Abstract
Introduction
The aim of this study was to determine the psychometric properties of the 12-Item Hypoglycemia Impact Profile (HIP12), a brief measure of the impact of hypoglycemia on quality of life (QoL) among adults with type 1 (T1D) or type 2 diabetes (T2D).
Research design and methods
Adults with T1D (n=1071) or T2D (n=194) participating in the multicountry, online study, ‘Your SAY: Hypoglycemia’, completed the HIP12. Psychometric analyses were undertaken to determine acceptability, structural validity, internal consistency, convergent/divergent validity, and known-groups validity.
Results
Most (98%) participants completed all items on the HIP12. The expected one-factor solution was supported for T1D, T2D, native English speaker, and non-native English speaker groups. Internal consistency was high across all groups (ω=0.91–0.93). Convergent and divergent validity were satisfactory. Known-groups validity was demonstrated for both diabetes types, by frequency of severe hypoglycemia (0 vs ≥1 episode in the past 12 months) and self-treated episodes (<2 vs 2–4 vs ≥5 per week). The measure also discriminated by awareness of hypoglycemia in those with T1D.
Conclusions
The HIP12 is an acceptable, internally consistent, and valid tool for assessing the impact of hypoglycemia on QoL among adults with T1D. The findings in the relatively small sample with T2D are encouraging and warrant replication in a larger sample.
Keywords: quality of life, hypoglycemia, psychosocial issues, psychometrics
WHAT IS ALREADY KNOWN ON THIS TOPIC
Hypoglycemia is commonly experienced and can have a negative impact on several areas of life among adults with type 1 or type 2 diabetes.
WHAT THIS STUDY ADDS
The study provides a new, brief, and valid measure of the impact of hypoglycemia on quality of life (QoL): the 12-Item Hypoglycemia Impact Profile (HIP12).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The HIP12 can be used in research to determine the impact of hypoglycemia on domains of QoL and overall QoL.
The HIP12 may be suitable for use in clinical care; further research is needed to explore this.
Introduction
Despite major advancements in the management of diabetes since the discovery of insulin 100 years ago, hypoglycemia (low blood glucose) remains a common1–7 and burdensome8–11 side effect of insulin therapy among adults with type 1 (T1D) or type 2 diabetes (T2D). Living with the risk and/or fear of severe hypoglycemia and the everyday disruptions caused by self-treated hypoglycemia can impact on a person’s quality of life (QoL). Recent qualitative studies show that hypoglycemia impacts an individual’s QoL in many domains, such as relationships, work or studies, sleep, leisure, and physical activities.12 13 Person-reported outcome measures (PROMs) can be used to quantify the extent of these impacts. However, recent systematic reviews of the quantified impact of hypoglycemia on QoL among adults with T1D or T2D showed substantial heterogeneity in methods used to assess both hypoglycemia and QoL outcomes.14 15 Most studies assessed single domains of QoL, such as emotional well-being or health status, with limited evidence for the impact of hypoglycemia on other domains of life.14 15 Furthermore, existing hypoglycemia-focused PROMs have limited content validity for assessment of the impact of hypoglycemia on QoL.16 Most PROMs focus on more specific issues such as fear of hypoglycemia or confidence in managing hypoglycemia.16 Thus, a measure of the impact of hypoglycemia on QoL is needed.
The DAWN-2 (Diabetes Attitudes, Wishes, and Needs 2) Impact of Diabetes Profile (DIDP) has been found to meet the need for a brief, contemporary measure of the impact of diabetes on QoL.17 The scale invites respondents to rate how diabetes currently impacts on six aspects of their life (physical health, finances, relationships, leisure activities, work or studies, and emotional well-being), and a seventh item was added recently to include the impact on ‘dietary freedom’.17 Given that the domains of life assessed by the DIDP are reasonably well matched with domains identified as important to overall QoL in recent qualitative research,13 it was hypothesized that minor modifications would be required to adapt this instrument to assess the impact of hypoglycemia on QoL.
Thus, the aim of the current study was to develop and validate a brief measure of the impact of hypoglycemia on QoL, informed by the previously validated DIDP, and to determine its psychometric properties among adults with T1D and adults with T2D in the large, multicountry ‘Your SAY (Self-management And You): Hypoglycemia’ study.
Research design and methods
Design
The Your SAY: Hypoglycemia study is a cross-sectional, multicountry survey about the impact of hypoglycemia on the QoL of people with T1D or T2D and their partners. The study was conducted as part of the Hypo-RESOLVE project.18
Participants, recruitment, and procedure
Eligible participants were adults (aged ≥18 years) with either T1D or T2D using insulin for a minimum of 6 months. Participants were recruited between May and August 2021 via social media (eg, Facebook, Twitter, blogs/online articles) and e-newsletters/mail-outs from diabetes organizations (eg, My Diabetes My Way, Juvenile Diabetes Research Foundation). They were directed to a study website, where they could read information about the study and access the survey, which was administered via the online platform Qualtrics (Provo, Utah).19 Participants completed eligibility items and, if eligible, were directed to read the participant information sheet. After providing informed consent, participants then self-reported demographic and clinical information and completed several questionnaires.
Measures
The 12-Item Hypoglycemia Impact Profile
The 12-Item Hypoglycemia Impact Profile (HIP12) was adapted from the original, validated DIDP17 20 by members of the Hypo-RESOLVE Consortium, including input from Hypo-RESOLVE’s Patient Advisory Committee (PAC). Online supplemental material 1 provides full details of the adaptation process. The DIDP assesses the impact of diabetes on six domains of life: physical health, finances, relationships, leisure activities, work or studies, and emotional well-being. A modified version of the DIDP contains a seventh item about dietary freedom.21 Items are rated on a 7-point scale (from 1=very positive impact to 7=very negative impact) or participants can select ‘not applicable’ (N/A). All seven items and the 7-point scale were retained in the HIP12, and five items were added, based on qualitative research12 13 and consultation with the PAC, to assess the impact of hypoglycemia on the following domains of life: sleep, sex life, independence, ability to be spontaneous, and ability to keep fit/be active. Composite scores are calculated by averaging the scores across applicable items, with scores <4 indicating a positive impact, a score of 4 no impact, and a score of >4 a negative impact of hypoglycemia on QoL.
bmjdrc-2022-002890supp001.pdf (306.9KB, pdf)
To explore the comprehensiveness of the HIP12,22 study participants were invited to use free-text fields to nominate up to three additional domains of life that are impacted by hypoglycemia. Participants were also required to rate the impact of hypoglycemia on nominated domains using the same 7-point scale.
Additional measures
Several additional measures were used to explore the construct and known-groups validity of the HIP12. Validated scales included the original DIDP, which assesses the impact of diabetes on seven domains of QoL17 23; the WHO-5 Well-Being Index, which assesses general well-being over the past 2 weeks24; the Hypoglycemia Confidence Scale, which assesses confidence in managing hypoglycemia in various scenarios25; the Hypoglycemia Fear Survey - Short Form (worry subscale), which assesses how often participants worried about several aspects of hypoglycemia over the past 6 months26; the Hypoglycemia Awareness Questionnaire, which assesses hypoglycemia frequency, severity, and awareness in the past 12 months27; and the Gold score, which provides a categorical assessment of hypoglycemia awareness.28 These measures are further described in online supplemental material 2. Participants self-reported demographic and clinical information (table 1). As this study was conducted in the context of the COVID-19 pandemic, they also provided ratings of the overall impact of the pandemic on their QoL.
Table 1.
Participants’ demographic and clinical characteristics
| Total sample (N=1265) | T1D (n=1071) | T2D (n=194) | P value (T1D vs T2D) | |
| Demographic characteristics | ||||
| Age, years | 49.6±15.8 (18–88) | 47.1±15.2 (18–86) | 63.3±11.5 (26–88) | <0.001 |
| Gender, female | 67.4 (853) | 71.3 (764) | 45.9 (109) | <0.001 |
| Native language | <0.001 | |||
| English | 87.0 (1101) | 85.6 (917) | 94.8 (184) | |
| Other* | 13.0 (164) | 14.4 (154) | 5.2 (10) | |
| Country of residence | <0.001 | |||
| USA | 29.4 (372) | 30.6 (328) | 22.7 (44) | |
| UK | 35.7 (452) | 30.3 (325) | 65.5 (127) | |
| Australia | 9.2 (116) | 9.8 (105) | 5.7 (11) | |
| Other | 25.7 (325) | 29.2 (313) | 6.2 (12) | |
| Current employment status | <0.001 | |||
| Full-time or part-time work, including self-employed | 56.0 (708) | 61.3 (656) | 26.8 (52) | |
| Student (full-time or part-time) | 7.1 (90) | 7.9 (85) | 2.6 (5) | |
| Not working (retired, not retired, unable to work) | 34.6 (438) | 27.9 (299) | 71.6 (139) | |
| Other | 10.4 (131) | 10.6 (113) | 9.3 (18) | |
| Financial difficulties† in the past 12 months | 21.9 (261) | 20.3 (205) | 30.6 (56) | 0.003 |
| Highest level of education | <0.001 | |||
| Secondary or lower | 12.8 (153) | 11.5 (116) | 20.2 (37) | |
| University | 68.2 (814) | 72.6 (733) | 44.3 (81) | |
| Other | 18.9 (226) | 15.9 (161) | 35.5 (65) | |
| Clinical characteristics | ||||
| Age of diabetes onset, years | 24.2±16.8 (1–78) | 20.5±14.9 (1–78) | 44.6±11.1 (15–74) | <0.001 |
| Diabetes duration, years | 25.5±15.6 (0.5–75) | 26.7±16.2 (0.5–75) | 18.7±8.9 (1–51) | <0.001 |
| Current diabetes management regimen | ||||
| Multiple daily injections | 53.0 (670) | 45.3 (485) | 48.5 (94) | |
| 1–2 daily injections | 7.1 (90) | – | 46.4 (90) | |
| Insulin pump | 47.0 (595) | 54.7 (586) | 4.6 (9) | |
| Blood glucose-lowering medications (oral) | 12.6 (160) | 5.1 (55) | 54.1 (105) | <0.001 |
| Commercial artificial pancreas/closed-loop systems | 9.1 (115) | 10.6 (114) | <1 (1) | <0.001 |
| Open-source artificial pancreas/closed-loop systems | 4.5 (57) | 5.3 (57) | – | <0.001 |
| Non-insulin injections | 3.1 (39) | 1.4 (15) | 12.4 (24) | <0.001 |
| Other | 3.2 (40) | 3.1 (33) | 3.6 (7) | 0.657 |
| Current glucose monitoring method | <0.001 | |||
| Continuous glucose monitor | 43.1 (545) | 48.9 (524) | 10.8 (21) | |
| Finger prick blood glucose | 26.1 (330) | 18.1 (194) | 70.1 (136) | |
| Freestyle Libre | 16.4 (207) | 17.3 (185) | 11.3 (22) | |
| Freestyle Libre 2 | 14.2 (180) | 15.5 (166) | 7.2 (14) | |
| None | <1 (2) | <1 (1) | <1 (1) | |
| Urine glucose monitor | <1 (1) | <1 (1) | – | |
| HbA1c, % | 7±1.2 (4–16) | 6.9±1.1 (4–16) | 7.7±1.5 (5–13) | <0.001 |
| HbA1c, mmol/mol | 53.3±13.2 (21–155) | 52.3±12.4 (21–155) | 60.5±16.3 (33–116) | <0.001 |
| Awareness of hypoglycemia | ||||
| HypoA-Q impaired awareness subscale | 8.7±3.7 (1–18) | 8.9±3.8 (1–18) | 7.4±3.2 (1–14) | <0.001 |
| Gold score ≥4 | 32.1 (390) | 33.7 (347) | 23.1 (43) | 0.005 |
| Hypoglycemia frequency | ||||
| Any episode of any severity in the past week, median (range) | 3 (0–52) | 3 (0–52) | 1 (0–10) | <0.001 |
| ≥1 self-treated episode per week over the past year | 63.1 (773) | 71.4 (740) | 17.6 (33) | <0.001 |
| ≥1 severe episode in the past year | 21.5 (262) | 22.4 (231) | 16.7 (31) | 0.099 |
| Diabetes complications | ||||
| Retinopathy | 20.4 (258) | 19.8 (212) | 23.7 (46) | 0.210 |
| Neuropathy | 16.0 (202) | 12.8 (137) | 33.5 (65) | <0.001 |
| Sexual dysfunction | 13.7 (173) | 10.2 (109) | 33.0 (64) | <0.001 |
| Kidney damage/renal failure | 7.8 (99) | 6.3 (68) | 16.0 (31) | <0.001 |
| Heart disease/heart attack | 6.6 (83) | 4.3 (48) | 19.1 (37) | <0.001 |
| Vascular disease | 6.6 (83) | 4.2 (45) | 19.6 (38) | <0.001 |
| Stroke | 1.1 (14) | <1 (4) | 5.2 (10) | <0.001 |
| Psychological comorbidities | ||||
| Anxiety | 27.6 (349) | 27.6 (296) | 27.3 (53) | >0.999 |
| Depression | 21.7 (275) | 20.9 (224) | 26.3 (51) | 0.108 |
| Impact of COVID-19 on QoL‡ | 2.7±1.1(1–7) | 2.7±1.1 (1–7) | 2.6±1.3 (1–7) | 0.123 |
Data presented as M±SD (range) or valid % (n) unless otherwise listed.
Independent samples t-tests were conducted for continuous variable comparisons and χ2 tests for categorical variable comparisons.
Not all ‘n’s add up to 100% due to missing data. Some ‘n’s add up to >100% due to multiple selections allowed.
*Afrikaans, Arabic, Bengali, Cantonese, Danish, Dutch, Finnish, French, German, Greek, Gujarati, Hebrew, Hindi, Hungarian, Iranian, Irish, Italian, Kikuyu, Luxembourgish, Macedonian, Marathi, Portuguese, Romanian, Russian, Scottish, Slovakian, Slovenian, Spanish, Swedish, Tamil, Turkish and Welsh.
†Financial difficulties were defined as not being able to pay for things on time (eg, rent, mortgage, bills), not being able to buy important things (eg, food, clothing), or not being able to afford services (eg, healthcare).
‡Scored on a Likert scale from 1 (very negative impact) to 7 (very positive impact).
HbA1c, hemoglobin A1c; HypoA-Q, Hypoglycemia Awareness Questionnaire; QoL, quality of life; T1D, type 1 diabetes; T2D, type 2 diabetes.
Statistical analyses
Statistical analyses were conducted using SPSS V.28 and R Studio V.2021.09.1. Acceptability, applicability, and response patterns on the HIP12 were summarized with descriptive statistics. Interitem correlations, internal consistency calculations (McDonald’s ω),29 and confirmatory factor analyses (CFA) were conducted in four subgroups: T1D, T2D, native English speakers, and non-native English speakers. Spearman’s correlations were conducted for construct validity (convergent and divergent validity) and Mann-Whitney U tests were conducted for known-groups validity. Statistical analyses are detailed in online supplemental material 3.
Results
Sample characteristics
The eligible sample for the current study consisted of 1452 adults with diabetes, of whom 187 (13%) were excluded because they exited the survey before attempting the HIP12. There were no statistically significant differences between those who did (and did not) attempt the HIP12 (for age, gender, diabetes type, diabetes duration, or native language (English vs non-English)).
The final sample comprised 1265 adults with diabetes (n=1071 with T1D; n=194 with T2D). Table 1 details their demographic and clinical characteristics. The mean±SD age was 47±15 years for people with T1D and 63±12 years for people with T2D. Of the sample, 87% were native English speakers. Participants lived in 44 countries, with most (74%) from the UK, USA, or Australia. Sample characteristics differed considerably by diabetes type. Most participants with T2D lived in the UK (66%), whereas those with T1D were more diverse geographically. Participants with T2D reported more diabetes complications/physical comorbidities than those with T1D, but equivalent psychological comorbidities (depression and anxiety). Those with T2D were older, had different employment and living arrangements, and had more financial difficulties than those with T1D.
Acceptability and response patterns
Most participants who began the HIP12 completed all 12 items (T1D: 98%; T2D: 96%). Table 2 presents the item response patterns by diabetes type. At an item level, there were little missing data across the total sample. Items were broadly applicable; on 10 of the 12 items, <3% of participants used the N/A option. ‘Work or studies’ was not applicable to 19% of participants and ‘sex life’ was not applicable to 16%. No floor or ceiling effects were evident; less than 15% of the sample endorsed the highest or lowest scores on the 7-point scale for each item (not including ‘N/A’ responses). Across the total sample, every response option was used for every item by at least one person, although negative options were endorsed more frequently than positive options. For participants with T2D, some positive response options were unused across six items: physical health, leisure activities, work or studies, emotional well-being, dietary freedom, and independence.
Table 2.
Item-level response patterns on the 12-Item Hypoglycemia Impact Profile
| Very negative impact | Negative impact | Slightly negative impact | No impact | Slightly positive impact | Positive impact | Very positive impact | N/A | Missing | ||
| Type 1 diabetes (n=1071) | ||||||||||
| 1 | Physical health | 9.5 (101) | 26.4 (282) | 44.0 (470) | 17.5 (187) | 1.2 (13) | 0.9 (10) | 0.5 (5) | – | 0.3 (3) |
| 2 | Financial situation | 2.7 (29) | 6.5 (70) | 14.2 (152) | 73.0 (780) | 0.3 (3) | 0.7 (8) | 0.4 (4) | 2.2 (23) | 0.2 (2) |
| 3 | Relationships | 2.5 (27) | 11.3 (121) | 36.2 (387) | 45.4 (485) | 2.1 (22) | 0.8 (9) | 0.9 (10) | 0.7 (7) | 0.3 (3) |
| 4 | Leisure activities | 8.3 (89) | 24.0 (256) | 49.9 (533) | 15.8 (169) | 0.7 (8) | 0.7 (8) | 0.4 (4) | 0.1 (1) | 0.3 (3) |
| 5 | Work or studies | 5.6 (60) | 16.4 (175) | 36.7 (392) | 24.1 (258) | 1.3 (14) | 0.4 (4) | 0.1 (1) | 15.4 (165) | 0.2 (2) |
| 6 | Emotional well-being | 10.8 (115) | 26.1 (279) | 41.1 (439) | 19.7 (210) | 0.8 (9) | 1.0 (11) | 0.4 (4) | 0.1 (1) | 0.3 (3) |
| 7 | Sleep | 13.7 (146) | 28.5 (304) | 41.3 (441) | 14.8 (158) | 0.4 (4) | 0.6 (6) | 0.6 (6) | 0.2 (2) | 0.4 (4) |
| 8 | Dietary freedom | 9.7 (104) | 21.7 (232) | 32.7 (349) | 27.9 (298) | 4.7 (50) | 2.2 (23) | 0.7 (7) | 0.4 (4) | 0.4 (4) |
| 9 | Sex life | 5.4 (58) | 11.0 (117) | 22.0 (234) | 45.4 (484) | 0.5 (5) | 0.6 (6) | 0.3 (3) | 14.9 (159) | 0.5 (5) |
| 10 | Independence | 7.2 (77) | 18.0 (192) | 37.6 (401) | 34.3 (366) | 0.9 (10) | 1.5 (16) | 0.3 (3) | 0.2 (2) | 0.4 (4) |
| 11 | Spontaneity | 12.9 (138) | 25.4 (271) | 39.6 (423) | 19.1 (204) | 1.0 (11) | 1.1 (12) | 0.2 (2) | 0.7 (7) | 0.3 (3) |
| 12 | Keep fit/be active | 12.4 (132) | 24.3 (259) | 42.8 (456) | 17.5 (187) | 0.7 (7) | 1.1 (12) | 0.9 (10) | 0.3 (3) | 0.5 (5) |
| Type 2 diabetes (n=194) | ||||||||||
| 1 | Physical health | 10.4 (20) | 15.5 (30) | 39.9 (77) | 31.6 (61) | 1.6 (3) | 1.0 (2) | – | – | 0.9 (1) |
| 2 | Financial situation | 3.6 (7) | 6.8 (13) | 14.1 (27) | 71.9 (138) | 0.5 (1) | 0.5 (1) | 0.5 (1) | 2.1 (4) | 1.0 (2) |
| 3 | Relationships | 3.6 (7) | 4.1 (8) | 23.7 (46) | 58.8 (114) | 2.6 (5) | 4.1 (8) | 1.0 (2) | 2.1 (4) | – |
| 4 | Leisure activities | 8.3 (16) | 17.7 (34) | 35.4 (68) | 35.4 (68) | 1.0 (2) | 1.6 (3) | – | 0.5 (1) | 1.0 (2) |
| 5 | Work or studies | 4.7 (9) | 9.8 (19) | 14.5 (28) | 32.6 (63) | – | – | – | 38.3 (74) | 0.9 (1) |
| 6 | Emotional well-being | 8.8 (17) | 13.5 (26) | 33.7 (65) | 39.9 (77) | 1.6 (3) | – | 1.6 (3) | 1.0 (2) | 0.9 (1) |
| 7 | Sleep | 11.3 (22) | 18.6 (36) | 27.8 (54) | 38.1 (74) | 0.5 (1) | 2.6 (5) | 0.5 (1) | 0.5 (1) | – |
| 8 | Dietary freedom | 13.5 (26) | 20.7 (40) | 30.6 (59) | 30.1 (58) | 2.1 (4) | 2.6 (5) | – | 0.5 (1) | 0.9 (1) |
| 9 | Sex life | 11.3 (22) | 10.3 (20) | 10.8 (21) | 41.2 (80) | 0.5 (1) | 0.5 (1) | 1.0 (2) | 24.2 (47) | – |
| 10 | Independence | 5.7 (11) | 12.9 (25) | 21.1 (41) | 56.2 (109) | 1.0 (2) | – | 1.5 (3) | 1.5 (3) | – |
| 11 | Spontaneity | 9.8 (19) | 16.5 (32) | 22.7 (44) | 46.4 (90) | 1.5 (3) | 0.5 (1) | 1.5 (3) | 1.0 (2) | – |
| 12 | Keep fit/be active | 11.0 (21) | 16.2 (31) | 30.9 (59) | 39.8 (76) | 1.0 (2) | 0.5 (1) | 0.5 (1) | – | 1.5 (3) |
Data are valid % (n).
N/A, not applicable.
Internal consistency and structural validity
Interitem correlations were acceptable with values ranging from rs=0.25 to rs=0.71, and the determinant indicating no multicollinearity (=0.00296). The Kaiser-Meyer-Olkin values (>0.92) indicated that the data were suitable for factor analysis. The minimum number of participants (n=120) per CFA was exceeded. Table 3 presents the factor loadings for each item, internal consistency statistics, and the model fit indices on the CFAs by diabetes type and for native versus non-native English speakers. The one-factor solution was generally supported across the subgroups. Standardized factor loadings were acceptable (≥0.5, except for the ‘dietary freedom’ item in the non-native speakers subgroup, which was marginal at 0.48). Internal consistency was excellent across all subgroups (ω=0.91–0.93) and remained acceptable (>0.7) with up to seven missing item scores. Robust model fit parameters were satisfactory overall, with comparative fit index (CFI) >0.95, Tucker-Lewis index (TLI) >0.95, root mean square error of approximation (RMSEA) CIs including values of ≤0.06, and standardized root mean square residual <0.08 for all subgroups, with a few exceptions for the subgroup with T2D (CFI=0.90, TLI=0.90, and RMSEA=0.11) and the non-native English subgroup (TLI=0.94). In an additional ad hoc exploratory factor analysis (EFA) examining whether a multifactor solution was more appropriate for the subgroup with T2D, eigenvalues and scree plots also indicated that a one-factor solution was the best fit for the data. From a theoretical perspective (ie, the inter-relatedness of the constructs the items measure), the one-factor solution was considered the most appropriate and was therefore retained.
Table 3.
Confirmatory factor analyses testing a one-factor solution of the 12-Item Hypoglycemia Impact Profile in four groups: factor loadings, fit indices, and internal consistency
| Type 1 diabetes (n=1071) |
Type 2 diabetes (n=194) |
Native English speaker (n=1101) |
Non-native English speaker (n=164) |
|
| Factor loadings | ||||
| Physical health | 0.69 | 0.74 | 0.68 | 0.72 |
| Financial situation | 0.51 | 0.61 | 0.52 | 0.49 |
| Relationships | 0.64 | 0.58 | 0.62 | 0.72 |
| Leisure activities | 0.79 | 0.75 | 0.78 | 0.84 |
| Work or studies | 0.73 | 0.73 | 0.72 | 0.79 |
| Emotional well-being | 0.78 | 0.81 | 0.77 | 0.84 |
| Sleep | 0.67 | 0.72 | 0.69 | 0.72 |
| Dietary freedom | 0.51 | 0.66 | 0.54 | 0.48 |
| Sex life | 0.53 | 0.62 | 0.53 | 0.59 |
| Independence | 0.76 | 0.85 | 0.79 | 0.76 |
| Spontaneity | 0.76 | 0.83 | 0.78 | 0.79 |
| Keep fit/be active | 0.72 | 0.76 | 0.74 | 0.72 |
| Model fit statistics | ||||
| McDonald’s ꙍ | 0.91 | 0.93 | 0.91 | 0.92 |
| χ2 test statistics | 228.41 | 135.91 | 231.55 | 63.56 |
| df | 54 | 54 | 54 | 54 |
| Robust CFI | 0.96 | 0.90 | 0.95 | 0.99 |
| Robust TLI | 0.95 | 0.88 | 0.94 | 0.99 |
| Robust RMSEA (CI) | 0.07 (0.06 to 0.08) | 0.11 (0.09 to 0.14) | 0.07 (0.06 to 0.08) | 0.04 (<0.001 to 0.07) |
| SRMR | 0.03 | 0.05 | 0.03 | 0.04 |
CFI, comparative fit index; RMSEA, robust root mean square error of approximation; SRMR, standardized root mean square residual; TLI, Tucker-Lewis index.
Convergent, divergent, and known-groups validity
Spearman’s correlations were largely consistent with the hypotheses, supporting the convergent and divergent validity of the HIP12. Table 4 presents the correlations between the HIP12 (composite and item) scores and the measures of convergent/divergent validity. The HIP12 composite score had strong correlations with DIDP composite scores for adults with T1D (r=0.70) or T2D (r=0.68). Moderate statistically significant correlations (r>0.3) were observed with other psychological measures. The findings were as expected, demonstrating convergent validity. Divergent validity was indicated by small, non-significant correlations between HIP12 composite scores and diabetes duration for adults with T1D (r=−0.05) or T2D (r=0.02), and hemoglobin A1c (HbA1c) for adults with T1D (r=−0.09). The correlation between HIP12 composite score and HbA1c for adults with T2D was larger than expected (r=0.33) but not statistically significant.
Table 4.
Spearman’s r correlations for convergent and divergent validity of the 12-Item Hypoglycemia Impact Profile
| Type 1 diabetes | Type 2 diabetes | |||||||||||
| DIDP | HFS-SF | HCS | WHO-5 | Diabetes duration | HbA1c | DIDP | HFS-SF | HCS | WHO-5 | Diabetes duration | HbA1c | |
| Composite score | 0.700*** | 0.567*** | −0.531*** | −0.441*** | −0.045 | −0.092 | 0.682*** | 0.478*** | −0.428*** | −0.343** | 0.019 | 0.330 |
| Physical health | 0.474*** | 0.398*** | −0.378*** | −0.332*** | −0.032 | −0.102 | 0.474*** | 0.398*** | −0.360*** | −0.313*** | −0.096 | −0.076 |
| Financial situation | 0.363*** | 0.293*** | −0.259*** | −0.212*** | 0.020 | −0.043 | 0.418*** | 0.334*** | −0.282*** | −0.265*** | −0.053 | −0.123 |
| Relationships | 0.449*** | 0.325*** | −0.305*** | −0.238*** | 0.024 | −0.088 | 0.423*** | 0.233*** | −0.168* | −0.177* | 0.037 | 0.207 |
| Leisure activities | 0.557*** | 0.389*** | −0.339*** | −0.345*** | −0.083** | 0.045 | 0.436*** | 0.298*** | −0.263*** | −0.213** | −0.005 | −0.179 |
| Work or studies | 0.535*** | 0.421*** | −0.365*** | −0.329** | −0.077* | 0.185 | 0.564*** | 0.397*** | −0.294** | −0.358*** | −0.008 | 0.233 |
| Emotional well-being | 0.559*** | 0.480*** | −0.409*** | −0.394*** | −0.087** | 0.026 | 0.616*** | 0.519*** | −0.475*** | −0.470*** | −0.095 | 0.095 |
| Sleep | 0.486*** | 0.449*** | −0.346*** | −0.385*** | −0.100** | −0.006 | 0.499*** | 0.335*** | −0.304*** | −0.358*** | −0.053 | 0.114 |
| Dietary freedom | 0.432*** | 0.285*** | −0.294*** | −0.200*** | 0.007 | −0.106 | 0.520*** | 0.279*** | −0.194** | −0.276*** | −0.048 | 0.105 |
| Sex life | 0.282*** | 0.215*** | −0.207*** | −0.202*** | 0.049 | 0.083 | 0.272*** | 0.121*** | −0.199** | −0.279** | 0.122 | 0.171 |
| Independence | 0.504*** | 0.423*** | −0.429*** | −0.313*** | 0.008 | −0.061 | 0.573*** | 0.394*** | −0.380*** | −0.364*** | −0.114 | 0.323 |
| Spontaneity | 0.520*** | 0.391*** | −0.339*** | −0.298*** | −0.078* | −0.154 | 0.539*** | 0.366*** | −0.379*** | −0.308*** | −0.128 | 0.212 |
| Keep fit/be active | 0.549*** | 0.384*** | −0.370*** | −0.377*** | −0.064* | 0.013 | 0.457*** | 0.302*** | −0.328*** | −0.282*** | 0.012 | 0.114 |
*P<0.05, **P<0.01, ***P< 0.001.
DAWN-2, Diabetes Attitudes, Wishes, and Needs 2; DIDP, DAWN-2 Impact of Diabetes Profile; HbA1c, hemoglobin A1c; HCS, Hypoglycemia Confidence Scale; HFS-SF, Hypoglycemia Fear Survey - Short Form; WHO-5, WHO-5 Well-Being Index.
Mann-Whitney U tests broadly showed that the HIP12 was able to discriminate between known groups. For both diabetes types, the composite score was significantly higher among those who had (vs had not) experienced ≥1 episode of severe hypoglycemia in the past 12 months (T1D: r=0.16; T2D: r=0.22) and those who had experienced 2–4 compared with 0–1 episodes of hypoglycemia (of any severity) in the past week (T1D: r=0.16; T2D: r=0.22). The composite score was also higher among participants with T1D who had experienced ≥5 episodes of hypoglycemia (of any severity) in the past week compared with 0–1 (r=0.27) or 2–4 (r=0.13) episodes, and those who had impaired versus intact awareness of hypoglycemia (r=0.22). Table 5 presents the results for the HIP item scores, which showed a similar pattern to the composite scores.
Table 5.
Rank serial–biserial correlations for known-groups validity of the 12-Item Hypoglycemia Impact Profile
| Any episode of any severity in the past week | Type 1 diabetes | Type 2 diabetes | ||||
| 0–1 episode (n=226) vs 2–4 episodes (n=445) | 0–1 episode (n=226) vs 5+ episodes (n=365) | 2–4 episodes (n=445) vs 5+ episodes (n=365) | 0–1 episode (n=132) vs 2–4 episodes (n=46) | 0–1 episode (n=132) vs 5+ episodes (n=10) | 2–4 episodes (n=46) vs 5+ episodes (n=10) | |
| Composite | 0.161*** | 0.274*** | 0.128*** | 0.222** | 0.182* | 0.126 |
| Physical health | 0.067 | 0.183*** | 0.109** | 0.231** | 0.167* | 0.069 |
| Financial situation | 0.036 | 0.149*** | 0.118*** | 0.071 | 0.135 | 0.139 |
| Relationships | 0.080* | 0.149*** | 0.073* | 0.214** | 0.025 | 0.117 |
| Leisure activities | 0.143*** | 0.213*** | 0.073* | 0.153* | 0.039 | 0.050 |
| Work or studies | 0.050 | 0.195*** | 0.153*** | 0.148 | 0.085 | 0.017 |
| Emotional well-being | 0.117** | 0.188*** | 0.073* | 0.250*** | 0.124 | 0.034 |
| Sleep | 0.181*** | 0.263*** | 0.100** | 0.194* | 0.207* | 0.118 |
| Dietary freedom | 0.091* | 0.153*** | 0.068 | 0.144 | 0.141 | 0.096 |
| Sex life | 0.063 | 0.130** | 0.069 | 0.018 | 0.109 | 0.140 |
| Independence | 0.100* | 0.183*** | 0.086* | 0.211** | 0.109 | 0.020 |
| Spontaneity | 0.128*** | 0.224*** | 0.106** | 0.184* | 0.163 | 0.093 |
| Keep fit/be active | 0.148*** | 0.212*** | 0.071* | 0.075 | 0.089 | 0.058 |
| Severe hypoglycemia in the past year† | 0 episode (n=802) vs ≥1 SHE (n=231) | 0 episode (n=155) vs ≥1 SHE (n=31) |
| Composite | 0.158*** | 0.221** |
| Physical health | 0.165*** | 0.226** |
| Financial situation | 0.182*** | 0.085 |
| Relationships | 0.183*** | 0.037 |
| Leisure activities | 0.067* | 0.205** |
| Work or studies | 0.128*** | 0.211* |
| Emotional well-being | 0.134*** | 0.265*** |
| Sleep | 0.122*** | 0.108 |
| Dietary freedom | 0.066* | 0.181* |
| Sex life | 0.097** | 0.106 |
| Independence | 0.161*** | 0.157* |
| Spontaneity | 0.049 | 0.093 |
| Keep fit/be active | 0.076* | 0.169* |
| Awareness status‡ | Intact (n=682) vs IAH (n=347) | Intact (n=143) vs IAH (n=43) |
| Composite | 0.215*** | 0.002 |
| Physical health | 0.191*** | 0.047 |
| Financial situation | 0.133*** | 0.098 |
| Relationships | 0.163*** | 0.084 |
| Leisure activities | 0.102** | 0.022 |
| Work or studies | 0.099** | 0.026 |
| Emotional well-being | 0.131*** | 0.033 |
| Sleep | 0.160*** | 0.026 |
| Dietary freedom | 0.143*** | 0.076 |
| Sex life | 0.127*** | 0.022 |
| Independence | 0.243*** | 0.029 |
| Spontaneity | 0.132*** | 0.042 |
| Keep fit/be active | 0.086** | 0.012 |
Effect sizes are interpreted as follows: 0.1=small, 0.3=medium, 0.5=large.
*P<0.05, **P<0.01, ***P<0.001.
†Defined as episodes where they needed help/were unable to treat themselves.
‡Intact awareness was defined as a self-reported Gold score of ≤3 and impaired awareness was defined as a self-reported Gold score of ≥4.
IAH, impaired awareness of hypoglycemia; SHE, severe hypoglycemia episode.
Comprehensiveness
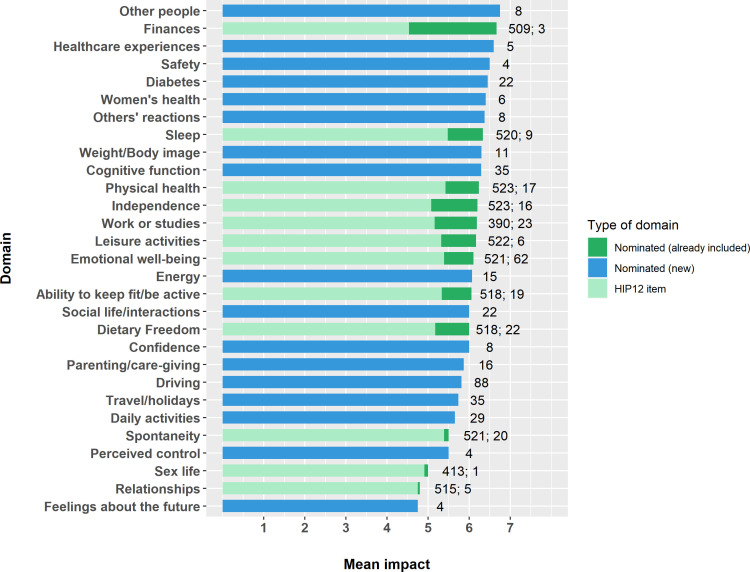
A complete description of the findings regarding the comprehensiveness of the HIP12 is provided in online supplemental material 4. Briefly, 27% of participants nominated at least one additional domain of life impacted by hypoglycemia. Several of the nominated domains aligned with already included domains on the HIP12 and 17 new areas were nominated. No single domain was nominated by >7% of the sample. The domain labels and the associated impact ratings are summarized in figure 1. For the 322 participants who rated the impact of at least one new domain, there was a marginal but statistically significant (p<0.001) difference between original composite scores (5.13±0.76) and composite scores that incorporated the rating of the new domain/s (5.19±0.75).
Figure 1.
Domains of life (HIP12 items and nominated) and their associated impact. A score above 4 indicates negative impact, 4 indicates no impact, and below 4 indicates positive impact of hypoglycemia. Numerals beside each bar represent the number of participants contributing to each mean score. HIP12, 12-Item Hypoglycemia Impact Profile.
Discussion
These psychometric analyses indicate that, overall, the HIP12 is an acceptable and valid tool for assessing the impact of hypoglycemia on QoL among adults with T1D or T2D. Almost all participants completed the entire HIP12; items were broadly applicable and no floor or ceiling effects were observed. Internal consistency was excellent for both diabetes types and for both native and non-native English speakers. The structural, construct, and known-groups validity of the HIP12 were all supported, with some exceptions for the sample with T2D, which need to be investigated in future psychometric studies with larger numbers.
Response patterns were largely as expected, with substantially more participants reporting a negative than positive impact of hypoglycemia on QoL. Among those with T1D, only 1%–8% reported any positive impact on each HIP12 item, and all response options were used by at least two participants. Holmes-Truscott et al 17 showed that the proportion of adults with T1D reporting a positive impact of diabetes on QoL on each item of the DIDP was somewhat higher (4%–15%), suggesting that hypoglycemia is perceived more consistently as negative than diabetes more broadly. Among those with T2D, 3%–15% reported some positive impact of hypoglycemia on each HIP12 item, although not all response options were used on all items. Holmes-Truscott et al 17 showed a similar proportion of their sample of 509 adults with insulin-treated T2D reporting a positive impact of diabetes on each DIDP item (5%–15%).17 The absence of responses on certain options of the HIP12 in this study may be due to the small sample size of people with T2D relative to that with T1D and to the above study.17 Further research is needed to explore the advantages and disadvantages of a bidirectional (positive–negative) versus a unidirectional (negative only) response scale for assessing the impact of hypoglycemia on QoL. However, it may remain important to present a balanced response scale in order to retain face validity and allow for the possibility of positive impact.
Although the CFAs evidenced structural validity for participants with T1D, native English speakers, and non-native English speakers, the model fit was less strong for the group with T2D, although TLI ≥0.90 in some instances has been considered acceptable.30 31 In this study, an EFA exploring whether a multidimensional structure was more appropriate suggested that a one-factor solution remained the most optimal. Less robust results in the sample with T2D may be due to subgroup differences; for example, older adults with T2D might have experienced that some items (eg, work or studies, sex life) were less relevant. Future studies are needed to test the one-factor model in larger independent samples and these should explore structural validity in older versus younger samples with T2D. It should also be noted that although the HIP12 composite score is likely appropriate for use in research, it has less relevance clinically than individual domain scores, which enable greater insight into how hypoglycemia impacts on QoL.32 33 Thus, although the composite score is psychometrically adequate, item-level analyses are recommended where possible.
Correlations between the HIP12 and measures of convergent/divergent validity were as expected and similar to correlations determining construct validity in similar studies.12 34 35 While a strong correlation was found between the DIDP (assessing diabetes-specific QoL) and the hypoglycemia-specific adaptation, the lack of multicollinearity suggests that the two scales assess different constructs. This provides support for the need for a hypoglycemia-specific measure of QoL, as it is clear that understanding the impact of diabetes on QoL is not a suitable proxy for understanding the impact of hypoglycemia on QoL.16 As expected, at an item level, correlations between HIP12 domains and validated scales were not as consistently large but were statistically significant. To establish construct validity for individual items, correlations with full scales assessing each respective domain (eg, sleep questionnaire for the sleep item) would be required. However, there is currently a lack of hypoglycemia-specific validated scales for individual life domains, so this is not feasible currently.
Although the HIP12 largely discriminated between known groups, effect sizes for significant differences were small. This finding is consistent with other QoL measures that discriminate based on hypoglycemia frequency,17 35 and compares favorably with the psychometric validation of the DIDP, as the HIP12 was largely better able to discriminate between those who had and had not experienced severe hypoglycemia in the past year, with larger effect sizes on most items. Known-groups validity was not confirmed for adults with T2D who had impaired versus intact awareness of hypoglycemia (assessed with the Gold score). It is possible that people in the ‘impaired awareness’ subgroup reported less awareness of the onset of hypoglycemia for reasons other than impaired awareness, for example, limited glucose monitoring or infrequent experience of hypoglycemia. Future research is needed to explore the sensitivity of the HIP12, particularly in T2D, and importantly to explore what constitutes a minimally important (clinical) change on the measure.
The HIP12 is a brief measure and as such there is a risk that comprehensiveness is sacrificed in favor of brevity. To explore comprehensiveness (a key aspect of face and content validity),22 after completing the HIP12, participants were invited to nominate additional domains of life affected by hypoglycemia and indicate the direction/extent of the impact. Although 17 additional domains were nominated, each was nominated by <7% of the total sample. When the impact ratings of these domains were incorporated into composite scores, scores were only slightly higher (although statistically significant). This difference is unlikely to be clinically meaningful; average scores were between ‘slightly negative’ and ‘negative’ before and after the addition of nominated domain ratings. Further research is needed to examine the utility of the additional domains in a large sample.
A strength of this study was the large, geographically diverse sample of adults with T1D. The sample with T2D was relatively small and more homogenous and it should be noted that the frequency of self-treated hypoglycemia reported by this group was slightly higher than in population-based studies.3 5 Additionally, the mean HbA1c of the sample was lower than would be expected in the broader population of adults with T1D or insulin-managed T2D.3 36 Thus, it would be prudent to confirm the psychometric properties in a representative sample, in a population-based study. Another strength of the study was the use of validated measures of hypoglycemia frequency, severity, and awareness, which were shown to be associated with HIP12 scores. Although these measures are subject to recall bias and no objective data on sensor-detected hypoglycemia were gathered, this study was focused on individuals’ perceptions of hypoglycemia and its impact on QoL; thus, objective indicators of hypoglycemia frequency are less relevant. The HIP12 is not designed to measure the direct impact of specific episodes of hypoglycemia. However, as part of Hypo-RESOLVE, a new app-based measure (using ecological momentary assessment methods) has been developed to assess the direct impact of episodes of hypoglycemia on aspects of daily functioning (eg, sleep, emotional well-being, work), many of which are relevant to QoL.37
A potential limitation of the HIP12 is that it was adapted from an existing measure of the impact of diabetes on QoL.23 However, the content was informed by recent qualitative research on the impact of hypoglycemia in adults with T1D13 and other relevant literature.38 39 Importantly, people with lived experience of diabetes (the Hypo-RESOLVE PAC) contributed to discussions about how to adapt the measure and reviewed the final adaptation for relevance, comprehensiveness, and comprehensibility. The use of free-text responses (and impact rating scales) further enabled some qualitative and quantitative investigation of the comprehensiveness of the HIP12, the findings of which can inform further development. A strength of this adaptation is that it has enabled rapid validation and demonstration of the suitability of a brief measure that can now fill a considerable gap in both research and clinical practice. The relative utility of the DIDP and the HIP12 for determining the impact of hypoglycemia on QoL can be compared directly in future research. The HIP12 was not developed for use in health economic evaluations or for cost utility analysis. However, as part of the Hypo-RESOLVE project, a new hypoglycemia-specific PROM and associated preference-based measure is being developed to address that need.18
In conclusion, this study demonstrates that the brief HIP12 is an acceptable, internally consistent, and valid tool for assessing the perceived impact of hypoglycemia on QoL in adults with T1D or T2D. It is appropriate for use in research and may have utility in clinical care. Further research is needed to investigate its acceptability and content validity, confirm the factor structure in larger independent and culturally diverse samples, and examine the responsiveness of the HIP12 in interventions designed to reduce the frequency and/or impact of hypoglycemia.
Acknowledgments
The authors would like to acknowledge the support from the Hypo-RESOLVE Patient Advisory Committee, including Renza Scibilia, Simon O’Neill, Laura Cooke, Ken Tait, and Bastian Hauck, for their input into the design of the HIP12 and the broader Your SAY: Hypoglycemia study and for their valuable assistance with recruiting participants for this study. The authors would also like to acknowledge the recruitment support received from Hypo-RESOLVE partners, including Novo Nordisk, Eli Lilly, Juvenile Diabetes Research Foundation, International Diabetes Federation, University of Sheffield, King’s College London, Nordsjællands Hospital Hillerød, University of Dundee, and Radboud University Medical Center.
Footnotes
Collaborators: The Hypo-RESOLVE Consortium members: Professor Cees Tack, Dr Bastiaan de Galan, Professor Stephanie Amiel, Dr Pratik Choudhary, Professor Thomas Pieber, Dr Julia Mader, Dr Mark Evans, Professor Eric Renard, Professor Frans Pouwer, Professor Jane Speight, Professor Bernard Thorens, Professor Simon Heller, Professor Alan Brennan, Professor Ulrik Pedersen-Bjergaard, Professor Rory McCrimmon, Jakob Haardt, Dr Mark Ibberson, Professor Giovanni Sparacino, Professor Helen Colhoun, Dr Stephen Gough, Dr Zvonko Milicevic, Dr Mahmood Kazemi, Dr Ohad Cohen, Dr Sanjoy Dutta, Dominique Robert, Dr Wendy Wolf, Dr Sean Sullivan.
Contributors: MB, HC, US, JS, and FP designed the study and created the manuscript plan. MB and JS wrote the first draft of the manuscript. MB, US, and HC conducted the data analysis. All authors critically reviewed the manuscript and were involved in the design of the Your SAY: Hypoglycemia study and/or the development of the Hypoglycemia Impact Profile-12. All authors approved the final version of the manuscript. MB is the guarantor of this work.
Funding: This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement number 777460. The JU receives support from the European Union’s Horizon 2020 research and innovation program and the EFPIA and T1D Exchange, JDRF, International Diabetes Federation (IDF), and The Leona M and Harry B Helmsley Charitable Trust. CH and JS are supported by core funding to the Australian Centre for Behavioural Research in Diabetes provided by the collaboration between Diabetes Victoria and Deakin University.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
the Hypo-RESOLVE Consortium:
Cees Tack, Bastiaan de Galan, Stephanie Amiel, Pratik Choudhary, Thomas Pieber, Julia Mader, Mark Evans, Eric Renard, Frans Pouwer, Jane Speight, Bernard Thorens, Simon Heller, Alan Brennan, Ulrik Pedersen-Bjergaard, Rory McCrimmon, Jakob Haardt, Mark Ibberson, Giovanni Sparacino, Helen Colhoun, Stephen Gough, Zvonko Milicevic, Mahmood Kazemi, Ohad Cohen, Sanjoy Dutta, Dominique Robert, Wendy Wolf, and Sean Sullivan
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request via contact with the corresponding author (http://orcid.org/0000-0003-4408-6304), provided that data are to be used for research projects related to health sciences. Data are deidentified participant responses from a web-based quantitative survey.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the University of Southern Denmark Research Ethics Committee (case no: 21/8758).
References
- 1. Akram K, Pedersen-Bjergaard U, Carstensen B, et al. Frequency and risk factors of severe hypoglycaemia in insulin-treated type 2 diabetes: a cross-sectional survey. Diabetic Medicine 2006;23:750–6. 10.1111/j.1464-5491.2006.01880.x [DOI] [PubMed] [Google Scholar]
- 2. Cariou B, Fontaine P, Eschwege E, et al. Frequency and predictors of confirmed hypoglycaemia in type 1 and insulin-treated type 2 diabetes mellitus patients in a real-life setting: results from the DIALOG study. Diabetes Metab 2015;41:116–25. 10.1016/j.diabet.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 3. Khunti K, Alsifri S, Aronson R, et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin‐treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab 2016;18:907–15. 10.1111/dom.12689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev 2004;20:479–86. 10.1002/dmrr.482 [DOI] [PubMed] [Google Scholar]
- 5. Ratzki-Leewing A, Harris SB, Mequanint S, et al. Real-world crude incidence of hypoglycemia in adults with diabetes: results of the InHypo-DM study, Canada. BMJ Open Diab Res Care 2018;6:e000503. 10.1136/bmjdrc-2017-000503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. UK Hypoglycaemia Study Group . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140–7. 10.1007/s00125-007-0599-y [DOI] [PubMed] [Google Scholar]
- 7. van Meijel LA, de Vegt F, Abbink EJ, et al. High prevalence of impaired awareness of hypoglycemia and severe hypoglycemia among people with insulin-treated type 2 diabetes: the Dutch diabetes pearl cohort. BMJ Open Diabetes Res Care 2020;8:e000935. 10.1136/bmjdrc-2019-000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brod M, Christensen T, Bushnell DM. The impact of non-severe hypoglycemic events on daytime function and diabetes management among adults with type 1 and type 2 diabetes. J Med Econ 2012;15:869–77. 10.3111/13696998.2012.686465 [DOI] [PubMed] [Google Scholar]
- 9. Brod M, Wolden M, Christensen T, et al. A nine country study of the burden of non‐severe nocturnal hypoglycaemic events on diabetes management and daily function. Diabetes Obes Metab 2013;15:546–57. 10.1111/dom.12070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabetes Metab Res Rev 2008;24:87–92. 10.1002/dmrr.796 [DOI] [PubMed] [Google Scholar]
- 11. Hendrieckx C, Gonder-Frederick L, Heller SR, et al. How has psycho-behavioural research advanced our understanding of hypoglycaemia in type 1 diabetes? Diabet Med 2020;37:409–17. 10.1111/dme.14205 [DOI] [PubMed] [Google Scholar]
- 12. Brod M, Højbjerre L, Bushnell DM, et al. Assessing the impact of non-severe hypoglycemic events and treatment in adults: development of the treatment-related impact measure—non-severe hypoglycemic events (TRIM-HYPO). Quality of Life Research 2015;24:2971–84. 10.1007/s11136-015-1023-6 [DOI] [PubMed] [Google Scholar]
- 13. Chatwin H, Broadley M, Valdersdorf Jensen M, et al. 'Never again will I be carefree': a qualitative study of the impact of hypoglycemia on quality of life among adults with type 1 diabetes. BMJ Open Diabetes Res Care 2021;9:e002322. 10.1136/bmjdrc-2021-002322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chatwin H, Broadley M, Speight J, et al. The impact of hypoglycaemia on quality of life outcomes among adults with type 1 diabetes: a systematic review. Diabetes Res Clin Pract 2021;174:108752. 10.1016/j.diabres.2021.108752 [DOI] [PubMed] [Google Scholar]
- 15. Matlock KA, Broadley M, Hendrieckx C, et al. Changes in quality of life following hypoglycaemia in adults with type 2 diabetes: a systematic review of longitudinal studies. Diabet Med 2022;39:e14706. 10.1111/dme.14706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carlton J, Leaviss J, Pouwer F, et al. The suitability of patient-reported outcome measures used to assess the impact of hypoglycaemia on quality of life in people with diabetes: a systematic review using COSMIN methods. Diabetologia 2021;64:1213–25. 10.1007/s00125-021-05382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmes-Truscott E, Skovlund SE, Hendrieckx C, et al. Assessing the perceived impact of diabetes on quality of life: psychometric validation of the DAWN2 impact of diabetes profile in the second diabetes miles – Australia (MILES-2) survey. Diabetes Res Clin Pract 2019;150:253–63. 10.1016/j.diabres.2019.03.020 [DOI] [PubMed] [Google Scholar]
- 18. de Galan BE, McCrimmon RJ, Ibberson M, et al. Reducing the burden of hypoglycaemia in people with diabetes through increased understanding: design of the hypoglycaemia redefining solutions for better liVEs (Hypo-RESOLVE) project. Diabet Med 2020;37:1066–73. 10.1111/dme.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qualtrics . Qualtrics: Provo, Utah, USA. 2020.
- 20. Peyrot M, Burns KK, Davies M, et al. Diabetes attitudes wishes and needs 2 (DAWN2): a multinational, multi-stakeholder study of psychosocial issues in diabetes and person-centred diabetes care. Diabetes Res Clin Pract 2013;99:174–84. 10.1016/j.diabres.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 21. Browne JL, Holmes-Truscott E, Ventura AD, et al. Cohort profiles of the cross-sectional and prospective participant groups in the second diabetes MILES—Australia (MILES-2) study. BMJ Open 2017;7:e012926. 10.1136/bmjopen-2016-012926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gagnier JJ, Lai J, Mokkink LB, et al. COSMIN reporting guideline for studies on measurement properties of patient-reported outcome measures. Quality of Life Research 2021;30:2197–218. 10.1007/s11136-021-02822-4 [DOI] [PubMed] [Google Scholar]
- 23. Nicolucci A, Kovacs Burns K, Holt RIG, et al. Diabetes attitudes, wishes and needs second study (DAWN2™): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med 2013;30:767–77. 10.1111/dme.12245 [DOI] [PubMed] [Google Scholar]
- 24. World Health Organisation . Wellbeing measures in primary health care/the depcare project. Copenhagen: WHO Regional Office for Europe, 1998. [Google Scholar]
- 25. Polonsky WH, Fisher L, Hessler D, et al. Investigating hypoglycemic confidence in type 1 and type 2 diabetes. Diabetes Technol Ther 2017;19:131–6. 10.1089/dia.2016.0366 [DOI] [PubMed] [Google Scholar]
- 26. Grabman J, Vajda Bailey K, Schmidt K, et al. An empirically derived short form of the hypoglycaemia fear survey II. Diabet Med 2017;34:500–4. 10.1111/dme.13162 [DOI] [PubMed] [Google Scholar]
- 27. Speight J, Barendse SM, Singh H, et al. Characterizing problematic hypoglycaemia: iterative design and preliminary psychometric validation of the hypoglycaemia awareness questionnaire (HypoA-Q). Diabet Med 2016;33:376–85. 10.1111/dme.12824 [DOI] [PubMed] [Google Scholar]
- 28. Gold AE, Macleod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703. 10.2337/diacare.17.7.697 [DOI] [PubMed] [Google Scholar]
- 29. McNeish D. Thanks coefficient alpha, we’ll take it from here. Psychol Methods 2018;23:412–33. 10.1037/met0000144 [DOI] [PubMed] [Google Scholar]
- 30. Fan Y, Chen J, Shirkey G, et al. Applications of structural equation modeling (SEM) in ecological studies: an updated review. Ecol Process 2016;5:1–12. 10.1186/s13717-016-0063-3 [DOI] [Google Scholar]
- 31. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care 2011;34:801–6. 10.2337/dc10-1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Speight J, Holmes-Truscott E, Hendrieckx C, et al. Assessing the impact of diabetes on quality of life: what have the past 25 years taught us? Diabet Med 2020;37:483–92. 10.1111/dme.14196 [DOI] [PubMed] [Google Scholar]
- 33. Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome-a review of quality of life measurement in adults with diabetes. Diabet Med 2009;26:315–27. 10.1111/j.1464-5491.2009.02682.x [DOI] [PubMed] [Google Scholar]
- 34. Cooke D, O'Hara MC, Beinart N, et al. Linguistic and psychometric validation of the Diabetes-Specific quality-of-life scale in U.K. English for adults with type 1 diabetes. Diabetes Care 2013;36:1117–25. 10.2337/dc12-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holmes-Truscott E, Cooke DD, Hendrieckx C, et al. A comparison of the acceptability and psychometric properties of scales assessing the impact of type 1 diabetes on quality of life—results of ‘YourSAY: quality of life’. Diabet Med 2021;38:e14524. 10.1111/dme.14524 [DOI] [PubMed] [Google Scholar]
- 36. Petersen E, Nielsen AA, Christensen H, et al. Vejle Diabetes Biobank - a resource for studies of the etiologies of diabetesand its comorbidities. Clin Epidemiol 2016;8:393–413. 10.2147/CLEP.S113419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Søholm U, Broadley M, Zaremba N, et al. Investigating the day-to-day impact of hypoglycaemia in adults with type 1 or type 2 diabetes: design and validation protocol of the Hypo-METRICS application. BMJ Open 2022;12:e051651. 10.1136/bmjopen-2021-051651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rankin D, Elliott J, Heller S, et al. Experiences of hypoglycaemia unawareness amongst people with type 1 diabetes: a qualitative investigation. Chronic Illn 2014;10:180–91. 10.1177/1742395313513911 [DOI] [PubMed] [Google Scholar]
- 39. Speight J, Barendse SM, Singh H, et al. Cognitive, behavioural and psychological barriers to the prevention of severe hypoglycaemia: a qualitative study of adults with type 1 diabetes. SAGE Open Medicine 2014;2:2050312114527443 10.1177/2050312114527443 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2022-002890supp001.pdf (306.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available on reasonable request via contact with the corresponding author (http://orcid.org/0000-0003-4408-6304), provided that data are to be used for research projects related to health sciences. Data are deidentified participant responses from a web-based quantitative survey.