Abstract
The will to live and the ability to maintain one’s well-being are crucial for survival. Yet, almost a million people die by suicide globally each year (Aleman and Denys, 2014), making premature deaths due to suicide a significant public health problem (Saxena et al., 2013). The expression of suicidal behaviors is a complex phenotype with documented biological, psychological, clinical, and sociocultural risk factors (Turecki et al., 2019). From a brain disease perspective, suicide is associated with neuroanatomical, neurophysiological, and neurochemical dysregulations of brain networks involved in integrating and contextualizing cognitive and emotional regulatory behaviors. From a symptom perspective, diagnostic measures of dysregulated mood states like major depressive symptoms are associated with over sixty percent of suicide deaths worldwide (Saxena et al., 2013). This paper reviews the neurobiological and clinical phenotypic correlates for mood dysregulations and suicidal phenotypes. We further propose machine learning approaches to integrate neurobiological measures with dysregulated mood symptoms to elucidate the role of inflammatory processes as neurobiological risk factors for suicide.
Keywords: Mood disorders, Brain, Body, Inflammation, Environmental adversity, Suicide
Highlights
-
•
Mood disorder symptoms are the highest risk factors for suicide.
-
•
The neurobiology of mood disorders and suicide risk behaviors are intricately linked.
-
•
Brain and peripheral inflammation in mood disorders are predictors of suicidal behaviors.
-
•
Our proposed framework illustrates links between environment and bodily homeostasis in influencing suicide risk biology.
-
•
Machine learning models can integrate bio-environmental and clinical risk prediction of suicide.
1. The interplay between mood disorders and suicide
The presence of severe mood symptoms poses the highest risk for suicide completion, the 10th leading cause of death, with up to 48,000 suicide deaths in 2018 in the U.S. alone. Across the most economically productive age group of 15–45 years olds, suicide is the third leading cause of death (Aleman and Denys, 2014), making suicide a cause of devastating economic loss and causing close to a million global deaths annually. Although various psychiatric symptoms and other factors increase suicide risk, about sixty percent of all suicides are associated with mood disorders, making mood symptoms the leading cause of suicide (Aleman and Denys, 2014; Turecki et al., 2019; Lu, 2015; Strakowski et al., 1996; Dilsaver et al., 1994; Chen and Dilsaver, 1996a). Because mood symptoms pose a considerable risk for suicide attempts and death, methods to elucidate the biological markers for mood dysregulatory indicators for suicide can facilitate the discovery of novel treatment, prediction, and prevention strategies for suicidal phenotypes.
Major depressive disorder (MDD) and bipolar disorder (BD), referred to as mood disorders, are etiologically complex. Given that these two disorders account for a larger share of suicide deaths, we will focus this review on mood disorders as they are both characterized by recurrent bouts of depressive illness and increased suicide risk. The extent to which the biological determinants for suicide phenotypes overlap with those underlying severe mood disorder symptomatology, especially depressive symptoms, remains unclear. However, complex pleiotropic and environmental risk factors are emerging as mediators of depressive symptoms (Henter et al., 2021). Emerging evidence further supports the existence of a robust phenotypic relationship between mood disorder severity and the risk for suicide at the clinically-relevant neurobiological levels (Jabbi et al., 2020a, 2020b).
Furthermore, recent genome-wide association studies support a close genetic relationship between mood disorder morbidity and suicide risk (Levey et al., 2019). However, suicidal behaviors are heterogeneous and not limited to mood disorders. Suicidal phenotypes are likely governed by many biological risk factors (Turecki et al., 2019), including diseases coupled with neuroanatomical, neurochemical, and neuroinflammatory risk markers awaiting discovery (Fig. 1). Complicating the complex etiologies of suicidal behaviors, myriad environmental factors, including the lack of social cohesion/support, early-life adversity and persistent traumatic experiences, and chronic substance misuse or other chronic diseases, can influence suicide outcomes (Turecki et al., 2019) (Fig. 1).
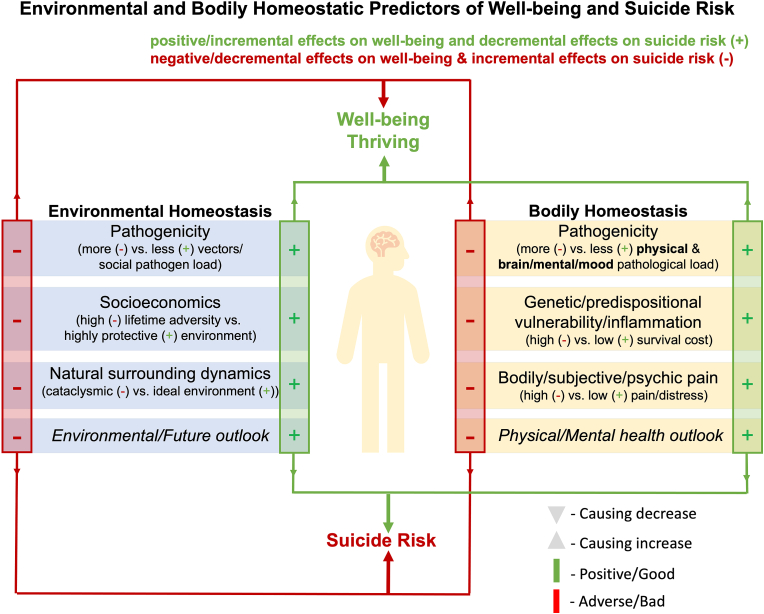
Fig. 1.
Conceptual Framework for the role of bodily & environmental homeostasis in suicide risk dynamics. It is important to note that the environmental/ecological and bodily homeostatic components can influence each other in this conceptual framework. Examples of cumulated negative environmental features/variables include physical environmental factors being ridden with adverse factors that can suppress individual well-being (e.g., adverse circumstances like hunger and starvation, extreme heat or cold, adverse socioeconomic status or events, socially caused physical or psychosocial distress/adversity/injury/extreme harm also akin to exposure to intraspecies ‘sociopathogenic’ and interspecies ‘zenopathogenic’ factors) (Salvadore et al., 2009, 2010). The framework further includes examples of negative values of biological homeostatic features: concerning the immediate bodily states that are detrimental to well-being over time (e.g., psychological or subjective experiences of pain or misery, psychosomatic pain, brain health/brain disorder ‘including cellular functional excitatory/inhibitory imbalance, limbic system dysregulation, parasympathetic/sympathetic response imbalance, neurological/psychologically challenged states, mild/moderate/severe neuropsychiatric disorder’; and systemic innate or adaptive immune imbalance (inflammation) stemming from intraspecies ‘sociopathogenic’ and interspecies ‘zenopathogenic’ factors, can all attenuate the wellness and well-being of an individual and decrease the value of staying alive in a bodily homeostatic extreme over time and thereby increase the risk for suicide (Salvadore et al., 2009, 2010; McGrath et al., 2013; Riva-Posse et al., 2018; Nauta, 1971; Goldman-Rakic, 1988; Joyce and Barbas, 2018; Jabbi et al., 2008; Craig, 2009; Harrison et al., 2009a; Khalsa et al., 2018; Dum et al., 2019; Sanvanson et al., 2019; Lerman et al., 2019; Hart, 1988; Watkins and Maier, 1999; Miller et al., 2009; Barbosa et al., 2013; Eisenberger et al., 2009; Gogolla, 2021; Koren et al., 2021; Gimeno et al., 2009; Tsigos and Chrousos, 2002; Scangos et al., 2021a) (Fig. 1, Fig. 2). Cumulatively, sustained accumulation of negative survival values in environmental and bodily/physical homeostatic frameworks can negatively impact existential outlook/perspectives and increase suicidal ideation and thoughts. For instance, when an individual is faced with such negative existential outlook, combined with with that same individual having access to means for carrying out a suicidal act such as weapons (e.g., guns, etc.), chemicals (e.g., medication or substance overdose, etc.), or mechanical facilitators (e.g., ropes, etc.), these combination of factors can increase the risk for suicide completion (Turecki et al., 2019). Although presented as categorically distinct, it is important to note that our proposed homeostatic and environmental variables are strongly interrelated, comprising intersecting feedback loops within and between these internal homeostatic reactive states and external environmental systems. The green (+) signs represent survival enhancing values that can represent individual states for each of the listed environmental and bodily homeostatic components and can, therefore, at the individual level, cumulative result in varying degrees of positive survival values that could enhance well-being and enable (see green arrow) the individual to achieve sustained states of well-being/thrive and minimize the likelihood for suicidal thinking and behaviors in a given homeostatic context. In contrast, the red minus (−) signs present negative survival limiting values for each of the listed environmental and bodily homeostatic components that, at the individual level, can cumulatively result in negative survival values and diminish well-being or, in the extremes, pose devastating outcomes and thereby increase the risk for suicidal thinking and suicide death. In a scenario where the negative environmental and bodily homeostatic states are exponentially exacerbated, an individual could be driven to escape their perceived or experienced compounded misery by seeing death or suicide as an escape from suffering. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Here, we briefly review the neurobiological correlates of mood disorder morbidity and suicide risk and illustrate the role of brain gene expression and inflammatory measures in mood disorder-related suicide, using data from post-mortem and clinical studies. In addition, we propose a novel suicide model based on a bioenvironmental perspective. Components of our proposed suicide model relate to the biopsychosocial model (Turecki et al., 2019). Still, the current model integrates environmental with bodily homeostatic drivers within a dynamic context that can afford individual risk prediction (Fig. 1). Additionally, we propose using a novel machine learning approach to integrate existing knowledge on the neurobiological and clinical risk correlates for mood disorder morbidity and suicide phenotypes to advance suicide risk prediction strategies using the proposed contextual framework.
2. The neuroanatomical basis for mood disorder-related suicidal phenotypes
There is growing evidence on the shared neuroanatomical bases for mood disorder severity and suicide risk. Neuroimaging studies of mood and comorbid disorders have identified predominantly reduced gray matter volume in prefrontal cortical networks, including the anterior insular and anterior cingulate (AIC-ACC) circuitry (Jabbi et al., 2020b; Goodkind et al., 2015; Wise et al., 2016). In addition, reduced gray matter volume and cortical thickness have also been observed in prefrontal, striatal, and temporal cortical networks (Turecki et al., 2019; Jabbi et al., 2020a, 2020b; Mann et al., 2012; van Heeringen and Mann, 2014; Taylor et al., 2015; Gosnell et al., 2016; Giakoumatos et al., 2013; Schmaal et al., 2020; Wagner et al., 2012; Mathews et al., 2013; Jabbi and Nemeroff, 2019; Jollant et al., 2018; Pan et al., 2013; Campos et al., 2021), bilateral thalamus, right pallidum, and lower surface area of the left interior parietal networks (Campos et al., 2021) in mood disorders and related suicide phenotypes (Jabbi et al., 2020a; Mann et al., 2012; van Heeringen and Mann, 2014; Taylor et al., 2015; Gosnell et al., 2016; Giakoumatos et al., 2013; Schmaal et al., 2020; Wagner et al., 2012; Mathews et al., 2013; Jabbi and Nemeroff, 2019; Jollant et al., 2018; Pan et al., 2013; Campos et al., 2021). These findings, especially relating to the AIC-ACC circuitry abnormalities in mood disorders, are relevant because the dorsal subgenual ACC component of the interoceptive network is involved in controlling emotional expression and emotional equilibrium/inhibition. In contrast, the anterior insula is documented to encode the embodiment of feeling states in health and disease states like depression (Melhem et al., 2019; Chen and Dilsaver, 1996b; Kloiber et al., 2020; McIntyre et al., 2020; Haarman et al., 2014; Hellwig and Domschke, 2019; Arasappan et al., 2021; Scaini et al., 2019; Hess et al., 2020; Salvadore et al., 2009, 2010; McGrath et al., 2013; Riva-Posse et al., 2018).
Within the human brain, primary sensory and higher-order associative subcortical and cortical networks coordinate the exteroceptive sensing of external audiovisual, social, chemosensory, and tactile stimuli (Nauta, 1971; Goldman-Rakic, 1988; Joyce and Barbas, 2018; Jabbi et al., 2008). In addition, based on the valence of incoming stimuli, the frontolimbic interoceptive brain cortical network processes the survival valuation of the environmental contexts and translates this information into inner feeling states (Jabbi et al., 2008; Craig, 2009; Harrison et al., 2009a; Khalsa et al., 2018). Together, the brain’s feedforward-feedback integration of the sensorimotor-to-frontotemporal exteroceptive cortices (Nauta, 1971; Goldman-Rakic, 1988; Joyce and Barbas, 2018; Jabbi et al., 2008) via the subcortical and prefrontal interoceptive AIC-ACC circuitry (Nauta, 1971; Goldman-Rakic, 1988; Joyce and Barbas, 2018; Jabbi et al., 2008) is critical for adaptive behaviors like the regulation of body temperature, mood, and ensuing fight or flight responses. Based on these inter-dependent adaptive functions, the coordinated interaction between exteroceptive and interoceptive brain systems is likely needed to maintain bodily and affective homeostatic stability. Thus, successful evaluation (Nauta, 1971; Goldman-Rakic, 1988; Joyce and Barbas, 2018; Jabbi et al., 2008) of how exposure to sociopathogens (e.g., trauma or extremely hurtful social interactions, as well as states of anxiety, regret, and loss) or zenopathogens (e.g., natural disasters or infectious diseases), but also favorable and survival enhancing stimuli, is critical for translating the impact of perceived or experienced environmental experiences into an individual’s bodily or affective feeling states. To summarize, the ventral AIC component of the interoceptive network is involved in coding the physiological and affective tone of individual experiences and integrating social-affective and painful interoceptive and affective states (i.e., sensing of the inner bodily/physiological conditions) (Nauta, 1971; Goldman-Rakic, 1988; Joyce and Barbas, 2018; Jabbi et al., 2008; Craig, 2009; Harrison et al., 2009a; Khalsa et al., 2018).
In line with the crucial role of this AIC-ACC circuitry in integrating exteroceptive-interoceptive senses, we have shown that the AIC-ACC circuitry’s involvement in regulating the body’s physiological conditions like the experience of tasting unpleasant and pleasant liquids is also mapped onto imagined social or one’s environmental contexts relating to the same tastants (Jabbi et al., 2008). This functional role of the AIC-ACC brain circuitry in embodying feeling states is facilitated by its interconnection with the peripheral adrenal medulla, adrenal cortex, gut, and cardiovascular systems (Dum et al., 2019). The AIC-ACC is interconnected with the gut, circulatory, and adrenal cortical systems (Dum et al., 2019) through the vagal nerve and related descending spinothalamic nerve fibers from the brain to the periphery (Dum et al., 2019; Sanvanson et al., 2019; Lerman et al., 2019), and anatomically situated to embody and integrate bodily (i.e., gut feelings) and affective feeling states and thereby chart moment-to-moment physiological and emotional well-being of the individual (Joyce and Barbas, 2018; Jabbi et al., 2008; Craig, 2009; Harrison et al., 2009a; Khalsa et al., 2018). This embodiment role of the AIC-ACC circuitry is supported by evidence of this brain network’s involvement in coding feelings of sickness and pain (Hart, 1988; Watkins and Maier, 1999; Miller et al., 2009; Barbosa et al., 2013; Eisenberger et al., 2009; Gogolla, 2021; Koren et al., 2021; Gimeno et al., 2009; Tsigos and Chrousos, 2002), including inflammatory sensing and regulation of cytokine-induced depressed mood and social pain (Hart, 1988; Watkins and Maier, 1999; Miller et al., 2009; Barbosa et al., 2013; Eisenberger et al., 2009; Gogolla, 2021; Koren et al., 2021; Gimeno et al., 2009; Tsigos and Chrousos, 2002), as well as in coding the awareness of feeling states, including depressive symptoms (Hart, 1988; Watkins and Maier, 1999; Miller et al., 2009; Barbosa et al., 2013; Eisenberger et al., 2009; Gogolla, 2021; Koren et al., 2021; Gimeno et al., 2009; Tsigos and Chrousos, 2002).
Given the reciprocal connections between the AIC-ACC network with the peripheral organ systems, including the adrenal gland secretory stress hormone system (Chen and Dilsaver, 1996b; Dum et al., 2019; Hart, 1988; Tsigos and Chrousos, 2002), and sympathetic and parasympathetic nervous systems (Chen and Dilsaver, 1996b), it is likely that an overdrive of inflammatory processes in the AIC-ACC and interconnected peripheral pathways could lead to profound dysregulation of mood states. Future research needs to study inflammatory abnormalities in the AIC-ACC network and peripheral blood in the same postmortem donors, including those who died by suicide, to better understand the mechanistic link between brain and peripheral inflammation and mood disorder outcomes. Emerging evidence points to an anatomical and physiological influence of the AIC-ACC network and interconnected peripheral body systems on affective feeling states (Hart, 1988; Watkins and Maier, 1999; Miller et al., 2009; Barbosa et al., 2013; Eisenberger et al., 2009; Gogolla, 2021; Koren et al., 2021; Gimeno et al., 2009; Tsigos and Chrousos, 2002). How this brain network influences the onset and lifetime trajectory of mood disorder and documented therapeutic responses (Sanvanson et al., 2019; Lerman et al., 2019; Hart, 1988; Watkins and Maier, 1999; Scangos et al., 2021a, 2021b, 2021c) needs to be characterized at the molecular level.
Identifying the precise neuroanatomical abnormalities associated with mood disorder phenotypes is crucial for the mechanistic understanding of how such structural alterations in specific brain networks can lead to the expression of suicidal behaviors. For instance, better localization of clinically-relevant network abnormalities can guide targeted brain stimulation techniques and advance diagnostic and prognostic studies. Furthermore, a better understanding of anatomical abnormalities, especially within the AIC-ACC circuitry that is more proximate to mood symptom severity, treatment response, and suicidal risk behaviors (see below and Figs. 1 and 2), can guide future studies of the molecular and physiological mechanisms associated with severe mood symptoms and suicide risk phenotypes.
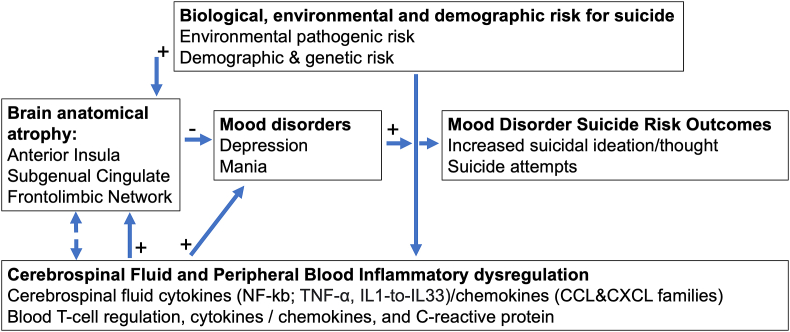
Fig. 2.
Integrative Bio-environmental and Demographic Correlate for Mood Disorder-Suicide risk over the lifespan. The model illustrates how increased environmental (i.e., adverse environmental factors) and demographic risk factors can impact localized brain anatomical integrity, cerebrospinal fluid (CSF), and inflammatory blood dysregulation, influencing mood disorder onset and disease course over the lifespan. Accordingly, increased brain anatomical atrophy, in interaction with increased CSF & peripheral blood immune/inflammation processes across sex and age, can collectively influence mood disorders and related suicidal risk outcomes.
3. Mood disorder-related suicide model
We present a suicide model that integrates environmental (including social) contexts with bodily (internal bodily states) and homeostatic states or experiences to predict survival valuation endpoints. Survival valuation endpoints include a) individual subjective values of being alive within a specific homeostatic range from thriving or in optimal well-being ‘top, Fig. 1’ to suffering, or b) a state in which one is suffering to the extent that their survival valuation endpoint becomes negative and an escape from more suffering by ending one’s life becomes an appealing option ‘bottom, Fig. 1’). In this model/conceptual framework, cumulative positive survival values could increase well-being, whereas compounding negative survival values could increase suicide risk (Fig. 1). The green plus (+) signs and the red (−) signs denote the contextual positives and negatives that could influence overall environmental or bodily homeostatic drivers/factors to maintain equilibrium or shift towards either extreme positives (thriving) or extreme negatives (increased suicide risk).
This proposed suicide model is based on data across the behavioral, clinical, neuroanatomical, and peripheral blood studies in mood disorders. For instance, recent research has shown that sickness behavior is a critical behavioral component of the immune and inflammatory response. Specifically, sickness behavior is well-documented to mediate the redirecting mechanism of energy expenditure towards context-dependent adaptive behaviors such as self-care and contextual information processing, rather than appetitive behaviors like foraging or mate-seeking.
Although neuroendocrine, monoamine, and inflammatory mechanisms are collectively implicated in mediating mood symptoms, this review focuses on immune and inflammatory processes, given the strong neuro-immune link with general sickness behavior and mood symptoms. It is important to note that environmental factors can bidirectionally cause bodily homeostatic changes and influence mood dysregulation states like bipolar depression or manic episodes. Furthermore, repeated exposures to adverse ecological (e.g., low socioeconomic status or socially inflicted physical, psychosocial, and emotional trauma) experiences akin to exposure to intraspecies adversity (i.e., sociopathogenic factors) can cause inflammatory pathologies. Moreover, interspecies pathogenic exposures, including bacterial, viral, and other physically or environmentally injurious vectors or even disasters (i.e., zenopathogenic factors), can all suppress well-being, inflict high inflammatory costs, cause diseases including physical and mental disorders and predict poorer health outcomes, including mood disorders and suicide.
For instance, acute or sustained bodily states such as psychological or subjective pain or misery, or brain disorder, including cellular functional excitatory/inhibitory imbalance, can all exert a toll on bodily homeostatic balances and trigger mood disturbances. Furthermore, systemic innate or adaptive immune imbalances can trigger inflammatory cascades stemming from sociopathogenic (Chen and Dilsaver, 1996b; Slavich et al., 2010; Kiecolt-Glaser et al., 2003; Glaser and Kiecolt-Glaser, 2005; Sloan et al., 2007; Cole et al., 2007, 2011; Cole, 2014; Cacioppo et al., 2015) and zenopathogenic (Gosnell et al., 2016; Finch, 2010; Decker et al., 2005; Rhen and Cidlowski, 2005) factors. Therefore, environmental pathogens and genetically determined neurodevelopmental vulnerabilities can compound well-being and profoundly impact physical and mental health outcomes such as mood disorders (Syed et al., 2018; Nemeroff, 2020; Teicher et al., 2021; Kirsch et al., 2021). The accumulation of negative biological and environmental pathogenic factors (Gosnell et al., 2016; Scangos et al., 2021a, 2021b, 2021c; Syed et al., 2018; Nemeroff, 2020; Teicher et al., 2021; Kirsch et al., 2021), including those noted above, can decrease the subjective value of staying alive in a highly imbalanced homeostatic states and thereby increase the risk of suicide. Thus, sustained accumulation of negative survival values perceived from adverse environmental experiences coupled with genetic vulnerability can depreciate the level of positive existential outlook and increase psychological pain and misery (Nemeroff, 2021; Shneidman, 1993). Such negative valuation processes can, in turn, trigger suicidal ideation and attempts as a maladaptive solution to escape current and future unbearable pain and misery mortally.
Environmental pathogens and sickness behavior (Chen and Dilsaver, 1996b; Slavich et al., 2010; Kiecolt-Glaser et al., 2003; Glaser and Kiecolt-Glaser, 2005; Sloan et al., 2007; Cole et al., 2007, 2011; Cole, 2014; Cacioppo et al., 2015) have a close link. For instance, repeated exposures to sociopathogens can induce components of sickness behavior such as fatigue, disruptions in normal sleep and anhedonia, impaired cognition, attenuated social behaviors, and unstable mood (Dantzer, 2004; Eisenberger et al., 2010; Krabbe et al., 2005; Reichenberg et al., 2001). Although these examples of environmentally induced sickness behaviors are not linearly compatible with manic illnesses, they could lead to excessive foraging and hypersexuality, underscoring the biological complexity underlying sickness behaviors. Therefore, it is not surprising that sickness behavior coincides, albeit temporarily, with the production and release of cytokines and chemokines (Moieni et al., 2015; Lasselin et al., 2016; Grigoleit et al., 2011; Wang and Miller, 2018). Thus, successful evaluation of how exposure to potential sociopathogens (e.g., human inflicted trauma, extremely hurtful social interactions, and perceived anxiety) (Chen and Dilsaver, 1996b; Slavich et al., 2010; Kiecolt-Glaser et al., 2003; Glaser and Kiecolt-Glaser, 2005; Sloan et al., 2007; Cole et al., 2007, 2011; Cole, 2014; Cacioppo et al., 2015) or zenopathogens (e.g., natural disasters, infections/disease, pollutants) (Gosnell et al., 2016; Finch, 2010; Decker et al., 2005; Rhen and Cidlowski, 2005) can cause sickness is a critical ability for survival. It is likely that the brain’s translation of perceived or experienced harmful environmental contexts into negative bodily or affective feeling states may, in part, be a mechanism for feeling depressed. Indeed, the direct connectivity between frontolimbic networks with peripheral adrenal cortex, gut, and cardiovascular systems (Dantzer et al., 2000), via the hypothalamus could be necessary for mediating depressive sickness (Goldman-Rakic, 1988; Dantzer et al., 2000). As such, dysregulated frontolimbic connections with striatal reward pathways (leading to anhedonia and, in turn, affecting inflammation) or dysregulated frontolimbic connections with the extra-striate pathways (leading to psychomotor inhibition) may serve as biological determinants of depression and suicidal urges. Furthermore, dysregulated frontolimbic links with the hypothalamus can lead to endocrine dysregulation, inflammation, and low mood (Inagaki et al., 2015; Harrison et al., 2009b, 2016; Lekander et al., 2016). In summary, evidence suggests that frontolimbic-related dysregulations could affect three distinct mechanisms: 1) frontolimbic-vagus nerve links to the peripheral systems, or 2) frontolimbic mediated cytokine diffusion into the cerebrospinal fluid, and 3) frontolimbic through blood-brain barrier endothelial transmission (Vitkovic et al., 2000; Inagaki et al., 2012).
The specific inflammatory processes mediating depressive sickness within the context of our suicide model are not well-defined (Fig. 1), and future studies need to characterize the inflammatory biological mechanisms for suicide risk in a broader context (see Fig. 2).
4. Postmortem brain studies of mood disorders and suicide-related gene expression signatures
Multiple studies using brain gene expression analyses, which measure genetic functions by capturing transcript abundance for specific genes and molecular pathways, have identified differentially expressed genes involved in microglial and immune system functions in mood disorders. In Pantazatos et al., differentially expressed immune system genes were identified in the prefrontal cortex of depressed individuals who died by suicide compared to non-suicide deaths (Pantazatos et al., 2017). These authors additionally found the IL8 gene to be downregulated in MDD (Pantazatos et al., 2017). Another study found that the mRNA expression of chemokines, a family of immunity modulating chemoattractant cytokines or small proteins secreted by cells, namely CXCL1, CXCL2, CXCL3, and CCL2 chemokine families, were significantly downregulated in the prefrontal cortex of depressed suicides compared with controls (Pandey et al., 2021). In contrast to these chemokine findings, another study found increased chemokine gene expression in the dorsal anterior cingulate of depressed suicides (Torres-Platas et al., 2014). However, CCL4 was downregulated in mood disorders when comparing high vs. low psychiatric morbidity and suicide mortality (Jabbi et al., 2020b). Jabbi et al. (2020b) replicated the Pantazatos finding (Pantazatos et al., 2017) of CCL4 downregulion in MDD and suicide. Together, these postmortem brain gene expression results (Jabbi et al., 2020b; Pantazatos et al., 2017) align with findings of lower cerebrospinal fluid and blood plasma levels of IL8 in suicide attempters versus non-attempters (Janelidze et al., 2014). In Jabbi et al., N.F.- κB pathway genes necessary for cellular-immune response to infections (Jabbi et al., 2020b) were also downregulated in mood disorder morbidity and suicide mortality, in line with the role for innate immunity immune and inflammatory abnormalities in the pathophysiology of depression (van Amerongen and Nusse, 2009; Caviedes et al., 2017). Furthermore, overexpression of ZC3H12D and POU2F1, a DNA-binding transcription factor involved in inflammation via stress-induced modulation of tissue-specific gene expression, was associated with suicide. This result is noteworthy because ZC3H12D is thought to a) suppress inflammatory cytokines, b) increase ubiquitination-associated immunological dysfunctions, and c) play a role in toll-like receptor signaling and host immunity (Huang et al., 2012).
Despite this mixed evidence regarding the up or downregulation of chemokine expression, other evidence suggests that chemokines are pleiotropic, having beneficial and deleterious attributes, thereby explaining the putative discrepancy between the studies mentioned above. Additionally, gene expression may fluctuate in different brain regions and tissue-type (brain versus blood) based on varying anatomical and environmental factors, yielding potential differences between results from specific brain regional studies or tissue-specific findings with different underlying molecular mechanisms. For example, lower expression of genes associated with chemokine activity and regulation in depression might suggest altered microglia-mediated synaptic pruning in MDD (Zhan et al., 2014). Furthermore, because chemokine and immune-related genes are expressed in microglia and astrocytes, reduced chemokine and immune-related gene expression could reflect cellular abnormalities such as reduced astrocytes and impaired neuroprotection, as well as impaired neuron-glia communication in depressed suicide brains (Barres, 2008). Together, these results point to innate/adaptive immune and related inflammatory abnormalities in mood disorders and suicide. More research is needed to elucidate the specific contribution of neuro-immune and inflammatory gene pathways to advance our understanding of the specific roles of immune and inflammatory abnormalities in mood disorder morbidity and suicide.
In summary, many avenues of research have explored the interdependencies between mood disorder morbidity, suicide risk, and aberrant gene expression in the brain. One example study used RNA-seq to show how gene expression patterns across the whole transcriptome are associated with major depressive disorder (MDD) and related suicide phenotypes (Pantazatos et al., 2017). Another study explored the neurotranscriptomic linkage between suicidal behaviors and mood disorders in the anterior insula (Jabbi et al., 2020b). The latter study used factor analysis to identify a possible higher-order factor explaining variance in post-mortem variables. i.e., how groups of variables cluster. This work assessed psychiatric morbidity and suicide mortality risk factors on a dimensional continuum so that clinical designations of mood disorder subtypes (i.e., MDD, BD) don’t incorrectly segregate patients into MDD versus BD diagnostic groups that share underlying gene expression profiles. Future research will need to extend and translate brain gene expression abnormalities, across multiple brain regions, to living populations by identifying related peripheral blood-based predictive biomarkers for suicide risk.
5. Inflammatory gene expression profiles in mood disorders and suicide
Major depressive disorder linked inflammatory signatures: Recent studies show the relationship between stress-related neuroinflammation and mood disorders. For example, stress/adversity can cause major depression and related alterations in innate and adaptive immunity (Beurel et al., 2020) and is paired with systemic immune activation, changing inflammatory markers, immune cell numbers, and antibody titers (Beurel et al., 2020; Tantin et al., 2005). Depressed patients have increased interleukin-6(IL-6) plasma concentrations and circulatory proinflammatory cytokines (Beurel et al., 2020). Various studies have shown that proinflammatory cytokines and acute-phase proteins are increased in depressed individuals. For instance, IL-6, tumor necrosis factor (TNF), and C-reactive protein (CRP) are upregulated, and patients with increased suicidal risk have increased interleukin concentrations in their blood and cerebrospinal fluid (Beurel et al., 2020; Tantin et al., 2005; Musselman et al., 2001).
Moreover, stress, MDD, and cancer are associated with an immunocompromised state marked by T cell response, natural killer cell activity, and the number of T helper cells (Tantin et al., 2005; Musselman et al., 2001; Brundin et al., 2017). A relatively new but promising area of research is the bidirectional relationship between autoimmune and other medical diseases and depression. For instance, people with MDD have an increased risk of developing autoimmune thyroiditis, multiple sclerosis, lupus, and irritable bowel syndrome (Beurel et al., 2020; Tantin et al., 2005). Additionally, cytokines, thought to be requisite mediators of inflammation, are associated with elevated depressive symptoms and suicide (Brundin et al., 2017; Podlipný et al., 2010). While evidence exists of a profound immune and inflammatory pathway abnormality in depressive disorders and related comorbid conditions, specific brain peripheral and cellular mechanisms by which immune and inflammatory processes influence depressive symptoms and disease outcomes need to be further characterized by future studies.
Bipolar disorder linked inflammatory signatures: Similar to depression, bipolar disorder is associated with elevated inflammatory cytokine levels (O'Brien et al., 2006; Black and Miller, 2015). Corticolimbic brain areas such as the amygdala, insula, and ACC play an important role in immune functional regulation and signaling (Jabbi et al., 2020b; Irwin and Cole, 2011). Therefore, abnormal corticolimbic activity might cause immune dysfunction, and more studies of the possible mechanisms by which corticolimbic networks mediate neuroimmune functions are needed (Jabbi et al., 2020b; Maletic and Raison, 2014). In several studies, IL-4 level was elevated in bipolar disorder patients relative to controls. Raised IL-4 in bipolar disorder is expected to compensate for the condition-dependent increase in proinflammatory cytokines (Munkholm et al., 2013). Other studies have also found increased tumor necrosis factor-alpha and IL-6 in bipolar depressive and manic patients (Brietzke et al., 2009; Kim et al., 2007).
Bipolar depressive states are characterized by an imbalanced ratio of proinflammatory IL-6 to anti-inflammatory IL-10. Additionally, inflammatory cytokines are known to decrease the sensitivity of glucocorticoid receptors (Tsigos and Chrousos, 2002), and inflammatory cytokines activate microglia cells in the brain, which boost inflammation by releasing reactive oxygen species, chemokines, and cytokines (Maletic and Raison, 2014). Inflammatory cytokines further influences dopamine receptor expression, impeding monoamine signaling (Felger and Lotrich, 2013) suggesting that mood disorders and related inflammatory abnormalities may be linked with other biological pathways.
Suicide-linked inflammatory signatures: Inflammation is particularly elevated in suicide (Musselman et al., 2001; Black and Miller, 2015). In specific terms, suicide risk behaviors are associated with global brain molecular changes affecting several functional pathways, including monoamine, immune and inflammatory, and GABAergic and glutamatergic excitatory/inhibitory dysfunctions (Lutz et al., 2017). The extent to which any functional pathway dysfunctions are specific to suicide and not shared with depression or other psychiatric disorders is challenging to resolve, given the interrelatedness of these phenotypes. Nevertheless, there are connections between immune responses and inflammation and depression (Turecki et al., 2019; Beurel et al., 2020; Tantin et al., 2005; Raison et al., 2006) (Fig. 2). Individuals with chronic medical conditions (i.e., cardiovascular and metobolic diseases) and those receiving cytokine therapy are more likely to develop inflammation (Kayser and Dalmau, 2011; Courtet et al., 2016). Furthermore, proinflammatory cytokines (IL-6, IL-8) are higher in individuals who attempted suicide (Serafini et al., 2013; Janelidze et al., 2015), underscoring the possibility that high levels of inflammatory markers may be indicators of suicide risk in depression (Bergmans et al., 2019).
In multiple studies, microglial and immune system genes were differentially expressed in suicide and depression groups. Differentially expressed immune system genes were identified between depressed subjects with and without suicide (Jabbi et al., 2020b; Pantazatos et al., 2017). CCL2 and CCL4, chemokine ligands 2 and 4, respectively (Semple et al., 2010), were further downregulated in depressed suicides, whereas increased chemokine gene expression was found in the dorsal anterior cingulate of depressed suicides (Torres-Platas et al., 2014). In addition to increased chemokine signaling, CCL4 was downregulated in BD, MDD, and the pooled mood disorder group in relation to high vs. low mood disorder morbidity and suicide (Jabbi et al., 2020b). In light of the potential pleiotropic properties of inflammasomes like cytokines and chemokines, future studies need to ascertain the role of inflammatory dosage in disease and suicide phenotypes.
6. Using machine learning with clinical features to predict suicide risk
Given the profound disease burden and premature deaths attributable to suicide phenotypes, predicting suicide risk is paramount but remains challenging due to its biological heterogeneity. Much research has been conducted to identify clinical records of suicide attempts, depression symptomatology, and comorbid substance use disorders as key predictors of suicide death (Melhem et al., 2017). Separately, high levels of psychopathology are a risk factor for suicide (Gvion and Levi-Belz, 2018), and logistic regression models have identified self-report metrics and clinically relevant health data to predict suicide with high accuracy (Simon et al., 2018).
Burgeoning machine learning research has shown potential for incremental advances in suicide prediction (Rakesh, 2017). For example, one machine-learning study on mood disorder outpatients found previous MDD-related hospitalizations, a history of psychosis and cocaine use, and comorbid PTSD to be strong clinical predictors of suicide (Passos et al., 2016). In this study, variables were chosen a priori based on previous findings, which might inhibit discovering latent features that could be important in predicting suicide. However, clinical heterogeneity might limit the discovery of underlying biological mechanisms, i.e., clinical factors may not explain or complement the suicide predictive values of biologically meaningful measures. Another study of soldiers and veterans after inpatient hospitalization found that male sex, late enlistment age, a history of criminal activity, and previous suicidal ideation were the strongest predictors of suicide completion (Kessler et al., 2015). However, these studies achieved a sensitivity and specificity of 70%, suggesting that the clinical markers used in these studies were insufficient to predict suicide with high accuracy, reinforcing the idea that suicide is phenotypically complex.
Because of the multi-dimensionality of suicide risk factors, we propose that machine learning tools enable a more robust prediction of mood disorder-related suicide outcomes at the population level. Furthermore, there is increasing evidence that elements of our proposed approach are emerging as a blossoming field in psychiatric and behavioral research. For instance, machine learning algorithms based on clinical and biological features have been developed to predict suicide with mixed success (Bernert et al., 2020; Bhak et al., 2019). Indeed, prediction models that rely solely on self-report metrics can be inadequate in their predictive power due to the subjectivity and lack of individual transparency about expressing troubling and often stigmatizing suicidal thoughts (Busch et al., 2003). Still, machine learning approaches have shown promise in discovering genetic biomarkers for disease and suicide outcomes (Bhak et al., 2019; Libbrecht and Noble, 2015).
Herein, we propose that machine learning enables researchers to integrate multiple data types (e.g., transcriptomics, clinical behavioral, environmental/ecological, and demographic variables) to facilitate a more gestalt understanding of suicide risk outcome measures across the lifespan and different global settings. Additionally, machine learning allows the detection of essential features not informed by a priori information, which can advance the identification of novel brain-related genetic risk components of suicide. Using machine learning to study the interplay between brain gene expression and history of mood symptoms and suicide phenotypes in post-mortem samples will advance the understanding of suicide etiology and guide the identification of potential biomarkers. Such biomarkers could, in turn, inform the development of novel drugs to treat suicidal phenotypes. By combining diagnostic, demographic, adversity history, and brain gene expression data in a discovery sample of post-mortem donors, researchers will more robustly develop sensitive models to predict suicide in mood disorders. Such models using postmortem samples will uncover previously unknown brain genetic and related biomolecular determinants of suicide outcomes across disease phenotypes.
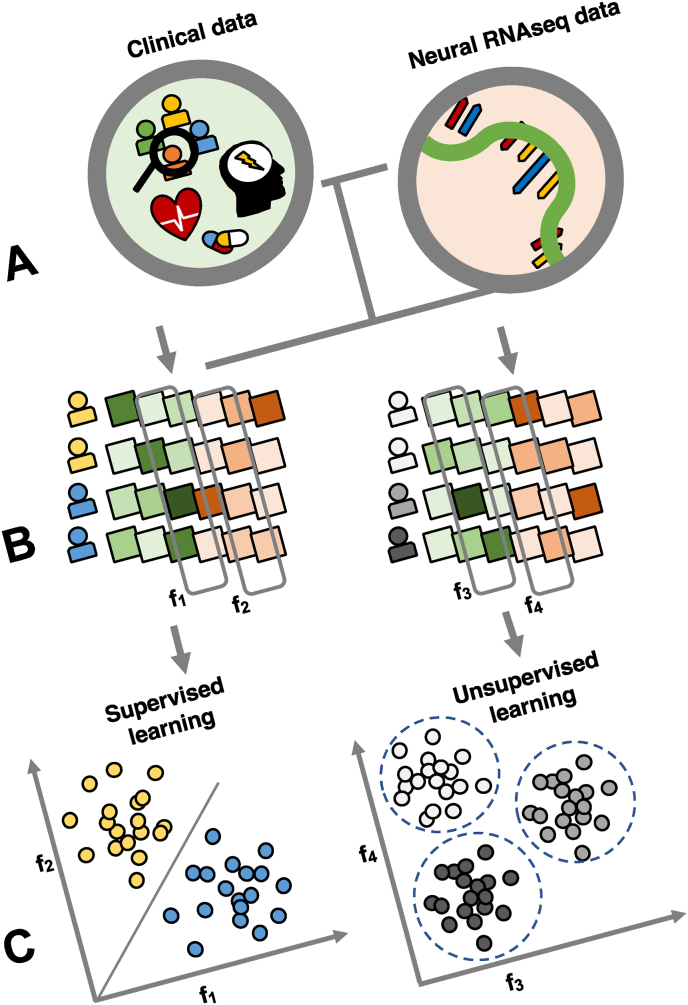
We suggest using a two-pronged machine learning approach (Fig. 3) in future studies. The first is to use supervised machine learning tools with post-mortem RNA-seq and clinical data to develop a more robust predictive model for suicide attempts. The second is to use unsupervised machine learning tools to uncover clusters of unique mood disorder and suicide predictive phenotypes. For the predictive model, once brain-based biomarkers for suicide are identified, those same markers can be validated in blood measures of the same subjects in follow-up studies. If successful, this will provide a framework of cross-tissue validated disease morbidity and mortality risk marker identification and enable clinicians to test for blood-based biomarkers in living patients to identify their risk profiles for mood disorder severity and imminent suicide risk. Secondly, using unsupervised learning will allow the identification of subtypes of suicide. Such unsupervised machine learning approaches can enable researchers to analyze what variables drive similarity, and advance understanding and identification of distinct suicide phenotypes. Integrating brain imaging studies into predictive suicide models could further bolster their efficacy, improving personalized neuropsychiatry.
Fig. 3.
Supervised and unsupervised learning methods for suicide research. A) Clinical and demographic data combined with RNA-sequencing data from post-mortem brain tissue improve suicide prediction models and neurobiological understanding of suicide risk. B) Features are automatically selected that facilitate suicide classification on the left and suicide subtype aggregation on the right C) Supervised learning classifies suicides from non-suicides with a boundary line (red) using input features (x1 and x2). Colored clusters represent suicide and non-suicide groups. Unsupervised learning clusters represent suicide phenotypes based on input features such as demographics, clinical, and biological and tissue qualitative variables. Colors are arbitrary and represent groupings of suicide subtypes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
7. Considerations for mood disorder and suicide heterogeneity in suicide risk prediction
This review shows that neurobiological measures such as brain gene expression profiles in postmortem mood disorders and suicide can vary significantly across individuals and contexts. This heterogeneity might partly be due to how mood disorders are defined incompatibly from biology to psychiatry. Depression, as defined by the Diagnostic and Statistical Manual of Mental Disorders Version V, can manifest in more than 10000 ways (Cai et al., 2020). Subtypes of depression, based on theory and data-driven approaches, have been proposed to reconcile this heterogeneity. Furthermore, the time course of mental illness generally varies substantially between individuals; depression subtypes with temporal dissimilarities could have different underlying biological pathways. Additionally, though clinically distinct, mood disorders such as MDD and bipolar disorder might not always be necessarily differentiable at a molecular level, as demonstrated by several overlapping gene expression profiles in previous research (Jabbi et al., 2020b).
While more analytically feasible, most RNA-seq studies that conduct group-level analysis of mood disorders have so far failed to capture individual differences in mood disorder-related gene expression abnormalities. One reason is that mildly depressed individuals who barely pass the symptom threshold are lumped together with severe cases with above threshold symptom scores. Furthermore, different molecular mechanisms could potentially confer symptom severity. Thus, future studies need to identify molecular substrates for mood disorder morbidity and suicide risks and conduct further analyses on homogeneous diagnostic cohorts as undertaken recently (Jabbi et al., 2020b; Arasappan et al., 2021). Such approaches could advance the mechanistic understanding of phenotypic specificity (i.e., mood disorder symptoms relative to suicide risk phenotypes).
Identifying quantitative biological traits and novel symptom cluster definitions of psychiatric disorders that better correlate with underlying biological abnormalities will advance the goals of developing better methods for identifying the underlying neurobiological mechanisms. Moreover, various genes and gene networks mediating specific molecular pathways have been identified, but the extent to which these molecular mechanisms are measurable at the individual level is underexplored. Finally, identifying unique molecular properties that scale with mood disorder severity and suicide lethality indices will enable research collaborations with clinical practices toward personalized and precision treatment and prevention strategies. Such interdisciplinary approaches will advance the biological understanding of suicide risk behaviors and thereby help decriminalize (Aleman and Denys, 2014) the expression of suicidal phenotypes as suicidal behaviors are still treated as a crime in at least 20 countries.
8. Example studies of machine learning prediction of suicide risk
In a recent study, Passos et al. (Brietzke et al., 2009, Passos et al., 2016) explored suicide prediction with machine learning techniques by identifying clinical risk factors for a suicide attempt at the individual level. One hundred forty-four patients with mood disorders were included in the study with exclusion criteria including head trauma, neurological disease, and uncontrolled primary medical conditions. Mood and anxiety symptoms were assessed with the Hamilton Depression Rating Scale (HDRS), the Young Mania Rating Scale (YMRS), and Hamilton Anxiety Rating Scale (HARS). Clinical and demographic variables were chosen according to a priori identification of suicide risk factors, including age, gender, race, years of education, current employment status, mood disorder diagnosis (i.e., bipolar disorder or major depression), anxiety spectrum disorders, obsessive-compulsive disorder, alcohol/cocaine dependence, number of lifetime depressive episodes, prior hospitalizations by depressive episodes, and psychotic symptoms (Brietzke et al., 2009, Passos et al., 2016).
Passos et al. (Brietzke et al., 2009, Passos et al., 2016) applied three machine learning algorithms, including 1) support vector machine (SVM), which performs linear classification by identifying a decision boundary that separates two classes (in this case, suicide and non-suicide); 2) relevance vector machine (RVM), which uses a Bayesian framework and linear kernel to provide probabilistic classification from a sparse use of data points; and 3) the least absolution shrinkage and selection operator (LASSO), which is a linear regression analysis method that uses variable selection and regularization, assigning some predictor variables to zero coefficients. The machine learning approach separates data into training and testing sets and utilizes cross-validation to generalize the data. The authors used leave-one-out cross-validation (LOOCV), which involves training an algorithm on all subject data except one, and repeated the LOOOCV process until all subjects are left out at least once. To avoid class imbalance, where observations in one class outnumber those in another, biasing classification of new observations, Passos et al., 2016 under-sampled the majority class and then resampled for the training stage for 1000 iterations to include each observation in the majority class in training at least once. In essence, each algorithm adopted in Passos et al.‘s study was designed to distinguish suicide attempters from non-attempters with greater than chance accuracy (Brietzke et al., 2009, Passos et al., 2016). RVM was most successful, yielding 72% classification accuracy (Brietzke et al., 2009, Passos et al., 2016). The most relevant predictor variables were as follows: 1) increased number of previous hospitalizations for depression, 2) history of psychosis, 3) cocaine dependence, and 4) PTSD. Additionally, a reduction in age and mood diagnosis, i.e., low age of onset for bipolar disorder diagnosis, was relevant in identifying suicide attempters. When removing the number of previous hospitalizations from the algorithm, the RVM performed with 68.9% accuracy, indicating that this variable was not the only driver of the predictive power of the applied algorithm. Although Passos et al.‘s study predicted suicide risk at the individual level using clinical and demographic variables with machine learning algorithms, their method did not include biological variables needed to identify mechanisms and treatment targets (Passos et al., 2016).
In another study, Bhak et al. (Bhak et al., 2019) constructed random forest binary classification models for three diagnostic group comparisons (i.e., suicide attempters vs. major depressive non-suicide attempters, suicide attempters vs. controls, and major depressive non-suicide attempters vs. controls) to identify differentially expressed genes and differentially methylated predictive features from whole blood using a sample of 56 suicide attempters with diagnosed major depression, 39 non-suicide attempters diagnosed with major depression, and 87 healthy controls. For Bhak et al.’ s machine learning prediction study, all participants were alive at the time of data collection and provided written informed consent before donating blood. Differentially expressed genes from whole blood with a fold change over 1.2 and a Benjamini-Hochberg adjusted p-value <0.05 were chosen as features in each model (Bhak et al., 2019). A tree-based feature selection method using a LOOCV method was used to eliminate irrelevant features that didn’t contribute to prediction accuracy. Using blood-derived multi-omics data, these authors found that suicide attempters had different characteristics than major depressive non-suicidal individuals with 92.6% accuracy. Major depressive individuals were further successfully discriminated from controls with 87.3% accuracy. In addition, suicide attempters were discriminated from controls with 86.7% accuracy (Bhak et al., 2019). The authors additionally built linear regression models based on multi-omics data that could predict psychiatric scale scores successfully using the Hamilton Rating Scale for Depression-17 (HAMD-17) and Scale for Suicidal Ideation (SSI) ratings. Predictive features were selected if differential methylation and gene expression significantly correlated with HAMD-17 or SSI. After predictive feature selection, 48 and 51 differentially expressed genes remained very predictive for HAMD-17 and SSI regression models, respectively. The linear regression models for the depressed and bipolar groups yielded an Rˆ2 of 0.961 for HAM17 and 0.943 for SSI (Bhak et al., 2019). In sum, Bhak et al.‘s study reveals that blood-based multi-omics data can be used to predict suicide risk behaviors successfully.
Furthermore, the applied integrative bio-behavioral methods by Bhak and colleagues could provide a framework for individualized predictive approaches (Bhak et al., 2019; Lutz et al., 2017). Emerging studies using clinical symptomatology (Melhem et al., 2017; Gvion and Levi-Belz, 2018; Simon et al., 2018; Rakesh, 2017; Passos et al., 2016; Kessler et al., 2015; Bernert et al., 2020; Bhak et al., 2019; Busch et al., 2003; Edavally et al., 2021; Gradus et al., 2020), health informatics (Gradus et al., 2020), multi-omic (Bhak et al., 2019; Cai et al., 2020; Han et al., 2019), or integration of clinical and biological datasets (Bhak et al., 2019) are being pursued for more accurate predictions of suicide risk with machine learning algorithms. Given the phenotypic complexity of suicide risk behaviors that are not specific to mood disorders, identifying highly reliable predictive clinical features for suicide risk will guide more reliable biological risk predictive markers in high-risk populations. Combining machine learning with postmortem brain biological studies and related records of lifetime clinical and endpoint suicide outcome measures can identify powerful predictors of suicide risk, albeit retrospectively.
9. Conclusions and future directions
Despite the high prevalence of suicide, accounting for close to a million global premature deaths annually (Aleman and Denys, 2014; Saxena et al., 2013; Turecki et al., 2019), especially in individuals with severe mood disorders, the need for better-prediction of suicide risk remains unmet. Pragmatic and individually precise research solutions are needed (Aleman and Denys, 2014). Evidence suggests that integrative biological and clinical research can identify the underlying biological mechanisms for mood disorder severity and suicide risk phenotypes. Once such clinically relevant biomarkers are characterized, prediction of suicidal intent and acts can be facilitated with integrative machine learning approaches (Edavally et al., 2021).
We propose using machine learning methods to comprehensively integrate environmental history, demographics and clinical symptomatology (Melhem et al., 2017; Gvion and Levi-Belz, 2018; Simon et al., 2018; Rakesh, 2017; Passos et al., 2016; Kessler et al., 2015; Bernert et al., 2020; Bhak et al., 2019; Busch et al., 2003; Edavally et al., 2021; Gradus et al., 2020), with physical homeostatic measures and molecular neurobiological outcomes such as gene expression data (Bhak et al., 2019; Cai et al., 2020; Han et al., 2019) as proposed in our suicide model (Fig. 1) using post-mortem studies. While such post-mortem methods in deceased individuals have low preventative value because disease and mortality outcomes are known for each donor, such postmortem studies can ultimately help identify suicide risk predictive features and related biological risk markers in a deterministic fashion (Jabbi et al., 2020b; Pantazatos et al., 2017). In addition, such studies could guide clinical translation of postmortem brain findings into peripheral blood markers that are less invasive to access in clinical populations. In line with cancer research that has increasingly integrated mixed data types to improve cancer patient stratification and disease prognostics (Han et al., 2019), such integrative mixed data approaches (Fig. 3) hold promise for the field of neuropsychiatry. Using biomarkers from postmortem studies with predictive values, future identification of diagnostic, prognostic, and predictive risk biomarkers for mood disorders and related suicide risk will advance mechanistic understanding and help identify biologically valid phenotypic markers for suicide risk prediction.
Declaration of competing interest
Authors have no conflict of interest to declare.
Acknowledgements
Authors would like to thank Dr. Bret Rutherford, Columbia University, for his helpful guidance and feedback on the conceptual model relating to the environmental influences on suicide risk, as well as his guidance on the simplified suicide model in Fig. 2.
Data availability
No data was used for the research described in the article.
References
- Aleman A., Denys D. A roadmap for suicide research and prevention. Science. 2014;509:421–423. doi: 10.1038/509421a. [DOI] [PubMed] [Google Scholar]
- Arasappan D., Eickhoff S.B., Nemeroff C.B., Hofmann H.A., Jabbi M. Transcription factor motifs associated with anterior insula gene-expression in mood disorders Brains. Mol. Neurobiol. 2021;58(5):1978–1989. doi: 10.1007/s12035-020-02195-8. [DOI] [PubMed] [Google Scholar]
- Barbosa I.G., Rocha N.P., Bauer M.E., de Miranda A.S., Huguet R.B., Reis H.J., et al. Chemokines in bipolar disorder: trait or state? Eur. Arch. Psychiatr. Clin. Neurosci. 2013;263:159–165. doi: 10.1007/s00406-012-0327-6. [DOI] [PubMed] [Google Scholar]
- Barres B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Bergmans R.S., Kelly K.M., Mezuk B. Inflammation as a unique marker of suicide ideation distinct from depression syndrome among U.S. adults. J. Affect. Disord. 2019;245:1052–1060. doi: 10.1016/j.jad.2018.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert R.A., Hilberg A.M., Melia R., Kim J.P., Shah N.H., Abnousi F. Artificial intelligence and suicide prevention: a systematic review of machine learning investigations. Int. J. Environ. Res. Publ. Health. 2020;17(16):1–25. doi: 10.3390/ijerph17165929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E., Toups M., Nemeroff C.B. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhak Y., Jeong H. oh, Cho Y.S., Jeon S., Cho J., Gim J.A., Jeon Y., Blazyte A., Park S.G., Kim H.M., Shin E.S., Paik J.W., Lee H.W., Kang W., Kim A., Kim Y., Kim B.C., Ham B.J., Bhak J., Lee S. Depression and suicide risk prediction models using blood-derived multi-omics data. Transl. Psychiatry. 2019;9(1) doi: 10.1038/s41398-019-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C., Miller B.J. Meta-analysis of cytokines and chemokines in suicidality: Distinguishing suicidal versus nonsuicidal patients. Biol. Psychiatr. 2015 Jul 1;78(1):28–37. doi: 10.1016/j.biopsych.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Brietzke E., Stertz L., Fernandes B.S., Kauer-Sant’anna M., Mascarenhas M., Escosteguy Vargas A., et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J. Affect. Disord. 2009;116:214–217. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Brundin L., Bryleva E.Y., Thirtamara Rajamani K. Role of inflammation in suicide: from mechanisms to treatment. Neuropsychopharmacology. 2017;42:271–283. doi: 10.1038/npp.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch K.A., Fawcett J., Jacobs D.G. Clinical correlates of inpatient suicide. J. Clin. Psychiatr. 2003;64:14–19. doi: 10.4088/jcp.v64n0105. [DOI] [PubMed] [Google Scholar]
- Cacioppo J.T., Cacioppo S., Capitanio J.P., Cole S.W. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 2015;66:733–767. doi: 10.1146/annurev-psych-010814-015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N., Choi K.W., Fried E.I. Reviewing the genetics of heterogeneity in depression: operationalizations, manifestations, etiologies. Hum. Mol. Genet. 2020;29:R10–R18. doi: 10.1093/hmg/ddaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos A.I., et al. Brain correlates of suicide attempt in 18,925 participants across 18 international cohorts. Biol. Psychiatr. 2021;90(4):243–252. doi: 10.1016/j.biopsych.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviedes A., Lafourcade C., Soto C., Wyneken U. BDNF/NF-κB signaling in the neurobiology of depression. Curr. Pharmaceut. Des. 2017;23(21):3154–3163. doi: 10.2174/1381612823666170111141915. [DOI] [PubMed] [Google Scholar]
- Chen Y.W., Dilsaver S.C. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other Axis I disorders. Biol. Psychiatr. 1996;39(10):896–899. doi: 10.1016/0006-3223(95)00295-2. [DOI] [PubMed] [Google Scholar]
- Chen Y.W., Dilsaver S.C. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other Axis I disorders. Biol. Psychiatr. 1996;39(10):896–899. doi: 10.1016/0006-3223(95)00295-2. [DOI] [PubMed] [Google Scholar]
- Cole S.W. Human social genomics. PLoS Genet. 2014;10(8) doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.W., Hawkley L.C., Arevalo J.M., Sung C.Y., Rose R.M., Cacioppo J.T. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.W., Hawkley L.C., Arevalo J.M., Cacioppo J.T. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc. Natl. Acad. Sci. U. S. A. 2011;108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtet P., et al. Neuroinflammation in suicide: toward a comprehensive model. World J. Biol. Psychiatr. 2016;17:564–586. doi: 10.3109/15622975.2015.1054879. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel - now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dantzer Robert. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004;500(1–3):399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer Robert, Konsman Jan-Pieter, Bluthe Rose-Marie, Kelley Keith. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton. Neurosci.: Basic and Clinical. 2000;85(1–3):60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- Decker T., Müller M., Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 2005;5(9):675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- Dilsaver S.C., et al. Suicidality in patients with pure and depressive mania. Am. J. Psychiatr. 1994;151(9):1312–1315. doi: 10.1176/ajp.151.9.1312. [DOI] [PubMed] [Google Scholar]
- Dum R.P., Levinthal D.J., Strick P.L. The mind-body problem: circuits that link the cerebral cortex to the adrenal medulla. Proc. Natl. Acad. Sci. U. S. A. 2019;116(52):26321–26328. doi: 10.1073/pnas.1902297116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edavally S., Miller D.D., Youssef N.A. Artificial intelligence to aid detection and diagnostic accuracy of mood disorders and predict suicide risk: a systematic review. Ann. Clin. Psychiatr. 2021;33(4):270–281. doi: 10.12788/acp.0041. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger Naomi I., Inagaki Tristen, Mashal Nehjla, Irwin Michael. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav. Immun. 2010;24(4):558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Lotrich F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch C.E. Evolution in health and medicine Sackler colloquium: evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl. Acad. Sci. U. S. A. 2010;107(Suppl. 1):1718–1724. doi: 10.1073/pnas.0909606106. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giakoumatos C.I., et al. Are structural brain abnormalities associated with suicidal behavior in patients with psychotic disorders? J. Psychiatr. Res. 2013;47:1389–1395. doi: 10.1016/j.jpsychires.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D., et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Gogolla N. The brain remembers where and how inflammation struck. Cell. 2021;184(24):5851–5853. doi: 10.1016/j.cell.2021.11.002. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Topography of cognition: parallel Distributed networks in primate association cortex. Annu. Rev. Neurosci. 1988;11(1):137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goodkind M., et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatr. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell S.N., et al. Prefrontal cortex, temporal cortex, and hippocampus volume are affected in suicidal psychiatric patients. Psychiatry Res. Neuroimaging. 2016;256:50–56. doi: 10.1016/j.pscychresns.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradus J.L., Rosellini A.J., Horváth-Puhó E., Street A.E., Galatzer-Levy I., Jiang T., Lash T.L., Sørensen H.T. Prediction of sex-specific suicide risk using machine learning and single-payer health care registry data from Denmark. JAMA Psychiatr. 2020;77(1):25–34. doi: 10.1001/jamapsychiatry.2019.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoleit Jan-Sebastian, Kullmann Jennifer, Wolf Oliver, Hammes Florian, Wegner Alexander, Jablonowski Stephanie, Engler Harald, Gizewski Elke, Oberbeck Reiner, Schedlowski Manfred. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gvion Y., Levi-Belz Y. Serious suicide attempts: systematic review of psychological risk factors. Front. Psychiatr. 2018;9(MAR):1–17. doi: 10.3389/fpsyt.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarman B.C., et al. Neuroinflammation in bipolar disorder - a [(11)C]-(R)-PK11195 positron emission tomography study. Brain. Behav Immun. 2014;40:219–225. doi: 10.1016/j.bbi.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Han Y., Ye X., Wang C., Liu Y., Zhang S., Feng W., Huang K., Zhang J. Integration of molecular features with clinical information for predicting outcomes for neuroblastoma patients. Biol. Direct. 2019;14(1):1–16. doi: 10.1186/s13062-019-0244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N.A., et al. Inflammation causes mood changes through alterations in the subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatr. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison Neil A., Brydon Lena, Walker Cicely, Gray Marcus, Steptoe Andrew, Critchley Hugo. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatr. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison Neil A., Voon Valerie, Cercignani Mara, Cooper Ella, Pessiglione Mathias, Critchley Hugo. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol. Psychiatr. 2016;80(1):73–81. doi: 10.1016/j.biopsych.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hellwig S., Domschke K. Update on PET imaging biomarkers in the diagnosis of neuropsychiatric disorders. Curr. Opin. Neurol. 2019;32(4):539–547. doi: 10.1097/WCO.0000000000000705. [DOI] [PubMed] [Google Scholar]
- Henter I.D., Park L.T., Zarate C.A., Jr. Novel glutamatergic modulators for the treatment of mood disorders: current status. CNS Drugs. 2021 May;35(5):527–543. doi: 10.1007/s40263-021-00816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J.L., et al. Transcriptomic abnormalities in peripheral blood in bipolar disorders, and discrimination of the major psychoses. Schizophr. Res. 2020 Mar;217:124–135. doi: 10.1016/j.schres.2019.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Qi D., Liang J., Miao R., Minagawa K., Quinn T., Matsui T., Fan D., Liu J., Fu M. The putative tumor suppressor Zc3h12d modulates toll-like receptor signaling in macrophages. Cell. Signal. 2012;24(2):569–576. doi: 10.1016/j.cellsig.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki Tristen K., Muscatell Keely, Irwin Michael, Cole Steve, Eisenberger Naomi. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59(4):3222–3227. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki Tristen K., Muscatell Keely, Irwin Michael, Moieni Mona, Dutcher Janine, Jevtic Ivana, Breen Elizabeth, Eisenberger Naomi. The role of the striatum in inflammatory-induced approach toward support figures. Brain Behav. Immun. 2015;44:247–252. doi: 10.1016/j.bbi.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M.R., Cole S.W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M., Nemeroff C.B. Convergent neurobiological predictors of mood and anxiety symptoms and treatment response. Expert Rev. Neurother. 2019;19(6):587–597. doi: 10.1080/14737175.2019.1620604. [DOI] [PubMed] [Google Scholar]
- Jabbi M., et al. A common anterior insula representation of disgust observation, experience, and imagination shows divergent functional connectivity pathways. PLoS One. 2008;3(8) doi: 10.1371/journal.pone.0002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M., et al. Frontolimbic brain volume abnormalities in bipolar disorder with suicide attempts. Psychiatr. Res. 2020;294 doi: 10.1016/j.psychres.2020.113516. [DOI] [PubMed] [Google Scholar]
- Jabbi M., Arasappan D., Eickhoff S.B., Strakowski S.M., Nemeroff C.B., Hofmann H.A. Neuro-transcriptomic signatures for mood disorder morbidity and suicide mortality. J. Psychiatr. Res. 2020;127:62–74. doi: 10.1016/j.jpsychires.2020.05.013. [DOI] [PubMed] [Google Scholar]
- Janelidze S., Suchankova P., Ekman A., Erhardt S., Sellgren C., Samuelsson M., et al. Low IL-8 is associated with anxiety in suicidal patients: genetic variation and decreased protein levels. Acta Psychiatr. Scand. 2014;131(4):269–278. doi: 10.1111/acps.12339. [DOI] [PubMed] [Google Scholar]
- Janelidze S., et al. Low IL-8 is associated with anxiety in suicidal patients: genetic variation and decreased protein levels. Acta Psychiatr. Scand. 2015;131:269–278. doi: 10.1111/acps.12339. [DOI] [PubMed] [Google Scholar]
- Jollant F., et al. Neuroimaging-informed phenotypes of suicidal behavior: a family history of suicide and the use of a violent suicidal means. Transl. Psychiatry. 2018;8(1):120. doi: 10.1038/s41398-018-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce M.K.P., Barbas H.J. Cortical connections position primate area 25 as a keystone for interoception, emotion, and memory. J. Neurosci. 2018;38(7):1677–1698. doi: 10.1523/JNEUROSCI.2363-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser M.S., Dalmau J. The emerging link between autoimmune disorders and neuropsychiatric disease. J. Neuropsychiatry Clin. Neurosci. 2011;23:90–97. doi: 10.1176/appi.neuropsych.23.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Warner C.H., Ivany C., et al. Predicting suicides after psychiatric hospitalization in U.S. Army soldiers: the army study to assess risk and resilience in service-members (army STARRS) JAMA Psychiatr. 2015;72(1):49–57. doi: 10.1001/jamapsychiatry.2014.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S.S., et al. Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(6):501–513. doi: 10.1016/j.bpsc.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Preacher K.J., MacCallum R.C., Atkinson C., Malarkey W.B., Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. U. S. A. 2003;100(15):9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.K., Jung H.G., Myint A.M., Kim H., Park S.H. Imbalance between proinflammatory and anti-inflammatory cytokines in bipolar disorder. J. Affect. Disord. 2007;104:91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Kirsch D.E., Tretyak V., Radpour S., Weber W.A., Nemeroff C.B., Fromme K., Strakowski S.M., Lippard E.T.C. Childhood maltreatment, prefrontal-paralimbic gray matter volume, and substance use in young adults and interactions with risk for bipolar disorder. Sci. Rep. 2021;11(1):123. doi: 10.1038/s41598-020-80407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloiber S., Rosenblat J.D., Husain M.I., Ortiz A., Berk M., Quevedo J., Vieta E., Maes M., Birmaher B., Soares J.C., Carvalho A.F. Neurodevelopmental pathways in bipolar disorders. Neurosci. Biobehav. Rev. 2020;112:213–226. doi: 10.1016/j.neubiorev.2020.02.005. [DOI] [PubMed] [Google Scholar]
- Koren T., et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184(24):5902–5915. doi: 10.1016/j.cell.2021.10.013. e17. [DOI] [PubMed] [Google Scholar]
- Krabbe Karen S., Abraham Reichenberg, Yirmiya Raz, Annelise Smed, Pedersen Bente, Bruunsgaard Helle. Low-dose endotoxemia and human neuropsychological functions. Brain Behav. Immun. 2005;19(5):453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Lasselin Julie, Elsenbruch Sigrid, Lekander Mats, Axelsson John, Karshikoff Bianka, Grigoleit Jan-Sebastian, Engler Harald, Schedlowski Manfred, Benson Sven. Mood disturbance during experimental endotoxemia: predictors of state anxiety as a psychological component of sickness behavior. Brain Behav. Immun. 2016;57:30–37. doi: 10.1016/j.bbi.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Lekander Mats, Karshikoff Bianka, Johansson Emilia, Soop Anne, Peter Fransson, Johan Lundstrom, Anna Andreasson, Martin Ingvar, Petrovic Predrag, Axelsson John, et al. Intrinsic functional connectivity of insular cortex and symptoms of sickness during acute experimental inflammation. Brain Behav. Immun. 2016;56:34–41. doi: 10.1016/j.bbi.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Lerman I., et al. Noninvasive vagus nerve stimulation alters neural response and physiological autonomic tone to noxious thermal challenge. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0201212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey D.F., et al. Genetic associations with suicide attempt severity and genetic overlap with major depression. Transl. Psychiatry. 2019;9(1):22. doi: 10.1038/s41398-018-0340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht M.W., Noble W.S. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015;16(6):321–332. doi: 10.1038/nrg3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R.B. Scientific World Journal; 2015. Mood Disorders: from Psychopathogenesis to Treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz P.E., Mechawar N., Turecki G. Neuropathology of suicide: recent findings and future directions. Mol. Psychiatr. 2017;22:395–1412. doi: 10.1038/mp.2017.141. [DOI] [PubMed] [Google Scholar]
- Maletic V., Raison C. Integrated neurobiology of bipolar disorder. Front. Psychiatr. 2014;5:98. doi: 10.3389/fpsyt.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J.J., Currier D., Dwivedi Y., editors. The Neurobiological Basis of Suicide. CRC Press/Taylor & Francis; 2012. [PubMed] [Google Scholar]
- Mathews D.C., et al. Neurobiological aspects of suicide and suicide attempts in bipolar disorder. Transl. Neurosci. 2013;4(2) doi: 10.2478/s13380-013-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath C.L., et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatr. 2013;70(8) doi: 10.1001/jamapsychiatry.2013.143. 821ñ829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre R.S., et al. Bipolar disorders. Lancet. 2020;396(10265):1841–1856. doi: 10.1016/S0140-6736(20)31544-0. [DOI] [PubMed] [Google Scholar]
- Melhem N.M., Porta G., Oquendo M.A., et al. Severity and variability of depression symptoms predicting suicide attempt in high-risk individuals. JAMA Psychiatr. 2017;6(6):603–613. doi: 10.1001/jamapsychiatry.2018.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem N.M., et al. The severity and variability depression symptoms predicting suicide attempt in high-risk individuals. JAMA Psychiatr. 2019;76(6):603–613. doi: 10.1001/jamapsychiatry.2018.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatr. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni Mona, Irwin Michael, Jevtic Ivana, Olmstead Richard, Breen Elizabeth, Eisenberger Naomi. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology. 2015;40(7):1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkholm K., Brauner J.V., Kessing L.V., Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J. Psychiatr. Res. 2013;47:1119–1133. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Musselman D.L., Miller A.H., Porter M.R., et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am. J. Psychiatr. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Nauta W.J.H. The problem of the frontal lobe: a reinterpretation. J. Psychiatr. Res. 1971;8(3–4):167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B. The state of our understanding of the pathophysiology and optimal treatment of depression: glass half full or half empty? Am. J. Psychiatr. 2020;177(8):671–685. doi: 10.1176/appi.ajp.2020.20060845. [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B. The trifecta of misery and disease vulnerability: poverty, childhood maltreatment, and inflammation. Am. J. Psychiatr. 2021;178(4):282–284. doi: 10.1176/appi.ajp.2020.21010087. [DOI] [PubMed] [Google Scholar]
- O'Brien S.M., Scully P., Scott L.V., Dinan T.G. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. J. Affect. Disord. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Pan L.A., et al. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol. Med. 2013;43(10):2129–2142. doi: 10.1017/S0033291712002966. [DOI] [PubMed] [Google Scholar]
- Pandey G.N., Rizavi H.S., Bhaumik R., Zhang H. Chemokines gene expression in the prefrontal cortex of depressed suicide victims and normal control subjects. Brain Behav. Immun. 2021;94:266–273. doi: 10.1016/j.bbi.2021.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazatos S.P., Huang Y.Y., Rosoklija G.B., Dwork A.J., Arango V., Mann J.J. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol. Psychiatr. 2017;22(5):760–773. doi: 10.1038/mp.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos I.C., Mwangi B., Cao B., et al. Identify a clinical signature of suicidality among patients with mood disorders: a pilot study using a machine learning approach. J. Affect. Disord. 2016;193:109–116. doi: 10.1016/j.jad.2015.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlipný J., Hess Z., Vrzalová J., Rosolová H., Beran J., Petrlová B. Lower serum levels of interleukin-6 in a population sample with symptoms of depression than in a population sample without symptoms of depression. Physiol. Res. 2010;59(1):121–126. doi: 10.33549/physiolres.931695. [DOI] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh G. Suicide prediction with machine learning. Am. J. Psychiatry Residents’ J. 2017;12(1):15–17. [Google Scholar]
- Reichenberg Abraham, Yirmiya Raz, Schuld Andreas, Kraus Thomas, Haack Monika, Abraham Morag, Pollmächer Thomas. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatr. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Rhen T., Cidlowski J.A. Anti-inflammatory action of glucocorticoids--new mechanisms for old drugs. N. Engl. J. Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Riva-Posse P., et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol. Psychiatr. 2018;23(4):843–849. doi: 10.1038/mp.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G., et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol. Psychiatr. 2009;65(4):289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G., et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35(7):1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvanson P., Li Z., Mei L., Kounev V., Kern M., Ward B.D., Medda B., Shaker R. Interplay of spinal and vagal pathways on esophageal acid-related anterior cingulate cortex functional networks in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;316(5):G615–G622. doi: 10.1152/ajpgi.00228.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena Shekhar, Funk Michelle, Chisholm Dan. The Lancet; 2013. World Health Assembly Adopts Comprehensive Mental Health Action Plan 2013–2020; p. 381. [DOI] [PubMed] [Google Scholar]
- Scaini G., Barichello T., Fries G.R., Kennon E.A., Andrews T., Nix B.R., Zunta-Soares G., Valvassori S.S., Soares J.C., Quevedo J. TSPO upregulation in bipolar disorders and concomitant downregulation of mitophagic proteins and NLRP3 inflammasome activation. Neuropsychopharmacology. 2019 Jun;44(7):1291–1299. doi: 10.1038/s41386-018-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos K.W., Makhoul G.S., Sugrue L.P., Chang E.F., Krystal A.D. State-dependent responses to intracranial brain stimulation in a patient with depression. Nat. Med. 2021;27(2):229–231. doi: 10.1038/s41591-020-01175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos K.W., et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat. Med. 2021;27(10):1696–1700. doi: 10.1038/s41591-021-01480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos K.W., et al. Distributed subnetworks of depression defined by direct intracranial neurophysiology. Front. Hum. Neurosci. 2021;15 doi: 10.3389/fnhum.2021.746499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L., et al. Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol. Psychiatr. 2020;25(2):408–427. doi: 10.1038/s41380-019-0587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B.D., Kossmann T., Morganti-Kossmann M.C. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cerebr. Blood Flow Metabol.: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30(3):459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini G., et al. The role of inflammatory cytokines in suicidal behavior: a systematic review. Eur. Neuropsychopharmacol. 2013;23:1672–1686. doi: 10.1016/j.euroneuro.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Shneidman E.S. Suicide as psychache. J. Nerv. Ment Dis. 1993;181(3):145–147. doi: 10.1097/00005053-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Simon G.E., Johnson E., Lawrence J.M., Rossom R.C., Ahmedani B., Lynch F.L., Beck A., Waitzfelder B., Ziebell R., Penfold R.B., Shortreed S.M. Predicting suicide attempts and suicide deaths following outpatient visits using electronic health records. Am. J. Psychiatr. 2018;175(10):951–960. doi: 10.1176/appi.ajp.2018.17101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G., et al. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci. U. S. A. 2010;107(33):14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan E.K., Capitanio J.P., Tarara R.P., Mendoza S.P., Mason W.A., Cole S.W. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J. Neurosci. 2007;27(33):8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski S.M., et al. Suicidality among patients with mixed and manic bipolar disorder. Am. J. Psychiatr. 1996;153(5):674–676. doi: 10.1176/ajp.153.5.674. [DOI] [PubMed] [Google Scholar]
- Syed S.A., et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron. 2018;99(5):914–924. doi: 10.1016/j.neuron.2018.08.001. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantin D., Schild-Poulter C., Wang V., Haché R.J., Sharp P.A. The octamer binding transcription factor Oct-1 is a stress sensor. Cancer Res. 2005;65(23):10750–10758. doi: 10.1158/0008-5472.CAN-05-2399. [DOI] [PubMed] [Google Scholar]
- Taylor W.D., et al. Widespread white matter but focal gray matter alterations in depressed individuals with thoughts of death. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;62:22–28. doi: 10.1016/j.pnpbp.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Gordon J.B., Nemeroff C.B. Recognizing the importance of childhood maltreatment as a critical factor in psychiatric diagnoses, treatment, research, prevention, and education. Mol. Psychiatr. 2021;4:1–8. doi: 10.1038/s41380-021-01367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas S.G., Cruceanu C., Chen G.G., Turecki G., Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors, and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Turecki G., Brent D.A., Gunnell D., O'Connor R.C., Oquendo M.A., Pirkis J., Stanley B.H. Suicide and suicide risk. Nat. Rev. Dis. Prim. 2019;5(1):74. doi: 10.1038/s41572-019-0121-0. [DOI] [PubMed] [Google Scholar]
- van Amerongen R., Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- van Heeringen K., Mann J.J. The neurobiology of suicide. Lancet Psychiatr. 2014;1(1):63–72. doi: 10.1016/S2215-0366(14)70220-2. [DOI] [PubMed] [Google Scholar]
- Vitkovic L., Konsman J., Bockaert J., Dantzer R., Homburger V., Jacque C. Cytokine signals propagate through the brain. Mol. Psychiatr. 2000;5(6):604–615. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- Wagner G., et al. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J. Psychiatr. Res. 2012;46(11):1449–1455. doi: 10.1016/j.jpsychires.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Wang Alexandre, Miller Brian. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 2018;44(1):75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins L.R., Maier S.F. Implications of immune-to-brain communication for sickness and pain. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T., et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatr. 2016;22(10):1455–1463. doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Paolicelli R., Sforazzini F., et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.