Abstract
Introduction: COVID-19 patients frequently experience headaches, malaise, and fatigue. For patients with shunted hydrocephalus, these signs and symptoms can often be indicative of shunt failure. Thus, it can be challenging to determine if shunt failure has occurred in this patient population. Therefore, we explored the question of how a diagnosis of COVID-19 in shunted hydrocephalus patients influences the rate of shunt revision.
Methods: We used a deidentified database network (TriNetX) to gather information on patients with shunted hydrocephalus and COVID-19 versus no COVID-19 from January 20, 2020, through September 26, 2021. Our primary outcome of interest was shunt revision at 90 days, with secondary outcomes of mortality, hospitalization, ICU admission, mechanical ventilation, tracheostomy, PEG tube placement, fall, seizure, acute kidney injury (AKI), venous thromboembolism (VTE), ischemic stroke (I.S.), myocardial infarction (MI), and sepsis. Cohorts were propensity score-matched for common comorbidities and demographics.
Results: After propensity score matching, 10,600 patients with shunted hydrocephalus and COVID-19 (cohort 1) and 10,600 patients with shunted hydrocephalus and no COVID-19 (cohort 2) were identified. Average age was 38.5 years. Eight hundred and thirty-four patients (7.869%) in cohort 1 and 180 (1.698%) patients in cohort 2 underwent shunt revision (p=<0.0001, OR 4.978, 95% CI 4.198, 5.821). Mortality was 4.642% vs. 2.113% (p<0.0001, OR 2.255, 95% CI 1.921, 2.647). Hospitalization rates were 27.72% vs. 10.303% (p<0.0001), and ICU admission rates 11.567% vs. 3.463% (p<0.0001). Ventilator dependence was 3.529% vs. 0.953% (p<0.0001), tracheostomy 1.142% vs. 0.236% (p<0.0001), PEG tube insertion 2.472% vs. 0.585% (p<0.0001), falls 2.321% vs. 1.076% (p<0.0001), seizure 11.369% vs. 5.953% (p<0.0001), AKI 4.416% vs. 1.717% (p<0.0001), VTE 3.538% vs. 1.293% (p<0.0001), sepsis 3.887% vs. 1.179% (p<0.0001), IS 0.585% vs. 0.16% (p<0.0001), and MI 1.34% vs. 0.519% (p<0.0001).
Conclusion: COVID-19 infection is associated with an almost five-fold increase in shunt revisions.
Keywords: covid-19, mortality rate, shunt revision, outcomes, ventriculoperitoneal shunt, shunt, covid, hydrocephalus, neurosurgery
Introduction
COVID-19 patients frequently experience headaches, malaise, and fatigue [1-5]. For patients with shunted hydrocephalus, these signs and symptoms can often be indicative of shunt failure. Thus, it can be difficult to determine if shunt failure has occurred in this patient population. We explored the question of how a diagnosis of COVID-19 in shunted hydrocephalus patients influences the rate of shunt revision using a multicenter research network with matched controls.
Materials and methods
This was a retrospective comparative case-control study. We used a de-identified database network (TriNetX) to retrospectively query via ICD-10 and current procedural terminology codes to evaluate all patients with a diagnosis of COVID-19 and a shunt (cohort 1) versus no COVID-19 and a shunt (cohort 2). Data came from 62 health care organizations (HCOs). Data includes demographics, diagnoses, medications, laboratory values, genomics, and procedures. The identity of the HCOs and patients is not disclosed to comply with ethical guidelines against data re-identification. Because of the database's federated nature, an IRB waiver was granted. The data is updated daily. Our use of this database and its validity were informed by previous literature, and exact details of the network have been previously described [6-9]. Diagnosis of COVID-19 was based on ICD-10 codes (U07.1, U07.2, J12.81, B34.2, B97.21, B97.29) and/or SARS-CoV-2 polymerase chain reaction (PCR) positivity. Data spanned January 20, 2020 - September 21, 2021. The index date was set at the date of COVID-19 diagnosis with a shunt (cohort 1) versus a shunt (cohort 2). Our primary outcome of interest was shunt revision at 90 days, with secondary outcomes of mortality, hospitalization, ICU admission, mechanical ventilation, tracheostomy, percutaneous endoscopic gastrostomy (PEG) tube placement, fall, seizure, acute kidney injury (AKI), venous thromboembolism (VTE), ischemic stroke (I.S.), myocardial infarction (MI), and sepsis.
The medical information included age at index date, as well as sex, race, and comorbidities of hypertension, acute kidney injury, diabetes, ischemic heart disease, heart failure, atrial fibrillation, disorders of lipoprotein metabolism and other lipidemias, obesity, history of nicotine dependence, chronic respiratory disease, cirrhosis, alcohol abuse or dependence, and peripheral vascular disease, recorded up to the date of the index date. Analysis was performed using unmatched and propensity score-matched cohorts, with the greedy-nearest neighbor algorithm with a caliper of 0.1 pooled standard deviations. Hazard ratios were calculated using R's survival package v3.2-3 and validated, comparing the output to SAS version 9.4. Chi-square analysis was performed on categorical variables. Significance was defined as a p-value less than 0.05.
Results
A total of 12,235 patients with shunted hydrocephalus and who were COVID-19 positive were identified, versus 14,944 with a shunt and no COVID-19. After propensity score matching, 10,599 patients were identified in each cohort. After matching, age at index was 38.5+-25.9 and 38.4+-25.6 for cohorts 1 and 2, respectively. 48.901% of cohort 1 were male, and 48.903% were in cohort 2. 68.19% vs. 66.147% of patients were white, 19.224% vs. 17.131% were black or African American, and 1.332% vs. 1.470% were Asian. Baseline demographics and characteristics are shown in Table 1. The table also includes ICD-10 codes.
Table 1. Baseline demographics and characteristics after propensity score matching.
Cohort 1: COVID-19 and a ventricular shunt
Cohort 2: Ventricular shunt without COVID-19
| Before Matching | After Matching | ||||||
| Code | Diagnosis | Cohort 1, n (%) | Cohort 2, n (%) | Std diff. | Cohort 1, n (%) | Cohort 2, n (%) | Std diff. |
| AI | Age at Index | 39.39 (100) | 38.23 (100.00) | - | 38.54 (100.00) | 38.46 (100.00) | - |
| 2106-3 | White | 8328 (68.16) | 9900 (66.17) | 0.040 | 7223 (68.14) | 7256 (68.45) | 0.0066 |
| F | Female | 6241 (51.08) | 7642 (51.08) | 0.00 | 5406 (51.00) | 5374 (50.69) | 0.0060 |
| M | Male | 5974 (48.89) | 7317 (48.91) | 0.0002 | 5191 (48.97) | 5224 (49.28) | 0.0062 |
| 2054-5 | Black or African American | 2352 (19.25) | 2560 (17.111) | 0.056 | 1937 (18.27) | 1933 (18.24) | 0.00098 |
| 2131-1 | Unknown Race | 1323 (10.83) | 2205 (14.74) | 0.12 | 1244 (11.74) | 1223 (11.54) | 0.0062 |
| 2028-9 | Asian | 163 (1.33) | 221 (1.48) | 0.012 | 147 (1.39) | 139 (1.31) | 0.0065 |
| I10-I16 | Hypertensive diseases | 5005 (40.96) | 4247 (28.39) | 0.27 | 3790 (35.76) | 3869 (36.50) | 0.016 |
| R53 | Malaise and fatigue | 3927 (32.14) | 3092 (20.67) | 0.26 | 2846 (26.85) | 2903 (27.39) | 0.012 |
| E78 | Disorders of lipoprotein metabolism and other lipidemias | 3094 (25.32) | 2631 (17.59) | 0.19 | 2292 (21.62) | 2343 (22.10) | 0.012 |
| R63 | Symptoms and signs concerning food and fluid intake | 3024 (24.75) | 2225 (14.87) | 0.25 | 2182 (20.59) | 2176 (20.53) | 0.0014 |
| J40-J47 | Chronic lower respiratory diseases | 2723 (22.29) | 2261 (15.11) | 0.18 | 1996 (18.83) | 2045 (19.29) | 0.012 |
| R13 | Aphagia and dysphagia | 2850 (23.33) | 1947 (13.01) | 0.27 | 1952 (18.42) | 1898 (17.91) | 0.013 |
| R40 | Somnolence, stupor and coma | 2271 (18.59) | 1524 (10.19) | 0.24 | 1507 (14.22) | 1471 (13.88) | 0.0098 |
| E08-E13 | Diabetes mellitus | 1973 (16.15) | 1533 (10.25) | 0.18 | 1414 (13.34) | 1411 (13.31) | 0.00083 |
| N17-N19 | Acute kidney failure and chronic kidney disease | 2122 (17.37) | 1338 (8.94) | 0.25 | 1344 (12.68) | 1311 (12.37) | 0.0094 |
| Z87.891 | Personal history of nicotine dependence | 1672 (13.69) | 1407 (9.40) | 0.13 | 1240 (11.69) | 1244 (11.74) | 0.0012 |
| F17 | Nicotine dependence | 1470 (12.03) | 1286 (8.59) | 0.11 | 1118 (10.55) | 1097 (10.35) | 0.0065 |
| I20-I25 | Ischemic heart diseases | 1498 (12.26) | 1016 (6.79) | 0.19 | 1006 (9.49) | 975 (9.19) | 0.010 |
| I50 | Heart failure | 941 (7.70) | 572 (3.82) | 0.17 | 568 (5.36) | 556 (5.25) | 0.0051 |
| I48 | Atrial fibrillation and flutter | 760 (6.22) | 556 (3.72) | 0.12 | 526 (4.96) | 504 (4.76) | 0.0097 |
| I73 | Other peripheral vascular diseases | 502 (4.11) | 338 (2.26) | 0.11 | 321 (3.028) | 317 (2.99) | 0.0022 |
| F10.1 | Alcohol abuse | 344 (2.82) | 221 (1.48) | 0.092 | 216 (2.038) | 217 (2.05) | 0.00067 |
| F10.2 | Alcohol dependence | 218 (1.78) | 150 (1.00) | 0.067 | 140 (1.32) | 133 (1.26) | 0.0059 |
| K74 | Fibrosis and cirrhosis of liver | 158 (1.29) | 85 (0.57) | 0.076 | 90 (0.85) | 85 (0.80) | 0.0052 |
Eight hundred and thirty-four patients (7.869%) in cohort 1 and 180 (1.698%) patients in cohort 2 underwent shunt revision (p=<0.0001, OR 4.978, 95% CI 4.198, 5.821). Mortality was 4.642% vs. 2.113% (p<0.0001, OR 2.255, 95% CI 1.921, 2.647). Hospitalization rates were 27.72% vs. 10.303% (p<0.0001), and ICU admission rates 11.567% vs. 3.463% (p<0.0001). Ventilator dependence was 3.529% vs. 0.953% (p<0.0001), tracheostomy 1.142% vs. 0.236% (p<0.0001), PEG tube insertion 2.472% vs. 0.585% (p<0.0001), falls 2.321% vs. 1.076% (p<0.0001), seizure 11.369% vs. 5.953% (p<0.0001), AKI 4.416% vs. 1.717% (p<0.0001), VTE 3.538% vs. 1.293% (p<0.0001), sepsis 3.887% vs. 1.179% (p<0.0001), IS 0.585% vs. 0.16% (p<0.0001), and MI 1.34% vs. 0.519% (p<0.0001). This is summarized in Table 2.
Table 2. Outcomes after propensity score matching.
Cohort 1: COVID-19 and a ventricular shunt
Cohort 2: Ventricular shunt without COVID-19
| Outcome | Cohort 1, n (%) | Cohort 2, n (%) | Odds ratio (95% CI) | P-value |
| Mortality | 492 (4.64) | 224 (2.11) | 2.26 (1.92,2.65) | <0.0001 |
| Shunt revision | 834 (7.87) | 180 (1.69) | 4.94 (4.19,5.82) | <0.0001 |
| Ventilator dependence | 374 (3.53) | 101 (0.95) | 3.80 (3.047,4.74) | <0.0001 |
| Tracheostomy | 121 (1.14) | 25 (0.24) | 4.88 (3.17,7.52) | <0.0001 |
| PEG | 262 (2.47) | 62 (0.59) | 4.31 (3.26,5.69) | <0.0001 |
| Falls | 246 (2.32) | 114 (1.08) | 2.19 (1.75,2.73) | <0.0001 |
| Seizures | 1205 (11.37) | 631 (5.95) | 2.02 (1.83,2.22) | <0.0001 |
| Hospitalization | 2938 (27.72) | 1092 (10.30) | 3.34 (3.09,3.60) | <0.0001 |
| Intensive care unit | 1226 (11.57) | 367 (3.46) | 3.65 (3.23,4.11) | <0.0001 |
| Acute kidney injury | 468 (4.42) | 182 (1.72) | 2.64 (2.22,3.15) | <0.0001 |
| Venous thromboembolism | 375 (3.54) | 137 (1.29) | 2.80 (2.29,3.41) | <0.0001 |
| Sepsis | 412 (3.887) | 125 (1.18) | 3.39 (2.77,4.15) | <0.0001 |
| Ischemic stroke | 62 (0.59) | 17 (0.16) | 3.66 (2.14,6.27) | <0.0001 |
| Myocardial infarction | 142 (1.34) | 55 (0.52) | 2.60 (1.91,3.56) | <0.0001 |
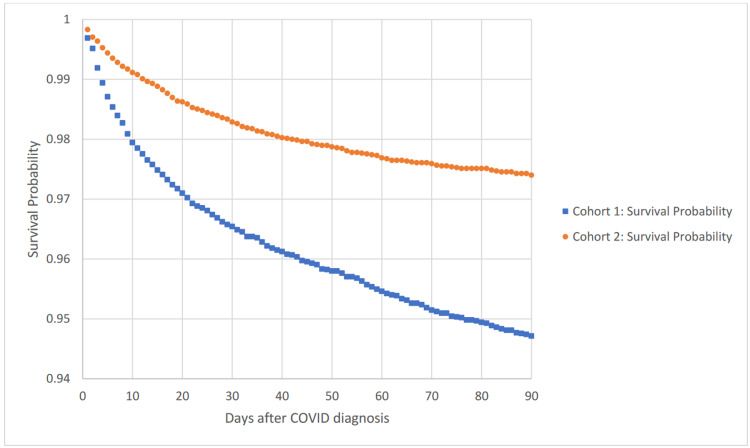
Figure 1 shows a Kaplan-Meier survival curve for outcome deceased to 90 days comparing cohorts 1 and 2. The hazard ratio was 2.035, with 95% CI 1.737, 2.383, p<0.0001.
Figure 1. Kaplan-Meier survival analysis for outcome: deceased.
Cohort 1: COVID-19 and a ventricular shunt
Cohort 2: Ventricular shunt without COVID-19
Discussion
Our results demonstrate a significant increase in shunt revisions associated with COVID-19 infection. As COVID-19 infection has been reported to cause neurological symptoms such as headache, weakness, altered mental status, seizure, stroke, drowsiness, malaise, fatigue, hypotonia, and peripheral neuropathy, and these symptoms can often be found in shunt failure, it begs the question on if COVID-19 is causing shunts to malfunction, or the infection is masquerading as a shunt malfunction [1,4,10]. Furthermore, rates of avoidable shunt revisions are high [11,12]. It is, therefore, important that clinicians become well-versed in the neurologic manifestations and complications of COVID-19 [13].
Silva et al. in 2020 reported 56 patients who had headaches and COVID-19 and subsequently underwent lumbar puncture (L.P.) for cerebrospinal fluid (CSF) analysis. Eleven of the 56 patients had an opening pressure of greater than 200 mmH2O, six of which had pressures greater than 250 mmH2O. Two patients had papilledema. All patients had normal CSF analysis. They concluded that COVID-associated coagulopathy might be an explanation for the increased intracranial pressure (ICP) [4].
Baccarella et al., in 2021, reported a case series of two patients with multi-inflammatory syndrome associated with COVID-19 infection who had evidence of increased intracranial pressure. The first patient presented with a headache, a right abducens palsy, and no papilledema. An L.P. was performed and revealed an opening pressure of 34 cm H2O and CSF without abnormality. The patient's headache improved after the L.P. The second patient also presented with a headache and was found to have bilateral papilledema and right abducens palsy. An L.P. was performed one week after the patient presented and revealed an opening pressure of 14 cm H2O and CSF without abnormality. The authors concluded that the multi-inflammatory syndrome associated with COVID-19 might cause increased ICPs, as has been noted in other systemic inflammatory disorders such as Lupus, Sjogren's, and Kawasaki disease [5].
Eden et al. in 2020 published a case series of CSF biomarkers in patients with neurological symptoms who also were COVID-19 positive. They found that CSF neopterin, a marker of inflammation, was elevated in all patients. They likewise noted that CSF beta-2-microglobulin was similarly elevated in all CSF samples. CSF neurofilament light chain protein, a marker of axonal injury, was elevated in one-third of patients, and SARS-CoV-2 RNA was also found in the CSF of one-third of patients. The white blood cell count, albumin ratios, and immunoglobulin G were all normal. This suggested that COVID-19 likely causes inflammation and possible axonal disruption of the central nervous system [2].
Lewis et al., in 2021, published a literature review of CSF in COVID-19-positive patients. Overall, the literature examined reported 430 patients with neurologic symptoms that prompted CSF testing. The authors found that SARS-CoV-2 in CSF is rare, at 6%. In addition, they found that 7% of patients had elevated WBC count in the CSF, and 40% of patients had elevated protein, indicative of inflammation or axonal injury. Thus, the authors conclude that most neurological complications of COVID-19 were unlikely related to direct viral neuroinvasion but instead could be due to toxic-metabolic changes, hypoxic-ischemic injury, and/or inflammatory response [3].
One recent paper by Laxpati et al. examined the number of shunt surgeries performed during the COVID-19 pandemic. This study was done at a single institution, looking at the number of elective and emergent shunt revisions from 2015 to 2020. They found that during 28 days in March of 2020, only 32 shunt surgeries were performed. As compared to numbers before the onset of the COVID-19 pandemic, this was a statistically significant decrease in the number of surgeries performed. The authors concluded that there was an environmental factor related to the pandemic that might be altering the presentation rate of shunt malfunctions [14]. A 2022 study by Lee et al. likewise showed a decreased total number of neurosurgery emergency patients from January 2020 to September 2020 [15].
Our analysis was not without limitations. The major limitation of this study was that it was retrospective. Furthermore, due to the nature of the database, we were unable to collect patient-level data on specific outcomes. We were unable to report on radiology information. We do not have information on the type of diagnostic test used for confirmation of disease. We do not have information on the type of shunt malfunction (proximal, distal, valve, or no shunt malfunction) found intraoperatively. In addition, some misidentification is inevitable in database studies.
Conclusions
COVID-19 is associated with an almost five-fold increase in shunt revisions. Likewise, patients with shunts who are COVID positive had higher mortality rates, hospitalization, ICU admission, ventilator dependence, tracheostomy, PEG tube insertion, falls, seizures, AKI, VTE, sepsis, I.S., and MI. This increase in shunt revisions could be due to associated increases in intracranial pressures from COVID-19 infection related to direct viral neuroinvasion, toxic-metabolic changes, multiinflammatory syndrome, coagulopathy, or hypoxic-ischemic injury.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Neurological issues in children with COVID-19. Lin JE, Asfour A, Sewell TB, et al. Neurosci Lett. 2021;743:135567. doi: 10.1016/j.neulet.2020.135567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CSF biomarkers in patients with COVID-19 and neurologic symptoms: A case series. Edén A, Kanberg N, Gostner J, et al. Neurology. 2021;96:0–300. doi: 10.1212/WNL.0000000000010977. [DOI] [PubMed] [Google Scholar]
- 3.Cerebrospinal fluid in COVID-19: A systematic review of the literature. Lewis A, Frontera J, Placantonakis DG, Lighter J, Galetta S, Balcer L, Melmed KR. J Neurol Sci. 2021;421:117316. doi: 10.1016/j.jns.2021.117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isolated intracranial hypertension associated with COVID-19. Silva MT, Lima MA, Torezani G, et al. Cephalalgia. 2020;40:1452–1458. doi: 10.1177/0333102420965963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Increased intracranial pressure in the setting of multisystem inflammatory syndrome in children, associated with COVID-19. Baccarella A, Linder A, Spencer R, et al. Pediatr Neurol. 2021;115:48–49. doi: 10.1016/j.pediatrneurol.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerebral venous thrombosis and portal vein thrombosis: A retrospective cohort study of 537,913 COVID-19 cases. Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. EClinicalMedicine. 2021;39:101061. doi: 10.1016/j.eclinm.2021.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Using a federated network of real-world data to optimize clinical trials operations. Topaloglu U, Palchuk MB. JCO Clin Cancer Inform. 2018;2:1–10. doi: 10.1200/CCI.17.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Outcomes of COVID-19 in patients with rheumatoid arthritis: A multicenter research network study in the United States. Raiker R, DeYoung C, Pakhchanian H, Ahmed S, Kavadichanda C, Gupta L, Kardeş S. Semin Arthritis Rheum. 2021;51:1057–1066. doi: 10.1016/j.semarthrit.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neurological involvement in COVID-19 and potential mechanisms: A review. Aghagoli G, Gallo Marin B, Katchur NJ, Chaves-Sell F, Asaad WF, Murphy SA. Neurocrit Care. 2021;34:1062–1071. doi: 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Application of the preventable shunt revision rate to an adult population. Venable GT, Dave P, Gordon WE, et al. World Neurosurg. 2020;134:0–21. doi: 10.1016/j.wneu.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Revision surgery following CSF shunt insertion: how often could it be avoided? Kaestner S, Poetschke M, Kehler U, Antes S, Krause M, Deinsberger W. Acta Neurochir (Wien) 2020;162:9–14. doi: 10.1007/s00701-019-04083-0. [DOI] [PubMed] [Google Scholar]
- 13.Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) Harapan BN, Yoo HJ. J Neurol. 2021;268:3059–3071. doi: 10.1007/s00415-021-10406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unexpected decrease in shunt surgeries performed during the shelter-in-place period of the COVID-19 pandemic. Laxpati N, Bray DP, Wheelus J, et al. Oper Neurosurg (Hagerstown) 2021;20:469–476. doi: 10.1093/ons/opaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comparative analysis of COVID-19 outbreak and changes in neurosurgical emergency patients. Lee MH, Jang SR, Lee TK. J Korean Neurosurg Soc. 2022;65:130–137. doi: 10.3340/jkns.2021.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]