Abstract
Introduction
The prevention and cure of postoperative infections has been a source of study over the years and is currently being studied. In this bibliographic review, a comparison between the different products used for the prevention and treatment of postsurgical infections has been procured, likewise, being able to determine which would be the best option for the treatment of post-surgical infections. In this bibliographic review we focus on Onychocryptosis because it is an emerging problem today. Many surgeries are performed to fix this condition, which increases the risk of infections.
Material
databases, including PubMed and Cochrane Library, as well as websites of international organizations, were searched up to January 2021. The search included studies and trials in humans on the use of hyaluronic acid and antibacterial ointments in various conditions or diseases.
Results
18 articles were analyzed individually, which included randomized studies of Hyaluronic Acid, various antibiotics and honey, and variables used topically. 3 articles were also selected to explain onychocryptosis and postoperative infections.
Conclusion
Despite being able to determine which antibiotic would be the best, and whether hyaluronic acid can be used for the prevention and/or cure of post-surgical infections, this review emphasizes that there is still a need for more specific studies on its use of these variables, both in post-surgical infections in general and in post-surgical onychocryptosis infections.
Keywords: Onychocryptosis, Postsurgical infections, Hyaluronic acid, Antibacterial ointments
Highlights
-
•
It is unclear what is the best AO to treat and/or prevent wound infections following ingrown toenail surgery.
-
•
The purpose of the present study was to review the main AO currently used to prevent or treat postoperative skin infections and to compare these with HA to establish which would be the best option.
-
•
HA has great anti-inflammatory capacities, the ability to create a stable environment for the healing of ulcers and wounds, it provides hydration, elasticity, and firmness to the skin.
-
•
The HA combined with antibiotics, specifically SS, is a very good option for partial burns.
Onychocryptosis; Postsurgical infections; Hyaluronic acid; Antibacterial ointments.
1. Introduction
1.1. Onychocryptosis
Onychocryptosis (OC) accounts for 20% of foot problems (Reyzelman et al., 2000). OC is when the nail plate grows into the periungual folds, causing infection and inflammation. It is more frequent in young adults and teenagers and appears more in the hallux (DeLauro and DeLauro, 2004).
Causes of OC include: trauma, poor hygiene, hyperhidrosis, medications for cancer (Gefitinib, Cetuximab), abnormal shape of the nail plate, anatomical abnormalities (Park and Singh, 2012; Langford et al., 1989) and improper cut (DeLauro and DeLauro, 2004). It is important to highlight the male predominance in OC (2:1). Also, OC occurs twice as frequently in the lateral nail fold than in the medial fold (Ezekian et al., 2017).
Regarding signs and symptoms, in Stage 1, the affected finger is swollen, erythema, and painful. Then, in Stage 2, there is an acute infection with seropurulent drainage and ulceration of the fold. Stage 3 is characterized by chronic infection with hypertrophic granulation tissue, increased secretion, and swelling (Gera et al., 2019; Romero-Pérez et al., 2017).
There are two approaches to treating OC: non-surgical therapy (appropriate for stages 1 and 2) (Mayeaux et al., 2019); and surgical therapy (appropriate for Stage 3 and relapses). Surgical therapy aims to suppress the interaction between the nail fold and the nail plate by partially or completely removing the plate, the fold, or both (Eekhof et al., 2012). Complications associated with surgical therapy for OC include lateralization or complete loss of the nail plate, cysts, recurrences, or infection processes due to poor asepsis (Carmona, 2003).
1.2. Hyaluronic acid
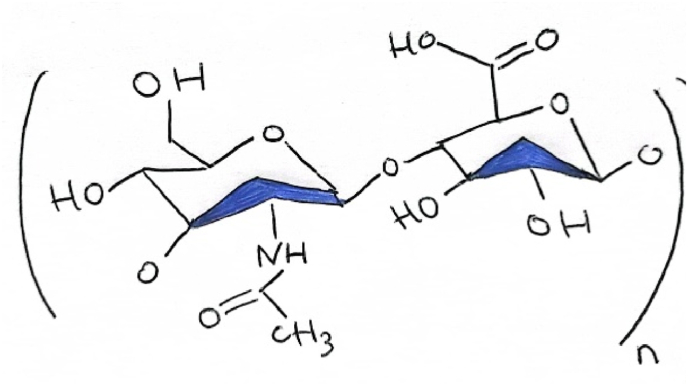
Hyaluronic acid (HA) (C28H44N2O23) is a glucosaminoglycan that consists of D-glucuronic acid and N-acetyl-D-glucosamine disaccharide units linked via a glycoside bond in the arrangement of alternating β-(1 → 4) and β-(1 → 3) bonds (Chen et al., 2018).
Figure 1: Illustration of hyaluronic acid in which we observe formula (C14H21NO11)n. N is Nitrogen, C is Carbon, O is Oxygen and H is Hydrogen.
Figure 1.
HA Formulation.
The HA is found in the body, joints, ligaments, skin, and tendons (Zhu et al., 2020). HA can be used in various formulations and has the potential to treat various inflammatory skin diseases, such as actinic keratosis, foot ulcer, psoriatic arthritis, acute radio-epithelioid, rosacea, superficial keratitis, facial seborrheic dermatitis, epidermal tumour, UV irradiation-induced skin diseases, melanoma, erythema, soft tissue augmentation, and postoperative adhesion (Chen et al., 2018).
The most important characteristics of AH are its biodegradability, biocompatibility, and viscoelasticity. AH has healing, lubricating, anti-inflammatory, moisturizing, and tissue regenerating activities, among others. The acid collaborates with the skin to preserve elasticity, turgor, and moisture (Zhu et al., 2020).
1.3. Antibacterial ointments
Antibiotic prophylaxis is important because it has been demonstrated that patients who do not receive antibiotic prophylaxis are much more likely to suffer a post-surgical infection (PSI) than those who do receive it (Bartella et al., 2018).
Topical antibiotics (TAs) can be found in various forms; they are placed in direct contact with the wound (reviewed in Cochrane Library Cochrane Database of Systematic Reviews Dressings and Topical Agents for Treating Pressure Ulcers, 2017). Also, for superficial wounds, TAs are typically used before systemic antibiotics (Schultz et al., 2003).
Silver sulfadiazine (SS) is an excellent, commercially-available antibiotic and is a standard treatment for burns (Rashaan et al., 2016; Black and Drake, 2015).
Collagenase can degrade necrotic collagen in wounds, helping to form granulation tissue and epithelialization, achieving healing (reviewed in Cochrane Library Cochrane Database of Systematic Reviews Dressings and Topical Agents for Treating Pressure Ulcers, 2017).
Mupirocin has been used for cutaneous infections and nasal decolonization of Staphylococcus aureus. Mupirocin can be considered broad-spectrum, although resistance has been detected (Williamson et al., 2017).
The main uses of fusidic acid are to treat superficial infections and S. Aureus infections. Gram-negative bacteria are often resistant to this antibiotic (Williamson et al., 2017). Neomycin is considered toxic by the systemic route, so it is only used topically (Williamson et al., 2017). Bacitracin is used for superficial bacterial infections. Bacitracin is also an antibiotic that is considered toxic by the systemic route (Williamson et al., 2017). There is little available information for polyhexanide; lengthy application times are necessary to achieve an acceptable bacterial reduction (Agents et al., 2017). Polyhexanide has a healing effect, reduces bacteria, and does not generate resistance (Wessels and Ingmer, 2013).
Table 1: Abstract of Topical Antibiotics, in which are included the bacteria to which they are active or inactive to know in what situations can be used.
Table 1.
Abstract of AO proposed, which will be compared with HA.
| Topical Antibiotics | ACTIVE | INACTIVE |
|---|---|---|
| Silver Sulfadiazine | Gram + (all), Gram –, Candida | – |
| Collagenase | Gram + (all), Gram –, Chlamydia | – |
| Mupirocin | Gram + (Staphylococcus), Streptococcus, Gram – | Streptococcus Bovis, Some Staphylococcus |
| Fusidic Acid | Gram + (all), Corynebacterium, anaerobes Gram +, Neisseria, Moraxella | Gram – |
| Neomicin | Gram + (Staphylococcus), Gram – aerobic | Streptococcus, Bacilli Gram + (all) |
| Bacitracin | Gram + (Staphylococcus Aureus, Streptococcus Pyogenes), Neisseria, Haemophilus | Streptococcus beta-hemolytics, Gram - |
| Polyhexanide | Gram + (all), Gram -, Staphylococcus Aureus, Pseudomonas Aeruginosa | Adenovirus, Candida Albicans |
1.4. Post-surgical infections
PSIs are a common problem, can be difficult to treat, and are associated with high mortality; good PSI prevention and treatment protocols are needed (Dellinger, 2015). PSIs that occur within the first 30 days can be classified as (Dellinger, 2015):
-
1.
Superficial infection of the incision: affects the skin and subcutaneous tissues; it must include at least one of the following things: purulent discharge, bad smell, fever, pain, redness, or increased temperature.
-
2.
Deep infection of the incision: affects deeper tissues. It must include at least one of the following things: purulent discharge, dehiscence, and abscesses.
-
3.
Infection of organs or spaces: affect any organ other than the incision site. It must include at least one of the following things: purulent discharge, abscesses, or organism isolated from the organ.
PSIs risk factors can be classified into two types: risk factors of the patient (e.g., advanced age, steroids, obesity, diabetes, and smoking) and risk factors of the process (e.g., drainage, previous infection, poor asepsis, contamination, hypothermia) (Vitiello et al., 2020). The risk of PSI was much greater before the aseptic techniques became common (Dellinger, 2015).
1.5. Pathophysiology
The contamination of the incision area can be caused by endogenous and endogenous factors (Dellinger, 2015). Endogenous factors include skin, mucous membranes, and viscera. The most common Endogenous factors are S. Aureus, S-negative coagulase, Enterococcus, and Escherichia coli (Owens and Stoessel, 2008). Exogenous factors include surgical room, air, and instrumental factors. The most common exogenous factors are Staphylococcus and Streptococcus (Young and Khadaroo, 2014).
1.5.1. Symptomatology
The symptoms usually appear within 3–7 days after the surgery. Early signals include inflammation, erythema, localized pain, pyrexia, discharge, dehiscence, and poor healing. When infection is suspected, dressings should be removed, a discharge sample collected, and antibiotic treatment started (Dellinger, 2015).
1.5.2. Evaluation
PSIs are diagnosed by clinical examination, but a sample with a swab should always be taken (Dellinger, 2015).
1.5.3. Treatment
Treatment is difficult, which is why there are many prevention protocols (e.g., preparation of the skin, sterility, dressings, and prophylaxis). The patient having good nutritional status and early mobilization can also help prevent PSIs (Berriós-Torres et al., 2017). If the infection is superficial, it can be approached conservatively, but if it is deep, a mixture of measures and interventions is needed to save the patient’s life (Yin et al., 2018).
1.5.4. Complications
Complications can be local or systemic. Locals complications include delayed healing, cellulite, abscesses, the increased opening of the incision, and osteomyelitis. Systemic complications include bacteremia, which can lead to sepsis (Dellinger, 2015).
1.5.5. Perioperative period
Patients are in contact with many professionals who are responsible for the PSI. It is important to recognize the risk factors that can be changed (Dellinger, 2015). During the surgery, all staff are responsible for sterility (Dellinger, 2015). In the postoperative period, all specialists are involved in recovery and PSI rates (Anderson et al., 2017).
2. Methods
2.1. Search processes
A search was made using the PubMed online database to carry out the bibliographic review. To collect the information, the following search terms related to the topic were used: “onychocryptosis”, “ingrown foot nail”, “hyaluronic acid”, “antibacterial ointments”, “mupirocin”, “collagenase”, “silver sulfadiazine”, “fusidic acid”, “neomycin”, “bacitracin”, “polyhexanide”, “post-surgical infection”, and “topic”. As Boolean operators, we used &.
2.1.1. Inclusion and exclusion criteria
The inclusion criteria were: (1) Reviews and systematic reviews were published in the last 5 years, except articles used to expose the information on PSI, for which the search was extended to 10 years. We chose this period of years because the more updated the information is, the more reliable the conclusions we can draw. (2) Content that includes information about HA. (3) Content that includes information about AO.
The exclusion criteria that were used were: (1) All works, studies or articles written in Spanish (except Carmona FjavierG “Surgical treatment of onychocryptosis”, for the importance of the information that has been really useful for this work). (2) All those works, studies or articles that used the products in any way another tan topically.
3. Results
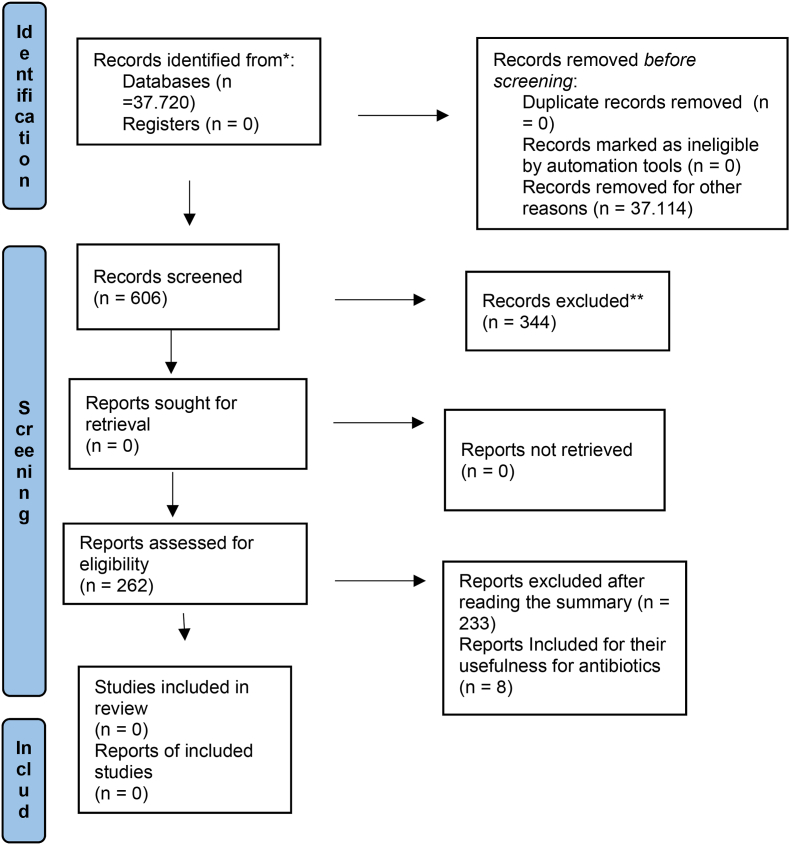
When performing the bibliographic search in the PubMed database and before implementing any filter, many articles were found.
HA: 30,932 articles were found. Introducing the filter “review, systematic review”, 28,072 were excluded. Then, 2823 were eliminated by applying the terms “topic & injuries”. The remaining 37 were filtered to include only the last 5 years and those with full text available, in addition to reading the abstract. Finally, five articles were selected after reading the articles and decided that it was necessary to include them due to their importance.
AO: 3001 articles were found. Introducing the filter “review, systematic review”, 2790 were excluded. One hundred sixty-six articles were eliminated by applying the term “topic”. The remaining 45 were filtered to include only the last 5 years and those with full text available, in addition to reading the abstract. Finally, five articles were selected due to their importance and trying to match the amount of information obtained in HA. Also, seven topical antibiotics were selected within this search, of which an individualized search was made. Two articles were selected from each of them, adding 16 articles to the total count, of which eight were excluded due to their content. The other eight were selected due to the importance of their information and the need for individual information on each article.
OC: 888 articles were found. Introducing the filter “review, systematic review”, 823 articles were excluded. Of the remaining 78, two were selected after narrowing the search to those published in the last 5 years, with the full text available, and reviewing the abstracts.
PSI: 2899 articles were found. Introducing the filter “review, systematic review”, 2440 were excluded. When using the full-text filter and articles published in the last 10 years (the search was expanded due to interest in an article from 2014 from which all the necessary information was obtained), 263 articles remained. After reading the abstracts, only one article was selected. This article was sufficient to obtain all the necessary information to explain the PSIs.
Ultimately, to carry out this bibliographic review, 21 articles were used.
Figure 2: FLOWCHART of the articles that were used to carry out this bibliographic review. Each of the steps taken for the selection of the perfect items are explained in this flowchart.
Figure 2.
Flowchart.
4. Discussion
4.1. hyaluronic acid
Several studies were conducted on humans diagnosed with five or more inflammatory skin lesions (Chen et al., 2018). Rivers et al. (2002) carried out a trial on 195 patients. Half of them then applied an ointment made up of 3% Diclofenac +2.5% HA gel, which was very effective vs. the placebo.
Wolf et al. (2001) carried out a trial on 96 patients. In half, the applied ointment included 3% Diclofenac +2.5% HA gel (vs. placebo). These authors obtained the same results as Rivers et al. (2002).
The following studies were conducted on patients with ulcers due to uncontrolled diabetes. One study compared HA with iodine (Chen et al., 2018). Sobotka et al. (2006) carried out a clinical trial on 22 patients; 18 achieved a completed closure in 2–6 weeks. This group published another article (Sobotka et al., 2007) in which they conducted a study on 18 patients using the same compound, demonstrating the efficacy of HA for wound closure.
Brenes et al. (2011) carried out another study with the HA + iodine compound in 14 patients. Most of the samples obtained a complete closure in 18 weeks; this supports the previous results, in addition to making it clear that iodine + HA provides a stable environment for healing foot ulcers and wounds.
Liguori et al. (1997) conducted a study on 152 patients suffering from acute radio epithelitis. They applied cream with HA that reduced signs and symptoms, reporting exceptional protective effects of HA. Schlesinger and Powell (2013) carried out a study on rosacea in 15 patients. They used a sodium salt cream + HA at 0.2%. As a result, the efficacy was demonstrated with good tolerance.
Koller J. (Topical Treatment of Partial Thickness Burns by Silver Sulfadiazine plus Hyaluronic Acid Compared to Silver Sulfadiazine Alone: A Double-Blind, Clinical Study - PubMed, n.d.) conducted a trial with 33 patients comparing the efficacy of 1.5% silver sulfadiazine (SS) + 0.2% HA and the efficacy of 1% SS alone. Patients 18–80 years old were included, and the mean healing time in the first group was 8167 days (vs. 13,067 for the second group).
Costagliola and Agrosì (2005) conducted a trial with 111 patients comparing the efficacy of 1.5% SS + 0.2% HA and the efficacy of 1% SS alone. Patients 18–75 years old were included, and the mean healing time was 9.5 days in the first group (vs. 14 days in the second group).
Samples from patients with partial burns were used, concluding that adding HA to regularly used topical formulations significantly decreases the mean time needed for healing (Fátima et al., 2016).
Table 2: In this table we observe all the information of the authors of HA that it is Hyaluronic Acid that we have collected during the review. Collaborators is reflected with the acronym Al. and Silver sulfadiazine is described with SS. Finally, we used the acronym VS (versus) to compared treatments.
Table 2.
Abstract of the Authors who speak and study the HA based on articles in the bibliography.
| Authors | Patients | Disease or condition | Product | Results |
|---|---|---|---|---|
| Rivers et al. (Rivers et al., 2002) | 195 | Actinic Keratosis | Ointment of Diclofenac 3% + HA 2,5% VS placebo | Effective results |
| Wolf et al. (Wolf et al., 2001) | 96 | Actinic Keratosis | Diclofenac 3% + HA 2,5% VS placebo | Effective results |
| Gebauer et al. (Gebauer et al., 2003) | 150 | Actinic Keratosis | Diclofenac 3% + HA 2,5% VS placebo | Effective results |
| Sobotka et al. (Sobotka et al., 2006, 2007) | 22/18 | Diabetic foot ulcers | Ha topical + Iodine | Complete closure in 2–6 weeks |
| Brenes et al. (Brenes et al., 2011) | 14 | Diabetic foot ulcers | Ha topical + Iodine | Complete closure in 18 weeks, provides a stable environment |
| Liguori et al. (Liguori et al., 1997) | 152 | Acute radioepithelitis | Cream of HA | Significant reduction in signs and symptoms |
| Schlesinger y Powell. (Schlesinger and Powell, 2013) | 15 | Rosacea | Sodium salt cream with 0,2% HA | Remarkable efficacy and Good tolerance |
| Jegasothy et al. (Jegasothy et al., 2014) | 33 | Wrinkles and erythema | HA topical use 8 weeks | Improved elasticity, hydration and firmness. Decrease in erythema, wrinkles and roughness |
| Varalakshmi (Varalakshmi, n.d.) | – | Facial seborrheic dermatitis | Low molecular weight HA compound gel | Proven efficacy and safety |
| Koller J. (Topical Treatment of Partial Thickness Burns by Silver Sulfadiazine plus Hyaluronic Acid Compared to Silver Sulfadiazine Alone: A Double-Blind, Clinical Study - PubMed, n.d.) | 33 | Partial burns | SS al 1,5% + HA al 0,2% VS SS al 1% | Healing average 8167 days for the group with HA and 13,067 for the group without HA |
| Costagliola y Agrosi. (Costagliola and Agrosì, 2005) | 111 | Partial burns | SS al 1,5% + HA al 0,2% VS SS al 1% | Healing average 9,5 days for the group with HA and 14 for the group without HA |
4.2. Antibacterial ointments
Concerning AO, various studies have been collected on humans. Grothe et al. (2016) conducted nine studies that included 187 patients receiving dialysis, studying the effect of different compounds on catheter insertion site prophylaxis. They made three types of comparisons: Topical mupirocin vs. placebo or no treatment; AO unspecified vs. placebo or no treatment; and Topical mupirocin vs. AO unspecified. The results were 74%, 56%, and 52% less likely to develop an infection, respectively.
In addition, they carried out more studies with other antibiotics; using gentamicin and cefazolin together decreased episodes of infections. Fusidic acid was evaluated separately, showing significant results, but it is contraindicated as monotherapy.
Heal et al. (2014) carried out several trials on various wounds made surgically. AO vs. non-topical antibiotic: eight trials were conducted with 5.427 participants. The results demonstrated the AO’s efficacy; infections were reduced in the first group. The second group received an inert antibiotic. AO vs. topical antiseptic: Five random trials involving 1299 participants were conducted, using one product or the other. The result was less PSI in the patients treated with AO than in those treated with antiseptics. AO vs. alternative topical antibiotic: a study of 99 patients was carried out comparing mupirocin and neomycin. There was no significant difference between these antibiotics in the risk of PSI. Another study was carried out on 219 participants, using a combined ointment of neomycin sulfate, bacitracin zinc and polymyxin sulfate vs. bacitracin zinc; no significant differences were found.
Several studies and trials have been conducted on prophylaxis at the dialysis catheter insertion site; Johnson et al. (2002), Sesso et al. (Staphylococcus Aureus Prophylaxis in Hemodialysis Patients Using Central Venous Catheter: Effect of Mupirocin Ointment - PubMed, n.d.), and Wong et al. (Prophylaxis against Gram-Positive Organisms Causing Exit-Site Infection and Peritonitis in Continuous Ambulatory Peritoneal Dialysis Patients by Applying Mupirocin Ointment at the Catheter Exit Site - PubMed, n.d.) made a comparison between mupirocin and no prophylaxis; while Bernardini et al. (2005) made a comparison between topical mupirocin and gentamicin. Bernardini et al. (1996) also made a comparison with oral rifampicin.
In the first studies, mupirocin showed a significant reduction in infections concerning the use of no prophylaxis. It acted mainly against Gram-positive bacteria, specifically S. aureus, so it was concluded that mupirocin prevents this infection. In the following, no significant differences were found in the prevention of infections, so it was concluded that mupirocin, gentamicin and rifampicin similarly prevented catheter infections. Gentamicin reduced the chances of getting Gram-negative bacterial infections, and rifampicin caused many secondary effects.
Several trials were conducted on surgical patients to study the effectiveness of prophylactic mupirocin in preventing nosocomial disease. Two types of studies were carried out in which both carriers and non-carriers of S. aureus were used. Perl et al. (2002), Kalmeijer et al. (2002), Konvalinka et al. (2006), García et al. ((PDF) Use of Nasal Mupirocin for Staphylococcus Aureus: Effect on Nasal Carriers and Nosocomial Infections, n.d.), and Suzuki et al. (2003) compared mupirocin in the form of intranasal ointment and placebo or no prophylaxis. When comparing carriers and non-carriers of S. aureus, mupirocin did not show a significant reduction in the risk of contracting a nosocomial disease. On the contrary, when compared only in carriers of S. aureus, Perl et al. showed that mupirocin was more effective than the placebo.
Shuman et al. (2012), Tai et al. (2013), and Bode et al. (2010) compared intranasal mupirocin combined with chlorhexidine gel and no prophylaxis. Bode et al. (2010) concluded that mupirocin with chlorhexidine was more effective than placebo in patients with S. aureus. Tai et al. (2013) reported similar results, so they also concluded that mupirocin with chlorhexidine is more effective than no prophylaxis.
In summary, Troeman et al. (2019) state that intranasal mupirocin shows evidence of its effectiveness against nosocomial diseases in carriers of S. aureus. Although a statistically significant reduction in PSI caused by S. aureus was not achieved, the use of mupirocin as preoperative prophylaxis was supported.
Studies were conducted to assess the effectiveness of chlorhexidine gluconate as prophylaxis to prevent nosocomial infections caused by S. aureus. Hayek et al. (1987) carried out a study in which chlorhexidine gluconate did not show significant differences compared to placebo; the patients received two baths prior to surgery. Therefore, it was concluded that chlorhexidine, without combination with another agent, cannot prevent PSIs. However, changes had to be made to the placebos used, and the results should be interpreted cautiously.
A later study by Climo et al. (2013) compared chlorhexidine body wash with a non-antimicrobial agent in preventing the acquisition of healthcare-associated-bloodstream infections (HA-BSIs) caused by coagulase-negative staphylococci and fungi. Chlorhexidine was found to significantly reduce HA-BSI acquisition compared to the non-antimicrobial agent. However, it did not show a reduction of HA-BSI caused by S. aureus.
Segers et al. (2006) included patients who were going to undergo sternotomy for cardiothoracic surgery; they were administered prophylactic therapy with chlorhexidine gluconate nasal ointment vs. placebo. It was administered from the first day until the day after the operation. Patients received antibiotic therapy as usual protocol. Four hundred eighty-five patients received chlorhexidine ointment and 469 placeboes; chlorhexidine was more effective than placebo in preventing nosocomial diseases in general, although it did not show statistically significant efficacy against nosocomial diseases caused by S. aureus. In conclusion, this ointment can reduce PSIs but may not reduce the risk of PSIs due to S. aureus.
Findlay et al. (2013) investigated polyhexanide vs. mupirocin in dialysis patients. They found a higher frequency of infections in the polyhexanide group, especially Pseudomonas and S. aureus, although it should be noted that there were many patients with diabetes relative to the other group.
About SP, several studies have been carried out on patients with burns. Wattanaploy et al. (2017) randomly assigned two groups of 23 patients and followed up for 22 days, comparing SP and polyhexanide + iodine gel (Prontosan). No significant differences were found. Huang et al. (2007) compared SP with acticoat in 98 patients. They obtained a shorter healing time in the group treated with acticoat than in the groups treated with SP (12.42 days vs. 15.79). The acticoat dressing can be kept for 3 days, so it was considered a good treatment option.
Maciel et al. (2019) reported that SP had shown excellent effectiveness as a bactericide, in addition to being well accepted by burn centres. Various products have been compared, such as aloe vera, aquacel, and centiderm, which have shown efficacy compared to SP, although it is considered too early to declare their efficacy.
Regarding collagenase, Soroff and Sasvary (1994) carried out a study on 15 patients with burns comparing collagenase with SP. The results were that the patients treated with collagenase took less time to cleanse and heal than those treated with SP. Hansbrough et al. (1995) carried out a study of 79 patients in which they compared collagenase + polymyxin sulfate vs. SP.
Koning et al. (2012) conducted a review in which they included studies by authors who treated patients with impetigo. The first studies were conducted on patients with non-bullous impetigo. The first comparisons were made between AOs and placebo: Mupirocin vs. placebo, more improvement with mupirocin (Eells et al., 1986), Gould (Gould: Mupirocin in General Practice: A Placebo-Controlled... - Google Académico, n.d.); fusidic acid vs. placebo, more improvement with fusidic acid (Koning et al., 2012); retapamulin vs. placebo, more improvement with retapamulin (Koning et al., 2012).
The second type of comparison was between AOs: gentamicin vs. neomycin, gentamicin showed superior results to neomycin (Farah et al. 1967); retapamulin vs. fusidic acid, with no significant differences (Oranje et al., 2007).
The third type of comparison was made between AO and oral antibiotics: mupirocin vs. erythromycin, with more improvement with mupirocin (Koning et al., 2012); mupirocin vs. dicloxacillin, with no significant differences (Arredondo, 1987); mupirocin vs. ampicillin, no significant differences (Welsh and Saenz, 1987); bacitracin vs. cefalexin, bacitracin was worse than oral cefalexin (Bass et al., 1997); bacitracin vs. penicillin, no significant differences (Ruby and Nelson, 1973).
The fourth type of comparison was between AO and antiseptics: bacitracin vs. hexachlorophene, with no significant differences (Ruby and Nelson, 1973); fusidic acid vs. hydrogen peroxide, the fusidic acid cream showed more efficacy, but a statistically significant efficacy was not reached (Christensen and Anehus, 1994).
The fifth type of comparison was between AO and topical antifungals: mupirocin vs. terbinafine, with no significant differences (Ciftci et al., 2002).
The sixth type of comparison was between AO + oral antibiotic and AO + oral antibiotic, using multiple combinations. No comparison showed significant differences (Kuniyuki et al., 2005): topical tetracycline + oral cefdinir vs. topical tetracycline + oral minomycin; topical tetracycline + oral cefdinir vs. topical tetracycline + oral fosfomycin; and topical tetracycline + oral minomycin vs. topical tetracycline + oral fosfomycin.
The following studies were performed on patients with bullous impetigo. Between AO vs. another AO (Moraes Barbosa: Comparative Study between Topical... - Google Académico, n.d.): fusidic acid vs. neomycin, fusidic acid was significantly more effective; fusidic acid vs. bacitracin, fusidic acid was significantly more effective; chloramphenicol vs. neomycin, no significant differences; chloramphenicol vs. bacitracin: no significant differences. Between AO vs. oral antibiotic (Moraes Barbosa: Comparative Study between Topical... - Google Académico, n.d.): neomycin vs. oral erythromycin, AO was significantly less effective; bacitracin vs. oral erythromycin, AO was significantly less effective; fusidic acid vs. oral erythromycin, no significant differences; chloramphenicol vs. oral erythromycin, no significant differences.
Finally, studies were carried out on patients with secondary impetigo: Wachs et al. and Wachs and Maibach (1976) compared mupirocin and oral cephalexin and detected no significant differences.
Table 3: In this table we observe all the information of the authors of antibiotical Ointments wich is reflected with the acronym AO that we have collected during the review. Collaborators are described with AL., Silver sulfadiazine use the acronym SS and Postsurgical infections are reflected with PSI. Finally, we used the acronym VS (versus) to compared treatments.
Table 3.
Abstract of the authors who speak and studies about topical antibiotics based on articles in the bibliography.
| Authors | Patients | Disease, condition or treatment | Product | Results |
|---|---|---|---|---|
| Grothe et al. (Grothe et al., 2016) | 187 | Dialysis | Topical Mupirocin VS placebo AO unspecified VS placebo Topical Mupirocin VS AO unspecified |
74%, 56% y 52% les likely to develop an infection respectively |
| Grothe et al. (Grothe et al., 2016) | – | Dialysis | Gentamicin + Cefazolin Fusidic Acid |
Decrease in infections Fusidic Acid showed significant results but is contraindicated as monotherapy |
| Heal et al. (Heal et al., 2016) | 5427 y 1034 | Surgical wound | AO VS non AO | Demonstrated efficacy of AO |
| Heal et al. (Heal et al., 2016) | 1299 | Surgical wound | AO VS topical antiseptic | Demonstrated efficacy of AO |
| Heal et al. (Heal et al., 2016) | 99 y 219 | Surgical wound | AO VS AO | No significant differences were found between Mupirocin, Neomycin, Bacitracin, Polymyxin |
| Bernardini et al. (Bernardini et al., 1996, 2005) | – | Dialysis | Mupirocin VS Gentamicin Mupirocin VS oral Rifampicin |
No significant differences were found in infection prevention. Gentamicin reduced Gram – infections and Rifampicin caused adverse reactions |
| Perl et al. (Perl et al., 2002), Kalmeijer et al. (Kalmeijer et al., 2002), Konvalinka et al. (Konvalinka et al., 2006), García et al. ((PDF) Use of Nasal Mupirocin for Staphylococcus Aureus: Effect on Nasal Carriers and Nosocomial Infections, n.d.), Suzuki et al. (Suzuki et al., 2003) | – | Surgical patients | Mupirocina intranasal ointment VS placebo or no prophylaxis | Mupirocin did not show significant effectiveness against nosocomial diseases |
| Perl et al. (Perl et al., 2002) | – | Surgical patients carrying Staphylococcus Aureus | Mupirocin intranasal ointment VS placebo | They demonstrated the efficacy of Mupirocin against placebo |
| Shuman et al. (Shuman et al., 2012), Tai et al. (Tai et al., 2013), Bode et al. (Bode et al., 2010) | – | Surgical patients carrying Staphylococcus Aureus | Intranasal Mupirocin + Chlorhexidine gel VS no prophylaxis | The efficacy of Mupirocin + Chlorhexidine was demonstrated |
| Hayek et al. (Hayek et al., 1987) | – | Surgical patients | Chlorhexidine Gluconate VS placebo Bath were held |
It did not show significant differences in the prevention of PSIs. He concluded that its use alone was not effective. |
| Climo et al. (Climo et al., 2013) | – | Surgical patients | Chlorhexidine gel VS non-antimicrobial agent | Chlorhexidine reduced HA-BSI acquisition by negative Staphylococci or fungi, but did not reduce HA-BSI causes by S. Aureus |
| Segers et al. (Segers et al., 2006) | 954 | Surgical patients | Chlorhexidine Gluconate Nasal Ointment VS placebo ointment | Chlorhexidine Gluconate was more effective in the prevention of nosocomial diseases but it was not as effective against the diseases produced by the S. Aureus. |
| Findlay et al. (Findlay et al., 2013) | – | Dialysis | Polyhexanide VS Mupirocin | More infections in the Polyhexanide group |
| Wattanaploy et al. (Wattanaploy et al., 2017) | 23 | Burns | SS VS Polyhexanide gel + iodine (Pontosan) | No significant differences were found |
| Muangman et al. (Muangman et al., 2010) | 70 | Burns | SS VS Aquacel | Less healing time in patients with SS |
| Huang et al. (Huang et al., 2007) | 98 | Burns | SS VS Acticoat | The dressing got les healing time and can be left on for several days |
| Soroff y Sasvary (Soroff and Sasvary, 1994) | 15 | Burns | Collagenase VS SS | Patients treated with Collagenase take les time to have the wound clean and healed |
| Hansbrough et al. (Hansbrough et al., 1995) | 79 | Burns | Collagenase + Polymyxin Sulfate VS SS | Those treated with Collagenase + Polymyxin Sulfate took les time to clean and heal the wound |
| Koning et al. (Koning et al., 2012) | – | Non-bullous impetigo | Fusidic Acid VS placebo Retapamulin VS placebo |
More improvement with AO. |
| Eells et al. (Eells et al., 1986), Gould. (Gould: Mupirocin in General Practice: A Placebo-Controlled... - Google Académico, n.d.) | – | Non-bullous impetigo | Mupirocin VS placebo | More improvement with Mupirocin. |
| Ruby et al. (Ruby and Nelson, 1973) | – | Non-bullous impetigo | Bacitracin VS placebo | No significant differences were found. |
| Farah et al. (Farah et al., 1967) | – | Non-bullous impetigo | Gentamicin VS Neomicin | Gentamicin showed better results. |
| Oranje et al. (Oranje et al., 2007) | 519 | Non-bullous impetigo | Retapamulin VS Fusidic Acid | No significant differences were found |
| Koning et al. (Koning et al., 2012) | – | Non-bullous impetigo | Mupirocin VS oral Erythromycin | More improvement with Mupirocin. |
| Arredondo. (Arredondo, 1987) | – | Non-bullous impetigo | Mupirocin VS oral Dicloxacillin | No significant differences were found |
| Bass et al. (Bass et al., 1997) | – | Non-bullous impetigo | Mupirocin VS oral Cephalexin Bacitracin VS oral Cephalexin |
No significant differences were found. Bacitracin was worse tan Oral Cephalexin |
| Welsh et al. (Welsh and Saenz, 1987) | – | Non-bullous impetigo | Mupirocin VS oral Ampicillin | No significant differences were found |
| Koranyi et al. (Evaluation of Bacitracin Ointment in the Treatment of Impetigo - PubMed, n.d.) | – | Non-bullous impetigo | Bacitracin VS oral Erythromycin | No significant differences were found |
| Ruby et al. (Ruby and Nelson, 1973) | – | Non-bullous impetigo | Bacitracin VS oral Penicillin | No significant differences were found |
| Ruby et al. (Ruby and Nelson, 1973) | – | Non-bullous impetigo | Bacitracin VS Hexachlorophene (antiseptic) | No significant differences were found |
| Christensen et al. (Christensen and Anehus, 1994) | – | Non-bullous impetigo | Fusidic Acid VS Hydrogen Peroxide (antiseptic) | Fusidic Acid showed more efficacy |
| Ciftci et al. (Ciftci et al., 2002) | – | Non-bullous impetigo | Mupirocin VS Terbinafine (antifungal) | No significant differences were found |
| Kuniyuki et al. (Kuniyuki et al., 2005) | – | Non-bullous impetigo | Topical Tetracycline + Oral Cefdinir VS Topical Tetracycline + Oral Minomycin Topical tetracycline + Oral Cefdinir VS Topical Tetracycline + Oral Fosfomycin Topical Tetracycline + Oral Minomycin VS Topical Tetracycline + Oral Fosfomycin |
No comparison showed significant differences |
| Moraes Barbosa (Moraes Barbosa: Comparative Study between Topical... - Google Académico, n.d.) | – | Bullous Impetigo | Fusidic Acid VS Neomycin Fusidic Acid VS Bacitracin Chloramphenicol VS Neomycin Chloramphenicol VS Bacitracin |
Fusidic Acid was more effective in both comparisons. In the following two, no significant differences were found between the AO. |
| Moraes Barbosa (Moraes Barbosa: Comparative Study between Topical... - Google Académico, n.d.) | – | Bullous Impetigo | Neomycin VS Oral Erythromycin Bacitracin VS Oral Erythromycin Fusidic Acid VS Oral Erythromycin Chloramphenicol VS Oral Erythromycin |
Neomycin and Bacitracin was significantly les effective. The rest of the comparisons did not find significant differences |
| Wachs et al. (Wachs and Maibach, 1976) | – | Secondary impetigo | Mupirocin VS Oral Cephalexin | No significant differences were found |
5. Conclusions
HA has great anti-inflammatory capacities, creating a stable environment for the healing of ulcers and wounds, and provides hydration, elasticity, and firmness to the skin. Also, HA combined with antibiotics, specifically SS, is a good option for partial burns. However, studies are needed before recommending HA over AO; we found no studies addressing PSI and HA.
Based on our review, mupirocin is the best available topical antibiotic for the prevention and treatment of PSIs. Collagenase is also very effective but is expensive. Therefore, it would be useful to carry out further studies comparing mupirocin and collagenase.
Various antibiotics are useful for treating impetigo (infectious skin disease). For treating bullous impetigo, mupirocin or fusidic acid are best; for non-bullous impetigo, fusidic acid is best, and for secondary impetigo, mupirocin is recommended.
However, all antibiotics demonstrate efficacy in preventing PSIs; due to their healing and antibiotic capacities, they are recommended to treat any PSI. Studies focused on the treatment of PSIs of OC might help clinicians choose specific antibiotics.
PSIs are a frequent problem that has long been studied. PSIs can be prevented and, when they occur, can be treated in multiple ways. Researchers are continuing to investigate alternatives to AOs, such as HA.
Declarations
Author contribution statement
Almudena Núñez Fernández, Rubén Sánchez-Gómez: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Ignacio Zaragoza García: Performed the experiments; Wrote the paper.
Álvaro Gómez Carrión: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Paola Sanz Wozniak, Arturo Gómez Lara, Alvaro Saura-Sempere:Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Carlos Martínez Sebastián: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Agents A., Williamson D., Carter G.P., Howden B.P. Antibacterianos y antisépticos tópicos actuales y emergentes: agentes, acción y patrones de resistencia. Clin. Microbiol. Rev. 2017;30(3):827–860. doi: 10.1128/CMR.00112-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P.A., Savage J.W., Vaccaro A.R., Radcliff K., Arnold P.M., Lawrence B.D., Shamji M.F. Prevention of surgical site infection in spine surgery. Clin. Neurosurg. 2017;80(3):S114–S123. doi: 10.1093/neuros/nyw066. [DOI] [PubMed] [Google Scholar]
- Arredondo J.L. Efficacy and tolerance of topical mupirocin compared with oral dicloxacillin in the treatment of primary skin infections. Curr. Ther. Res. 1987;41(1) [Google Scholar]
- Bartella A.K., Lemmen S., Burnic A., Kloss-Brandstätter A., Kamal M., Breisach T., Hölzle F., Lethaus B. Influence of a strictly perioperative antibiotic prophylaxis vs a prolonged postoperative prophylaxis on surgical site infections in maxillofacial surgery. Infection. 2018;46(2):225–230. doi: 10.1007/s15010-017-1110-4. [DOI] [PubMed] [Google Scholar]
- Bass J.W., Chan D.S., Creamer K.M., Thompson M.W., Malone F.J., Becker T.M., Marks S.N. Comparison of oral cephalexin, topical mupirocin and topical bacitracin for treatment of impetigo. Pediatr. Infect. Dis. J. 1997;16(7):708–710. doi: 10.1097/00006454-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Bernardini J., Piraino B., Holley J., Johnston J.R., Lutes R. A randomized trial of Staphylococcus aureus prophylaxis in peritoneal dialysis patients: mupirocin calcium ointment 2% applied to the exit site versus cyclic oral rifampin. Am. J. Kidney Dis. 1996;27(5):695–700. doi: 10.1016/s0272-6386(96)90105-5. [DOI] [PubMed] [Google Scholar]
- Bernardini J., Bender F., Florio T., Sloand J., PalmMontalbano L., Fried L., Piraino B. Randomized, double-blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J. Am. Soc. Nephrol. 2005;16(2):539–545. doi: 10.1681/ASN.2004090773. [DOI] [PubMed] [Google Scholar]
- Berriós-Torres S.I., Umscheid C.A., Bratzler D.W., Leas B., Stone E.C., Kelz R.R., Reinke C.E., Morgan S., Solomkin J.S., Mazuski J.E., Dellinger E.P., Itani K.M.F., Berbari E.F., Segreti J., Parvizi J., Blanchard J., Allen G., Kluytmans J.A.J.W., Donlan R., Schecter W.P. Centers for disease control and prevention guideline for the prevention of surgical site infection. JAMA Surg. 2017;152(8):784–791. doi: 10.1001/jamasurg.2017.0904. American Medical Association. [DOI] [PubMed] [Google Scholar]
- Black J.S., Drake D.B. A prospective randomized trial comparing silver sulfadiazine cream with a water-soluble polyantimicrobial gel in partial-thickness burn wounds. Plast. Surg. Nurs. 2015;35(1):46–49. doi: 10.1097/PSN.0000000000000081. [DOI] [PubMed] [Google Scholar]
- Bode L.G.M., Kluytmans J.A.J.W., Wertheim H.F.L., Bogaers D., Vandenbroucke-Grauls C.M.J.E., Roosendaal R., Troelstra A., Box A.T.A., Voss A., van der Tweel I., van Belkum A., Verbrugh H.A., Vos M.C. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 2010;362(1):9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- Brenes R.A., Ajemian M.S., Macaron S.H., Panait L., Dudrick S.J. Initial experience using a hyaluronate-iodine complex for wound healing. Am. Surg. 2011;77(3):355–359. [PubMed] [Google Scholar]
- Carmona F. Javier.G. Grupo Aula Médica; 2003. Tratamiento quirúrgico de la onicocriptosis. [Google Scholar]
- Chen L.H., Xue J.F., Zheng Z.Y., Shuhaidi M., Thu H.E., Hussain Z. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: a review of recent developments and critical appraisal of preclinical and clinical investigations. Int. J. Biol. Macromol. 2018;116:572–584. doi: 10.1016/j.ijbiomac.2018.05.068. [DOI] [PubMed] [Google Scholar]
- Christensen O.B., Anehus S. Hydrogen peroxide cream: an alternative to topical antibiotics in the treatment of impetigo contagiosa. Acta Derm. Venereol. 1994;74(6):460–462. doi: 10.2340/0001555574460462. [DOI] [PubMed] [Google Scholar]
- Ciftci E., Guriz H., Aysev A.D. Mupirocin vs terbinafine in impetigo. Indian J. Pediatr. 2002;69(8):679–682. doi: 10.1007/BF02722704. [DOI] [PubMed] [Google Scholar]
- Climo M.W., Yokoe D.S., Warren D.K., Perl T.M., Bolon M., Herwaldt L.A., Weinstein R.A., Sepkowitz K.A., Jernigan J.A., Sanogo K., Wong E.S. Effect of daily chlorhexidine bathing on hospital-acquired infection. N. Engl. J. Med. 2013;368(6):533–542. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Library . 2017. Cochrane Database of Systematic Reviews Dressings and Topical Agents for Treating Pressure Ulcers (Review) Dressings and Topical Agents for Treating Pressure Ulcers (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagliola M., Agrosì M. Second-degree burns: a comparative, multicenter, randomized trial of hyaluronic acid plus silver sulfadiazine vs. silver sulfadiazine alone. Curr. Med. Res. Opin. 2005;21(8):1235–1240. doi: 10.1185/030079905X56510. [DOI] [PubMed] [Google Scholar]
- DeLauro N.M., DeLauro T.M. Onychocryptosis. Clin. Podiatr. Med. Surg. 2004;21(4):617–630. doi: 10.1016/j.cpm.2004.05.009. W.B. Saunders. [DOI] [PubMed] [Google Scholar]
- Dellinger E.P. Clinical Infectious Disease. second ed. Cambridge University Press; 2015. Postoperative wound infections; pp. 729–733. [Google Scholar]
- Eekhof J.A., van Wijk B., Knuistingh Neven A., van der Wouden J.C. Interventions for ingrowing toenails. Cochrane Database Syst. Rev. 2012;4 doi: 10.1002/14651858.CD001541.pub3. [DOI] [PubMed] [Google Scholar]
- Eells L.D., Mertz P.M., Piovanetti Y., Pekoe G.M., Eaglstein W.H. Topical antibiotic treatment of impetigo with mupirocin. Arch. Dermatol. 1986;122(11):1273–1276. [PubMed] [Google Scholar]
- Ezekian B., Englum B.R., Gilmore B.F., Kim J., Leraas H.J., Rice H.E. Onychocryptosis in the pediatric patient: review and management techniques. Clin. Pediatr. 2017;56(2):109–114. doi: 10.1177/0009922816678180. SAGE Publications Inc. [DOI] [PubMed] [Google Scholar]
- Farah F.S., Kurban A.K., Malak J.A., Shehadeh N.H. The treatment oe pyodeema with gentamicin. Br. J. Dermatol. 1967;79(2):85–88. doi: 10.1111/j.1365-2133.1967.tb11460.x. [DOI] [PubMed] [Google Scholar]
- Fátima F. de, Petz C., Santos M.C., Dalmedico M.M. Hyaluronic acid covers in burn treatment; a systematic review. Rev. Esc. Enferm. USP. 2016;50(3):519–524. doi: 10.1590/S0080-623420160000400020. [DOI] [PubMed] [Google Scholar]
- Findlay A., Serrano C., Punzalan S., Fan S.L. Increased peritoneal dialysis exit site infections using topical antiseptic polyhexamethylene biguanide compared to mupirocin: results of a safety interim analysis of an open-label prospective randomized study. Antimicrob. Agents Chemother. 2013;57(5):2026–2028. doi: 10.1128/AAC.02079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García, et al. (PDF) Use of Nasal Mupirocin for Staphylococcus aureus: Effect on Nasal Carriers and Nosocomial Infections. https://www.researchgate.net/publication/10651372_Use_of_nasal_mupirocin_for_Staphylococcus_aureus_effect_on_nasal_carriers_and_nosocomial_infections n.d.; Retrieved April 20, 2021, from.
- Gebauer K., Brown P., Varigos G. Topical diclofenac in hyaluronan gel for the treatment of solar keratoses. Australas. J. Dermatol. 2003;44(1):40–43. doi: 10.1046/j.1440-0960.2002.00635.x. [DOI] [PubMed] [Google Scholar]
- Gera S.K., Pg Zaini D.H., Wang S., Abdul Rahaman S.H.B., Chia R.F., Lim K.B.L. Ingrowing toenails in children and adolescents: is nail avulsion superior to nonoperative treatment? Singap. Med. J. 2019;60(2):94–96. doi: 10.11622/smedj.2018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould Gould: Mupirocin in General Practice: a Placebo-Controlle... - Google Académico. https://scholar.google.com/scholar?cluster=14977840232457314197&hl=es&as_sdt=2005&sciodt=0 5; n.d.; Retrieved April 26, 2021, from.
- Grothe C., Taminato M., Belasco A., Sesso R., Barbosa D. Prophylactic treatment of chronic renal disease in patients undergoing peritoneal dialysis and colonized by Staphylococcus aureus: a systematic review and meta-analysis. BMC Nephrol. 2016;17(1):115. doi: 10.1186/s12882-016-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansbrough J.F., Achauer B., Dawson J., Himel H., Luterman A., Slater H., Levenson S., Salzberg C.A., Hansbrough W.B., Doré C. Wound healing in partial-thickness burn wounds treated with collagenase ointment versus silver sulfadiazine cream. J. Burn Care Rehabil. 1995;16(3):241–247. doi: 10.1097/00004630-199505000-00004. [DOI] [PubMed] [Google Scholar]
- Hayek L.J., Emerson J.M., Gardner A.M.N. A placebo-controlled trial of the effect of two preoperative baths or showers with chlorhexidine detergent on postoperative wound infection rates. J. Hosp. Infect. 1987;10(2):165–172. doi: 10.1016/0195-6701(87)90143-5. [DOI] [PubMed] [Google Scholar]
- Heal C.F., van Driel M.L., Lepper P.D., Banks J.L. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst. Rev. 2014;2014(12) doi: 10.1002/14651858.CD011426.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal C.F., Banks J.L., Lepper P.D., Kontopantelis E., van Driel M.L. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst. Rev. 2016;2016(11) doi: 10.1002/14651858.CD011426.pub2. John Wiley & Sons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Li X., Liao Z., Zhang G., Liu Q., Tang J., Peng Y., Liu X., Luo Q. A randomized comparative trial between Acticoat and SD-Ag in the treatment of residual burn wounds, including safety analysis. Burns. 2007;33(2):161–166. doi: 10.1016/j.burns.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Jegasothy S.M., Zabolotniaia V., Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J. Clin. Aesth. Dermatol. 2014;7(3):27–29. /pmc/articles/PMC3970829/ [PMC free article] [PubMed] [Google Scholar]
- Johnson D.W., MacGinley R., Kay T.D., Hawley C.M., Campbell S.B., Isbel N.M., Hollett P. A randomized controlled trial of topical exit site mupirocin application in patients with tunnelled, cuffed haemodialysis catheters. Nephrol. Dial. Transplant. 2002;17(10):1802–1807. doi: 10.1093/ndt/17.10.1802. [DOI] [PubMed] [Google Scholar]
- Kalmeijer M.D., Coertjens H., van Nieuwland-Bollen P.M., Bogaers-Hofman D., de Baere G.A.J., Stuurman A., van Belkum A., Kluytmans J.A.J.W. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin. Infect. Dis. 2002;35(4):353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- Koller J. Topical Treatment of Partial Thickness burns by Silver Sulfadiazine Plus Hyaluronic Acid Compared to Silver Sulfadiazine Alone: a Double-Blind, Clinical Study - PubMed. https://pubmed.ncbi.nlm.nih.gov/15700744/ n.d.; Retrieved April 11, 2021, from. [PubMed]
- Koning S., van der Sande R., Verhagen A.P., van Suijlekom-Smit L.W., Morris A.D., Butler C.C., Berger M., van der Wouden J.C. Interventions for impetigo. Cochrane Database Syst. Rev. 2012;2012(1) doi: 10.1002/14651858.CD003261.pub3. John Wiley & Sons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konvalinka A., Errett L., Fong I.W. Impact of treating Staphylococcus aureus nasal carriers on wound infections in cardiac surgery. J. Hosp. Infect. 2006;64(2):162–168. doi: 10.1016/j.jhin.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koranyi, et al. Evaluation of Bacitracin Ointment in the Treatment of Impetigo - PubMed. https://pubmed.ncbi.nlm.nih.gov/958658/ n.d.; Retrieved April 22, 2021, from. [PubMed]
- Kuniyuki S., Nakano K., Maekawa N., Suzuki S. Topical antibiotic treatment of impetigo with tetracycline. J. Dermatol. 2005;32(10):788–792. doi: 10.1111/j.1346-8138.2005.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Langford D.T., Burke C., Robertson K. Risk factors in onychocryptosis. Br. J. Surg. 1989;76(1):45–48. doi: 10.1002/bjs.1800760114. [DOI] [PubMed] [Google Scholar]
- Liguori V., Guillemin C., Pesce G.F., Mirimanoff R.O., Bernier J. Double-blind, randomized clinical study comparing hyaluronic acid cream to placebo in patients treated with radiotherapy. Radiother. Oncol. 1997;42(2):155–161. doi: 10.1016/s0167-8140(96)01882-8. [DOI] [PubMed] [Google Scholar]
- Maciel A.B. da S., Ortiz J.F., Siqueira B.S., Zanette G.F. Tissue healing efficacy in burn patients treated with 1% silver sulfadiazine versus other treatments: a systematic review and meta-analysis of randomized controlled trials. An. Bras. Dermatol. 2019;94(2):204–210. doi: 10.1590/abd1806-4841.20198321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeaux E.J., Carter C., Murphy T.E. Ingrown toenail management. Am. Fam. Physician. 2019;100(3):158–164. [PubMed] [Google Scholar]
- Moraes Barbosa Moraes Barbosa: Comparative study between topical... - Google Académico. (n.d.). Retrieved April 26, 2021, from https://scholar.google.com/scholar_lookup?journal=Arquivos+Brasileiros+de+Medicina&title=Comparative+study+between+topical+2%+sodium+fusidate+and+oral+association+of+chloramphenicol/neomycin/bacitracin+in+the+treatment+of+staphylococcic+impetigo+in+newborn&author=AD+Moraes+Barbosa&volume=60&issue=6&publication_year=1986&pages=509-11&
- Muangman P., Pundee C., Opasanon S., Muangman S. A prospective, randomized trial of silver containing hydrofiber dressing versus 1% silver sulfadiazine for the treatment of partial thickness burns. Int. Wound J. 2010;7(4):271–276. doi: 10.1111/j.1742-481X.2010.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje A.P., Chosidow O., Sacchidanand S., Todd G., Singh K., Scangarella N., Shawar R., Twynholm M. Topical retapamulin ointment, 1%, versus sodium fusidate ointment, 2%, for impetigo: a randomized, observer-blinded, noninferiority study. Dermatology. 2007;215(4):331–340. doi: 10.1159/000107776. [DOI] [PubMed] [Google Scholar]
- Owens C.D., Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J. Hosp. Infect. 2008;70(SUPPL. 2):3–10. doi: 10.1016/S0195-6701(08)60017-1. [DOI] [PubMed] [Google Scholar]
- Park D.H., Singh D. The management of ingrowing toenails. BMJ (Online) 2012;344(7851) doi: 10.1136/bmj.e2089. BMJ. [DOI] [PubMed] [Google Scholar]
- Perl T.M., Cullen J.J., Wenzel R.P., Zimmerman M.B., Pfaller M.A., Sheppard D., Twombley J., French P.P., Herwaldt L.A. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 2002;346(24):1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- Rashaan Z.M., Krijnen P., van den Akker- van Marle M.E., van Baar M.E., Vloemans A.F.P., Dokter J., Tempelman F.R.H., van der Vlies C.H., Breederveld R.S. Clinical effectiveness, quality of life and cost-effectiveness of Flaminal® versus Flamazine® in the treatment of partial thickness burns: study protocol for a randomized controlled trial. Trials. 2016;17(1):122. doi: 10.1186/s13063-016-1240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyzelman A.M., Trombello K.A., Vayser D.J., Armstrong D.G., Harkless L.B. Are antibiotics necessary in the treatment of locally infected ingrown toenails? Arch. Fam. Med. 2000;9(9):930–932. doi: 10.1001/archfami.9.9.930. [DOI] [PubMed] [Google Scholar]
- Rivers J.K., Arlette J., Shear N., Guenther L., Carey W., Poulin Y. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br. J. Dermatol. 2002;146(1):94–100. doi: 10.1046/j.1365-2133.2002.04561.x. [DOI] [PubMed] [Google Scholar]
- Romero-Pérez D., Betlloch-Mas I., Encabo-Durán B. Onychocryptosis: a long-term retrospective and comparative follow-up study of surgical and phenol chemical matricectomy in 520 procedures. Int. J. Dermatol. 2017;56(2):221–224. doi: 10.1111/ijd.13406. [DOI] [PubMed] [Google Scholar]
- Ruby R.J., Nelson J.D. The influence of hexachlorophene scrubs on the response to placebo or penicillin therapy in impetigo. Pediatrics. 1973;52(6) [PubMed] [Google Scholar]
- Schlesinger T.E., Powell C.R. Efficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosacea. J. Drugs Dermatol. JDD. 2013;12(6):664–667. https://europepmc.org/article/med/23839183 [PubMed] [Google Scholar]
- Schultz G.S., Sibbald R.G., Falanga V., Ayello E.A., Dowsett C., Harding K., Romanelli M., Stacey M.C., Teot L., Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(SUPPL. 1) doi: 10.1046/j.1524-475x.11.s2.1.x. [DOI] [PubMed] [Google Scholar]
- Segers P., Speekenbrink R.G.H., Ubbink D.T., van Ogtrop M.L., de Mol B.A. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. J. Am. Med. Assoc. 2006;296(20):2460–2466. doi: 10.1001/jama.296.20.2460. [DOI] [PubMed] [Google Scholar]
- Sesso, et al. Staphylococcus aureus Prophylaxis in Hemodialysis Patients Using central Venous Catheter: Effect of Mupirocin Ointment - PubMed. https://pubmed.ncbi.nlm.nih.gov/9621293/ n.d.; Retrieved April 20, 2021, from. [DOI] [PubMed]
- Shuman A.G., Shuman E.K., Hauff S.J., Fernandes L.L., Light E., Chenoweth C.E., Bradford C.R. Preoperative topical antimicrobial decolonization in head and neck surgery. Laryngoscope. 2012;122(11):2454–2460. doi: 10.1002/lary.23487. [DOI] [PubMed] [Google Scholar]
- Sobotka L., Velebný V., Šmahelová A., Kusalová M. Komplex hyaluronanu a jódu - hyiodine® - nová metoda při terapii diabetických defektů. Vnitr. Lek. 2006;52(5):417–422. [PubMed] [Google Scholar]
- Sobotka L., Smahelova A., Pastorova J., Kusalova M. A case report of the treatment of diabetic foot ulcers using a sodium hyaluronate and iodine complex. Int. J. Low. Extrem. Wounds. 2007;6(3):143–147. doi: 10.1177/1534734607304684. [DOI] [PubMed] [Google Scholar]
- Soroff H.S., Sasvary D.H. Collagenase ointment and polymyxin b sulfate/bacitracin spray versus silver sulfadiazine cream in partial-thickness burns: a pilot study. J. Burn Care Rehabil. 1994;15(1):13–17. doi: 10.1097/00004630-199401000-00003. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Kamigaki T., Fujino Y., Tominaga M., Ku Y., Kuroda Y. Randomized clinical trial of preoperative intranasal mupirocin to reduce surgical-site infection after digestive surgery. Br. J. Surg. 2003;90(9):1072–1075. doi: 10.1002/bjs.4269. [DOI] [PubMed] [Google Scholar]
- Tai Y.J., Borchard K.L.A., Gunson T.H., Smith H.R., Vinciullo C. Nasal carriage of Staphylococcus aureus in patients undergoing Mohs micrographic surgery is an important risk factor for postoperative surgical site infection: a prospective randomised study. Australas. J. Dermatol. 2013;54(2):109–114. doi: 10.1111/ajd.12028. [DOI] [PubMed] [Google Scholar]
- Troeman D.P.R., van Hout D., Kluytmans J.A.J.W. Antimicrobial approaches in the prevention of Staphylococcus aureus infections: a review. J. Antimicrob. Chemother. 2019;74(2):281–294. doi: 10.1093/jac/dky421. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varalakshmi T. An empirical study on perception of corporate professionals towards individual work vs group work. IOSR J. Bus. Manag. 2021;3(4) www.iosrjournals.org n.d. 4; Retrieved April 11, 2021, from. [Google Scholar]
- Vitiello R., Perna A., Peruzzi M., Pitocco D., Marco G. Clinical evaluation of tibiocalcaneal arthrodesis with retrograde intramedullary nail fixation in diabetic patients. Acta Orthop. Traumatol. Turcica. 2020;54(3):255–261. doi: 10.5152/j.aott.2020.03.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs G.N., Maibach H.I. Co-operative double-blind trial of an antibiotic/corticoid combination in impetiginized atopic dermatitis. Br. J. Dermatol. 1976;95(3):323–328. doi: 10.1111/j.1365-2133.1976.tb07021.x. [DOI] [PubMed] [Google Scholar]
- Wattanaploy S., Chinaroonchai K., Namviriyachote N., Muangman P. Randomized controlled trial of polyhexanide/betaine gel versus silver sulfadiazine for partial-thickness burn treatment. Int. J. Low. Extrem. Wounds. 2017;16(1):45–50. doi: 10.1177/1534734617690949. [DOI] [PubMed] [Google Scholar]
- Welsh O., Saenz C. Topical mupirocin compared with oral ampicillin in the treatment of primary and secondary skin infections. Curr. Ther. Res. 1987;41(1) [Google Scholar]
- Wessels S., Ingmer H. Modes of action of three disinfectant active substances: a review. Regul. Toxicol. Pharmacol. 2013;67(3):456–467. doi: 10.1016/j.yrtph.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Williamson D.A., Carter G.P., Howden B.P. 2017. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J.E., Taylor J.R., Tschen E., Kang S. Topical 3.0% diclofenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int. J. Dermatol. 2001;40(11):709–713. doi: 10.1046/j.1365-4362.2001.01324.x. [DOI] [PubMed] [Google Scholar]
- Wong, et al. Prophylaxis against Gram-Positive Organisms Causing Exit-Site Infection and Peritonitis in Continuous Ambulatory Peritoneal Dialysis Patients by Applying Mupirocin Ointment at the Catheter Exit Site - PubMed. https://pubmed.ncbi.nlm.nih.gov/17986538/ n.d.; Retrieved April 20, 2021, from. [PubMed]
- Yin D., Liu B., Chang Y., Gu H., Zheng X. Management of late-onset deep surgical site infection after instrumented spinal surgery. BMC Surg. 2018;18(1) doi: 10.1186/s12893-018-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P.Y., Khadaroo R.G. Surgical site infections. Surg. Clin. 2014;94(6):1245–1264. doi: 10.1016/j.suc.2014.08.008. W.B. Saunders. [DOI] [PubMed] [Google Scholar]
- Zhu J., Tang X., Jia Y., Ho C.T., Huang Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery – a review. Int. J. Pharm. 2020;578(September 2019) doi: 10.1016/j.ijpharm.2020.119127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.