Abstract
Background:
Patients with substance use disorders (SUDs) and severe bacterial infections requiring prolonged antibiotic therapy represent a significant challenge to providers due to complexity of care coordination required to ensure safe and effective treatment. Our institution developed a patient-centered multidisciplinary discharge planning conference, OPTIONS-DC, to address this challenge.
Methods:
We conducted a retrospective review to evaluates parameters between patients who received an OPTIONS-DC and those who did not.
Results:
We identified 73 patients receiving an OPTIONS-DC and 100 who did not. More patients with an OPTIONS-DC were < 40 years of age (76.7% versus 61.0%, OR = 2.3, 95% CI = 1.1–4.7, p = 0.02), had positive HCV antibody testing (58.9% versus 41.0%, OR = 2.1, 95% CI = 1.1–3.8, p = 0.02), injection drug use (93.2% versus 79.0%, OR = 3.6 95% CI = 1.3–10.1, p = 0.01), used methamphetamines (84.9% versus 72.0%, OR = 2.2, 95% CI = 1.0–4.8, p = 0.04), and started inpatient SUD treatment (80.8% versus 63%, OR = 2.5, 95% CI = 1.2–5.0, p = 0.04) compared with those without a conference. The OPTIONS-DC group was more likely to be diagnosed with bacteremia (74.0% versus 57.0%, OR = 2.1, 95% CI = 1.1–4.1, p = 0.02), endocarditis (39.7% versus21.0%, OR = 2.5, 95% CI = 1.3–4.9, p = 0.03), vertebral osteomyelitis (45.2% versus 15.0%, OR = 4.7, 95% CI = 2.3–9.6, p < 0.01), and epidural abscess (35.6% versus 10.0%, OR = 5.0, 95% CI = 2.2–11.2, p < 0.01) and require 4 weeks or more of antibiotic treatment (97.3% versus 51.1%, OR = 34.1, 95% CI = 7.9–146.7, p = 0.01). Patients with an OPTIONS-DC were also more likely to be admitted between 2019 and 2020 than between 2018 and 2019 (OR = 4.1, 95% CI = 2.1–7.9, p < 0.01).
Conclusion:
Patients with an OPTIONS-DC tended to have more complicated infections and longer courses of antibiotic treatment. While further research on outcomes is needed, patients receiving an OPTIONS-DC were able to successfully complete antibiotic courses across a variety of settings.
Keywords: multidisciplinary conference, serious infections in PWID, substance use disorder
Introduction
Over the last two decades, substance use disorders (SUDs), particularly opioid and methamphetamine use disorders, have been increasing in the United States, creating a substantial public health burden.1–3 In parallel, hospitals across the country are witnessing higher rates of admissions for injection-related infections in persons who inject drugs (PWID).4–6 A recent study demonstrates that admission for infections in PWID is associated with higher average hospital costs and longer lengths of stay compared with an inpatient cohort without SUDs. 7 Moreover, patients with housing instability and SUDs have low rates of infection treatment completion compared with those with only one or neither of these risk factors.8,9 Mortality rates are also higher in patients with unplanned or premature discharges. 10
Serious bacterial infections are commonly treated with long courses of intravenous (IV) antibiotics, and although outpatient parenteral antimicrobial therapy (OPAT) has been successful in PWID, application is limited. Concerns for misuse of the peripherally inserted central catheter (PICC), non-adherence, and feasibility still remain, with current guidelines suggesting that decisions to treat PWID with OPAT be made on a case-by-case basis.11–13 Infectious diseases (ID) providers note many obstacles in providing care for PWID with infections, which has led a number of institutions to establish tools to better incorporate SUD and infection treatment while optimizing safe discharges.7,14,15
The importance of a multidisciplinary treatment approach has been recognized in a variety of complex infections.16–19 In February 2018, our academic medical center instituted a multidisciplinary discharge planning conference, ‘OPTIONS-DC’. The goal of the conference is to improve outcomes in patients with SUDs and infections requiring long-term antibiotic therapy by incorporating harm-reduction principles and considering patient preferences and priorities when determining treatment and disposition recommendations.20,21 An OPTIONS-DC can be requested by anyone from the patient care team and on average occurs 2 weeks into the hospital stay once the patient is clinically stable and the ID team has recommended an antibiotic regimen. 22 Conference attendees include the ID consult service, and addiction medicine consult service comprised addiction medicine providers, social workers, and peers with lived experience in recovery, primary admitting team, case management, ID pharmacist, and an OPAT nurse who leads the meeting using a standardized conference tool. 22 This tool is a series of questions that guide participants to review factors that may affect infection treatment, substance use treatment, and the patients’ needs and goals, eliciting input from each team’s expertise before opening up the discussion for anybody to share concerns or solutions. A PICC safety assessment performed by the addiction medicine social worker prior to the conference is shared as well. 23 The conference serves as a decisional support tool for providers to review individual risk and protective factors and then tailor both SUD and infection treatments with the intent of creating a safe, patient-centered discharge plan. 24 The conferences last 28 min on average, and a detailed summary of the conference is entered into the patient’s electronic medical record (EMR) by the OPAT nurse and co-signed by the ID provider who was in attendance. Any patient with an SUD and an infection who may require long-term antibiotics is eligible for an OPTIONS-DC; however, not all eligible patients received a conference. We hypothesize that an OPTIONS-DC conference was offered to patients with more severe SUDs and complex infections.
The aims of this study are to evaluate factors associated with a patient receiving an OPTIONS-DC and to describe treatment completion, length of stay, and unplanned discharges between those who received a conference and those who did not.
Methods
Study population
We conducted a retrospective case–control study to identify factors associated with the presence or absence of OPTIONS-DC during hospital admission for a serious bacterial infection. The study period began in February 2018, when OPTIONS-DC was introduced, through March 2020, when the state of Oregon instituted COVID-19 emergency stay-at-home measures. OPTIONS-DC protocol details and conference description have been previously described. 22 Patients were identified via SAP BusinessObjects Enterprise Business Intelligence Platform 4.2 (SAP America, Inc., PA, USA) report of all inpatient admissions during the identified time period with both ID and addiction medicine consultation notes written during the same hospital encounter. We included patients aged 18 years or older, with an SUD and a bacterial infection requiring at least 2 weeks of antibiotics, and if both ID and addiction medicine teams completed an assessment with documented contact with the patient.
Patients were excluded if the infection included prosthetic material, they were receiving dialysis, they were pregnant, or the infection was managed primarily at an outside facility (transferred to study site hospital for source control procedure only). Patients were also excluded if the primary infection was due to a virus, fungus, or mycobacteria. If a patient had more than one admission during the study period, they were only counted once for the demographic data. For the case–control analysis, each admission for a different infection was counted as a unique event eligible for inclusion, but if it occurred within 90 days of another admission and was due to the same infection, it was measured as an outcome of the index admission.
Data collection
We performed chart review to collect variables of interest, including demographics, comorbidities, housing status, and type of insurance. Any psychiatric disorder included mood disorder, psychotic disorder, post-traumatic stress disorder, or personality disorder present on the patient’s problem list. SUD characteristics including type of substances used at the time of hospital encounter, number of substances used, route of use (injection, inhalation, or ingestion), most recent use, and associated tobacco use were largely identified from the addiction medicine service initial consult note, which also detailed the patient’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) severity score for each substance used. 25 Subsequent addiction medicine notes were used to determine SUD treatment such as buprenorphrine or methadone while inpatient. Key infection characteristics including infection type, infection organism, antibiotic regimen (type, route, and duration), and location of antibiotic administration [hospital, home infusion, infusion center, skilled nursing facility (SNF), respite program, or rehabilitation center] were identified from ID provider notes and discharge summary. For patients completing treatment outside the hospital, encounters from the OPAT nurse, ID providers, subsequent hospitalizations, and emergency department (ED) visits were utilized to track outcome variables until 90 days after antibiotic end date. Outcomes of interest included length of stay, change to antibiotic regimen including use of long-acting injectable (LAI) antibiotic, remaining antibiotic duration after discharge, treatment completion, reason for ED visit or readmission within 90 days, PICC misuse, and mortality. Lack of PICC misuse was determined by treatment completion along with the absence of misuse documentation by OPAT nurse or ED visit notes. Treatment completion was confirmed by discharge summary of those who remained in hospital or by ID provider outpatient notation and OPAT nurse encounter closure.
Statistics
We used SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) to perform descriptive analyses and compare categorical variables in univariable fashion by the chi-square or Fisher exact tests. We used the Cochran–Armitage test to evaluate trends in the proportion of OPTIONS-DC performed over time. We calculated crude and adjusted odds ratio (OR) and 95% confidence intervals (CIs) of cases (OPTIONS-DC) with each exposure variable compared with the proportion of controls (no OPTIONS-DC) with the exposure variable for each admission during our time period. We used the Student’s t test to evaluate continuous variables. For multivariable analysis, we performed a stepwise logistic regression analysis with all variables with a p value of 0.2 or less in univariable analysis considered for inclusion. Our final multivariable model included variables with a p value ⩽0.05 or if their addition significantly altered the OR of another variable present in the model. Variables considered for inclusion in the multivariable model are listed in Table 2.
Table 2.
Case–control analysis.
| OPTIONS-DC conference n (%) 73 (42.2) |
No OPTIONS-DC
conference n (%) 100 (57.8) |
Univariable odds ratio (95% confidence interval) |
p value a | Multivariable odds ratio (95% confidence interval) |
p value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Admission year 2019–2020 | 55 (75.3) | 43 (43.0) | 4.1 (2.1–7.9) | <0.01 | 8.4 (3.6–19.5) | <0.01 |
| Age < 40 years | 43 (58.9) | 41 (41.0) | 2.1 (1.1–3.8) | 0.02 | ||
| White | 67 (91.8) | 92 (92.0) | 1.0 (0.3–2.9) | 0.95 | ||
| Housing instability | 39 (53.4) | 64 (64.0) | 0.8 (0.8–2.9) | 0.16 | ||
| Psychiatric disorder | 48 (65.8) | 69 (69.0) | 1.2 (0.6–2.2) | 0.65 | ||
| HCV Ab or PCR positive | 56 (76.7) | 61 (61.0) | 2.3 (1.1–4.7) | 0.02 | ||
| Substance use disorder (SUD) parameters | ||||||
| Injection drug use | 68 (93.2) | 79 (79.0) | 3.6 (1.3–10.1) | 0.01 | 5.8 (1.7–20.0) | <0.01 |
| Methamphetamine use | 62 (84.9) | 72 (72.0) | 2.2 (1.1–4.8) | 0.04 | ||
| Inpatient SUD treatment | 59 (80.8) | 63 (63.0 | 2.5 (1.2–5.0) | 0.04 | ||
| Infection-related parameters | ||||||
| Bacteremia | 54 (74.0) | 57 (57.0) | 2.1 (1.1–4.1) | 0.02 | ||
| Endocarditis | 29 (39.7) | 21 (21.0) | 2.5 (1.3–4.9) | 0.03 | ||
| Vertebral osteomyelitis | 33 (45.2) | 15 (15.0) | 4.7 (2.3–9.6) | <0.01 | ||
| Epidural Abscess | 26 (35.6) | 10 (10.0) | 5.0 (2.2–11.2) | <0.01 | ||
| MRSA | 32 (43.8) | 31 (31.0) | 1.7 (0.9–3.3) | 0.19 | ||
| MSSA | 26 (35.6) | 27 (27.0) | 1.5 (0.8–2.9) | 0.34 | ||
| Other organism present | 16 (21.9) | 48 (48.0) | 0.3 (0.2–0.6) | <0.01 | ||
| >4 weeks of therapy b | 71 (97.3) | 51 (51.0) | 34.1 (7.9–146.7) | <0.01 | 59.3 (12.7–277.4) | <0.01 |
HCV, hepatitis C virus; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PCR, polymerase chain reaction.
p values in bold indicate variables were considered for inclusion in the multivariable model.
4 weeks of therapy, includes intravenous or oral route of administration.
This study was approved by the Oregon Health & Science University Institutional Review Board (IRB00003522) and conducted under an approved Waiver of Health Insurance Portability and Accountability Act Authorization Requirement.
Results
Descriptive analysis: cohort characteristics
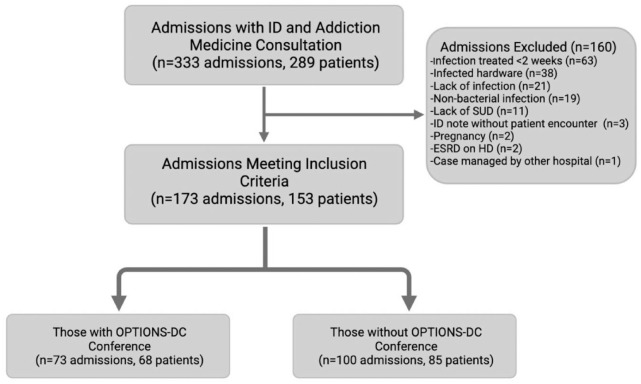
We identified 289 patients with 333 eligible admissions during the study period in which an ID and addiction medicine team evaluation was performed during the same hospital encounter (Figure 1). The most common reasons for exclusion were infection treated less than 2 weeks (63), hardware infection (38), no infection identified (21), non-bacterial infection (19), and no active SUD (11). Three patients were excluded because they had an ID note in the chart, but there was no direct patient contact. In all, 173 admissions comprising 153 patients met inclusion criteria and categorized into 73 admissions (65 total patients) that had at least one OPTIONS-DC, and 100 (85 total patients) admissions did not.
Figure 1.
Selection flowchart.
Overall, most patients were male (105, 68.6%) and White (142, 92.8%), with a median age of 40 years (range: 19–68 years) (Table 1). In addition, most patients (87, 56.9%) were unhoused, had Medicaid insurance (126, 82.4%), and had a diagnosis of at least one documented psychiatric disorder (98, 64.1%). Methamphetamines (118, 77.1%) and opiates (113, 73.9%) were the most common substances used. The majority of patients (101, 66.0%) had a positive hepatitis C virus (HCV) antibody or polymerase chain reaction (PCR) test on admission, and eight patients (5.2%) were living with human immunodeficiency virus (HIV). The most common infection diagnoses were bacteremia (96, 62.7%), endocarditis (44, 28.8%), vertebral osteomyelitis (44, 28.8%), epidural abscess (32, 20.9%), and septic arthritis (21, 13.7%). Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) were the most common organisms identified (55, 35.9% and 48, 31.4%, respectively). In addition, there was a significant trend of increasing proportion of OPTIONS-DC performed each year (p < 0.01).
Table 1.
Patient characteristics.
| OPTIONS-DC Conference (N = 68 patients totaling 73 admissions) | No OPTIONS-DC Conference (N = 85 patients totaling 100 admissions) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Age: median (range) | 38 | 19–65 | 42 | 19–65 |
| Male | 42 | 61.8 | 63 | 74.1 |
| White | 62 | 91.2 | 80 | 94.1 |
| Medicaid insurance | 59 | 86.8 | 67 | 78.8 |
| Unstable housing | 36 | 52.9 | 51 | 60.0 |
| Reported substance use | ||||
| Methamphetamine | 58 | 85.3 | 60 | 70.6 |
| Opioid | 56 | 82.4 | 57 | 67.0 |
| Alcohol | 7 | 10.3 | 17 | 20.0 |
| Cannabis | 21 | 30.9 | 30 | 35.3 |
| Benzodiazepine | 7 | 10.3 | 11 | 12.9 |
| Tobacco | 57 | 83.8 | 72 | 84.7 |
| Number of substances used | ||||
| 1 | 10 | 14.7 | 24 | 28.2 |
| 2 | 38 | 55.9 | 35 | 41.2 |
| >2 | 17 | 25.0 | 25 | 29.4 |
| Injection drug use reported | 63 | 92.6 | 69 | 81.2 |
| Any psychiatric disorder | 43 | 63.2 | 55 | 64.7 |
| HCV-positive antibody or PCR | 51 | 75.0 | 50 | 58.8 |
| Bacteremia | 50 | 73.5 | 46 | 54.1 |
| Endocarditis | 27 | 39.7 | 17 | 20.0 |
| Vertebral osteomyelitis | 31 | 45.6 | 13 | 15.3 |
| Epidural abscess | 24 | 35.3 | 8 | 9.4 |
| Septic arthritis | 13 | 19.1 | 8 | 9.4 |
| MRSA | 28 | 41.2 | 27 | 31.8 |
| MSSA | 25 | 36.8 | 23 | 27.1 |
HCV, hepatitis C virus; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PCR, polymerase chain reaction.
Case-control analysis
In univariable analysis, there were no significant differences between patients who had a conference and those who did not in terms of gender, race, insurance type, or housing status. Significantly more patients who had an OPTIONS-DC were less than 40 years of age (76.7% versus 61.0%, OR = 2.3, 95% CI = 1.1–4.7, p = 0.02) and had prior/positive HCV antibody testing on admission (58.9% versus 41.0%, OR = 2.1, 95% CI = 1.1–3.8, p = 0.02) (Table 2). The number of comorbid conditions including HIV, diabetes, heart disease, chronic lung disease, cirrhosis, chronic kidney disease, malignancy, and psychiatric disorders did not differ between the two groups. Patients who received an OPTIONS-DC conference were more likely to be admitted between the years 2019 and 2020 (OR = 4.1, 95% CI = 2.1–7.9, p < 0.01).
Patients who had a conference were significantly more likely to use methamphetamines (84.9% versus 72.0%, OR = 2.2, 95% CI = 1.0–4.8, p = 0.04) and endorse injection drug use (93.2% versus 79.0%, OR = 3.6, 95% CI = 1.3–10.1, p = 0.01). Otherwise, there was no difference between groups regarding additional substances used and the severity of their SUDs based on DSM-V criteria. More patients who received an OPTIONS-DC were started on medication for their SUDs than those who did not have a conference (80.8% versus 63%, OR = 2.5, 95% CI = 1.2–5.0, p = 0.04).
Patients who had a conference were significantly more likely to be diagnosed with bacteremia (74.0% versus 57.0%, OR = 2.1, 95% CI = 1.1–4.1, p = 0.02), endocarditis (39.7% versus 21.0%, OR = 2.5, 95% CI = 1.3–4.9, p = 0.03), vertebral osteomyelitis (45.2% versus 15.0%, OR = 4.7, 95% CI = 2.3–9.6, p < 0.01), and epidural abscess (35.6% versus 10.0%, OR = 5.0, 95% CI = 2.2–11.2, p < 0.01) and require 4 weeks or more of antibiotics to treat their infection (97.3% versus 51.1%, OR = 34.1, 95% CI = 7.9–146.7, p < 0.01). While there was no difference regarding the presence of MRSA or MSSA, an infection caused by an organism other than MRSA or MSSA was more likely in the group without a conference (21.9% versus 48.0%, OR = 0.3, 95% CI = 0.2–0.6, p < 0.01).
In multivariable analysis, requiring 4 weeks or more of antibiotics (OR = 59.3, 95% CI = 12.7–277.4, p < 0.01) and injection drug use (OR = 5.8, 95% CI = 1.7–20.0, p < 0.01) were more common in those who received an OPTIONS-DC. Patients who received an OPTIONS-DC were more likely to be admitted between the years 2019 and 2020 compared with 2018 and 2019 (OR = 8.4, 95% CI = 3.6–19.5, p < 0.01).
Descriptive analysis: outcomes
The median length of stay was 32 days (range: 7–66 days) for those who had an OPTIONS-DC and 15 days (range: 2–66 days) for those who did not (Table 3). The percentage of IV antibiotic-based regimens decreased from 98.6% to 80.8% in patients after an OPTIONS-DC was performed. Switching to an LAI antibiotic occurred in 27.4% in the OPTIONS-DC group in contrast to 9.0% in those without a conference. Patients with a conference were discharged without antibiotics prior to completing therapy in four (5.5%) instances compared with eight (8.0%) of those without a conference. The median days of antibiotic therapy remaining at discharge were 13 (range: 0–43 days) in the OPTIONS-DC group and 7 (range: 0–54 days) in the group that did not have an OPTIONS-DC. Overall, 130 (75%) patients completed their planned antibiotic regimen, 61 (83.6%) in the OPTIONS-DC group and 69 (69.0%) in the no OPTIONS-DC group. There were 12 patients who had unknown therapy completion in the no OPTIONS-DC group and zero in the OPTIONS-DC group.
Table 3.
Descriptive outcomes.
| OPTIONS-DC conference(N = 68 patients totaling 73 admissions) | No options-DC
conference (N = 85 patients totaling 100 admissions) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Length of stay in days – median (range) | 32 (7–66) | 15 (2–66) | ||
| IV antibiotics as initial regimen | 72 | 98.6 | 85 | 85.0 |
| IV antibiotics after OPTIONS-DC | 59 | 80.8 | n/a | |
| Changed to oral antibiotic at discharge | 15 | 20.6 | 19 | 19.0 |
| Changed to long-acting injectable antibiotic at discharge | 20 | 27.4 | 9 | 9.0 |
| Discharged without antibiotics prior to completing therapy | 4 | 5.5 | 8 | 8.0 |
| Antibiotic days remaining after discharge – median (range) | 13 (0–43) | 7 (0–54) | ||
| Antibiotic treatment completion | ||||
| Confirmed Completed | 61 | 83.6 | 69 | 69.0 |
| Not Completed | 12 | 16.4 | 19 | 19.0 |
| Unknown Completion | 0 | 0.0 | 12 | 12.0 |
| Discharge Location | ||||
| Hospital | 27 | 37.0 | 44 | 44.0 |
| Skilled nursing facility | 5 | 6.8 | 3 | 3.0 |
| Addiction treatment center | 2 | 2.7 | 1 | 1.0 |
| Home | 23 | 31.5 | 20 | 20.0 |
| Temporary housing | 3 | 4.1 | 1 | 1.0 |
| Street | 8 | 11.0 | 29 | 29.0 |
| Infusion center utilized for antibiotic therapy | 13 | 17.8 | 4 | 4.0 |
| PICC misuse suspected during treatment | 3 | 4.1 | 1 | 1.0 |
| Unplanned or premature discharge | 10 | 13.7 | 17 | 17.0 |
| Readmission or ED visit within 90 days of discharge | 9 | 12.3 | 20 | 20.0 |
| Readmission for relapse of same infection | 4 | 5.5 | 4 | 4.0 |
| Readmission for new infection | 2 | 2.7 | 12 | 12.0 |
| Readmission not related to infection | 3 | 4.1 | 4 | 4.0 |
| Death within 90 days of antibiotic end date | 6 | 8.2 | 6 | 6.0 |
ED, emergency department; IV, intravenous; PICC, peripherally inserted central catheter.
In terms of disposition, 37.0% of patients who received an OPTIONS-DC completed their therapy in the hospital compared with 44.0% of those who did not have a conference. Of the patients who received an OPTIONS-DC, 31.5% were discharged home and 11% were discharged to the street, whereas in patients who did not have a conference, 20% were discharged home and 29% were discharged to the street. Thirteen (17.8%) patients who had an OPTIONS-DC received their antibiotics at an infusion center, whereas in those who did not have a conference, only four (4.0%) received their therapy at an infusion center. An unplanned or premature discharge occurred on 10 (13.7%) occasions in the OPTIONS-DC group and 12 (12.0%) for the group without a conference.
A total of 9 patients (12.3%) with a conference and 20 patients (20.0%) without a conference had a readmission or ED visit documented within 90 days of discharge. Readmission due to relapse of infection occurred in 5.5% in the OPTIONS-DC group and in 4.0% in those without a conference. There were six documented deaths within 90 days of antibiotic end date in both groups.
Discussion
Although the criteria for requesting an OPTIONS-DC is a serious bacterial infection in a person with SUD, we found that over half of eligible admissions did not have a conference. We identified patient-specific factors associated with receiving an OPTIONS-DC, which included age <40 years old, positive HCV testing, methamphetamine use disorder, injection drug use, and initiating SUD treatment inpatient. The patients who received an OPTIONS-DC might be perceived as having a more severe SUD; however, the severity score of SUDs determined by addiction medicine documentation did not differ between the groups, indicating possible missed opportunities for more patients to benefit from an OPTIONS-DC. An OPTIONS-DC must be requested by someone from the patient’s care team. The need for this conference for an individual patient may be influenced by provider biases and attitudes or by nuanced discussions between the patient and multiple members of the healthcare team, and could not be adequately assessed via retrospective chart review. Prospectively surveying providers to evaluate requesting or not requesting an OPTIONS-DC for specific patients would be an important addition for future studies. Our data suggest that more time and experience with the OPTIONS-DC likely contributed to a greater proportion of patients receiving a conference toward the end of the study period. The severity of infection and prolonged treatment durations may prompt teams to seek a multidisciplinary approach to help evaluate safe discharge options versus continued in-hospital treatment. Further studies should compare outcomes for similar types of infections between patients who received a conference and those who did not to better assess the effect of an OPTIONS-DC on treatment outcomes for specific infections.
From the outcomes of the descriptive analysis, trends among patients receiving a conference included an increased proportion of antibiotic completion at home, an SNF, or in temporary housing, whereas higher percentages of patients who did not have a conference completed their therapy in the hospital or were discharged to the street. Despite recent data demonstrating patients with SUDs are being treated successfully with OPAT with no worse outcomes than patients without SUDs,12,26 stigma around PWID and treatment with IV antibiotics in the outpatient setting persists. In a survey conducted of ID physicians on the treatment of PWID, 43% of responding ID providers said they would never prescribe OPAT to PWID, and when asked whether they would prescribe for patients on stable opioid replacement therapy, 40% still responded never. 27 By design, the OPTIONS-DC tool reviews psychosocial and SUD risk and protective factors and establishes close monitoring of the patient with outpatient resources, including linkage to a primary care provider, an outpatient case manager, and the OPAT nurse care coordinators. These details may allow providers to feel more comfortable with a discharge from the hospital to home or temporary housing with antibiotic administration in a more monitored setting such as at an infusion center. At our institution, any patient considered for OPAT with an active or recent history of SUDs should have a PICC safety evaluation if a PICC is being considered. This assessment is reviewed at the OPTIONS-DC allowing for real-time discussion among the involved providers to assist the group in reaching a consensus on an individualized safe discharge plan. Our cohort showed similar results to past studies with low numbers of individuals suspected to manipulate their PICC. 12
Antibiotics were administered at an infusion center or changed to an LAI in a higher proportion of OPTIONS-DC cases. An OPTIONS-DC conference allows discussion of alternative antibiotic modalities and settings using a harm-reduction approach. The off-label use of LAI antibiotics as treatment for serious bacterial infections in patients with SUDs has been increasing to facilitate early discharge, assist with treatment completion without need for a PICC or safe home environment, and reduce hospital costs.28–31 Further studies are needed to better ascertain treatment outcomes for specific infectious conditions, such as endocarditis, in patients with SUDs receiving LAI antibiotics.
Patients who had an OPTIONS-DC had a trend for being discharged with more days remaining of their antibiotic therapy, less unplanned discharges, and higher treatment completion. Given our study design and the non-differential allocation of the conference between patients, we are unable to attribute these outcomes to the OPTIONS-DC alone as patient selection played a key role. However, in a previous study, 70% of patients with SUDs were found to complete Staphylococcus aureus bacteremia treatment, which is similar to the treatment completion in our cohort without a conference. 32 Of patients who had an OPTIONS-DC, 83.6% completed treatment. We hypothesize that OPTIONS-DC may increase the likelihood of long-term antibiotic treatment completion in patients with SUDs and would be important to evaluate in future studies.
Our study has several important limitations. It was conducted at a single academic medical center with ID, comprehensive OPAT, and multidisciplinary addiction medicine teams, so may not be generalizable to other institutions that do not have this robust infrastructure to conduct discharge planning conferences similar to OPTIONS-DC. Our study population was also mostly White, representative of the Oregon population, which also limits generalizability. The retrospective nature of our study allowed us to describe outcomes from chart review, but did not allow us to capture provider reception or patient perspectives on their discharge and final antibiotic plan or certain patient factors, such as desire to start SUD treatment or social supports that may impact outcomes. Patient and provider interviews are needed to determine whether the treatment plan for those who received an OPTIONS-DC aligned with patient priorities and preferences, a guiding principle of the conferences. Furthermore, we were not able to interview teams to determine why they may have opted for an OPTIONS-DC in certain patients, but not in others despite eligibility. Our data may also be skewed because the study period began right as we implemented OPTIONS-DC, but before wide-spread education and uptake of the conferences occurred, reflected by the increasing proportion of OPTIONS-DC over time. Finally, some patients may have left the hospital before a referral for an OPTIONS-DC could be made. Further constructive evaluation of the OPTIONS-DC model, now that most providers at our institution are aware of it, should be explored.
Our findings add to the growing body of literature investigating improving outcomes for patients admitted to the hospital with concurrent SUDs and severe bacterial infections, and have important implications for patient care and healthcare systems. A patient-centered discharge planning conference for patients with SUDs or others with potentially complex discharges could improve medication adherence, provider understanding of psychosocial factors, and connection to a primary care provider or other outpatient resources. For hospital systems, similar conferences could help improve safe discharges and decrease hospital readmission while also increasing bed availability. Similar discharge conferences may help increase the comfort of home infusion companies or SNFs in working with patients with SUDs, thus decreasing the frequent refusals experienced in this setting. Finally, these conferences could facilitate screening for and treatment of co-infections that sometimes are not addressed during a hospital stay. We are currently expanding our conference tool to include questions regarding co-infection testing and offering inpatient HCV treatment with linkage to outpatient follow-up. By using structured, non-stigmatizing language for the conferences, we also hope the conferences serve as a method to educate providers and reduce stigma in this patient population. An OPTIONS-DC has now become a recognized strategy in comprehensive discharge planning in patients with SUDs and severe infection at our institution, which we anticipate will continue to reach a higher proportion of eligible persons.
Conclusion
In conclusion, persons less than 40 years old, with 4 weeks or more of antibiotic therapy, PWID, initiation of SUD treatment while inpatient, positive HCV testing, and diagnosed with bacteremia, endocarditis, vertebral osteomyelitis, or epidural abscess were more likely to receive an OPTIONS-DC. While further research on outcomes is needed, patients receiving an OPTIONS-DC were able to successfully complete antibiotic courses across a variety of settings.
Acknowledgments
We would like to thank the OHSU OPAT nurses for conducting every OPTIONS-DC conference.
Footnotes
ORCID iDs: Cara D. Varley  https://orcid.org/0000-0003-3209-4489
https://orcid.org/0000-0003-3209-4489
Monica K. Sikka  https://orcid.org/0000-0001-8192-8019
https://orcid.org/0000-0001-8192-8019
Contributor Information
Michael Conte, Division of Infectious Diseases, School of Medicine, Oregon Health & Science University, Portland, OR, USA.
Brent Schneider, Division of Infectious Diseases, School of Medicine, Oregon Health & Science University, Portland, OR, USA.
Cara D. Varley, Division of Infectious Diseases, School of Medicine, Oregon Health & Science University, Portland, OR, USA School of Public Health, Oregon Health & Science University–Portland State University, Portland, OR, USA.
Amber C. Streifel, Department of Pharmacy, Oregon Health & Science University, Portland, OR, USA
Monica K. Sikka, Division of Infectious Diseases, School of Medicine, Oregon Health & Science University, Portland, OR 97239-3098, USA.
Declarations
Ethics approval and consent to participate: This study was approved by the Oregon Health & Science University Institutional Review Board (IRB00003522). All methods were carried out in accordance with relevant guidelines and regulations/Declaration of Helsinki. A waiver of informed consent was granted by the Oregon Health & Science Institutional Review Board due to minimal risk of the retrospective review.
Consent for publication: Not applicable.
Author contributions: Michael Conte: Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Brent Schneider: Conceptualization; Data curation; Investigation; Writing – original draft.
Cara D. Varley: Conceptualization; Data curation; Formal analysis; Investigation; Supervision; Validation; Writing – review & editing.
Amber C. Streifel: Conceptualization; Data curation; Investigation; Supervision; Writing – review & editing.
Monica K. Sikka: Conceptualization; Investigation; Resources; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The data used/analyzed for this study are available from the corresponding author on reasonable request.
References
- 1. Centers for Disease Control and Prevention. Understanding the epidemic: drug overdose, 2021, https://www.cdc.gov/drugoverdose/epidemic/index.html
- 2. Jones CM, Logan J, Gladden RM, et al. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep 2015; 64: 719–725. [PMC free article] [PubMed] [Google Scholar]
- 3. Winkelman TNA, Admon LK, Jennings L, et al. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open 2018; 1: e183758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capizzi J, Leahy J, Wheelock H, et al. Population-based trends in hospitalizations due to injection drug use-related serious bacterial infections, Oregon, 2008 to 2018. PLoS ONE 2020; 15: e0242165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood) 2016; 35: 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCarthy NL, Baggs J, See I, et al. Bacterial infections associated with substance use disorders, large cohort of United States Hospitals, 2012-2017. Clin Infect Dis 2020; 71: e37–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rapoport AB, Fine DR, Manne-Goehler JM, et al. High inpatient health care utilization and charges associated with injection drug use-related infections: a cohort study, 2012-2015. Open Forum Infect Dis 2021; 8: ofab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Appa A, Adamo M, Le S, et al. Patient-directed discharges among persons who use drugs hospitalized with invasive staphylococcus Aureus infections: opportunities for improvement. Am J Med 2022; 135: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beieler A, Magaret A, Zhou Y, et al. Outpatient parenteral antimicrobial therapy in vulnerable populations – people who inject drugs and the homeless. J Hosp Med 2019; 14: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med 2012; 125: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Callaghan K, Tapp S, Hajkowicz K, et al. Outcomes of patients with a history of injecting drug use and receipt of outpatient antimicrobial therapy. Eur J Clin Microbiol Infect Dis 2019; 38: 575–580. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki J, Johnson J, Montgomery M, et al. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis 2018; 5: ofy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Norris AH, Shrestha NK, Allison GM, et al. 2018 infectious diseases society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis 2019; 68: 1–4. [DOI] [PubMed] [Google Scholar]
- 14. Price CN, Solomon DA, Johnson JA, et al. Feasibility and safety of outpatient parenteral antimicrobial therapy in conjunction with addiction treatment for people who inject drugs. J Infect Dis 2020; 222(Suppl. 5): S494–S498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eaton EF, Mathews RE, Lane PS, et al. A 9-point risk assessment for patients who inject drugs and require intravenous antibiotics: focusing inpatient resources on patients at greatest risk of ongoing drug use. Clin Infect Dis 2019; 68: 1041–1043. [DOI] [PubMed] [Google Scholar]
- 16. O’Donnell M, Englander H, Strnad L, et al. Expanding the team: optimizing the multidisciplinary management of drug use-associated infective endocarditis. J Gen Intern Med 2022; 37: 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart 2017; 4: e000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burden M, Thornton M. Reducing the risks of surgical site infection: the importance of the multidisciplinary team. Br J Nurs 2018; 27: 976–979. [DOI] [PubMed] [Google Scholar]
- 19. Akgun D, Muller M, Perka C, et al. High cure rate of periprosthetic hip joint infection with multidisciplinary team approach using standardized two-stage exchange. J Orthop Surg Res 2019; 14: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strnad L, Douglass A, Young K, et al. The development, implementation, and feasibility of multidisciplinary treatment planning conference for individuals with unstable substance use disorders and active infections requiring prolonged antimicrobial therapy: the OPTIONS-DC model. Open Forum Infect Dis 2019; 6: S331–S332. [Google Scholar]
- 21. Gore SJ, Strnad L, Englander H, et al. Outcomes of multidisciplinary care conferences for patients with substance use disorders requiring prolonged antimicrobial therapy for severe infections. Open Forum Infect Dis 2019; 6: S349. [Google Scholar]
- 22. Sikka MK, Gore S, Vega T, et al. ‘OPTIONS-DC’, a feasible discharge planning conference to expand infection treatment options for people with substance use disorder. BMC Infect Dis 2021; 21: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the improving addiction care team. J Addict Med 2019; 13: 85–89. [DOI] [PubMed] [Google Scholar]
- 24. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the improving addiction care team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med 2017; 12: 339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Publishing, 2013. [Google Scholar]
- 26. Vazirian M, Jerry JM, Shrestha NK, et al. Outcomes of outpatient parenteral antimicrobial therapy in patients with injection drug use. Psychosomatics 2018; 59: 490–495. [DOI] [PubMed] [Google Scholar]
- 27. Rapoport AB, Fischer LS, Santibanez S, et al. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis 2018; 5: ofy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Streifel AC, Sikka MK, Bowen CD, et al. Dalbavancin use in an academic medical centre and associated cost savings. Int J Antimicrob Agents 2019; 54: 652–654. [DOI] [PubMed] [Google Scholar]
- 29. Bryson-Cahn C, Beieler AM, Chan JD, et al. Dalbavancin as secondary therapy for serious staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis 2019; 6: ofz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vazquez Deida AA, Shihadeh KC, Preslaski CR, et al. Use of a standardized dalbavancin approach to facilitate earlier hospital discharge for vulnerable patients receiving prolonged inpatient antibiotic therapy. Open Forum Infect Dis 2020; 7: ofaa293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive gram-positive infections. Infect Dis Ther 2019; 8: 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Appa A, Adamo M, Le S, et al. Comparative 1-year outcomes of invasive staphylococcus aureus infections among persons with and without drug use: an observational cohort study. Clin Infect Dis 2022; 74: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]