Abstract
Allogeneic hematopoietic stem cell transplantation (allo-SCT) remains the best curative option for the majority of patients with hematologic malignancies (HM); however, many elderly patients are excluded from transplant and outcome data in this population is still limited. The novel two-step graft engineering approach has been the main platform for allo-SCT at Thomas Jefferson University since 2006. Following administration of the preparative regimen, we infuse donor lymphocytes, followed by cyclophosphamide to induce bidirectional tolerance, then infusion of CD34-selected cells. A total of 76 patients ≥ 65 years old with HM underwent haploidentical (haplo) allo-SCT on the two-step transplant platform between 2007 and 2021. The median time to neutrophil engraftment was 11 days and platelet engraftment was 18 days. With a median follow up of 44 months, the 3-year overall survival (OS) and progression-free survival (PFS) were 36.3% and 35.6%, respectively. The cumulative incidences of non-relapse mortality (NRM) and relapse at 3 years were 43.5% and 21.0% at 3 years, respectively. The cumulative incidence of grade III-IV acute graft-versus-host-disease (GVHD) was 11.1% at 6 months, and chronic GVHD requiring treatment was 15.1% at 2 years. The two-step haplo allo-SCT is a novel alternative platform for high-risk older HM patients, achieving fast engraftment, low relapse rates and promising survival.
Subject terms: Acute myeloid leukaemia, Myelodysplastic syndrome, Haematopoietic stem cells, B-cell lymphoma
Introduction
Older patients are disproportionally affected by HM and are more likely to have worse outcomes due to adverse disease biology, poor performance status, and existing medical comorbidities. Allogenic hematopoietic stem cell transplantation remains the only curative option and offers the most potential for long-term disease control for many HM. Historically, many older patients were not offered allo-SCT due to concern for increased NRM, higher rates of relapse, and worse OS [1–3]. The development of reduced intensity (RIC) and nonmyeloablative (NMA) conditioning regimens [4, 5], expansion of donor availability via use of unrelated donor (URD) and haplo donors [6], and improved supportive care have allowed the extension of allo-SCT to older patients. This is reflected by an upward trend of allo-SCT in older adults in recent years, with 26% of procedures performed in patients aged 65 and older in 2019 as reported to the Center for International Blood and Marrow Transplant Research (CIBMTR), up from 2% in 2000 [7].
Despite the availability of more effective and tolerable treatment modalities, a recent study found more than one-half of patients with acute myeloid leukemia (AML) age ≥66 years in the United States do not receive any antileukemic therapy [8], and only about 6% of newly diagnosed AML patients aged 60-75 undergo allo-SCT [9]. Several studies have investigated the use of allo-SCT in older adults and reported comparable outcomes with those reported in younger patients with similarly advanced disease [10–14]. A CIBMTR analysis in older patients with AML in first complete remission or myelodysplastic syndrome (MDS) undergoing RIC allo-SCT compared outcomes in those aged 40 to 54 versus ≥65 years and found no significant differences in relapse or OS [14]. Given the recent advances in the treatment of HM in general and in allo-SCT in particular, additional studies in older patients undergoing allo-SCT in the modern era are urgently needed.
The two-step approach separates the lymphoid and myeloid portions of the graft to avoid the exposure of CD34 positive cells to cyclophosphamide (CY) and to allow a fixed T cell dosing to improve consistency in outcome comparisons. After conditioning, a fixed dose of donors’ lymphocytes is infused (DLI - first step), followed by infusion of CY for bidirectional lymphocyte toleration. Selected CD34 positive cells are infused one day after the completion of CY (CD34 - second step) [15]. The two-step approach to allo-SCT was initially developed at our institution for patients with haplo donors and has been extended to matched related donors (MRD) [16]. It has been associated with early blood count recovery, robust immune reconstitution, and excellent outcomes in over 400 transplant procedures performed to date. Here we report the clinical outcomes of patients ≥65 years old who received haplo allo-SCT using the two-step approach.
Methods
The two-step protocol
Patients received either myeloablative (MAC: 12 Gy total body irradiation [TBI]), or reduced intensity conditioning (RIC: fludarabine 30 mg/m2 for 3 doses and 4 Gy TBI or fludarabine 30 mg/m2 for 4 doses, busulfan 3.2 mg/kg for 2 doses or thiotepa 5 mg/kg for 3 doses, and 2 Gy TBI). After conditioning, patients were infused with a fixed dose of 2 × 108/kg of donor CD3+ T cells (step 1). Two days later, CY (60 mg/kg/day) was given on day -3 and -2 to deplete alloreactive cells and establish tolerance induction [17]. Tacrolimus and mycophenolate mofetil (MMF) were started on day -1 for GVHD prophylaxis. CD34 selected donor stem cells were infused 24 h after the completion of CY on day 0 (step 2). All patients received GM-CSF 250 mcg/m2/day from day + 1 until neutrophil engraftment. MMF was discontinued on day 28, and tacrolimus was tapered from day 60 in the absence of GVHD.
All patients received prophylaxis against viral, fungal, and encapsulated organisms. Patients were started on Bactrim DS 1 tab 3 times per week, Fluconazole 400 mg PO daily, and Valacyclovir 500 mg PO every 12 h on admission. Upon Posaconazole approval, Fluconazole was switched to Posaconazole 300 mg PO daily after infusion of cyclophosphamide on D-1 (since Posaconazole can block the conversion of cyclophosphamide to its active metabolite 4-hydroxycyclophosphamide). Patients were discharged on Posaconazole 300 mg PO daily, Valacyclovir 500 mg PO every 12 h, and Bactrim DS 1 tab once daily. Recent patients who were seropositive for CMV or received CMV positive grafts were placed on Letermovir 480 mg PO daily starting on D + 7 and discontinued on D + 100 if weekly CMV PCR remains negative. The treatment schema is shown in Fig. 1.
Fig. 1. Schema for the 2-step approach allogeneic stem cell transplantation.
In the myeloablative group (MA), recipients received total body irradiation (TBI) from day -10 to day -8 with total dose of 12 Gy. For the reduced intensity (RIC) group: RIC1 received fludarabine (Flu) 30 mg/m2 for 4 doses, busulfan (Bu) 3.2 mg/kg for 2 doses or thiotepa 5 mg/kg for 3 doses, and 2 Gy TBI; RIC2 received Flu 30 mg/m2 for 3 doses and 4 Gy TBI. Then donor lymphocyte infusion (DLI) which contains 2 × 108 CD3 + cells/kg was performed on day -6 after TBI. On day -3 and -2, recipients received cyclophosphamide (CY) 60 mg/kg/day. Tacrolimus (Tacro) and mycophenolate mofetil (MMF) were started on day -1. On day 0, CD34 + stem cells were infused, and growth factor (GF) was started on day + 1.
Study design and patient population
All two-step haplo allo-SCT protocols were approved by Thomas Jefferson University institutional review board before study initiation. Informed consents were obtained from all patients before enrollment. The study was conducted in accordance with the
Declaration of Helsinki. A total of 76 patients aged 65 and older with HM underwent 2-step haplo allo-SCT at Thomas Jefferson Hospital between April 2007 and June 2021. All patients were treated on one of eight two-step clinical trials. All of these trials have the same two-step framework, DLI dose, and GVHD prophylaxis, but differ in conditioning intensity. Supplementary Table 1 contains NCT registration number, conditioning regimen, and eligibility criteria for each of these trials.
Definitions and outcomes
Neutrophil engraftment was defined as an absolute neutrophil count (ANC) ≥ 0.5 × 109/L for at least 3 consecutive days after from the date of stem cell infusion. Platelet engraftment was defined as platelet ≥ 20 × 109/L without platelet transfusion in the preceding 7 days. Acute GVHD (aGVHD) was assessed by the Glucksberg grading criteria [18]. Chronic GVHD (cGVHD) was assessed by the National Institutes of Health consensus criteria for GVHD [19].
NRM was defined as death without disease relapse or progression. Progression free survival (PFS) was defined as the time from transplant until relapse, disease progression or death from any cause, whichever came first. Overall survival (OS) was defined as the time from transplant until death. Patients alive without evidence of disease relapse were censored at last follow-up. Disease risk, as calculated by the CIBMTR disease risk index (CIBMTR-DRI), was developed in 2014 and incorporates disease-related parameters developed for the primary outcome of overall survival after HCT [20]. Medical co-morbidities were calculated by the hematopoietic cell transplantation comorbidity index (HCT-CI) [21]. The HCT-CI, originally developed by Sorror et al. in 2005, is a measurement of comorbidity, and has been shown to predict survival and NRM after allo-SCT [21, 22].
Statistical analysis
Patients were followed from the date of transplant until death or end of follow up (July 13, 2021). Descriptive statistics were summarized, and categorical variables were presented as percentages. Median value and range were used for continuous variables with non-normal distribution. Both PFS and OS were calculated using the Kaplan-Meier method and the log-rank test was used to calculate statistical difference in OS with respect to selected characteristics. Probabilities of NRM, relapse rate, and GVHD were calculated using cumulative incidence estimates to accommodate competing risks.
Univariable and multivariable Cox regression analyses were used to calculate hazard ratio (HR). For univariate analysis, age (< 70 and ≥ 70 years), sex (male and female), disease type (AML/acute lymphoblastic leukemia [ALL], MDS/myeloproliferative neoplasms [MPN], non-Hodgkin lymphoma/chronic lymphocytic leukemia [NHL/CLL]), remission status (any complete remission [CR], not in CR, MDS/MPN), performance status (90–100% and 70–80%), HCT-CI (0–2 and ≥ 3), CIBMTR-DRI score (low/intermediate risk and high/very high risk), conditioning regimen (MAC and RIC), CMV serological status, donor age (< 45 and 45 + ), and transplant year (2007–2014 and 2015–2021) were analyzed. Multivariate Cox proportional hazard regression analysis was used to investigate the independent association between OS and PFS with covariates, using gender, age, disease type, remission status at transplant, HCT-CI, and DRI score as selected based on results of univariate analysis.
All statistical analyses were made using R version 4.1.1. A P-value of < 0.05 was considered to be statistically significant.
Results
Patient characteristics
From April 2007 to June 2021, a total of 76 patients aged ≥ 65 years old with HM underwent peripheral blood haplo allo-SCT using the two-step approach at the Thomas Jefferson Hospital. Median age of all patients at transplantation was 69 years (range 65–78 years), with 31 patients (41%) ≥ 70 years old; 70% of patients were males. Detailed patient and donor characteristics are described in Table 1.
Table 1.
Patient, disease and donor characteristics.
| Characteristics | All patients (n = 76) |
|---|---|
| Age at HCT | No (%) |
| < 70 years | 45 (59) |
| ≥ 70 years | 31 (41) |
| Male sex | 53 (70) |
| Race | |
| Caucasian | 62 (82) |
| African American | 9 (12) |
| Asian | 2 (3) |
| Hispanic | 3 (4) |
| Diagnosis | |
| AML | 36 (47) |
| ALL | 3 (4) |
| MDS | 20 (26) |
| CMML | 1 (1) |
| MPD | 4 (5) |
| CLL | 3 (4) |
| Follicular | 4 (5) |
| Mantle cell | 2 (3) |
| WM | 1 (1) |
| DLBCL | 1 (1) |
| Biphenotypic leukemia | 1 (1) |
| Remission status | |
| CR1 | 24 (32) |
| CR > = 2 | 8 (11) |
| Not in CR | 44 (58) |
| HCT-CI | |
| 0 | 12 (16) |
| 1 | 5 (7) |
| 2 | 19 (25) |
| 3 | 18 (24) |
| > = 4 | 22 (29) |
| DRI score | |
| Low | 4 (5) |
| Intermediate | 34 (45) |
| High | 36 (47) |
| Very high | 2 (3) |
| Performance status | |
| 90–100% | 60 (79) |
| 70–80% | 16 (21) |
| Conditioning regimen | |
| MA | 11 (14) |
| RIC | 65 (86) |
| CMV status, recipient/donor | |
| recipient + /donor+ | 18 (24) |
| recipient + /donor- | 30 (40) |
| recipient-/donor+ | 10 (13) |
| recipient-/donor- | 18 (24) |
| Donor age (median, range) | 41 (22–70) |
| Donor age 45 or above | 26 (34) |
| Donor age 44 or below | 50 (66) |
HCT Hematopoietic stem cell transplant, No (%) Number (percentage), AML Acute myeloid leukemia, ALL Acute lymphocytic leukemia, MDS Myelodysplastic syndrome, CMML Chronic myelomonocytic leukemia, MPD Myeloproliferative disease, DLBCL Diffuse large B-cell lymphoma, CLL Chronic lymphocytic leukemia, WM Waldenstrom macroglobulinemia, CR1 First complete remission, CR > = 2 Any complete remission except CR1, HCT-CI Hematopoietic cell transplantation-specific comorbidity index, DRI Disease risk index, MA Myeloablative, RIC Reduced intensity conditioning, CMV Cytomegalovirus infection.
AML and MDS constituted most of the primary diseases, 47% and 26%, respectively. Almost all AML patients (97%) had intermediate or adverse risk cytogenetics according to ELN-2017 risk stratification [23], and 65% of MDS patients were classified as high or very high risk according to revised International Prognostic Scoring System (IPSS-R) [24] (Supplementary Table 2). At the time of transplant, 24 (32%) were in first complete remission (CR1), 8 (11%) were in CR2 or more, and 44 patients (58%) were not in any CR. See Supplementary Table 3 for disease-specific CIBMTR response criteria for patients not in CR at the time of transplant [23, 25–27].
Twenty-two patients (29%) had HCT-CI ≥ 4; 34 patients (45%) had DRI intermediate risk disease, and 38 (50%) had high or very high-risk disease. Karnofsky performance status was 90–100% for 60 patients (79%). Most patients received RIC (86%). Four patients had received a second transplant, with 3 patients previously received an autologous transplant, and 1 patient previously received an allogeneic transplant.
All patients received haplo allo-SCT from their children, and patients with MRD were excluded. Median donor age was 41 years, with 66% less than 45 years. The median duration of inpatient stay for all patients was 33 days (range 14–104 days). The median CD34 cell dose infused was 7.08 × 108 CD 34+ cells (range 1.40–15.00 × 108 CD 34+ cells).
Engraftment
Successful engraftment was seen in all evaluable patients (3 patients died before engraftment). Neutrophils engrafted at a median of 11 days (range from 9 to 15 days) and platelets engrafted at a median of 18 days (range from 12 to 124 days). Two patients had secondary graft failure, both of whom had MDS; one patient died from transplant-related toxicity on D + 89, and the other patient died from CMV reactivation on D + 107.
Graft-versus-host disease
The cumulative incidence of grade II-IV aGVHD at 6 months was 39.5% (Fig. 2) and this remained the same at the 1-year timepoint. The cumulative incidence of grade III-IV aGVHD at 6 months was 11.1%. The majority of aGVHD involved the skin only (21 out of 27 patients, 78%), 10 patients had GI aGVHD and 3 patients had liver aGVHD. Four patients (5%) died from complications from acute GVHD, including 3 patients with skin and gut involvement, and 1 patient with skin and liver involvement.
Fig. 2. Cumulative incidence of acute GVHD Grade II–IV (left) and chronic GVHD Grade I–IV (right) with 95% confidence interval.
X-axis denotes time after transplantation in days.
The cumulative incidence of cGVHD of any grade was 16.0% at 1 year and 19.4% at 2 years (Fig. 2). The cumulative incidence of cGVHD requiring treatment was 15.1% at 2 years, and the cumulative incidence of moderate or severe cGVHD was 5.7%. Severe cGVHD (score 3) affected 3 out of 6 patients, with liver, ocular, and upper GI involvement, respectively. There was no death attributable to chronic GVHD.
Non-relapse mortality and relapse
The cumulative incidence of NRM and relapse was 35.2% and 12.6% at 1 year, 41.6% and 21.0% at 2 years, and 43.5% and 21.0% at 3 years, respectively (Fig. 3). Notably, no patient experienced relapse after year two with a median follow-up of 44 months. The median time to relapse was 230 days post-transplant (range 42–658 days). All patients who relapsed were in CR at one-month post-transplant and achieved > 99% donor chimerism (Supplementary Table 4). The main causes of death were infection (16 of 47 deaths), followed by relapsed disease (14 of 47 deaths), followed by toxicity from treatment regimen (10 of 47 deaths). For infection-related deaths, 3 were bacterial infections (Pneumocystis pneumonia, Pseudomonas bacteremia, E coli bacteremia), 9 were viral infections (CMV reactivation, HHV-6, COVID-19 infection, rhinovirus), 1 was fungal infection, and 3 were combined bacterial and viral infections.
Fig. 3. Cumulative incidence of non-relapse mortality and relapse.
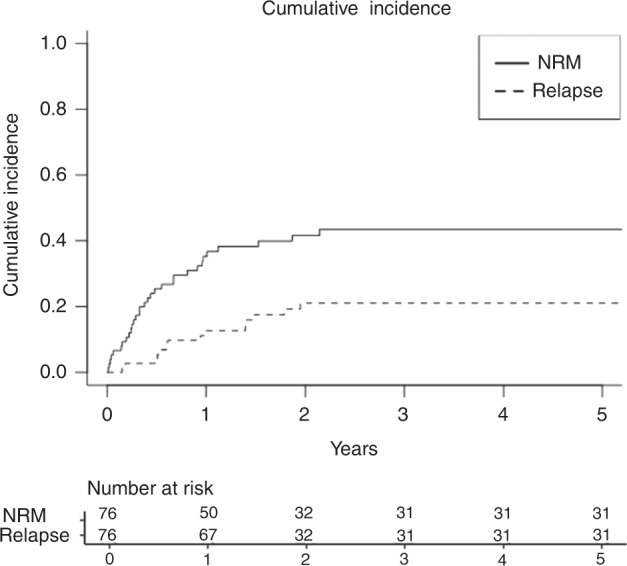
NRM Non-relapse mortality. X-axis denotes time after transplantation in years.
Survival outcomes
With a median follow-up of 44 months (range 1–164 months) among live patients, the 1-year OS was 52.2% [95% CI (41.9–65.1)] and the 3-year OS was 36.3% [95% CI (26.3–49.9)] (Fig. 4). The 1-year PFS was 51.1% [95% CI (40.8–63.9)] and the 3-year PFS was 35.6% [95% CI (25.7–49.3)] (Fig. 4).
Fig. 4. Kaplan-Meier overall survival (left) and progression-free survival (right) probability with 95% confidence interval.
X-axis denotes time after transplantation in years.
Patients transplanted for a diagnosis of NHL/CLL had an estimated OS of 63.6% [95% CI (40.7–99.5)] and 54.5% [95% CI (31.8–93.6)] at 1 and 3 years, respectively, whereas patients transplanted for acute leukemia, MDS or myeloproliferative neoplasm (MPN) had an estimated OS of 50.1% and 31.9% at 1 and 3 years, respectively (Fig. 5). Patients transplanted for acute leukemia who were in remission at the time of transplant had an estimated OS of 60.0% at 1 year, compared to 38.5% for those in primary induction failure (PIF) or relapse, and 45.5% for patients transplanted for MDS/MPN.
Fig. 5. Survival outcomes with respect to various characteristics.
Overall survival for 76 patients by age (a), gender (b), diagnosis (c), remission status at transplant (d), performance status (e), hematopoietic cell transplantation comorbidity index (f), disease risk index score (G), intensity of conditioning regimen (h), Cytomegalovirus status (i), and donor age (j). X-axis denotes time after transplantation in years. P-values from log-rank test.
Univariate analysis of OS
Survival outcomes for unadjusted associations of gender, diagnosis, remission status at transplant, performance status, HCT-CI, DRI score, intensity of conditioning regimen, CMV status, donor age, and transplant year for the entire patient population, older (age ≥ 70 years) and younger (age <70 years) cohorts are shown in Table 2. The following variables were associated with an inferior OS for the entire population: HCT-CI (0–2 vs 3 + , median OS 5.80 vs 0.67 years, P = 0.008), and DRI score (low/intermediate risk vs high/very high risk, median OS 2.15 vs 0.94 years, P = 0.046). Other variables including age, sex, diagnosis, intensity of conditioning regimen, performance status, CMV status, and donor age did not have an impact on outcome parameters (Fig. 5).
Table 2.
Univariate analysis of survival outcomes in the entire patient cohort and subgroups.
| Characteristics | All patients | > = 70 years | < 70 years | > = 70 vs < 70 years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n (c) | mOS | 95% CI | P-value | n | n (c) | mOS | 95% CI | P-value | n | n (c) | mOS | 95% CI | P-value | P-value | |
| Total | 76 | 47 | 1.12 | [0.81–2.15] | 31 | 19 | 0.97 | [0.54–NR] | 45 | 28 | 1.12 | [0.67–NR] | 0.63 | |||
| Age | 0.63 | |||||||||||||||
| >=70 years | 31 | 19 | 0.97 | [0.54–NR] | ||||||||||||
| <70 years | 45 | 28 | 1.12 | [0.67–NR] | ||||||||||||
| Gender | 0.49 | 0.76 | 0.31 | |||||||||||||
| Female | 23 | 14 | 1.79 | [0.61–NR] | 7 | 5 | 0.97 | [0.22–NR] | 16 | 9 | 1.95 | [0.61–NR] | 0.43 | |||
| Male | 53 | 33 | 1.00 | [0.67–5.80] | 24 | 14 | 1.41 | [0.44–NR] | 29 | 19 | 1.00 | [0.55–NR] | 0.97 | |||
| Disease | 0.11 | 0.34 | 0.02 | |||||||||||||
| AML/ALL | 40 | 23 | 1.12 | [0.67-NR] | 19 | 11 | 1.49 | [0.67–NR] | 21 | 12 | 1.12 | [0.51–NR] | 0.84 | |||
| MDS/MPN | 25 | 19 | 0.97 | [0.51–1.95] | 8 | 5 | 0.94 | [0.15–NR] | 17 | 14 | 1.00 | [0.51–NR] | 0.90 | |||
| NHL/CLL | 11 | 5 | NR | [0.67–NR] | 4 | 3 | 0.25 | [0.01–NR] | 7 | 2 | NR | [2.15–NR] | 0.07 | |||
| Remission status* | 0.12 | 0.48 | 0.01 | |||||||||||||
| Any CR | 27 | 13 | 1.87 | [0.96–NR] | 12 | 7 | 1.49 | [0.67–NR] | 15 | 6 | NR | [0.81–NR] | 0.40 | |||
| Not in CR | 13 | 10 | 0.54 | [0.25–NR] | 7 | 4 | 9.87 | [0.25–NR] | 6 | 6 | 0.42 | [0.06–NR] | 0.06 | |||
| MDS/MPN | 25 | 19 | 0.97 | [0.51–1.95] | 8 | 5 | 0.94 | [0.15–NR] | 17 | 14 | 1.00 | [0.51–NR] | 0.90 | |||
| Performance status | 0.22 | 0.55 | 0.21 | |||||||||||||
| 90–100% | 60 | 35 | 1.41 | [0.94–9.87] | 28 | 17 | 0.97 | [0.67–NR] | 32 | 18 | 1.95 | [0.91–NR] | 0.42 | |||
| 70–80% | 16 | 12 | 0.56 | [0.29–NR] | 3 | 2 | 0.25 | [0.01–NR] | 13 | 10 | 0.61 | [0.38–NR] | 0.77 | |||
| HCT-CI | 0.008 | 0.30 | 0.009 | |||||||||||||
| 0–2 | 36 | 16 | 5.80 | [0.97–NR] | 16 | 8 | 1.49 | [0.33–NR] | 20 | 8 | NR | [1.12–NR] | 0.32 | |||
| 3+ | 40 | 31 | 0.67 | [0.51–1.79] | 15 | 11 | 0.81 | [0.44–NR] | 25 | 20 | 0.61 | [0.41–1.95] | 0.87 | |||
| DRI-score | 0.046 | 0.91 | 0.02 | |||||||||||||
| Low/intermediate | 38 | 19 | 2.15 | [0.96–NR] | 14 | 8 | 1.49 | [0.44–NR] | 24 | 11 | 5.80 | [0.91–NR] | 0.21 | |||
| High/Very high risk | 38 | 28 | 0.94 | [0.54–1.79] | 17 | 11 | 0.96 | [0.33–NR] | 21 | 17 | 0.61 | [0.41–1.79] | 0.44 | |||
| Conditioning regimen | 0.90 | 0.93 | 0.83 | |||||||||||||
| MAC | 11 | 7 | 0.61 | [0.38–NR] | 11 | 7 | 0.61 | [0.38–NR] | ||||||||
| RIC1 | 49 | 31 | 1.01 | [0.60–9.87] | 21 | 14 | 0.97 | [0.33–NR] | 28 | 17 | 1.12 | [0.60–NR] | 0.64 | |||
| RIC2 | 16 | 9 | 1.53 | [1.00–NR] | 10 | 5 | 1.41 | [0.94–NR] | 6 | 4 | 1.95 | [1.53–NR] | 0.37 | |||
| Cytomegalovirus status | 0.27 | 0.05 | 0.93 | |||||||||||||
| R + /D + | 18 | 8 | 2.15 | [1.79–NR] | 10 | 3 | NR | [1.87–NR] | 8 | 5 | 1.79 | [1.40–NR] | 0.25 | |||
| R + /D- | 30 | 21 | 0.61 | [0.41–NR] | 8 | 7 | 0.29 | [0.22–NR] | 22 | 14 | 0.84 | [0.51–NR] | 0.12 | |||
| R-/D + | 10 | 7 | 0.97 | [0.48–NR] | 4 | 3 | 0.97 | [0.94–NR] | 6 | 4 | 0.80 | [0.33–NR] | 0.89 | |||
| R-/D- | 18 | 11 | 0.96 | [0.81–NR] | 9 | 6 | 0.67 | [0.27–NR] | 9 | 5 | 1.01 | [0.91–NR] | 0.22 | |||
| Donor age | 0.14 | 0.52 | 0.21 | |||||||||||||
| <45 | 50 | 28 | 1.41 | [0.91–NR] | 19 | 11 | 1.41 | [0.94–NR] | 31 | 17 | 1.12 | [0.67–NR] | 0.68 | |||
| 45+ | 26 | 19 | 1.00 | [0.41–NR] | 12 | 8 | 0.67 | [0.27–NR] | 14 | 11 | 1.20 | [0.41–NR] | 0.97 | |||
| Transplant year | 0.69 | 0.34 | 0.79 | |||||||||||||
| 2007–2014 | 38 | 26 | 0.84 | [0.48–NR] | 14 | 11 | 0.61 | [0.25–NR] | 24 | 15 | 1.21 | [0.51–NR] | 0.33 | |||
| 2015–2021 | 38 | 21 | 1.41 | [0.94–NR] | 17 | 8 | 1.41 | [0.94–NR] | 21 | 13 | 1.12 | [0.81–NR] | 0.73 | |||
*Excluding patients with NHL/CLL
AML Acute myeloid leukemia, ALL Acute lymphocytic leukemia, MDS Myelodysplastic syndrome, MPN Myeloproliferative disorder, NHL Non-hodgkin’s lymphoma, CLL Chronic lymphocytic leukemia, CR Complete remission, HCT-CI Hematopoietic cell transplantation comorbidity index, DRI-score Disease risk index score, MAC Myeloablative conditioning, RIC1 Flu/Bu plus 2 Gy TBI, RIC2 Flu plus 4 Gy TBI, R + /D + Recipient positive/donor positive, n(C) Number of censored individuals in each stratum, mOS Median overall survival in years, CI Confidence interval, NR Not reached. P-values from log-rank test, bold is statistically significant.
After stratification for age, HCT-CI 0-2 and low/intermediate DRI score continued to have statistically longer survival in patients aged 65-69. AML/ALL patients in CR at the time of transplant had longer survival [median OS not reached (NR), 95% CI (0.81-NR)] compared to AML/ALL patients not in CR and patients with MDS/MPN [median OS 0.42 years, 95% CI (0.06-NR); 1.00 year, 95% CI (0.51-NR); P = 0.01]; however, this difference was not observed in patients ≥70 years old (P = 0.48). In contrast, recipient + /donor + CMV status was significantly associated with higher OS in the older group only (median OS NR, P = 0.05).
Multivariate analysis of OS and PFS
Multivariate analysis adjusting for gender, age, disease, remission status at transplant, performance status, HCT-CI, DRI score, intensity of conditioning regimen, CMV status, donor age, and transplant year as covariates is depicted in Forrest plot (Fig. 6). Having an HCT-CI of 3 or more at the time of transplant was an independent adverse prognostic factor for death [HR = 3.10, 95% CI (1.38–6.97), P = 0.006], and the Flu/4 Gy TBI RIC regimen was an independent favorable prognostic factor for survival [HR = 0.093, 95% CI (0.02–0.43), P = 0.002], both of which were true for PFS as well. An independent influence on OS and PFS was not detected for any of the other variables.
Fig. 6. Forrest plot of multivariate analysis for all patients by selected characteristics.
AML Acute myeloid leukemia, ALL Acute lymphocytic leukemia, MDS Myelodysplastic syndrome, MPN Myeloproliferative disorder, NHL Non-Hodgkin lymphoma, CLL Chronic lymphocytic leukemia, CR Complete remission, PS Performance status, KPS Karnofsky, HCT-CI Hematopoietic cell transplantation comorbidity index, RDRI Disease risk index score, MAC Myeloablative, RIC2 Flu and 4 Gy TBI, RIC1 Flu/Bu and 2 Gy TBI, CMV Cytomegalovirus, CI confidence interval. **statistically significant.
Discussion
Patients ≥ 65 years old are often excluded from allo-SCT, due to perceived high treatment-related mortality and risk of relapse, multiple medical comorbidities, adverse disease biology with underlying treatment resistance, and provider and patient reluctance for such therapy [28–30]. The use of less intensive conditioning regimens and contemporary transplant modalities have allowed an increasing number of elderly patients to access this treatment modality, although the outcomes remain suboptimal. Most elderly patients do not have suitable MRD, leaving only haplo and unrelated donors as potential graft options. Since prospective studies that evaluate haplo allo-SCT in the elderly are limited with most studies only include up to 5–10% of mismatched related grafts, we aimed to evaluate the clinical outcomes of patients aged 65 and above who underwent the novel two-step haplo allo-SCT for HM at our institution.
Our study included 76 patients with 41% ≥ 70 years old, with a 3-year OS and PFS of 36.3% and 35.6%, respectively. As expected, AML/ALL patients in CR at the time of transplant had a superior 3-year OS (45%) compared to patients with active disease, but even PIF and relapsed AML patients benefited from transplant, with 31% surviving at 3 years after transplantation.
With a long follow up of median 44 months, our survival outcomes for haplo allo-SCT are comparable to prior published results in similar age group with HLA matched stem cell grafts [10, 11, 13, 31]. A retrospective analysis of patients aged 60 and older with AML and MDS transplanted between 1999 and 2014 where 82% received well matched grafts showed a three-year PFS and OS of 32% and 35%, respectively [11]. Similar results were found in a CIBMTR analysis, where patients aged 70 and older with HM had improved transplant outcomes over time, with a two-year PFS and OS of 32% and 39% in patients transplanted between 2008 and 2013 compared to 22% and 26% in patients transplanted between 2000-2007 [10]. In this study, 74% patients received HLA matched grafts, and only 7% received mismatched related grafts. Disease status and disease-specific risk factors at the time of transplant, performance status, and the presence of other medical comorbidities have been shown to be independent adverse factors for overall survival.
The cumulative incidence of NRM was high (43% at 3 years) in our cohort, with opportunistic infections accounting for the majority of deaths. However, the 1-year and 3-year NRM for patients transplanted between 2015-2021 were 29% and 37%, respectively, which is notably in line or better than previously reported studies, where 2-year NRM have ranged from 33% to 45% [10, 11, 14, 31]. This is likely a reflection of our center’s experience and improved supportive care (i.e. Posaconazole and Letermovir) in recent years. Additionally, the cumulative incidence of relapse was remarkably low at 21% at 3 years, despite the fact that 58% were not in a CR at the time of transplant, and 86% had received RIC. A recent CIBMTR analysis by Muffly et al reported a 2-year relapse rate of 37% among patients ≥ 70 years who underwent allo-SCT, with similar HCT-CI distribution as our cohorts, although 66% of patients had missing DRI score and 19% had unknown disease status at transplant [10]. Other published studies have reported relapse rates ranging from 40 to 60% at 3 years in elderly patients undergoing haplo allo-SCT using posttransplant CY [31–34].
We evaluated multiple prognostic factors that might predict better survival in our study. Age was not found to be a predictive factor for survival in the entire cohort and with respect to each covariate. Patients transplanted for NHL/CLL and AML patients in CR at the time of transplant were found to have an impact on outcome parameters in the younger cohort, but not in patients aged 70 years or greater. Studies regarding the impact of donor age and CMV serostatus on transplant survival in the literature have been conflicting. One large study looking at more than 10,000 donors showed a survival benefit in recipients with young donors, but no difference in terms of CMV serostatus [35], while another study done by the European Group for Blood and Marrow Transplantation (EBMT) showed a negative prognostic effect of CMV seropositivity of the donor and/or the recipient on OS, PFS and NRM in patients with AML and ALL [36]. Further reports suggest early CMV replication after allo-SCT substantially reduced relapse risk in AML patients secondary to a putative virus-versus-leukemia effect [37, 38]. In our study, CMV serological status had a prognostic impact on survival in the cohort older than 70 years only, with CMV seropositive patients receiving seropositive grafts having the best survival. Donor age did not have an impact on survival in our study.
Previously, various transplant-specific risk models have been used to predict transplant outcomes of patients undergoing allo-SCT. The HCT-CI and DRI have been shown to convey prognostic information on survival after transplant in some but not all studies [10, 11, 31]. We evaluated performance status, HCT-CI, and DRI score on outcome parameters in our study. We found both HCT-CI and DRI score to have an impact on survival in univariate analysis. After stratification for age, only patients < 70 years retained this prognostic impact. In contrast, performance status did not have an impact on survival, which may be due to it being a subjective measurement of one’s fitness, as opposed to HCT-CI and DRI which incorporate objective measures of organ function, disease type, and disease status at the time of transplant as factors influencing transplant survival. This suggests that HCT-CI and DRI may be more accurate determinants of survival than the subjective performance status and is likely more predictive of better survival in patients < 70 years old. A comprehensive geriatric assessment is currently being implemented in our program [39].
Our two-step approach to haplo allo-SCT allows for the administration of a fixed dose of T cells and prevents exposure of donor stem cells to the effects of CY. This was reflected in our study where all evaluable patients achieved rapid neutrophil and platelet engraftment at a median of 11 and 18 days, respectively. This is superior to other studies of haplo allo-SCT, where the neutrophils and platelets engrafted at a median of 17-18 and 22-37 days, respectively [40, 41]. Consequently, our patients generally experienced shorter duration of cytopenia, which may decrease risk of hospital-acquired infections, minimize transfusions, and reduce costs associated with hospitalization.
Limitations of the study include relatively small number of patients without a control group, and single institutional experience. Nevertheless, all patients were enrolled on prospective clinical trials ensuring the homogeneity of study treatment and supportive care. Additionally, this is the first report that evaluates outcomes of haplo allo-SCT in a cohort of patients aged 65 or greater using the two-step transplant approach.
Conclusion
Our study suggests that allo-SCT in elderly patients is tolerable and should be considered in eligible patients. The haploidentical two-step approach is a novel alternative platform for high risk HM patients, resulting in fast engraftment and low rates of disease relapse. Taken together, our results appear encouraging and suggest that select older patients can achieve promising disease control and long-term survival after allo-SCT. Further refinements of the two-step transplant protocol and better identification of suitable candidates are ongoing to decrease complication rates and improve transplant outcomes.
Supplementary information
Author contributions
XB, UG, NF, and LG designed the research. LG and XB collected data. XB analyzed the data and wrote the manuscript. All authors interpreted data and took part in critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-022-01780-w.
References
- 1.Armitage JO. Bone marrow transplantation. N. Engl J Med. 1994;330:827–38. doi: 10.1056/NEJM199403243301206. [DOI] [PubMed] [Google Scholar]
- 2.Kernan NA, Bartsch G, Ash RC, Beatty PG, Champlin R, Filipovich A, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N. Engl J Med. 1993;328:593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 3.Castro-Malaspina H, Harris RE, Gajewski J, Ramsay N, Collins R, Dharan B, et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood. 2002;99:1943–51. doi: 10.1182/blood.V99.6.1943. [DOI] [PubMed] [Google Scholar]
- 4.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. doi: 10.1182/blood.V97.11.3390. [DOI] [PubMed] [Google Scholar]
- 5.Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–9. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Total Body Irradiation-Based Myeloablative Haploidentical Stem Cell Transplantation Is a Safe and Effective Alternative to Unrelated Donor Transplantation in Patients Without Matched Sibling Donors. Biol Blood Marrow Transpl. 2015;21:1299–307. doi: 10.1016/j.bbmt.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Phelan R, Arora M, Chen M. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2020. Available at: https://www.cibmtr.org Accessed August 21, 2021.
- 8.Zeidan AM, Podoltsev NA, Wang X, Bewersdorf JP, Shallis RM, Huntington SF, et al. Temporal patterns and predictors of receiving no active treatment among older patients with acute myeloid leukemia in the United States: A population-level analysis. Cancer. 2019;125:4241–51. doi: 10.1002/cncr.32439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ustun C, Lazarus HM, Weisdorf D. To transplant or not: A dilemma for treatment of elderly AML patients in the twenty-first century. Bone Marrow Transpl. 2013;48:1497–505. doi: 10.1038/bmt.2013.67. [DOI] [PubMed] [Google Scholar]
- 10.Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017;130:1156–64. doi: 10.1182/blood-2017-03-772368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohlen M, Groth C, Sauer T, Gorlich D, Mesters R, Schliemann C, et al. Outcome of allogeneic stem cell transplantation for AML and myelodysplastic syndrome in elderly patients (60 years) Bone Marrow Transpl. 2016;51:1441–8. doi: 10.1038/bmt.2016.156. [DOI] [PubMed] [Google Scholar]
- 12.Wong R, Giralt SA, Martin T, Couriel DR, Anagnostopoulos A, Hosing C, et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood. 2003;102:3052–9. doi: 10.1182/blood-2003-03-0855. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich S, Ziagkos D, de Wreede LC, van Biezen A, Finke J, Platzbecker U, et al. Allogeneic Stem Cell Transplantation for Patients Age >/= 70 Years with Myelodysplastic Syndrome: A Retrospective Study of the MDS Subcommittee of the Chronic Malignancies Working Party of the EBMT. Biol Blood Marrow Transpl. 2017;23:44–52. doi: 10.1016/j.bbmt.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 14.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–87. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosso D, Gaballa S, Alpdogan O, Carabasi M, Filicko-O’Hara J, Kasner M, et al. A two-step approach to myeloablative haploidentical transplantation: low nonrelapse mortality and high survival confirmed in patients with earlier stage disease. Biol Blood Marrow Transpl. 2015;21:646–52. doi: 10.1016/j.bbmt.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Gaballa S, Palmisiano N, Alpdogan O, Carabasi M, Filicko-O’Hara J, Kasner M, et al. A Two-Step Haploidentical Versus a Two-Step Matched Related Allogeneic Myeloablative Peripheral Blood Stem Cell Transplantation. Biol Blood Marrow Transpl. 2016;22:141–8. doi: 10.1016/j.bbmt.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39:683–93. doi: 10.1053/j.seminoncol.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401 e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121:2854–63. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savona MR, Malcovati L, Komrokji R, Tiu RV, Mughal TI, Orazi A, et al. An international consortium proposal of uniform response criteria for myelodysplastic/myeloproliferative neoplasms (MDS/MPN) in adults. Blood. 2015;125:1857–65. doi: 10.1182/blood-2014-10-607341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tefferi A, Cervantes F, Mesa R, Passamonti F, Verstovsek S, Vannucchi AM, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122:1395–8. doi: 10.1182/blood-2013-03-488098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekeres MA, Guyatt G, Abel G, Alibhai S, Altman JK, Buckstein R, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4:3528–49. doi: 10.1182/bloodadvances.2020001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ossenkoppele G, Lowenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125:767–74. doi: 10.1182/blood-2014-08-551499. [DOI] [PubMed] [Google Scholar]
- 30.Thomas X, Le Jeune C. Treatment of elderly patients with acute myeloid leukemia. Curr Treat Options Oncol. 2017;18:2. doi: 10.1007/s11864-017-0445-5. [DOI] [PubMed] [Google Scholar]
- 31.Hsu J, Chen Z, Shore T, Gergis U, Mayer S, Phillips A, et al. Outcomes of allogeneic stem cell transplant for elderly patients with hematologic malignancies. Biol Blood Marrow Transpl. 2020;26:789–97. doi: 10.1016/j.bbmt.2019.12.766. [DOI] [PubMed] [Google Scholar]
- 32.Imus PH, Tsai HL, Luznik L, Fuchs EJ, Huff CA, Gladstone DE, et al. Haploidentical transplantation using posttransplant cyclophosphamide as GVHD prophylaxis in patients over age 70. Blood Adv. 2019;3:2608–16. doi: 10.1182/bloodadvances.2019000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasamon YL, Bolanos-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA, et al. Outcomes of Nonmyeloablative HLA-Haploidentical Blood or Marrow Transplantation With High-Dose Post-Transplantation Cyclophosphamide in Older Adults. J Clin Oncol. 2015;33:3152–61. doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slade M, DiPersio JF, Westervelt P, Vij R, Schroeder MA, Romee R. Haploidentical Hematopoietic Cell Transplant with Post-Transplant Cyclophosphamide and Peripheral Blood Stem Cell Grafts in Older Adults with Acute Myeloid Leukemia or Myelodysplastic Syndrome. Biol Blood Marrow Transpl. 2017;23:1736–43. doi: 10.1016/j.bbmt.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Kollman C, Spellman SR, Zhang MJ, Hassebroek A, Anasetti C, Antin JH, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127:260–7. doi: 10.1182/blood-2015-08-663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122:3359–64. doi: 10.1182/blood-2013-05-499830. [DOI] [PubMed] [Google Scholar]
- 37.Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–12. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 38.Goldsmith SR, Slade M, DiPersio JF, Westervelt P, Lawrence SJ, Uy GL, et al. Cytomegalovirus viremia, disease, and impact on relapse in T-cell replete peripheral blood haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide. Haematologica. 2016;101:e465–e8. doi: 10.3324/haematol.2016.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olin RL, Fretham C, Pasquini MC, Arora M, Bhatt VR, Derman B, et al. Geriatric assessment in older alloHCT recipients: association of functional and cognitive impairment with outcomes. Blood Adv. 2020;4:2810–20. doi: 10.1182/bloodadvances.2020001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugita J, Kagaya Y, Miyamoto T, Shibasaki Y, Nagafuji K, Ota S, et al. Myeloablative and reduced-intensity conditioning in HLA-haploidentical peripheral blood stem cell transplantation using post-transplant cyclophosphamide. Bone Marrow Transpl. 2019;54:432–41. doi: 10.1038/s41409-018-0279-1. [DOI] [PubMed] [Google Scholar]
- 41.Law AD, Salas MQ, Lam W, Michelis FV, Thyagu S, Kim DDH, et al. Reduced-Intensity Conditioning and Dual T Lymphocyte Suppression with Antithymocyte Globulin and Post-Transplant Cyclophosphamide as Graft-versus-Host Disease Prophylaxis in Haploidentical Hematopoietic Stem Cell Transplants for Hematological Malignancies. Biol Blood Marrow Transpl. 2018;24:2259–64. doi: 10.1016/j.bbmt.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.








