Abstract
The efficacy and safety of a novel Subtilisin protease from a Bacillus sp. produced in Bacillus licheniformis was investigated in broiler chickens, and a range of toxicological tests, respectively. The B. licheniformis production strain culture supernatant was not found cytotoxic in a Vero cell assay. Subtilisin was non-mutagenic and non-clastogenic in in-vitro tests, and did not exhibit irritating potential to the eye or skin in ex-vivo/in-vitro models. Oral administration of Subtilisin to rats did not cause any adverse effects in a 13-week sub-chronic toxicity study. In addition, a 35-day dose response broiler performance trial conducted with Subtilisin (30,000 and 60,000 NFP/kg diet), showed a significant linear improvement in both body weight gain and feed conversion ratio up to 35 days of protease supplementation.
In conclusion, there are no safety concerns using this novel Subtilisin as a feed additive, and the protease is efficient in improving broiler growth performance, making it a good candidate for use as a feed additive.
Keywords: Subtilisin, Bacillus licheniformis, Toxicity, Safety, Feed additive, Broiler chickens
Highlights
-
•
Subtilisin-treated broiler chickens showed a significant linear improvement in body weight gain and feed conversion ratio.
-
•
The B. licheniformis production strain was not found cytotoxic in a Vero cell assay.
-
•
Treatment with Subtilisin did not exhibit genotoxicity (Ames, micronucleus) or skin and eye irritation.
-
•
Oral administration of Subtilisin to rats did not cause any adverse effects in a 13-week sub-chronic toxicity study.
-
•
Subtilisin is a good candidate for use as a feed additive.
Subtilisin; Bacillus licheniformis; Toxicity; Safety; Feed additive; Broiler chickens.
1. Introduction
Enzymes added to animal feed have in modern days gained popularity, due to the increase in nutrient digestibility that leads to benefit in nutrition, while reducing the environmental impact. The use of various enzymes enhances nutrient utilization from feed ingredients (Adeola and Cowieson, 2011; Aureli et al., 2018; Cowieson and Roos, 2016; Dersjant-Li et al., 2015; Rebello et al., 2019). As summarized by Ojha and colleagues, in addition to the improvement of digestion of nutrients, enzymes can aid in the release of micronutrients (Ojha et al., 2019); and as a result of less undigested food reaching the lower gut and the intestinal microbiota, there is a general improvement of gut health (Bedford and Cowieson, 2012; Ojha et al., 2019). Generally, this leads to improved and more sustainable meat production and environmental benefits (Bundgaard et al., 2014; Leinonen and Kyriazakis, 2016; Rebello et al., 2019).
The primary purpose of adding proteases to animal feed is improving amino acid availability; however, exogenous proteases can also have other secondary benefits, such as increased digestibility of starch and fat, enteric resilience, improved gut health, as well as litter management (Cowieson and Roos, 2016).
Several enzymes currently used in feed are produced in host Bacillus licheniformis (Pariza and Johnson, 2001), since the microorganism is regarded as non-pathogenic and non-toxigenic (de Boer et al., 1994; Pariza and Johnson, 2001).
The Association of American Feed Control Officials has deemed source organism B. licheniformis and several enzymes produced by this microorganism as acceptable for use in animal feed (AAFCO, 2018); while the European Food Safety Agency (EFSA) has included B. licheniformis on qualified presumption of safety (QPS)-recommended biological agents intentionally added to food or feed list (EFSA, 2007), and QPS strains are not subject to full safety assessment (EFSA, 2017).
The B. licheniformis production strain used to produce the protease employed in the studies derives from Ca63 (DSM 9552) and is genetically modified to produce a novel subtilisin (hereon referred to as Subtilisin) from a Bacillus sp.
Subtilisin is from a sub-family of the S8 proteases. To the best of our knowledge, proteases from this sub-family have not previously been used in feed. The protease is stable, has an alkaline pH activity optimum and a high thermostability, and the broad specificity of the protease enables it to cleave at many sites in a potential feed substrate, such as soybean feed.
In order to assess the safety of the novel Subtilisin, a series of toxicological studies were performed as advised by Pariza (Pariza and Cook, 2010; Pariza and Johnson, 2001) and consistent with the safety assessment of enzymes performed by Lichtenberg and Aureli (Aureli et al., 2018; Lichtenberg et al., 2011, 2017). The studies reported here comply with the requirements of the European Union Regulation N° 1831/2003 on additives for use in animal nutrition and the corresponding guidelines (EFSA, 2012). A supernatant prepared from the B. licheniformis production strains was investigated for cytotoxicity in the Vero cell assay in accordance with the criteria defined by EFSA (2018). In addition, the enzyme preparation was subjected to genetic toxicity testing (bacterial reverse mutation test and in-vitro mammalian cell micronucleus test), irritation potential testing (ex-vivo irritation and in vitro skin irritation), and a sub-chronic oral toxicity study in rats. In addition, an efficacy study using broiler chickens was conducted.
This article provides the safety profile of Subtilisin as a feed additive, covering safety of the production strain, toxicological studies, as well as efficacy studies in broiler chickens following respective guidelines (EFSA, 2011).
2. Materials and methods
2.1. Construction of the production strain
The wild type subtilisin gene from a Bacillus sp. was modified to produce Subtilisin. The Subtilisin expression cassette was made using standard vectors with strictly defined and well-characterized DNA sequences, which do not encode or express any harmful or toxic substances. The Subtilisin expression cassette was introduced through recombination into the Bacillus licheniformis recipient strain derived from Ca63 (DSM 9552) using a standard transformation procedure. The transformants were subsequently evaluated by gene sequencing to assess incorporation of the expression cassette and to ensure that no unintended sequences were incorporated into the genome of the selected production strain. The Subtilisin protein expressed from the introduced genes in the final production strain was verified by mass spectroscopy to be 100% identical to the protein sequence encoded by the donor gene.
2.2. Production of Subtilisin
Subtilisin was produced in an industrial set-up, certified to ISO 9001 and in accordance with the procedures used for the manufacturing of commercial enzyme products. In brief, the B. licheniformis production strain described in Section 2.1, was cultivated in a bioreactor with pH-adjusted sterilized food-grade ingredients. After fermentation, the production organism was separated from the fermentation broth through a series of filtration and concentration steps. A filtered liquid fermentation broth was used for the toxicological studies.
2.3. Characterization of Subtilisin
Subtilisin activity is expressed in subtilisin units NFP. The activity is determined relative to an enzyme standard using an enzyme assay. The result is given in NFP/g. One NFP unit is defined as the amount of enzyme that releases approximately 1 μmol of p-nitroaniline from 1 mM substrate (N-Succinyl-Ala-Ala-Pro-Phe p-nitroanilide) per minute at pH 9.0 and 37 °C. Subtilisin was also analyzed for chemical and microbial status using standard methods (Table 1). Total organic solids (TOS) from the fermentation consists mainly of protein and carbohydrate components.
Table 1.
Composition analyzes of Subtilisin.
| Composition analysis of Subtilisin | |
|---|---|
| Enzyme activity NFP/g | 5.81 × 105 |
| Total Organic Solids (% w/w) | 9.3 |
| Total viable count CFU/g | <100 |
2.4. Assessment of cytotoxicity of the QPS production strain
A cytotoxicity test was performed using the culture supernatants to determine if the production strain produces high levels of non-ribosomal synthesized peptides, which is one of the qualifications of the QPS approach (EFSA, 2018). To investigate the cytotoxic potential of the B. licheniformis production strain, Vero cells (ATCC CCL-81) were exposed to culture supernatants of the production strain, negative and positive control, respectively (Bacillus subtilis, DSM10 and Bacillus cereus, DSM31, purchased from DSMZ, Braunschweig, Germany) and assessment of cytotoxicity was done by measuring lactate dehydrogenase release (LDH), which is an intracellular enzyme produced by living cells. Detection of LDH activity in the medium is therefore indicative of potential cytotoxicity. A commercial kit was used to measure LDH activity (Roche Diagnostics GmbH, Mannheim, Germany), as described by the manufacturer. The supernatant derived from the production strain was prepared according to the EFSA recommendations (EFSA, 2018). Supernatants from cultures containing >1 × 108 CFU/mL were used. The culture was centrifuged at 8000 rpm for 15 min and the supernatants were transferred to a fresh tube and passed through a 0.22 μm filter. Cells were seeded and cultured overnight to 80–90% confluence in 96-well plates. Addition of culture supernatant was done in quadruplicate wells at a 1:10 (vol:vol) ratio (bacterial supernatant: Vero growth medium). After an exposure period of 2 h, the supernatants from the wells were harvested and transferred to new plates. Measurement of LDH activity was performed by determining tetrazolium dye conversion (absorbance at 490), using an EnSpire multimode plate reader by PerkinElmer. As a positive control, lysed cells representing total LDH release and cells treated with B. cereus (DSM 31) supernatants were used. Relative cytotoxicity was calculated. Relative cytotoxicity values > 20% of total lysis indicate cytotoxicity.
2.5. Toxicological evaluations
The toxicological studies described below were performed in compliance with GLP and according to current OECD test guidelines (TG). The in vivo studies were performed in agreement with the regulation and ethical guidelines on the use of animals for experimental purposes of the local authorities of the countries where the studies were performed, as well as internal Novozymes guidelines.
2.5.1. Ex-vivo eye irritation test
Subtilisin was evaluated eye irritation potential in the Isolated Chicken Eye (ICE) test in accordance with OECD TG 438 (OECD, 2018a). The study was carried out at Covance CRS Research Limited (Shardlow, UK). Chicken eyes (Spring chickens (Gallus Gallus e.g. Ross 308 Broiler)), were obtained from heads of animals that were humanly slaughtered for human consumption, and used within the same day. The test included a negative control (sodium chloride 0.9% w/v) and 5% benzalkonium chloride (Acros Organice, Belgium, Lot: A0366813) as a positive control. The isolated chicken eyes were applied 30 μL of Subtilisin onto the cornea of each of three enucleated eyes for 10 s, and rinsed with 20 mL isotonic saline. Treated corneas were evaluated prior to treatment and at 30, 75, 120, 180 and 240 min (±5 min) after the eyes had been rinsed with the isotonic saline. Toxic effects to the cornea are measured by (i) a qualitative assessment of opacity (ii) a qualitative assessment of damage to epithelium based on application of fluorescein to the eye (fluorescein retention) (iii) a quantitative measurement of increased thickness (swelling) and (iv) a qualitative evaluation of macroscopic morphological damage to the surface.
2.5.2. In-vitro skin irritation test
In-vitro skin irritation potential of Subtilisin was assessed using the EPISKIN™ Reconstructed Human Epidermis Model (EpiSkin Laboratories, Lyon, France). The study followed OECD TG 439 (OECD, 2015), and was carried out at Covance CRS Research Limited (Shardlow, UK). Triplicate tissues were treated with Subtilisin 10 μL (26.3 μL/cm2) applied to the epidermis surface) for an exposure period of 15 min. Triplicate tissues treated with 10 μL of DPBS (Gibco™) served as the negative control and triplicate tissues treated with 10 μL of SDS 5% w/v (Sigma Aldrich, UK) served as the positive control. At the end of the exposure period, each tissue was rinsed using a wash bottle containing DPBS, and transferred to wells containing 2 mL of maintenance medium, before incubating for 42 h. At the end of the incubation period, each tissue was taken for MTT-loading. The maintenance medium from beneath each tissue was transferred to pre-labeled micro tubes and stored in a freezer for possible inflammatory mediator determination. After MTT-loading, a total biopsy of each epidermis was performed and placed into micro tubes containing acidified isopropanol for extraction of formazan crystals out of the MTT-loaded tissues. At the end of the formazan extraction period each tube was mixed thoroughly and duplicate 200 μL samples were transferred to the appropriate wells of a pre-labeled 96-well plate. The optical density was measured at 570 nm. Data are presented in the form of percentage viability (MTT reduction in the test item treated tissues relative to negative control tissues).
2.5.3. Bacterial reverse mutation assay
To assess for any mutagenic activity Subtilisin, an Ames bacterial reverse mutation test was performed according to OECD TG 471 (OECD, 1997) at Covance CRS Research Limited (Cambridgeshire, UK).
The study utilized histidine-dependent auxotrophic mutants of Salmonella typhimurium, strains TA1535 (hisG46 rfa uvrB), TA1537 (hisC3076 rfa uvrB), TA98 (hisD3052 rfa uvrB pKM10) and TA100 (hisG46 rfa uvrB pKM101), and a tryptophan-dependent mutant of Escherichia coli, strain WP2 uvrA (trpE ochre uvrA pKM101), obtained from MolTox Inc. Boone, NC, USA. The genotypes were confirmed by standard procedures (Green and Muriel, 1984; Maron and Ames, 1983). Subtilisin may contain small amounts of histidine and tryptophan, which may increase the apparent number of revertants in the test. To avoid growth facilitated by exogenous histidine and tryptophan, the ‘treat and wash’ protocol was used (Mahon, 1989; Pedersen and Broadmeadow, 2000; Thompson et al., 2005).
Two independent experiments were conducted. For both experiment 1 and 2, the bacterial strains were exposed to a range of concentrations of Subtilisin, vehicle (water purified by reverse osmosis), and appropriate positive control substances Sodium azide, 2-Nitrofluorene, 4-Nitroquinoline-1-oxide, 2-Aminoanthracene or Benzo[a]pyrene (Sigma Aldrich/Merck Life Science UK Limited, Gillingham, Dorset, UK), 9-Aminoacridine (Fluka, UK), as detailed in Table 6) in phosphate buffered nutrient broth liquid cultures (incubation mixtures).
Table 6.
Bacterial reverse mutation assay; data from experiment 2 only. Mean number of revertant colonies per plate and fold increase relative to vehicle (in parentheses). Abbreviations: TOS: Total Organic Solids, NaN3: Sodium azide, 2-NF: 2-Nitrofluorene, NQO: 4-Nitroquinoline-1-oxide, AAC: 9-Aminoacridine, B[a]P: Benzo[a]pyrene, AAN: 2-Aminoanthracene.
| Compound | μg TOS/mL | S9 | TA98 | TA100 | TA1535 | TA1537 | WP2 uvrA pKM101 |
|---|---|---|---|---|---|---|---|
| Purified water | – | − | 40.7 | 112.3 | 13.3 | 14.3 | 112.3 |
| Subtilisin | 50 | − | 39.7 (1.0) | 105.0 (0.9) | 10.7 (0.8) | 13.0 (0.9) | 116.3 (1.0) |
| Subtilisin | 150 | − | 47.3 (1.2) | 117.0 (1.0) | 10.3 (0.8) | 11.0 (0.8) | 119.0 (1.1) |
| Subtilisin | 500 | − | 40.0 (1.0) | 126.3 (1.1) | 12.7 (0.9) | 17.7 (1.2) | 113.7 (1.0) |
| Subtilisin | 1500 | − | 41.3 (1.0) | 128.3 (1.1) | 13.7 (1.0) | 12.3 (0.9) | 147.3 (1.3) |
| Subtilisin | 5000 | − | 40.0 (1.0) | 128.7 (1.1) | 17.7 (1.3) | 11.3 (0.8) | 150.7 (1.3) |
| 2NF | 1.25 | − | 100.7 (2.5) | ||||
| NQO | 0.3 | − | 597 (5.3) | ||||
| NaN3 | 1.9 | − | 111.7 (8.4) | ||||
| AAC | 31.3 | − | 58.7 (4.1) | ||||
| NQO | 0.3 | − | 2560 (22.8) | ||||
| Purified water | – | + | 47.3 | 107.3 | 10.7 | 12.7 | 150.3 |
| Subtilisin | 50 | + | 29.7 (0.6) | 114.0 (1.1) | 17.3 (1.6) | 7.3 (0.6) | 178.7 (1.2) |
| Subtilisin | 150 | + | 39.0 (0.8) | 115.7 (1.1) | 17.7 (1.7) | 11.7 (0.9) | 178.0 (1.2) |
| Subtilisin | 500 | + | 32.3 (0.7) | 118.7 (1.1) | 19.3 (1.8) | 9.7 (0.8) | 161.0 (1.1) |
| Subtilisin | 1500 | + | 41.7 (0.9) | 126.0 (1.2) | 17.0 (1.6) | 9.7 (0.8) | 182.3 (1.2) |
| Subtilisin | 5000 | + | 44.3 (0.9) | 109.3 (1.0) | 14.7 (1.4) | 13.7 (1.1) | 178.3 (1.2) |
| B[a]P | 9.4 | + | 134.7 (2.8) | ||||
| AAN | 6.3 | + | 677.7 (6.3) | 160.3 (15) | |||
| AAN | 9.4 | + | 106 (8.4) | 447.3 (3.0) |
In experiment 1, seven concentrations of Subtilisin, separated by approximately half-log10 intervals were tested up to a maximum concentration of 5000 g TOS/mL, which is the recommended maximum. The maximum concentration chosen for experiment 2 was 5000 μg TOS/mL, but with a smaller factor between the concentrations (50, 150, 500, 1500 and 5000 μg TOS/mL). The tests were carried out with and without a metabolic activation system. S9 fraction was prepared from male Sprague-Dawley derived rats, (PB/BNF induced – Moltox Inc. Boone, NC, USA). For testing with S9 mix, Subtilisin was heat-treated (90 °C for 30 min) prior to testing to avoid proteolytic degradation of the S9 mix.
2.5.4. In-vitro micronucleus test in cultured human lymphocytes
Subtilisin was tested in an in-vitro micronucleus assay, using duplicate human lymphocyte cultures prepared from pooled blood of two male donors, at Covance Laboratories Ltd. (Harrogate, UK). The study was conducted according to OECD TG 487 (OECD, 2016). Treatments were performed both with and without metabolic activation system, S9 mix (S9 fraction obtained from Molecular Toxicology Incorporated, Boone, USA). For testing in the presence of S9 mix, formulations with heat-treated (90 °C for 30 min) Subtilisin were prepared to avoid proteolytic degradation of the S9 mix. The maximum Subtilisin concentration for micronucleus analysis was selected based on a preliminary cytotoxicity range-finder experiment where concentrations up to 5000 μg TOS/mL (the recommended maximum concentration) were tested.
Cell cultures were mitogen stimulated by phytohaemagglutinin (PHA) (Life Technologies, UK) for 48 h prior to Subtilisin exposure. Cells were exposed to the test article for 3 h followed by 21 h of recovery (3 + 21 h) in the absence or presence of S9. In addition, a continuous 24 h treatment followed by 24 h of recovery (24 + 24 h) in the absence of S9 was performed. The following positive control chemicals were used: cyclophosphamide (CPA) (Acros Organics, Belgium) dissolved in dimethyl sulphoxide (DMSO) (Sigma–Aldrich, UK), vinblastine (VIN) (Sigma–Aldrich, UK) and mitomycin C (MMC) (Sigma–Aldrich, UK).
2.5.5. Sub-chronic oral toxicity in rat
Subtilisin was administered daily by oral gavage to rats over 13 weeks to assess the systemic toxic potential. The study was designed according to OECD test guideline 408 (OECD, 2018b), including endocrine-sensitive endpoints intended to improve detection of potential endocrine activity of test chemicals. The study was conducted by Covance CRS Limited (Suffolk, UK), and in accordance with the applicable sections of the United Kingdom Animals (Scientific Procedures) Act 1986, Amendment Regulations 2012 (the Act), and approved by the Animal Welfare Ethical Review Body at the contract laboratory and the Home Office Inspectorate (UK). Four groups of Han Wistar (RccHan™; WIST) rats (obtained from Envigo RMS Limited) each comprising ten males and ten females were included in the study. The rats were 5–6 weeks old at commencement of treatment and were fed an expanded rodent diet throughout the study Teklad 2014C, pelleted diet (Envigo Teklad Diets, Wisconsin USA). Potable water was freely available. The animals were housed five of the same gender in each cage. Autoclaved wood shavings were used as bedding and aspen chew blocks and plastic shelters were provided as environmental enrichment. Each animal was identified uniquely by microchip and randomly allocated to a treatment group. The environment was kept at temperature within the range 20–24 °C and relative humidity within the range 40–70%, with a 12-hour light and 12-hour dark cycle except during designated procedures. The animals were acclimatized to these conditions for 14 days before treatment commenced.
Subtilisin dose formulations were prepared by dilution with reverse osmosis water for each exposure group to 10%, 33% and 100% of the test item. These doses corresponded to 0, 48.4, 159.6 and 483.6 mg TOS/kg body weight/day equivalent to 0, 3.02 × 105, 9.97 × 105 and 3.0 × 106 NFP/kg body weight/day, respectively. Animals received vehicle control (reverse osmosis water) or the test item formulations orally by gavage at a total volume-dose of 5 mL/kg body weight, using a graduated syringe and a rubber catheter inserted via the mouth. Doses were sampled at weeks 1, 6 and 13 to perform analysis of content check.
Clinical signs were recorded daily. Body weights and food consumption were recorded once weekly. Water consumption was monitored daily by visual inspection. Before treatment began and on weekly basis during the study, animals were removed from the home cage and examined for physical condition and behavior during handling and after being placed in a standard arena. Assessment of approach response, auditory startle response, tail-pinch response, righting reflex and touch response and measurement of grip-strength, were made prior to treatment start and again in week 12. Likewise, motor activity for each individual animal over a 1-hour period was assessed (6-minutes intervals), before commencement of treatment and in week 12, by automated sensor equipment. Ophthalmoscopy was performed on all animals before start of treatment and at termination on all control rats and high dose. The assessment of estrus cycles was conducted by taken wet smear from the vagina of the all females using pipette lavage for 4 days before scheduled necropsy.
Blood was sampled, after overnight food deprivation, in week 13 in BD Microtainers internally coated with spray-dried K2 EDTA (BD Cat#365974, Becton Dickinson UK Limited, Berkshire, UK) and in coagulation tubes prepared at Envigo (plain polypropylene tubes pre-filled with sodium citrate at a ratio of 9 parts blood to 1 part citrate). EDTA treated samples were analyzed using a Bayer Advia 120 Analyzer for a range of hematological parameters. Derived values (Hct, MCH and MCHC) were calculated in ClinAxys the Rectic gated (g) parameters were used: HCTg = (Retic RBC × Retic MCV)/1000 and MCHg = (Retic CHCM × Retic MCV)/100. Citrate treated samples were analyzed for prothrombin and activated partial thromboplastin times using a Stago STA Compact Max analyzer. Additional blood samples were obtained at the same time, using BD Microtainers internally coated with spray-dried lithium heparin, also containing an inert polymer gel (for plasma separation) (BD Cat#365985, Becton Dickinson UK Limited, Berkshire, UK). Using a Roche P Modular Analyzer with an ion-specific electrode, the concentrations of glucose, urea, creatinine, total cholesterol, total proteins, albumin (with calculation of the albumin to globulin ratio), sodium, and potassium were analyzed in the plasma. Further, the activities of alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate amino-transferase (AST) were analyzed in the plasma. Thyroid hormone analysis (T3, T4 and TSH) samples were taken after overnight food deprivation, at termination, in Grenier Minicollect tubes (Fisher Scientific, Leicestershire, UK) with clotting activator. Separation of serum was done by centrifugation at 4 °C for 10 min at 2000g. The T3 and T4 was determined using liquid chromatography with tandem mass spectrometry detection LC-MS/MS) method validated by Envigo. The TSH was analyzed using the Luminex Thyroid Hormone Magnetic Bead Panel, provided as a Milliplex MAP Rat TSH Pituitary Panel Assay kit (Merck Life Science UK Limited, Dorset, UK).
After completion of the treatment period the rats were euthanized by carbon dioxide inhalation followed by exsanguination. They were dissected and examined macroscopically, and a range of tissues collected. Weights were recorded for the various organs. Histopathological examination was performed on the high dose and control animals. Grossly abnormal tissues were examined for all groups whereas histopathological examination of the mid- and low-dose groups were only done if abnormalities were identified.
A parametric analysis was performed if Bartlett's test (Bartlett, 1937) was not significant at the 1% level. The F1 approximate test was applied. This test is designed to detect significant departure from monotonicity of means when the main test for the comparison of the means is a parametric monotonic trend test, such as Williams' test (Williams, 1971, 1972). If the F1 approximate test for monotonicity of dose-response was not significant at the 1% level, Williams' test for a monotonic trend was applied. If the F1 approximate test was significant, suggesting that the dose response was not monotone, Dunnett's test (Dunnett, 1955, 1964) was performed instead.
A non-parametric analysis was performed if Bartlett's test was still significant at the 1% level following both logarithmic and square-root transformations. The H1 approximate test, the non-parametric equivalent of the F1 test described above, was applied. This test is designed to be used when the main test for comparison of the means is a non-parametric monotonic trend test, such as Shirley's test (Shirley, 1977). If the H1 approximate test was significant, suggesting that the dose-response was not monotone, Steel's test (Steel, 1959) was performed instead. For grip strength, motor activity and clinical pathology data, if 75% of the data (across all groups) were the same value, for example c, Fisher’s exact tests (Fisher, 1973) were performed. For organ weight data, analysis of covariance was performed using terminal body weight as covariate (Angervall and Carlström, 1963), unless non-parametric methods were applied. Significant differences between the groups compared were expressed at the 5% (p < 0.05) or 1% (p < 0.01) level.
2.6. Broiler efficacy study
Subtilisin was used in a broiler growth performance experiment. The objective was to evaluate the efficacy of the protease on growth performance (body weight gain, feed intake and feed conversion ratio) of broiler chickens from 0 to 35 days of age. The trial was carried out at the Research Centre for Animal Nutrition and Health (CRNA, DSM Nutritional Products France, F-68128 Village-Neuf). All applied procedures were approved by the CRNA ethical committee (CEEA-123) and complied with the official French regulation on use of animals for experimental purposes under the EU regulation (European Parliament, 2010).
A total of 864, day-old male Cobb 500 broiler chicks supplied by a commercial hatchery (Couvoir de la Seigneurtière Orvia, 44116 Vieillevigne, France) were used. The experiment started on day 0 of broiler age and was completed on day 35. On the day of arrival (day 0), the chickens were randomly allocated to one of the dietary treatments using body weight as criteria, such that homogenous inter-replicate and inter-treatment was obtained. The experiment is a dose response study involving 3 doses (0, 30,000 and 60,000 NFP/kg) of Subtilisin added to the same basal diets. Each treatment was replicated with 16 pens and each pen contained 18 broiler birds, housed in an environmentally controlled room.
During the first week of the trial, feed was offered to the birds as crumbled pellets, and afterwards as pelleted feed until the end of the trial on day 35 of broiler age. Birds were offered ad libitum access to feed and water throughout the experiment. The composition of the basal diets is shown in Table 2. The diets were composed of maize and soybean meal as main ingredients and contained 205, 200 and 195 g/kg crude protein for the starter, grower and finisher phases respectively. To include the product in the basal diet, the appropriate amount of the product was mixed with 5 kg of the basal feed as a premix, which was then mixed to the feed to get the final concentration required for the treatment. After mixing, the feed was pelleted (3 × 25 mm) at 70 °C and feed samples were taken for analysis of the enzyme activity.
Table 2.
Composition of the basal experimental diets.
| Ingredient | 0–14 d |
14–28 d |
28–35 d |
|---|---|---|---|
| Starter | Grower | Finisher | |
| Maize | 60.20 | 60.96 | 63.88 |
| SoyaBean Meal | 33.48 | 32.60 | 30.12 |
| Soy Oil | 1.21 | 1.92 | 2.12 |
| Dicalcium Phosphate | 2.23 | 1.88 | 1.55 |
| Premix Broiler VN(1) | 1.00 | 1.00 | 1.00 |
| Limestone | 0.70 | 0.65 | 0.60 |
| Sodium Bicarbonate | 0.36 | 0.36 | 0.16 |
| DL Methionine | 0.29 | 0.26 | 0.20 |
| Salt (NaCl) | 0.21 | 0.16 | 0.25 |
| l-Lysine HCL | 0.18 | 0.10 | 0.04 |
| Avatec –(coccidiostat) | 0.06 | 0.06 | 0.06 |
| l-Threonine | 0.08 | 0.05 | 0.02 |
| Total |
100.00 |
100.00 |
100.00 |
|
Calculated nutrient content (g/kg) | |||
| ME Poultry (MJ/kg) | 12.75 | 13.15 | 13.35 |
| Crude Protein | 205.00 | 200.00 | 190.00 |
| Crude protein (Measured) | 208.00 | 201.00 | 191.00 |
| Lysine | 11.72 | 10.85 | 9.80 |
| Methionine | 5.95 | 5.62 | 4.96 |
| T.S.A.A. | 8.92 | 8.54 | 7.79 |
| Threonine | 7.80 | 7.35 | 6.79 |
| Tryptophan | 2.31 | 2.26 | 2.13 |
| Valine | 9.10 | 8.92 | 8.50 |
| dLys | 11.10 | 10.30 | 9.30 |
| dMet | 5.40 | 5.10 | 4.50 |
| dThr | 7.00 | 6.60 | 6.10 |
| dTSAA | 8.48 | 8.13 | 7.42 |
| dVal | 8.41 | 8.25 | 7.85 |
| Avl Phosphorus | 4.50 | 4.00 | 3.50 |
| Calcium | 9.00 | 8.00 | 7.00 |
| Chloride | 2.00 | 1.58 | 2.00 |
| DEB (Na+K−Cl) meq/kg | 225.72 | 220.00 | 183.36 |
| Potassium | 8.98 | 8.80 | 8.37 |
| Sodium | 2.00 | 1.80 | 1.60 |
| Total Phosphorus | 7.80 | 7.14 | 6.47 |
| Fat | 41.33 | 53.88 | 56.20 |
| Fibre | 32.54 | 32.14 | 31.65 |
1Vitamin-mineral premix provided per kilogram of diet: Vitamin A: 11’000 I.U.; vitamin E: 40 I.U.; vitamin K3: 3.0 mg; vitamin C: 100 mg; vitamin B1: 2.50 mg; vitamin B2: 8.00 mg; vitamin B6: 5.00 mg; vitamin B12: 0.03 mg; niacin: 50.0 mg; pantothenate calcium: 12.0 mg; folic acid: 1.50 mg; biotin 0.15 mg; choline: 450 mg; Na: 1.17 g; Mg: 0.8 g; Mn: 80 mg; Fe: 60 mg; Cu: 30 mg; Zn: 54 mg; I: 1.24 mg; Co: 0.6 mg; Se: 0.3 mg.
Body weight gain, feed intake (FI) and feed conversion ratios (FCR) were the main endpoint parameters in this study. The body weight of birds and feed intake were recorded per cage (as replicate group) on day 0, 14, 28 and 35.
Body weight gain (g/bird) was calculated as the difference between the start body weight and end body weight for each period. The feed consumption was estimated as a difference between the weight of the feed offered and weight of the feed left over. Feed conversion ratio was calculated as the feed consumed/body weight gain for each phase.
A simple linear regression according to the model below was used to assess the effect of the protease on body weight gain (g/bird), feed intake (g/bird) and the feed conversion ratio (g:g).
| Y = a + bX + ε |
where Y = measure variable of BWG, FI and FCR; X = protease dose; a = Intercept; b = Slope; ε – Residual (error). All statistical analyses were performed using JMP®, Version 15. SAS Institute Inc., Cary, NC, 1989–2021.
3. Results & discussion
3.1. Assessment of cytotoxicity of the QPS production organism
The relative cytotoxicity of the supernatant prepared from the production strain B. licheniformis was found to be below 20% (Table 3).
Table 3.
Cytotoxic effect of production strain culture supernatant using Vero cells lactate dehydrogenase (LDH) activity assay. N = 4.
| Samples | Relative cytotoxicity % |
|---|---|
| Naïve (background) | 0 |
| Positive control strain | 89 |
| B. licheniformis (production strain) culture supernatant | 0.7 |
All acceptance criteria were fulfilled, and the assay was considered valid. Dilution series of total lysed cell displayed linear relations (R2 ≥ 0.980) and the relative cytotoxicity value for the positive control (B. cereus type strain) was >80% of total lysis.
Under the conditions employed in this test, the supernatant of the production strain did not show any cytotoxic potential.
3.2. Ex-vivo isolated chicken eye test
Maximal ocular irritation observations recorded for Subtilisin treated eyes are given in Table 4. Following assessment of the data for all endpoints the test item was considered unlikely to have the potential to cause ocular corrosivity/severe irritancy ex-vivo at the tested dose (UN GHS Classification: No Category).
Table 4.
Results from Subtilisin ex-vivo Isolated Chicken Eye Test. N = 3.
| Mean Corneal Opacity (ICE class) |
Mean Fluorescein Retention (ICE class) |
Mean Corneal Thickness compared to time zero % (ICE class) |
Combination of the 3 Endpoints | ||||
|---|---|---|---|---|---|---|---|
| 30 min |
75 min |
120 min |
180 min |
240 min |
|||
| 1.2 | 0.2 | −2.55 | 2.55 | 1.02 | 5.61 | 0.51 | |
| (II) | (I) | (I) | (I) | (I) | (II) | (I) | |
| (II) | 1 × I, 2 × II | ||||||
| Classification: | No Category | ||||||
3.3. In-vitro skin irritation test using EpiSkin™ reconstructed human epidermis model
The test item did not directly reduce MTT, and the solution containing the test item was colorless. It was therefore unnecessary to run color correction tissues. The criteria required for acceptance of results in the test were satisfied. The individual and mean OD570 values, standard deviations and tissue viabilities are given in Table 5. The relative mean viability of the test item treated tissues was 78.4%. It was considered unnecessary to perform IL-1α analysis, as the results of the MTT test were unequivocal. Based on these data, Subtilisin was classified as non-irritant.
Table 5.
Subtilisin irritation potential in the EpiSkin Reconstructed Human Epidermis Model. Mean OD570 Values and Viabilities for the Negative Control Item (Dulbecco’s Phosphate Buffered Saline (DPBS)), Positive Control Item (Sodium dodecyl sulphate (SDS), prepared as a 5% w/v aqueous solution) and Test Item. N = 3.
| Item | OD570 of tissues | Mean OD570 of triplicate tissues | ± SD of OD570 | Relative individual tissue viability (%) | Relative mean viability (%) | ± SD of Relative mean viability (%) |
|---|---|---|---|---|---|---|
| Negative Control Item | 0.705 | 0.718 | 0.096 | 98.2 | 100 | 13.4 |
| 0.629 | 87.6 | |||||
| 0.82 | 114.2 | |||||
| Positive Control Item | 0.212 | 0.148 | 0.06 | 29.5 | 20.6 | 8.3 |
| 0.14 | 19.5 | |||||
| 0.093 | 13.0 | |||||
| Subtilisin | 0.465 | 0.563 | 0.098 | 64.8 | 78.4 | 13.7 |
| 0.564 | 78.6 | |||||
| 0.661 | 92.1 |
3.4. Bacterial reverse mutation assay
The results of the bacterial reverse mutation assay (Ames test) are presented in Table 6. No signs of cytotoxicity were observed after exposure to Subtilisin. Revertant colony numbers for the vehicle controls were consistent with the normal ranges for the laboratory.
The positive control substances induced significant increases in revertant colony numbers relative to the vehicle control, thereby verifying the sensitivity of the assay and the metabolizing activity of the S9 mix. No concentration-related or reproducible increases in revertant colonies were obtained with any of the bacterial strains exposed to Subtilisin, either in the presence or absence of S9 mix, up to 5000 μg TOS/mL were tested.
It was concluded that Subtilisin showed no evidence of mutagenic activity in this bacterial system under the test conditions employed.
3.5. In-vitro micronucleus test in cultured human lymphocytes
Micronuclei frequencies in human lymphocytes exposed to Subtilisin in three treatment regimes, (3 + 21 h with and without S9 mix) and (24 + 24 h without S9 mix), are summarized in Table 7. The proportion of micronucleated binucleate (MNBN) cells from vehicle controls cultures fell within the current 95th percentile of the historical vehicle control ranges in the laboratory. In the absence of S9 mix, Mitomycin C (MMC) and Vinblastine (VIN) were used as clastogenic and aneugenic positive control chemicals, respectively. In the presence of S9 mix, Cyclophosphamide (CPA) was used as a clastogenic positive control chemical. Statistically significant increases in the proportion of cells with micronuclei were seen for all positive control compounds, demonstrating the sensitivity of the test system and the metabolizing activity of the S9 mix. All acceptance criteria were considered met and the study was therefore accepted as valid.
Table 7.
In-vitro micronucleus test in cultured human lymphocytes.
| Treatment | Concentration (μg TOS/mL) | Cytotoxicity (%) $ | Mean MNBN Cell Frequency (%) | Historical Control Range (%)# | Statistical Significance |
|---|---|---|---|---|---|
| 3 + 21 h -S-9 | Vehiclea | – | 0.35 | 0.00 to 0.70 | – |
| 250.0 | 12 | 0.40 | NS | ||
| 1000 | 21 | 0.25 | NS | ||
| 3000 | 50 | 0.45 | NS | ||
| 4000 | 45 | 0.25 | NS | ||
| ∗MMC, 0.30 | 49 | 4.85 | p ≤ 0.001 | ||
| 3 + 21 h + S-9 | Vehiclea | – | 0.35 | 0.10 to 0.90 | – |
| 1000 | 7 | 0.38 | NS | ||
| Subtilisin, batch | 3000 | 11 | 0.50 | NS | |
| PPA55402 | 4000 | 20 | 0.43 | NS | |
| 5000 | 11 | 0.63 | NS | ||
| ∗CPA, 3.00 | 43 | 2.45 | p ≤ 0.001 | ||
| 24 + 24 h -S-9 | Vehiclea | – | 0.45 | 0.00 to 0.80 | – |
| 15.00 | 14 | 0.35 | NS | ||
| 25.00 | 27 | 0.45 | NS | ||
| 30.00 | 40 | 0.55 | NS | ||
| 40.00 | 56 | 0.50 | NS | ||
| ∗VIN, 0.04 | 80 | 6.95 | p ≤ 0.001 |
a Vehicle control was purified water.
∗ Positive control: concentration given in μg/mL.
# 95th percentile of the observed range.
$ Based on replication index.
NS Not significant.
The MNBN cell frequencies of all Subtilisin-treated cultures (all concentrations) were similar to and not significantly (p ≤ 0.05) higher than the concurrent vehicle control values, which fell within the 95th percentile of the historical vehicle control (normal) ranges of the laboratory. Concentrations were either tested up to 5000 μg TOS/mL (3 + 21-hour treatments) or were limited by cytotoxicity to 40 μg TOS/mL (24 + 24 h treatment).
It was concluded that Subtilisin did not induce biologically relevant increases in micronuclei in cultured human peripheral blood lymphocytes following treatment in the absence and presence of S9 mix.
3.6. Sub-chronic oral toxicity in rat
The systemic toxic potential of Subtilisin at doses of 48.4, 159.6 and 483.6 mg TOS/kg/day, corresponding to 0, 3.02 × 105, 9.97 × 105 and 3.0 × 106 NFP/kg body weight/day, respectively was assessed by dosing Subtilisin orally by gavage to Wistar rats over a period of 13 weeks. Analyses of test item dose formulations, based on total nitrogen content, N-total %, were performed on three occasions during the study and confirmed that all animals were exposed to Subtilisin at expected doses.
Clinical observations were made continuously and no adverse signs of toxicity or changes in the general behavior or appearance were observed that could be attributed to the exposure to Subtilisin. However, one animal died in week 11, and the death of this animal was attributed to the dose administration procedure and not to toxicity.
The sensory activity and grip strength assessments in Week 12 did not reveal any findings that were treatment related. Similarly, motor activity was unaffected by treatment. There were no treatment-related ophthalmoscopic findings.
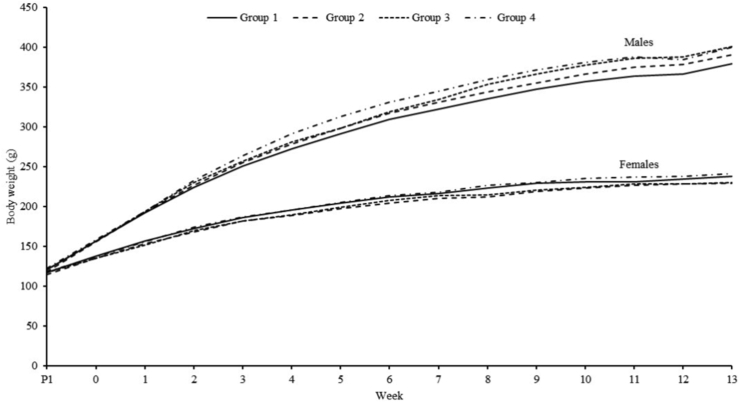
There was a non-statistically significant trend towards slightly higher body weight for males compared to control (visual presententation in Figure 1). This was attributed to the low group mean value for the control males, as one control animal showed poor body weight gain. There was no similar finding in females. The variations of body weight gain in males were therefore considered non-adverse and were attributed to normal biological variation.
Figure 1.
Weight gain during 13-week toxicity study in rats. The animals were exposed to Subtilisin at doses of 0, 48.4, 159.6 and 483.6 mg TOS/kg/day in groups 1–4, respectively. N = 10 per group; Group 4M, N = 9.
The animals were subjected to a macroscopic necropsy after 13 weeks of treatment. The macroscopic examination performed revealed no test-item related lesions. Specified organs and tissues were weighed, fixed and prepared for histopathological examination. The organ weight analysis after 13 weeks of treatment did not identify any differences from controls that were attributable to treatment. Further, the microscopic examination performed after 13 weeks of treatment revealed no test-item related findings. The incidence and distribution of all findings were unrelated to treatment.
All intergroup organ weight differences were minor and not statistically significant, and were considered to represent normal biological variation (Table 8).
Table 8.
13-week toxicity study in rats. Absolute organ weights (g) group mean values for animals after 13 weeks of treatment and standard deviation (SD) of males (M) and females (F). N = 10 per group; Group 4M, N = 9.
| Terminal Body weight | Adrenals | Brain | Epididymides | Heart | Kidneys | Liver | Prostate | Spleen | Testes | Thymus | Thyroids and Parathyroids | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1M | Mean | 379 | 0.056 | 2.053 | 1.364 | 0.987 | 1.970 | 11.446 | 2.166 | 0.689 | 3.778 | 0.313 | 0.017 |
| SD | 52 | 0.010 | 0.092 | 0.217 | 0.123 | 0.336 | 1.962 | 0.251 | 0.084 | 0.321 | 0.103 | 0.004 | |
| 2M | Mean | 390 | 0.059 | 2.005 | 1.418 | 0.981 | 1.991 | 11.609 | 2.172 | 0.677 | 3.744 | 0.313 | 0.019 |
| SD | 37 | 0.014 | 0.076 | 0.158 | 0.087 | 0.172 | 1.424 | 0.214 | 0.091 | 0.271 | 0.069 | 0.003 | |
| 3M | Mean | 400 | 0.057 | 2.044 | 1.341 | 1.052 | 2.055 | 11.640 | 2.359 | 0.732 | 3.825 | 0.278 | 0.017 |
| SD | 38 | 0.009 | 0.069 | 0.136 | 0.127 | 0.172 | 1.236 | 0.299 | 0.092 | 0.443 | 0.090 | 0.003 | |
| 4M | Mean | 400 | 0.057 | 2.132 | 1.473 | 1.066 | 2.116 | 11.522 | 2.392 | 0.714 | 3.928 | 0.270 | 0.017 |
| SD | 34 | 0.011 | 0.096 | 0.164 | 0.151 | 0.173 | 1.090 | 0.203 | 0.117 | 0.345 | 0.051 | 0.003 |
| Terminal Body weight | Adrenals | Brain | Heart | Kidneys | Liver | Ovaries | Spleen | Thymus | Thyroids and Parathyroids | Uterus and Cervix | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1F | Mean | 237 | 0.068 | 1.927 | 0.777 | 1.399 | 7.997 | 0.092 | 0.540 | 0.287 | 0.015 | 1.062 |
| SD | 14 | 0.009 | 0.135 | 0.048 | 0.142 | 0.852 | 0.016 | 0.073 | 0.060 | 0.003 | 0.587 | |
| 2F | Mean | 229 | 0.064 | 1.877 | 0.740 | 1.366 | 7.281 | 0.093 | 0.539 | 0.283 | 0.014 | 0.668 |
| SD | 17 | 0.006 | 0.087 | 0.061 | 0.150 | 0.851 | 0.010 | 0.058 | 0.095 | 0.003 | 0.178 | |
| 3F | Mean | 228 | 0.068 | 1.917 | 0.727 | 1.387 | 7.539 | 0.093 | 0.523 | 0.293 | 0.016 | 0.591 |
| SD | 16 | 0.012 | 0.077 | 0.037 | 0.103 | 0.752 | 0.012 | 0.041 | 0.046 | 0.003 | 0.077 | |
| 4F | Mean | 242 | 0.074 | 1.953 | 0.770 | 1.492 | 8.040 | 0.083 | 0.557 | 0.284 | 0.017 | 0.930 |
| SD | 13 | 0.012 | 0.049 | 0.054 | 0.124 | 0.786 | 0.013 | 0.054 | 0.056 | 0.003 | 0.448 |
The hematological examination during Week 13 did not identify any differences from controls that were attributable to treatment. All inter-group differences from controls, including those that attained statistical significance, were minor, occurred in one sex only or were without dose-relationship and were therefore considered to represent normal biological variation (Table 9). Such differences included the slightly high erythrocyte counts (RBC) at all dose levels in females, where all the individual values were within the background range and most values for the controls were at or below the background range. The low mean cell volume (MCV) in low dosage group females showed no dose-response and all the individual values were within the background range. The low red cell distribution width (RDW) in mid and high group females were also considered incidental, since none of the individual values was outside the normal background range and no similar trend occurred in the males. The slightly low reticulocyte counts (Retic) in the high dose group males, did not associate with any alteration of other erythrocyte indices and only two animals were just below the background range, hence, the observed difference was considered of no toxicological significance. They also included the slightly low lymphocyte (L) counts for males and females receiving 100% Subtilisin, since none of the individual values was outside the background range and there was no histopathological evidence for any inflammatory change in any of the tissues examined.
Table 9.
13-week toxicity study in rats. Hematology. Group mean values and standard deviation of males (M) and females (F). Significantly different from the controls: ∗: P < 0.05; ∗∗: P < 0.01. N = 8–10 for females; N = 6–9 for males.
| Hct |
Hb |
RBC |
Retic |
MCH |
MCHC |
MCV |
RDW |
WBC |
N |
L |
E |
B |
M |
LUC |
Plt |
PT |
APTT |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L/L | g/dL | x1012/L | x1012/L | pg | g/dL | fL | % | x109/L | x109/L | x109/L | x109/L | x109/L | x109/L | x109/L | x109/L | sec | sec | ||
| 1M | Mean | 0.454 | 15.6 | 8.31 | 0.148 | 18.8 | 34.5 | 54.7 | 12.7 | 8.27 | 1.17 | 6.67 | 0.10 | 0.06 | 0.21 | 0.07 | 610 | 22.0 | 14.5 |
| SD | 0.0156 | 0.47 | 0.299 | 0.0222 | 0.72 | 0.60 | 2.03 | 0.84 | 1.213 | 0.276 | 1.048 | 0.025 | 0.019 | 0.070 | 0.014 | 58.7 | 1.15 | 2.17 | |
| 2M | Mean | 0.457 | 16.0 | 8.21 | 0.141 | 19.5 | 35.0 | 55.7 | 12.5 | 9.11 | 2.20 | 6.42 | 0.11 | 0.06 | 0.24 | 0.07 | 545 | 25.0 | 16.3 |
| SD | 0.0146 | 0.43 | 0.320 | 0.0167 | 0.82 | 0.88 | 1.67 | 0.65 | 2.658 | 2.280 | 1.050 | 0.045 | 0.033 | 0.128 | 0.016 | 93.2 | 6.00 | 1.86 | |
| 3M | Mean | 0.470 | 15.9 | 8.65 | 0.146 | 18.4 | 33.9 | 54.4 | 12.7 | 7.77 | 1.64 | 5.69 | 0.11 | 0.06 | 0.21 | 0.05 | 601 | 27.7 | 15.0 |
| SD | 0.0162 | 0.39 | 0.403 | 0.0160 | 0.65 | 0.71 | 1.33 | 0.41 | 2.467 | 1.311 | 1.270 | 0.079 | 0.041 | 0.142 | 0.028 | 57.2 | 14.12 | 1.48 | |
| 4M | Mean | 0.454 | 15.7 | 8.50 | 0.126∗ | 18.4 | 34.5 | 53.5 | 12.5 | 9.56 | 3.84 | 5.20∗∗ | 0.10 | 0.07 | 0.27 | 0.07 | 559 | 24.5 | 16.2 |
| SD | 0.0165 | 0.66 | 0.372 | 0.0124 | 0.63 | 0.71 | 0.89 | 0.49 | 3.567 | 3.099 | 0.742 | 0.042 | 0.041 | 0.116 | 0.032 | 61.9 | 4.48 | 2.36 | |
| 1F | Mean | 0.415 | 14.8 | 7.40 | 0.167 | 20.0 | 35.6 | 56.0 | 10.9 | 5.03 | 0.90 | 3.95 | 0.11 | 0.01 | 0.12 | 0.03 | 828 | 20.0 | 19.9 |
| SD | 0.0223 | 0.68 | 0.355 | 0.0259 | 0.48 | 0.71 | 1.12 | 0.24 | 1.105 | 0.216 | 1.101 | 0.046 | 0.005 | 0.043 | 0.014 | 131.4 | 3.93 | 6.54 | |
| 2F | Mean | 0.426 | 15.1 | 7.85∗ | 0.152 | 19.2 | 35.4 | 54.3∗∗ | 10.9 | 4.09 | 0.60 | 3.21 | 0.06 | 0.01 | 0.09 | 0.02 | 836 | 21.5 | 18.1 |
| SD | 0.0121 | 0.59 | 0.277 | 0.0194 | 0.93 | 1.11 | 1.37 | 0.86 | 1.054 | 0.387 | 0.702 | 0.020 | 0.011 | 0.031 | 0.008 | 89.1 | 2.59 | 5.68 | |
| 3F | Mean | 0.429 | 15.0 | 7.69∗ | 0.149 | 19.5 | 35.0 | 55.8 | 10.6∗ | 5.83 | 1.31 | 4.23 | 0.07 | 0.02 | 0.16 | 0.04 | 836 | 19.4 | 18.5 |
| SD | 0.0113 | 0.29 | 0.285 | 0.0142 | 0.53 | 0.60 | 1.25 | 0.15 | 1.336 | 1.055 | 0.734 | 0.026 | 0.012 | 0.066 | 0.010 | 145.0 | 1.67 | 2.48 | |
| 4F | Mean | 0.424 | 15.0 | 7.62∗ | 0.164 | 19.7 | 35.4 | 55.7 | 10.6∗ | 4.06 | 0.91 | 2.92∗ | 0.09 | 0.02 | 0.10 | 0.03 | 812 | 21.0 | 19.1 |
| SD | 0.0194 | 0.64 | 0.319 | 0.0284 | 0.63 | 1.02 | 0.88 | 0.34 | 1.642 | 0.692 | 1.091 | 0.032 | 0.008 | 0.060 | 0.023 | 75.3 | 3.87 | 6.02 |
Hematocrit (Hct), Hemoglobin concentration (Hb), Erythrocyte count (RBC), Absolute reticulocyte count (Retic), Mean cell hemoglobin (MCH), Mean cell hemoglobin concentration (MCHC), Mean cell volume (MCV), Red cell distribution width (RDW), Total leucocyte count (WBC), Differential leucocyte count: Neutrophils (N), Lymphocytes (L), Eosinophils (E), Basophils (B), Monocytes (M), Large unstained cells (LUC), Platelet count (Plt), Prothrombin time (PT), Activated partial thromboplastin time (APTT).
The biochemical analyses of plasma performed after 13 weeks of treatment did not reveal any changes in response to treatment with Subtilisin. All inter-group differences from control, including those attaining statistical significance, were minor, lacked dose-relationship or were confined to one sex and were therefore attributed to normal biological variation (Table 10). Such differences included: the marginally high potassium (K) concentrations in mid and high group males which was considered incidental, since all individual values were within the background range and four controls had individual values that were below the background range; and there was no similar trend in females. Slightly low alanine amino transferase (ALT) activities were reported in mid and high group males, but only one animal from these groups had a value marginally lower than the concurrent controls and this value was well within the background range, hence, the observed difference was considered attributable to normal biological variation. Moreover, reduced plasma transaminase activities are generally considered of no toxicological importance as tissue damage or injury generally causes an increase in plasma enzymes. Plasma albumin (Alb) concentrations in high group males were also slightly low, leading to a small statistically significant reduction of the albumin to globulin ratio, but all individual plasma albumin values were well within the background range, females were unaffected and there was no effect upon liver function that would have accounted for this trend, hence, the observed difference was considered attributable to normal biological variation.
Table 10.
13-week toxicity study in rats. Clinical Chemistry. Group mean values and standard deviation (in brackets) of males (M) and females (F). Significantly different from the controls: ∗: P < 0.05; ∗∗: P < 0.01. N = 9–10 for females; Group 4M, N = 9. Alkaline phosphatase (ALP), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Urea, Creatinine (Creat), Glucose (Gluc), total cholesterol (Chol), low-density lipoproteins (LDL), high-density lipoproteins (HDL), Sodium (Na), Potassium (K), Total protein (Total Prot), Albumin (Alb), Albumin/globulin ratio (A/G Ratio).
| ALP |
ALT |
AST |
Bi Ac |
Urea |
Creat |
Gluc |
Chol |
HDL |
LDL |
Na |
K |
Total Prot |
Alb |
A/G |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U/L | U/L | U/L | μmol/L | mmol/L | μmol/L | mmol/L | mmol/L | mmol/L | mmol/L | mmol/L | mmol/L | g/L | g/L | Ratio | ||
| 1M | Mean | 91 | 58 | 76 | 26.5 | 7.61 | 32 | 8.35 | 2.51 | 2.39 | 0.46 | 142 | 4.2 | 66 | 36 | 1.18 |
| SD | 15.2 | 16.2 | 26.1 | 11.37 | 1.018 | 3.7 | 1.097 | 0.453 | 0.427 | 0.090 | 0.8 | 0.25 | 1.7 | 0.6 | 0.055 | |
| 2M | Mean | 97 | 57 | 65 | 18.1 | 8.13 | 34 | 9.76 | 2.65 | 2.59 | 0.52 | 142 | 4.3 | 67 | 36 | 1.17 |
| SD | 20.0 | 15.5 | 14.5 | 6.18 | 1.818 | 2.5 | 1.249 | 0.536 | 0.447 | 0.194 | 1.2 | 0.26 | 2.1 | 0.7 | 0.047 | |
| 3M | Mean | 97 | 45∗ | 70 | 18.6 | 8.03 | 36 | 8.97 | 2.55 | 2.42 | 0.45 | 142 | 4.4∗ | 67 | 36 | 1.13 |
| SD | 17.1 | 6.6 | 14.3 | 3.18 | 1.039 | 3.1 | 0.622 | 0.358 | 0.336 | 0.093 | 1.0 | 0.15 | 1.7 | 1.0 | 0.051 | |
| 4M | Mean | 81 | 38∗∗ | 87 | 19.0 | 6.62 | 34 | 9.03 | 2.19 | 2.10 | 0.46 | 142 | 4.4∗ | 65 | 34∗∗ | 1.12∗ |
| SD | 10.2 | 7.4 | 40.5 | 5.38 | 0.837 | 1.6 | 0.854 | 0.503 | 0.435 | 0.146 | 1.0 | 0.27 | 2.5 | 1.0 | 0.082 | |
| 1F | Mean | 31 | 34 | 66 | 32.8 | 7.44 | 38 | 7.14 | 2.19 | 1.98 | 0.19 | 141 | 3.8 | 71 | 41 | 1.32 |
| SD | 8.5 | 9.1 | 23.3 | 21.16 | 1.112 | 3.5 | 0.646 | 0.342 | 0.179 | 0.032 | 0.9 | 0.30 | 3.0 | 2.0 | 0.069 | |
| 2F | Mean | 31 | 30 | 71 | 28.2 | 7.80 | 39 | 6.86 | 1.87 | 1.79 | 0.18 | 142 | 3.9 | 68 | 39 | 1.32 |
| SD | 6.6 | 7.8 | 16.5 | 20.04 | 0.943 | 5.0 | 1.092 | 0.289 | 0.211 | 0.067 | 0.5 | 0.33 | 2.5 | 1.9 | 0.081 | |
| 3F | Mean | 29 | 34 | 69 | 31.5 | 6.90 | 34 | 7.22 | 1.93 | 1.78 | 0.19 | 142 | 3.9 | 72 | 40 | 1.25 |
| SD | 6.6 | 7.4 | 16.0 | 9.11 | 0.533 | 3.4 | 1.004 | 0.530 | 0.425 | 0.101 | 1.5 | 0.22 | 5.1 | 2.7 | 0.092 | |
| 4F | Mean | 29 | 33 | 69 | 24.2 | 6.85 | 39 | 7.27 | 1.95 | 1.76 | 0.20 | 141 | 3.8 | 71 | 40 | 1.27 |
| SD | 6.9 | 5.1 | 15.4 | 13.21 | 1.008 | 2.9 | 0.838 | 0.443 | 0.340 | 0.105 | 0.8 | 0.37 | 4.2 | 2.7 | 0.061 |
The analyses of endocrine-sensitive endpoints did not show any treatment-related effects. There was no effect of treatment on estrous cycles at the end of the treatment period. Since no treatment-related changes were identified in the testes from organ weight or histopathology investigations, assessments of sperm count and sperm morphology were not performed. The serum concentrations of triiodothyronine (T3), thyroxine (T4) and thyroid stimulating hormone (TSH) concentrations in Week 13 were considered to have been unaffected by treatment. Statistical analysis revealed no inter-group significant differences in triiodothyronine (T3) or thyroxine (T4) concentrations for either males or females. The thyroid stimulating hormone (TSH) concentrations were statistically significantly higher than those of the controls for all groups of treated males but there was no dose response and females were not affected. An assessment of the individual results demonstrated that the T4 and T3 concentrations in animals with the highest TSH concentrations were similar to those reported for the other animals in this study. A chronic physiologically significant increase in TSH would be expected to also result in stimulation of the thyroid gland resulting in an increase in thyroid gland weight and the presence of follicular cell hypertrophy or hyperplasia. There was no effect on thyroid weights or any pathology changes. Thyroid effects are generally determined on the sum of total findings rather than isolated changes, and thyroid weight and histopathology are ultimately the defining factors. The variations observed for TSH were, in the absence of any physiological influence on thyroid gland weight or pathology, considered to represent normal biological variation.
It is concluded that daily oral administration of Subtilisin to Han Wistar rats at dose levels up to 100% of the test batch for 13 weeks was well-tolerated, with no evidence of any adverse finding at any of the administered doses. Consequently, the no-observed-adverse-effect level (NOAEL) was considered to be the highest dose tested, 483.6 mg TOS/kg bwt/day (equivalent to 3.0 × 106 NFP/kg bwt/day).
3.7. Broiler chicken efficacy study
The recovery of the active enzyme Subtilisin protease is presented in Table 11. Generally, diets had good recovery 75–99%, with the high concentration feed having a higher recovery, and the average recovery in the diet was 88%.
Table 11.
Recovery of the Subtilisin in the experimental diets.
| Trial phase | Treatment | In-feed analytics (NFP/kg) |
||
|---|---|---|---|---|
| declared | measured | Recovery | ||
| Starter | A/Control | – | – | |
| B/Protease | 30,000 | 27,000 | 89% | |
| C/Protease | 60,000 | 56,000 | 93% | |
| Grower | A/Control | – | – | |
| B/Protease | 30,000 | 22,000 | 74% | |
| C/Protease | 60,000 | 58,000 | 97% | |
| Finisher | A/Control | – | – | |
| B/Protease | 30,000 | 23,000 | 75% | |
| C/Protease | 60,000 | 59,000 | 99% | |
The average body weight of the day-old birds upon arrival was 40 g. The growth performance of the birds during the study reached 2.5 kg and it was in agreement with the expectation (Cobb500, 2018). Average mortality during the experimental period (0–35 d) was 2.1%, 2,4% and 2.1% for treatments receiving 0, 30,000 and 60,000 NFP/kg feed. respectively. This can be considered within the normal range in this facility. No specific cause of mortality of birds was observed.
Results of the growth performance are summarized in Table 12 for the three individual periods (starter period, day 0–14; grower period, day 14–28; finisher period, day 28–35) and for the whole experimental period from day 0 to day 35.
Table 12.
Summary of feed intake (FI), body weight gain (BWG) and feed conversion ratio (FCR) of male broiler chickens fed control diet supplemented with the protease at 0, 30,000 and 60,000 NFP/kg diet. 16 replicates, 8 birds per replicate.
| Parameter | Age (days) | Predictor | Estimate | Std Error | t Ratio | P value | Lower 95% | Upper 95% |
|---|---|---|---|---|---|---|---|---|
| Body weight gain (g/b) | 0–14 | Intercept | 397.5 | 4.09 | 97.31 | <.0001 | 389 | 406 |
| Protease (mg/kg) | 0.8 | 1.05 | 0.71 | 0.479 | −1 | 3 | ||
| 14–28 | Intercept | 1152.7 | 13.51 | 85.31 | <.0001 | 1125 | 1180 | |
| Protease (mg/kg) | 5.0 | 3.47 | 1.43 | 0.160 | −2 | 12 | ||
| 28–35 | Intercept | 737.9 | 15.05 | 49.04 | <.0001 | 708 | 768 | |
| Protease (mg/kg) | 11.9 | 3.87 | 3.07 | 0.004 | 4 | 20 | ||
| Overall 0–35 | Intercept | 2287.8 | 19.14 | 119.52 | <.0001 | 2249 | 2326 | |
| Protease (mg/kg) | 17.6 | 4.92 | 3.57 | 0.001 | 8 | 28 | ||
| Feed intake (g/b) | 0–14 | Intercept | 507.5 | 11.56 | 43.88 | <.0001 | 484 | 531 |
| Protease (mg/kg) | 3.9 | 2.97 | 1.32 | 0.193 | −2 | 10 | ||
| 14–28 | Intercept | 1818.2 | 31.25 | 58.18 | <.0001 | 1755 | 1881 | |
| Protease (mg/kg) | −18.6 | 8.04 | −2.31 | 0.026 | −35 | −2 | ||
| 28–35 | Intercept | 1213.4 | 21.18 | 57.30 | <.0001 | 1171 | 1256 | |
| Protease (mg/kg) | −3.0 | 5.44 | −0.55 | 0.584 | −14 | 8 | ||
| Overall 0–35 | Intercept | 3539.1 | 44.22 | 80.04 | <.0001 | 3450 | 3628 | |
| Protease (mg/kg) | −17.6 | 11.37 | −1.55 | 0.128 | −41 | 5 | ||
| FCR (g:g) | 0–14 | Intercept | 1.277 | 0.026 | 48.764 | <.0001 | 1.224 | 1.330 |
| Protease (mg/kg) | 0.007 | 0.007 | 1.111 | 0.273 | −0.006 | 0.021 | ||
| 14–28 | Intercept | 1.580 | 0.030 | 52.450 | <.0001 | 1.519 | 1.641 | |
| Protease (mg/kg) | −0.022 | 0.008 | −2.877 | 0.006 | −0.038 | −0.007 | ||
| 28–35 | Intercept | 1.660 | 0.041 | 40.270 | <.0001 | 1.577 | 1.743 | |
| Protease (mg/kg) | −0.029 | 0.011 | −2.770 | 0.008 | −0.051 | −0.008 | ||
| Overall 0–35 | Intercept | 1.548 | 0.018 | 84.499 | <.0001 | 1.511 | 1.585 | |
| Protease (mg/kg) | −0.019 | 0.005 | −4.008 | 0.000 | −0.028 | −0.009 |
During the overall period of the trial, a significant linear protease effect on BWG and the FCR was observed. FI was not significantly impacted, though numerically lower. The efficacy of protease supplementation was more pronounced during the finisher period in all the measured production parameters. The results of this study are in agreement with previous research (Cowieson and Roos, 2014), which showed the efficacy of proteases in improving the efficiency of feed utilisation in poultry and swine via the improved nitrogen and amino acid digestibility. Although digestibility was not in the scope of the current study there were two indications of a clear evidence of the positive benefit of Subtilisin in broiler chickens: the linear relationship between the Subtilisin dose and the efficiency of feed utilisation, and the lack of difference in mortality between the control and the Subtilisin-treated group.
4. Conclusion
The present work summarizes the toxicological studies performed to evaluate the safety of a novel microbial Subtilisin as a feed additive, as well as the efficacy study of Subtilisin in broiler chickens. A toxicological evaluation of Subtilisin was carried out as recommended by the European Food Safety Authority (EFSA, 2012), in accordance with respective OECD guidelines.
The supernatant from the production strain was not found to be cytotoxic in the Vero cell assay. Subtilisin did not exhibit irritation potential when applied to the eye using the ex-vivo isolated chicken eye test or to the skin using the in-vitro EPISKIN™ Reconstructed Human Epidermis Model. Subtilisin was found not to represent mutagenic or clastogenic potential when tested in relevant genotoxicological assays (Ames and micronucleus). Subtilisin administered by oral gavage to rats for 13 weeks did not cause any adverse effects. Based on the toxicological data and the fact that the Bacillus licheniformis production organism derives from a safe strain lineage, it is concluded that there are no reasons for safety concerns for this novel Subtilisin. The results of the dose response performance trial in broiler chickens showed a significant linear improvement of both the growth rate and feed conversion ratio. In conclusion, Subtilisin is a good candidate for use as a feed additive.
Declarations
Author contribution statement
Denisa Cupi, Michael Thorsen: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jose-Otavio Berti-Sorbara, Aaron J. Cowieson: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Signe Gry Elvig-Jørgensen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Murtala Umar Faruk: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Linda Wulf-Andersen: Conceived and designed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- AAFCO . Association of American Feed Control Officials; 2018. Section 30.1. Enzymes/Source Organisms Acceptable for Use in Animal Feeds. Official Publication. [Google Scholar]
- Adeola O., Cowieson A. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011;89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Angervall L., Carlström E. Theoretical criteria for the use of relative organ weights and similar ratios in biology. J. Theor. Biol. 1963;4:254–259. doi: 10.1016/0022-5193(63)90004-3. [DOI] [PubMed] [Google Scholar]
- Aureli R., et al. A novel glucuronoxylan hydrolase produced by fermentation is safe as feed additive: toxicology and tolerance in broiler chickens. Regul. Toxicol. Pharmacol. 2018;99:213–224. doi: 10.1016/j.yrtph.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Bartlett M.S. Properties of sufficiency and statistical tests. Proc. Roy. Soc. Lond. Math. Phys. Sci. 1937;160:268–282. [Google Scholar]
- Bedford M., Cowieson A. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci. Technol. 2012;173:76–85. [Google Scholar]
- Bundgaard A.M., et al. Assessment of the potential of digestibility-improving enzymes to reduce greenhouse gas emissions from broiler production. J. Clean. Prod. 2014;73:218–226. [Google Scholar]
- Cobb500 . 2018. Broiler Performance and Nutrition Supplement. [Google Scholar]
- Cowieson A.J., Roos F.F. Bioefficacy of a mono-component protease in the diets of pigs and poultry: a meta-analysis of effect on ileal amino acid digestibility. J. Appl. Anim. Nutr. 2014;2:1–8. [Google Scholar]
- Cowieson A.J., Roos F.F. Toward optimal value creation through the application of exogenous mono-component protease in the diets of non-ruminants. Anim. Feed Sci. Technol. 2016;221:331–340. [Google Scholar]
- de Boer A.S., et al. On the industrial use of Bacillus licheniformis: a review. Appl. Microbiol. Biotechnol. 1994;40:595–598. [Google Scholar]
- Dersjant-Li Y., et al. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015;95:878–896. doi: 10.1002/jsfa.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett C.W. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 1955;50:1096–1121. [Google Scholar]
- Dunnett C.W. New tables for multiple comparisons with a control. Biometrics. 1964;20:482–491. [Google Scholar]
- EFSA Introduction of a qualified presumption of safety (QPS) approach for assessment of selected microorganisms referred to EFSA-opinion of the scientific committee. EFSA J. 2007;5:587. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed. Technical guidance: tolerance and efficacy studies in target animals. EFSA J. 2011;9:2175. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed. Guidance for establishing the safety of additives for the consumer. EFSA J. 2012;10:2537. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed. Guidance on the assessment of the safety of feed additives for the consumer. EFSA J. 2017;15(10):5022. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018;16 doi: 10.2903/j.efsa.2018.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuropeanParliament . 2010. Directive 2010/63/EU Revising Directive 86/609/EEC on the protection of Animals Used for Scientific Purposes. [Google Scholar]
- Fisher R. fourteenth ed. Hafner Publishing Company; New York: 1973. Statistical Methods for Research Workers. [Google Scholar]
- Green M., Muriel W. 1984. Mutagen testing using trp+ reversion in Escherichia coli; pp. 161–187. (Handbook of Mutagenicity Test Procedures). [Google Scholar]
- Leinonen I., Kyriazakis I. How can we improve the environmental sustainability of poultry production? Proc. Nutr. Soc. 2016;75:265–273. doi: 10.1017/S0029665116000094. [DOI] [PubMed] [Google Scholar]
- Lichtenberg J., et al. Safety evaluation of a novel muramidase for feed application. Regul. Toxicol. Pharmacol. 2017;89:57–69. doi: 10.1016/j.yrtph.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Lichtenberg J., et al. Toxicological studies on a novel phytase expressed from synthetic genes in Aspergillus oryzae. Regul. Toxicol. Pharmacol. 2011;60:401–410. doi: 10.1016/j.yrtph.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Mahon G. 1989. Analysis of data from microbial colony assays; pp. 26–65. (Statistical Evaluation of Mutagenicity Test Data). [Google Scholar]
- Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Environ. Mutagen Relat. Subj. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- OECD . 1997. Test No. 471: Bacterial Reverse Mutation Test. [Google Scholar]
- OECD . 2015. Test No. 439. (Vitro Skin Irritation: Reconstructed Human Epidermis Test Method). [Google Scholar]
- OECD . 2016. Test guideline 487. (Vitro Mammalian Cell Micronucleus Test). [Google Scholar]
- OECD . 2018. Test Guideline 438: Isolated Chicken Eye Test Method for Identifying I) Chemicals Inducing Serious Eye Damage and II) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage. [Google Scholar]
- OECD . 2018. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. [Google Scholar]
- Ojha B., et al. Elsevier; 2019. Enzymes in the animal feed industry; pp. 93–109. (Enzymes in Food Biotechnology). [Google Scholar]
- Pariza M.W., Cook M. Determining the safety of enzymes used in animal feed. Regul. Toxicol. Pharmacol. 2010;56:332–342. doi: 10.1016/j.yrtph.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Pariza M.W., Johnson E.A. Evaluating the safety of microbial enzyme preparations used in food processing: update for a new century. Regul. Toxicol. Pharmacol. 2001;33:173–186. doi: 10.1006/rtph.2001.1466. [DOI] [PubMed] [Google Scholar]
- Pedersen P., Broadmeadow A. Toxicological studies on Thermomyces lanuginosus xylanase expressed by Fusarium venenatum, intended for use in food. Food Addit. Contam. 2000;17:739–747. doi: 10.1080/026520300415273. [DOI] [PubMed] [Google Scholar]
- Rebello S., et al. Springer; 2019. Industrial Enzymes as Feed Supplements—Advantages to Nutrition and Global Environment. Green Bio-Processes; pp. 293–304. [Google Scholar]
- Shirley E. A non-parametric equivalent of Williams' test for contrasting increasing dose levels of a treatment. Biometrics. 1977:386–389. [PubMed] [Google Scholar]
- Steel R.G. Biometrics; 1959. A Multiple Comparison Rank Sum Test: Treatments versus Control; pp. 560–572. [Google Scholar]
- Thompson C., et al. Modified bacterial mutation test procedures for evaluation of peptides and amino acid-containing material. Mutagenesis. 2005;20:345–350. doi: 10.1093/mutage/gei045. [DOI] [PubMed] [Google Scholar]
- Williams D. A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics. 1971:103–117. [PubMed] [Google Scholar]
- Williams D. The comparison of several dose levels with a zero dose control. Biometrics. 1972:519–531. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.