Abstract
We report boronate-caged guanidine-lipid 1 that activates liposomes for cellular delivery only upon uncaging of this compound by reactive oxygen species (ROS) to produce cationic lipid products. These liposomes are designed to mimic the exceptional cell delivery properties of cell-penetrating peptides (CPPs), while the inclusion of the boronate cage is designed to enhance selectivity such that cell entry will only be activated in the presence of ROS. Boronate uncaging by hydrogen peroxide was verified by mass spectrometry and zeta potential (ZP) measurements. A microplate-based fluorescence assay was developed to study the ROS-mediated vesicle interactions between 1-liposomes and anionic membranes, which were further elucidated via dynamic light scattering (DLS) analysis. Cellular delivery studies utilizing fluorescence microscopy demonstrated significant enhancements in cellular delivery only when 1-liposomes were incubated with hydrogen peroxide. Our results showcase that lipid 1 exhibits strong potential as an ROS-responsive liposomal platform for targeted drug delivery applications.
Keywords: Reactive Oxygen Species, Liposomes, Drug Delivery, Caged Guanidine, Targeted Cellular Delivery
Graphical Abstract
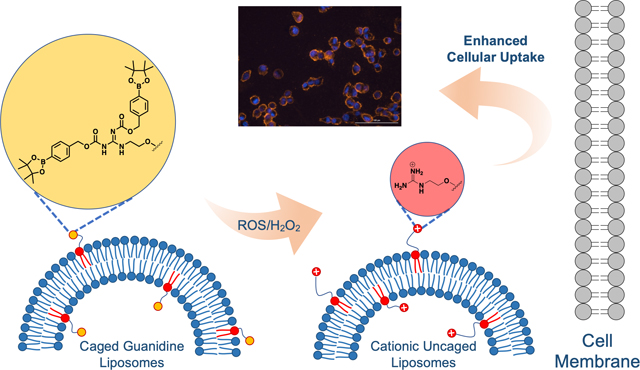
We report a boronate-caged guanidine-lipid for enhancing liposome cellular delivery mediated by reactive oxygen species (ROS). Our results showcase that this lipid enables cell delivery only after being activated in the presence of ROS. This approach exhibits strong potential as an ROS-responsive liposomal platform for targeted drug delivery applications.
Introduction
Liposomes are effective drug delivery nanocarriers due to their ability to encapsulate and enhance the cellular delivery of therapeutics with wide-ranging physicochemical properties. There are at least fifteen FDA-approved liposomal drug delivery formulations,[1] and the importance of related lipid nanocarriers is showcased by instrumental roles in the rapid development of COVID-19 vaccines.[2] Nevertheless, further improvements in liposomal delivery properties are required to fully exploit their therapeutic potential. Smart liposomes containing caged targeting moieties or triggered release mechanisms have thus received increased attention in recent years, which can enhance diseased-cell specificity and drug release/potency.[3] The selective targeting of liposomes to diseased cells is a key aspect that requires improvement. While moieties including antibodies and small molecules (i.e., folate, peptides) enhance delivery to diseased cells by binding overexpressed cell-surface receptors, these are not economically feasible or tunable to different cell types, respectively.
Cell-penetrating peptides (CPPs), polycationic peptide sequences inspired by the HIV tat protein,[4] have emerged as powerful transport vectors for intracellular delivery.[5] Despite their great potential in clinical uses and the fact that numerous CPPs have entered clinical trials since the late 1980s, none of these have been approved by the US FDA for clinical use.[5c] Typical CPPs possess positively charged amino acid sequences that bind to negatively charged cell membranes through electrostatic interactions at physiological pH and leverage these associations to traverse cell membranes.[4] As a result, most CPPs do not exhibit cell type or tissue specificity. Non-specific CPP-mediated cell-penetration into normal tissues can result in reduced therapeutic efficacy and increased toxicity, which limits applicability.[6] To address this problem, smart delivery systems utilizing activable CPPs have been developed, which are designed to be responsive to a certain stimulus that can enhance the selectivity of CPPs towards certain diseased cells.[7] External stimuli such as light have been widely used for photocleavable CPPs.[8] Pathophysiological conditions of diseased cells, including enhanced acidity[9] and aberrant enzyme expression[10] have been harnessed for targeted delivery. However, these internal stimuli are hindered by minimal differences between healthy and diseased cells (i.e., pH ~6.5–6.7 (cancer) vs. ~7.2–7.4 (healthy)),[11] providing a narrow window for differentiation. The delivery of external stimuli to the desired diseased sites is also problematic, exemplified by UV light irradiation exhibiting limited tissue penetration and potentially causing damage to nearby healthy tissue.[12]
Among potential stimuli, reactive oxygen species (ROS) that are overproduced by diseased cells have been significantly underexplored, providing an exciting avenue for site-specific liposomal targeting purposes. ROS,[13] including hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide (O2·−) and hydroxyl radicals (HO·) are normally maintained at low levels[14] since they play crucial roles in metabolism[15] and regulate key physiological processes.[16] Overly abundant ROS cause oxidative stress that culminates in pathophysiological conditions including cancer,[17] Alzheimer’s disease,[18] atherosclerosis,[19] hypertension,[20] diabetic nephropathy,[21] and lung fibrosis.[22] In particular, ROS concentrations in cancer cells (~100 μM) have been measured to be approximately three orders of magnitude higher than in healthy cells (~20 nM).[23] One rare H2O2-activated CPP example was reported by Tsien et al.,[24] where they incorporated a boronic acid-containing cleavable linker between polycationic and polyanionic CPPs separately labeled with a FRET pair. This system selectively reacts with endogenous H2O2, acting effectively as a H2O2 sensor for cellular targeting and imaging purposes. While this elegant design showcased that ROS could be applied to activable CPP design, reports of liposomal delivery platforms using similar strategies remain sparse.
Results and Discussion
Herein, we report the development of a smart liposomal delivery system containing masked lipid 1 bearing boronate-caged guanidinium headgroups to activate cell entry only in the presence of ROS. This provides a key advancement in which neutral, latent liposomes will circulate, while encountering upregulated ROS will unveil cationic groups to promote cell entry (Scheme 1A). The polycationic characteristics of typical CPPs result from the inclusion of arginine or lysine, which contain a guanidine and an amine group, respectively. Although they carry the same net charge under physiological conditions, the guanidine groups can interact more effectively with cell membranes due to hydrogen bonding interactions with phosphate and sulfate moieties presented on the cell surface, leading to enhanced internalization.[25] For example, Aida and co-workers developed dendritic photoactivable guanidinium molecular glues that could efficiently adhere to anionic biomolecules, such as DNA and phospholipid membranes, upon light irradiation.[26]
Scheme 1.
Design for caged guanidine liposomes containing lipid 1 for ROS-mediated targeted delivery. A. Cartoon depicting uncaging when lipid 1 is incorporated in the membrane. Positively charged membranes will be generated in the presence of ROS/H2O2. B. Structure of caged guanidine lipid 1 and the uncaging mechanism after adding H2O2. The addition of H2O2 cleaves the boronate ester headgroup, leading to a QM self-immolation process that ultimately produces cationic guanidinium lipid 2.
The structural design for lipid 1 is shown in Scheme 1B. The guanidine headgroup of lipid 1 is masked by two arylboronic ester groups, which have been widely applied in ROS-responsive materials due to their fast degradation kinetics upon H2O2 contact.[27] In the presence of H2O2, lipid 1 can be oxidized by insertion of an oxygen atom into the carbon-boron bond, leading to the formation of a phenoxide intermediate. This serves as part of a quinone-methide (QM)-generating self-immolation linker (SIL), which has been intensively applied in the development of nanomedicine[28] and sensors.[29] In our design, after oxidative cleavage, the remaining structure undergoes a QM elimination reaction that releases cationic guanidium lipid 2 designed to turn on cell entry. Since CPPs typically contain several repeating cationic groups, it is noteworthy that our compound contains only one caged guanidine. This is because it was envisioned that liposome membranes would provide a fluid multivalent environment in which multiple individual guanidinium-lipid conjugates would conspire to mimic CPP structural features. Furthermore, the density of charged moieties can be modulated based on the percentage of 1 that is incorporated within liposome formulations. Lipid 1 also contains a tetra ethylene glycol (TEG) linker between the caged guanidinium headgroup and the lipid backbone to prevent the headgroups from being buried inside the membrane bilayer and thus inaccessible to react with added H2O2. Furthermore, a key triazole functionality is included as a lipid headgroup mimetic since this polar moiety is known to prefer the aqueous interface rather than the hydrophobic membrane environment.[30] This also facilitates synthesis of 1 by exploiting the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction.
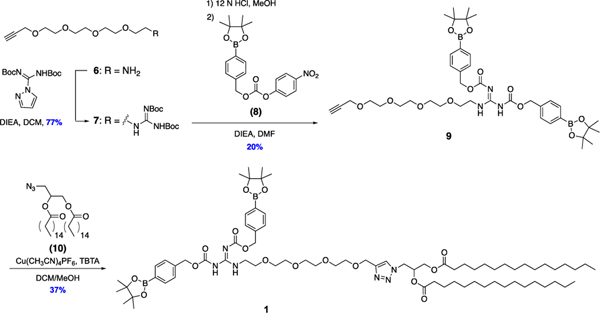
The synthetic route to lipid 1 is detailed in Scheme 2. Amino and alkynyl functionalized TEG linker 6 was first synthesized in four steps from tetra ethylene glycol, including mono-ether coupling with propargyl bromide to 3, alcohol tosylation to 4, substitution with azide to 5, and a Staudinger reduction to reduce the azide into the amine group of 6 without reducing the alkyne group (Scheme S1).[31] Compound 6 was next reacted with N,N′-di-Boc-1H-pyrazole-1-carboxamidine to introduce the Boc-protected guanidine headgroups of 7. Deprotection of the Boc groups with concentrated hydrochloric acid followed by coupling to arylboronate ester p-nitrophenol carbonate compound 8[32] resulted in boronate caged guanidine intermediate 9, which was reacted via CuAAC with azido-lipid 10 (route shown in Scheme S2)[33] to produce boronate-caged guanidine lipid 1.
Scheme 2.
Synthetic route to caged guanidine lipid 1. A guanidinylation reaction was performed on alkynyl and amino functionalized TEG intermediate 6 to introduce the Boc-protected guanidine moiety of 7. Boc deprotection, boronate caging group introduction, and CuAAC with azido-lipid 10 yielded the final caged guanidine lipid 1.
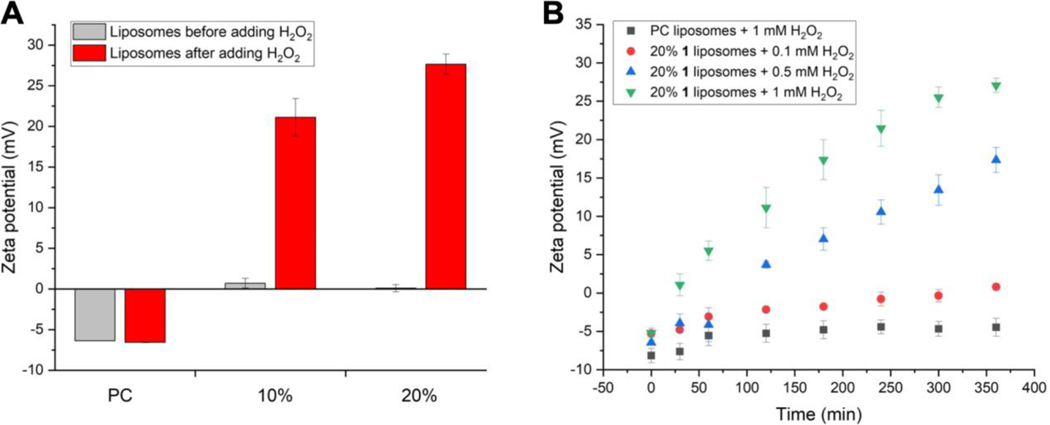
After completion of the synthesis of ROS-responsive caged guanidine lipid 1, we first verified that the uncaging process occurred upon H2O2 addition using high-resolution mass spectrometry (HRMS). Lipid 1 was dissolved in a chloroform/methanol mixture and incubated with H2O2 for 1 hour before HRMS analysis. Direct infusion positive mode electrospray ionization (ESI+) HRMS showed successful boronate uncaging and formation of positively charged guanidinium lipid 2 (Figure S1). Next, to study uncaging and guanidinium formation within a membrane environment, zeta potential (ZP) measurements were conducted to verify the expected introduction of positive charge within liposomes. Unilamellar liposomes containing 0–20% of lipid 1 and otherwise composed of L-⍺-phosphatidylcholine (PC mixed isomers from chicken egg) were prepared using standard thin-film hydration methods, including lipid film preparation, hydration, freeze-thaw cycling, and extrusion through 200 nm polycarbonate membranes. The formation of uniform liposomes with desired size was determined by dynamic light scattering (DLS) (distribution curves shown in Figure S2, grey traces). ZP values were first measured for PC liposomes including 0%, 10%, or 20% of 1 before and after ~25 mM H2O2 incubation for one hour. For 1-liposomes, dramatic increases in positive charge were detected that correlated with the percentages of 1 within liposomes (Figure 1A). PC negative control liposomes lacking 1 initially showed a slight negative charge, in agreement with literature,[34] and did not change upon H2O2 incubation.
Figure 1.
Zeta potential measurements for liposomes containing 0%, 10%, and 20% lipid 1 before and after H2O2 addition. A. End point studies indicate strongly positively charged liposomes were generated after H2O2-induced uncaging of 1, while 100% PC control liposomes did not show any changes in ZP values. B. Time-dependent ZP analysis for PC-based liposomes containing 0% or 20% 1 after adding 0.1 mM, 0.5 mM, or 1 mM H2O2. 1-Liposomes treated with 0.5 mM and 1 mM H2O2 both showed increases in ZP values over time, while 0.1 mM H2O2 treatment only resulted in a slight ZP increase. Error bars denote standard errors from at least three independent trials.
Next, a detailed study of ZP changes upon H2O2 treatment over time was conducted by treating 1 mM liposomes containing 20% 1 (200 μM final concentration of 1) with 0.1 mM, 0.5 mM, or 1 mM H2O2. As shown in Figure 1B, increasingly positive ZP values were observed over time for liposomes treated with 0.5 mM or 1 mM H2O2, with 1 mM H2O2 showing slightly faster kinetics. Whereas PC liposomes treated with 1 mM H2O2 showed minimal changes in ZP values. Interestingly, 20% liposomes incubated with 0.1 mM H2O2 also only exhibited a modest increase in ZP values. Considering that oxidative cleavage of the boronate headgroups requires stoichiometric amounts of peroxide, insufficient H2O2 could lead to incomplete uncaging, resulting in limited changes to liposome surface charge that could affect subsequent cellular entry. These results show that 20% 1-liposomes begin to produce cationic liposomes when subjected to ROS concentrations in the range previously cited for cancer cells (~100 μM), although the response is fairly weak at this level. Further optimization would be beneficial to increase activity at lower H2O2 concentrations. Nevertheless, these results support uncaging of 1 and confirm the generation of cationic liposomes.
To study binding interactions with anionic membranes after boronate uncaging, we developed a fluorescence-based microplate vesicle binding/fusion assay by modifying protocols for immobilizing intact liposomes that we reported previously.[33b,35] This assay provides an effective model system for gauging cell entry by determining the extent to which the presence of uncaged 1 promotes liposome binding/fusion. Anionic liposomes targeted for binding/fusion contained phosphatidylserine (PS), which is of particular interest since cancer cells are known to overexpress this negatively charged lipid on the outer leaflets of their cell membranes, which could be exploited for promoting cell entry.[36] This approach analyzes the binding and/or fusion of two different samples of liposomes (Figure 2A). The first batch of liposomes was immobilized onto 96-well streptavidin-coated microplates by including biotin-PE (1%) within a PC formulation doped with PS. The resulting functionalized microplate surfaces were then treated with a second liposome batch that includes a varying percentage of 1 and the fluorescently labeled lipid rhodamine-L-⍺-phosphatidylethanolamine (Rd-PE) for detection purposes. Washes were performed after each step to remove liposomes that did not interact with the surface or with immobilized liposomes, respectively. Next, microplate fluorescence readings were acquired to evaluate binding/fusion based on expected fluorescence enhancements.
Figure 2.
Schematic for microplate assay to detect liposome fusion/binding promoted by H2O2 uncaging of 1. A. Cartoon illustration for fluorescence-based microplate binding assay. Anionic PS liposomes containing biotin-PE were immobilized onto a streptavidin-coated plate, then liposomes containing lipid 1 (labeled with Rd-PE), either treated with H2O2 or untreated was added. The plate was then washed several times to remove non-interacting liposomes. Increased fluorescence is expected to result from 1 uncaging that activates fusion/binding to immobilized liposomes (fusion is depicted in the cartoon). B. Dose-dependent fluorescence increases were observed only when 10% or 20% 1/PC liposomes were incubated with 5% PS/PC liposomes after treatment with H2O2. Minimal changes were observed without H2O2, when PS/PC liposomes were treated with liposomes lacking 1, or with just TBS buffer addition. Error bars denote standard errors from at least three independent trials.
Initially, we sought to show that liposomes were successfully immobilized onto microplates, for which experiments only required one batch of liposomal samples. To do so, we prepared PC-based liposomes doped with 5% PS and 1% biotin-PE that were fluorescently labeled with 1% Rd-PE. After treating each well with different concentrations of these samples, and conducting washes to remove unbound liposomes, microplate fluorescence readings were acquired. We observed a dose-dependent increase in Rd-PE fluorescence, indicating that liposomes were successfully immobilized, while liposomes without biotin-PE only resulted in minimal fluorescence enhancements, acting as effective negative controls (Figure S3).
To apply microplate functionalization for a vesicle binding/fusion assay, the first batch of liposomes composed of PC doped with 5% PS and 1% biotin-PE were immobilized onto streptavidin-coated microplates. The resulting microplate wells were then treated with the second batch of samples. These consisted of PC liposomes containing 0%, 10%, or 20% 1 along with 1% Rd-PE in which half of the samples were treated with 10 mM H2O2, while the other half were untreated controls. The resulting liposomes were incubated for 3 hours with the previously functionalized microplates to interact with immobilized liposomes and unbound fluorescent liposomes were removed via washing with TBS buffer. Fluorescence analysis showed that 1-liposomes yielded a significant dose-dependent increase in fluorescence (Figure 2B), with 20% 1-liposomes showing greater enhancements compared to 10% 1-liposomes. In negative control experiments, 1-liposomes not treated with H2O2, PC liposomes lacking 1, or only buffer addition to immobilized liposomes all yielded minimal fluorescence signal changes. A positive control experiment was also performed by treating 5% PS liposome-functionalized microplate surfaces with Rd-PE-labeled PC liposomes containing 10% or 20% of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), which is a cationic lipid widely used in cell transfection.[37] Since DOTAP carries the same net charge as uncaged lipid 1, this experiment serves as an ideal positive control that mimics the expected endpoint conditions of the previous assay. In line with our expectations, we again observed increases in Rd-PE fluorescence that correlated with DOTAP percentages, which also match well with the experimental data obtained from 1-liposomes that were incubated with H2O2 (Figure S4). These results support that ROS treatment leads to uncaging of 1-liposomes, which promotes association with anionic liposomes that are in this case immobilized on microplate surfaces.
These conclusions were further evidenced by solution-phase DLS analysis. DLS data first supported that 20% 1/PC formulations form stable liposomes and that H2O2 treatment does not alter their morphology (Figure S2). However, when these liposomes were incubated with 10% PS/PC or 10/40/50 L-⍺-phosphatidic acid (PA)/PC/dioleoyl-PE (DOPE) liposomes along with H2O2, increases in particle sizes were observed that we attribute to liposome fusion and/or binding. As shown in Figure 3 and Figure S5A (raw distribution curves in Figure S5B-D), particles with larger diameters and greater polydispersity indices (PDI) were only detected after 1-liposomes were incubated with H2O2 and subjected to anionic liposomes. In contrast, negative control experiments in which liposomes were incubated with TBS buffer showed minimal changes in particle sizes. Here, the addition of DOPE appears to result in the production of particles with larger sizes, which likely results in enhanced fusion due to the non-bilayer properties of this lipid.
Figure 3.
DLS results for 20% 1/PC liposomes mixed with 10% PS/PC or PA/PE/PC=10/50/40 liposomes before and after addition of H2O2. Larger particles were detected upon H2O2 treatment in both cases. Error bars denote standard errors from at least three independent trials.
After successful demonstration of H2O2-mediated uncaging of lipid 1 and elucidation of subsequent interactions with anionic membranes microplate binding assays, we moved on to fluorescence microscopy studies to evaluate whether 1-liposomes enhance the cellular delivery of liposomes in the presence of H2O2. For these experiments, we prepared 20% 1/PC liposomes labeled with 0.08% Rd-PE, which enables detection of cellular infiltration by liposomes via fluorescence microscopy. These study liposomes (2 mM) were pre-incubated with 1 mM H2O2 for uncaging at room temperature for six hours before being diluted with buffer to 1 mM (H2O2 concentration = 0.5 mM) and added to human epithelial breast cancer cells (MDA-MB-231) in a 96-well plate. After 1 h incubation (37 °C and 5 % CO2), liposome solutions were removed, and cells were washed, fixed, labeled with DAPI, and imaged (20X images shown in Figure 4). As negative controls, cells were treated with PBS++ buffer (Figure 4A) or 0.5 mM H2O2 (Figure 4B) in the absence of any liposome formulations. Additionally, cells were treated with PC/Rd-PE liposomes lacking 1 with and without H2O2 incubation (Figures 4C-D). Minimal Rd-PE fluorescence could be seen in all of these cases, indicating no non-specific cell entry under these conditions. Cells treated with only 20% 1/PC liposomes similarly showed minimal Rd-PE fluorescence (Figure 4E), whereas a significant increase in Rd-PE fluorescence was observed for cells incubated with H2O2 pre-treated 20% 1/PC liposomes (Figure 4F). Additionally, the 4X Images were also captured (Figure S6) and the Rd-PE fluorescence intensities were quantified using Gen5 software. When the background fluorescence is account for, we observed a ~400-fold increase in cellular delivery when the cells were treated with uncaged 20% 1 liposomes (Figure 4G). The same experiment was conducted with a slightly different protocol where the liposomes were diluted with media before incubation with cells, since the excess H2O2 could potentially be quenched by the catalase from fetal bovine serum (10 % of cell culture medium). As shown in Figures S7-8, comparable results were observed in which only uncaged 1-liposomes enhanced the cellular entry. The same experiments were conducted with 10% 1-liposomes (Figures S9-10). These liposomes with lower percentages of uncaged 1 did not lead to significant cell delivery, which matched the vesicle assays (Figure 2B). While our microplate data show that guanidine uncaging drives liposome binding and/or fusion, this doesn’t necessarily mean that interactions with lipids drive cell entry. For example, a plethora of carbohydrates and other molecules on the cell surface could act as contacts between liposomes and cell membranes. Nevertheless, these results demonstrated that 1-liposomes yield dramatic enhancements in cellular delivery only in the presence of H2O2, showcasing their potential in the ROS-mediated liposomal target delivery.
Figure 4.
Fluorescence images indicating enhanced liposomal delivery to MDA-MB-231 cells with liposomes containing H2O2-uncaged 1 under buffer dilution conditions. Representative 20X images of cells treated with (A) PBS++ buffer, (B) 0.5 mM H2O2, (C) PC/Rd-PE liposomes, (D) PC/Rd-PE liposomes pre-incubated with H2O2, (E) 20% 1/PC/Rd-PE liposomes, and (F) 20% 1/PC/Rd-PE liposomes pre-incubated with H2O2. Blue: DAPI; red: Rd-PE. Scale bar, 100 μm. (G) Quantified Rd-PE fluorescence intensities with 4X images (Figure S6). A significant increase in Rd-PE fluorescence could only be seen for cells treated with H2O2-uncaged 1-liposomes. Error bars denote standard errors from at least three independent trials.
Conclusion
In conclusion, we developed a ROS-responsive liposome platform for targeted delivery with boronate-caged guanidine lipid 1. This compound contains three major structural components, including a H2O2-responsive boronate ester-caged guanidinium headgroup bearing a QM SIL, a hydrophilic TEG linker, and a triazole lipid anchor. Upon H2O2 incubation, lipid 1 undergoes decomposition to unveil guanidinium-containing cationic lipid product 2. Mass spectrometry as well as ZP measurements verified the expected uncaging process in the presence of H2O2 in the solution state and within a membrane environment, respectively. Liposomes containing 1 could efficiently fuse/bind to anionic membranes, as demonstrated by a microplate-based fluorescence assay as well as dynamic light scattering (DLS) analyses. Cellular fluorescence microscopy experiments verified enhanced cellular delivery with 1-liposomes pre-treated with H2O2. While ROS are often generated intracellularly, these changes can lead to tissue-associated increases that could be harnessed to uncage liposomes outside of the cell membrane.[38] Our data showcase a smart delivery system where liposomes are designed to initially be neutral and inactive, while encountering upregulated ROS unveils cationic characteristics that promote cell entry. This approach shows strong prospects for enhancing the targeting of liposomes to diseased rather than healthy cells in a manner mediated by local ROS abundance.
Experimental Section
Experimental procedures, synthesis and characterizations of new compounds are described in the Supplemental Information.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number NIH R15GM146193. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also thank Mahshid Mokhtarnejad and Dr. Bamin Khomami for assistance with DLS experiments.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supporting information for this article is given via a link at the end of the document
References
- [1].a) Dawidczyk CM, Kim C, Park JH, Russell LM, Lee KH, Pomper MG, Searson PC, J. Control. Release 2014, 187, 133–144; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Pharm. Res 2016, 33, 2373–2387; [DOI] [PubMed] [Google Scholar]; c) Crommelin DJA, van Hoogevest P, Storm G, J. Control. Release 2020, 318, 256–263. [DOI] [PubMed] [Google Scholar]
- [2].Hou X, Zaks T, Langer R, Dong Y, Nat. Rev. Mater 2021, 6, 1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Pattni BS, Chupin VV, Torchilin VP, Chem. Rev 2015, 115, 10938–10966; [DOI] [PubMed] [Google Scholar]; b) Oude Blenke E, Mastrobattista E, Schiffelers RM, Expert Opin. Drug Deliv 2013, 10, 1399–1410; [DOI] [PubMed] [Google Scholar]; c) Bibi S, Lattmann E, Mohammed AR, Perrie Y, Microencapsul J. 2012, 29, 262–276; [DOI] [PubMed] [Google Scholar]; d) Lee Y, Thompson DH, WIREs Nanomed. Nanobi 2017, 9, e1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Madani F, Lindberg S, Langel Ü, Futaki S, Gräslund A, Biophys J. 2011, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Zorko M, Langel Ü, Adv. Drug Deliv. Rev 2005, 57, 529–545; [DOI] [PubMed] [Google Scholar]; b) Foged C, Nielsen HM, Expert Opin. Drug Deliv 2008, 5, 105–117; [DOI] [PubMed] [Google Scholar]; c) Koren E, Torchilin VP, Trends Mol. Med 2012, 18, 385–393. [DOI] [PubMed] [Google Scholar]
- [6].a) Saar K, Lindgren M, Hansen M, Eiríksdóttir E, Jiang Y, Rosenthal-Aizman K, Sassian M, Langel Ü, Anal. Biochem 2005, 345, 55–65; [DOI] [PubMed] [Google Scholar]; b) Kilk K, Mahlapuu R, Soomets U, Langel Ü, Toxicology 2009, 265, 87–95. [DOI] [PubMed] [Google Scholar]
- [7].a) He Y, Li F, Huang Y, in Advances in Protein Chemistry and Structural Biology, Vol. 112 (Ed.: Donev R), Academic Press, 2018, pp. 183–220; [DOI] [PubMed] [Google Scholar]; b) Huang Y, Jiang Y, Wang H, Wang J, Shin MC, Byun Y, He H, Liang Y, Yang VC, Adv. Drug Deliv. Rev 2013, 65, 1299–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Lin Y, Mazo MM, Skaalure SC, Thomas MR, Schultz SR, Stevens MM, Chem. Sci 2019, 10, 1158–1167; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hansen MB, van Gaal E, Minten I, Storm G, van Hest JCM, Löwik DWPM, Control J. Release 2012, 164, 87–94; [DOI] [PubMed] [Google Scholar]; c) Shamay Y, Adar L, Ashkenasy G, David A, Biomaterials 2011, 32, 1377–1386. [DOI] [PubMed] [Google Scholar]
- [9].a) Jin E, Zhang B, Sun X, Zhou Z, Ma X, Sun Q, Tang J, Shen Y, Van Kirk E, Murdoch WJ, J. Am. Chem. Soc 2013, 135, 933–940; [DOI] [PubMed] [Google Scholar]; b) Lee ES, Gao Z, Bae YH, Control J. Release 2008, 132, 164–170; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH, Control J. Release 2008, 129, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) He H, Sun L, Ye J, Liu E, Chen S, Liang Q, Shin MC, Yang VC, Control J. Release 2016, 240, 67–76; [DOI] [PubMed] [Google Scholar]; b) Zhu L, Kate P, Torchilin VP, ACS Nano 2012, 6, 3491–3498; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Harris TJ, von Maltzahn G, Lord ME, Park JH, Agrawal A, Min DH, Sailor MJ, Bhatia SN, Small 2008, 4, 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Webb BA, Chimenti M, Jacobson MP, Barber DL, Nat. Rev. Cancer 2011, 11, 671–677. [DOI] [PubMed] [Google Scholar]
- [12].Matsumura Y, Ananthaswamy HN, Toxicol. Appl. Pharmacol 2004, 195, 298–308. [DOI] [PubMed] [Google Scholar]
- [13].Nathan C, Ding A, Cell 2010, 140, 951-951. e952. [DOI] [PubMed] [Google Scholar]
- [14].Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M, Antioxid. Redox Signal 2013, 18, 1208–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fransen M, Nordgren M, Wang B, Apanasets O, Biochim. Biophys. Acta, Mol. Basis Dis 2012, 1822, 1363–1373. [DOI] [PubMed] [Google Scholar]
- [16].Apel K, Hirt H, Annu. Rev. Plant Biol 2004, 55, 373–399. [DOI] [PubMed] [Google Scholar]
- [17].Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, LLeonart ME, Ageing Res. Rev 2013, 12, 376–390. [DOI] [PubMed] [Google Scholar]
- [18].Yang J, Yang J, Liang SH, Xu Y, Moore A, Ran C, Sci. Rep 2016, 6, 35613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H, Am. J. Cardiol 2003, 91, 7–11. [DOI] [PubMed] [Google Scholar]
- [20].Harrison DG, Gongora MC, Guzik TJ, Widder J, J. Am. Soc. Hypertens 2007, 1, 30–44. [DOI] [PubMed] [Google Scholar]
- [21].Li J-M, Shah AM, J. Am. Soc. Nephrol 2003, 14, S221–S226. [DOI] [PubMed] [Google Scholar]
- [22].Fubini B, Hubbard A, Free Radical Biol. Med 2003, 34, 1507–1516. [DOI] [PubMed] [Google Scholar]
- [23].Szatrowski TP, Nathan CF, Cancer Res. 1991, 51, 794–798. [PubMed] [Google Scholar]
- [24].Weinstain R, Savariar EN, Felsen CN, Tsien RY, J. Am. Chem. Soc 2014, 136, 874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].a) Saczewski F, Balewski Ł, Expert Opin. Ther. Pat 2009, 19, 1417–1448; [DOI] [PubMed] [Google Scholar]; b) Li L, Vorobyov I, Allen TW, J. Phys. Chem. B 2013, 117, 11906–11920; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Carlson PM, Schellinger JG, Pahang JA, Johnson RN, Pun SH, Biomater. Sci 2013, 1, 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Arisaka A, Mogaki R, Okuro K, Aida T, J. Am. Chem. Soc 2018, 140, 2687–2692. [DOI] [PubMed] [Google Scholar]
- [27].Lippert AR, Van de Bittner GC, Chang CJ, Acc. Chem. Res 2011, 44, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].a) Gisbert-Garzarán M, Manzano M, Vallet-Regí M, Chem. Eng. J 2018, 340, 24–31; [Google Scholar]; b) Zalipsky S, Qazen M, Walker JA, Mullah N, Quinn YP, Huang SK, Bioconjugate Chem. 1999, 10, 703–707. [DOI] [PubMed] [Google Scholar]
- [29].Yan J, Lee S, Zhang A, Yoon J, Chem. Soc. Rev 2018, 47, 6900–6916. [DOI] [PubMed] [Google Scholar]
- [30].O’Neil EJ, DiVittorio KM, Smith BD, Org. Lett 2007, 9, 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hwang D, Nilchan N, Nanna AR, Li X, Cameron MD, Roush WR, Park H, Rader C, Cell Chem. Biol 2019, 26, 1229–1239.e1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].a) Jourden JLM, Daniel KB, Cohen SM, Chem. Commun 2011, 47, 7968–7970; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lou J, Best MD, Bioconjugate Chem. 2020, 31, 2220–2230. [DOI] [PubMed] [Google Scholar]
- [33].a) Whitehead SA, McNitt CD, Mattern-Schain SI, Carr AJ, Alam S, Popik VV, Best MD, Bioconjugate Chem. 2017, 28, 923–932; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Losey EA, Smith MD, Meng M, Best MD, Bioconjugate Chem. 2009, 20, 376–383. [DOI] [PubMed] [Google Scholar]
- [34].Fatouros DG, Antimisiaris SG, Colloid Interface Sci J. 2002, 251, 271–277. [DOI] [PubMed] [Google Scholar]
- [35].a) Gong D, Smith MD, Manna D, Bostic HE, Cho W, Best MD, Bioconjugate Chem. 2009, 20, 310–316; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rowland MM, Bostic HE, Gong D, Lucas N, Cho W, Best MD, Chem. Phys. Lipids 2012, 165, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].a) Bevers EM, Williamson PL, Physiol. Rev 2016, 96, 605–645; [DOI] [PubMed] [Google Scholar]; b) Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ, Cancer Res. 1991, 51, 3062–3066. [PubMed] [Google Scholar]
- [37].Simberg D, Weisman S, Talmon Y, Barenholz Y, Crit. Rev. Ther. Drug 2004, 21, 62. [DOI] [PubMed] [Google Scholar]
- [38].Meitzler JL, Antony S, Wu Y, Juhasz A, Liu H, Jiang G, Lu J, Roy K, Doroshow JH, Antioxid. Redox Signal. 2014, 20, 2873–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.