Abstract
Introduction
Productivity loss may contribute to a large proportion of costs of health conditions in an economic evaluation from a societal perspective, but there is currently a lack of methodological consensus on how productivity loss should be measured and valued. Despite the research progress surrounding this issue in other countries, it has been rarely discussed in China.
Methods
We reviewed the official guidelines on economic evaluations in different countries and regions and screened the literature to summarise the extent to which productivity loss was incorporated in economic evaluations and the underlying methodological challenges.
Results
A total of 48 guidelines from 46 countries/regions were included. Although 32 (67%) guidelines recommend excluding productivity loss in the base case analysis, 23 (48%) guidelines recommend including productivity loss in the base case or additional analyses. Through a review of systematic reviews and the economic evaluation studies included in these reviews, we found that the average probability of incorporating productivity loss in an economic evaluation was 10.2%. Among the economic evaluations (n=478) that explicitly considered productivity loss, most (n=455) considered losses from paid work, while only a few studies (n=23) considered unpaid work losses. Recognising the existing methodological challenges and the specific context of China, we proposed a practical research agenda and a disease list for progress on this topic, including the development of the disease list comprehensively consisting of health conditions where the productivity loss should be incorporated into economic evaluations.
Conclusion
An increasing number of guidelines recommend the inclusion of productivity loss in the base case or additional analyses of economic evaluation. We optimistically expect that more Chinese researchers notice the importance of incorporating productivity loss in economic evaluations and anticipate guidelines that may be suitable for Chinese practitioners and decision-makers that facilitate the advancement of research on productivity loss measurement and valuation.
Keywords: review, health economics
What is already known on this topic
A societal perspective recommends the inclusion of productivity loss in health economic evaluations. An exclusion approach may lead to an underestimation of the actual costs. However, this issue has been rarely discussed in China.
What this study adds
Though most official guidelines recommend excluding productivity loss in the base case analyses, there are almost half of guidelines (23 out of 48) accepting the inclusion of productivity loss in the base case or additional analyses. In practice, among the studies that explicitly considered productivity loss, most considered only the losses from paid work, while a few considered unpaid work losses, due to the existing methodological challenges that are hard to overcome in practice.
How this study might affect research, practice or policy
It might be wise for the Chinese to learn from the guidelines of other countries and the mature methods for productivity measurement and valuation and bypass the existing methodological challenges temporarily. Therefore, we propose a practical research agenda to facilitate the advancement of research on productivity loss in China.
Background
Health economic evaluations aim to inform decision makers for efficient resource allocation. Through the comparison of the health benefits (eg, life-years gained) and costs of different healthcare interventions, economic evaluation demonstrates the optimal choice for decision makers. A societal perspective, recommended by health economists to facilitate policies aimed at maximising the welfare gains to society, commonly incorporates all relevant costs to the society, including the losses due to the reduced productivity of patients.1 The productivity, according to economic theory, measures the aggregate output of a single input or a combination of different inputs, such as workforce and capital input. In health economics, it is important to account for the impact of health conditions on the workforce, which may be negatively affected in terms of quantity (eg, time) and quality (eg, skills and concentration).2 Ultimately, the reduced workforce may lead to a decrease in the aggregate output, known as productivity loss. Productivity loss may contribute to a large proportion of costs of health conditions to the society (eg, depression of the workforce).3 4 Therefore, in an economic evaluation using a societal perspective, excluding productivity loss could lead to an underestimation of the actual costs.5
China published new guidelines in 2020 in response to the increasing volume of health economic evaluations in China.6 In the new guidelines, both societal and healthcare system perspectives are recommended. A societal perspective is preferred when the decision problem concerns the publicly funded healthcare expenditure. Since the outbreak of the pandemic in 2019, it has been evident how substantial the impacts of health risks and associated interventions can be to the whole society and every person’s daily life. Therefore, the Chinese health economists reinforced the recommendation of adopting a societal perspective in economic evaluations in the postpandemic era to incorporate all relevant costs and benefits.7 8 From a societal perspective, all direct, indirect and intangible costs should be included. The indirect costs mainly refer to productivity loss in the Chinese guidelines. However, this type of cost has been rarely incorporated in health economic evaluations in China, even among those that claimed to take a societal perspective.9 A possible cause is the lack of appropriate and credible data on productivity loss. A more relevant reason is that the National Healthcare Security Administration (NHSA), which manages the publicly funded healthcare insurance in China, does not require the incorporation of productivity loss, as it advocates a public payer perspective. This inconsistency between the Chinese guidelines and the internal requirement by NHSA, together with the lack of credible data, is hindering the incorporation of productivity loss in economic evaluations. In some cases, the failure of the incorporation may have significantly underestimated the societal costs of some health conditions (eg, depression and arthritis).
Additionally, there is still a lack of detailed methodological guidance on how productivity loss should be measured and valued in the Chinese context. In contrast, the line of research has been undergoing in other countries for decades and leads to the official recommendation of including productivity loss.10 Given the Chinese guidelines and health economists prioritising the societal perspective, the inclusion of productivity loss deserves a more extensive discussion. In this article, we aim to review the guidelines of different countries and regions and summarise their recommendations on productivity loss. Additionally, through a review of the systematic reviews of health economic evaluations, we aim to summarise the incorporation of productivity loss in practice and the methodological challenges and, based on that, suggest a practical research agenda for China to move forward on this topic.
Methods
We first referred to the official website of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) to search for guidelines in different jurisdictions published before the end of December 2021. The website of Health Technology Assessment International and the official websites of country-specific health technology assessment (HTA) organisations were also referred to as supplementary sources. In the countries where no governmental guidelines exist, we searched for guidelines issued by professional research organisations. The process ensured a comprehensive coverage of HTA guidelines that may influence economic evaluations in different countries. If the original guidelines were not written in English, we referred to the literature (eg, reviews summarising guidelines) or the summary information on the ISPOR website to examine their recommendations on the incorporation of productivity loss. We extracted their bibliographic information (ie, country, year, organisation and title) and the recommendations on perspective(s), whether to include productivity loss and approaches for productivity loss identification and measurement.
We also reviewed the systematic reviews of the economic evaluations published in the last decade (ie, 2011–2021) to examine how frequently the productivity loss was incorporated and its impact on economic results. The frequency was calculated as the proportion of studies incorporating productivity loss among all the studies included in a review. We also pooled the frequencies of different reviews and calculated the average probability of an economic evaluation incorporating productivity loss, which referred to the proportion of studies considering productivity loss relative to the total number of studies included in all the reviews.
The search for systematic reviews was conducted in EMBASE and MEDLINE on 31 December 2021. Other sources from the grey literature were searched using Google and Google Scholar. The following keywords were used in the search: ‘productivity loss’, ‘productivity cost’, ‘work loss’, ‘presenteeism’, ‘absenteeism’, ‘time off’, ‘sick leave’, ‘days off’, ‘absence’, ‘unemployment’ etc, accompanied by terms on economic evaluation, such as ‘cost-effectiveness analysis (CEA)’, ‘cost-utility analysis (CUA)’ and ‘cost-benefit analysis (CBA)’. Only the full-length systematic reviews in English were considered. We included the reviews investigating the incorporation of productivity loss in CEA, CUA or CBA and excluded the reviews without evidence synthesis and those focused on the cost of illness. The selection process included two rounds, first by the title and abstract of each document and then by the full text.
In the selection process, we screened the individual economic evaluations that were included in the reviews and identified a brief list of diseases to showcase the examples where researchers were likely to consider productivity loss. We included a disease if the productivity loss it caused accounted for no less than 50% of the total cost. The identification of the cut-off level was through a discussion among coauthors of this review who had experience as external consultants for reimbursement decisions. Failing to incorporate productivity loss caused by these diseases would cause significant underestimates in the total costs and may alter the results for decision making, for example, incremental cost-effectiveness ratios (ICERs).
We identified some methodological challenges of incorporating productivity loss in economic evaluation by scanning the discussions of the systematic reviews we selected and their references. We summarised the challenges from the aspects of identification, measurement and valuation of loss in paid and unpaid work. According to the requirement on reporting patient and public involvement in research, we clarify that patients or the public were not involved in the design, conduct, reporting or dissemination plans of this study.
Results
Recommendations on the inclusion of productivity loss
A total of 48 guidelines from 46 countries or regions were included and reviewed in this study. We found that the inclusion or exclusion of productivity loss largely depends on the suggested perspectives for the economic evaluations.
Among the 48 guidelines, 32 (67%) guidelines for 31 countries or regions recommend the healthcare system or payer’s perspective, which requires the inclusion of only direct costs (table 1). These guidelines suggest the exclusion of productivity loss in the base case as they are defined as indirect costs and irrelevant to the system or payer. Four guidelines (8%) (in Austria, Brazil, Cuba and Mercosur) do not clearly recommend a perspective in economic evaluations and state that the researchers should choose an appropriate perspective according to the decision problem.
Table 1.
Recommendations on the inclusion of productivity loss in economic evaluations
| Country/region* | Publication year | Perspective | Inclusion of PL in the base case | Identification of PL | Measurement of PL |
| Africa | |||||
| South Africa45 | 2013 | Payer perspective | No, indirect costs should be excluded. | NA | NA |
| Egypt46 | 2013 | Healthcare perspective | No. But it could be included in separate analysis. | NA | NA |
| Latin America | |||||
| Brazil47 | 2014 | No preferred perspective | It depended on the selection of perspective. | NA | NA |
| Colombia48 | 2014 | Healthcare perspective | No, indirect costs and direct non-medical costs should be excluded. | NA | NA |
| Cuba49 | 2003 | No preferred perspective | It depended on the selection of perspective. | NA | NA |
| Mexico50 | 2015 | Healthcare perspective | No, indirect costs should be excluded. | NA | NA |
| MERCOSUR51 | 2015 | No preferred perspective | It depended on the selection of perspective. | NA | NA |
| North America | |||||
| USA17 | 2020 (ICER) | Healthcare perspective | No. But it could be included in separate analysis. Healthcare sector and modified societal perspectives should be presented together in the base case if indirect costs is substantial, and these costs are considered largely relevant to direct costs. |
Paid work loss; unpaid work loss | NA |
| USA52 | 2016 (second panel) | Both healthcare perspective and societal perspective | Yes, future productivity and consumption should be included. | Paid work loss; unpaid work loss | NA |
| USA53 | 2020 (AMCP) | Healthcare perspective | No specific statement. | NA | NA |
| Canada20 | 2017 | Public payer perspective | No, PL should not be included. | Paid work loss (absenteeism and presenteeism); unpaid work loss; costs of hiring and training new workers for replacement | FCA for base case; other approaches for additional analyses |
| Asia | |||||
| Mainland China6 | 2020 | Both healthcare perspective and societal perspective | Yes, from the societal perspective, indirect costs should be included. | Paid work loss; PL due to premature death | HCA |
| Taiwan (China)54 | 2008 | Societal perspective | Yes. From the societal perspective, indirect costs should be included. | Paid work loss; PL due to premature death | HCA |
| Japan55 | 2019 | Healthcare perspective | No. But PL could be included in a separate analysis, if it can be estimated using Japanese data. | Paid work loss | HCA |
| Malaysia56 | 2019 | Payer perspective | No, PL should be excluded. | NA | NA |
| South Korea57 | 2013 | Limited societal perspective | No, PL should be excluded. | NA | NA |
| Iran58 | NA | Societal perspective | Yes | NA | NA |
| Israel59 | 2010 | Healthcare perspective | No | NA | NA |
| Thailand11 | 2014 | Societal perspective | Yes, indirect costs should be included. | Paid work loss (absenteeism and presenteeism) | HCA |
| Indonesia60 | 2017 | Societal perspective | Yes, indirect costs should be included. | NA | NA |
| Philippines61 | 2020 | Payer perspective | No. Only costs related to the healthcare system should be included. | NA | NA |
| Singapore62 | 2019 | Healthcare perspective | No. But indirect costs are permitted in the additional analyses. | NA | NA |
| Europe | |||||
| Austria63 | 2006 | No preferred perspective | It depended on the selection of perspective. | NA | NA |
| Denmark13 | 2007 | Societal perspective | Yes, production loss/gains should be included. | Paid work loss (absenteeism and presenteeism); PL due to premature death | NA |
| Hungary64 | 2017 | Payer perspective | No, productivity costs must be disregarded. | NA | NA |
| Italy65 | 2020 | Healthcare perspective | No. But indirect costs and non-health care costs could be considered in a supplementary analysis from the societal perspective. | NA | NA |
| Russia66 | 2016 | Healthcare perspective | No | NA | NA |
| Spain67 | 2010 | Societal perspective | Yes. The results of healthcare costs, PL/lost time and care costs should be expressed separately. | NA | NA |
| Croatia68 | 2011 | Payer perspective | No | NA | NA |
| Baltic†69 | 2002 | Healthcare perspective | No. If relevant, include all costs outside healthcare system and present separately. | NA | NA |
| Belgium16 | 2015 | Healthcare perspective | No. But it could be included in separate analysis. | Paid work loss; unpaid work loss | HCA for short-term PL; FCA for long-term PL |
| France70 | 2020 | Healthcare perspective | No. But it could be included in separate analysis. Indirect costs can be identified when health interventions concern life-threatening conditions with total or partial incapacity in carrying out an activity. | NA | NA |
| Germany71 | 2009 | Payer perspective | No. But it could be included in separate analysis, if PL is substantially affected by a new health technology. | NA | HCA for base case; FCA for sensitivity analysis |
| Ireland12 | 2019 | Healthcare perspective | No. But it could be included in separate analysis. | Paid work loss (absenteeism and presenteeism) | NA |
| The Netherlands72 | 2016 | Societal perspective | Yes, if illness or treatment prevents people from being productive, the productivity losses (or gains) involved must be specified and valued. | Paid work loss (absenteeism and presenteeism); unpaid work loss | FCA |
| Norway73 | 2018 | Healthcare perspective | No, PL should be excluded. | NA | NA |
| Portugal74 | 1998 | Societal perspective | Yes, all indirect costs should be identified. | NA | NA |
| Slovak75 | 2011 | Healthcare perspective | No. Only direct health costs should be included. | NA | NA |
| Slovenia76 | 2013 | Payer perspective | No. Only direct health costs should be included. | NA | NA |
| Sweden77 | 2018 | Societal perspective | Yes, all relevant indirect costs should be included. | Paid work loss (absenteeism and presenteeism); PL due to premature death | HCA and FCA |
| Switzerland78 | 2011 | Healthcare perspective | No | NA | NA |
| Czech79 | 2017 | Healthcare perspective | No | NA | NA |
| England and Wales80 | 2013 | Healthcare perspective | No, productivity costs are not included in either the reference case or non-reference case analyses. | NA | NA |
| Scotland81 | 2020 | Healthcare perspective | No | NA | NA |
| Finland82 | 2019 | Payer perspective | No. But if productivity losses are included in the cost inventory, the results should be interpreted. | Paid work loss (absenteeism and presenteeism); PL due to premature death | NA |
| Poland83 | 2016 | Payer perspective | No. Only direct health costs should be included. | NA | FCA for base case; HCA for sensitivity analysis |
| Oceania | |||||
| Australia19 | 2016 | Healthcare perspective | No, costs and outcomes that are not specifically related to ‘health and/or provision of healthcare’ should not be included in the base case. PL could be included in the supplementary analyses. | NA | NA |
| New Zealand18 | 2015 | Healthcare perspective | No, indirect patient costs should be excluded. | NA | NA |
*We included health technology assessment (HTA) guidelines by official HTA agencies and other organisations that conduct HTA within countries (eg, ICER in the USA).
†Baltic includes Latvia, Lithuania and Estonia.
AMCP, Academy of Managed Care Pharmacy; FCA, friction cost approach; HCA, human capital approach; ICER, Institute for Clinical and Economic Review; MERCOSUR, officially refers to Southern Common Market, including Argentina, Brazil, Paraguay, and Uruguay as full members, and Bolivia, Chile, Colombia, Ecuador, Guyana, Peru, and Suriname as associated countries; NA, not available; PL, productivity loss.
In contrast, 10 (21%) guidelines (in Denmark, Indonesia, Iran, Netherlands, Portugal, Spain, South Korea, Sweden, Taiwan and Thailand) recommend the use of a societal perspective to cover all the direct and indirect costs (table 1). All the guidelines suggest the inclusion of productivity loss in the cost calculation, except for the one in South Korea as it adopts a ‘limited’ societal perspective.
Two guidelines (4%) (in China and the USA) recommend two perspectives (healthcare system and societal) for economic evaluations, which could allow decision makers to have a more comprehensive overview of the impacts of indirect costs (mainly productivity loss). For example, the Institute for Clinical and Economic Review guidelines in the USA specifically suggest adopting both the healthcare system perspective and societal perspective in the base case analysis, when two conditions are satisfied: (1) the impact of the intervention on the indirect costs such as patient and caregiver’s productivity is judged to be substantial, and (2) these costs are considered large relative to the direct costs associated with the intervention.
Moreover, 11 guidelines (in Australia, Egypt, Baltic, Belgium, France, Finland, Germany, Ireland, Japan, Singapore and the USA) indicate that additional analyses (eg, scenario analysis and sensitivity analysis) with the inclusion of productivity loss are acceptable if they are perceived as important and relevant for certain interventions. To conclude, there are 23 (48%) guidelines in total accepting a societal perspective and recommending the incorporation of productivity loss in the base case or additional analyses.
Arguments in guidelines supporting inclusion or exclusion
The guidelines recommending the societal perspective and the inclusion of productivity loss provide the following justifications. First, a broader perspective could give more informative results to decision makers than a healthcare system perspective.11 12 A narrow scope of costs in consideration may bias the healthcare policies and fail to optimise the health resource allocation. Second, productivity loss is unavoidable in society, due to morbidity (reduced capability to work in the case of illness and disability) and mortality (lost capability to work in the case of premature death).13 In some specific situations such as mental diseases, the consideration of productivity costs may cause noticeable differences in cost-effectiveness results and lead to a different medical decision.5 14 15
On the contrary, other guidelines provide different reasons for excluding productivity loss. First, the decision makers may be more interested in the costs of an intervention incurred within the healthcare system.16 The inclusion of productivity loss, which commonly requires assumptions on uncertainty, may decrease the credibility of the results.17 Second, although health conditions may reduce the productivity of the affected individual, it may not necessarily lead to a decrease in productivity in the society, because the affected individual could be replaced by others.18 Third, it may be unethical to incorporate productivity loss in economic evaluations: the inclusion of productivity loss may lead to favouring those who are more ‘productive’ and neglecting those who are not paid labour force, such as children, homemakers, retired people, the unemployed and those unable to work due to disability, frailty or disease.17–19 Consequently, health resources are more likely to be allocated to the well-paid working people, which is unfair to other members of society. Fourth, the inclusion of productivity loss may cause a double-counting issue, as suggested in some guidelines.13 19 20 They explain that the changes in health-related quality of life can capture the impact of reduced productivity for the affected individual. As a result, the incorporation of productivity loss may overestimate the negative impact of health conditions.
Recommendations on measurement and valuation
Loss from paid work due to illness or premature death of patients is the primary component of productivity loss. Seven guidelines specify that the paid work loss should consist of absenteeism and presenteeism, including the guidelines from Canada, Denmark, Finland, Ireland, the Netherlands, Sweden and Thailand (table 1). Absenteeism refers to the patient’s absence from work due to the affected productivity, while presenteeism refers to the patient’s presence at work with reduced productivity.
To calculate the loss from paid work, two approaches are commonly recommended: the human capital approach and the friction cost approach. The human capital approach is recommended in five guidelines (in mainland China, Germany, Japan, Taiwan and Thailand in table 1). It multiplies the average salary rate per unit of time from the labour market with the length of time off work when the productivity is affected, sometimes plus the fringe benefits (eg, pension benefits, health and life insurance). This approach implicitly assumes the labour market is fully or nearly fully employed such that the work of the affected individual would not be replaced by other workers. However, the full employment assumption is frequently unrealistic. In the real-world context, the work of the affected individual could be replaced if other eligible workers are hired during the period when the patient is affected. It is not rare that the replacement would maintain the productivity level and prevent a significant productivity loss. In this case, the human capital approach may overestimate the productivity loss from paid work.
The friction cost approach is recommended in three guidelines (in the Netherlands, Canada and Poland in table 1). This approach assumes that when away from work, a person is eventually replaced with a previously unemployed person, so that the productivity loss occurs during the time before the affected employee is replaced, namely the friction period. The productivity loss is the product of the average salary rate multiplied by the length of the friction period. The friction period varies across countries. For example, it is specified as 85 days in the Netherlands, 3 months in Poland and 2–6 months in Belgium. The advocates of this approach argue that when someone is away from work, productivity falls only during the friction period, and the productivity loss is minimised.
When the time off work is short, the estimates from the two approaches may have no significant difference. For longer periods of unemployment, the friction cost approach will result in a lower cost estimate compared with the human capital approach. Some guidelines support a mixed use of the two approaches. In Belgium, the human capital approach is recommended for a short-term productivity loss and the friction cost approach is recommended for a long-term absence from work or death. In Germany, the human capital approach is suggested for the base case analysis, while the friction cost approach is recommended for the sensitivity analyses. Poland has a contrary suggestion that the friction cost approach should be used in the base case and the human capital approach in the sensitivity analysis.
In the calculation of productivity loss, the salary rate is determined by the gross domestic product per capita, adjusted by the marginal productivity in different countries. For example, the rate is determined at €257 per working day in Belgium and €34.75 per working hour in the Netherlands. The average salary rate, regardless of economic sector, age and gender, is recommended in five guidelines (in Japan, Germany, Sweden, Belgium and the Netherlands in table 1) to prevent the prioritisation of some subgroups of people in the economic evaluation. Only the guidelines in Denmark recommend the use of the average salary rate adjusted by age and sex.
Loss from unpaid work (eg, housework and voluntary job) is another component of productivity loss. The guidelines in Canada, the Netherlands and the USA (from the Institute for Clinical and Economic Review and the Second Panel on Cost-Effectiveness in Health and Medicine) explicitly suggest the inclusion of unpaid work loss (table 1). The guidelines in Belgium recommend presenting the unpaid working days in economic evaluations but not including unpaid work loss in the cost estimates.16 The ‘mixed’ recommendation is because there is no consensus between guidelines on how to measure the value of unpaid work. The valuation difficulty is also the reason why other guidelines recommend an exclusion approach in the base case. However, the exclusion may be questioned in situations where the affected length is considerably long, for example, rheumatoid arthritis.
Two common methods could facilitate the measurement of productivity loss from unpaid work, the opportunity cost approach and the replacement cost approach. The former approach is based on forgone wage, while the latter approach assesses the costs of purchasing the service (eg, housekeeping) by the informal caregiver or patient. Very few guidelines except that in Canada make recommendations related to the measurement of unpaid work. The Canadian guidelines recommend the opportunity cost method, as this approach values time spent on unpaid work based on the value of spending this time in an alternative capacity (eg, paid work) rather than relying on the value of a market substitute (eg, hired housekeeper). This approach is less likely to bias the estimation of the value of unpaid work by the patient and informal caregivers.
Incorporation of productivity loss in practice
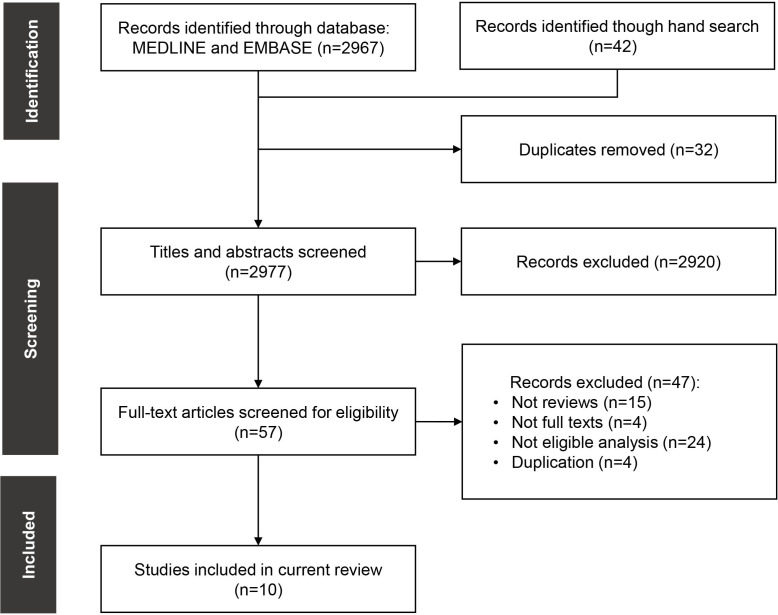
A total of 10 systematic reviews, published in the last decade (ie, 2011–2021), were finally included and reviewed for information retrieval (figure 1). Five reviews included different types of diseases, while the other five focused on specific disease categories (two in depression, one in rheumatic and musculoskeletal diseases, one in diabetes and one in rare diseases). Table 2 presents the reviews and associated information retrieved from them. Among the three reviews recruiting economic evaluations without a specific focus on the incorporation of productivity loss (ie, Krol et al 2011, Krol et al 2016 and Aranda-Reneo et al 2021 in table 2), the average probability of incorporating productivity loss was 10.2%. If we excluded one review focusing on depressive disorders where productivity loss was frequently considered,5 the probability dropped to 6.8%. Even if we removed the duplicates between the two reviews, the probability would be no greater than 9% (ie, the frequency in Krol et al 2016). In a summary made by Krol and colleagues in 2013,21 191 out of 1695 (11.3%) economic evolutions considered productivity loss. The probability remains low after decades of debate on this issue. It could be partly explained by the fact that most (69%) official guidelines recommend the healthcare system or payer’s perspective, with which social costs such as productivity loss are usually excluded.
Figure 1.
The selection process of eligible studies.
Table 2.
An overview of the inclusion of productivity loss in economic evaluations and its impact, with information retrieved from the identified reviews in the last decade (2011–2021)
| Review study | Topic | Total no. of studies in the review | No. and % studies including PL* | Identification* | Valuation approach* | % PL in total costs | Impact of including PL | |
| Paid work | Unpaid work | |||||||
| Krol et al (2011)84 | Depression | 81 | 25 (31) | Ab (n=25) Pr (n=2) |
n=1 | HCA (n=24) FCA (n=1) |
HCA: 61% FCA: 56% |
Incremental costs changed: decreased in 43 cases, increased in 16 cases and remained equal in two cases. |
| Krol et al (2016)85 | Expensive drugs | 249 | 22 (9) | Ab (n=22) Pr (n=0) |
n=1 | HCA (n=22) FCA and HCA (n=3) |
HCA: 45% FCA: 24% |
11 out of 36 ICERs (31%) altered decision making based on a fixed €40 000 threshold. |
| Kigozi et al86 | Not specific | 46 | 46 (100)‡ | Ab (n=46) Pr (n=1) |
n=15 | FCA (n=46)† | NA | NA |
| Kigozi et al (2017)87 | Not specific | 28 | 28 (100)‡ | Ab (n=15) Pr (n=28) |
Not considered | HCA (n=26) FCA (n=1) Not stated (n=1) |
NA | Presenteeism costs averagely comprised 52% of the total costs. The proportion was the highest in rheumatoid arthritis, back pain and insomnia conditions. |
| Jones et al (2019)88 | Rheumatic and musculoskeletal diseases | 21 | 21 (100)‡ | Ab (n=21) Pr (n=5) |
n=3 | HCA (n=12) FCA (n=5) |
NA | NA |
| Duevel et al (2020)14 | Depression | 50 | 50 (100)‡ | Ab (n=50) Pr (n=13) |
NA | HCA (n=18) FCA (n=15) |
NA | 22 studies (24%) witnessed the change in the CE quadrant when the societal perspective was applied. In nine studies, the inclusion of PL changed the decision making. |
| Rodriguez-Sanchez et al (2021)89 | Diabetes | 47 | 45 (96)‡ | Ab (n=30) Pr (n=5) |
n=3 | HCA (n=34) FCA (n=4) HCA and FCA (n=1) |
NA | Eight estimations from seven studies changed conclusions. The inclusion in six estimations led to the intervention becoming cost effective. |
| Aranda-Reneo et al (2021)90 | Rare diseases | 249 | 12 (5) | Ab (n=11) Pr (n=1) |
Not considered | HCA (n=11) | NA | One study led to changes in the conclusions. |
| Yuasa et al (2021)91 | Vaccines | 88 | 71 (81)‡ | Ab (n=70) Pr (n=3) |
Not considered | HCA (n=16) FCA (n=8) |
NA | 76 studies reported the impact of including PL on ICER. 71 (93%) studies reported more favourable ICERs with the inclusion of productivity losses. |
| Yuasa et al (2021)22 | Not specific | 208 | 208 (100)‡ | Ab (n=159) Pr (n=30) |
Not considered | HCA (n=43) FCA (n=9) |
NA | 110 of 144 studies reported more favourable ICERs with the inclusion of productivity losses. |
*In some review studies, the authors did not specify the type of productivity loss from paid work (ie, absenteeism and presenteeism) and unpaid work and the valuation approaches of some included cost-effectiveness analysis (CEA) studies, because the authors of the CEAs did not identify the types and approaches. It leads to a possibility that the number of studies including productivity loss is larger than the sum of studies identified by absenteeism and presenteeism, or larger than the sum of studies using HCA and FCA.
†Kigozi et al (2016) investigated only the studies that estimated productivity costs using the friction cost approach.
‡The selection criteria of these reviews included the incorporation of productivity losses or social costs in the economic evaluations, such that almost all studies included considered at least one aspect of productivity loss.
Ab, absenteeism; CE, cost-effectiveness; FCA, friction cost approach; HCA, human capital approach; ICER, incremental cost-effectiveness ratio; NA, not available; PL, productivity loss; Pr, presenteeism.
Among all the economic evaluations (n=478) that considered productivity loss, most studies (n=455) considered productivity losses from paid work, while few studies (n=23) considered unpaid work losses. Among those incorporating paid work losses, the vast majority of studies (n=399, average probability=83.5%) incorporated absenteeism, while only 75 studies (average probability=15.7%) considered presenteeism. The human capital approach was more often used than the friction cost approach (n=192 vs. n=78). As indicated by two reviews,3 5 the use of the human capital approach might result in a higher estimate of productivity loss compared with the use of the friction cost approach in terms of the percentage of productivity loss in total costs.
The impact of including productivity loss varied among the economic evaluations. The inclusion may result in an increase or decrease of the incremental costs and thus alter the decision making. A recent review found that, in 71 out of 76 economic evaluations on vaccines, the inclusion of productivity loss led to more favourable ICERs.15 Another recent review found that, in 110 out of 144 studies on medical interventions except for vaccines, the inclusion led to more favourable ICERs.22 The two reviews implied that the inclusion of productivity loss would probably lead to more favourable ICERs, although the inclusion may change the ICERs in both directions theoretically.
Table 3 presents a list of diseases where researchers would probably consider the productivity losses in economic evaluations. The list consists of seven diseases, including rheumatoid arthritis, shoulder osteoarthritis, chronic musculoskeletal pain, depression, coronary artery disease, laryngeal cancer and influenza. All the diseases might cause significant productivity loss to society, accounting for at least 50% of the total costs. The largest number appeared in chronic musculoskeletal pain. Reneman et al23 found that the proportion caused by this disease accounted for 85% of the total cost. Schwarzkopf et al24 found that, in the scenario of depression, the proportion of productivity loss in the total cost could be as high as 81%. Two studies found that productivity loss caused by rheumatoid arthritis accounted for 76% and 54% of the total costs, respectively.25 26 Even influenza, a common infectious disease, might cause significant productivity loss, accounting for about 57% of the total cost.27
Table 3.
A list of diseases where researchers are likely to consider the associated productivity losses in economic evaluations
| Typical disease | Source | Proportion of productivity loss in the total cost (%) |
| Rheumatoid arthritis | Nathalie et al25 | 76 |
| Mulligen et al26 | 54 | |
| Shoulder osteoarthritis | Grobet et al92 | 64 |
| Chronic musculoskeletal pain | Reneman et al23 | 85 |
| Depression | Schwarzkopf et al24 | 81 |
| Brettschneider et al93 | 58 | |
| Coronary artery disease | Brouwers et al94 | 53 |
| Laryngeal cancer | Johansson et al95 | 73 |
| Influenza | Kohli et al27 | 57 |
Methodological challenges
Measurement of paid work
Many instruments for the measurement of productivity loss from paid work have been developed.4 Selecting an appropriate instrument, however, remains challenging.4 28 Among the reviews we scrutinised, we found that the original economic evaluations used different instruments, which led to significant heterogeneity in outcomes. The inconsistency in selecting instruments and the heterogeneous outcomes are concerning, as they may threaten the validity and comparability of incorporating productivity loss in economic evaluations. The first cause of the inconsistency is the lack of standardisation of measurement methods for losses from paid work.4 For example, when using the friction cost approach, researchers do not have a consensus on how to measure the duration and frequency of friction periods. Second, most measurement instruments were designed for data collection from the patients directly, without considering the possibility that many patients have limited/no capability to answer the questions due to mental or physical conditions. In practice, the ignorance of these patients may bias the estimates, and the various approaches to deploying agents for these patients may further add to the inconsistency. Third, most instruments focus on either absenteeism or presenteeism, reflecting either time or capacity loss in paid work, while both should be accounted for but few did for both.28–30 If researchers select an instrument from dozens of comparators (eg, 42 unique instruments identified by a previous review)4 without a consensus on the best choice, inconsistency occurs. Therefore, we conclude that the current instruments for productivity loss from paid work are not always appropriate for economic evaluations. It is similar to the findings in a previous review.4
Work quality
Health conditions have impacts on both the quantity and quality of work. Employees with diseases may be absent from work more frequently (ie, quantity) and perform worse (ie, quality) than usual time. Although the concept of presenteeism aims to capture the impacts of health conditions on work quality, its measurement is challenging, if not impossible, because of the extremely heterogeneous working contexts. We found some attempts in the literature trying to directly measure the change in work quality, for example, the Quantity and Quality instrument (QQ) and the Work Limitation Questionnaire (WLQ).31–33 The QQ instrument asks patients to rate their work quality on recent workdays compared with that on ordinary days. The WLQ asks patients about their recent work performances such as the difficulty in doing work carefully without making mistakes. We also identified instruments using an indirect approach to measure the change in work quality, for example, the Health and Labour Questionnaire,34 and the Work Productivity and Activity Impairment Questionnaire.35 These instruments ask patients to translate the quality change into time loss in paid work and then monetary equivalent. No consensus exists on which approach (ie, direct vs indirect) is more reliable and which instrument provides the best estimate on presenteeism. More research is needed to examine the validity and reliability of different approaches that measure the impact of health conditions on work quality.
Measurement frequency
From the studies we scanned, we found that it was difficult to determine the most appropriate frequency for the measurement of productivity loss. When the losses were measured more frequently, patients were more likely to recall their memories precisely. Meanwhile, the survey costs would increase, and the compliance of respondents would decrease. Since the valuation of productivity loss is unlikely made on a single measurement, the ideal frequency of measurement should be determined by balancing the loss in precision and the increase in survey cost and patient burden. So far, no studies have investigated the effect of different frequencies on response accuracy and patient burden. Further research should fill the gap and investigate the approach to determine the best frequency in different contexts.
Objective measures
Objective measures of productivity loss come from the accumulation of real-world data in the working contexts, for example, the records of sick leave and the rating of working performance. In our review, we found no studies applied objective measures. It may be because the reliability of objective measures for productivity loss measurement has not been tested.21 32
We noticed one previous empirical study used objective measures to validate the subjective measures (ie, self-reported questionnaires).36 Validating the subjective instruments might be a workable way to use the objective measures. However, similar attempts have been rare in the literature, and there are no generalisable approaches to guide the validation between objective and subjective measures. Research is needed to investigate the feasibility and validity of using objective measures.
Measurement and valuation of unpaid work
Unpaid work usually includes household work, voluntary work, childcare, etc. The time spent on unpaid work varies significantly between individuals and families. Our review found that very few economic evaluations incorporated productivity losses from unpaid work. It is possibly because very few instruments have been developed for the measurement of unpaid work losses.4 Consequently, this type of loss has very limited influence on decision making.
Although two common methods are available for estimating productivity losses from unpaid work (ie, opportunity cost and replacement cost), both methods have drawbacks in practice that are difficult to overcome. For example, both require the patients to recall how much time they usually spend on unpaid work (eg, household work) when they are healthy. However, this recall is usually imprecise, especially when the recall period is long and/or the responses are from those with limited memory capacity. So far, little is known about how to precisely capture losses from unpaid work and relevant research is needed for extensive investigation.
Discussion
Research agenda in China
In this review, we summarised the recommendations of HTA guidelines in different countries and regions on the inclusion of productivity loss in economic evaluations and how frequently the losses were incorporated in the evaluation practices. We noticed the difficulties in maintaining the comparability and transparency of the measurement and valuation of productivity loss by identifying several (out of many) unresolved methodological issues. The most urgent task is to address the lack of standardisation and guidance regarding the inclusion of productivity loss. Other tasks include measuring unpaid work losses, developing comprehensive instruments covering both absenteeism and presenteeism in paid and unpaid work, investigating the appropriate measurement frequency for different types of productivity loss, exploring the feasibility of objective measures and achieving consensus on using the measurement methods such as the friction cost and replacement cost approaches.
Since the societal perspective is gaining increasing support from the Chinese health economists in the postpandemic era,7 8 the consideration of productivity loss in economic evaluations becomes preferable. However, due to the lack of fundamental research infrastructures such as established data sets and validated questionnaires in China, the research progresses in China on productivity loss is challenging and no basis exists for adequately resolving the methodological issues we identified from the systematic reviews. At this stage, a realistic solution for the Chinese is to bypass the existing methodological challenges temporarily and learn from the guidelines of other countries and the mature methods for the identification, measurement and valuation of productivity loss. The Chinese health economists may also conduct explorative studies to develop measurement and valuation methods suitable for China. By acknowledging the specific methodological and ethical challenges8 37 and the recent efforts in defining a clear cost inventory in economic evaluations for the Chinese population,38–40 we propose a down-to-earth research agenda in China to facilitate the advancement of research on productivity loss.
Developing a disease list
The Chinese health economists could establish a consensus on the list of diseases where productivity loss should be incorporated in economic evaluations to avoid underestimating total costs to society. We have proposed a brief list (table 3) from this review and call for attention to rheumatoid arthritis, shoulder osteoarthritis, chronic musculoskeletal pain, depression, coronary artery disease, laryngeal cancer and influenza. This is not a complete list, however. Future research should screen the economic evaluation literature for a complete list of diseases, identify the proportion of productivity loss caused by the diseases in the total costs and examine whether the incorporation of productivity loss alters the decision making in disease-specific scenarios. If the productivity losses caused by the diseases are large enough to influence the decision making, economic evaluations targeting these diseases should consider productivity loss. To develop such a disease list, explicit inclusion and exclusion criteria should be established. The rationale should be provided in the list that, without the incorporation of productivity loss, how the total cost may be underestimated. Also, a dynamic list is recommended, which could be updated regularly to account for the possibility that some health conditions cause time-varying productivity losses to society.
From specific to generic instruments
Chinese health economists may start with developing disease-specific questionnaires for productivity loss measurement. Although many instruments in current practice are generic, we found that disease-specific questionnaires are commonly used in the literature. They are preferable because patients are more familiar with the questions tailored to the disease context and it is easier for them to translate the questions into a reliable assessment of the losses from work.2 21 However, Chinese economists should keep in mind that the losses from work are from the disease itself and the impact of its comorbidities.2 The use of specific questionnaires may therefore underestimate the overall productivity losses incurred by the disease as they usually cannot capture the impacts of comorbidities. Therefore, the pros and cons of the specific questionnaires should be adequately recognised, which may pave the way for the establishment of generic instruments in China.
Quality of life (QoL) and productivity loss
The correlation between QoL valuation and productivity loss makes it possible to indirectly estimate the losses based on QoL measures, on occasions when it is extremely difficult to directly collect the required information for productivity loss.41 An explorative study shows promising results by establishing a prediction model using EQ-5D data.42 Since the Chinese health economists are familiar with the application of established QoL instruments among the Chinese population,43 44 they could explore the correlation between productivity loss and health state valuation in China and establish a prediction model. The validation process is necessary by comparing the predicted productivity losses with the conventionally measured losses, for which a translated and validated direct measurement questionnaire is required.
It is worth noting that the indirect estimation approach has unresolved issues. For example, it is unclear how to predict productivity loss from unpaid work using QoL data, and little is known about how to tease out the concerns of double-counting when using the productivity loss predicted by QoL data. Although further studies are required to resolve these issues and validate the approach, the indirect estimation is a practical solution and preferable to ignoring the productivity loss in China where the direct measures are usually unavailable.
Instruments for China
Since the measurement of absenteeism in paid work is more frequently conducted in economic evaluations than presenteeism and unpaid work loss, the Chinese experts could translate some instruments for absenteeism and conduct validation studies among Chinese employees. If the instruments are proven valid, researchers could use them in practice directly. If they are found invalid in the Chinese context, new instruments should be developed specifically for China. It should be noted that the use of country-specific instruments should not hamper international comparability. That is, the measured productivity losses in China using the China-specific instruments should be able to be compared with the results in other countries, using some mapping algorithms. The measurement of the losses in unpaid work seems not an urgent task in China due to the existing methodological issues, but we encourage exploratory attempts.
The previous recommendations are necessary steps towards the improvement of research on productivity loss in China. Since economic evaluations have increasingly been used to inform public-funded insurance for reimbursement decisions, we are optimistic that more researchers will recognise the importance of incorporating productivity loss in economic evaluations.
Limitations
This review has several limitations. First, we primarily searched guidelines written in English and then referred to supplementary sources for guidelines not written in English. We completely depended on the supplementary sources regarding their interpretations for the latter category, but we recognise the risk of importing inaccuracy interpretations. Second, when pooling the frequencies of different reviews and calculating the average probability of an economic evaluation incorporating productivity loss, we did not remove the duplicates between different reviews. We recognise the limitation may impact the accuracy of pooled probabilities. Third, we applied an ad hoc approach of selecting the cut-off level for the establishment of a brief disease list. Though we had a thorough discussion among coauthors, we did not know the impact of using other cut-off levels. More studies are warranted to explore the impact of using different cut-off levels to determine the scenarios where the incorporation of productivity loss should be recommended.
Conclusions
This review summarised the recommendations of HTA guidelines in different countries and regions on the inclusion of productivity loss in economic evaluations and the arguments supporting inclusion and exclusion. Though most guidelines recommend excluding productivity loss in the base case analysis, an increasing number of guidelines recommend an inclusion approach in the base case or additional analyses. In the postpandemic era, a broader perspective for medical decision making is gaining an increasing supportive voice. We expect that more guidelines will shift future economic evaluations from narrower perspectives to a societal perspective or adopt a both-perspective approach, allowing the decision makers to have a more comprehensive consideration of the impact of productivity loss.
We identified the existing methodological challenges in the measurement and valuation of productivity loss that hamper the comparability and transparency of economic evaluations. Recognising these issues and the specific research context in China, we proposed a practical research agenda for the inclusion of productivity loss in health economic evaluations. We anticipate the additional guidelines that may be suitable for Chinese practitioners and decision makers that facilitate the progress on productivity loss measurement and valuation. The progress would contribute to the harmonisation of methods of including, measuring and valuing productivity loss within international studies.
Footnotes
Handling editor: Seye Abimbola
SJ and YW contributed equally.
Contributors: Conceptualisation and writing: SJ. Review and information extraction: SJ and YW. Summary of guidelines: YW. Discussion and manuscript revision: SJ, YW, XZ, YJ, GG-EL and JW. All authors approved the submitted version. SJ is the guarantor responsible for the overall content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Byford S, Raftery J. Perspectives in economic evaluation. BMJ 1998;316:1529. 10.1136/bmj.316.7143.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Bansback N, Anis AH. Measuring and valuing productivity loss due to poor health: a critical review. Soc Sci Med 2011;72:185–92. 10.1016/j.socscimed.2010.10.026 [DOI] [PubMed] [Google Scholar]
- 3.Krol M, Papenburg J, Tan SS, et al. A noticeable difference? Productivity costs related to paid and unpaid work in economic evaluations on expensive drugs. Eur J Health Econ 2016;17:391–402. 10.1007/s10198-015-0685-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubens K, Krol M, Coast J, et al. Measurement instruments of productivity loss of paid and unpaid work: a systematic review and assessment of suitability for health economic evaluations from a societal perspective. Value Health 2021;24:1686–99. 10.1016/j.jval.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 5.Krol M, Papenburg J, Koopmanschap M, et al. Do productivity costs matter? Pharmacoeconomics 2011;29:601–19. 10.2165/11539970-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 6.China Guidelines for Pharmacoeconomic Evaluations Working Group . China guidelines for pharmacoeconomic evaluations, 2020. Available: https://tools.ispor.org/PEguidelines/source/China-Guidelines-for-Pharmacoeconomic-Evaluations-2020.pdf [Accessed Oct 2021].
- 7.Jiang S, Wang Y, Zhou J, et al. Incorporating future unrelated medical costs in cost-effectiveness analysis in China. BMJ Glob Health 2021;6:e006655. 10.1136/bmjgh-2021-006655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang S, Chen Z, Wu J, et al. Addressing methodological and ethical issues in practicing health economic evaluation in China. J Glob Health 2020;10:020322. 10.7189/jogh.10.020322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorat T, Lin P-J, Neumann PJ. The state of cost-utility analyses in Asia: a systematic review. Value Health Reg Issues 2015;6:7–13. 10.1016/j.vhri.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 10.National Health Care Institute . Guideline for economic evaluations in healthcare, 2016. Available: https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare [Accessed May 2021].
- 11.Medical association of Thailand . Guidelines for health technology assessment in Thailand, 2014. Available: http://www.hitap.net/wp-content/uploads/2017/06/Thai-HTA-guideline-UPDATES-Jmed-with-Cover.pdf [Accessed Oct 2021].
- 12.Health Information and Quality Authority . Guidelines for the economic evaluation of health technologies in Ireland, 2019. Available: https://www.hiqa.ie/reports-and-publications/health-technology-assessment/guidelines-economic-evaluation-health [Accessed Oct 2021].
- 13.National board of health . Health technology assessment handbook Copenhagen: Danish centre for health technology assessment, 2007. Available: http://aaz.hr/resources/pages/57/1.%20HTA%20Handbook%20DACEHTA.pdf [Accessed Oct 2021].
- 14.Duevel JA, Hasemann L, Peña-Longobardo LM, et al. Considering the societal perspective in economic evaluations: a systematic review in the case of depression. Health Econ Rev 2020;10:32. 10.1186/s13561-020-00288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuasa A, Yonemoto N, LoPresti M, et al. Productivity loss/gain in cost-effectiveness analyses for vaccines: a systematic review. Expert Rev Pharm Out 2021;21:235–45. 10.1080/14737167.2021.1881484 [DOI] [PubMed] [Google Scholar]
- 16.Belgian Health Care Knowledge Centre . Belgian guidelines for economic evaluations and budget impact analyses: second edition, 2015. Available: https://kce.fgov.be/en/belgian-guidelines-for-economic-evaluations-and-budget-impact-analyses-second-edition [Accessed Oct 2021].
- 17.Institute for Clinical and Economic Review . ICER’s base case for economic evaluations: principles and rationale, 2020. Available: https://icer.org/wp-content/uploads/2020/10/ICER_Reference_Case_013120.pdf [Accessed Oct 2021].
- 18.Pharmaceutical Management Agency . Prescription for pharmacoeconomic analysis - methods for cost-utility analysis, 2015. Available: https://www.pharmac.govt.nz/medicines/how-medicines-are-funded/economic-analysis/pfpa/ [Accessed Oct 2021].
- 19.Pharmaceutical Benefits Advisory Committee . Guidelines for preparing a submission to the pharmaceutical benefits Advisory Committee version 5.0, 2016. Available: https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf [Accessed Oct 2021].
- 20.The Canadian Agency for Drugs and Technologies in Health . Guidelines for the economic evaluation of health technologies: Canada 4th edition, 2017. Available: https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada [Accessed Oct 2021].
- 21.Krol M, Brouwer W, Rutten F. Productivity costs in economic evaluations: past, present, future. Pharmacoeconomics 2013;31:537–49. 10.1007/s40273-013-0056-3 [DOI] [PubMed] [Google Scholar]
- 22.Yuasa A, Yonemoto N, LoPresti M, et al. Use of productivity loss/gain in cost-effectiveness analyses for drugs: a systematic review. Pharmacoeconomics 2021;39:81–97. 10.1007/s40273-020-00986-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reneman MF, Beemster TT, Welling SJ, et al. Vocational rehabilitation for patients with chronic musculoskeletal pain with or without a work module: an economic evaluation. J Occup Rehabil 2021;31:84–91. 10.1007/s10926-020-09921-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarzkopf L, Dorscht L, Kraus L, et al. Is bouldering-psychotherapy a cost-effective way to treat depression when compared to group cognitive behavioral therapy - results from a randomized controlled trial. BMC Health Serv Res 2021;21:1162. 10.1186/s12913-021-07153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathalie L-M, Mulligen VE, Maria WAEA, et al. Comparing cost-utility of DMARDs in autoantibody-negative rheumatoid arthritis patients. Rheumatology 2021;60:keab251 10.1093/rheumatology/keab251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Mulligen E, Weel AE, Kuijper TM, et al. Two-year cost effectiveness between two gradual tapering strategies in rheumatoid arthritis: cost-utility analysis of the TARA trial. Ann Rheum Dis 2020;79:1550–6. 10.1136/annrheumdis-2020-217528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohli MA, Maschio M, Mould-Quevedo JF, et al. The cost-effectiveness of expanding vaccination with a cell-based influenza vaccine to low risk adults aged 50 to 64 years in the United Kingdom. Vaccines 2021;9:598. 10.3390/vaccines9060598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad M, Wahlqvist P, Shikiar R, et al. A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics 2004;22:225–44. 10.2165/00019053-200422040-00002 [DOI] [PubMed] [Google Scholar]
- 29.Mattke S, Balakrishnan A, Bergamo G, et al. A review of methods to measure health-related productivity loss. Am J Manag Care 2007;13:211–7. [PubMed] [Google Scholar]
- 30.Lofland JH, Pizzi L, Frick KD. A review of health-related workplace productivity loss instruments. Pharmacoeconomics 2004;22:165–84. 10.2165/00019053-200422030-00003 [DOI] [PubMed] [Google Scholar]
- 31.Koopmanschap M, Burdorf A, Jacob K, et al. Measuring productivity changes in economic evaluation. Pharmacoeconomics 2005;23:47–54. 10.2165/00019053-200523010-00004 [DOI] [PubMed] [Google Scholar]
- 32.Meerding WJ, IJzelenberg W, Koopmanschap MA, et al. Health problems lead to considerable productivity loss at work among workers with high physical load jobs. J Clin Epidemiol 2005;58:517–23. 10.1016/j.jclinepi.2004.06.016 [DOI] [PubMed] [Google Scholar]
- 33.Lerner D, Reed JI, Massarotti E, et al. The work limitations questionnaire's validity and reliability among patients with osteoarthritis. J Clin Epidemiol 2002;55:197–208. 10.1016/s0895-4356(01)00424-3 [DOI] [PubMed] [Google Scholar]
- 34.van Roijen L, Essink-Bot ML, Koopmanschap MA, et al. Labor and health status in economic evaluation of health care. the health and labor questionnaire. Int J Technol Assess Health Care 1996;12:405–15. 10.1017/S0266462300009764 [DOI] [PubMed] [Google Scholar]
- 35.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–65. 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 36.Lerner D, Amick BC, Lee JC, et al. Relationship of employee-reported work limitations to work productivity. Med Care 2003;41:649–59. 10.1097/01.MLR.0000062551.76504.A9 [DOI] [PubMed] [Google Scholar]
- 37.Chen Z, Jiang S, Wang Y, et al. Pharmacoeconomics of obesity in China: a scoping review. Expert Rev Pharmacoecon Outcomes Res 2021;21:173–81. 10.1080/14737167.2021.1882306 [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Cai D, Chen D. Economic evaluation of remdesivir for the treatment of severe COVID‐19 patients in China under different scenarios. Brit J Clin Pharmaco 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y, Cai D, Chen D, et al. The cost-effectiveness of conducting three versus two reverse transcription-polymerase chain reaction tests for diagnosing and discharging people with COVID-19: evidence from the epidemic in Wuhan, China. BMJ Glob Health 2020;5:e002690. 10.1136/bmjgh-2020-002690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, Jiang S, Ni W. Burden of cardiovascular diseases associated with fine particulate matter in Beijing, China: an economic modelling study. BMJ Glob Health 2020;5:e003160. 10.1136/bmjgh-2020-003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamers LM, Meerding W-J, Severens JL, et al. The relationship between productivity and health-related quality of life: an empirical exploration in persons with low back pain. Qual Life Res 2005;14:805–13. 10.1007/s11136-004-0800-4 [DOI] [PubMed] [Google Scholar]
- 42.Krol M, Stolk E, Brouwer W. Predicting productivity based on EQ-5D: an explorative study. Eur J Health Econ 2014;15:465–75. 10.1007/s10198-013-0487-y [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Xie S, He X, et al. Valuation of SF-6Dv2 health states in China using time trade-off and discrete-choice experiment with a duration dimension. Pharmacoeconomics 2021;39:521–35. 10.1007/s40273-020-00997-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang F, Jiang S, X-n H. Do rural residents in China understand EQ-5D-5L as intended? Evidence from a qualitative study. Pharmacoeconomics - Open 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Department of Health . Guidelines for pharmacoeconomic evaluations of medicines and scheduled substances, 2013. Available: https://tools.ispor.org/PEguidelines/source/PEGazette_February2013_SouthAfrica.pdf [Accessed Oct 2021].
- 46.Elsisi GH, Kaló Z, Eldessouki R, et al. Recommendations for reporting pharmacoeconomic evaluations in Egypt. Value Health Reg Issues 2013;2:319–27. 10.1016/j.vhri.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 47.Brazil Ministry of Health . Methodological guidelines: economic evaluation of health technologies, 2014. Available: https://tools.ispor.org/PEguidelines/source/Avaliacao_Economica_Brazil2014.pdf [Accessed Oct 2021].
- 48.Institute of Health Technology Assessment . Manual para La elaboración de evaluaciones económicas en salud, 2014. Available: http://www.iets.org.co/Archivos/64/Manual_evaluacion_economica.pdf [Accessed Oct 2021].
- 49.Cuba Ministry of Health . Methodological guidelines for health economic evaluation, 2003. Available: https://tools.ispor.org/PEguidelines/source/Methodological-Guidelines-for-Health-Economic-Evaluations-in-Cuba.pdf [Accessed Oct 2021].
- 50.General Health Council . Economic assessment study guideline for updating the National formulary in Mexico, 2015. Available: http://www.csg.gob.mx/descargas/pdfs/2015/GCEEE_2015.pdf [Accessed Oct 2021].
- 51.Common Market of the Southern Cone (MERCOSUR) . Guideline for economic evaluation of health technologies, 2015. Available: https://www.mercosur.int/ [Accessed Oct 2021].
- 52.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093–103. 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 53.Academy of Managed Care Pharmacy . AMCP format for formulary submissions guidance on submission of pre-approval and post-approval clinical and economic information and evidence, 2020. Available: https://www.amcp.org/sites/default/files/2019-12/AMCP_Format%204.1_1219_final.pdf [Accessed Oct 2021].
- 54.Center for Drug Evaluation . Guidelines of methodological standards for pharmacoeconomic evaluations version 1.1, 2008. Available: https://www3.cde.org.tw/Content/Files/HTA/3/7_PE%E8%A9%95%E4%BC%B0%E6%96%B9%E6%B3%95%E5%AD%B8%E6%8C%87%E5%8D%97.pdf [Accessed Oct 2021].
- 55.Study team for “establishing evaluation methods, data standardization, and assessment systems toward the application of economic evaluation of healthcare technologies to governmental policies”. guideline for preparing cost-effectiveness evaluation to the central social insurance medical council, 2019. Available: https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf [Accessed Oct 2021].
- 56.Ministry of Health . Pharmacoeconomic guidelines for Malaysia second edition, 2019. Available: https://www.pharmacy.gov.my/v2/en/documents/pharmacoeconomic-guideline-malaysia-2nd-edition.html [Accessed Oct 2021].
- 57.Bae S, Lee S, Bae EY, et al. Korean guidelines for pharmacoeconomic evaluation (second and updated version) : consensus and compromise. Pharmacoeconomics 2013;31:257–67. 10.1007/s40273-012-0021-6 [DOI] [PubMed] [Google Scholar]
- 58.Iran FDA, Medicine Selecting Committee Secretriate . Criteria for developing an economic evaluation file - 2017 to 2019. Available: https://tools.ispor.org/PEguidelines/countrydet.asp?c=48&t=2 [Accessed Oct 2021].
- 59.Pharmaceutical Administration of the Ministry of Health . Guidelines for the submission of a request to include a pharmaceutical product in the national list of health services, 2010. Available: https://tools.ispor.org/PEguidelines/source/Israel-Guidelines-for-submission_2010.pdf [Accessed Oct 2021].
- 60.Indonesian Health Technology Assessment Committee (InaHTAC) . Health Technology Assessment (HTA) guideline, 2017. Available: http://adphealth.org [Accessed Oct 2021].
- 61.Department of Health - Philippines . Philippine methods guide for health technology assessment, 2020. Available: https://hta.doh.gov.ph/philippine-hta-methods-guide/ [Accessed Oct 2021].
- 62.Agency for Care Effectiveness . Drug evaluation methods & process guide, 2019. Available: https://www.ace-hta.gov.sg/resources/process-methods [Accessed Oct 2021].
- 63.Institute for Pharmaeconomic Research (IPF) in Cooperation with An Expert Group . Guidelines on health economic evaluation consensus paper, 2006. Available: https://tools.ispor.org/PEguidelines/source/Guidelines_Austria.pdf [Accessed Oct 2021].
- 64.National Institute of Pharmacy and Nutrition . Professional healthcare guideline on the methodology of health technology assessment, 2017. Available: https://tools.ispor.org/PEguidelines/source/HTA_Guideline_HUN_eng.pdf [Accessed Oct 2021].
- 65.Italian Medicines Agency . Guidance to applicants for the submission of pharmacoeconomic analysis within the pricing and reimbursement dossier, 2020. Available: https://www.aifa.gov.it/documents/20142/1028586/Guidance_pharmacoeconomic_analyses_UVE_24.7.2020.pdf/98f7b3fc-b705-fc81-5a45-31bf9cc513e8 [Accessed Oct 2021].
- 66.Center for Healthcare Quality Assessment and Control of the Ministry of Health of the Russian Federation . Guidelines for conducting a comparative clinical and economic evaluation of drugs, 2016. Available: https://rosmedex.ru/wp-content/uploads/2016/12/MR-KE%60I-23.12.2016.pdf [Accessed Oct 2021].
- 67.Canary Islands Health Services, Castilla La Mancha University, La Rioja University, Public Health Agency of Barcelona, Vic University, Alto Deba Hospital & Pompeu Fabra University Spanish . Recommendations on economic evaluation of health technologies, 2010. Available: https://tools.ispor.org/PEguidelines/source/Spain-Guidelines-Abstract_English-Version-2010.pdf [Accessed Oct 2021].
- 68.Agency for Quality and Accreditation in Health Care . The Croatian guideline for health technology assessment process and reporting, 2011. Available: http://www.aaz.hr/sites/default/files/hrvatske_smjernice_za_procjenu_zdravstvenih_tehnologija.pdf [Accessed Oct 2021].
- 69.Experts from health authorities of the Baltic countries . Baltic guideline for economic evaluation of pharmaceuticals (pharmacoeconomic analysis), 2002. Available: https://tools.ispor.org/PEguidelines/source/Baltic-PE-guideline.pdf [Accessed Oct 2021].
- 70.Commission for Economic Evaluation and Public Health . Choices in methods for economic evaluation-HAS, 2020. Available: https://www.has-sante.fr/jcms/r_1499251/en/choices-in-methods-for-economic-evaluation [Accessed Oct 2021].
- 71.German national institute for quality and efficiency in health care (IQWiG) . Working paper cost estimation, 2009. Available: https://tools.ispor.org/PEguidelines/source/Germany_WorkPaperCostEst.pdf [Accessed Oct 2021].
- 72.National Health Care Institute . Guideline for economic evaluations in healthcare, 2016. Available: https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare [Accessed Oct 2021].
- 73.Norwegian Medicines Agency . Guidelines for the submission of documentation for single technology assessment (STA) of pharmaceuticals, 2018. Available: https://legemiddelverket.no/Documents/English/Public%20funding%20and%20pricing/Documentation%20for%20STA/Guidelines%20151018.pdf [Accessed Oct 2021].
- 74.Ministry of health . Guidelines for economic drug evaluation studies, 1998. Available: https://tools.ispor.org/PEguidelines/source/PE%20guidelines%20in%20English_Portugal.pdf [Accessed Oct 2021].
- 75.Ministry of Health of the Slovak Republic . Guidelines for economic evaluation of health care interventions, 2011. Available: https://www.zakonypreludi.sk/zz/2011-422 [Accessed Oct 2021].
- 76.Health Insurance Institute of Slovenia . Rules on the classification of medicine on the list, 2013. Available: https://www.uradni-list.si/glasilo-uradni-list-rs/vsebina/112932 [Accessed Oct 2021].
- 77.Swedish Council on Health Technology Assessment . Assessment of methods in health care and social services: a handbook, 2018. Available: https://www.sbu.se/contentassets/76adf07e270c48efaf67e3b560b7c59c/eng_metodboken.pdf [Accessed Oct 2021]. [PubMed]
- 78.Swiss Federal Office of Public Health (BAG) . Document Operationalisierung Der Begriffe Wirksamkeit, Zweckmässigkeit und Wirtschaftlichkeit, 2011. Available: https://tools.ispor.org/peguidelines/countrydet.asp?c=25&t=1 [Accessed Oct 2021].
- 79.State Institute for Drug Control . Cost-effectiveness guidelines, 2017. Available: http://www.sukl.cz/file/85788_1_1 [Accessed Oct 2021].
- 80.National Institute for Health and Care Excellence . Guide to the methods of technology appraisal, 2013. Available: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 [Accessed Oct 2021]. [PubMed]
- 81.Scottish Medicines Consortium . Guidance to submitting companies for completion of new product assessment form, 2020. Available: https://www.scottishmedicines.org.uk/media/4990/guidance-supplement-ultra-orphan-updated-011119.pdf [Accessed Oct 2021].
- 82.Health Insurance Review and Assessment Service . Preparing a health economic evaluation to be attached to the application for reimbursement status and wholesale price for a medicinal product, 2019. Available: https://www.hila.fi/content/uploads/2020/01/Instructions_TTS_2019.pdf [Accessed Oct 2021].
- 83.The Agency for Health Technology Assessment and Tariff System . Health technology assessment guidelines version 3.0, 2016. Available: https://www.aotm.gov.pl/www/wp-content/uploads/wytyczne_hta/2016/20161104_HTA_Guidelines_AOTMiT.pdf [Accessed Oct 2021].
- 84.Krol M, Papenburg J, Koopmanschap M, et al. Do productivity costs matter?: the impact of including productivity costs on the incremental costs of interventions targeted at depressive disorders. Pharmacoeconomics 2011;29:601–19. 10.2165/11539970-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 85.Krol M, Papenburg J, Tan SS, et al. A noticeable difference? Productivity costs related to paid and unpaid work in economic evaluations on expensive drugs. Eur J Health Econ 2016;17:391–402. 10.1007/s10198-015-0685-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kigozi J, Jowett S, Lewis M, et al. Estimating productivity costs using the friction cost approach in practice: a systematic review. Eur J Health Econ 2016;17:31–44. 10.1007/s10198-014-0652-y [DOI] [PubMed] [Google Scholar]
- 87.Kigozi J, Jowett S, Lewis M, et al. The estimation and inclusion of Presenteeism costs in applied economic evaluation: a systematic review. Value Health 2017;20:496–506. 10.1016/j.jval.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 88.Jones C, Verstappen SMM, Payne K. A systematic review of productivity in economic evaluations of workplace interventions: a need for reporting criteria? Appl Health Econ Health Policy 2019;17:591–613. 10.1007/s40258-019-00473-8 [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez-Sanchez B, Aranda-Reneo I, Oliva-Moreno J, et al. Assessing the effect of including social costs in economic evaluations of diabetes-related interventions: a systematic review. Clinicoecon Outcomes Res 2021;13:307–34. 10.2147/CEOR.S301589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aranda-Reneo I, Rodríguez-Sánchez B, Peña-Longobardo LM, et al. Can the consideration of societal costs change the recommendation of economic evaluations in the field of rare diseases? an empirical analysis. Value Health 2021;24:431–42. 10.1016/j.jval.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 91.Yuasa A, Yonemoto N, LoPresti M, et al. Productivity loss/gain in cost-effectiveness analyses for vaccines: a systematic review. Expert Rev Pharmacoecon Outcomes Res 2021;21:235–45. 10.1080/14737167.2021.1881484 [DOI] [PubMed] [Google Scholar]
- 92.Grobet CE, Glanzmann MC, Eichler K, et al. Cost-utility analysis of total shoulder arthroplasty: a prospective health economic study using real-world data. J Shoulder Elbow Surg 2021;30:1998–2006. 10.1016/j.jse.2021.03.136 [DOI] [PubMed] [Google Scholar]
- 93.Brettschneider C, Heddaeus D, Steinmann M, et al. Cost-effectiveness of guideline-based stepped and collaborative care versus treatment as usual for patients with depression - a cluster-randomized trial. BMC Psychiatry 2020;20:427. 10.1186/s12888-020-02829-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brouwers RWM, van der Poort EKJ, Kemps HMC, et al. Cost-Effectiveness of cardiac Telerehabilitation with relapse prevention for the treatment of patients with coronary artery disease in the Netherlands. JAMA Netw Open 2021;4:e2136652. 10.1001/jamanetworkopen.2021.36652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johansson M, Finizia C, Persson J, et al. Cost-effectiveness analysis of voice rehabilitation for patients with laryngeal cancer: a randomized controlled study. Support Care Cancer 2020;28:5203–11. 10.1007/s00520-020-05362-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.