Abstract
Sleep deprivation (SD) causes significant deficits in multiple aspects of cognition, including sustained attention and working memory. Investigating the neural processes underpinning these cognitive losses has proven challenging due to the confounds of current animal tasks; many employ appetitive or aversive stimuli to motivate behavior, while others lack task complexity that translates to human studies of executive function. We established the Lux Actuating Search Task (LAST) to circumvent these issues. The LAST is performed in a circular, open-field arena that requires rats to find an unmarked, quasi-randomly positioned target. Constant low-level floor vibrations motivate ambulation, while light intensity (determined by the rodent's proximity to the target destination) provides continuous visual feedback. The task has two paradigms that differ based on the relationship between the light intensity and target proximity: the Low Lux Target (LLT) paradigm and the High Lux Target paradigm (HLT). In this study, on days 1–6, the rats completed nine trials per day on one of the two paradigms. On day 7, the rats were either sleep deprived by gentle handling or were left undisturbed before undertaking the opposite (reversal) paradigm on days 7–9. Our results showed that SD significantly impeded the ability of Long Evans rats to learn the reversal paradigm, as indicated by increased times to target and increased failure percentages compared to rats whose sleep was undisturbed. Rats also showed reduced learning with the HLT paradigm, as the initial task or as the reversal task, likely due to the rodents' photophobia limiting their motivation to navigate toward a bright light, which is required to succeed.
Keywords: Light, Cognition, Rodent models, Attention, Sleep loss, Cognitive flexibility, Reversal learning
Highlights
-
•
A continuous feedback paradigm examining the effects of sleep loss on cognitive flexibility in rats is introduced.
-
•
Floor vibrations motivate and variable light intensity directs navigation to an unmarked location in an open field arena.
-
•
The reversal of light intensity cues from light to dark and vice versa is disrupted by sleep deprivation.
Abbreviations:
- CONT
undistrbed sleep control animal
- HLT
High Lux Target paradigm
- LAST
Lux Actuating Search Task
- LLT
Low Lux Target paradigm
- PVT
Psychomotor Vigilance Task
- SD
Sleep deprivation
- VAST
Vibration Actuating Search Task
1. Introduction
High-pressure decision-making scenarios, requiring sustained attention and executive functions, are common to demanding occupations (e.g., military, emergency services, and healthcare). To adequately attend to the demands of these scenarios, individuals must partner knowledge acquired from previous decisions with new information presented in the context of a dynamic environment. Thus, successful decision-making requires cognitive flexibility — the ability to adjust behavior in response to changing contingencies. Unfortunately, many cognitively demanding occupations require long work hours and shift work, which is associated with poor sleep, errors in decision-making, and reduced cognitive flexibility capabilities (James et al., 2017; Ganesan et al., 2019; McHill and Wright., 2019).
Reversal learning paradigms have been integral to identifying the neural correlates of cognitive flexibility. Several studies suggest that reversal learning is mediated by cortico-striatal networks (Remijnse et al., 2005; Ghahremani et al., 2010). Although lesions in these regions have led to reversal learning deficits (Fellows and Farah, 2003; Hornak et al., 2004), modulating cortico-striatal neurotransmitter activity has had limited success in clinical settings (Marinova et al., 2017; Robbins et al., 2019). The specific mechanisms underpinning reversal learning are still under investigation; however, the detrimental impact of sleep deprivation (SD) on reversal learning is consistent across human (Ghahremani et al., 2010; Pilcher and Huffcutt, 1996), primate (Chau et al., 2015; Dias et al., 1996) and rodent models (Palchykova et al., 2006; Marti et al., 2020).
Elucidating the neurobiological basis of SD-induced deficits in cognitive flexibility requires a robust animal paradigm which yields SD-mediated declines in performance that are comparable to the complexities of human decision making. Studies in humans have demonstrated that sleep loss and fatigue critically impact sustained attention (Gunzelmann et al., 2011; Chua et al., 2014); thus, to be an accurate translational model, a rodent paradigm must be capable of demonstrating and quantifying this key aspect of cognition. Such a paradigm should also be simple enough to allow for rapid task acquisition – thereby reducing resource and time requirements – while maintaining sufficient complexity to accurately model the dynamic and continuous performance feedback that is crucial to many applications of empirical and real-world human tasks.
Current rodent reversal-learning paradigms use appetitive or aversive incentives to reinforce task-related behavioral responses. Appetitive rewards, such as food pellets and water given in response to successful performance, can be confounded by satiation, which reduces reward salience (Bissonette et al., 2014; Goltstein et al., 2018). To overcome this, rodents are typically food- or water-restricted prior to task initiation — though this naturally leads to a gradual decrease in motivation as the hunger or thirst is reduced, which introduces changes to the animals' physiological state (Iivonen et al., 2003; Oonk et al., 2015). Meanwhile, aversive stimuli, such as forced swim and foot shock, induce physiological and psychological confounds, such as fatigue and stress induction, which can impede a clear interpretation of results (Agterberg and Versnel, 2014 Armario, 2021; Hurtubise and Howland, 2017).
The Lux Actuating Search Task (LAST) is a novel, open-field spatial navigation paradigm described herein. It replaces traditional motivational stimuli with gradient visual feedback used to guide rats to an unmarked target destination (Fig. 1). Our group introduced an earlier iteration of this task as a mouse paradigm (Bushana et al., 2020) and later as a rat paradigm (Lawrence-Sidebottom et al., 2021), called the Vibration Actuating Search Task (VAST), which used floor vibration as continuous haptic and audio feedback for guidance. Here, we have successfully implemented the LAST to examine how SD affects cognitive flexibility, as evidenced by performance decrements in a cued reversal spatial navigation task. In these experiments, the rat's proximity to the target destination determined the light intensity, providing continuous feedback. With movement toward the target, the lights either diminished or increased in intensity, corresponding with the Low Lux Target (LLT; Fig. 2A and B) paradigm and the High Lux Target (HLT; Fig. 2C and D) paradigm, respectively. Floor vibrations were delivered continuously, and without change in intensity, throughout the task. Once the rat reached the unmarked target destination, the lights and vibration ceased, and the rat was removed from the apparatus. Thus, the rat's distance from the target destination modulated the visual feedback intensity in a smooth, graded fashion (Fig. 2B and D). The instantaneous rodent spatial position-based continuous feedback on the LAST and earlier VAST (Bushana et al., 2020; Lawrence-Sidebottom et al., 2021) is unprecedented compared to contemporary rodent goal-oriented navigational tasks.
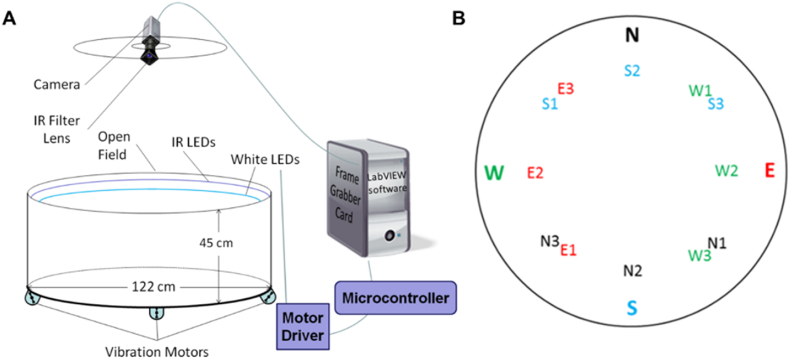
Fig. 1.
Schematic of the LAST arena and insertion/target map.
The open field arena is encircled by an acrylic wall, with two LED SMD 5050-300-IR infrared Tri-Chip flexible LED strips (LEDLightsWorld) mounted around the arena perimeter (B). There are also four, 45 mm electric rotary motors (model 345–400; Precision Microdrives) located at the base of each leg of the arena platform. The apparatus also includes a Manta G-201 digital camera with an infrared filter (Allied Vision), two TB6612FNG dual motor driver carriers (Pololu), a chipKIT WF32 microcontroller (Digilent), and a computer with LabVIEW software and a PCIe-6341 multifunction I/O card (National Instruments). (B) The LAST arena insertion points (N=North, W=West, E = East, and S=South) are shown with their associated, equidistant target destinations (N1-3 for entry point N, W1-3 for entry point W, S1-3 for entry point S, E1-3 for entry point E).
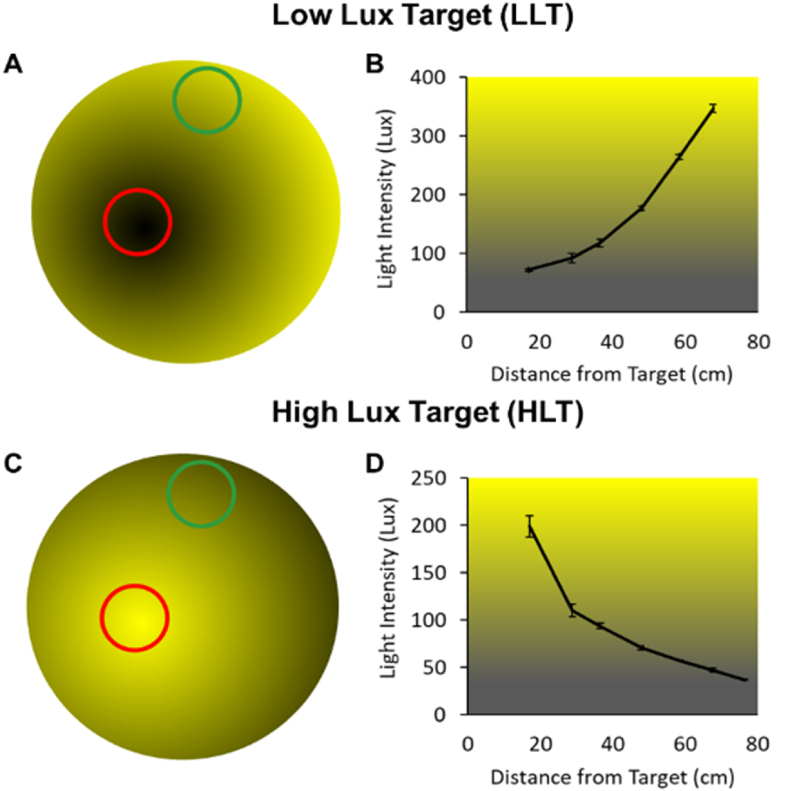
Fig. 2.
Light intensity gradients for the LLT and HLT paradigms.
A schematic of the ambient light intensity produced for any given XY coordinate of rat position in the open field arena with light intensity either decreasing (A; LLT) or increasing (C; HLT) as the rat approaches the target (red circle). The green circle identifies the pre-determined entry location. The LAST light intensities are shown as mean (+/-SEM) functions of the distances from the target for the LLT (B) and HLT (D) paradigms. Lux was measured with a luminometer (sampled in triplicate), while a stationary object was incrementally moved from the outer edge of a target destination. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2. Methods
2.1. Animals
56 male Long Evans rats aged 9–12 weeks were used for these studies. The rats were progeny from our onsite colony generated from breeder stock (Envigo). Rats were housed two to a cage at 23 ± 1 °C and placed on a 12:12hr light:dark cycle with access to food and water ad libitum. Cages were also enriched with nesting materials and a Nylabone chew. All LAST-related experimentation (including handling) was performed during the final 2 h of the light cycle, zeitgeber time (ZT) 10–12, except for the 10-h SD which occurred from ZT0-10 in those cohorts. The length of SD was selected from the SD dose response curve of response latency in the 5-choice serial reaction time test, which shows 10 h to be more effective than four or 7 h of SD (Córdova et al., 2006). This timing of LAST training in the current study was selected because the majority of rat sleep during the light phase had occurred (i.e., training had minimal interference with sleep), and also to allow for sufficient sleep pressure to build over the 10 h SD condition. Rats completed the experiment in serial cohorts of four. Cohorts were randomly assigned to SD or control (CONT) groups. All animal procedures were approved by the Washington State University Institutional Animal Care and Use Committee and were compliant with National Institutes of Health guidelines. At the end of the study, rats were euthanized by CO2 inhalation in a manner consistent with the Panel on Euthanasia of the American Veterinary Medical Association.
2.2. Apparatus
The LAST was delivered on a circular open-field arena (122 cm diameter), which was encircled by a 45 cm wall of white acrylic (Fig. 1A). The open-field arena was painted flat tan to reduce glare. Attached to the top of the wall were two LED strips: one white (850 nm, type 5050) and one infrared. Four support legs elevated the arena, with vibration motors mounted at the base of each leg. This elevation also served to obscure any extra-maze visual cues from the rats.
2.2.1. Position and motion tracking
A downward-angled strip of infrared LEDs mounted around the top of the wall provided platform coverage adequate for tracking throughout the surface area of the arena. A Manta Vision camera video, mounted above the open-field arena, recorded each trial through an infrared filter, allowing the rat's position to be determined and recorded for data analysis of trial performance. The camera recorded position at ten frames per second, and image acquisition was implemented in LabVIEW using NI-IMAQdx 4.0 driver software (National Instruments, Austin, TX). The video feed was filtered based on a predetermined pixel intensity threshold, enabling us to distinguish the rat from the platform. A center mass algorithm was used to define the rat's centroid to estimate and track the rat's position from the resulting image. A criterion of at least 1 pixel (approximately 1 mm) change in position was used to determine whether the rat moved between frames (Dias et al., 1996). The data were saved using the LIVE HDF5 software package (UPVI LLC, Hancock, MI).
2.2.2. Visual LED feedback
The DC output was governed by a pulse width modulator that controlled the duty cycle of the second downward-angled LED strip of white lights via the LINX vi package (LabVIEW, MakerHub). A LabVIEW software subroutine calculated the distance between the centroid position and the target destination. These values drove the microcontroller to govern the intensity of the lights (range: 35–350 lux). The LED strips utilized in the LAST are PWM dimmable SMD5050 capable of 900 lumens per meter with a natural white color temperature (4000–4500K) drawing approximately 16 W while in use. Fig. 2 shows the relationship between distance to target and light intensity across the entire field, as measured with a lux meter (sampled in triplicate for each X, Y coordinate and averaged).
2.3. Experimental design
2.3.1. Handling/habituation protocol
The rats were habituated over ten days (H1–H10). On the first five days (H1–H5), each rat received 5 min of gentle handling to acclimate them to the experimenters. The handling consisted of gentle stroking, holding, and repeatedly removing and replacing the rats in their home cages to acclimate them to the LAST process. On H6, the entire cohort was placed in the LAST arena for 300 s in the absence of lights and vibration. Then, each individual rat was placed in the LAST arena for 300 s without lights or vibration. On H7, the rats were individually exposed to the arena for 150 s with no lights or vibration, then for 150 s with only lights and no vibration. On H8, the rats were exposed to the arena for 300 s without lights but with continuous, low-level vibration delivered whenever they were on the arena's perimeter (defined as 15 cm from the edge of the arena) to encourage movement toward the center. On H9 and H10, the rats were exposed to the arena for 300 s in the presence of ambient white light (63 ± 3 lux) and constant, low-level vibration when they were on the arena's perimeter.
2.3.2. LAST sessions and sleep deprivation
Prior to LAST performance testing, the rats' home cages were moved from the colony room to the experiment room. For each trial, each rat was positioned to face the apparatus wall at one of four predetermined entry locations: A, B, C, or D. Each entry point had three potential target destinations (depicted as A1-3, B1-3, C1-3, D1-3; Fig. 1B), all of which were 25 cm in diameter and equidistant from their corresponding entry point. Target destination borders were at least 15 cm from the wall to prevent unintentional discovery from thigmotaxis. Entry points and target destinations were quasi-randomly selected for each performance testing day using a Latin square. Once a rat entered the apparatus, the motors began vibrating at a constant 2500 RPM (41.6 Hz) and the LED strips illuminated at an intensity determined by the rat's proximity to the target destination (Fig. 2). In the LLT paradigm, the illumination intensity diminished as the rat approached the target (Fig. 2A and B). In the HLT paradigm, the illumination intensity increased as the rat approached the target (Fig. 2C and D). The vibration and lights shut off immediately once the rat entered the target, or after 90 s of searching without locating the target. The latter was classified as a “failed” trial. Rats were tested with nine trials each on either the LLT or HLT paradigm on each of the first six days (54 trials total). On day 7, at light onset (ZT0), the rats either remained in their home cage for spontaneous sleep (CONT group) or were subjected to total SD by gentle handling for 10 h (SD group). At ZT10, immediately following spontaneous sleep or 10-h SD, rats were tested on the reversed paradigm for the remaining three days.
Rats alternated trials with their cage mate, and the second pair from a cohort began trials immediately after the first pair completed all nine trials. The arena was cleaned with 10% ethanol between each trial. After completing all nine trials, two Froot Loops were given to each rat as a reward. After each cohort completed their nine trials for the day, the rats were returned to the colony room in their home cages. Sessions occurred once per day, beginning at ZT10, on five successive days before a two-day weekend break, with the remaining four sessions carried out the following week.
2.4. Statistical analyses
Data were analyzed using SAS 9.4. All type I error thresholds were set to α = 0.05. Group trends are reported as mean ± standard error of the mean (SEM), as calculated using estimates within each analysis of variance (ANOVA). Rats with more than two failures (<78% success) on day 6 were eliminated from the analysis. 23 rats did not successfully complete >78% of the trials on day 6, indicating inadequate task acquisition and retention (HLT n = 16 vs. LLT n = 3), and were therefore excluded from the data analyses. Thus, 32 rats were included in the data analyses, of which, 18 were in the CONT group (LLT reversal n = 10; HLT reversal n = 8), and 14 were in the SD group (LLT reversal n = 7; HLT reversal n = 7).
2.4.1. Dependent variables
For each trial, “failure” in task performance was based on whether the rat located the target within 90 s. Time to target was the time it took for the rat to end the trial, which is the time from the entry to the target destination for successful trials or 90 s for failed trials. Path distance was the cumulative movement of a rat's centroid from the trial start to finish. Time still was operationally defined as the total amount of time the rat spent immobile or moving a negligible amount (e.g., a position change due to a non-ambulatory head movement), operationalized as any 0.1 s interval during which the change in position was less than 15 pixels (approximately 1.5 cm).
2.4.2. Between-session analyses
Data acquired from the nine trials per day were averaged for each rat on each dependent variable (viz. failure percentage, time to target, path distance, and time still) and used for the between-session data analyses. Data were analyzed using mixed-effects ANOVA with fixed effects of day, group (SD and CONT), task paradigm (HLT – LLT or LLT – HLT), and a random effect of the subject on the intercept. For each task paradigm, planned contrasts were performed between consecutive days – i.e., day 1 was compared to day 2, day 2 was compared to day 3, etc. – to assess for changes in performance over days. All rats were analyzed as a single group for comparisons between day 1 and day 6. For comparisons between days 6–9 the rats were differentiated into SD and CONT groups. Also, for each task paradigm, between-groups planned contrasts were performed for days 7–9 to determine the effect of SD on post-reversal performance.
2.4.3. Within-session analyses
For the within-session data analyses, time to target and path distance results from each block of three trials (i.e., trials 1–3, 4–6, and 7–9) were averaged per day for each subject over days 1–6 to assess within-session daily performance. Data were analyzed using mixed-effects ANOVA with fixed effects of day, trial block, task paradigm, and a random effect of the subject on the intercept. For each task paradigm, planned contrasts were performed between the first trial block (trials 1–3) and the last trial block (trials 7–9) on each day.
3. Results
3.1. Between-session data analyses
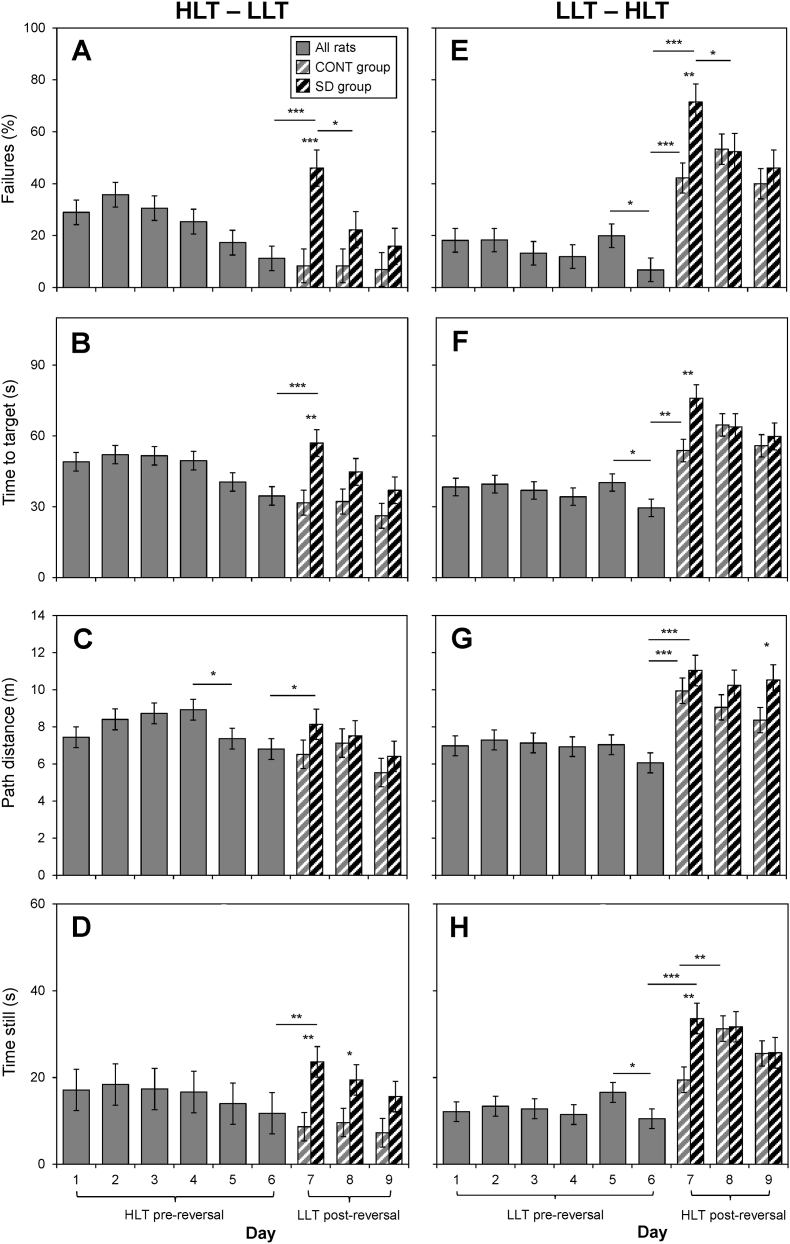
For percent failures, significant main effects of day (F8,224 = 9.48, p < 0.001), group (F1,224 = 7.93, p = 0.005), and a significant interaction between day and group (F8,224 = 2.80, p = 0.006) indicate that SD impedes task completion in reversal learning (Fig. 3A and E). Task paradigm (LLT vs. HLT) also affected trial failures per significant interactions with day (F8,224 = 13.22, p < 0.001) and with group (F1,224 = 8.46, p = 0.004). A main effect of day (F8,224 = 7.00, p < 0.001) and a day by task paradigm interaction (F8,224 = 13.45, p < 0.001) were detected for time to target (Fig. 3B and F). Significant interactions between day and group (F1,224 = 3.88, p < 0.001) and group and task paradigm (F1,224 = 4.11, p = 0.044) suggest that SD delayed task completion times. Task performance efficiency changed over sessions as demonstrated by a significant main effect of day (F8,224 = 4.60, p < 0.001) and significant interactions of day with task paradigm (F8,224 = 9.62, p < 0.001) and with group (F8,224 = 3.06, p = 0.003) on path distance (Fig. 3C and G). Finally, time still was different across days (F8,224 = 6.54, p < 0.001), groups (F1,224 = 7.07, p = 0.008) and this effect was also reflected by a day and group interaction (F8,224 = 2.41, p = 0.017; Fig. 3D and H). Other significant interactions on time still occurred between task paradigm and day (F8,224 = 9.39, p < 0.001) and group. (F1,224 = 7.78, p = 0.006). Representative traces for each paradigm are provided (Fig. 4).
Fig. 3.
LAST performance for rats in the LLT – HLT reversal and HLT – LLT reversal groups. Data are plotted as group means ± SEM for all rats (solid gray) on days 1–6 (pre-reversal days), and for the CONT (gray stripes) and SD (black stripes) groups on days 7–9 (post-reversal days). Between-day comparisons between days 1–6 included all rats (not differentiated by group) and between-day comparisons between days 6–9 were made within-group (CONT and SD differentiated). Statistically significant between-day differences are indicated by lines and asterisks. Between-group comparisons were performed for days 7–9. Statistically significant within-day differences between the CONT and SD groups are indicated by asterisks alone. *p < 0.05, **p < 0.01. ***p < 0.001.
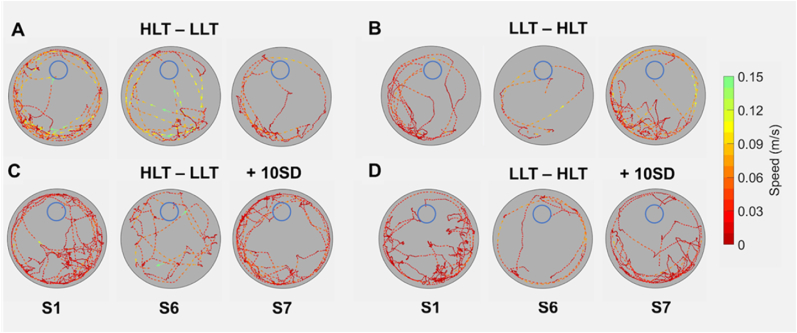
Fig. 4.
Representative LAST performance traces for rats in the LLT – HLT and HLT – LLT reversal groups with and without sleep deprivation. Path traces of trials 6–9 of sessions 1, 6 and 7 (S1, S6 and S7, respectively) from a rat in each of the four groups (A–D). The three session traces are aligned on target destination (blue circles) and traces depict position, direction (arrows) and speed (color). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.1.1. HLT– LLT reversal paradigm
For rats subjected to the HLT paradigm during the pre-reversal phase, the incremental decreases from day 1 to day 6 on percent failures were not statistically significant (between-day comparisons p > 0.05; Fig. 3A). Time to target and time still changes across days were also not detected (Fig. 3B and D, solid gray bars). However, we did observe a 1.5 m decrease in path distance from day 4 to day 5 (F1,224 = 5.07, p = 0.025) (Fig. 3C, solid gray bars).
Failure percentage, time to target, path distance, and time still following reversal to LLT were unchanged (day 6 vs. day 7) (Fig. 3A–D gray striped bars) in the CONT group. These results indicate that well-rested rats rapidly adapt to the more ethologically relevant behavior of low lux preference when approximating the target in the LLT reversal paradigm following the HLT pre-reversal paradigm. Furthermore, the performance of well-rested rats was not affected by reversal to the LLT paradigm from the HLT counter-preferent paradigm.
SD group performance was significantly impaired on the first day of reversal to the LLT paradigm. From day 6 to day 7, the percentage of failures increased by about three-fold (F1,224 = 12.93, p < 0.001), time to target increased by 25.3 s (F1,224 = 52.97, p < 0.001), path distance increased by 2.2 m (F1,224 = 4.82, p = 0.029), and in time still increased by 11.9 s (F1,224 = 7.44, p = 0.007; Fig. 3A–D black striped bars). While the failure percentage, time to target, path distance, and time still decreased over the reversal days (day 7 to day 9) for the SD group, the only statistically significant change observed was a decrease in the percentage of failures from day 7 to day 8 (F1,224 = 6.60, p = 0.011). Despite moving to a much easier LLT paradigm, the lack of sleep disrupted cognitive flexibility on all performance measures.
Between-groups comparisons confirmed that the SD group performed worse than the CONT group on the first day of reversal (day 7), with the SD group failing the task more than the CONT group (46.0% vs. 8.3%; F1,224 = 15.64, p < 0.001), spending 31.4 s more time finding the target (F1,224 = 10.57, p = 0.001), and staying still for 15.1 s longer (F1,224 = 9.69, p = 0.002) than the CONT group. Path distance did not differ between-groups on the first day of reversal. The percentage of failures, time to target, and path distance also did not differ between groups on the subsequent reversal days (days 8 and 9) (Fig. 3A–C). However, time still was greater for the SD group than the CONT group on day 8 (F1,224 = 4.18, p = 0.042) and day 9 (comparison approached statistical significance; F1,224 = 3.02, p = 0.084). These results indicate that SD impaired the rats' ability to perform the LLT paradigm after being subjected to the HLT paradigm. There appear to be improvements in LLT paradigm performance over the reversal days for the SD group, suggesting a recovery in the performance impairments induced by SD, but these changes were not statistically significant.
3.1.2. LLT – HLT reversal paradigm
Increased response efficiencies in rats initially performing the LLT paradigm were more subtle but evident from day 5 to day 6, based on a 13.7 point decrease in percentage of failures (F1,224 = 4.69, p = 0.031), a 10.5 s decrease in time to target (F1,224 = 5.22, p = 0.023) and a 5.6 s decrease in time still (F1,224 = 4.52, p = 0.035; solid gray bars on Fig. 3E, F and H, respectively).
Following reversal to the HLT paradigm, the well-rested CONT group exhibited impairments in performance. From day 6 to day 7, the percentage of failures increased by about four-fold (F1,224 = 18.47, p < 0.001), time to target increased by 18.0 s (F1,224 = 8.91, p = 0.003), path distance increased by 3.1 m (F1,224 = 13.66, p < 0.001), and time still increased by 6.6 s (comparison approached statistical significance; F1,224 = 3.21, p = 0.075; Fig. 3E–H, gray striped bars). For the CONT group, the percentage of failures was stable from day 7–8 but decreased from day 8 to day 9 (comparison approached statistical significance; F1,224 = 2.96, p = 0.087). Time to target increased from day 7–8 (comparison approached statistical significance; F1,224 = 3.25, p = 0.073), as did time still (F1,224 = 10.35, p = 0.002), then both variables were stable from day 8–9. Path distance was stable over the post-reversal days (days 8 and 9).
For the SD group, performance was significantly impaired on the first reversal day. Most notably, from day 6 to day 7, the percentage of failures increased by approximately fourteen-fold (F1,224 = 51.71, p < 0.001). Additionally, time to target increased by 52.4 s (F1,261 = 52.97, p < 0.001), path distance increased by 5.7 m (F1,261 = 31.58, p < 0.001), and time still increased by 25.5 s (F1,261 = 33.91, p=<0.001; Fig. 3E–H, black striped bars). The percentage of failures observed for the SD group decreased from day 7 to day 8 (F1,224 = 4.22, p = 0.041), but remained stable from day 8 to day 9. Time to target, path distance, and time still were stable from day 7 to day 9 for the SD group, though the comparison between day 7 and 8 approached statistical significance for time to target (F1,224 = 2.85, p = 0.093).
Between-groups comparisons revealed that the SD group performed worse than the CONT group on the first day of reversal (day 7). Indeed, the SD group failed the task more than the CONT group (71.4% vs. 42.2%; F1,224 = 10.36, p = 0.002), spent 32.1 s more time reaching the target (F1,224 = 8.96, p = 0.003), and spent 14.3 s more time still (F1,224 = 9.51, p = 0.002). However, no difference in path distance was observed between SD and CONT on day 7. On days 8 and 9, the CONT and SD groups did not differ in failure percentage, time to target, or time still, but the SD group did have a significantly greater path distance than the CONT group on day 9 (F1,224 = 4.12, p = 0.044). These results indicate that when compared to the well-rested CONT group, SD impaired the rats' ability to perform the HLT paradigm after reversal.
3.2. Within-session data analyses
In the analysis of trial blocks for time to target, there were statistically significant effects of group (F1,510 = 5.93, p = 0.015), day (F5,510 = 6.00, p < 0.001), and trial block (F2,510 = 40.97, p < 0.001). The interaction of group and day was also significant (F5,510 = 2.44, p = 0.034). For path distance, there were significant effects of group (F1,510 = 4.89, p = 0.027), day (F5,510 = 3.68, p = 0.003), and trial block (F2,510 = 21.82, p < 0.001). These data suggest that rat response efficiency increases across trials. Within-session data for time to target and trial block separated by task paradigm are reported in Table 1.
Table 1.
LAST pre-reversal performance over trial blocks for the HLT and LLT paradigms. Data are reported as mean ± SEM for each trial block over the six pre-reversal days for each task paradigm. A statistically detected difference between trial block 1 and trial block 3 is indicated after the trial block 3 data. *p < 0.05. **p < 0.01. ***p < 0.001.
| Day | HLT |
LLT |
|||
|---|---|---|---|---|---|
| Trial block | Time to target (s) | Path distance (m) | Time to target (s) | Path distance (m) | |
| 1 | 1 | 58.3 ± 5.3 | 7.9 ± 0.8 | 44.2 ± 5.0 | 8.3 ± 0.7 |
| 2 | 42.6 ± 5.3 | 7.2 ± 0.8 | 35.2 ± 5.0 | 6.3 ± 0.7 | |
| 3 | 45.3 ± 5.3 | 7.3 ± 0.8 | 35.6 ± 5.0 | 6.0 ± 0.7 * | |
| 2 | 1 | 58.9 ± 5.3 | 8.8 ± 0.8 | 49.2 ± 5.0 | 8.5 ± 0.7 |
| 2 | 48.7 ± 5.3 | 8.7 ± 0.8 | 36.3 ± 5.0 | 6.5 ± 0.7 | |
| 3 | 47.1 ± 5.3 | 7.6 ± 0.8 | 33.0 ± 5.0 * | 6.4 ± 0.7 * | |
| 3 | 1 | 59.0 ± 5.3 | 9.7 ± 0.8 | 44.9 ± 5.0 | 8.5 ± 0.7 |
| 2 | 48.4 ± 5.3 | 8.9 ± 0.8 | 38.8 ± 5.0 | 7.3 ± 0.7 | |
| 3 | 46.2 ± 5.3 | 7.6 ± 0.8 * | 31.1 ± 5.0 * | 5.9 ± 0.7 ** | |
| 4 | 1 | 59.9 ± 5.3 | 10.5 ± 0.8 | 41.5 ± 5.0 | 7.7 ± 0.7 |
| 2 | 44.3 ± 5.3 | 8.2 ± 0.8 | 31.9 ± 5.0 | 6.4 ± 0.7 | |
| 3 | 43.8 ± 5.3 * | 8.2 ± 0.8 * | 31.2 ± 5.0 | 6.7 ± 0.7 | |
| 5 | 1 | 58.9 ± 5.3 | 9.7 ± 0.8 | 46.0 ± 5.0 | 6.8 ± 0.7 |
| 2 | 29.5 ± 5.3 | 6.0 ± 0.8 | 35.8 ± 5.0 | 6.7 ± 0.7 | |
| 3 | 33.5 ± 5.3 *** | 6.6 ± 0.8 ** | 41.9 ± 5.0 | 7.9 ± 0.7 | |
| 6 | 1 | 48.9 ± 5.3 | 7.8 ± 0.8 | 47.5 ± 5.0 | 8.3 ± 0.7 |
| 2 | 29.5 ± 5.3 | 6.8 ± 0.8 | 23.7 ± 5.0 | 5.4 ± 0.7 | |
| 3 | 26.5 ± 5.3 *** | 6.1 ± 0.8 | 21.2 ± 5.0 *** | 4.9 ± 0.7 *** | |
3.2.1. HLT paradigm
Rats completing the HLT paradigm in the initial phase, on average, lowered their time to target from the first to the third trial block by 16.1 s (F1,510 = 5.77, p = 0.017), 25.4 s (F1,510 = 14.22, p < 0.001) and 22.4 s (F1,510 = 11.19, p < 0.001) on days 4, 5 and 6, respectively. In addition, path distance was shortened from the first the third block by 2.1 m (F1,510 = 4.13, p = 0.043), 2.2 m (F1,510 = 4.72, p = 0.030) and 3.1 m (F1,510 = 8.81, p = 0.003), respectively. These results demonstrate that, especially as the number of days increased, the rats learned to perform the HLT paradigm more efficiently from the start to the end of each day.
3.2.2. LLT paradigm
Likewise, rats completing the LLT paradigm in the initial phase, time to target decreased on days 2 (F1,510 = 6.61, p = 0.010), 3 (F1,510 = 4.78, p = 0.029), and 6 (F1,510 = 17.32, p < 0.001) from the first and the third block by 16.2 s, 13.8 s, and 21.2 s, respectively. Path distance was shorter in the third block compared to the first block on days 1 (F1,510 = 5.75, p = 0.017), 2 (F1,510 = 4.57, p = 0.033), 3 (F1,510 = 7.10, p = 0.008), and 6 (F1,510 = 11.90, p < 0.001) by 2.3 m, 2.1 m, 2.6 m and 3.4 m, respectively. As with the HLT paradigm, these results indicate that the rats learned to perform the LLT paradigm more efficiently from the start to the end of each day.
4. Discussion
This study introduces a rapidly learned, ambient light-cued navigational task that demonstrates significant performance deficits incurred due to SD. We used the LAST to assess cognitive flexibility in rats, while avoiding confounds that are common to reversal learning paradigms. Indeed, the implementation of lights and vibration in lieu of foot shock, forced swim, and other aversive stimuli are extremely favorable given the impact of stress (LeBlanc, 2009; Yaribeygi et al., 2017) and fatigue (Van Dongen and Dinges, 2005; Chen et al., 2021) on behavior and performance. Other paradigms have also been designed to circumvent the common confounds of rodent paradigms. For example, similar to the LAST, the Barnes maze utilizes bright lights and open spaces to encourage rodents to locate an unmarked escape box. However, this task has faced criticism due to the high light intensity, ranging between 600 and 1000 lux. Long-term exposure to white LEDs with intensities of as little as 500 lux can induce retinal damage in albino rats and pigmented rats (Kelliher et al., 2000). Furthermore, our previous study showed that exposure to 900 lux incurred corticosteroid levels comparable to both foot shock and forced swim (Lawrence-Sidebottom et al., 2021).
In contrast, in the LAST, the rats averaged only 6.83 min per session, with the maximum light intensity in the HLT paradigm approaching 350 lux. This upper lux range is lower than the recommended lux for domestic lighting in a vivarium. We also recently demonstrated that rats exposed to vibration and bright light had lower corticosterone levels than those exposed to forced swimming in water, and that rats exhibited place preference for vibration over foot shock, water, and bright light environments (Lawrence-Sidebottom et al., 2021). Although the rats selected to spend more time in an environment with floor vibrations over bright light, the number of entrances to bright light vs. vibration were similar. Additionally, the place preference observed for the floor vibration environment was more pronounced for comparisons between vibration vs. foot shock and water. Notably, in the previous study of place preference, we used more than double the brightest light intensity than was used in the LAST (900 lux vs. 350 lux). Within the context of these previous findings, our present application of bright light in the LAST uses light that is likely to be much less aversive and is below the threshold for retinal damage (Kelliher et al., 2000).
Another criticism of the Barnes maze is that there is little incentive to attempt to complete the trial, due to the lack of a strong motivational stimulus. The LAST addresses this criticism because it pairs visual cues with haptic motivation delivered via continuous low-level floor vibrations. It has been demonstrated that rodents are sensitive to vibrations (Norton et al., 2011), resulting in effects on breeding efficacy (Atanasov et al., 2015; Rasmussen et al., 2009) and digestion (Toraason et al., 1980). Furthermore, long-term, whole-body vibrations (240 min) produce increases in circulating glucocorticoid levels and nominal changes in brain serotonin and noradrenaline levels in rats (Ariizumi and Okada, 1983, Ariizumi and Okada, 1985). However, the LAST uses a low intensity of vibration (41.6 Hz), which is safely outside the 70–100 Hz range that consistently induces behavioral responses in rats (Garner et al., 2018). This low intensity, paired with the relatively short duration of exposure and intensive habituation protocol, sufficiently motivates behavior while minimizing anxiety/stress-related neurochemical responses. In support of this, the rats exhibited reduced thigmotaxis (a proxy for rodent stress) from the second habituation day relative to the third and fourth habituation day. This was evidenced by 25 s longer dwell time in the center (the inner 92 cm) from the arena edge (the outer 15 cm; p < 0.001) and on average 4 more entries (p = 0.002) into the arena center on the third and fourth habituation days compared with the second habituation day.
While the floor vibration provided a motivational stimulus with only modest stress induction, the ambient light cues in the LAST also confer moment-to-moment visual feedback on performance to aid the rat in locating the target destination. Commonly used rodent task paradigms that incorporate motivational stimuli lack real-time directional feedback. Thus, the LAST more accurately models real-world settings and traditional human paradigms measuring sustained attention, attentional control, and cognitive flexibility, which often provide feedback after each decision opportunity (Whitney et al., 2015, 2017). As a relatively complex and more nuanced search task, the LAST is poised to be a powerful tool used for assessing the underlying neurological processes required for more elaborate assessment of decision-making and the impact of SD on these functions. An added benefit of the LAST is that it can accurately measure many behavioral metrics beyond trial success. For example, the moment-to-moment measurement of position and speed allows for in-depth analysis of specific search strategies and path directions available in path trace outputs. Time spent moving toward and away from the target, time still, path distance, and average speed are also candidate metrics. Furthermore, the LAST can accommodate electrophysiological monitoring and optogenetic manipulation to delve into the biochemical correlates that underpin the behavior.
Operant conditioning paradigms provide the versatility to examine tasks of increasing complexity and to query cognitive processes such as attention and cognitive flexibility (Brady and Floresco, 2015). For example, the rat psychomotor vigilance test (PVT) was developed as an animal analog to the human PVT, which is the favored paradigm to assess the effects of sleep loss on vigilant attention. In the rat PVT, rats are trained to respond to a pseudorandomly presented visual stimulus with a nose poke. Christie et al. (2008) found that 24 h of SD led to slower responses and a higher frequency of lapses. Though these particular SD-induced effects are similar to those observed with the human PVT, the rat PVT has several notable limitations. First, the rat PVT does not manifest the characteristic performance decrement with time-on-task and response-stimulus interval following SD as are observed with the human PVT (Oonk et al., 2015). Second, unlike the human PVT performance on the rat PVT is motivated by the restriction of water before performing the task, which is expected to produce physiological changes in the animal. Finally, training rats to perform the rat PVT takes several weeks; 3–5 weeks of training were reported by Christie et al. In the 5-choice serial reaction time operant task, rodents respond with a nose poke to a visual stimulus presented above one of 5 holes. Upon response to the correct hole, the animal receives a reward. SD produces slower responses and increases in omission errors as reported by Córdova et al. (2006). Similar to the rat PVT, behavior on this task is motivated by a reward (i.e., sucrose pellets); and to increase the salience of this reward, rodents are food-restricted prior to task performance. Additionally, as this task is relatively complex, training time is on the order of 3–5 months. Finally, reversal-learning performance after SD on an operant discrimination task reversal was not observed (Leenaars et al., 2012), which suggests that cognitive flexibility is preserved with SD. In comparison to these operant tasks, the LAST is superior in terms of detecting SD-induced cognitive flexibility deficits, does not require food restriction, and takes a fraction of the time to execute.
The Morris Water Maze is a commonly used spatial search task, in which rodents swim in an open tub in search of a hidden rescue platform (Morris, 1984). Rodents show increasing escape efficiency over several days, which is a relatively short window for observable behavioral change as opposed to weeks in operant paradigms. SD impairs some aspects of performance on the maze, as evidenced by less time spent in the target quadrant following SD (Saygin et al., 2017). While the task is useful for assessing hippocampal-dependent memory, it utilizes aversive conditions (e.g., forced swim) to motivate escape behavior, thereby introducing stress as a potential confound (see Lawrence-Sidebottom et al., 2021). Also, water exposure may alter thermoregulation, and performing electrophysiological recordings in swimming rodents is problematic. Moreover, the ability to examine the effects of SD task reversal in the water maze is not ideal, as selective REM SD or maze reversal had little effect on performance (Walsh et al., 2011). In perhaps a more ethologically relevant rodent learning paradigm, attentional set shifting uses odor or digging material as a predictor for the presence of a food reward. While this task requires minimal training (1–2 h), rewards are appetitive and food-restriction is used to incentivize reward retrieval. McCoy et al. (2007) found that sleep loss, produced by interrupting sleep, impaired performance following an extra-dimensional shift, but not with simple or complex discrimination, intra-dimensional shifts, or cue reversal suggesting that cognitive flexibility was still largely intact. Other quickly learned and ethologically relevant tasks include novel object recognition and object location recognition. These tasks utilize the rodent's innate drive to explore by allowing rodents to explore two or more objects in an arena and, after an initial exposure to the objects, then observing behavior when one of the objects is moved. While recognition of object relocation appears to be compromised by sleep loss, the recognition of a new object is intact depending on the time of day, duration of SD, or method of SD (Chen et al., 2014; Ishikawa et al., 2014; Palchykova et al., 2006, 2009; Prince et al., 2014; Wadhwa et al., 2015). While these behavioral assays can be fast and relatively easy to execute, task performance can be altered by other factors such as estrus (Cordeira et al., 2018), habituation and task complexity (Gessner et al., 2022). In the LAST, reversal is compromised by SD, despite the interference of photophobic behaviors. Furthermore, the LAST allows for more nuanced and detailed assessments of moment-to-moment performance than most other paradigms.
While the LAST was quickly acquired and revealed performance deficits induced by SD, the LAST reversal paradigm also has limitations that must be addressed. The HLT paradigm is a naturally ‘more aversive’ paradigm, as successful performance requires rats to navigate in a direction that increases ambient light and goes against their innate aversion to exploring brightly lit and open areas (Crawley et al., 1985). This results in a greater disparity between- and within-groups, with individual differences in bright light tolerance leading to more variable pre-reversal task performance. Hence, a high number of rats were excluded with the day 6 failure criterion (HLT n = 16 vs. LLT n = 3). The HLT – LLT reversal paradigm showed rapid adaptation to the less adverse directional cue via successful navigation that dimmed ambient lighting such that post-reversal performance outcomes in well rested rats mirrored day 6 values – as if a reversal had not taken place. Several metrics also demonstrated that the HLT– LLT paradigm switch blunted reversal learning following sleep loss, and significantly worsened performance on each parameter measured. Taken together, these effects indicate that LLT – HLT reversal may be a more robust paradigm that allows for better between-group comparisons, although multiple day 7 performance outcomes achieved statistical significance, regardless of the reversal paradigm. Comparing the two paradigms also suggests that the application of more noxious stimuli may blunt task acquisition, regardless of vigilance state, and that such effects are exaggerated by insufficient sleep.
Godsil and Fanselow (2004) demonstrated that rats have a clear dark preference to bright light when tested during the latter part of the light cycle. Importantly, they reported increased locomotion/activity regardless of ambient light-to-dark or dark-to-light transitions. In addition, order effects were observed with elevated locomotion/activity when the initial condition was dark as compared to light. While they used a much brighter light range than we use in the LAST, the rats in the present study also had concurrent floor vibrations during performance testing. It is possible that rats would perform the LAST more efficiently if the training occurred during the dark phase (rat's subjective day), rather than at ZT10. Exposure to intense light decreases natural behaviors in rats (Castelhano-Carlos and Baumans, 2009), and retinal damage from bright lights is greater when exposure occurred during the light period (rat's subjective night) compared to dark period (Organisciak et al., 2000). Moreover, rats are more sensitive to foot shock in the light period versus the dark period, as evidenced by longer avoidance latencies in passive avoidance conditioning (Yamada and Iwasaki, 1994) and also forced swim (Kelliher et al., 2000). This light period sensitivity to aversive stimuli appears to apply to foot shock, and restraint and shaking stress (Gattermann and Weinandy, 1996). Taken together this may indicate that the rat's performance on the LAST reversal would have been optimized given the exposure to gradient light intensities and floor vibration exposure. However, time of day had little effect on performance on the Barnes maze (which is bright light incentivized) or the Morris water maze, when performance was measured at two different times (12 h apart; Snider et al., 2016; Valentinuzzi et al., 2004).
In summary, the LAST is a novel, goal-oriented, rodent spatial navigation task. It is rapidly learned and easily modified to become a reversal-learning task, which is advantageous compared with other rodent paradigms. Utilizing the LAST, we have demonstrated that cognitive flexibility is impeded by SD and that more noxious stimuli disrupt cognitive flexibility to a greater degree, regardless of vigilance state. Moreover, due to the task complexity and continuous feedback, the LAST parallels human tasks of sustained attention and decision making. Finally, the experimental apparatus and system can accommodate multi-sensory detection, sustained cognitive evaluation, and motor response efficiency in an associative spatial task. Indeed, the LAST is a promising behavioral assay to investigate the underlying neurobiological mechanisms of information processing and how these mechanisms are impacted by sleep.
CRediT authorship contribution statement
Callum Foakes: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Darian Lawrence-Sidebottom: Data curation, Formal analysis, Writing – original draft, Visualization. Aseru T. Dralega: Data curation, Investigation, Writing – review & editing. Daniel O. Harvey: Conceptualization, Project administration, Software, Methodology, Writing – review & editing, Visualization, Validation. Michelle A. Schmidt: Formal analysis, Supervision, Project administration, Resources, Methodology, Writing – review & editing, Validation. Christopher J. Davis: Conceptualization, Funding acquisition, Supervision, Project administration, Methodology, Writing – original draft, Visualization, Validation.
Declaration of competing interest
The authors have no interests to declare.
Acknowledgments
This work was funded by a Washington State University Faculty Seed grant awarded to CJD and a Congressionally Directed Medical Research Program grant W81XWH-16-1-0319 awarded to Hans Van Dongen. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of Defense. We thank Amy Sullivan at Obrizus Communications for helpful insights during the preparation of this manuscript.
References
- Agterberg M.J.H., Versnel H. Behavioral responses of deafened Guinea pigs to intracochlear electrical stimulation: a new rapid psychophysical procedure. Hear. Res. 2014;313:67–74. doi: 10.1016/j.heares.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Atanasov N.A., Sargent J.L., Parmigiani J.P., Palme R., Diggs H.E. Characterization of train-induced vibration and its effect on fecal corticosterone metabolites in mice. Journal of the American Association for Laboratory Animal Science:JAALAS. 2015;54(6):737–744. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4671789/ [PMC free article] [PubMed] [Google Scholar]
- Ariizumi M., Okada A. Effect of whole body vibration on the rat brain content of serotonin and plasma corticosterone. Eur. J. Appl. Physiol. Occup. Physiol. 1983;52(1):15–19. doi: 10.1007/BF00429019. [DOI] [PubMed] [Google Scholar]
- Ariizumi M., Okada A. Effects of whole body vibration on biogenic amines in rat brain. Br. J. Ind. Med. 1985;42(2):133–136. doi: 10.1136/oem.42.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A. The forced swim test: historical, conceptual and methodological considerations and its relationship with individual behavioral traits. Neurosci. Biobehav. Rev. 2021;128:74–86. doi: 10.1016/j.neubiorev.2021.06.014. [DOI] [PubMed] [Google Scholar]
- Bissonette G.B., Gentry R.N., Padmala S., Pessoa L., Roesch M.R. Impact of appetitive and aversive outcomes on brain responses: linking the animal and human literatures. Front. Syst. Neurosci. 2014;8:24. doi: 10.3389/fnsys.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady A.M., Floresco S.B. Operant procedures for assessing behavioral flexibility in rats. Journal of visual experiments. 2015;96 doi: 10.3791/52387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushana P.N., Koberstein J.N., Nguyen T., Harvey D.O., Davis C.J. Performance on the mouse vibration actuating search task is compromised by sleep deprivation. J. Neurophysiol. 2020;123(2):600–607. doi: 10.1152/jn.00826.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelhano-Carlos M.J., Baumans V. The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats. Lab animals. 2009;43(4):311–327. doi: 10.1258/la.2009.0080098. [DOI] [PubMed] [Google Scholar]
- Chau B.K., Sallet J., Papageorgiou G.K., Noonan M.P., Bell A.H., Walton M.E., Rushworth M.F. Contrasting roles for orbitofrontal cortex and amygdala in credit assignment and learning in macaques. Neuron. 2015;87(5):1106–1118. doi: 10.1016/j.neuron.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tian S., Ke J. Rapid eye movement sleep deprivation disrupts consolidation but not reconsolidation of novel object recognition memory in rats. Neurosci. Lett. 2014;563:12–16. doi: 10.1016/j.neulet.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Chen Y., Fang W., Guo B., Bao H. Fatigue-related effects in the process of task interruption on working memory. Front. Hum. Neurosci. 2021;15 doi: 10.3389/fnhum.2021.703422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M.A., McKenna J.T., Connolly N.P., McCarley R.W., Strecker R.E. 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. J. Sleep Res. 2008;17(4):376–384. doi: 10.1111/j.1365-2869.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua E.C., Yeo S.C., Lee I.T., Tan L.C., Lau P., Cai S., Zhang X., Puvanendran K., Gooley J.J. Sustained attention performance during sleep deprivation associates with instability in behavior and physiologic measures at baseline. Sleep. 2014;37(1):27–39. doi: 10.5665/sleep.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeira J., Kolluru S.S., Rosenblatt H., Kry J., Strecker R.E., McCarley R.W. Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav. Brain Res. 2018;339:124–129. doi: 10.1016/j.bbr.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdova C.A., Said B.O., McCarley R.W., Baxter M.G., Chiba A.A., Strecker R.E. Sleep deprivation in rats produces attentional impairments on a 5-choice serial reaction time task. Sleep. 2006;29(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- Crawley J.N. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev. 1985;9(1):37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Dias R., Robbins T.W., Roberts A.C. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav. Neurosci. 1996;110(5):872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Fellows L.K., Farah M.J. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain: J. Neurol. 2003;126(Pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Ganesan S., Magee M., Stone J.E., Mulhall M.D., Collins A., Howard M.E., Lockley S.W., Rajaratnam S., Sletten T.L. The impact of shift work on sleep, alertness and performance in healthcare workers. Sci. Rep. 2019;9(1):4635. doi: 10.1038/s41598-019-40914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A.M., Norton J.N., Kinard W.L., Kissling G.E., Reynolds R.P. Vibration-induced behavioral responses and response threshold in female C57bl/6 mice. JAALAS: JAALAS. 2018;57(5):447–455. doi: 10.30802/AALAS-JAALAS-17-00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattermann R., Weinandy R. Time of day and stress response to different stressors in experimental animals. Part I: golden hamster (Mesocricetus auratus Waterhouse, 1839) J. Exp. Anim. Sci. 1996;38(2):66–76. [PubMed] [Google Scholar]
- Gessner N., Shinbashi M., Chuluun B., Heller C., Pittaras E. Handling, task complexity, time-of-day, and sleep deprivation as dynamic modulators of recognition memory in mice. Physiol. Behav. 2022;251 doi: 10.1016/j.physbeh.2022.113803. [DOI] [PubMed] [Google Scholar]
- Ghahremani D.G., Monterosso J., Jentsch J.D., Bilder R.M., Poldrack R.A. Neural components underlying behavioral flexibility in human reversal learning. Cerebr. Cortex. 2010;20(8):1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsil B.P., Fanselow M.S. Light stimulus change evokes an activity response in the rat. Learn. Behav. 2004;32(3):299–310. doi: 10.3758/bf03196029. [DOI] [PubMed] [Google Scholar]
- Goltstein P.M., Reinert S., Glas A., Bonhoeffer T., Hübener M. Food and water restriction lead to differential learning behaviors in a head-fixed two-choice visual discrimination task for mice. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0204066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzelmann G., Moore L.R., Gluck K.A., Van Dongen H.P.A., Dinges D.F. In: Cognitive Fatigue: Multidisciplinary Perspectives on Current Research and Future Applications. Ackerman P.L., editor. American Psychological Association; 2011. Fatigue in sustained attention: generalizing mechanisms for time awake to time on task; pp. 83–101. [DOI] [Google Scholar]
- Hurtubise J.L., Howland J.G. Effects of stress on behavioral flexibility in rodents. Neuroscience. 2017;345:176–192. doi: 10.1016/j.neuroscience.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Hornak J., O'Doherty J., Bramham J., Rolls E.T., Morris R.G., Bullock P.R., Polkey C.E. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J. Cognit. Neurosci. 2004;16(3):463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Iivonen H., Nurminen L., Harri M., Tanila H., Puoliväli J. Hypothermia in mice tested in Morris water maze. Behav. Brain Res. 2003;141(2):207–213. doi: 10.1016/s0166-4328(02)00369-8. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Yamada K., Pavlides C., Ichitani Y. Sleep deprivation impairs spontaneous object-place but not novel-object recognition in rats. Neurosci. Lett. 2014;580:114–118. doi: 10.1016/j.neulet.2014.08.004. [DOI] [PubMed] [Google Scholar]
- James S.M., Honn K.A., Gaddameedhi S., Van Dongen H.P.A. Shift work: disrupted circadian rhythms and sleep-implications for health and well-being. Curr Sleep Med Rep. 2017;3(2):104–112. doi: 10.1007/s40675-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher P., Connor T.J., Harkin A., Sanchez C., Kelly J.P., Leonard B.E. Varying responses to the rat forced-swim test under diurnal and nocturnal conditions. Physiology & behaviour. 2000;69:531–539. doi: 10.1016/s0031-9384(00)00213-4. [DOI] [PubMed] [Google Scholar]
- Lawrence-Sidebottom D., Schmidt M.A., Harvey D.O., Van Dongen H., Davis C.J. Floor vibrations for motivation and feedback in the rat vibration actuating search task. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0257980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc V.R. The effects of acute stress on performance: implications for health professions education. Acad. Med. : journal of the Association of American Medical Colleges. 2009;84(10 Suppl. l):S25–S33. doi: 10.1097/ACM.0b013e3181b37b8f. [DOI] [PubMed] [Google Scholar]
- Leenaars C.H., Joosten R.N., Kramer M., Post G., Eggels L., Wuite M., Dematteis M., Feenstraae M.G.P., Van Someren E.J.W. Spatial reversal learning is robust to total sleep deprivation. Behav. Brain Res. 2012;230(1):40–47. doi: 10.1016/j.bbr.2012.01.047. [DOI] [PubMed] [Google Scholar]
- Marinova Z., Chuang D.M., Fineberg N. Glutamate-modulating drugs as a potential therapeutic strategy in obsessive-compulsive disorder. Curr. Neuropharmacol. 2017;15(7):977–995. doi: 10.2174/1570159X15666170320104237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti A.R., Pedersen T.T., Wisor J.P., Mrdalj J., Holmelid Ø., Patil S., Meerlo P., Bramham C.R., Grønli J. Cognitive function and brain plasticity in a rat model of shift work: role of daily rhythms, sleep and glucocorticoids. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-69969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy J.G., Tartar J.L., Bebis A.C., Ward C.P., McKenna J.T., Baxter M.G., McGaughy J., McCarley R.W., Strecker R.E. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30(1):52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- McHill A.W., Wright K.P., Jr. Cognitive impairments during the transition to working at night and on subsequent night shifts. J. Biol. Rhythm. 2019;34(4):432–446. doi: 10.1177/0748730419848552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.G. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscientific methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Norton J.N., Kinard W.L., Reynolds R.P. Comparative vibration levels perceived among species in a laboratory animal facility. JAALAS : JAALAS. 2011;50(5):653–659. https://pubmed.ncbi.nlm.nih.gov/22330711/ [PMC free article] [PubMed] [Google Scholar]
- Oonk M., Davis C.J., Krueger J.M., Wisor J.P., Van Dongen H.P. Sleep deprivation and time-on-task performance decrement in the rat psychomotor vigilance task. Sleep. 2015;38(3):445–451. doi: 10.5665/sleep.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisciak D.T., Darrow R.M., Barsalou L., Kutty R.K., Wiggert B. Circadian-dependent retinal light damage in rats. Investigative ophthalmology & visual sciences. 2000;41(3):694–701. [PubMed] [Google Scholar]
- Palchykova S., Winsky-Sommerer R., Meerlo P., Dürr R., Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol. Learn. Mem. 2006;85(3):263–271. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Palchykova S., Winsky-Sommerer R., Tobler I. Sleep deprivation in the dark period does not impair memory in OF1 mice. Chronobiol. Int. 2009;26(4):682–696. doi: 10.1080/07420520902926025. [DOI] [PubMed] [Google Scholar]
- Pilcher J.J., Huffcutt A.I. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19(4):318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Prince T., Wimmer M., Choi J., Havekes R., Aton S., Abel T. Sleep deprivation during a specific 3-hour time window post-training impairs hippocampal synaptic plasticity and memory. Neurobiol. Learn. Mem. 2014;109:122–130. doi: 10.1016/j.nlm.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S., Glickman G., Norinsky R., Quimby F.W., Tolwani R.J. Construction noise decreases reproductive efficiency in mice. JAALAS. 2009;48(4):363–370. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2715925/ [PMC free article] [PubMed] [Google Scholar]
- Remijnse P.L., Nielen M.M., Uylings H.B., Veltman D.J. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26(2):609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Robbins T.W., Vaghi M.M., Banca P. Obsessive-compulsive disorder: puzzles and prospects. Neuron. 2019;102(1):27–47. doi: 10.1016/j.neuron.2019.01.046. [DOI] [PubMed] [Google Scholar]
- Saygin M., Ozguner M.F., Onder O., Doguc D.K., Ilhan I., Peker Y. The impact of sleep deprivation on hippocampal-mediated learning and memory in rats. Bratisl. Lek. Listy. 2017;118(7):408–416. doi: 10.4149/BLL_2017_080. [DOI] [PubMed] [Google Scholar]
- Snider K.H., Dziema H., Aten S., Loeser J., Norona F.E., Hoyt K., Obrietan K. Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav. Brain Res. 2016;308:222–235. doi: 10.1016/j.bbr.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraason M.A., Badger D.W., Wright G.L. Gastrointestinal response in rats to vibration and restraint. Environ. Res. 1980;23(2):341–347. doi: 10.1016/0013-9351(80)90069-9. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi V.S., Menna-Barreto L., Xavier G. Effect of circadian phase on performance of rats in the Morris water maze task. J. Biol. Rhythm. 2004;19:312–324. doi: 10.1177/0748730404265688. [DOI] [PubMed] [Google Scholar]
- Van Dongen H.P., Dinges D.F. Sleep, circadian rhythms, and psychomotor vigilance. Clin. Sports Med. 2005;24(2):237–viii. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Wadhwa M., Sahu S., Kumari P., Kauser H., Ray K., Panjwani U. Caffeine and modafinil given during 48h sleep deprivation modulate object recognition memory and synaptic proteins in the hippocampus of the rat. Behav. Brain Res. 2015;294:95–101. doi: 10.1016/j.bbr.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Walsh C.M., Booth V., Poe G.R. Spatial and reversal learning in the Morris water maze are largely resistant to six hours of REM sleep deprivation following training. Learn. Mem. 2011;18(7):422–434. doi: 10.1101/lm.2099011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P., Hinson J.M., Jackson M.L., Van Dongen H.P.A. Feedback blunting: total sleep deprivation impairs decision making that requires updating based on feedback. Sleep. 2015;38(5):745–754. doi: 10.5665/sleep.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P., Hinson J.M., Satterfield B.C., Grant D.A., Honn K.A., Van Dongen H.P.A. Sleep deprivation diminishes attentional control effectiveness and impairs flexible adaptation to changing conditions. Sci. Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-16165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Iwasaki T. Diurnal variation in passive avoidance response and serum corticosterone in rats. Shinrigaku Kenkyu. 1994;65:173–180. doi: 10.4992/jjpsy.65.173. [DOI] [PubMed] [Google Scholar]
- Yaribeygi H., Panahi Y., Sahraei H., Johnston T.P., Sahebkar A. The impact of stress on body function: a review. EXCLI journal. 2017;16:1057–1072. doi: 10.17179/excli2017-480. [DOI] [PMC free article] [PubMed] [Google Scholar]