Introduction
Mucous membrane pemphigoid (MMP), previously known as cicatricial pemphigoid, is an autoimmune blistering disease predominantly affecting mucosae of the mouth and eyes. In severe cases, complications such as blindness and esophageal/tracheal strictures cause significant morbidity.1 Herein, we present the case of an elderly woman with recalcitrant MMP with oral, ocular, and esophageal involvement, recalcitrant to several therapies now responding to baricitinib and methotrexate.
Case report
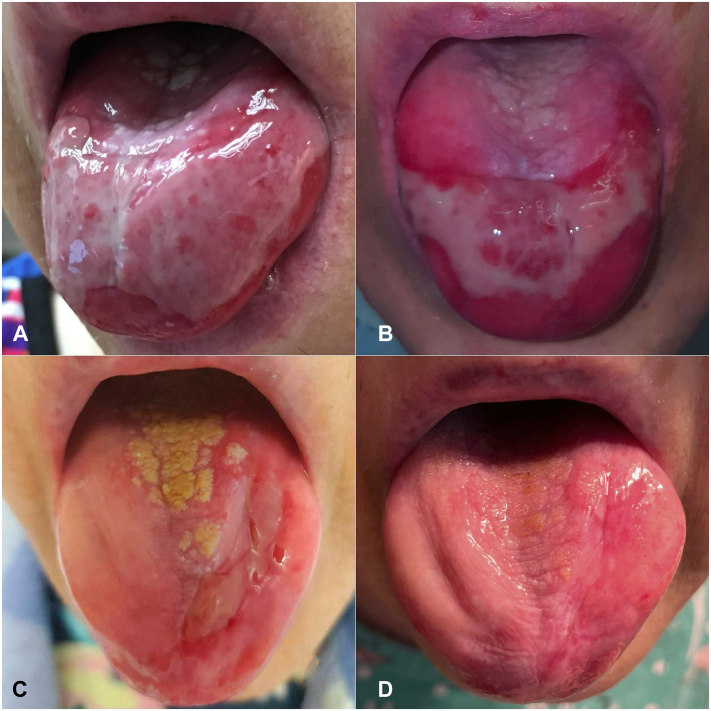
In 2016, a 69-year-old woman with a history of type 2 diabetes and estrogen receptor–positive breast cancer treated in 1995 by mastectomy, adjuvant tamoxifen, and radiation therapy developed eye redness, oral ulcers, odynophagia, and dysphagia leading to a 50-pound weight loss. Esophagogastroduodenoscopy was significant for ulcerations and strictures leading to percutaneous endoscopic gastrostomy tube placement. She was edentulous with painful erosions on the hard palate, oropharynx, buccal mucosa, and dorsal tongue (Fig 1, A). An initial ophthalmologic evaluation in July 2017 was significant for 2+ inflammation and Foster stage III conjunctival scarring bilaterally. Serological studies included elevated anti-BP180 and BP230 IgG antibodies on enzyme-linked immunosorbent assay and negative indirect immunofluorescence performed on the salt-split normal human skin. Conjunctival biopsy demonstrated 2+ positivity for IgA and IgG at the basement membrane zone on direct immunofluorescence, and she was diagnosed with MMP with ocular, oral, and esophageal involvement.
Fig 1.
A, July 2017 initial evaluation at our institution: Persistent tongue ulceration despite 7 months of prednisone 20 mg daily and methotrexate 20 mg weekly. B, October 2018. Persistent ulcers on the tongue after 12 months of rituximab/IVIG and 5 months of oral MMF 1000 mg twice daily. C, October 2021. Improvement in tongue ulceration after 20 months of baricitinib 2 mg daily and 8 months of methotrexate weekly. D, June 2022. Minimal ulceration on the tongue, after 27 months of baricitinib 2 mg daily and 16 months of methotrexate weekly and 2 intralesional triamcinolone 10 mg/cc injections. IVIG, Intravenous immunoglobulin; MMF, mycophenolate mofetil.
Treatment previously included topical corticosteroid therapy, prednisone 20 mg daily and methotrexate 15 mg weekly for 10 months. In September 2017, methotrexate was discontinued, and she began the Foster-Ahmed protocol of intravenous immunoglobulin (IVIG) at 2 g/kg monthly and rituximab 375 mg/m2 weekly for 8 weeks, monthly for 4 months, and every 4 months thereafter.2 After 6 months, ocular inflammation decreased, but oral and esophageal disease persisted. In January 2018, chronic prednisone therapy led to the development of vertebral compression fractures. She had not been on oral bisphosphonate therapy due to esophageal involvement of MMP. Subsequently, she was started on intravenous zoledronic acid infusions. Other complications during her first year of treatment included fungal keratitis treated with topical voriconazole and amphotericin and localized varicella zoster.
In May 2018, mycophenolate mofetil 1000 mg twice a day was added to IVIG and rituximab due to persistent activity in all sites. After 5 months, this regimen was changed to cyclophosphamide due to partial response (Fig 1, B). Cyclophosphamide was titrated to 2 mg/kg daily with mild improvement in ocular and esophageal disease. Severe diarrhea and cytopenias necessitated a dose reduction to 0.5 mg/kg daily, and her disease flared. In May 2019, she developed new nodules on her right breast; biopsy revealed recurrence of breast cancer, her first since finishing tamoxifen therapy in 2000. Positron emission tomography-computed tomography was negative for metastatic disease, and she was started on anastrozole in July 2019. By January 2020, the breast nodules had regressed in size, with no new nodules formed.
In February 2020, cyclophosphamide was discontinued due to persistent oral and esophageal disease and concern that it contributed to her breast cancer recurrence, and baricitinib 2 mg daily was begun. Within 1 month, she reported improvement in oral and ocular symptoms. Additionally, oral intake improved, and her percutaneous endoscopic gastrostomy tube was discontinued in September 2020. Due to persistent tongue ulceration and mild esophageal disease, methotrexate 12.5 mg weekly was added in February 2021 and increased to 20 mg weekly in August 2021. At her last endoscopy in September 2021, there was minimal inflammation without stenoses. Her conjunctival inflammation has been inactive since October 2020. She tapered off prednisone in early 2022 for the first time in 6 years. Her persistent tongue ulceration has decreased (Fig 1, C) and the recent addition of intralesional steroid injections have led to almost complete healing (Fig 1, D).
Discussion
Due to the lack of United States Food and Drug Administration (FDA)-approved therapies, treatment of MMP relies on the use of “off-label” immunosuppressive agents. “Low-risk” cases involving only the oral mucosa and/or the skin can be managed with topical therapy, but “high-risk” cases involving ocular, genital, nasopharyngeal, esophageal, or laryngeal mucosae are generally treated with cyclophosphamide or rituximab and IVIG.3 The European (S3) 2021 guidelines for the management of MMP also recommend dapsone and tumor necrosis factor inhibitors.4 Bortezomib has been used for recalcitrant cases.5
These therapies carry significant adverse effects and costs. Cyclophosphamide has gastrointestinal effects, causes cytopenia requiring close monitoring, and has a long-term cancer risk. Systemic steroids are ineffective for inducing remission and cause unacceptable complications with chronic use. Rituximab, IVIG, and bortezomib infusions require significant health care resources and cost patients time, travel, and have risks of complications related to long-term IV access. Rituximab is associated with increased risk of severe complications of coronavirus disease 2019 infection and interferes with coronavirus disease 2019 vaccine efficacy. Tumor necrosis factor inhibitors require self-injection and cold storage and eventually develop antibodies. Alternative treatments for severe MMP are sorely needed.
Baricitinib, a Janus kinase (JAK) inhibitor, blocks the JAK1 and JAK2-STAT signaling pathway and inhibits interleukin 6 (IL-6), IL-12, and IL-23, resulting in the suppression of pathogenic Th17 cells differentiation. Studies have described a role for IL-6 and IL-17 in MMP.6,7 Baricitinib, in combination with methotrexate, is FDA-approved for treatment of moderate-to-severe rheumatoid arthritis and has garnered recent attention as an off-label treatment for severe coronavirus disease 2019 pneumonia.8 Importantly, baricitinib is orally bioavailable and has milder side effects and fewer long-term risks than cyclophosphamide. While JAK inhibitors bear an FDA black box warning for venous thromboembolism, a recent meta-analysis suggested that this risk may be overstated.9
In 2018, Sarny et al10 reported the case of a patient with recalcitrant MMP with severe ocular involvement that was successfully managed with baricitinib and methotrexate. Citing this case, a phase II randomized clinical trial examining the efficacy of baricitinib plus methotrexate for the treatment of ocular MMP is ongoing.11 Tofacitinib, a JAK1 and JAK3 inhibitor, was recently reported to also be effective in 2 patients with refractory ocular MMP.12
In this case of recalcitrant multisystem MMP, treatment with baricitinib and methotrexate induced a corticosteroid-free near-complete remission. Baricitinib’s favorable side effect profile and oral bioavailability make it an exciting alternative to current treatments. Prospective studies are needed to further evaluate JAK inhibitors and define their place within the treatment algorithm for severe MMP.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Informed Consent: Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient.
References
- 1.Du G., Patzelt S., van Beek N., Schmidt E. Mucous membrane pemphigoid. Autoimmun Rev. 2022;21(4) doi: 10.1016/j.autrev.2022.103036. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A.R., Kaveri S. Reversing autoimmunity combination of rituximab and intravenous immunoglobulin. Front Immunol. 2018;9:1189. doi: 10.3389/fimmu.2018.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan L.S., Ahmed A.R., Anhalt G.J., et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138(3):370–379. doi: 10.1001/archderm.138.3.370. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt E., Rashid H., Marzano A.V., et al. European Guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology - Part II. J Eur Acad Dermatol Venereol. 2021;35(10):1926–1948. doi: 10.1111/jdv.17395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeed L., Schmidt T.H., Gensler L.S., et al. Successful treatment of mucous membrane pemphigoid with bortezomib. JAAD Case Rep. 2017;4(1):81–83. doi: 10.1016/j.jdcr.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choy E., Rose-John S. Interleukin-6 as a multifunctional regulator: inflammation, immune response, and fibrosis. J Scleroderma Relat Disord. 2017;2(suppl 2):1–5. doi: 10.5301/jsrd.5000265. [DOI] [Google Scholar]
- 7.Suelves A.M., Zhao T.Z., Siddique S.S., Foster C.S. Profile of local interleukin expression in a cohort of ocular cicatricial pemphigoid patients. Invest Ophthalmol Vis Sci. 2012;53(13):8112–8117. doi: 10.1167/iovs.11-9322. [DOI] [PubMed] [Google Scholar]
- 8.Roskoski R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2022 update. Pharmacol Res. 2022;175 doi: 10.1016/j.phrs.2021.106037. [DOI] [PubMed] [Google Scholar]
- 9.Yates M., Mootoo A., Adas M., et al. Venous thromboembolism risk with JAK inhibitors: a meta-analysis. Arthritis Rheumatol. 2021;73:779–788. doi: 10.1002/art.41580. [DOI] [PubMed] [Google Scholar]
- 10.Sarny S., Hucke M., El-Shabrawi Y. Treatment of mucous membrane pemphigoid with Janus kinase inhibitor baricitinib. JAMA Ophthalmol. 2018;136(12):1420–1422. doi: 10.1001/jamaophthalmol.2018.3789. [DOI] [PubMed] [Google Scholar]
- 11.Washington University School of Medicine Baricitinib for the treatment of ocular mucous membrane pemphigoid. Identifier NCT05263505. 2022. https://clinicaltrials.gov/ct2/show/NCT05263505
- 12.James H., Paley G.L., Brasington R., Custer P.L., Margolis T.P., Paley M.A. Tofacitinib for refractory ocular mucous membrane pemphigoid. Am J Ophthalmol Case Rep. 2021;22 doi: 10.1016/j.ajoc.2021.101104. [DOI] [PMC free article] [PubMed] [Google Scholar]