (Table 3) had a median age of 67 years, 91% were male; 18 (56%) had ischemic heart disease, of whom 72% had a prior myocardial infarction and 38% had undergone a prior CABG. Nonischemic heart disease producing cardiomyopathy were present in 14 (44%) (Table 3). The mean LVEF was 37%, and the majority of patients had class II (50%) or III (22%) heart failure. These patients had received an average of 45 ICD (shock or ATP) therapies in the preceding 3 to 6 months. Of the 32 patients, 59% had 1 prior ablation procedure, 22% had 2, and 19% had 3 – 5 prior ablation procedures, respectively. All had previously received endocardial procedures and 22 % had also undergone epicardial ablation. One of the patients also underwent trans-coronary ethanol ablation and a needle ablation using another investigational catheter.
Table 3:
Characteristics of the 32 Patients Enrolled in the SERF Trial
| Patients (n) | 32 |
| Age (years) | 67± 9 |
| Gender | |
| Male | 29 (91%) |
| Female | 3 (9%) |
| Ischemic Cardiomyopathy | 18 (56%) |
| Prior MI | 72% |
| Prior CABG | 36% |
| Nonischemic-Cardiomyopathy | 14 (44%) |
| Idiopathic | 8 |
| Sarcoid | 3 |
| Lamin A/C | 1 |
| Lymphocytic CM | 1 |
| Late stage HCM | 1 |
| EF | 37% |
| NYHA Class | |
| Class I | 29% |
| Class II | 45% |
| Class III | 26% |
| VT Subtype | |
| Monomorphic (32) | 100% |
| Storm (n=3) | 9% |
| Incessant | 0% |
| Prior VT Ablation | |
| Endocardialy | 100% |
| Epicardialy | 27% |
| Endo- and Epicardialy | 27% |
| Diabetes Mellitus | |
| Chronic Renal Disease | 22% |
| Peripheral Vascular Disease | 25% |
| Prior stroke (CVA/TIA) | 16% |
| COPD | 22% |
| AF/AFL | 28% |
n = Number of subjects; MI = myocardial infarction; CABG = Coronary artery bypass grafting; A/C = arrhythmogenic cardiomyopathy; HCM = hypertrophic cardiomyopathy; EF = ejection fraction; NYHC = New York Heart Association; = ; CVA = cerebral vascular accident; TIA = transient ischemic attack; COPD = chronic obstructive pulmonary disease; AF/AFL = atrial fibrillation or atrial flutter
Mapping and Structural Characterization
Pre-procedure imaging was obtained in 25 patients with CTA, including delayed contrast assessment, while 1 underwent MR assessment including late gadolinium enhancement.
Patients had a median of two different inducible VTs. At least one clinical VT was inducible in 88%, and nonclinical VTs were inducible in 45% of patients. The relevant VT was mappable in 49% of patients. Ablation was guided by substrate assessment combined with mapping during VT in the majority of patients (Figures 3 and 4). In 3 patients ablation was guided only by substrate assessment and use of the VT QRS morphology to determine the likely region within extensive scar areas that gave rise to the VT.
Figure 3.
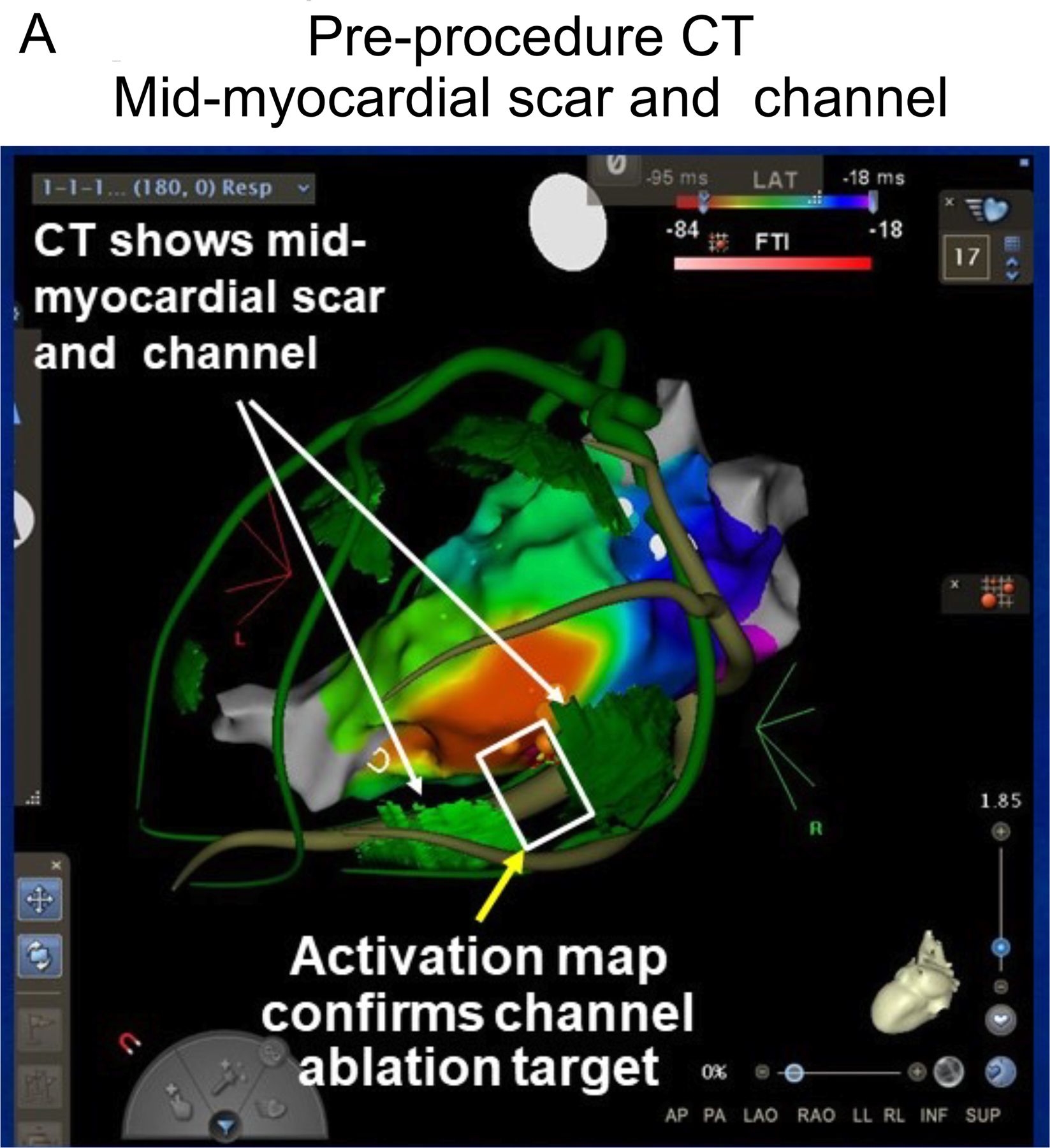
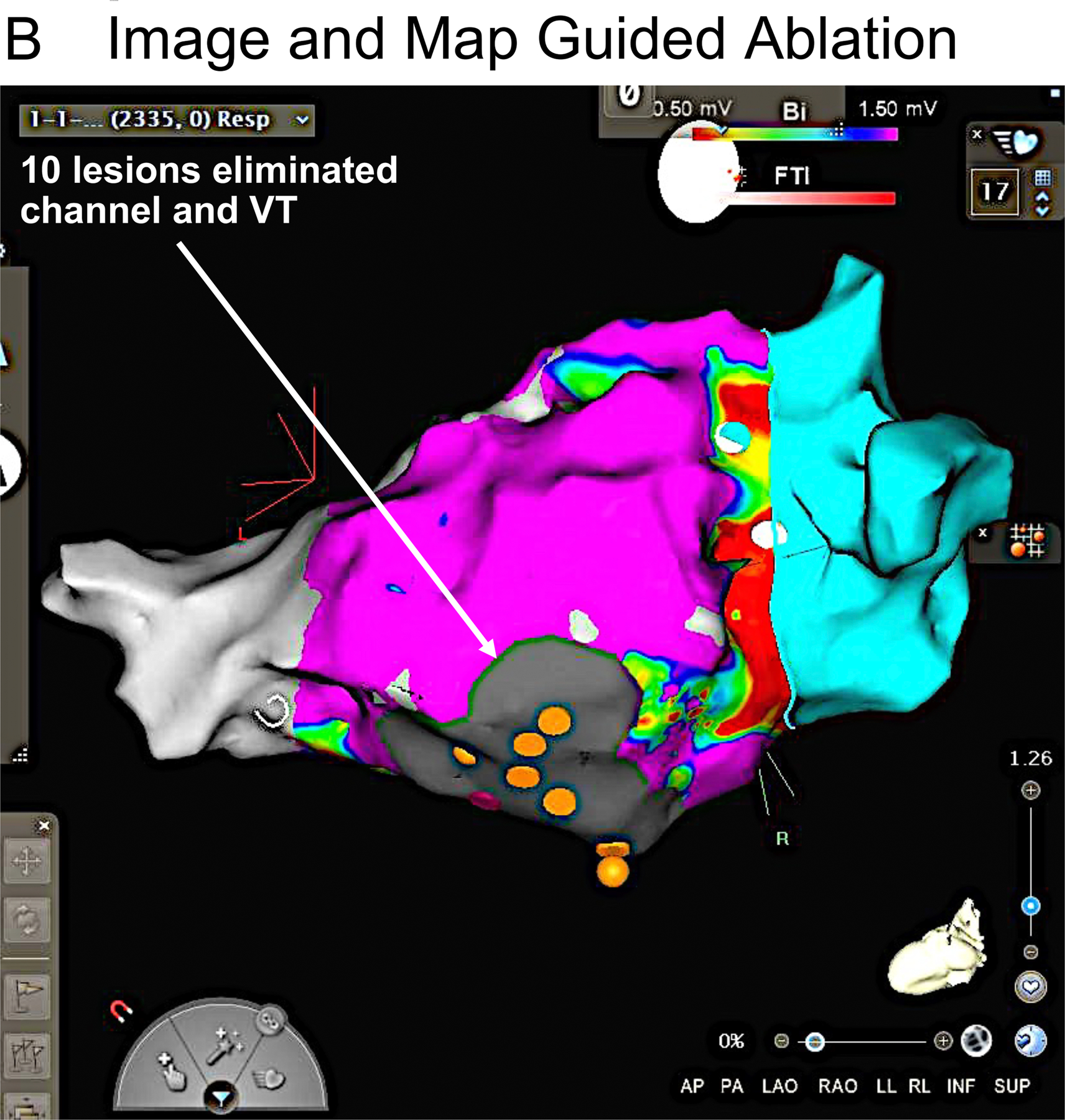
A patient with recurrent VT due to nonischemic cardiomyopathy had 4 prior ablation procedures, with the last procedure using both endocardial and epicardial approaches. Panel A shows a pre-procedural CT registered with an electroanatomic endocardial activation map of VT. In the CT, green areas indicate thin regions of likely scar whose borders define a potential intramural channel (white rectangle). The activation map of VT (red earliest) shows an area of focal endocardial break out (earliest ventricular activation) overlying this channel. Within this region several areas of fractionated activity were present. The orange markers are ablation lesion sites. Following ablation, VT was no longer inducible. B. Endocardial voltage map suggested a large region of scar. Needle lesion sites are identified by the orange markers. Purple indicates electrogram amplitude > 1.5 mV.
Figure 4.
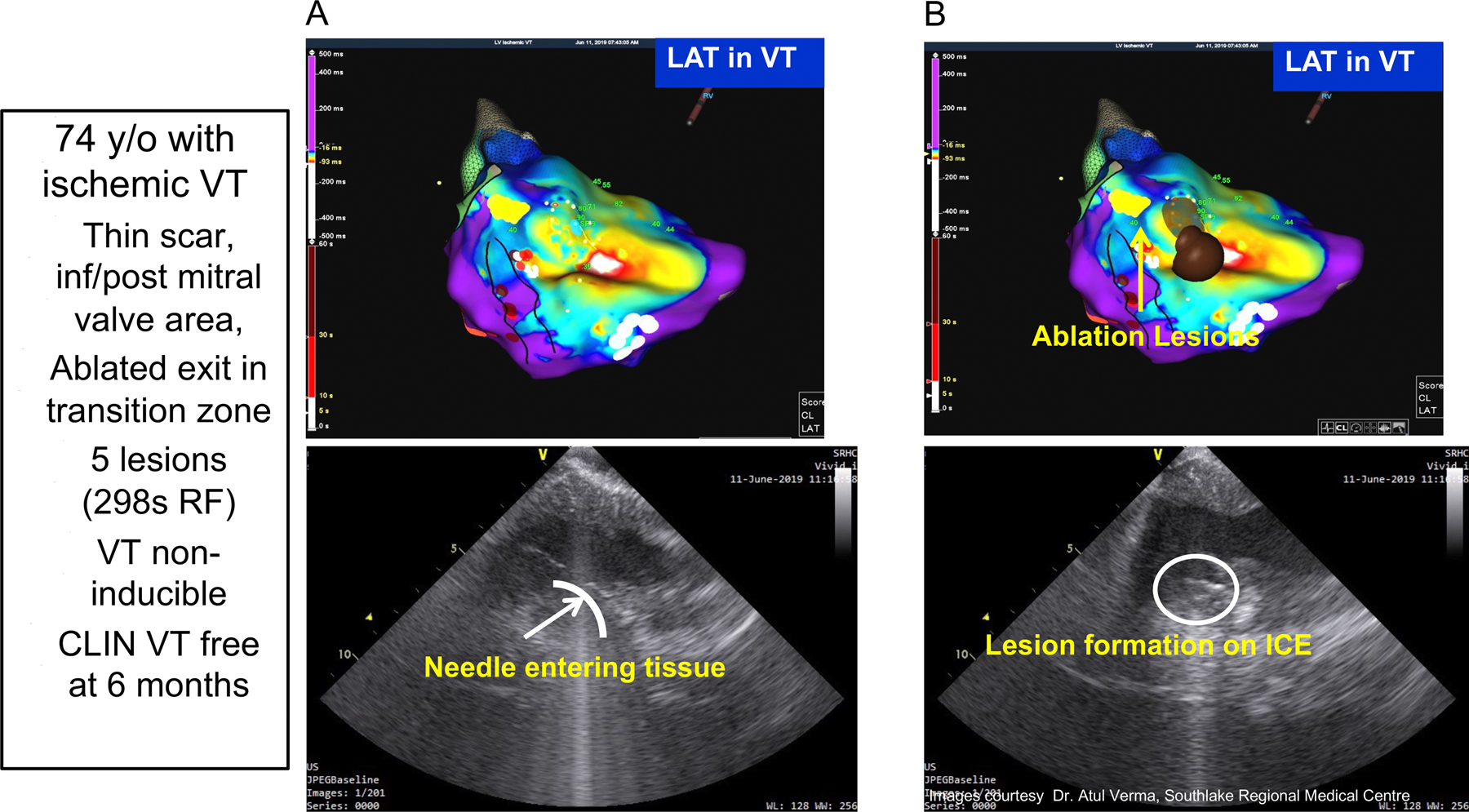
Mapping and Intracardiac Ultrasound Imaging from a patient with prior infero-posterior myocardial infarction and recurrent VT that had failed to be controlled by prior ablation procedures. Panel A shows the activation map of the targeted VT. Panel B shows the site of ablative lesions. Panel C shows a pre-ablation ICE image of the catheter tip/issue positioning and the inserted needle into the underlying myocardium. Panel D show the evolving ablation lesion indicated by the increasing contrast and intensity of the local tissue seen on ICE. Five needle ablation lesions were applied at the endocardial break out region and abolished this inducible VT, which remained absent during 6 month follow-up.
Ablation
Subsequent positioning of the ablation catheter is shown by real time ultrasound in Figure 4. Also seen is the catheter tip and the extended needle. The number of RF deliveries averaged 10 ± 5 per patient. The depth of needle penetration was 4 or 8 mm as shown in Figure 5A. The ablation profile settings are shown in 5B, with locations in 5C. Median total ablation time was 6.5 minutes, with a median saline volume of 91 mls delivered. The SERF catheter was in place an average of 105 minutes and the total procedure time averaged 4 hours and 42 minutes. Standard RF lesions were used in 14 patients.
Figure 5.
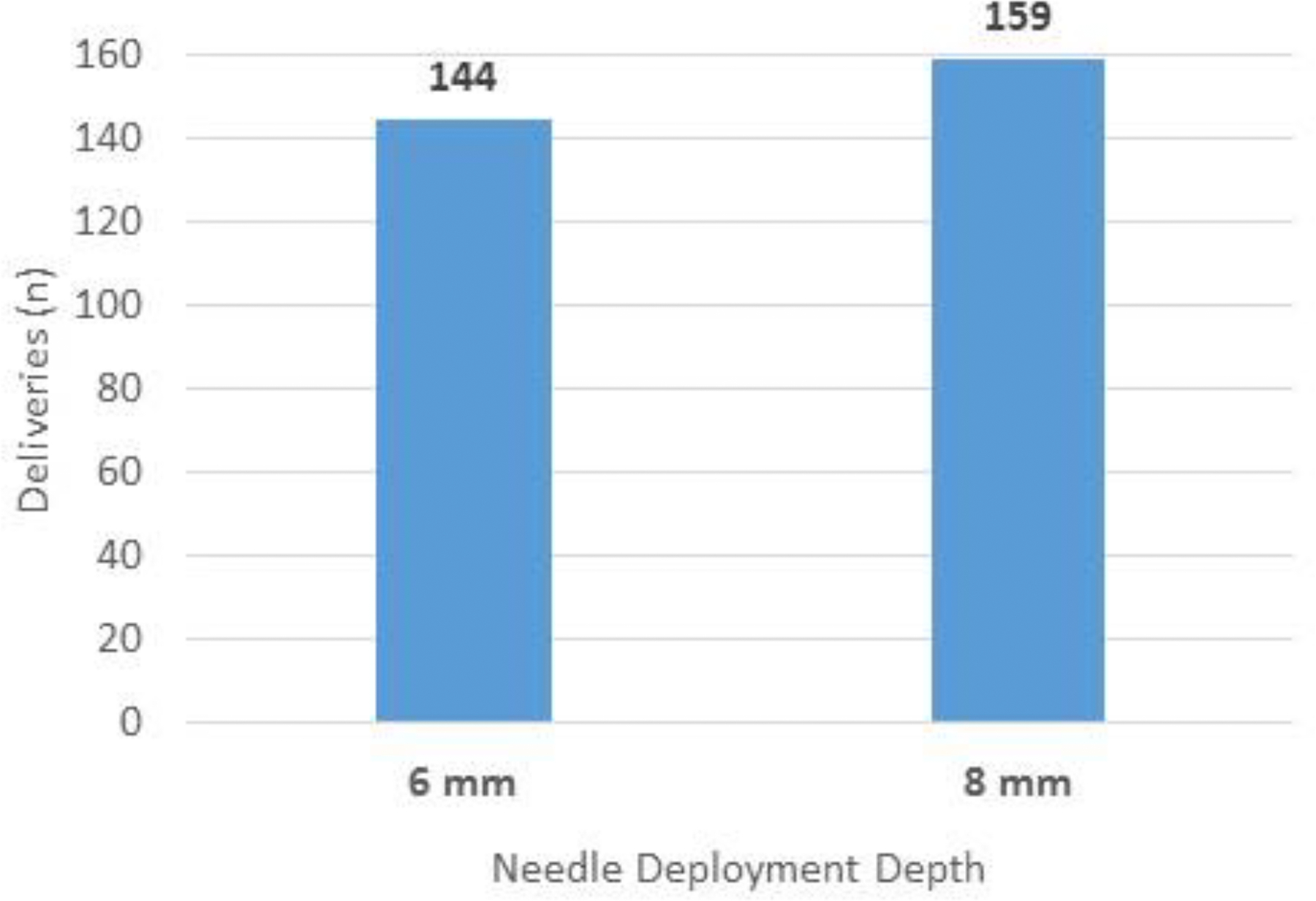
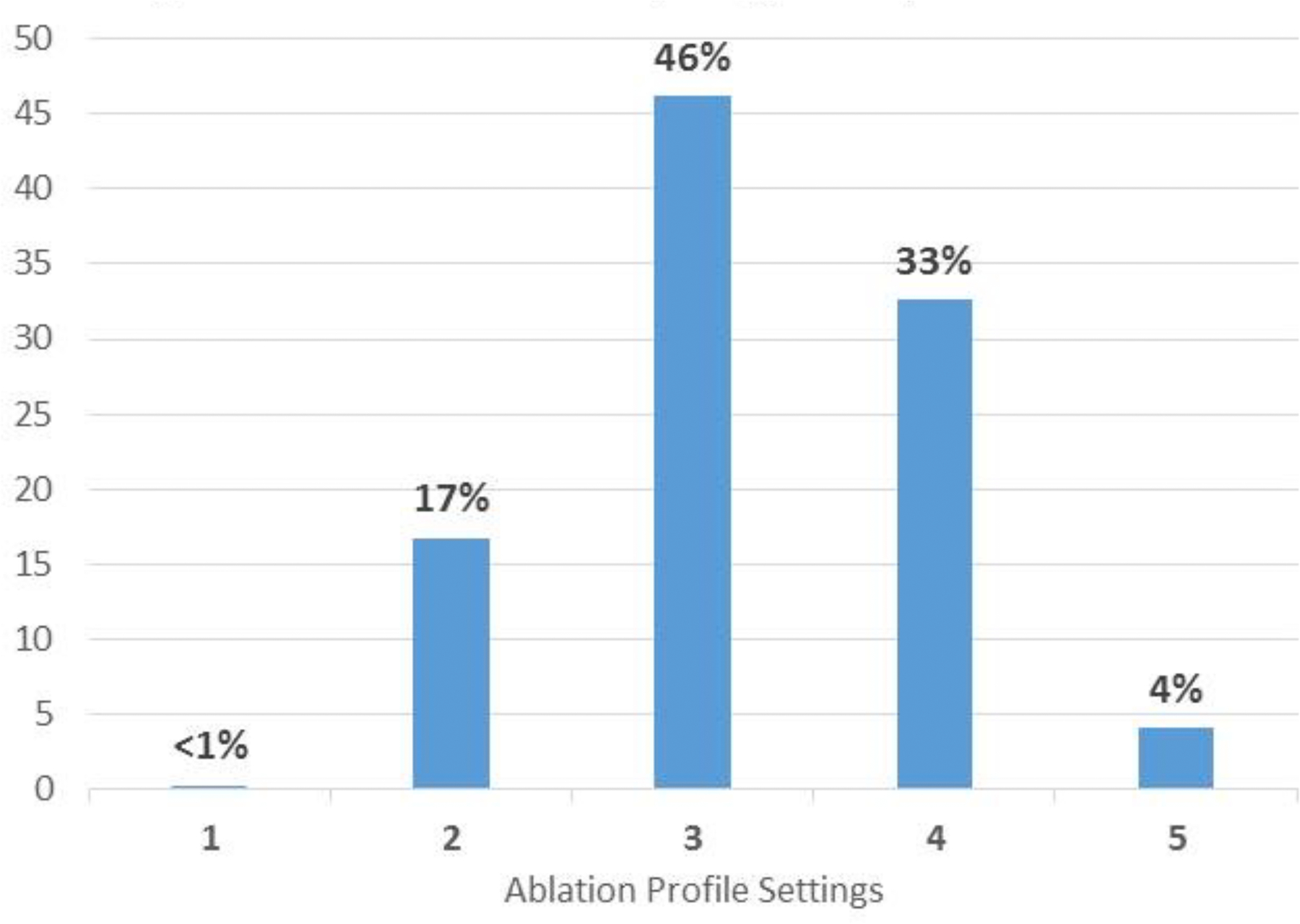
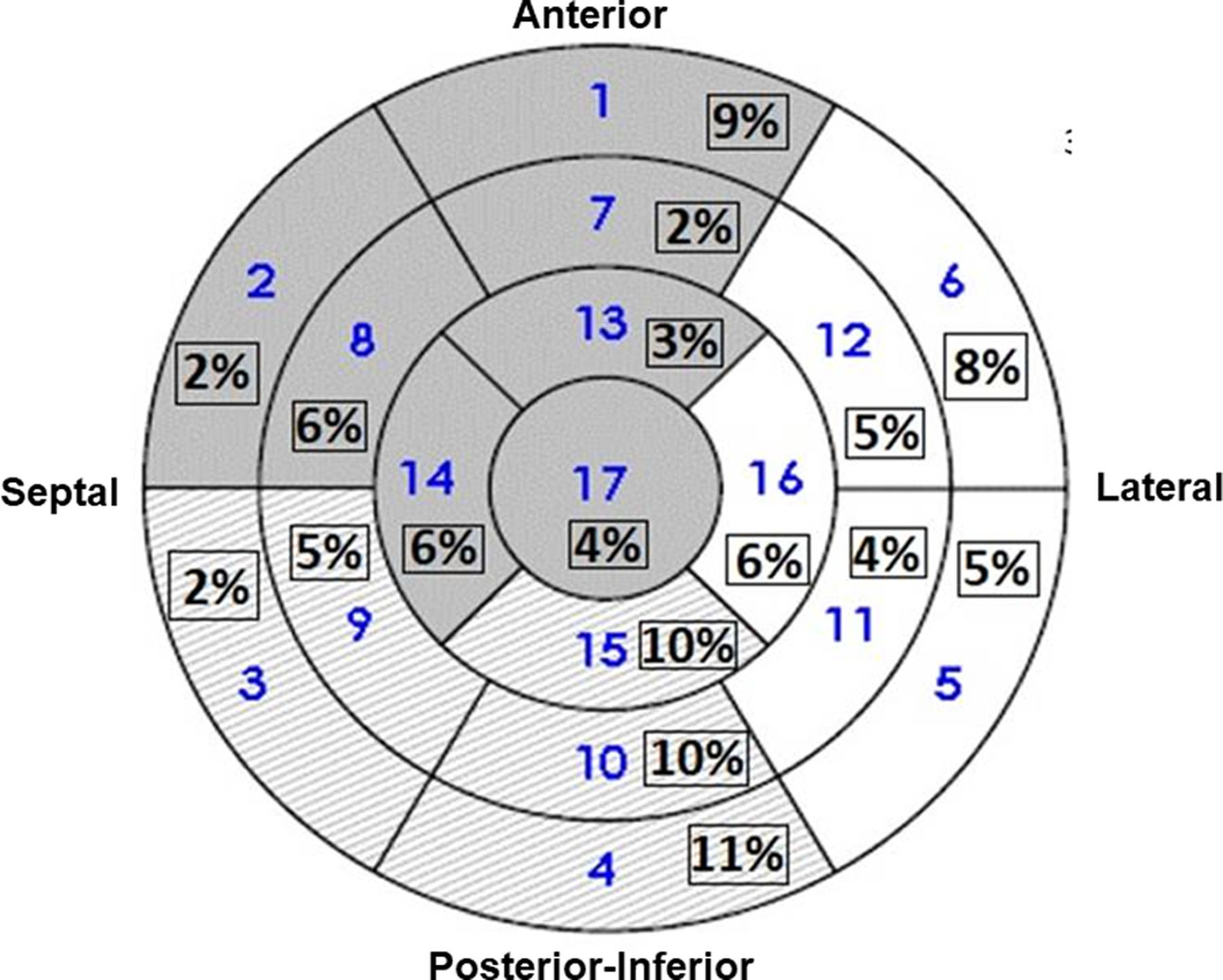
Characteristics of Needle Ablation of VT. A shows the number of energy deliveries at each depth. B shows the number of deliveries at each ablation setting seen in Table 2. C shows an LV polar map indicating the anatomic distribution of energy deliveries in all patients.
Efficacy
Acutely, 97% of testable patients were non-inducible for their clinical VT at the end of the procedure, while no repeat programmed stimulation was performed in one patient due to reduced BP. Median follow-up time was 5 months and the impact of ablation of VT is shown in Figure 6. Prior to needle ablation, these patients required up to 720 shocks or ATPs to treat recurrent VT, as shown in Figure 6B. At 6 months follow up after the procedure, recurrent VT occurred in 50% of patients with 78% of patients remaining on an antiarrhythmic drug at the end of follow up. During the 6 months before the needle ablation, there were 33 ± 81 VT episodes and 18 ± 45 after ablation [p = .0003]. 18 patients receiving shocks before and 9 after (p = .029) [Fig. 6]. Of these patients, 2.0 ± 4.4 (range 0–23) received shocks before and 0.9 ± 2.1 (range 0–10) [p = .0075] after the needle ablation. There was a significant difference in ATPs before 31.5 ± 81.5 (range 0–414) versus 17.1 ± 45.3 (range 0–167) after ablation (p = <.026).
Figure 6.
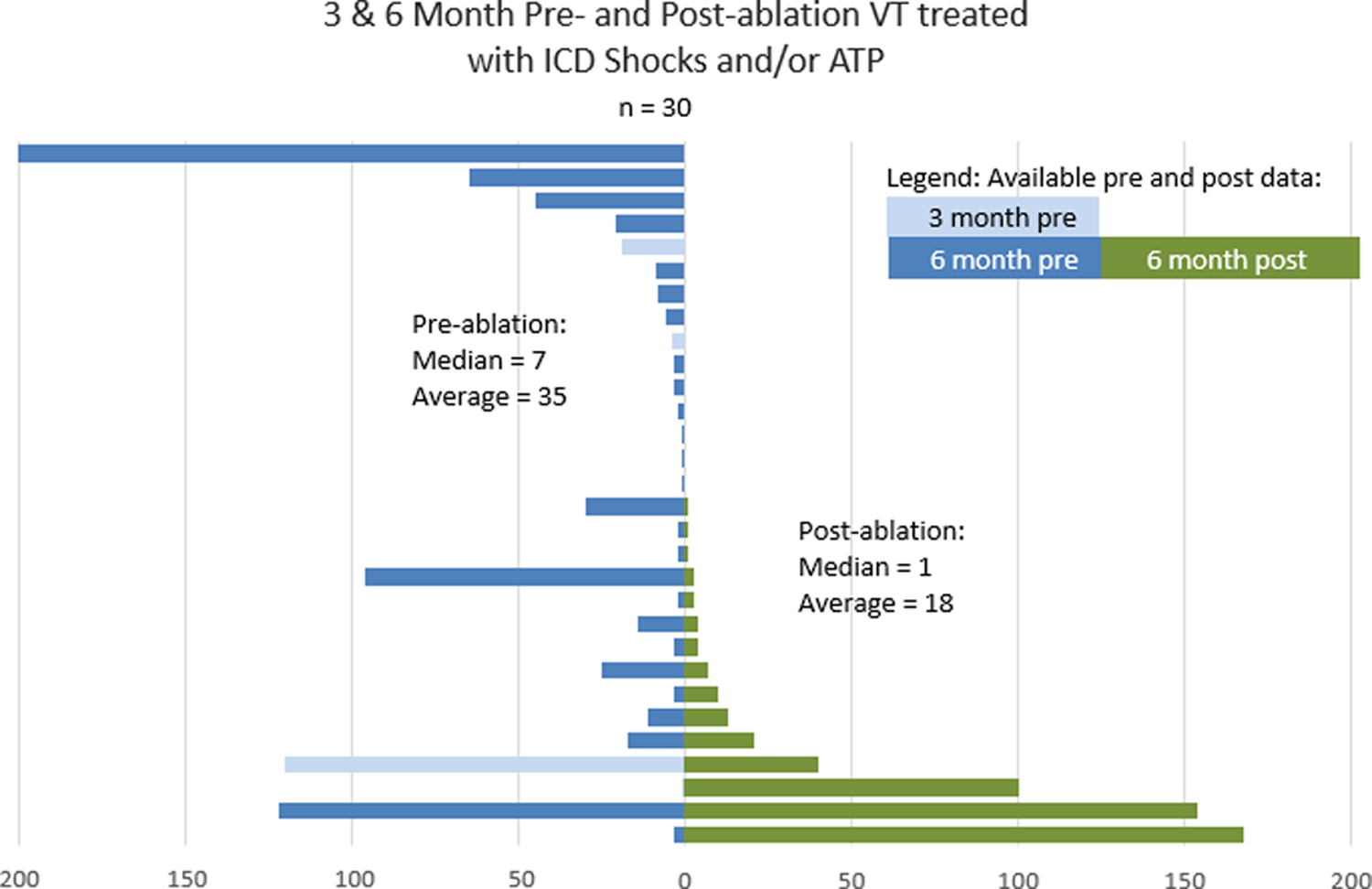
Pre- and Post-Procedure VT Occurrences, and ICD/ ATP Therapies Under Both Circumstances. The blue bars indicate the pre-ablation frequencies of ICD shocks and ATP, while the green bars show the recurrences of the clinical events after ablation. ICD/ATP therapies were totally eliminated in 50% of all patients; there was a more than 90% reduction in 61% and more than 75% reduction in 78% of all patients, respectively. The spread in the standard deviation described on page 11 reflects the observation that several patients had ongoing ATPs, although this was seen in only 3 patients.
Adverse Events
Adverse events are shown in Table 4. Embolic events occurred in 3 patients. One patient suffered a mesenteric embolic infarct that was fatal. Although unproven, this event may have been related to an air embolus via the trans-septal sheath. A second patient reported a visual field cut, which had been present prior to the ablation, but unreported by the patient. There was no evidence of air emboli, and the event was more likely due to a classical embolic CVA event. One patient sustained a minor stroke and recovered without extensive sequelae over 4 months. Another patient developed a femoral pseudo-aneurysm in addition to an increase in a preexisting pericardial effusion from a prior ablation requiring pericardiocentesis of 300cc of blood. Two patients died within 7 days of the ablation, including the patient with the embolic bowel infarction. The other had ischemic cardiomyopathy and developed refractory cardiogenic shock after extensive ablation for multiple VTs, exiting from a large inferolateral to septal infarct scar. Autopsy showed extensive intramural bleeding through the infarct region dissecting into adjacent myocardium. Seven patients required recurrent hospitalization for VT. Overall, six percent of patients had serious adverse events (Table 4), probably or definitely related to the device, all within 30 days of the ablation, while serious adverse events probably or definitely related to the device after 30 days were not observed. Of the serious adverse events possibly related to the device, 83% were seen within the first 14 patients, while only one occurring in the subsequent 18 patients, possibly reflecting a favorable learning curve with the system.
Table 4:
Adverse Events with Needle Electrode Ablation
| Serious Adverse Events | |
|---|---|
| Stroke/TIA | 2 |
| Pericardial effusion requiring drainage | 1 |
| Bowel infarct - Fatal | 1 |
| Cardiogenic shock - Fatal | 1 |
| Femoral pseudoaneurysm | 1 |
| Recurrent VT requiring hospitalization (n=7) | 7 |
| Heart failure requiring hospitalization | 1 |
| Death 6 mos. post ablation: Due to chronic heart failure and sepsis | 1 |
| Acute asthma exacerbation | 1 |
One death occurred after the bowel infarction on day 2 after ablation; one death occurred at day 1 after ablation due to refractory cardiogenic shock and endocardial dissection; one death occurred at 6 months due to heart failure and sepsis.
Discussion
This study presents the initial clinical outcomes of patients undergoing ablation with a novel deployable needle and heated saline-enhanced RF ablation (SERF) system to eliminate VT. It demonstrates that this system can be effective in reducing VT burden in a high-risk group of patients with severe disease and difficult VT that failed to be controlled by prior antiarrhythmic drugs, ICD therapy and conventional catheter ablation. In addition, adverse events were seen to decrease with experience. These results are encouraging, suggesting that SERF ablation has the potential to improve ablation efficacy over conventional RF ablation, and supports continued evaluation of SERF VT ablation in a larger number of patients.
Traditional Ablation for Ventricular Tachycardia
The utility of RF ablation for reducing VT recurrence is well established1–3, 23. VT in patients with ischemic heart disease is eliminated in 50–80% of patients1–3, 5, 13, 17, 23, 38–41. In non-ischemic dilated cardiomyopathy success rates are somewhat lower in the range of 40–75%, 1, 5, 18, 42–45, although they may have higher VT recurrence and all-cause mortality40, 46, 47. Still, the recurrence rate in both ischemic and non-ischemic patients remain significant. VT in patients with structural heart disease is usually due to reentry in regions of scar. Ablation seeks to destroy viable strands of muscle participating in these reentry circuits or channels2, 47–50. Fibrous scar tissue surrounds areas of both surviving and damaged myocytes and creates the reentry substrate, which can be subendocardial, subepicardial, intramural or transmural. Portions of reentry circuits commonly extend deep to the endocardium and are a recognized reason for failure of VT ablation50. RF ablation has been the favored approach, in part because of ease of use27, 29, 30, 34–36, 49. Despite these innovations, radiofrequency (RF) ablation has remained the mainstay of ablation, albeit with disappointing success rates for eliminating VT depending on the number and type of VTs3, 7. A wide range of approaches from DC shock delivery, or particle or photon therapy have been more recently developed with the goal of improving ablation outcomes25, 51–53.
SERF-Mediated VT Ablation and Mechanisms
Traditional RF ablation relies on creation of an area of resistive heating immediately adjacent to the electrode, with conduction of that heat into deeper tissue. Heat transfer by thermal conductivity through tissue is an inefficient process and lesion size is limited by the temperature that can be achieved in the area of resistive heating without producing char or steam pops. The SERF catheter heats saline near the tip of the catheter such that heated saline is injected directly into the myocardium through the side holes in the intramural needle during RF delivery. The saline flow is confined between the endo and epicardial borders. The resulting dramatic increase in “in wall” convective heating can markedly increase lesion size. Preclinical studies have shown creation of controllable lesions that can be large and transmural, extending from the endocardial to the epicardial surface, when desired27, 29, 30, 34–36, 49. The anticipated flow of saline follows in a direction along fiber orientation27, 29, 30, 34–36, 49.
By histologic inspection in animal models, needle lesions are highly uniform, with reliable creation of full-thickness lesions covering scar and ventricular epicardium, without creating potentially pro-arrhythmic irregular border zones. The lesions size ranged from 10×10 to 22×22 mms, depending on the power utilized and the duration of the deliveries, with these data used to develop parameters to predict lesion volumes (Table 2)30, 34. The lesion size in this study was identified acutely on intracardiac ultrasound. In the absence of follow-up CT and MR studies, further definition of lesion size was not possible, thereby excluding the specification of the actual volumes achieved with ablation in these patients. These will be evaluated in subsequent components of this study. Preclinical studies have nevertheless shown an 89% likelihood of eliminating scar channels seen on histology27, 48, 50.
Catheter Deployment
Effective lesion creation requires insertion of the needle into the myocardium. It should be noted that catheter placement for lesion creation could be guided by either biplane fluoroscopy or intracardiac ultrasound. No other ancillary technology was specifically required. Nevertheless, the ability to identify a perpendicular catheter tip/tissue orientation at the endocardial surface is thought to be important to ensure direct delivery of the heated saline into the tissue rather than losing it into the blood pool. With incomplete insertion, some of the side pores of the needle can inject saline into the blood pool, producing microbubbles that are readily evident on ultrasound inspection, and providing a further means of assessing appropriate positioning of the needle. The success of the energy delivery was also likely related to the excellent tissue contact facilitated by insertion of the needle into myocardium.
Identification and Elimination of Intramural VT Substrate
The VT origins in these patients are chronicled in Figure 5c in both the LV septum and free walls, with deeper or sub-epicardial locations, as shown by cumulative needle extensions (Figure 5A). The majority of VT cases were related to ischemic heart disease, thought to be present in 75% of patients dying of SCD1. VT in patients with coronary artery disease can arise in areas of thin tissue in the border zones of the infarction. In some cases, the site of endocardial break out appears to be a reasonable marker for underlying arrhythmia substrate (Figures 3 and 4). Pre-procedure imaging with CT or MRI may also be helpful in identifying intramural channels (Figure 3)7, 11, 54. Intracardiac ultrasound information may also be helpful to suggest a general target region and for monitoring ablation lesion size4, 49, 54–56.
Safety
Although it might be thought that the insertion of a needle into the myocardium would be accompanied by an increased risk of perforation, this preliminary study demonstrated that this occurrence was rare. There was only 1 patient who had a prior pericardial effusion in whom the effusion increased, requiring drainage. A previous trial with a longer needle also observed that needle insertion into the myocardium is relatively safe57.
SERF ablation can produce very large lesions27, 34, 49, raising concern for a risk to viable myocardium outside of the VT substrate scar and worsening ventricular function. One patient in this study, who had extensive substrate and received the largest number of RF applications in multiple areas around an extensive infarct scar did develop fatal cardiogenic shock. Areas of dissecting intramural hemorrhage were seen on autopsy. This led to guidance limiting the total SERF ablation volume to be delivered in one procedure. This study shows, however, that the procedure can be done without excessive injury to residual myocardium outside of the scar or target region. Monitoring catheter placement with intracardiac ultrasound and limiting the number of ablation applications to those required for the targeted VT is prudent. As with standard VT ablation it is important to deliver in regions with pre-existing scar when possible. Use of real-time imaging by intracardiac ultrasound and electroanatomic mapping to delineate regions of scarred vs viable myocardium to correctly identify VT substrate should be critical. There were no steam pops or perforations observed in this study, as is consistent with the findings from preclinical investigations that defined the parameters used. Avoidance of residual myocardium as “an organ at risk” remains critical in reducing risk of the procedure. Furthermore, as the operators gained experience with the device, the occurrence of adverse events decreased.
Three embolic events occurred. Two were middle cerebral artery events. One of two strokes likely occurred prior to ablation, based on symptoms, and was concealed by the patient from investigators for fear it would cancel his procedure. One embolic event (bowel infarct) could have been due to air embolization. It remains unclear whether these were related to the ablation itself, or specifically related to the catheter under study. These emphasize the critical importance of anticoagulation through the course of the procedure and the importance of pre-procedural risk of embolic from the left atrial appendage in patients with AF or the ventricle in patients with poor ventricular function. Overall, the rate of complications is consistent with that seen in other studies of conventional and epicardial RF ablation despite the fact that this was a high-risk population who had already failed prior conventional ablation8, 19. Nevertheless, 7 patients required rehospitalization for recurrent VT.
Limitations
This study has limitations by the nature of its feasibility trial design. Subsequent studies of a greater number of patients will be needed to further establish longer term efficacy and safety. The study did not provide data on the correlation of actual lesion sizes to those predicted from preclinical studies as post-procedural CT or MRI scans were not required. While the lesions were seen acutely on intracardiac ultrasound, the final lesion size can only be inferred from prior animal studies27, 29, 35–37, 49.
In 14 cases, endocardial ablation with a standard catheter was also performed, often when additional VTs were encountered that were not felt to require deep ablation lesions. This endocardial ablation could also have had an impact on outcome. Of note, it is possible to have shallow ablation requiring a limited needle depth. Over time, however, the use of other non-needle catheters decreased as investigators gained familiarity with lesion titration with the SERF system.
The patients in this study were limited to those who had already failed a prior ablation. It is unclear whether the efficacy or safety would be different in patients receiving a first-time SERF ablation. All of the patients also had ischemic or non-ischemic cardiomyopathy. It is unknown if this approach would be equally useful in patients with focal intramural arrhythmias in the absence of structural heart disease, or other VT etiologies and less severe ventricular dysfunction. Further study is required to define the optimal means to select endocardial deployment sites for ablation of intramural VT substrate. Finally, the needle electrode studies were performed by highly skilled investigators. It therefore remains unclear, if those clinicians undertaking the original pre-needle ablations, before the ablations described here, would have had equivalent outcomes as seen by the experienced investigators in this needle ablation study, were it performed first.
We targeted VTs designated as clinically relevant by the investigator as the preferred approach. Limitations in defining clinical VTs are well recognized in patients with ICDs that generally terminate the arrhythmia before an ECG can be obtained2. Finally, although the ablation of all inducible VTs in this population might be considered desirable, and might be associated with a lower risk of recurrence than in other studies40, it is difficult to predict longer-term outcome. The extent to which recurrences are consistent with previously identified clinical VTs versus new or previously identified non-clinical VTs is difficult to assess, partially given the limitations of VT characterization from ICD electrograms. Long-term studies with better 12-lead detectors will be required to clarify the utility of this surrogate endpoint for completely predicting late outcome. Non-inducibility is not necessarily the only reliable predictor of long-term outcome, particularly given the inability to parse out what VT is present upon recurrence based solely on device interrogation.
Conclusion
This study demonstrates effective performance of a novel catheter ablation method in a population refractory to previous, conventional catheter ablation. The targeted inducible VT was eliminated acutely in nearly all patients and during follow-up VT was eliminated in more than 50% of patients with substantial reduction in VT burden in the remaining patients. These initial efficacy data are beyond that anticipated from a further standard RF ablation. This method may obviate the need for epicardial ablation and accompanying risk. To clarify this, larger studies will nevertheless be required.
What is Known:
Ventricular tachycardia is common in patients with coronary artery and idiopathic myocardium disease
The ability to successfully eliminate arrhythmia sites is difficult depending on their location
Sometimes this is because the actual arrhythmia site is internal at the middle or outside of the heart muscle
What the Study Adds:
The needle electrode can be inserted 6–8mm into tissue
Arrhythmia sites in the mid-myocardium and epicardium are readily ablated
5–20mm large lesions are typically created
Excessive ablation with this system can create heart muscle injury
Sources of Funding:
This trial was Funded in part by: R44HL132746 NIH grant
Nonstandard Abbreviations and Acronyms
- AAD
Anti-arrhythmic drugs
- AE
Adverse events
- ATP
Anti-tachycardia pacing
- BPM
Beats per minute
- CTA
Computed tomography (angiographic delayed enhancement)
- ECG
Electrocardiogram
- EF
Ejection fraction
- ICD
Implantable cardioverter defibrillator
- LV
Left ventricular
- MI
Myocardial infarction
- MRS
Modified Rankin Scale
- NICM
Non-ischemic cardiomyopathy
- NYHA
New York Heart Association
- RF
Radiofrequency
- RFA
Radiofrequency catheter ablation
- SA
Sinoatrial
- SAE
Serious adverse event
- SHD
Structural hear disease
- STEMI
ST elevation myocardial infarction
- VF
Ventricular fibrillation
- VT
Ventricular tachycardia
Footnotes
Clinical Trial Registration: ClinicalTrials.gov; Unique Identifiers: NCT03628534 & NCT02994446
Disclosure: Douglas Packer in the past 12 months has provided consulting services for Abbott $0, AtriFix $0, Biosense Webster $0, Inc., Biotronik <$5000, Boston Scientific $0, CardioFocus $0, Johnson & Johnson $0, MediaSphere Medical, LLC<$5000, Medtronic $0, St. Jude Medical $0, and Siemens $0, SigNum Preemptive Healthcare, Inc.$0, Spectrum Dynamics $0, and Thermedical $0. Douglas Packer received research funding from NIH/NHLBI during the conduct of this study, as well as Abbott, Biosense Webster, Boston Scientific/EPT, CardioInsight, CardioFocus, Endosense, German Heart Foundation, Medtronic, Robertson Foundation, St. Jude Medical, Siemens and Thermedical. Mayo Clinic and Dr. Richard Robb have a financial interest in Analyze-AVW technology that may have been used to analyze some of the heart images in this research. In accordance with the Bayh-Dole Act, this technology has been licensed to commercial entities, and both Mayo Clinic, Dr. Robb and Dr. Packer have received royalties greater than $10,000, the federal threshold for significant financial interest. In addition, Mayo Clinic holds an equity position in the company to which the AVW technology has been licensed. Dr. Packer and Mayo Clinic jointly have equity in a privately held company, External Beam Ablation Medical Devices. This is not related to this study. Atul Verma reports grants from Bayer, Biosense Webster, Biotronik and Medtronic. He serves on advisory boards for Biosense Webster, Bayer, Medtronic, Thermedical, Volta Medical, Ablacon and Adagio Medical. He has received speaking fees from Bayer and Servier. William Stevenson reports speaking Honoria from Biosense Webseter, Abbott, Medtronic, Biotronik, and Boston Scientific. He holds a patent for irrigated needle ablation ($0) that is consigned to Brigham Hospital. Suraj Kapa reports grants from Abbott, Boston Scientific, and Toray. He serves on advisory boards for Abbott, Boston Scientific, Pfizer, and Biosig. He also serves as a consultant for Affera, Boston Scientific, Abbott, and Biosig. He holds patents on electroporation technology ($0). David Wilber reports lecture fees (Biotronik, Medtronic, Biosense Webster); research (Abbott, Boston Scientific, Biosense Webster, Thermedical); consultant (ACC Foundation, Biosense Webster, Thermedical). All of these are minor. Katia Dyrda reports, in the past 12 months, speaking Honoraria from Biosense Webster, Medtronic, Bayer, Servier, BMS-Pfizer. She serves as a consultant for Thermedical. Isabelle Nault reports, in the past 12 months, Speaker honoraria: Servier, BMS Pfizer, Medtronic, Biosense Webster. Consultant honoraria: Servier, Bayer, BMS-Pfizer, Novartis. Michael Curley reports grants from the NIH/NHLBI for the conduct of the study. Michael Curley holds shares in and is a founder of Thermedical, Inc. Arvindh Kanagasundram reports, in the past 12 months, Speaking honoraria: Johnson and Johnson; Janssen Pharmaceuticals. Ammar M. Killu reports, in the past 12 months, Consulting – Boston Scientific. All others have none.
References:
- 1.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC et al. : 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 2.Aliot E, Stevenson W, Almendral-Garrote J, Bogun F, Calkins H, Delacretaz E, Bella P, Hindricks G, Jais P, Josephson M et al. : EHRA/HRS Expert concensus on catheter ablation of ventricular arrhythmias: Developed in a partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Europace 2009,11:771–817. [DOI] [PubMed] [Google Scholar]
- 3.Bunch T, Weiss J, Crandall B, Day J, May H, Bair T, Osborn J, C M, Fischer A, Brunner J et al. : Patients treated with catheter ablation for ventricular tachycardia after an ICD shock have lower long-term rates of death and heart failure hospitalization than do patients treated with medical management only. Heart Rhythm 2014;11:533–540. [DOI] [PubMed] [Google Scholar]
- 4.Garcia F, Bazan V, Zado E, Ren J, Marchlinski F: Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2009;120:366–375. [DOI] [PubMed] [Google Scholar]
- 5.Natale A, Raviele A, Al-Ahmad A, Alfieri O, Aliot E, Almendral J, Breithardt G, Brugada J, Calkins H, Callans D et al. : Venice Chart International Consensus Document on ventricular tachycardia/ventricular filbrillation ablation. J Cardiovasc Electrophysiol 2010;21:339–379. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H, Epstein A, Packer D, Arria A, Hummel J, Gilligan D, Trusso J, Carlson M, Luceri R, Kopelman H et al. : Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF multicenter investigators group. J Am Coll Cardiol 2000;35:1905–1914. [DOI] [PubMed] [Google Scholar]
- 7.Jais P, Maury P, Khairy P, Sacher F, Nault I, Komatsu Y, Hocini M, Forclaz A, Jadidi A, Weerasooryia R et al. : Elimination of local abnormal ventricular activities: A new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation 2012;125:2184–2196. [DOI] [PubMed] [Google Scholar]
- 8.Killu AM, Friedman PA, Mulpuru SK, Munger TM, Packer DL, Asirvatham SJ: Atypical complications encountered with epicardial electrophysiological procedures. Heart Rhythm 2013;10:1613–1621. [DOI] [PubMed] [Google Scholar]
- 9.Killu AM, Mulpuru SK, Al-Hijji MA, Sugrue A, Munger TM, Hodge DO, McLeod CJ, Packer DL, Kapa S, Asirvatham SJ et al. : Outcomes of Combined Endocardial-Epicardial Ablation Compared With Endocardial Ablation Alone in Patients Who Undergo Epicardial Access. Am J Cardiol 2016;118:842–848. [DOI] [PubMed] [Google Scholar]
- 10.Lim H, Sacher F, Cochet H, Berte B, Yamashita S, Mahida S, Zellerhoff S, Komatsu Y, Denis A, Derval N et al. : Safety and prevention of complications during percutaneous epicardial access for the ablation of cardiac arrhythmias. Heart Rhythm 2014;11:1658–1665. [DOI] [PubMed] [Google Scholar]
- 11.Sacher F, Roberts-Thomson K, Maury P, Tedrow U, Nault I, Steven D, Hocini M, Koplan B, Leroux L, Derval N et al. : Epicardial ventricular tachycardia ablation: a multicenter safety study. J Am Coll Cardiol 2010;55:2366–2372. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson WG, Tedrow UB, Reddy V, AbdelWahab A, Dukkipati S, John RM, Fujii A, Schaeffer B, Tanigawa S, Elsokkari I et al. : Infusion Needle Radiofrequency Ablation for Treatment of Refractory Ventricular Arrhythmias. J Am Coll Cardiol 2019;73:1413–1425. [DOI] [PubMed] [Google Scholar]
- 13.Tanner H, Hindricks G, Volkmer M, Furniss S, Kuhlkamp V, Lacroix D, DE Chillou C, Almendral J, Caponi D, Kuck K et al. : Catheter ablation of recurrent scar-related ventricular tachycardia using electroanatomical mapping and irrigated ablation technology: Results of the prospective multicenter Euro-VT study. J Cardiovasc Electrophysiol 2010;21:47–53. [DOI] [PubMed] [Google Scholar]
- 14.Vergara P, Trevisi N, Ricco A, Petracca F, Baratto F, Cireddu M, Bisceglia C, Maccabelli G, Della Bella P: Late potential ablation as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol 2012;23:621–627. [DOI] [PubMed] [Google Scholar]
- 15.Sarkozy A, Tokuda M, Tedrow UB, Sieria J, Michaud GF, Couper GS, John R, Stevenson WG: Epicardial ablation of ventricular tachycardia in ischemic heart disease. Circ Arrhythm Electrophysiol 2013;6:1115–1122. [DOI] [PubMed] [Google Scholar]
- 16.Tung R, Michowitz Y, Yu R, Mathuria N, Vaseghi M, Buch E, Bradfield J, Fujimura O, Gima J, Discepolo W et al. : Epicardial ablation of ventricular tachycardia: an institutional experience of safety and efficacy. Heart Rhythm 2013;10:490–498. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson W, Wilber D, Natale A, Jackman W, Marchlinski F, Talbert T, Gonzalez M, Worley S, Daoud E, Hwang C et al. : Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation 2008;118:2773–2782. [DOI] [PubMed] [Google Scholar]
- 18.Dinov B, Fiedler L, Schonbauer R, Bollmann A, Rolf S, Piorkowski C, Hindricks G, Arya A: Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Center of Leipzig VT (HELP-VT) study. Circulation 2014;129:728–736. [DOI] [PubMed] [Google Scholar]
- 19.Di Biase L, Burkhardt J, Lakkireddy D, Carbucicchio C, Mohanty S, Mohanty P, Trivedi C, Santangeli P, Bai R, Forleo G et al. : Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol 2015;66:2872–2882. [DOI] [PubMed] [Google Scholar]
- 20.Sapp J, Wells G, Parkash R, Stevenson W, Blier L, Sarrazin J, Thibault B, Rivard L, Gula L, Leong-Sit P et al. : Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med 2016;375:111–121. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson W, Khan H, Sager P, Saxon L, Middlekauff H, Natterson P, Wiener I: Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation 1993;88(4 pt 1):1647–1670. [DOI] [PubMed] [Google Scholar]
- 22.Bala R, Ren J, Hutchinson M, Desjardins B, Tschabrunn C, Gerstenfeld E, Deo R, Dixit S, Garcia F, Cooper J et al. : Assessing epicardial substrate using intracardiac echocardiographyduring VT ablation. Circ Arrhythm Electrophysiol 2011;4:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury P, Maccabelli G, Vergara P, Baratto F, Berruezo A et al. : Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol 2011;4:653–659. [DOI] [PubMed] [Google Scholar]
- 24.Tung R, Raiman M, Liao H, Zhan X, Chung FP, Nagel R, Hu H, Jian J, Shatz DY, Besser SA et al. : Simultaneous Endocardial and Epicardial Delineation of 3D Reentrant Ventricular Tachycardia. J Am Coll Cardiol 2020;75:884–897. [DOI] [PubMed] [Google Scholar]
- 25.Hartzler GO: Electrode catheter ablation of refractory focal ventricular tachycardia. J Am Coll Cardiol 1983;2:1107–1113. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki A, Lehmann HI, Wang S, Parker K, Rettmann ME, Viker KB, Johnson S, Packer DL: Impact of RF application time and power on lesion volume created by a novel irrigated RF balloon ablation catheter in the canine heart. Circulation 2016;134:A19881. [Google Scholar]
- 27.Henz B, Okumura Y, Johnson S, Miller D, Curley M, Packer D: Deep ablation candidate of intra-scar circuit component: Effect of scar penetrating ablation with a novel, saline irrigated needle catheter. Heart Rhythm 2008;5:S364. [Google Scholar]
- 28.Packer D, Kapa K, Killu A, Wilber D, Stevenson W, Richardson T, Kanagasandrum A, Verma A, Dyrda K, Rivard L: Ablation of refractory VT with a hHeated-saline (SERF) needle catheter For enhanced radiofrequency ablation for creation of transmural lesions: A First-in-Man feasibility study 2020.
- 29.Suzuki A, Deisher AJ, Rettmann ME, Lehmann HI, Hohmann S, Wang S, Konishi H, Kruse JJ, Cusma JT, Newman LK et al. : Catheter-Free Arrhythmia Ablation Using Scanned Proton Beams: Electrophysiologic Outcomes, Biophysics, and Characterization of Lesion Formation in a Porcine Model. Circ Arrhythm Electrophysiol 2020;13:e008838. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki A, Lehmann H, Deisher A, Wang S, Kruse J, Cusma J, Parker K, Rettmann M, Viker K, Packer D: Comparison of newly proposed scanned proton beam catheter-free ablation vs. x-ray/photon beams for creation of atrioventricular node block in a porcine model. Heart Rhythm 2017;14(5 suppl):S5. [Google Scholar]
- 31.Hohmann S, Deisher A, Suzuki A, Konishi H, Kruse J, Lehmann H, Rettmann M, Parker K, Newman L, Herman M et al. : Targeting ischemic myocardial scar with pencil-beam scanning proton therapy in a porcine model. Heart Rhythm 2018;15:S3. [Google Scholar]
- 32.Hohmann S, Deisher A, Suzuki A, Konishi H, Rettmann ME, Lehmann HI, Kruse J, Parker KD, Newman LK, Herman MG et al. : Safety of catheter-free VT ablation: Dose-dependent LVEF changes after proton beam therapy of the LV in a porcine model. In: European Society of Cardiology. 2018; 2018.
- 33.Spartalis M, Spartalis E, Tzatzaki E, Tsilimigras DI, Moris D, Kontogiannis C, Livanis E, Iliopoulos DC, Voudris V, Theodorakis GN: Novel approaches for the treatment of ventricular tachycardia. World J Cardiol 2018;10:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki A, Deisher A, Rettie A, Lehmann H, Wang S, Kruse J, Cusma J, Parker K, Herman M, Packer D: Preliminary investigation of time-course MRI assessment for lesion maturation created by a newly proposed scanned proton beam catheter-free ablation in the porcine left ventricle. Circulation 2017;136:A15876. [Google Scholar]
- 35.Suzuki A, Lehmann HI, Wang S, Parker K, Rettmann M, Viker KB, Johnson S, Packer DL: Characterization of lesions created by a heated, saline irrigated needle-tip catheter in the normal and infarcted canine heart. Circulation 2016;134:A19810. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki A, Lehmann HI, Wang S, Parker K, Rettmann M, Viker KB, Johnson S, Packer DL: Impact of RF application time and power on lesion volume created by a novel irrigated RF balloon ablation catheter in the canine heart. Circulation 2016;134:A19881. [Google Scholar]
- 37.Takami M, Lehmann HI, Parker K, Welker K, Johnson S, Packer D: Effect of left atrial ablation process and strategy on microemboli formation during irrigated RF catheter ablation in an in vivo model. Circulation Arrhythm and Electrophysiol 2016;9(e003226). [DOI] [PubMed] [Google Scholar]
- 38.Hsia H, Callans D, Marchlinski F: Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation 2003;108:704–710. [DOI] [PubMed] [Google Scholar]
- 39.Marchlinski F, Haffajee C, Beshai J, Dickfeld T, Gonzalez M, Hsia H, Schuger C, Beckman K, Bogun F, Pollak S et al. : Long-term success of irrigated radiofrequency catheter ablation of sustained ventricular tachycardia: Post-approval THERMOCOOL VT trial. J Am Coll Cardiol 2016;67:674–683. [DOI] [PubMed] [Google Scholar]
- 40.Ghanbari H, Baser K, Yokokawa M, Stevenson W, Della Bella P, Vergara P, Deneke T, Kuck K, Kottkamp H, Fei S et al. : Noninducibility in postinfarction ventricular tachycardia as an end point for ventricular tachycardia ablation and its effects on outcomes: a meta-analysis. Circ Arrhythm Electrophysiol 2014;7:677–683. [DOI] [PubMed] [Google Scholar]
- 41.Kosimdou I, Inada K, Seiler J, Koplan B, Stevenson W, Tedrow U: Role of repeat procedures for catheter ablation of postinfarction ventricular tachycardia. Heart Rhythm 2011;8:1516–1522. [DOI] [PubMed] [Google Scholar]
- 42.Dinov B, Arya A, Schratter A, Fiedler L, Sommer P, Bollmann A, Rolf S, Piorkowski C, Hindricks G: Catheter ablation of ventricular tachycardia and mortality in patients with nonischemic dilated cardiomyopathy: can noninducibility after ablation be a predictor for reduced mortality? Circ Arrhythm Electrophysiol 2015;8:598–605. [DOI] [PubMed] [Google Scholar]
- 43.Tung R, Vaseghi M, Frankel D, Vergara P, Di Biase L, Nagashima K, Yu R, Vangala S, Tseng C, Choi E et al. : Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An international VT ablation center collaborative group study. Heart Rhythm 2015;12:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Romero J, Mehta N, Fujii A, Kapur S, Baldinger S, Barbhaiya C, Koplan B, John R, Epstein L et al. : Long-term outcomes after catheter ablation of ventricular tachycardia in patients with and without structural heart disease. Heart Rhythm 2016;13:1957–1963. [DOI] [PubMed] [Google Scholar]
- 45.Muser D, Santangeli P, Pathak RK, Castro SA, Liang JJ, Magnani S, Hayashi T, Garcia FC, Hutchinson MD, Supple GE et al. : Long-Term Outcomes of Catheter Ablation of Ventricular Tachycardia in Patients With Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol 2016;9(8). [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Baldinger S, Romero J, Fujii A, Mahida S, Tedrow U, Stevenson W: Substrate-based ablation versus ablation guided by activation and entrainment mapping for ventricular tachycardia: a systematic review and meta-analysis. J Cardiovasc Electrophysiol 2016;27:1437–1447. [DOI] [PubMed] [Google Scholar]
- 47.Mallidi J, Nadkarni G, Berger R, Calkins H, Nazarian S: Meta-analysis of catheter ablation as an adjunct to medical therapy for treatment of ventricular tachycardia in patients with structural heart disease. Heart Rhythm 2011;8:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR: Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation 1993;88:915–926. [DOI] [PubMed] [Google Scholar]
- 49.Dickow J, Suzuki A, Henz B, Madhavan M, Lehmann HI, Wang S, Parker K, Monahan K, Rettmann ME, Curley M et al. : Characterization of lesions created by a heated, saline irrigated needle-tip catheter in the normal and infarcted canine heart. Circ Arrhythm Electrophysiol 2020: Accepted for publication. [DOI] [PubMed]
- 50.Hsia HH, Lin D, Sauer WH, Callans DJ, Marchlinski FE: Anatomic characterization of endocardial substrate for hemodynamically stable reentrant ventricular tachycardia: identification of endocardial conducting channels. Heart Rhythm 2006;3:503–512. [DOI] [PubMed] [Google Scholar]
- 51.Cuculich PS, Robinson CG: Noninvasive Ablation of Ventricular Tachycardia. N Engl J Med 2018;378:1651–1652. [DOI] [PubMed] [Google Scholar]
- 52.Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, Faddis M, Gleva M, Noheria A, Smith TW et al. : Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med 2017;377:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehmann H, Graeff C, Constantinescu A, Deisher A, Takami M, Lugenbiel P, Richter D, Prall M, Kaderka R, Eichhorn A et al. : Comparison of photon versus scanned carbon particle irradiation for catheter-free arrhythmia ablation in a porcine model. Heart Rhythm 2016;13:S213. [Google Scholar]
- 54.Esposito A, Palmisano A, Antunes S, Maccabelli G, Colantoni C, Rancoita PMV, Baratto F, Di Serio C, Rizzo G, De Cobelli F et al. : Cardiac CT With Delayed Enhancement in the Characterization of Ventricular Tachycardia Structural Substrate: Relationship Between CT-Segmented Scar and Electro-Anatomic Mapping. JACC Cardiovasc Imaging 2016;9:822–832. [DOI] [PubMed] [Google Scholar]
- 55.Berruezo A, Fernandez-Armenta J, Andreu D, Penela D, Herczku C, Evertz R, Cipolletta L, Acosta J, Borras R, Arbelo E et al. : Scar dechanneling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythm Electrophysiol 2015;8:326–336. [DOI] [PubMed] [Google Scholar]
- 56.Kottkamp H, Wetzel U, Schirdewahn P, Dorszewski A, Gerds-Li J, Carbucicchio C, Kobza R, Hindricks G: Catheter ablation of ventricular tachycardia in remote myocardial infarction: Substrate description guiding placement of individual linear lesions targeting noninducibility J Cardiovasc Electrophysiol 2003;14:675–681. [DOI] [PubMed] [Google Scholar]
- 57.Sapp J, Beeckler C, Pike R, Parkash R, Gray C, Zeppenfeld K, Kuriachan V, Stevenson W: Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia. Circulation 2013;128:2289–2295. [DOI] [PubMed] [Google Scholar]


