Abstract
Objectives:
There is a paucity of literature evaluating new-onset diabetes mellitus (NODM) following resection of pancreatic cystic lesions (PCLs). We sought to characterize the incidence and risk factors associated with NODM following partial pancreatectomy for PCLs.
Methods:
We utilized the IBM MarketScan Database (2012–2018) to identify all non-diabetic adults who underwent partial pancreatectomy for PCLs. Patients with any other pancreatic disease were excluded. We performed Kaplan-Meier analysis and multivariable Cox proportional hazards regression to define the incidence and risk factors of postoperative NODM.
Results:
Among 311 patients, the overall risk (95% confidence interval) of NODM was 9.1% (6.3–12.9%), 15.1% (11.3–20.2%), and 20.2% (15.3–26.4%) at six, 12 and 24 months, respectively. Multivariable analysis (adjusted hazard ratio, 95% confidence interval) revealed that older age (1.97, 1.04–3.72; 55–64 vs 18–54 years), obesity (2.63, 1.35–5.12), hypertension (1.79, 1.01–3.17), and cardiovascular disease (2.54, 1.02–6.28) were independent predictors of NODM. Rates of NODM were similar following distal pancreatectomy versus pancreaticoduodenectomy.
Conclusions:
Within two years, one in five patients without any other pancreatic disease will develop NODM following partial pancreatectomy for PCLs. Those with advanced age, metabolic syndrome features, and/or cardiovascular disease may benefit from preoperative counselling and intensive postoperative monitoring, education, and treatment for DM.
Keywords: pancreatic cystic lesion, pancreatectomy, diabetes mellitus, population database, intraductal papillary mucinous neoplasm
Introduction
Pancreatogenic diabetes, also described as type 3c diabetes mellitus (DM), refers to DM secondary to a disease of the exocrine pancreas, including acute and chronic pancreatitis, pancreatic cancer, cystic fibrosis, and pancreatectomy.1,2 Regarding pancreatic surgery, pancreaticoduodenectomy (PD) and distal pancreatectomy (DP) can result in DM for a subset of patients, primarily due to loss of islet cell mass; a total pancreatectomy causes obligatory DM. Nearly 3800 elective pancreatectomies are performed each year in the US for a variety of indications.3,4 The incidence and associated risk factors for new onset diabetes mellitus (NODM) following pancreatectomy varies widely, and may be related, in part, to differences in the underlying pancreatic disorder.5–7
Previous single and multi-center studies have noted that the risk of NODM following PD or DP in patients with normal preoperative endocrine function ranges from 18–40%.8–18 Differences in the type and extent of resection, as well as variations in preoperative patient characteristics, may contribute to the observed variability in NODM. In the most recent and largest systematic review, the pooled estimates for NODM were 21% following DP, 16% following PD, and 6% following central pancreatectomy.19 The increased density of insulin-producing β-islet cells in the pancreatic body and tail is believed to contribute to the higher risk of NODM following distal pancreatic resections, however, this is an ongoing area of investigation.20–22
Pancreatic cystic lesions (PCLs) are increasingly diagnosed due to advancements in cross-sectional imaging techniques, detected in 2.5% of all adults using computed tomography and 13.5–19.5% using magnetic resonance imaging.23–25 The current standard of care for PCLs with advanced features (symptomatic, presence of a mural nodule or solid component, main pancreatic duct dilation >5 mm or change in duct diameter with upstream atrophy, size ≥3 cm, rapid increase in size, or high grade dysplasia) is surgical resection for patients with satisfactory operative fitness.26 Patient selection is of particular importance considering the relatively high morbidity (30–40%) of pancreatic surgery and potential perioperative mortality (1–2%).27 The broad biologic behavior, lack of diagnostic tools, difficulty in the accurate diagnosis of cyst type and risk of malignancy, and the risk of complications following pancreatectomy necessitates a multidisciplinary team approach for appropriate management decisions. Despite the growing need for individualized care planning, preoperative counselling and intensified post-operative monitoring, to our knowledge, there are no studies evaluating the incidence or risk factors for NODM following elective pancreatectomy specifically for PCLs. Hence, we conducted a retrospective cohort study using a nationally representative population database to evaluate the incidence and identify risk factors for post-operative NODM among patients undergoing partial pancreatectomy for PCLs.
MATERIALS AND METHODS
Study Design and Data Source
This analysis is a retrospective, longitudinal study of the IBM MarketScan Research Database® from 2012 to 2018. This database is a publicly available population-based dataset. As such, the study was exempt from approval by the Ohio State University Institutional Review Board.
The MarketScan Database is compiled and maintained by Truven Health Analytics® and IBM Watson Health (Armonk, NY). MarketScan is one of the largest collections of claims data from insured employees and their dependents, early retirees, Consolidated Omnibus Budget Reconciliation Act (COBRA) continuers and Medicare-eligible retirees with employer-provided Medicare Supplemental plans in the US. These databases capture individual-level claims data on healthcare utilization, expenditures, diagnoses and pharmaceutical claims at both inpatient and outpatient levels to longitudinally track over 250 million unique, de-identified patients from all 50 states across the continuum of healthcare.28, 29
The database was queried using International Classification of Diseases, Ninth Edition Clinical Modification (ICD-9-CM) diagnostic codes from January, 2012 to September, 2015 and International Classification of Diseases, Tenth Edition Clinical Modification (ICD-10-CM) diagnostic codes from October, 2015 to December, 2018 to define variables of interest (Supplemental Table 1). The transition from ICD-9-CM to ICD-10-CM clinical practice coding in 2015 was replicated in this study with appropriate code conversion. Current Procedural Terminology (CPT) as well as ICD-9/10 Procedural Coding System codes were used to comprehensively capture all medical procedures. Adult patients (age ≥18) with an inpatient or outpatient diagnosis of a PCL who underwent partial pancreatectomy were eligible for inclusion. Rigorous stepwise multi-level exclusion criteria (Fig. 1) were then applied to identify a selective cohort of patients without existing DM prior to surgery. Patients with pregnancy, total pancreatectomy, necrosectomy following acute pancreatitis (AP), islet cell transplantation, prior pancreatic surgery, AP within 6 months prior to surgery, and other causes of pancreatogenic DM (chronic pancreatitis, cystic fibrosis, hemochromatosis, pancreatic cancer) were also excluded. Patients without prior data or sufficient follow up (<1 month after surgery) were further excluded (Supplemental Table 2).
FIGURE 1.
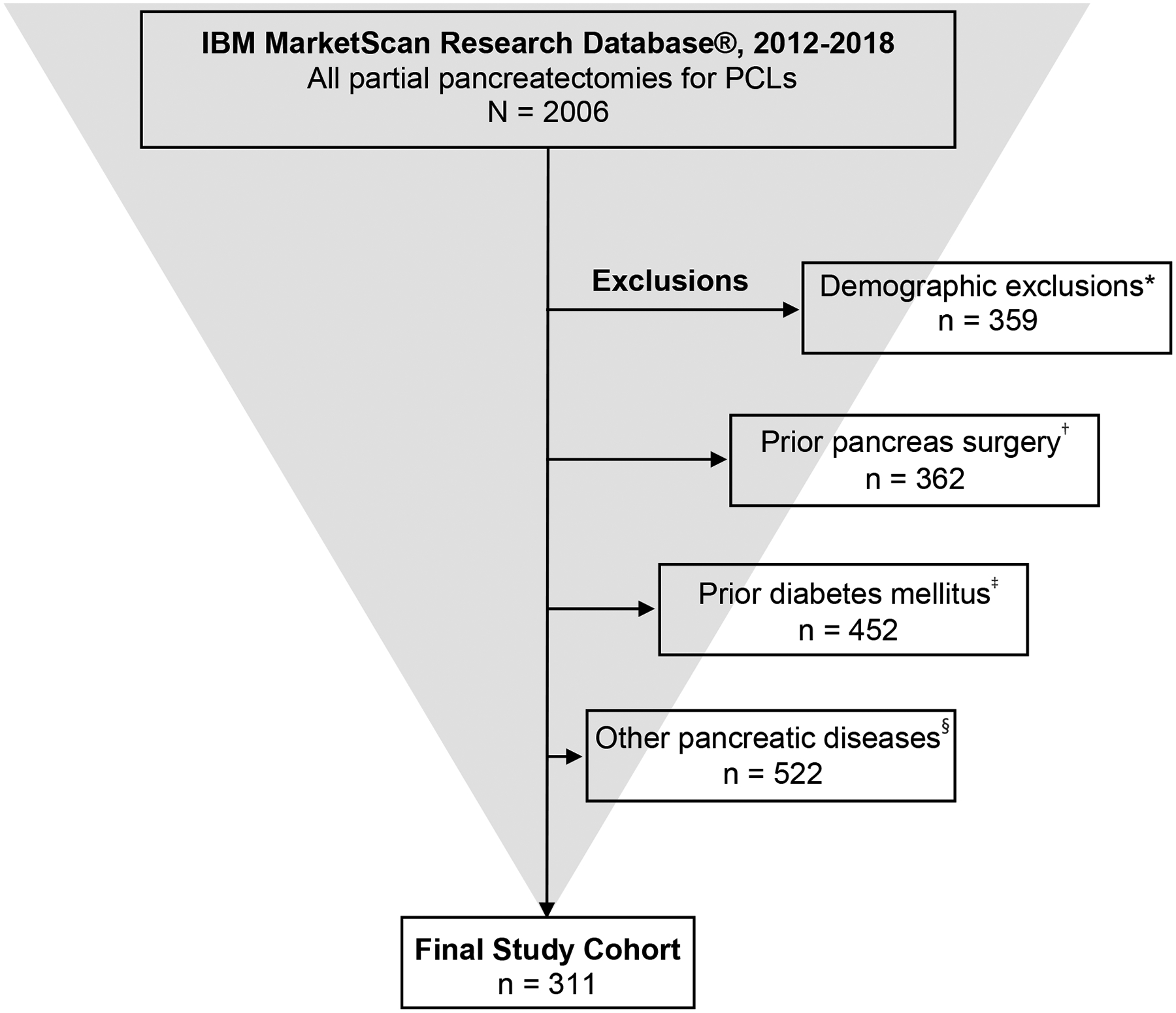
Selection of the final study cohort. *No enrollment information, <18 years of age, <3 months pre-surgery enrollment, <1 month post-surgery enrollment, pregnancy, no data prior to surgery. †Total pancreatectomy, islet cell auto-transplantation, necrosectomy, other prior pancreas surgery. ‡Diabetes mellitus or use of anti-glycemic medications within 12 months prior to surgery. §Cystic fibrosis, hemochromatosis, prior pancreatic enzyme use, acute pancreatitis within 6 months prior to surgery, prior chronic pancreatitis, prior pancreatic cancer. PCL, pancreatic cystic lesion.
The MarketScan Commercial Claims and Encounters Database includes claims data on patients 18–64 years of age with private or employer-based health insurance. Data from Medicare claims are not included in the Commercial Claims and Encounters database. The Medicare Supplemental Database contains data from patients age 65 years and older who have supplemental insurance. The Medicare Supplemental Database contains both Medicare claims and supplemental insurance claims for included patients. Of the 311 patients included in final analysis, 77 (24.7%) were derived from the Medicare supplemental database.
Demographic and Clinical Data
Baseline characteristics included age, sex, demographic region, and comorbidities. Other variables included type of resection (distal, proximal, enucleation), open versus laparoscopic surgery, duration of follow-up available, and Charlson Comorbidity Index.30 Specific ICD and CPT coding for all procedures and variables of interest are noted in Supplemental Table 1. For the purpose of this study, proximal pancreatectomy with duodenectomy (ie, Whipple procedure) was not differentiated from proximal pancreatectomy without duodenectomy.
Statistical Analysis
Demographics were summarized as mean and standard deviation or median and interquartile range for continuous variables and count and percentage for categorical variables. The primary statistical outcome was time from surgery to development of NODM (Fig. 2). Patients who did not develop NODM during the available follow-up period were censored at the latest available follow-up date. For the purposes of this study, NODM was defined as two or more outpatient visits or one inpatient admission with a diagnosis of DM, or starting a new diabetic medication (Supplemental Table 3).31 A Kaplan-Meier curve was generated to visualize time to NODM and the Kaplan-Meier estimator was used to estimate incidence of NODM at the selected time points, overall and by patient characteristics.
FIGURE 2.
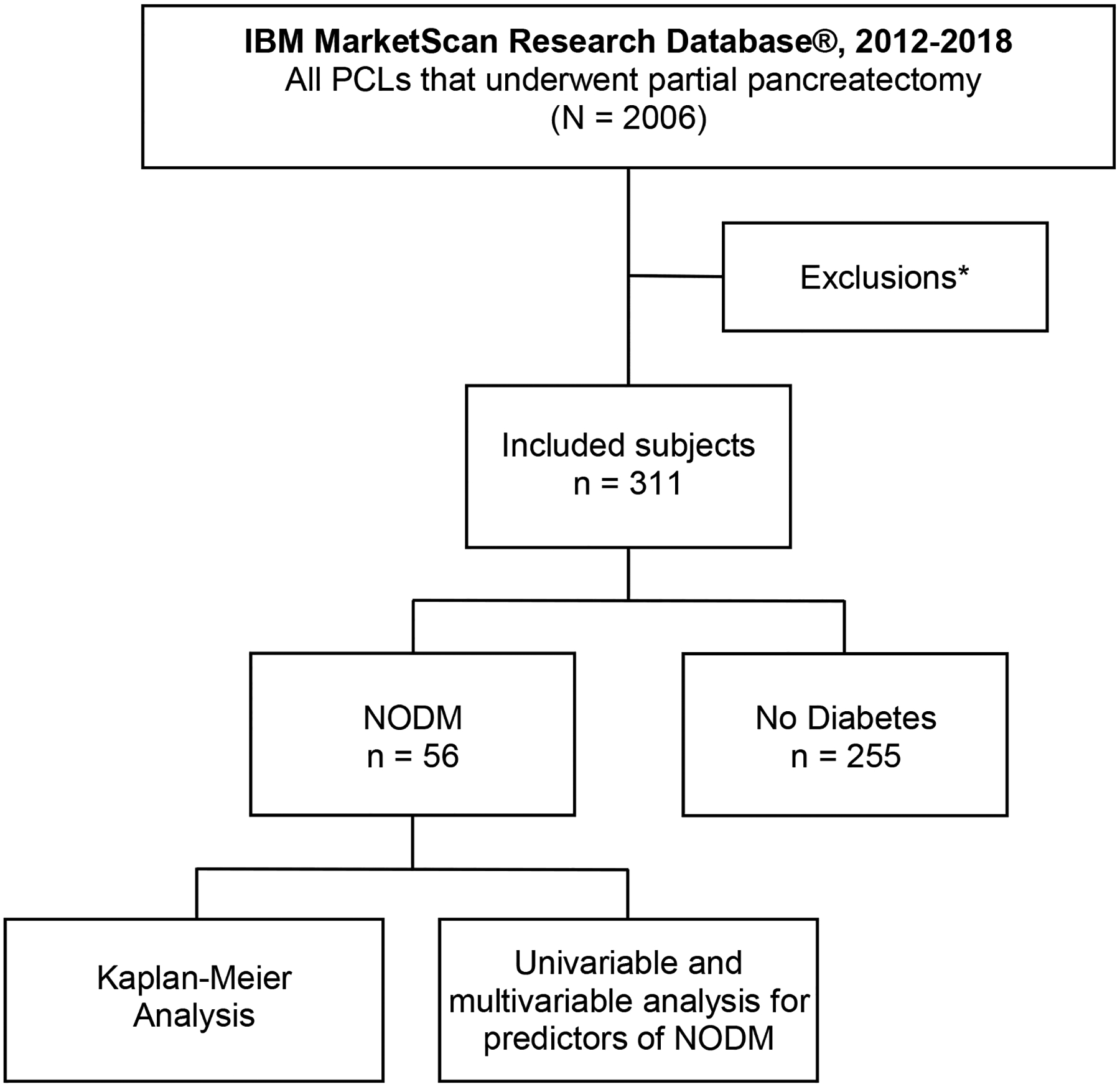
Study flowchart. *Exclusions: No enrollment data, no prior encounters, age <18 years, <3 months prior enrollment, <1 month post-operative enrollment, total pancreatectomy, pregnancy, islet cell auto-transplantation, cystic fibrosis, hemochromatosis, pancreatic malignancy, necrosectomy, pre-existing diabetes mellitus or use of anti-glycemic medications, acute pancreatitis within 6 months, prior chronic pancreatitis, and prior pancreatic surgery. PCL, pancreatic cystic lesion; NODM, new-onset diabetes mellitus
The secondary outcomes were risk factors associated with NODM. Univariable Cox proportional hazards models were fit to evaluate associations between patient characteristics and time to NODM. A multivariable Cox proportional hazards model was fit to identify independent risk factors for NODM. Variables with P < 0.1 in univariable modeling (age, sex, region, surgery type, hypertension, hyperlipidemia, obesity, and cardiovascular disease/heart failure) were considered as candidates for the final multivariable model. Backward selection with inclusion criteria P < 0.05 was used to select the final multivariable model. Variables with P < 0.01 in univariable modeling were kept in the multivariable model regardless of P value. All two-way interactions were tested. Proportional hazards assumptions were evaluated by Kaplan-Meier plots and Schoenfeld residual plots. All two-way interactions were tested using the P < 0.05 criteria. Statistical analyses were performed using SAS version 9.4 (Cary, NC).
RESULTS
Baseline Characteristics
A total of 2006 partial pancreatectomies were performed for PCLs from 2012–2018. After the application of stepwise multi-level exclusion criteria, 311 unique patients with otherwise normal pancreatic parenchyma and without pre-existing DM were identified (Fig. 1, Supplemental Table 2). Patient characteristics are demonstrated in Table 1. The median follow-up time was 16.8 months (interquartile range, 7.2–32.4 months). The mean age of the study cohort was 56.2 (standard deviation, 14.1) years. The majority of patients was female (76.2%). While 218 (70.1%) patients underwent DP, 68 (21.9%) underwent PD, and the excision type was unknown in the remaining 25 (8%).
TABLE 1.
Summary of Demographics and Baseline Characteristics for all Patients who Underwent Partial Pancreatectomy for PCLs Included in the Final Cohort: Analysis of the IBM MarketScan Research Database®, 2012–2018
| Variable | All PCL Surgeries (n = 311) |
|---|---|
| Age, mean (SD), y | 56.2 (14.1) |
| Age group, y, n (%) | |
| 18–44 | 63 (20.3) |
| 45–54 | 69 (22.2) |
| 55–64 | 102 (32.8) |
| 65+ | 77 (24.8) |
| Sex, n (%) | |
| Male | 74 (23.8) |
| Female | 237 (76.2) |
| Region, n (%) | |
| Northeast | 79 (25.4) |
| North Central | 69 (22.2) |
| South | 98 (31.5) |
| West | 61 (19.6) |
| Unknown | 4 (1.3) |
| Surgery type, n (%) | |
| Distal pancreatectomy | 218 (70.1) |
| Proximal pancreatectomy | 68 (21.9) |
| Excision of pancreatic lesion | 24 (7.7) |
| Unspecified partial pancreatectomy | 1 (0.3) |
| Laparoscopic surgery, n (%) | 33 (10.6) |
| Follow up available, median (IQR), mo | 16.8 (7.2–32.4) |
| Charlson Comorbidity Index, mean (SD) | 3.4 (2.4) |
| Charlson Comorbidity Index ≥3, n (%) | 185 (59.5) |
| Hypertension, n (%) | 115 (37.0) |
| Hyperlipidemia, n (%) | 51 (16.4) |
| Obesity, n (%) | 37 (11.9) |
| Tobacco use, n (%) | 34 (10.9) |
| Alcohol use, n (%) | 1 (0.3) |
| CV disease/heart failure, n (%) | 18 (5.8) |
| Cerebrovascular disease, n (%) | 0 (0) |
| Chronic kidney disease, n (%) | 10 (3.2) |
| Alcoholic liver disease, n (%) | 0 (0) |
| Chronic hepatitis B, n (%) | 0 (0) |
| Chronic hepatitis C, n (%) | 1 (0.3) |
| COPD, n (%) | 29 (9.3) |
| Depression, n (%) | 14 (4.5) |
PCL indicates pancreatic cystic lesion; SD, standard deviation; IQR, interquartile range; CV, cardiovascular; COPD, chronic obstructive pulmonary disease.
Time-to-Event Analysis
Kaplan-Meier analysis was utilized to assess time to development of NODM following partial pancreatectomy for a PCL. The curve is shown in Figure 3 and corresponding incidence rates at select time points are listed in Table 2. The estimated incidence of NODM was 9.1% (95% confidence interval [CI], 4.5–10.3%) at 6 months, 15.1% (95% CI, 11.3–20.1%) at 12 months, and 20.2% (95% CI, 15.3–26.4%) at 24 months.
FIGURE 3.
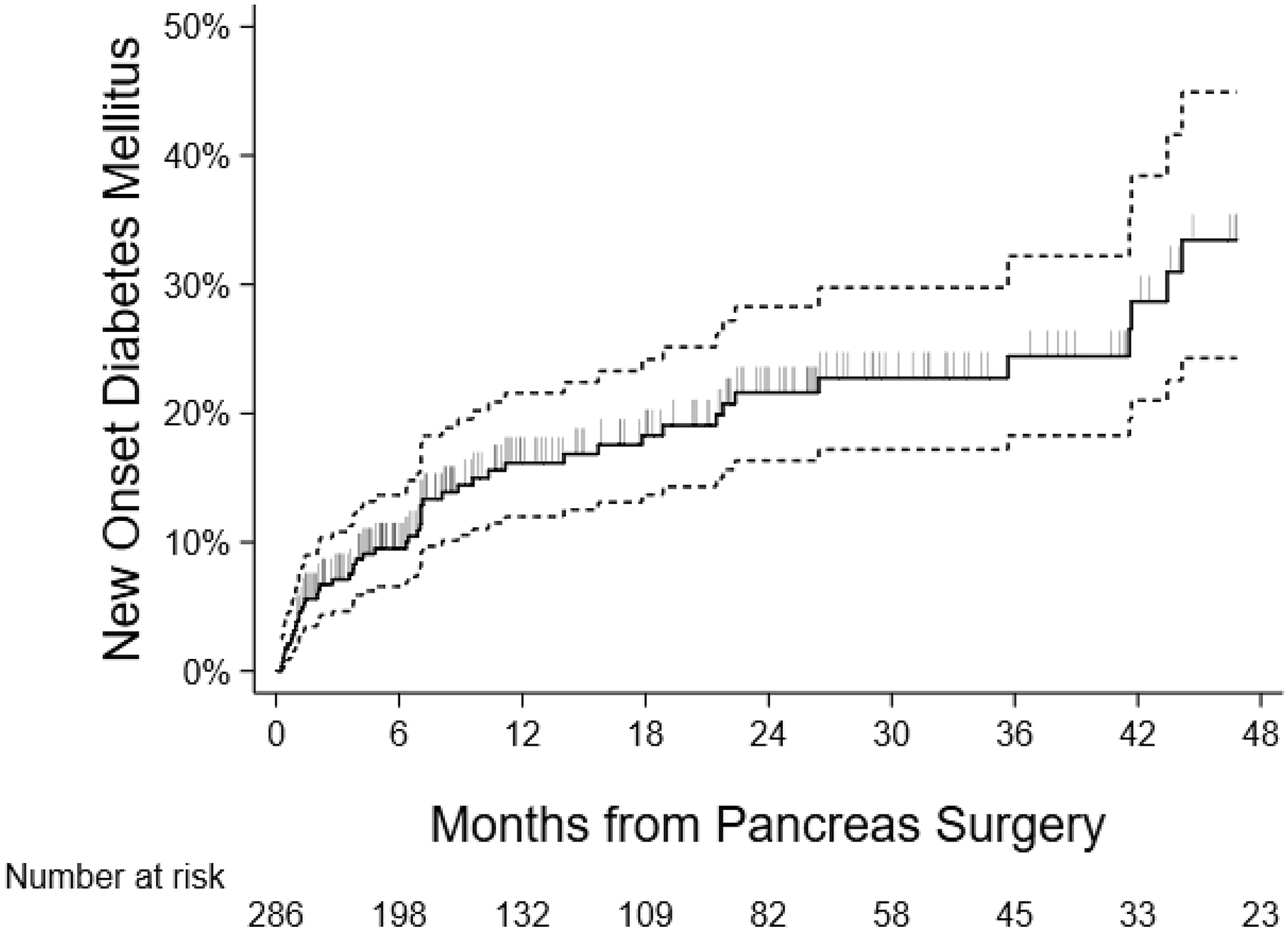
Kaplan-Meier curve for time to NODM for patients undergoing partial pancreatectomy for PCLs: Analysis of the IBM MarketScan Research Database®, 2012–2018. Vertical slashes indicate censored observations (end of follow-up with no DM observed). Dotted lines represent the 95% confidence bands for Kaplan-Meier estimate. NODM, new-onset diabetes mellitus; PCL, pancreatic cystic lesion.
TABLE 2.
Time to NODM at Selected Time Points for Patients Undergoing Partial Pancreatectomy for PCLs: Analysis of the IBM MarketScan Research Database®, 2012–2018
| Time Post-Surgery, mo | Estimated DM Incidence (95% CI) |
|---|---|
| 3 | 6.9% (4.5–10.3%) |
| 6 | 9.1% (6.3–12.9%) |
| 12 | 15.1% (11.3–20.2%) |
| 18 | 17.1% (12.8–22.6%) |
| 24 | 20.2% (15.3–26.4%) |
| 36 | 22.7% (17.0–29.8%) |
| 48 | 32.4% (23.7–43.2%) |
NODM indicates new-onset diabetes mellitus; PCL, pancreatic cystic lesion; DM, diabetes mellitus; CI, confidence interval.
Predictors of NODM Following Surgery
Univariable Cox proportional hazard models (Table 3) for time to DM were utilized to identify characteristics associated with NODM and produce corresponding hazard ratios (HR). Patients age 55–64 years (HR, 2.42, 95% CI, 1.33–4.41), male sex (HR, 2.23, 95% CI, 1.29–3.84), hypertension (HR, 2.23, 95% CI, 1.31–3.79), obesity (HR, 2.32, 95% CI, 1.22–4.42), hyperlipidemia (HR, 1.90, 95% CI, 0.99–3.63), and presence of ≥3 Charlson Comorbidity Index (HR, 1.78, 95% CI, 1.01–3.15) were associated with increased risk of NODM. Multivariable results are shown in Table 4. Patients in the age group of 55–64 years (adjusted hazard ratio [aHR], 1.97, 95% CI, 1.04–3.72), obesity (aHR, 2.63, 95% CI, 1.35–5.12), hypertension (aHR, 1.79, 95% CI, 1.01–3.17) and cardiovascular disease/heart failure (aHR, 2.54, 95% CI, 1.02–6.28) were identified as independent risk factors of NODM following pancreatectomy for PCLs.
TABLE 3.
Univariable Analysis and Incidence of NODM at 6, 12, and 24 Months Post-Surgery in Patients Undergoing Partial Pancreatectomy for PCLs: Analysis of the IBM MarketScan Research Database®, 2012–2018
| Incidence of New Onset Diabetes (95% CI), % | Unadjusted HR (95% CI) | ||||
|---|---|---|---|---|---|
| Variable | 6 mo | 12 mo | 24 mo | P | |
| Age group, y | |||||
| 18–54 | 5.4 (2.6–11.0) | 8.8 (4.8–15.9) | 16.4 (9.9–26.5) | Reference | |
| 55–64 | 16.7 (10.5–25.8) | 26.6 (18.3–37.6) | 26.6 (18.3–37.6) | 2.42 (1.33–4.41) | 0.004 |
| 65+ | 5.4 (2.1–13.7) | 11.2 (5.4–22.5) | 16.6 (8.7–30.5) | 1.07 (0.51–2.25) | 0.86 |
| Sex | |||||
| Male | 17.0 (10.0–28.1) | 25.7 (16.3–39.0) | 32.2 (20.8–47.6) | 2.23 (1.29–3.84) | 0.004 |
| Female | 6.6 (4.0–10.7) | 12.0 (8.1–17.5) | 16.6 (11.6–23.4) | Reference | |
| Region | |||||
| Northeast | 8.1 (3.7–17.2) | 12.1 (6.1–23.0) | 14.8 (7.7–27.4) | Reference | |
| North Central | 10.2 (5.0–20.2) | 17.8 (10.2–30.1) | 23.7 (14.0–38.2) | 1.87 (0.86–4.07) | 0.12 |
| South | 8.5 (4.3–16.3) | 15.6 (9.3–25.6) | 25.0 (15.7–38.3) | 1.74 (0.83–3.64) | 0.14 |
| West | 10.4 (4.8–21.7) | 15.4 (7.9–29.0) | 15.4 (7.9–29.0) | 1.27 (0.52–3.07) | 0.60 |
| Unknown | n/a | n/a | n/a | n/a | n/a |
| Surgery type | |||||
| Distal pancreatectomy | 9.6 (6.3–14.5) | 17.5 (12.6–24.0) | 24.4 (18.1–32.5) | 1.41 (0.69–2.88) | 0.35 |
| Proximal pancreatectomy | 9.2 (4.2–19.4) | 11.3 (5.5–22.5) | 11.3 (5.5–22.5) | Reference | |
| Excision of pancreatic lesion | 4.2 (0.6–26.1) | 4.2 (0.6–26.1) | 4.2 (0.6–26.1) | 0.25 (0.03–1.95) | 0.18 |
| Unspecified partial pancreatectomy | n/a | n/a | n/a | n/a | n/a |
| Laparoscopic surgery | 9.5 (3.2–26.7) | 9.5 (3.2–26.7) | 9.5 (3.2–26.7) | 0.66 (0.21–2.12) | 0.49 |
| Open surgery | 9.0 (6.1–13.1) | 15.6 (11.5–21.0) | 20.9 (15.7–27.5) | ||
| Charlson comorbidity index | 1.78 (1.01–3.15) | 0.05 | |||
| <3 | 4.9 (2.2–10.6) | 8.5 (4.4–15.8) | 15.4 (8.9–25.9) | ||
| ≥3 | 11.9 (7.9–17.7) | 19.8 (14.2–27.1) | 23.4 (17.0–31.8) | ||
| Hypertension | 2.23 (1.31–3.79) | 0.003 | |||
| Present | 14.4 (9.1–22.5) | 20.9 (14.0–30.5) | 29.1 (20.0–41.3) | ||
| Absent | 5.9 (3.3–10.4) | 11.7 (7.6–17.9) | 15.1 (10.0–22.5) | ||
| Hyperlipidemia | 1.90 (0.99–3.63) | 0.05 | |||
| Present | 12.5 (5.8–25.8) | 25.0 (14.0–42.2) | 35.1 (20.5–55.7) | ||
| Absent | 8.4 (5.5–12.6) | 13.5 (9.5–18.8) | 17.6 (12.7–24.1) | ||
| Obesity | 2.32 (1.22–4.42) | 0.01 | |||
| Present | 17.0 (8.0–34.0) | 27.5 (15.2–46.6) | 31.8 (18.2–51.6) | ||
| Absent | 8.0 (5.3–12.0) | 13.4 (9.5–18.6) | 18.5 (13.4–25.2) | ||
| Tobacco use | 1.03 (0.41–2.60) | 0.94 | |||
| Present | 6.3 (1.6–23.0) | 20.3 (8.8–42.9) | 20.3 (8.8–42.9) | ||
| Absent | 9.4 (6.4–13.6) | 14.6 (10.7–19.9) | 20.1 (15.0–26.7) | ||
| CV disease/heart failure | 2.33 (0.99–5.46) | 0.05 | |||
| Present | 22.2 (9.0–48.9) | 22.2 (9.0–48.9) | 22.2 (9.0–48.9) | ||
| Absent | 8.2 (5.5–12.1) | 14.6 (10.7–19.8) | 19.9 (14.9–26.3) | ||
| Chronic kidney disease | 0.58 (0.08–4.21) | 0.59 | |||
| Present | 0 | 12.5 (1.9–61.3) | 12.5 (1.9–61.3) | ||
| Absent | 9.3 (6.5–13.3) | 15.2 (11.3–20.4) | 20.4 (15.4–26.7) | ||
| COPD | 1.02 (0.41–2.57) | 0.96 | |||
| Present | 10.7 (3.6–29.8) | 19.7 (8.6–41.3) | 19.7 (8.6–41.3) | ||
| Absent | 8.8 (6.0–12.9) | 14.6 (10.6–19.9) | 20.1 (14.9–26.7) | ||
| Depression | 0.78 (0.19–3.21) | 0.73 | |||
| Present | 7.1 (1.0–40.9) | 7.1 (1.0–40.9) | 22.6 (5.5–68.5) | ||
| Absent | 9.1 (6.3–13.1) | 15.5 (11.5–20.7) | 19.9 (15.0–26.2) | ||
Incidence rates are derived from the Kaplan-Meier Estimator except the Charlson Index, which is derived from the Cox proportional hazard model as it was modeled as a continuous variable. Hazard ratios are from univariable Cox proportional hazards models for time to NODM. Variables with very low counts were excluded to avoid unintentional identification of individual patients.
NODM indicates new-onset diabetes mellitus; n/a, not applicable; PCL, pancreatic cystic lesion; CI, confidence interval; CV, cardiovascular; COPD, chronic obstructive pulmonary disease.
TABLE 4.
Multivariable Cox Proportional Hazard Model for Time to NODM in Patients Undergoing Partial Pancreatectomy for PCLs: Analysis of the IBM MarketScan Research Database®, 2012–2018
| Variable | Adjusted Hazard Ratio (95% CI) | P |
|---|---|---|
| Age, y | ||
| 18–54 | Reference | |
| 55–64 | 1.97 (1.04–3.72) | 0.04 |
| 65+ | 0.74 (0.33–1.62) | 0.44 |
| Sex, male vs female | 1.62 (0.91–2.88) | 0.10 |
| Obesity vs no | 2.63 (1.35–5.12) | 0.004 |
| Hypertension vs no | 1.79 (1.01–3.17) | 0.04 |
| CV disease/heart failure vs no | 2.54 (1.02–6.28) | 0.04 |
Backward selection with inclusion criteria P ≤ 0.05 was used to select the final multivariable model. Candidates for the multivariable model included age, sex, region, surgery type, hypertension, hyperlipidemia, obesity, and cardiovascular disease/heart failure. Variables with P < 0.01 in univariable modeling were kept in the multivariable model regardless of P. All two-way interactions were tested.
NODM indicates new-onset diabetes mellitus; PCL, pancreatic cystic lesion, CI, confidence interval; CV, cardiovascular.
Influence of Surgery Type
The impact of distal vs. proximal resection was assessed relative to risk of developing NODM. Based on the univariate analysis, there was insufficient evidence to conclude any difference in NODM following DP vs PD (HR, 1.41, 95% CI, 0.69–2.88). Incidence rates for NODM following DP vs PD were 17.5% (95% CI, 12.6–24.0%) vs 11.3% (95% CI, 5.5–22.5%) at 12 months and 24.4% (95% CI, 18.1–32.5%) vs. 11.3% (95% CI, 5.5–22.5%) at 24 months, respectively. This was further investigated with Kaplan-Meier analysis (Fig. 4, Supplemental Table 4). A log-rank test for difference in the curves for DP vs PD did not demonstrate a significant difference, including when follow-up was limited to 24 months and extended to 72 months.
FIGURE 4.
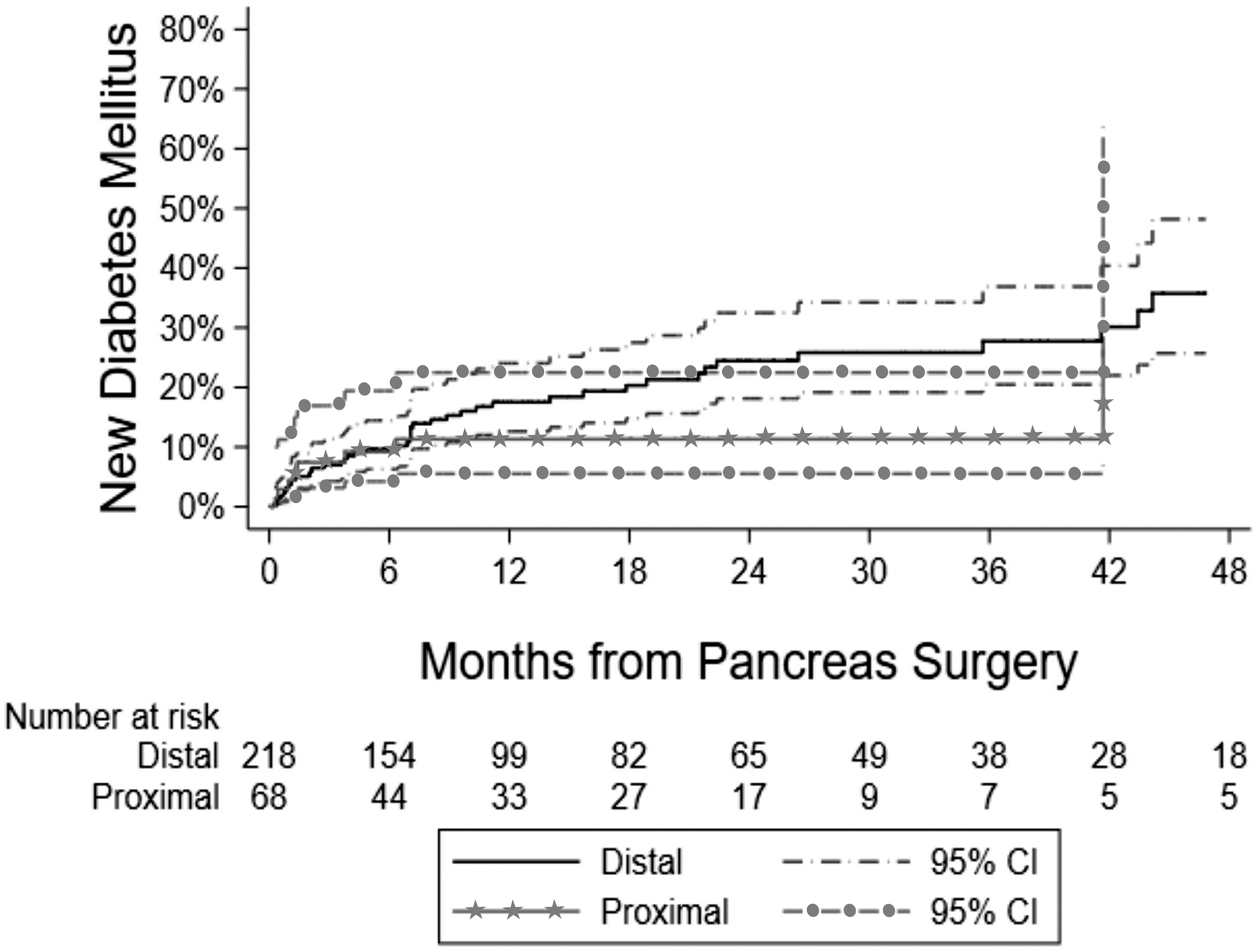
Kaplan-Meier curve for time to NODM for patients undergoing distal compared to proximal pancreatectomy for PCLs: Analysis of the IBM MarketScan Research Database®, 2012–2018. The number at risk indicates how many patients have follow-up at that point and have not yet developed NODM. Some separation exists in the curves after 6 months up to 2, however the overlapping confidence intervals at all time points indicates no significant difference. NODM, new-onset diabetes mellitus; PCL, pancreatic cystic lesion; CI, confidence interval.
DISCUSSION
In this population-based analysis of patients undergoing partial pancreatectomy for pancreatic cysts, approximately 1 in 5 patients developed NODM within 24 months following surgery. The risk of developing NODM was independently associated with increasing age, two components of metabolic syndrome (obesity and hypertension), and cardiovascular disease/heart failure, with the highest risk attributed to comorbid obesity. There was no observed difference in the risk of NODM comparing DP to PD. To our knowledge, this is the first study characterizing the risk of NODM after pancreatectomy for PCLs and specifically among patients without any other pancreatic disease. The current study provides important information for patient counselling prior to surgery, including identifying potentially reversible contributing factors and a subset of patients who may benefit from more intensive post-operative education and follow-up.
The overall incidence of NODM following partial pancreatectomy for PCLs was 20.2% at 24 months, which is similar to other high-level studies involving resections of pancreata for combined etiologies.19,31–34 The most recent systematic review noted a pooled estimate of NODM following PD and DP to be 16% (95% CI, 14–17%) and 21% (95% CI, 16–25%), respectively, though these data represent risk following pancreatectomy for all indications.19 Among other recent meta-analyses in which the indication for pancreatectomy was primarily limited to benign or malignant lesions (after exclusion of chronic pancreatitis), the risk of NODM was reported to be 16% following PD and 14% following DP.33,34 While one systematic review has reported an increased incidence of NODM after resection of malignant tumors compared with benign lesions,32 others have not noted a difference.8,18,33 Neoplastic classifications of PCLs follow well-defined sexual distribution patterns. Mucinous cystic neoplasms and solid pseudopapillary neoplasms are almost exclusively seen in women and are preferentially located in the body or tail of the pancreas.35 Moreover, branch duct intraductal papillary mucinous neoplasms, which account for one-third of all PCLs, are also more common in women.35,36 This readily explains the preponderance of females and the predominance of distal versus proximal pancreatectomies in our study.35
Patients aged 55–64 years were at higher risk of post-operative NODM. Age has previously been identified as a risk factor for NODM following other pancreatic insults, such as acute pancreatitis.37–39 A retrospective review of 472 patients undergoing DP or Whipple procedure for any pancreas pathology also identified age >65 years to be a significant risk factor.40 One potential explanation is reduced pancreatic reserve in older patients leading to increased susceptibility to sustained pancreatic dysfunction and poorer post-operative recovery. Non-diabetic components of metabolic syndrome, including hypertension and obesity, were also independent predictors of NODM. Hyperlipidemia was significant on univariate analysis and trended towards being a predictor of NODM on the multivariate analysis. Prior studies have also linked dyslipidemia and obesity to NODM following resection for various pancreatic pathologies.10,15,16,31 The pathophysiologic relationship between cardiovascular disease/heart failure and risk of NODM illustrated in our study remains somewhat unclear. Components of metabolic syndrome including DM are implicated in cardiovascular disease, and DM displays robust relationships with both heart failure and coronary artery disease, as well as worse patient outcomes when co-prevalent.41–43
Distal pancreatectomy has historically been attributed to higher risk of post-operative endocrine insufficiency than PD due to the increased density of insulin-producing β-islet cells in the body and tail versus the head of the pancreas.20,22,44 However, prior studies remain inconsistent. Our analysis did not observe a significant difference in risk of NODM following DP versus PD for PCL resection. Although there was a trend towards a higher risk in those with a DP, there is risk for a type 2 error due to the statistically small sample size (as reflected by the wide confidence intervals). At the same time, there may be a potential biological explanation for these similarities based on our growing understanding of the hormonal regulation of metabolism. In addition to insulin, other hormones such as the incretins, gastric-inhibitor peptide (GIP) and glucagon-like peptide (GLP), play an important role glucose metabolism via intestinal stimulation of insulin secretion. GLP-1-producing L cells and GIP-producing K cells both reside primarily in the human duodenum.45 Duodenal resection results in reduced GIP expressions and paradoxically increased GLP-1 expression from other intestinal sources, though with an overall reduction of post-prandial insulin release.46–48 Furthermore, pancreatic polypeptide, secreted from PP cells in the ventral pancreas, plays a role in appetite suppression and weight management, which may also contribute to the risk for NODM following PD.49,50
In light of this, alternatives to traditional PD and DP can be considered, including central pancreatectomy, pylorus-preserving PD, and pancreatic enucleation. Most recently, however, with advancements in endoscopic ultrasound, chemo- and thermal ablative techniques using fine-needle injection or radiofrequency ablation have been developed as surgical alternatives for PCL treatment. Despite initial concern for serious adverse events, recent studies investigating ethanol-free chemoablation report reduced rates of adverse events without reduced efficacy.51,52 Although larger trials are warranted before widespread implementation, the pooled complete resolution rate for paclitaxel based regimens is 63.9%.53 Further, 98% of cysts achieving complete resolution retain resolution at 6-year follow up.54 These techniques provide an exciting possibility for alternative management of PCLs, particularly in patients at high risk of post-operative complications such as those described by this study.
Several limitations should be considered when interpreting results of the current study. First, MarketScan, like other large databases, utilizes diagnostic and procedural ICD-9, ICD-10 and CPT coding, restricting which variables can be studied, and introducing risk for an unidentified confounding variable. As such, traditional risk factors for DM (eg, pre-operative hemoglobin A1c, family history of DM) could not be included, nor could other potential contributors such as ethnicity, other laboratory parameters, or volume of resected pancreas, which has previously been identified as a significant predictor of post-operative NODM.11,17,19,55,56 Second, the database does not reveal the specific diagnosis of the resected cyst other than being diagnosed with adenocarcinoma as a peri-operative finding; and these fall within our exclusions. The final diagnosis of PCLs is confirmed histologically and may be unknown at the time of resection, reducing the real-world effect of this limitation since the purpose of this risk-profile remains primarily for pre-operative planning. Third, a significant constraint of the database is the exclusion of federally funded Medicare insurance claims. Due to this limitation, only a small fraction of adults >65 years with Medicare supplemental insurance are included in the MarketScan database and hence patient age >65 years was not a significant risk factor in this study. The other risk factors identified are expected to translate to this older age group although additional risk factors and difference in disease incidence may exist. Finally, the final sample size (n = 311) limited the statistical power of subset analyses. Consequent to this, the number of subjects with data beyond two years remains relatively small. However, stringent selection criteria were necessary to maintain a rigorous design and this sample size would be challenging to reach using a traditional single or multicenter study design. Although not a limitation of study design, it is worth mentioning that, as post-AP screening for NODM is not routinely performed, subclinical DM cannot be captured in this study, likely resulting in an underestimate of the true incidence of NODM. Despite these considerations, such a study isolating risk of PCL resection would not be possible without the use of such a database and the results remain a valuable contribution to current practice.
In conclusion, NODM following pancreatic resection of PCLs occurs in a significant proportion of patients (20.2% at 24 months) with otherwise normal pancreata. Risk of NODM is most strongly associated with advanced age, components of metabolic syndrome (obesity and hypertension), and cardiovascular disease/heart failure. These data are informative for preoperative counselling and risk factor modification. The associated factors also identify subsets of patients who may benefit from more intensive post-operative education and follow-up as well as consideration for novel, non-surgical treatment strategies. Additional studies regarding the hormonal changes of glucose metabolism following pancreatic surgery across different disease etiologies may provide the opportunity for a more tailored approach for the patients who develop post-operative diabetes mellitus.
Supplementary Material
Conflicts of Interest and Sources of Funding:
The project described was supported by Award Number UL1TR002733 (SAF) from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. No other potential conflicts of interest exist for any author in relationship to this publication.
Abbreviations:
- ADA
American Diabetes Association
- aHR
adjusted hazard ratio
- AP
acute pancreatitis
- ASGE
American Society for Gastrointestinal Endoscopy
- CI
confidence interval
- COBRA
Consolidated Omnibus Budget Reconciliation Act
- CPT
Current Procedural Terminology
- DM
diabetes mellitus
- DP
distal pancreatectomy
- GIP
gastric inhibitor peptide
- GLP
glucagon-like peptide
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, Ninth Edition Clinical Modification
- ICD-10-CM
International Classification of Diseases, Tenth Edition Clinical Modification
- NODM
new onset diabetes mellitus
- PCL
pancreatic cystic lesion
- PD
pancreaticoduodenectomy
References
- 1.Hart PA, Bellin MD, Andersen DK, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81–90. [DOI] [PubMed] [Google Scholar]
- 3.Lyu HG, Sharma G, Brovman E, et al. Risk factors of reoperation after pancreatic resection. Dig Dis Sci. 2017;62:1666–1675. [DOI] [PubMed] [Google Scholar]
- 4.Zafar SN, Shah AA, Nembhard C, et al. Readmissions after complex cancer surgery: Analysis of the nationwide readmissions database. J Oncol Pract. 2018;14:e335–e345. [DOI] [PubMed] [Google Scholar]
- 5.Dresler CM, Fortner JG, McDermott K, et al. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991;214:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller MW, Friess H, Kleeff J, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–974; discussion 974–975. [DOI] [PubMed] [Google Scholar]
- 7.Jethwa P, Sodergren M, Lala A, et al. Diabetic control after total pancreatectomy. Dig Liver Dis. 2006;38:415–419. [DOI] [PubMed] [Google Scholar]
- 8.Elliott IA, Epelboym I, Winner M, et al. Population-level incidence and predictors of surgically induced diabetes and exocrine insufficiency after partial pancreatic resection. Perm J. 2017;21:16–095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roeyen G, Jansen M, Hartman V, et al. The impact of pancreaticoduodenectomy on endocrine and exocrine pancreatic function: A prospective cohort study based on pre- and postoperative function tests. Pancreatology. 2017;17:974–982. [DOI] [PubMed] [Google Scholar]
- 10.Kim KJ, Jeong CY, Jeong SH, et al. Pancreatic diabetes after distal pancreatectomy: incidence rate and risk factors. Korean J Hepatobiliary Pancreat Surg. 2011;15:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon JH, Kim SC, Shim IK, et al. Factors affecting the development of diabetes mellitus after pancreatic resection. Pancreas. 2015;44:1296–1303. [DOI] [PubMed] [Google Scholar]
- 12.Burkhart RA, Gerber SM, Tholey RM, et al. Incidence and severity of pancreatogenic diabetes after pancreatic resection. J Gastrointest Surg. 2015;19:217–225. [DOI] [PubMed] [Google Scholar]
- 13.Kang JS, Jang JY, Kang MJ, et al. Endocrine function impairment after distal pancreatectomy: Incidence and related factors. World J Surg. 2016;40:440–446. [DOI] [PubMed] [Google Scholar]
- 14.Tariq M, Jajja MR, Maxwell DW, et al. Diabetes development after distal pancreatectomy: results of a 10 year series. HPB (Oxford). 2020;22:1034–1041. [DOI] [PubMed] [Google Scholar]
- 15.Hwang HK, Park J, Choi SH, et al. Predicting new-onset diabetes after minimally invasive subtotal distal pancreatectomy in benign and borderline malignant lesions of the pancreas. Medicine (Baltimore). 2017;96:e9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusakabe J, Anderson B, Liu J, et al. Long-term endocrine and exocrine insufficiency after pancreatectomy. J Gastrointest Surg. 2019;23:1604–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirakawa S, Matsumoto I, Toyama H, et al. Pancreatic volumetric assessment as a predictor of new-onset diabetes following distal pancreatectomy. J Gastrointest Surg. 2012;16:2212–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah KP, Baugh KA, Brubaker LS, et al. Long-term assessment of pancreatic function after pancreatectomy for cystic neoplasms. J Surg Res. 2020;247:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Nahm CB, Jamieson NB, et al. Risk factors for development of diabetes mellitus (Type 3c) after partial pancreatectomy: A systematic review. Clin Endocrinol (Oxf). 2020;92:396–406 [DOI] [PubMed] [Google Scholar]
- 20.Ionescu-Tirgoviste C, Gagniuc PA, Gubceac E, et al. A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep. 2015;5:14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittingen J, Frey CF. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann Surg; 1974. p. 412–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahier J, Guiot Y, Goebbels RM, et al. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10 Suppl 4:32–42. [DOI] [PubMed] [Google Scholar]
- 23.Kromrey ML, Bülow R, Hübner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138–145. [DOI] [PubMed] [Google Scholar]
- 24.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XM, Mitchell DG, Dohke M, et al. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–553. [DOI] [PubMed] [Google Scholar]
- 26.Elta GH, Enestvedt BK, Sauer BG, et al. ACG clinical guideline: Diagnosis and management of pancreatic cysts. Am J Gastroenterol. 2018;113:464–479. [DOI] [PubMed] [Google Scholar]
- 27.Scheiman JM, Hwang JH, Moayyedi P. American Gastroenterological Association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824–848.e22. [DOI] [PubMed] [Google Scholar]
- 28.IBM MarketScan Research Databases. 2020. Available at: https://www.ibm.com/products/marketscan-research-databases. Accessed April 1, 2020.
- 29.Sellers ZM, MacIsaac D, Yu H, et al. Nationwide trends in acute and chronic pancreatitis among privately insured children and non-elderly adults in the United States, 2007–2014. Gastroenterology. 2018;155:469–478.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–1294. [DOI] [PubMed] [Google Scholar]
- 31.Wu JM, Ho TW, Yang CY, et al. Changes in glucose metabolism after distal pancreatectomy: a nationwide database study. Oncotarget. 2018;9:11100–11108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beger HG, Poch B, Mayer B, et al. New onset of diabetes and pancreatic exocrine insufficiency after pancreaticoduodenectomy for benign and malignant tumors: A systematic review and meta-analysis of long-term results. Ann Surg. 2018;267:259–270. [DOI] [PubMed] [Google Scholar]
- 33.Scholten L, Mungroop TH, Haijtink SAL, et al. New-onset diabetes after pancreatoduodenectomy: A systematic review and meta-analysis. Surgery. 2018;164:6–16. [DOI] [PubMed] [Google Scholar]
- 34.De Bruijn KM, van Eijck CH. New-onset diabetes after distal pancreatectomy: a systematic review. Ann Surg. 2015;261:854–861. [DOI] [PubMed] [Google Scholar]
- 35.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malleo G, Marchegiani G, Borin A, et al. Observational study of the incidence of pancreatic and extrapancreatic malignancies during surveillance of patients with branch-duct intraductal papillary mucinous neoplasm. Ann Surg. 2015;261:984–990. [DOI] [PubMed] [Google Scholar]
- 37.Lee YK, Huang MY, Hsu CY, et al. Bidirectional relationship between diabetes and acute pancreatitis: A population-based cohort study in Taiwan. Medicine (Baltimore). 2016;95:e2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soo DHE, Pendharkar SA, Jivanji CJ, et al. Derivation and validation of the prediabetes self-assessment screening score after acute pancreatitis (PERSEUS). Dig Liver Dis. 2017;49:1146–1154. [DOI] [PubMed] [Google Scholar]
- 39.Ma JH, Yuan YJ, Lin SH, et al. Nomogram for predicting diabetes mellitus after the first attack of acute pancreatitis. Eur J Gastroenterol Hepatol. 2019;31:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen A, Demirjian A, Yamamoto M, et al. Development of postoperative diabetes mellitus in patients undergoing distal pancreatectomy. Am Surg. 2017;83:1050–1053. [PubMed] [Google Scholar]
- 41.Giugliano D, Meier JJ, Esposito K. Heart failure and type 2 diabetes: From cardiovascular outcome trials, with hope. Diabetes Obes Metab. 2019;21:1081–1087. [DOI] [PubMed] [Google Scholar]
- 42.Zareini B, Rørth R, Holt A, et al. Heart failure and the prognostic impact and incidence of new-onset of diabetes mellitus: a nationwide cohort study. Cardiovasc Diabetol. 2019;18:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wittingen J, Frey CF. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann Surg. 1974;179:412–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550–559. [DOI] [PubMed] [Google Scholar]
- 46.Beger HG, Mayer B, Poch B. Resection of the duodenum causes long-term endocrine and exocrine dysfunction after Whipple procedure for benign tumors - Results of a systematic review and meta-analysis. HPB (Oxford). 2020;22:809–820 [DOI] [PubMed] [Google Scholar]
- 47.Beger HG, Nakao A, Mayer B, et al. Duodenum-preserving total and partial pancreatic head resection for benign tumors--systematic review and meta-analysis. Pancreatology. 2015;15:167–178. [DOI] [PubMed] [Google Scholar]
- 48.Muscogiuri G, Mezza T, Prioletta A, et al. Removal of duodenum elicits GLP-1 secretion. Diabetes Care. 2013;36:1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asakawa A, Inui A, Yuzuriha H, et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124:1325–1336. [DOI] [PubMed] [Google Scholar]
- 50.Maxwell JE, O’Dorisio TM, Bellizzi AM, et al. Elevated pancreatic polypeptide levels in pancreatic neuroendocrine tumors and diabetes mellitus: causation or association? Pancreas. 2014;43:651–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeWitt JM, Al-Haddad M, Sherman S, et al. Alterations in cyst fluid genetics following endoscopic ultrasound-guided pancreatic cyst ablation with ethanol and paclitaxel. Endoscopy. 2014;46:457–464. [DOI] [PubMed] [Google Scholar]
- 52.Moyer MT, Sharzehi S, Mathew A, et al. The safety and efficacy of an alcohol-free pancreatic cyst ablation protocol. Gastroenterology. 2017;153:1295–1303. [DOI] [PubMed] [Google Scholar]
- 53.Attila T, Adsay V, Faigel DO. The efficacy and safety of endoscopic ultrasound-guided ablation of pancreatic cysts with alcohol and paclitaxel: a systematic review. Eur J Gastroenterol Hepatol. 2019;31:1–9. [DOI] [PubMed] [Google Scholar]
- 54.Choi JH, Seo DW, Song TJ, et al. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy. 2017;49:866–873. [DOI] [PubMed] [Google Scholar]
- 55.Yun SP, Seo HI, Kim S, et al. Does the pancreatic volume reduction rate using serial computed tomographic volumetry predict new onset diabetes after pancreaticoduodenectomy? Medicine (Baltimore). 2017;96:e6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh AN, Pal S, Kilambi R, et al. Diabetes after pancreaticoduodenectomy: can we predict it? J Surg Res. 2018;227:211–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


