Abstract
Purpose
We aimed to determine whether there was a difference in access to cancer-related healthcare between people living in Sweden and the United Kingdom (UK) during the COVID-19 pandemic. We also describe how the pandemic affected social contact of patients undergoing treatment.
Methods
This cross-sectional study used survey data collected through the War on Cancer mobile phone application between September 5, 2020, and January 6, 2021. We included individuals with cancer diagnoses living in Sweden or the UK. The association between difficulty accessing cancer-related healthcare and country was examined using logistic regression. Frequencies were used to describe the effect of the pandemic on social contact.
Results
Of 491 individuals included in the study, 183 were living in the UK and 308 in Sweden. Living in the UK was associated with greater difficulty accessing cancer-related healthcare (n = 99/183, 54.1%) than living in Sweden (n = 100/308, 32.5%) (odds ratio 2.12, 95% CI 1.39–3.23, p < 0.001). The pandemic affected social contact for almost all patients (n = 218/238, 91.6%) undergoing treatment.
Conclusion
This study highlights the differential impact that the pandemic may have had on patients’ access to cancer-related care in the UK and Sweden. In both countries, the pandemic overwhelmingly affected social contact of individuals undergoing cancer treatment. New ways must be found to improve access to cancer-related care and reduce social isolation for patients with cancer during a pandemic.
Keywords: Cancer patient, Coronavirus disease, UK, Sweden, Social isolation, Healthcare access
Introduction
The COVID-19 pandemic has challenged healthcare systems in unprecedented ways. By putting pressure on medical resources and isolating us from one another, the coronavirus has indirectly affected people with other illnesses, including those living with cancer. In 2018, over 17 million people were diagnosed with cancer, and one in roughly two people living in high-income countries are expected to be diagnosed with the disease within their lifetime [1, 2]. Patients with cancer face additional stressors during the pandemic such as delays and interruptions in treatment schedules and depression due to social isolation [3]. Reports from oncologists across Europe indicate that access to cancer-related care has been affected, and models predict a surge in delayed cancer diagnoses and reduced cancer survival in the aftermath of the pandemic [4–6]. Despite these unique effects of the pandemic on access to care and cancer outcomes, the effects of the COVID-19 crisis from the perspective of those living with cancer remain largely unknown.
National responses to COVID-19 have varied along a continuum, and it is important to consider the regulatory context when characterizing experiences for those living with cancer in different countries. This is particularly true when comparing Sweden and the UK, two countries with similar healthcare systems but that took markedly different approaches to managing the pandemic. Beginning in the fall of 2020, the Swedish government prohibited large-scale public gatherings, asked citizens to refrain from gathering in private homes with more than eight people, and recommended that adults and many students work and study remotely. With these predominantly voluntary measures, Sweden did not go into national “lockdown”. Rather, the Swedish government maintained their belief in citizens’ collectivist values and had a more “open” approach to the COVID-19 pandemic. In contrast, in November 2020, the UK instated a national lockdown, which prohibited gathering indoors. Those living alone were exempt from this mandate and were allowed a support “bubble” linking two households. Towards the end of this study in December 2020, many regions of the UK were once again allowed gatherings of up to six people. The UK’s response represents a more legislatively stringent approach to the pandemic than Sweden’s approach, as the UK’s measures were mandatory rather than voluntary.
In both scenarios, face-to-face data collection for public health purposes became more difficult. Mobile phone applications (apps) are increasingly being used to collect survey data because they do not require physical contact and cover large geographic areas quickly and at low cost [7]. This study used the War on Cancer app, which aims to support those living with cancer. We administered a survey through the app to explore and compare perceptions of how the pandemic affected access to cancer-related care and social contacts from the perspective of individuals living with cancer in Sweden and the UK.
Objectives
Determine whether there was a difference in patient perspectives of their access to cancer-related healthcare between those living in Sweden and those living in the UK during the pandemic.
Identify the types of difficulties that participants had accessing cancer-related care.
Describe the effect of the pandemic on social contacts for those undergoing cancer treatment.
Determine whether employers adapted to the needs of patients who considered themselves at high risk of contracting or getting seriously ill from COVID-19.
Methods
Study design
This cross-sectional study used survey data collected through the mobile phone app “War on Cancer” (waroncancer.com). This app, launched in 2016, aims to establish a digital community where people share and connect with others also experiencing cancer. During the study period, War on Cancer had just under 6000 members from across the globe, including those with cancer and loved ones of those with cancer. Most members are female and between the ages of 30 and 50. The majority are living in Sweden and the UK. Content is available in two languages, Swedish and English, although it may be translated to additional languages within the app. The platform is free of charge. Once registered, members can post their own stories, react and respond to others’ stories, send messages in private groups, and take part in webinars, podcasts, and health surveys. Members can also search for relevant clinical trials.
This study included those who had been diagnosed with cancer living in Sweden or the UK during part of the first and second waves of the COVID-19 pandemic (defined as being before or after November 15, 2020, respectively). Data were collected from September 5, 2020, to January 5, 2021. A team of researchers from the UK and Sweden, including epidemiologists and cancer care clinicians, formulated the survey. Questions focused on how the COVID-19 pandemic affected participants’: (1) access to cancer-related healthcare, (2) access to social contacts for those undergoing cancer treatment, and (3) employer support for those who were employed.
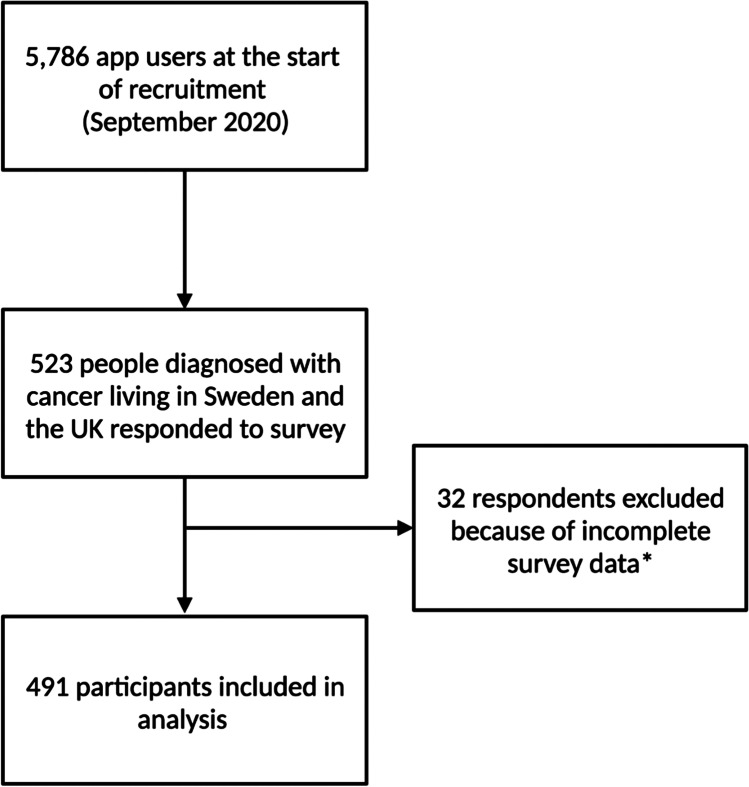
All app users were welcome to complete the survey (Fig. 1), but only those who indicated that they were living in the UK or Sweden were included in this study. Participants who were loved ones of those with cancer were excluded, as were those who completed fewer than eight of the 12–14 survey questions. There were no exclusion criteria with respect to age, gender, or cancer type. All participants provided informed consent before completing the questionnaire.
Fig. 1.
War on Cancer in-app survey interface
The primary outcome was reported difficulty accessing cancer-related healthcare. The type of difficulty was identified, including difficulties getting questions answered; postponed examinations (e.g., blood samples, specialist visits); postponed treatments (e.g., chemotherapy, radiation); and other types of difficulties. Responses to these questions were coded 0 (no) or 1 (yes). Multiple responses were permitted, but any one indication of a difficulty was considered as having a difficulty accessing cancer-related healthcare.
Whether the pandemic influenced visits from social contacts for those undergoing cancer treatment were also coded 0 (no) or 1 (yes). Participants had the opportunity to select multiple ways in which the pandemic affected their contact socially. Similarly, for those who were employed and considered themselves at high risk of contracting COVID-19 or becoming seriously ill from the virus, whether their employer met their needs was coded as 0 (no) and 1 (yes). Demographic data, such as age, country of residence, gender, and type of cancer, were also collected.
The number of participants who responded to the survey during the study timeframe determined sample size. It is accepted that logistic regression models should have a minimum of 10 outcome events per variable included in the model. With 199 events of difficulties accessing cancer-related healthcare in this cohort, our sample size was adequate to build a stable model with the four selected variables [8].
Statistical analyses
Descriptive analyses of the study population were conducted using frequencies for categorical variables and mean and standard deviation (SD) for continuous variables. Histograms and measures of skewness and kurtosis were used to assess normality of continuous variables before summarizing the data using means. For the primary analysis, a univariable analysis was used to examine the crude association between country and difficulty accessing cancer-related healthcare. Then the following potentially confounding variables were considered: age (≤ 45 years v. > 45 years); sex (male v. female); wave of the pandemic (first wave v. second wave); and type of cancer (breast, cervical, lung, other). These variables were included in an initial logistic regression multivariable model and retained in the final model if their p-value was ≤ 0.25. Odds ratios (OR) alongside 95% confidence intervals and p-values are reported. A two-sided p-value of less than 0.05 was considered significant. We performed descriptive analyses for secondary outcomes and reported the results as frequencies and proportions. Descriptive analyses were conducted in SPSS 28 (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, version 27.0. Armonk, NY: IBM Corp), and the logistic regression model was built in Stata 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
Between September 5, 2020, and January 6, 2021, 523 people living in Sweden or the UK who identified as having been diagnosed with cancer completed the survey, 491 of whom were included in the study (Fig. 2). More participants were from Sweden (62.7%) than the UK (37.3%), and the mean age of respondents was 44 years old (SD 10.93). Most participants were female (84.7%). Additionally, 56.8% of responses were submitted during the first wave of the pandemic, while 43.2% were submitted during the second wave (Table 1).
Fig. 2.
Participant flow chart. Incomplete responses were defined as those with less than 8 of the 12–14 survey questions answered
Table 1.
Baseline characteristics
| Sweden (n = 308) | UK (n = 183) | |
|---|---|---|
| Gender | ||
| Female | 266 (86.36%) | 150 (81.97%) |
| Male | 41 (13.31%) | 33 (18.03%) |
| Other/rather not say | 1 (0.32%) | 0 (0.00%) |
| Age | ||
| Mean (standard deviation) | 44.41 (11.72) | 43.83 (9.48) |
| ≤ 45 | 158 (51.30%) | 92 (50.27%) |
| > 45 | 150 (48.70%) | 91 (49.73%) |
| Cancer diagnosis | ||
| Breast | 110 (35.71%) | 82 (44.81%) |
| Cervical | 16 (5.19%) | 12 (6.56%) |
| Lung | 12 (3.90%) | 9 (4.92%) |
| Other | 161 (52.27%) | 77 (42.08%) |
| Missing data | 9 (2.92%) | 3 (1.64%) |
| COVID-19 pandemic wave | ||
| First | 125 (40.58%) | 154 (84.15%) |
| Second | 183 (59.42%) | 29 (15.85%) |
| COVID-19 test | ||
| Positive test | 22 (7.14%) | 8 (4.37%) |
| Negative test | 164 (53.25%) | 113 (61.75%) |
| No test | 117 (37.99%) | 61 (33.33%) |
| Missing data | 5 (1.62%) | 1 (0.55%) |
| Treatment | ||
| Undergoing treatment* | 141 (45.78%) | 97 (53.01%) |
| Not undergoing treatment | 159 (51.62%) | 80 (43.72%) |
| Missing data | 8 (2.60%) | 6 (3.28%) |
| Employment status | ||
| Employed | 230 (74.68%) | 113 (61.75%) |
| Not employed | 67 (21.75%) | 63 (34.43%) |
| Missing data | 11 (3.57%) | 7 (3.83%) |
*Of the 141 participants undergoing treatment in Sweden, 21 (14.89%) reported doing so exclusively at the hospital, 93 (65.96%) exclusively at home, and 27 (19.15%) at both. Of the 97 participants undergoing treatment in the UK, 16 (16.49%) reported doing so exclusively at the hospital, 52 (53.61%) exclusively at home, and 29 (29.90%) at both
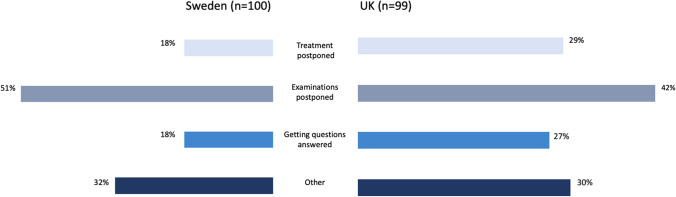
Those living in the UK had more difficulties accessing cancer-related healthcare than those in Sweden. In the UK, 54.7% of respondents had difficulties compared to 33.3% respondents living in Sweden. Data were missing for 10 participants. Patients in the UK were more likely to have had difficulty accessing cancer-related care than those in Sweden (crude odds ratio 2.41, 95% confidence interval 1.65–3.53, p < 0.001) (Table 2). After adjustment for age and wave of the pandemic, this finding remained significant (adjusted odds ratio 2.12, 95% CI: 1.39–3.23; p < 0.001). Sex and type of cancer did not significantly contribute to the model. The primary difficulty participants had accessing cancer-related healthcare in both countries was their examinations being postponed (51.0% in Sweden and 42.4% in the UK) (Fig. 3).
Table 2.
Multivariable logistic regression results for country and difficulty accessing cancer-related care during the COVID-19 pandemic
| Factor | Crude OR (95% CI), p-value | Adjusted* model: OR (95% CI), p-value | Final model†: OR (95% CI), p-value |
|---|---|---|---|
| UK vs Sweden | 2.41 (1.65–3.53), < 0.001 | 2.19 (1.42–3.36), < 0.001 | 2.12 (1.39–3.23), < 0.001 |
*Adjusted for gender, age, pandemic wave, and cancer diagnosis
†Adjusted for age and pandemic wave
Fig. 3.
Type of difficulty accessing cancer-related healthcare* by country. *Answers are not mutually exclusive
Next, we looked at the pandemic affected participants’ access to their social contacts while undergoing treatment. Of those included in the study, 238 were receiving cancer treatment: 201 at hospital, 93 at home, and 56 both at the hospital and at home. The pandemic affected 91.6% of participants’ access to social contacts, with 92.2% (n = 130/141) and 90.7% (n = 88/97) of respondents reporting that their social contact was affected in Sweden and the UK, respectively. Fear appeared to play a larger role in people’s access to social contacts for those being treated at home than it did for those being treated in hospital, with 33.8% (n = 68/201) of home-treated patients compared to 8.6% (n = 8/93) of hospital-treated patients reporting fear of contracting the virus from visitors (Table 3).
Table 3.
Access to social contacts by treatment location and country
| Sweden | UK | |
|---|---|---|
| Overall | (n = 141) | (n = 97) |
| Social contact not affected | 11 (7.8%) | 9 (9.3%) |
| Social contact affected | 130 (92.2%) | 88 (90.7%) |
| Treated at hospital | (n = 48) | (n = 45) |
| Visitors are allowed | 2 (4.2%) | 4 (8.9%) |
| Only a few visitors are allowed | 7 (14.6%) | 9 (20.0%) |
| No visitors are allowed | 33 (68.8%) | 26 (57.8%) |
| Fear of contracting the virus from visitors | 3 (6.3%) | 5 (11.1%) |
| Visitors fearful of transmitting the virus to the participant | 7 (14.6%) | 12 (26.7%) |
| Other | 5 (10.4%) | 1 (2.2%) |
| Treated at home | (n = 120) | (n = 81) |
| People are allowed to visit as before | 13 (10.8%) | 16 (19.8%) |
| Only allow a few visitors | 71 (59.2%) | 41 (50.6%) |
| Doesn’t allow visitors because of fear of contracting the virus | 40 (33.3%) | 28 (34.6%) |
| Fear transmitting the virus to visitors | 2 (1.7%) | 4 (4.9%) |
Of those who were employed and considered themselves at high-risk (n = 274/491, 55.8%), 89.8% (n = 246/274) of individuals felt that their employer catered to their needs. The proportion who felt that their employer was supportive was similar in both settings, with 91.0% of respondents in Sweden and 87.6% of respondents in the UK agreeing with this statement. The question on employment was not applicable to 40.5% (n = 199/491) of the respondents because they were either not employed or did not consider themselves high risk of contracting COVID-19 or getting seriously ill from the virus. Additionally, data were missing for 18/491 (3.7%) participants.
Discussion
In this study, respondents from the UK experienced more difficulties accessing cancer-related healthcare than their Swedish counterparts. The greatest difficulty reported by participants in both countries was having their examinations postponed. In Sweden and the UK, the pandemic overwhelmingly affected participants’ access to their social contacts, regardless of where participants received treatment. Most employed respondents in both nations felt that their employer catered to their needs.
While Sweden and the UK’s healthcare systems differ, they are comparable in some ways. Both nations have universal healthcare systems and face similar population-level health challenges, such as an aging population and high rates of cancer. In response to the pandemic, both nations’ healthcare systems reprioritized resources in attempt to curb the spread of the virus and to provide care for those who became ill with COVID-19. Such adjustments impacted all medical services, including those receiving care along the cancer care continuum, comprised of screening, early detection, diagnosis, treatment, survivorship, and end-of-life care [9]. Numerous high-income countries in Europe, including Sweden and the UK, have reported decreased access to other medical and emotional resources during the pandemic [10]. As such, the potential for a post-pandemic surge in reduced cancer survival appears likely [11]. This study adds a patient perspective to these reports, with patients from both countries having difficulties accessing cancer-related care, primarily related to having their examinations postponed [12, 13]. These findings are supported by a systematic review indicating that people who have cancer experienced significant delays in their follow-up appointments, treatment, and lack of access to other aspects of cancer care during the COVID-19 pandemic [14]. In this study, differences in access to cancer-related care between Sweden and the UK during the pandemic may be related to pre-pandemic differences in cancer care access between the two countries. Research suggests that cancer care in the UK lags behind that in Sweden, and that late diagnoses, delayed access to treatment, and age bias are likely factors for England’s poor cancer survival rates [15, 16]. Our results suggest that this gap in access to cancer care between these two countries persisted through the COVID-19 pandemic.
More than 90% of respondents in Sweden and the UK reported that the pandemic affected their access to social contacts, which is concerning, as previous research has linked social connectedness with improved cancer survival [17]. In 2019, Braun et al. found that frequent contact with family, friends, and religious communities decreased the risk of death from any cause by 15–28% [18]. Other populations in Europe, including those surveyed using the European Organization for Research and Treatment of Cancer quality of life questionnaire, also experienced a significant decline in social functioning and social isolation during the pandemic [19, 20]. Despite the differences in Sweden’s and the UK’s approaches to managing social gatherings during the pandemic, patient reports of reduced access to social support appear similar between the two countries. This finding may suggest that in this case, access to social contacts is mediated more by individuals themselves than by national regulation. As access to social contacts did not differ much between Sweden and the UK, it appears that voluntary recommendations were enough to establish social norms of social distancing in Sweden. Nonetheless, the problem of finding ways to safely maintain access to social support during apandemic needs to be addressed, particularly as a lack of social support is a risk factor for loneliness [21]. Clinicians may help alleviate the detrimental impact of the pandemic on social support by informing patients of new ways to ensure continued support. This includes informational support through telehealth programs, on-line peer support and group psychosocial support programs, and encouragement to maintain the support of family and friends via telephone and on-line [22, 23].
For many cancer survivors, work plays a key role in maintaining feelings of normalcy, control, and financial security. Working, and feeling supported at work, has been shown to improve quality of life for cancer survivors and those around them [24]. Because the immunosuppressed status of some patients living with cancer puts them at greater risk of developing serious complications if they were to become infected with COVID-19, it may be of particular benefit for this population to work remotely for their health and safety, even after other employees return to work in-person [25]. Other studies have found that people with cancer experienced job loss or a reduction of hours during the pandemic [26], and that they were less likely to work from home than people without cancer and other disabilities [27]. In our study, most participants in both Sweden and the UK felt that their employers catered to their needs, which is encouraging. Maintaining this type of support for those living with cancer during the ongoing COVID-19 crisis and future pandemics is critical to their well-being and financial stability [28].
This study has several strengths. First, while there are many comparative studies of cancer treatment and outcomes between countries, and studies of single-country experiences of those with cancer during the COVID-19 pandemic, ours is the first to contrast the experiences of those living with cancer in two countries during the pandemic [29, 30]. Second, the two countries compared in the present study have important similarities and differences — both Sweden and the UK are classified as high income and have national healthcare systems, yet they differed markedly in their national response strategies to COVID-19. This study highlights the usefulness of mobile applications, such as the War on Cancer app, as a means of data collection during times of decreased social contact. Limitations of this study include the potential for volunteer bias. Those who subscribe to the War on Cancer app may have had different experiences than the general population of those with cancer living in Sweden or the UK, which may have affected their experiences accessing cancer-related healthcare or changes to their social contacts during the pandemic. Furthermore, while we were able to adjust our analyses for confounders such as age and wave of the pandemic, we were unable to control for other potentially important confounding factors such as ethnicity and education.
Our comparison between patients with cancer living in Sweden and the UK allows us to better understand how the COVID-19 pandemic impacted their perspectives on access to cancer care, social contact, and employer support. Like people with cancer, those who have a chronic disease require regular disease management and follow-up, which was likely affected by the pandemic. Social contact with others outside of their household also provides benefits to those with chronic diseases compared to the general population [31]. Our findings may be generalizable to those with chronic disease, although research specific to these populations may be more informative. Further research is required to understand the unique fears and worries of patients with cancer during the pandemic as well as the long-term impacts of treatment delays and reduced social contact on their mental health and clinical outcomes. This study emphasizes the importance of finding new ways to ensure that individuals living with cancer are supported during the ongoing COVID-19 pandemic and future public health crises.
Acknowledgements
We would like to thank the study participants for their contribution to the study and Caroline Weibull for her valuable comments on the final manuscript. Funding organizations include War on Cancer and the Karolinska Institutet.
Author contributions
Lisen Dahlström and Anna Mia Ekström conceived of and designed the study. Lisen Dahlström and Karolina Edlund were responsible for data collection. Karolina Edlund was responsible for cleaning the data. Mia van der Kop performed the data analysis. Karolina Edlund wrote the first draft of the manuscript, and all authors contributed important intellectual content to the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by the Karolinska Institutet. This study was funded by War on Cancer and the Karolinska Institutet.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
All participants in this study provided informed consent via the mobile application War on Cancer.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Cancer Society (2018) Global Cancer Facts & Figures 4th Edition. https://www.cancer.org/research/cancer-facts-statistics/global.html. Accessed 4 July 2022.
- 2.Ahmad AS, Ormiston-Smith N, Sasieni PD. Trends in the lifetime risk of developing cancer in Great Britain: comparison of risk for those born from 1930 to 1960. Br J Cancer. 2015;112(5):943–947. doi: 10.1038/bjc.2014.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Momenimovahed Z, Salehiniya H, Hadavandsiri F, Allahqoli L, Günther V, Alkatout I (2021) Psychological distress among cancer patients during COVID-19 pandemic in the world: a systematic review. Front Psychol 1210.3389/fpsyg.2021.682154 [DOI] [PMC free article] [PubMed]
- 4.Crul M, Boşnak AS, Astier A, Meier K (2021) The effect of COVID-19 on oncology pharmacy services. Results of a 3 month long weekly global survey. Eur J Oncol Pharm 4(1):e027
- 5.Crul M, Lawler M, Aapro M (2020) The impact of COVID-19 on cancer in Europe: the 7-point plan to address the urgency and build back better. European Cancer Organisation. file:///C:/Users/User/Downloads/Impact%20of%20COVID-19%20on%20Cancer_7-Point%20Plan_Final%20(1).pdf Accessed 4 July 2022.
- 6.Sud A, Jones ME, Broggio J, Loveday C, Torr B, Garrett A, Nicol DL, Jhanji S, Boyce SA, Gronthoud F, Ward P, Handy JM, Yousaf N, Larkin J, Suh YE, Scott S, Pharoah P, Swanton C, Abbosh C, Williams M, Turnbull C. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncology. 2020;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcano Belisario JS, Jamsek J, Huckvale K, O’Donoghue J, Morrison CP, Car J (2015) Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database Syst Rev 7:MR000042. 10.1002/14651858.MR000042.pub2 [DOI] [PMC free article] [PubMed]
- 8.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 9.Dulaney C, Wallace AS, Everett AS, Dover L, McDonald A, Kropp L. Defining health across the cancer continuum. Cureus. 2017;9(2):e1029. doi: 10.7759/cureus.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinmohamed AG, Visser O, Verhoeven R, Louwman M, van Nederveen FH, Willems SM, Merkx M, Lemmens V, Nagtegaal ID, Siesling S. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra S, Scott JA, Laydon DJ, Flaxman S, Gandy A, Mellan TA, Unwin H, Vollmer M, Coupland H, Ratmann O, Monod M, Zhu HH, Cori A, Gaythorpe K, Whittles LK, Whittaker C, Donnelly CA, Ferguson NM, Bhatt S. Comparing the responses of the UK, Sweden and Denmark to COVID-19 using counterfactual modelling. Sci Rep. 2021;11(1):16342. doi: 10.1038/s41598-021-95699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lou E, Teoh D, Brown K, Blaes A, Holtan SG, Jewett P, Parsons H, Mburu EW, Thomaier L, Hui J, Nelson HH, Vogel RI. Perspectives of cancer patients and their health during the COVID-19 pandemic. PLoS ONE. 2020;15(10):e0241741. doi: 10.1371/journal.pone.0241741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treiman K, Kranzler EC, Moultrie R, Arena L, Mack N, Fortune E, Garcia R, Street RL. Patients’ experiences with cancer care: impact of the COVID-19 pandemic. J Patient Exp. 2022;9:23743735221092567. doi: 10.1177/23743735221092567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jammu AS, Chasen MR, Lofters AK. Bhargava R (2021) Systematic rapid living review of the impact of the COVID-19 pandemic on cancer survivors: update to August 27. Support Care Cancer. 2020;29(6):2841–2850. doi: 10.1007/s00520-020-05908-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kmietowicz Z. Cancer care in England lags behind other countries, confirms review. BMJ. 2011;342:d3571. doi: 10.1136/bmj.d3571. [DOI] [PubMed] [Google Scholar]
- 16.Munro AJ. Comparative cancer survival in European countries. Br Med Bull. 2014;110(1):5–22. doi: 10.1093/bmb/ldu009. [DOI] [PubMed] [Google Scholar]
- 17.Boen CE, Barrow DA, Bensen JT, Farnan L, Gerstel A, Hendrix LH, Yang YC. Social relationships, inflammation, and cancer survival. Cancer Epidemiol Biomarkers Prev. 2018;27(5):541–549. doi: 10.1158/1055-9965.EPI-17-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun LA, Zomorodbakhsch B, Keinki C, Huebner J. Information needs, communication and usage of social media by cancer patients and their relatives. J Cancer Res Clin Oncol. 2019;145(7):1865–1875. doi: 10.1007/s00432-019-02929-9. [DOI] [PubMed] [Google Scholar]
- 19.Amaniera I, Bach C, Vachani C, Hampshire M, Arnold-Korzeniowski K, Healy M, Rodriguez A, Misher C, Kendrick L, Metz JM, Hill-Kayser CE. Psychosocial impact of the COVID-19 pandemic on cancer patients, survivors and caregivers. J Psychosoc Oncol. 2021;39(3):485–492. doi: 10.1080/07347332.2021.1913780. [DOI] [PubMed] [Google Scholar]
- 20.Ciążyńska M, Pabianek M, Szczepaniak K, Ułańska M, Skibińska M, Owczarek W, Narbutt J, Lesiak A. Quality of life of cancer patients during coronavirus disease (COVID-19) pandemic. Psycho-Oncol. 2020;29(9):1377–1379. doi: 10.1002/pon.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deckx L, van den Akker M, Buntinx F. Risk factors for loneliness in patients with cancer: a systematic literature review and meta-analysis. Eur J Oncol Nurs. 2014;18(5):466–477. doi: 10.1016/j.ejon.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Gentry MT, Lapid MI, Clark MM, Rummans TA. Evidence for telehealth group-based treatment: a systematic review. J Telemed Telecare. 2019;25(6):327–342. doi: 10.1177/1357633X18775855. [DOI] [PubMed] [Google Scholar]
- 23.Usta YY (2012) Importance of social support in cancer patients. Asian Pac JCancer Prev Asian Pacific Organization for Cancer Prevention10.7314/apjcp.2012.13.8.3569Accessed4July [DOI] [PubMed]
- 24.Wells M, Williams B, Firnigl D, Lang H, Coyle J, Kroll T, MacGillivray S. Supporting ‘work-related goals’ rather than ‘return to work’ after cancer? A systematic review and meta-synthesis of 25 qualitative studies. Psycho-Oncol. 2013;22(6):1208–1219. doi: 10.1002/pon.3148. [DOI] [PubMed] [Google Scholar]
- 25.Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020;43(6):452–455. doi: 10.1097/COC.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaniera I, Bach C, Vachani C, Hampshire M, Arnold-Korzeniowski K, Healy M, Rodriguez A, Misher C, Kendrick L, Metz JM, Hill-Kayser CE (2021) Psychosocial impact of the COVID-19 pandemic on cancer patients, survivors and caregivers. J Psychosoc Oncol 39(3):485–492 10.1080/07347332.2021.1913780 [DOI] [PubMed]
- 27.Kruse D, Park SR, van der Meulen RY, Schur L. Disability and remote work during the pandemic with implications for cancer survivors. J Cancer Surviv. 2022;1:183–199. doi: 10.1007/s11764-021-01146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blinder VS, Gany FM. Impact of cancer on employment. J Clin Oncol. 2020;38(4):302–309. doi: 10.1200/JCO.19.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TÅ, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, De P, McClure C, Ramanakumar AV, Stuart-Panko H, Engholm G, Bray F. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez GM, Ferguson JM, Kurian A, Bondy M, Patel MI. The impact of COVID-19 on patients with cancer: a national study of patient experiences. Am J Clin Oncol. 2021;44(11):580–587. doi: 10.1097/COC.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penninx BWJH, van Tilburg T, Boeke AJP, Deeg DJH, Kriegsman DMW, van Eijk JTM. Effects of social support and personal coping resources on depressive symptoms: different for various chronic diseases? Health Psychol. 1998;17(6):551–558. doi: 10.1037/0278-6133.17.6.551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.