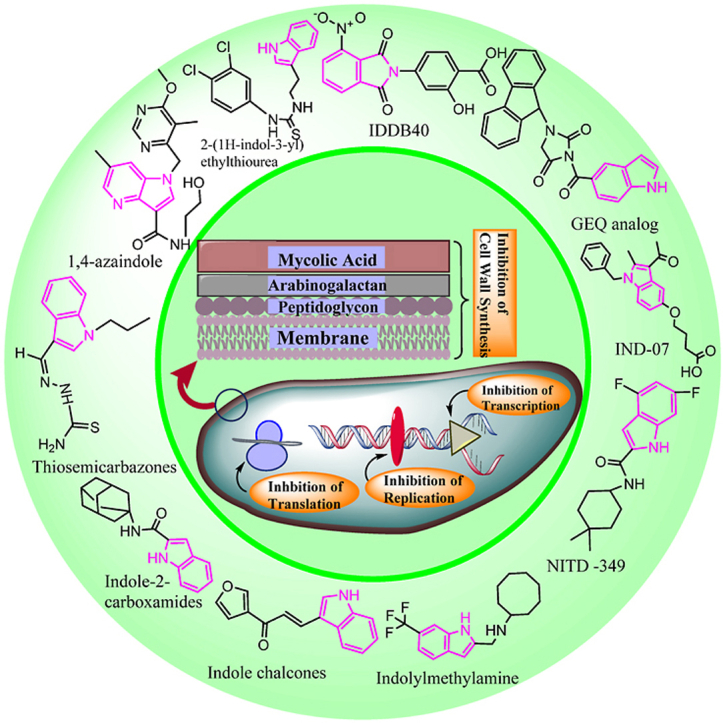
Abstract
Indole-containing small molecules have been reported to have diverse pharmacological activities. The aromatic heterocyclic scaffold, which resembles various protein structures, has received attention from organic and medicinal chemists. Exploration of indole derivatives in drug discovery has rapidly yielded a vast array of biologically active compounds with broad therapeutic potential. Nature is the major source of indole scaffolds, but various classical and advanced synthesis methods for indoles have also been reported. One-pot synthesis is widely considered an efficient approach in synthetic organic chemistry and has been used to synthesize some indole compounds. The rapid emergence of drug-resistant tuberculosis is a major challenge to be addressed. Identifying novel targets and drug candidates for tuberculosis is therefore crucial. Researchers have extensively explored indole derivatives as potential anti-tubercular agents or drugs. Indole scaffolds containing the novel non-covalent (decaprenylphosphoryl-β-D-ribose2′-epimerase) DprE1 inhibitor 1,4-azaindole is currently in clinical trials to treat Mycobacterium tuberculosis. In addition, DG167 indazole sulfonamide with potent anti-tubercular activity is undergoing early-stage development in preclinical studies. Indole bearing cationic amphiphiles with high chemical diversity have been reported to depolarize and disrupt the mycobacterial membrane. Some indole-based compounds have potential inhibitory activities against distinct anti-tubercular targets, including the inhibition of cell wall synthesis, replication, transcription, and translation, as summarized in the graphical abstract. The success of computer-aided drug design in the fields of cancer and anti-viral drugs has accelerated in silico studies in antibacterial drug development. This review describes the sources of indole scaffolds, the potential for novel indole derivatives to serve as anti-tubercular agents, in silico findings, and proposed actions to facilitate the design of novel compounds with anti-tubercular activity.
Keywords: Indole, Tuberculosis, Computer-aided drug design, Clinical trials, In vitro, In silico, Synthesis, Indole alkaloids, Drug discovery, Drug development
Abbreviations: DprE1, decaprenylphosphoryl-β-D-ribose2′-epimerase; KasA, β-ketoacyl ACP synthase I; MmpL3, mycobacterial membrane protein large 3; CM, chorismate mutase; DHFR, Dihydrofolate reductase; SBDD, Structure-based drug design
Graphical abstract
Highlights
-
•
The Indole derivatives emerged as an efficient bioactive compoundes with wide range of therapeutic potential.
-
•
Identifying novel drug candidates with indole derivatives can curtail the rapid emergence of drug-resistant tuberculosis.
-
•
The current review highlights the sources of indole scaffolds, their derivatives, and in silico findings as anti-tubercular agents.
-
•
Currently, DprE1 inhibitor 1,4-azaindole and DG167 indazole sulfonamide are in clinical trials to treat Mycobacterium tuberculosis.
1. Introduction
Tuberculosis (TB), an infectious disease caused by the bacillus Mycobacterium tuberculosis (Mtb), remains a leading cause of morbidity and mortality worldwide (Kontsevaya et al., 2021). In the year 2017, TB was announced as matter of high priority by the World Health Organization (Khawbung et al., 2021). Streptomycin was the first drug discovered to treat TB, and the subsequent entry of thiacetazone and para-aminosalicylic acid into the market improved the cure rate of the disease. The development of resistance to streptomycin resulted in the discovery of new anti-tubercular drugs such as isoniazid (INH), pyrazinamide, cycloserine, ethionamide, rifampicin, and ethambutol. (Chetty et al., 2017). The World Health Organization recommends directly observed treatment, short-course anti-TB therapy including INH, rifampicin, pyrazinamide, and ethambutol as first-line drugs. The Mycobacterium genome, after undergoing mutation and several other structural changes, can evade the drugs commonly used to treat the infection. This drug resistance can also decrease drug accumulation in the bacterium or inactivation due to mutation. A decrease in the drug's binding affinity to target genes has also been observed. The control measures and medications for the disease have been complicated by the outbreak of resistance (Rivers and Mancera, 2008).
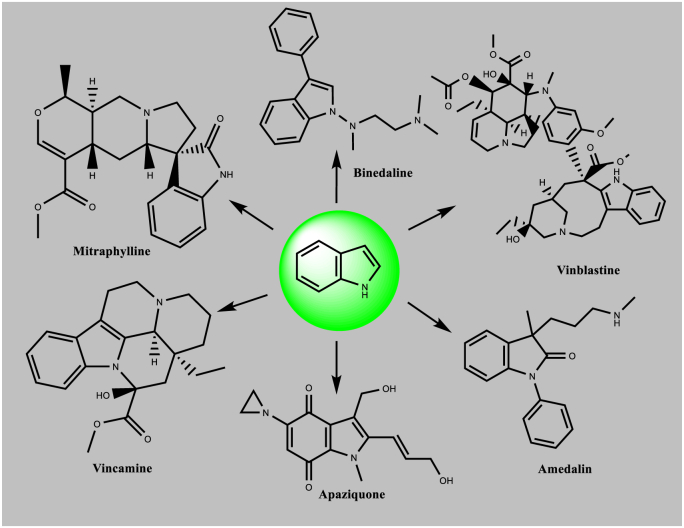
The emergence of drug-resistant bacteria is the primary concern regarding the infectiveness of first-line drugs. Several new drug candidates with novel mechanisms of action are currently under development (Rivers and Mancera, 2008). Thus, new drugs with novel and existing mechanisms of action with minimal or no drug resistance are currently needed. Indoles (C8H7N) are aromatic heterocyclic structures consisting of a benzene ring fused to a five-membered nitrogen-containing pyrrole ring. The therapeutic potential of indole-containing small molecules has been reported, on the basis of their various pharmacological activities (Kumari and Singh, 2019). Many drug molecules containing an indole ring have been marketed for the treatment of various disease conditions. These drugs include the anticancer drugs vincristine, vinblastine, vinorelbine, vindesine, mitraphylline, and apaziquone (marketed as an anti-microbial agent), and also anti-hypertensive drugs, such as vincamine, reserpine, and perindopril. Some anti-depressant drugs have also been marketed, such as binedaline, trandolapril, amedalin, pindolol, siramesine, and indalpine (Fig. 1) (Biswal et al., 2012). Several mechanisms that inhibit mycobacterial strains have been explored, such as inhibition of cell wall synthesis, mycolic acid synthesis, protein synthesis, RNA synthesis, ATP synthase, DNA topoisomerase, and DNA gyrase (Chetty et al., 2017; Rivers and Mancera, 2008; Mitchison and Davies, 2008). Mechanistic and computational studies of novel indoles derivatives against mycobacteria have revealed the modes of action of various compounds. Here, we summarize the sources of indole scaffolds and the proposed modes of action of novel indole-based compounds (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7), which may serve as anti-tubercular drugs against various targets. We also highlight the potential findings from an in silico approach to discovering and developing anti-tubercular agents.
Fig. 1.
Indole scaffolds in several marketed drugs.
Table 1.
Indole-based cell wall inhibitors.
| Sr. No | Compound | Structure | % Inhibition/IC50 | Ref. |
|---|---|---|---|---|
| 1. | 2-Hydroxy-4-(4-nitro-1,3-dioxoisoindolin-2-yl) benzoic acid (IDDB40) | 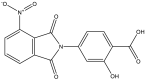 |
0.39 μg/ml | (Islam et al., 2021) |
| 2. | GEQ analog, 3-(9H-fluoren-9-yl)-1-(1H-indol-5-ylcarbonyl)- 2,5-pyrrolidinedione | 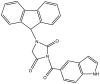 |
39.4 μM | (Matviiuk et al., 2013) |
| 3. | Spiro[indole-thiazolidine] derivatives | 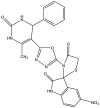 |
12.5 μg/ml | (Borad et al., 2018) |
| 4 | 3-(3-(5-Chloro-2-phenyl-1H-indol-3-yl)-1-isonicotinoyl-1H-pyrazol-5-yl)-2H-chromen-2-one | 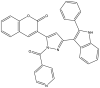 |
12.5 μg/ml | (Rathod et al., 2017) |
| 5 | Indole-3-thiosemicarbazone (H1L) | 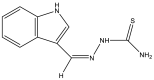 |
1.6 μg/ml | (Khan et al., 2018) |
| 6. | Indole-based 1,3,4-oxadiazole derivatives | 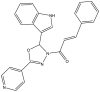 |
28.72 μg/ml | (Desai et al., 2016) |
| 7. | N-(2-hydroxyethyl)-1-((6-methoxy-5-methylpyrimidin-4-yl) methyl)-6-methyl-1H pyrrolo[3,2-b] pyridine-3-carboxamide | 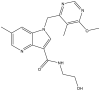 |
10 nM | (Chatterji et al., 2014) |
| 8. | N-(4-(4-(bis(5-bromo-1H-indol-3-yl)methyl)-1H-1,2,3-triazol-1-yl)phenyl)acetamide | 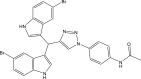 |
1 μg/mL | Danne et al. (2018) |
| 9. | DG167, indazole sulfonamide | 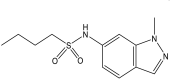 |
0.39 μM | (Inoyama et al., 2020) |
| 10. | Indole chalcones | 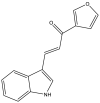 |
210 μM | (Ramesh et al., 2020) |
Table 2.
Indole-based cell membrane depolarizers.
| Sr. No | Compound | Structure | % Inhibition/IC50 | Ref. |
|---|---|---|---|---|
| 11. | n-Octyl side chain containing 4-fluoro-3-[(azepan-1-yl)methyl] indole |  |
2 μM | (Yang et al., 2017) |
| 12. | Indolylpropyl-triphenylphosphonium | 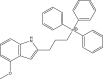 |
3 μM | (Li et al., 2017) |
Table 3.
Indole-based MmpL3 inhibitors.
| Sr. No. | Compound | Structure | % Inhibition/IC50 | Ref. |
|---|---|---|---|---|
| 13. | Indole-2-carboxamides | 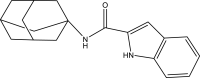 |
0.2 μg/ml | (Franz et al., 2017) |
| 14. | N-cyclooctyl-6-trifluoromethylindol-2- ylmethylamine | 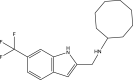 |
0.13 μM | (Tan et al., 2020) |
| 15. | N-(1-(adamantan-1-yl) ethyl)-4,6-dichloro-1H-indole-2- carboxamide | 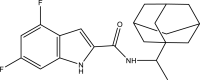 |
0.32 μM | (Alsayed et al., 2021) |
| 16. | Adamantanol analogs | 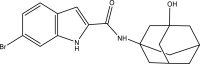 |
NA | (Alsayed et al., 2021) |
| 17. | NITD-349, N-(4,4-dimethylcyclohexyl)-4,6-difluoro-1H-indole-2-carboxamide | 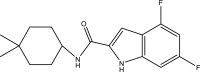 |
NA | (Yang et al., 2020) |
| 18. | Indolamide | 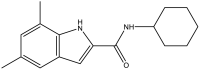 |
>64 μg/mL | (Lun et al., 2013) |
Table 4.
Indole-based chorismate mutase inhibitors.
| Sr. No. | Compound | Structure | % Inhibition/IC50 | Ref. |
|---|---|---|---|---|
| 19. | Isatin-indole | 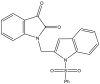 |
74.20% at 30 μM | (Reddy et al., 2020) |
| 20. | Indole containing o-(RSO2) C6H4 group | 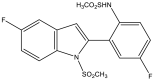 |
17.02 mM | (Nakhi et al., 2011) |
| 21. | 3-{(5-Chloro-1-tosyl-1H-indol-2-yl)methyl}benzo[d][1,2,3] triazin-4(3H)-one | 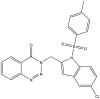 |
78% at 30 μM | (Reddy et al., 2019) |
| 22. | 7-Sulfonyl indole | 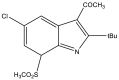 |
45% at 30 μM | (Prasad et al., 2012) |
| 23. | 2-Chloro-3-(5,6-difluoro-1H-indol-3-yl)quinoxaline | 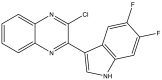 |
19.74 μM | (Kumar et al., 2012) |
Table 5.
Indole-based compounds targeting DNA replication machinery.
| Sr. No. | Compound | Structure | % Inhibition/IC50 | Ref. |
|---|---|---|---|---|
| 24. | 3-((1-Oxoindolin-2-ylidene)methyl)phenyl benzoate | 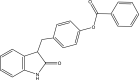 |
NA | (Kumar et al., 2012) |
| 25. | 2-(1H-indol-3-yl) ethylthiourea | 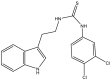 |
72.6 ± 1.2 μg/mL | (Sanna et al., 2018) |
| 26. | 3-(1-Benzyl-5-chloro-2-(ethoxycarbonyl)-4-(trifluoromethyl)-1H-indol-3-yl) propanoic acid | 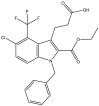 |
86.6 μM | (Singh et al., 2020) |
| 27. | Indole-3-acetic acid-based inhibitor | 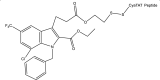 |
3.9 μM | (Dewangan et al., 2021) |
| 28. | Tetracyclic indole derivatives |  |
NA | (Yadav et al., 2015) |
| 29. | 4-((3-Acetyl-1-benzyl-2-methyl-1H-indol-5-yl) oxy) butanoic acid | 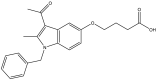 |
150 μM | (Sharma et al., 2018) |
| 30. | (1-(1-Benzyl-5-((1-(4- bromophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-methyl-1H-indol-3-yl) ethanone) | 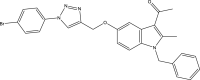 |
6.79 μM | (Sharma et al., 2019) |
Table 6.
Miscellaneous indole-based compounds.
| Sr. No. | Compound | Structure | % Inhibition/IC50 | Ref. |
|---|---|---|---|---|
| 31. | (E)-1-(furan-3-yl)-3-(1H-indol-3-yl) prop2-en-1-one | 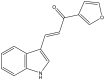 |
87.67% | (Ramesh et al., 2020) |
| 32. | 2-(Indol-3-yl)-2-oxo-acetamide | 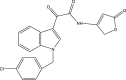 |
0.9 μg/mL | (Mashayekhi et al., 2021) |
| 33. | 3-(1H-indol-3-yl) propanoic acid | 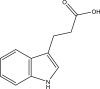 |
68 μM | (Negatu et al., 2018) |
| 34. | Hybrid hydrazides and hydrazide-hydrazones indoles | 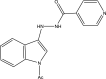 |
0.2 μg/mL | (Velezheva et al., 2016) |
| 35. | Indole-benzimidazole-based 1,2,3-triazole hybrids | 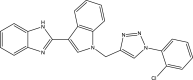 |
3.125–6.25 μg/mL |
(Ashok et al., 2018) |
| 36. | Indole-based thiosemicarbazones | 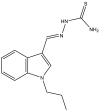 |
1.9 μg/mL | (Mashayekhi et al., 2021) |
| 37. | Indole diketo acids | 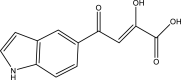 |
0.02 μM | (Libardo et al., 2018) |
Table 7.
Indole-based compounds as co-crystallized ligands with Mtb proteins/enzymes.
| Sr. No. | Proteins/enzymes with PDB IDs | Structure model of protein in complex with ligand | Co-crystallized ligand | Ref. |
|---|---|---|---|---|
| 1. 1. |
Mtb tryptophan synthase (MtTrpAB) with inhibitor GSK2 ((1-[2-fluorobenzoyl]-N-methyl-2,3-dihydro-1H-indole-5-sulfonamide) PDB ID: 6U6C |
 |
 |
(Michalska et al., 2020) |
| 2. | Pantothenate synthetase in complex with 2-(5-methoxy-2-(5-methylpyridin-2-ylsulfonylcarbamoyl)-1H-indol-1-yl) acetic acid PDB ID: 3IUE |
 |
 |
(Hung et al., 2009) |
| 3. |
Mtb malate synthase in complex with 2-hydroxy-3-(1H-indol-3-yl) propanoic acid PDB ID: 5CAK |
 |
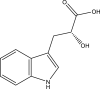 |
(Huang et al., 2016) |
| 4. |
Mtb ArgB in complex with 1H-indole-3-carbonitrile PDB ID: 7NN8 |
 |
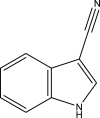 |
(Gupta et al., 2021) |
| 5. | Mtb cytochrome P450 CYP121 in complex with (3S,6S)-3-(4-hydroxybenzyl)-6-(1H-indol-3-ylmethyl) piperazine-2,5-dione. PDB ID: 4IQ9. |  |
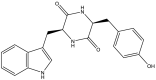 |
(Fonvielle et al., 2013) |
2. Sources of indole scaffolds
Indole moieties are found in various bioactive molecules of natural and synthetic origin. Nature is a major source of small molecules with a wide range of biological activities and structural complexities that enable the design of novel therapeutic agents (de Sa Alves et al., 2009; Bajad et al., 2021a). Indole alkaloids have been described to have high therapeutic importance. The alkaloids are widely distributed in plant families such as Apocynaceae, Rubiaceae, Loganiaceae, and Nyssacease. The principal genera containing alkaloids include Geissospermum, Alstonia, Rauvolfia, Aspidosperma, Tabernaemontana, Psychotria, Kopsia, Hexalobus, Gelsemium, and Vinca (Rosales et al., 2020). The important pharmacologically active classes of indole alkaloids include the antitumor drugs vinblastine and vincristine from Catharanthus roseus, and the antihypertensive drug reserpine from Rauvolfia serpentina (Hamid et al., 2017). Several indole alkaloids of marine origin have been isolated from sponges, seaweeds, ascidians, and mollusks (Kochanowska-Karamyan and Hamann, 2010). The marine-derived indole alkaloids are promising molecules with various biological activities, including anti-inflammatory, anti-parasitic, cytotoxic, antiviral, serotonin modulating, and antagonistic functions (Dey et al., 2020). The structural similarity of indole alkaloids to endogenous neurotransmitters endows the molecules with neurological activity and affinity toward serotonin receptors (Kochanowska-Karamyan and Hamann, 2010). In 1866, Adolf von Baeyer first synthesized indole from oxindole by using zinc dust (Baeyer and Knop, 1866). Various classical and advanced synthesis methods for indoles have since been reported, including Fischer indole synthesis, the classical and widely used method for synthesizing indole and its derivatives (Gassman et al., 1974; Bhakhar et al., 2022). Other modern versions of classical synthetic methods include Nenitzescu synthesis, Reisert synthesis, Ullmann reaction, Leimgruber-Batcho synthesis, Madelung synthesis, the Bartoli reaction, and the Cadogan-Sundberg reaction (Bugaenko et al., 2019; Humphrey and Kuethe, 2006). The transition metal-catalyzed cyclization reactions of unsaturated substrates have become a powerful tool for constructing indole rings (Neto and Zeni, 2020).
3. Indole-based anti-tubercular compounds
3.1. Cell wall inhibitors
The architecture of the mycobacterial cell wall is uniquely composed of a double-membrane cell envelope rich in lipids and carbohydrates, which forms a permeability barrier that blocks the passage of several drug molecules (Hett and Rubin, 2008). The first challenge of any anti-mycobacterial drug is to penetrate this barrier before reaching its target and exerting its potential action. Cell wall biosynthesis inhibition has been demonstrated to be a promising and validated drug target of many discovered anti-mycobacterial drugs (Bugg et al., 2011). The cell envelope comprises three major domains: the characteristic long-chain mycolic acids, highly branched arabinogalactan polysaccharide, and crossed-linked peptidoglycan network (Jankute et al., 2015). The involvement of multiple enzymes in the complex cell wall architecture provides several potential targets for developing new drugs against drug-resistant forms of Mtb (Chen et al., 2018). Targeting of different proteins, e.g., alanine racemase, L, D-transpeptidase (LdtMt2), L, D-transpeptidase (LdMt1), galactofuranosyl transferase, Pks13, Acyl-MP ligase, FabH, InhA (E), MabA, and DprE1, can inhibit cell wall synthesis (Chetty et al., 2017). Several indole scaffold containing agents have been reported to inhibit cell wall biosynthesis by targeting specific enzymes or proteins. Mycolic acids are the most essential primary constituent of the mycobacterial cell wall, contributing to its integrity and permeability. Several key enzymes that are involved in the biosynthesis of mycolic acids, including fatty acid synthesis, and are excellent drug targets include KaS, MabA, KasB, InhA, and HadABC; mycolic acid modifying enzymes; aNAT, SAM-dependent methyltransferases; fatty acid activating and condensing enzymes; and Pks13, FadD32, Acc, transporters (MmpL3), and transferases (antigen 85A-C) (Jeffrey North et al., 2014). The incidence of drug resistance increases with the currently clinically available mycolic acid inhibitors, including isoniazid, ethionamide, and nitroimidazoles. Therefore, the development of new drugs, particularly those inhibiting mycolic acids, may be a promising approach. Some potential indole-containing mycolic acid inhibitors, including their synthesis and biological evaluation against different targets, have been reported. The iso-indole-based compound 2-hydroxy-4-(4-nitro-1,3-dioxoisoindolin-2-yl) benzoic acid (IDDB40) (Kontsevaya et al., 2021) exhibits strong activity against all strains of Mtb with mycolic acid inhibition. The 2-hydroxy-4-(4-nitro-1,3-dioxoisoindolin-2-yl) benzoic acid (IDDB40) (Kontsevaya et al., 2021) compound has been found to have isoniazid activity in a preliminary study of mycolic acid inhibition and may be a potential anti-TB agent (Islam et al., 2021).
3.1.1. Enoyl acyl carrier protein reductase (InhA) inhibitors
The two main elongation systems that control mycolic acid biosynthesis are fatty acid synthase type I (FAS-I) and fatty acid synthase type II (FAS-II). Because eukaryotic cells use FAS-1 enzymes, inhibition of FAS-II enzymes is a viable target in drug development. The enoyl acyl carrier protein reductase (InhA or ENR) of the FAS-II system is involved in the catalysis of NADH-dependent reduction of the double bond at the second position of the growing fatty acid chain associated with ACP (Rožman et al., 2017). The Mtb ENR, commonly known as InhA or Fab I, has been shown to be a potential target for developing anti-tubercular drugs (Kuo et al., 2003). The succinimide and indole scaffold containing the GEQ analog 3-(9H-fluoren-9-yl)-1-(1H-indol-5-ylcarbonyl)-2,5-pyrrolidinedione (Khawbung et al., 2021) potentially inhibits InhA from Mtb with an IC50 of 39.4 μM. The 5-indole carbonyl derivatives have been found to moderately inhibit InhA (Matviiuk et al., 2013). Several in silico findings with respect to InhA inhibition have been reported for indole derivatives. A series of novel spiro[indole-thiazolidines] (Chetty et al., 2017) derivatives have been synthesized from 5-substituted isatin derivatives and thioglycolic acid, with ZrSiO2 as an efficient catalyst under microwave irradiation, and have been evaluated against the Mtb H37Rv strain. The in silico analysis of synthesized compounds with the target long-chain enoyl acyl carrier protein reductase (InhA) has identified several promising compounds (Borad et al., 2018). Various indole, coumarinyl, pyridinyl, and INH derivatives (Rivers and Mancera, 2008) have been synthesized and evaluated against the Mtb H37Rv strain. The computational data from molecular docking studies of all compounds against the InhA enzyme, as well as data from in vitro assays, have been found to replicate the experimental anti-TB activity (Rathod et al., 2017). The synthesized complexes of indole-3-thiosemicarbazone (H1L) (Kumari and Singh, 2019), 5-methoxy indole-3-thiosemicarbazone (H3L), indole-N1-methyl-3-thiosemicarbazone (HIntsc-N1-Me, H2 L), and 5-methoxy indole-N1-methyl-3-thiosemicarbazone (H4L) with copper (I) and silver (I) have been tested for their antimicrobial activity. The compounds have also been evaluated for their binding affinity by docking against ENR, and the active indole-3-thiosemicarbazone and its complexes have shown favorable binding affinity toward the target (Khan et al., 2018). The evaluation of a series of indole- and pyridine-based 1,3,4-oxadiazole derivatives (Biswal et al., 2012) has been performed to determine the in vitro anti-tubercular activity against Mtb H37Ra and Mycobacterium bovis BCG, in both inactive and dormant states, and some of the compounds have shown favorable anti-TB activity. Additional in silico studies against InhA have suggested that the compounds have excellent potential for further optimization and development (Desai et al., 2016).
3.1.2. Decaprenylphosphoryl-β-D-ribose2′-epimerase (DprE1) inhibitors
The target DprE1 is a crucial enzyme in cell wall synthesis in Mycobacterium. DprE1 is a flavoprotein that plays a crucial role in the synthesis of arabinogalactan and lipoarabinomannan, the critical mycobacterium cell-wall polysaccharides (Chikhale et al., 2018). The 1,4-azaindoles are potential drug candidates for the management of TB through the non-covalent inhibition of DprE1. One molecule in the 1,4-azaindole class (TBA 7371) (Mitchison and Davies, 2008) is currently in clinical trials for the treatment of Mtb (NCT04176250). These compounds inhibit DprE1 with an IC50 value of 10 nM and have also been shown to be active against Mtb with a minimal inhibitory concentration (MIC) of 0.78–3.12 μM (Robertson et al., 2021; Shirude et al., 2013) (Chatterji et al., 2014). A triazole–diindolylmethane conjugate (de Sa Alves et al., 2009) compound has been found to have substantial in vitro activity against Mtb H37Ra. The favorable binding of compounds in the active site of the DprE1 enzyme has also been demonstrated with molecular docking studies (Danne et al., 2018).
3.1.3. β-ketoacyl ACP synthase I (KasA) inhibitors
KasA is an essential member of three β-ketoacyl synthases encoded in the genome of Mtb. This enzyme actively participates in the catalysis of 2-carbon elongation of growing fatty acyl chains in the FAS-II pathway, which is essential in the biosynthesis of mycolic acids (Kumar et al., 2018) DG167, an indazole sulfonamide (Bajad et al., 2021a) inhibitor of KasA, is undergoing early-stage development in preclinical studies and has been reported to have an MIC of 0.39 μM against the wild-type H37Rv strain and to lack cross-resistance with front line TB agents. Another preclinical candidate, JSF-3285, has been developed to optimize DG167 (Inoyama et al., 2020; Kumar et al., 2021). Mtb indole chalcones (Rosales et al., 2020) have been demonstrated to inhibit the H37Rv strain of Mtb with an MIC of 210 μM and to show favorable in silico binding affinity toward KasA (Ramesh et al., 2020).
3.2. Selective cell membrane depolarization with the indole bearing motif
The selective targeting of the bacterial cell membrane is considered a novel targeting method for anti-tubercular drugs. Targeting of the intact cell membrane, which is crucial for the survival of bacteria, has been found to disrupt a multitude of embedded targets and to have lethal pleiotropic consequences (Chen et al., 2018). Furthermore, those antibacterial agents selectively inhibit the function of the mycobacterial membrane, thus delaying the emergence of resistance, decreasing relapse, and shortening the treatment period for TB. The selective cell membrane permeabilization and depolarization is based on the incorporation of a cationic amphiphilic motif in the form of a lipophilic n-octyl side chain and positively charged azepanyl in the 4-fluoro and 6-methoxyindoles (Hamid et al., 2017), which have shown potent anti-mycobacterial activity, solubility, and metabolic stability (Yang et al., 2017). Furthermore, other indole bearing lipophilic moieties and extensively charge-delocalized triphenylphosphonium cations have been used as a membrane-targeting motif. The indolylalkyltriphenylphosphonium (Kochanowska-Karamyan and Hamann, 2010) analogs have shown potent antibacterial activity through sustained depolarization and disruption of mycobacterial membranes (Li et al., 2017).
3.3. Mycolic acid transporter MmpL3 inhibitors
The mycobacterial membrane protein large 3 (MmpL3) is involved primarily in the transport of mycolic acids in trehalose-monomycolates, precursors of trehalose-dimycolates, and mycolates, which are essential components of the mycobacterial outer membrane (Bolla, 2020). The inhibition of MmpL3 weakens the mycobacterial cell wall and leads to cell death, thus serving as a new potential target for developing novel anti-tubercular drugs (Rayasam, 2014). MmbL3 inhibitors are identified primarily through whole cell-based screening and mutant generation coupled with whole-genome sequencing (Rayasam, 2014). Franz et al. have designed, synthesized, and evaluated indole-2-carboxamides (Dey et al., 2020) for their anti-mycobacterial activity. The indole-2-carboxamides have been tested against several fast- and slow-growing Mycobacterium species, including M. massiliense, M. abscessus, M. bolletii, Mtb, M. chelonae, M. avium, M. xenopi, and M. smegmatis. The compounds have been found to inhibit MmpL3 and to have potent pan-activity against mycobacterial species (Franz et al., 2017). Amide-amine replacement in indole-2-carboxamides yields the potent mycobactericidal compound N-cyclooctyl-6-trifluoromethylindol-2-ylmethylamine (Baeyer and Knop, 1866), which has favorable water solubility. The activity is observed after the replacement of carboxamide, but not in the absence of the indole moiety (Tan et al., 2020). Indoleamide analogs have been reported to be MmpL3 inhibitors with anti-tubercular activity. The most potent adamantanol analogs (Gassman et al., 1974; Bhakhar et al., 2022) are highly selective toward drug-sensitive and drug resistant Mycobacterium strains and show no cytotoxicity (Alsayed et al., 2021). The crystal structure of MmpL3 complexed with the inhibitors NITD-349 (N-(4,4-dimethylcyclohexyl)-4,6-difluoro-1H-indole-2-carboxamide) (Bugaenko et al., 2019), comprising an indole-2-carboxamide, has been studied. NITD-349 binds deep in the central channel of the transmembrane domain and causes conformational changes to the protein. The indole nitrogen of NITD-349 mainly interacts with Asp645, and targets the proton relay pathway, thereby blocking the activity of MmpL3 (Yang et al., 2020). The targeting of MmpL3 with indoleamides (Humphrey and Kuethe, 2006) has shown potent activity against both drug-susceptible and drug-resistant strains of Mtb. The in vivo evaluation of indolamide derivatives has also shown dose-dependent anti-mycobacterial activity after oral administration to mice infected with Mtb (Lun et al., 2013).
3.4. Chorismate mutase (CM) inhibitors
The Mtb chorismate mutase (MtbCM) catalyzes the conversion of chorismate to prephenate in the shikimate biosynthetic pathway in the synthesis of the essential amino acids phenylalanine and tyrosine. The secretory form, MtbCM, encoded by Rv1885c, is believed to actively participate in the pathogenesis of TB. Therefore, the inhibition of CM may directly hinder the supply of nutrients to the organism (Khanapur et al., 2017). A series of isatin-indole (Neto and Zeni, 2020) derivatives synthesized through the one-pot coupling method have been reported to have potential CM inhibitory activity at nanomolar concentrations. Strong interaction has also been observed through in silico docking studies with CM (Reddy et al., 2020). A novel indole containing an o-(RSO2) C6H4 group at C-2 (Hett and Rubin, 2008), synthesized via in situ desilylation-Sonogashira strategies, has been evaluated for in vitro Mtb H37Rv CM inhibition activity. One compound has shown potent and dose-dependent CM inhibition with an IC50 value of 17.02 μM (Nakhi et al., 2011). The substantial in vitro inhibition of CM at 30 μM and the potential in silico inhibitory activity of 3-indolylmethyl substituted pyrazolotriazinone derivatives (Bugg et al., 2011) have been demonstrated. These compounds were synthesized in a single pot through the Pd/Cu-catalyzed coupling-cyclization approach (Reddy et al., 2019). The potential CM inhibitory activity of 7-sulfonyl indoles (Jankute et al., 2015) synthesized via AlCl3 has been found to mediate unexpected regioselective migration of the sulfonyl group (Prasad et al., 2012). Promising CM inhibiting agents have been developed by combining the structural features of indole and the quinoxaline moiety in a single molecule, 2-chloro-3-(5,6-difluoro-1H-indol-3-yl)quinoxaline (Chen et al., 2018), through one pot synthesis of AlCl3 induced (hetero)arylation of 2,3-dichloroquinoxaline (Kumar et al., 2012).
3.5. Targeting DNA replication machinery
The large multi-protein replisome synthesizes leading and lagging strands in bacterial DNA replication. Replisome proteins catalyze several events such as DNA unwinding, RNA primer synthesis, clamp loading, and DNA synthesis (Ditse et al., 2017). The main components of the DNA replication machinery include the DnaA replication initiator, PriA helicase loader, DnaB helicase, DnaG primase, SSB, clamp loader subunits (τ/γ, δ, and δ ′), DNA polymerases I and III, DnaN β-clamp, DNA ligase I, and type I and II topoisomerases.
3.5.1. DNA gyrase inhibitors
DNA gyrase is the only clinically validated target of the fluoroquinolones, which inhibit replication and are used to treat multi-drug resistant TB (Reiche et al., 2017). Efforts to design novel DNA gyrase inhibitors are accelerating because of resistance to fluoroquinolones. The pharmacophore model of GyrB has also been used to screen an in-house database. Docking analysis, MM-GBSA, and MD simulations of selective GyrB inhibitors have been performed to validate the selectivity and potency of the molecules toward the GyrB domain and have also been evaluated for their anti-potential activity (Jeffrey North et al., 2014) (Kashyap et al., 2018; Dighe and Collet, 2020). A series of 2-(1H-indol-3-yl) ethylthiourea (Islam et al., 2021) derivatives have been synthesized and evaluated for their antimicrobial activity against Gram-positive cocci, Gram-negative rods, and fungi. One of the compounds, 1-(2-(1H-indol-3-yl)ethyl)-3-(3,4-dichlorophenyl)thiourea, inhibits S. aureus topoisomerase IV decatenation activity and S. aureus DNA gyrase supercoiling activity (Sanna et al., 2018). A series of potent inhibitors (Rožman et al., 2017) have been developed to target the two enzymes i.e., DnaG primase and DNA gyrase, involved in DNA replication of Mtb. Two of the obtained novel selective inhibitors exhibited bacteriostatic activity against Mycobacterium smegmatis, a fast-growing non-pathogenic model species of mycobacteria. Impairment of biofilm development has also been observed, thus indicating the therapeutic potential of the molecules against infections caused by planktonic and sessile forms of Mycobacterium species (Dewangan et al., 2021). The cell-penetrating peptide conjugates of indole-3-acetic acid-based DNA primase/gyrase 2 inhibitors (Kuo et al., 2003) have been found to be potent anti-tubercular agents. The synthetic conjugates have been evaluated on M. smegmatis (a non-pathogenic mycobacteria), and the MIC has been determined. Unexpectedly, the conjugates have been found to be more potent than the free inhibitors (Dewangan et al., 2021; Singh et al., 2020). The tetracyclic indole derivatives (Matviiuk et al., 2013) target the bacterial DNA ligases and distinguish them from human DNA ligases. The compounds bind the cofactor binding site and compete with the cofactor, according to in silico docking and enzyme inhibition assays (Yadav et al., 2015).
3.5.2. Dihydrofolate reductase (DHFR) inhibitors
DHFR is a key enzyme involved in producing tetrahydrofolate, which is essential for bacterial survival. DHFR is required for the biosynthesis of purine and pyrimidine nucleic acids, which have a major role in cell reproduction (Kleinhans et al., 2010). The selective and potent inhibition of Mtb-DHFR by 4-((3-acetyl-1-benzyl-2-methyl-1H-indol-5-yl) oxy) butanoic acid (Borad et al., 2018) has been demonstrated in a structural comparison of Mtb-DHFR and human DHFRs (Sharma et al., 2018). The bioisosteric replacement of these compounds has resulted in new series of 1-(1-benzyl-2-methyl-5-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)-1H-indol-3-yl)ethanone (Rathod et al., 2017) and ethyl 1-benzyl-2-methyl-5-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)-1H-indole-3-carboxylate derivatives, some of which have shown selectivity and favorable in vitro activity against Mtb-DHFR (Sharma et al., 2019).
3.6. Miscellaneous indole-based compounds
Several indole-based compounds have also been reported for their activity against various strains of Mtb with diverse mechanisms of action. Ramesh et al. have designed, synthesized, and evaluated a series of indole chalcones against the H37Rv strain. Some compounds in the series, including (E)-1-(furan-3-yl)-3-(1H-indol-3-yl)prop2-en-1-one (Khan et al., 2018), (E)-3-(1H-indol-3-yl)-1-(thiophene-2-yl)prop-2-en-1-one, and (E)-2-((1H-indol-2-yl)methylene)cyclopentane-1-one, have shown potential anti-tubercular activity at 50 mg/ml with MIC values of 210, 197, and 236 μM, respectively (Ramesh et al., 2020). Novel 1-substituted indole-3-carboxaldehyde thiosemicarbazones (Desai et al., 2016) have been synthesized and evaluated for their anti-mycobacterial activity. Compounds substituted with a propyl and 4-nitrobenzyl group have been found to have the highest potential and selective inhibition, with IC50 values of 0.9 and 1.9 μg/mL, respectively (Mashayekhi et al., 2021). The gut microbiota metabolite indole propionic acid [3-(1H-indol-3-yl) propanoic acid] (Chikhale et al., 2018) has been purified in vivo and evaluated in a mouse model of acute Mtb infection. This compound has been found to decrease the bacterial load in the spleen by sevenfold and is worthy of further exploration (Negatu et al., 2018). A series of novel hybrid hydrazides and hydrazide-hydrazones combining indole (Robertson et al., 2021) and pyridine nuclei have been designed, synthesized, and tested in vitro for their anti-mycobacterial activity. Some of the compounds have been found to have favorable activity against the INH-sensitive strain Mtb H37Rv, with promising MIC values (Velezheva et al., 2016). Triheterocycles containing indole-benzimidazole-based 1,2,3-triazole hybrid compounds (Shirude et al., 2013) have shown in vitro anti-tubercular activity against the strain, along with antioxidant activity (Ashok et al., 2018). A series of novel indole-based thiosemicarbazones (Chatterji et al., 2014) with potential anti-mycobacterial activity have also been reported (Mashayekhi et al., 2021). Indole diketo acids (Danne et al., 2018) have been identified to inhibit Mtb malate synthase (GlcB), part of the glyoxylate shunt, through high-throughput screening and structure-guided design (Libardo et al., 2018).
4. Structure-based drug design approach
Structure-based drug design approaches have been demonstrated to be successful in the field of non-transmissible and viral diseases but less effective in antibacterial drug development. Nevertheless, notable progress has been made in the past 20 years in understanding the pathogen molecular physiology of TB, beginning with the seminal publication of the Mtb genome in 1998 (Bruch et al., 2020). The availability of crystal structures of target proteins is essential in the structure-based drug design approach. The 3D crystal structure of a target protein is determined through various techniques, such as X-ray crystallography, cryo-electron microscopy, or nuclear magnetic resonance (Bajad et al., 2021b). Some crystal structures of Mtb proteins in complex with indole-based compounds have been deposited in the Protein Data Bank. The functional Mtb tryptophan synthase (MtTrpAB) is essential for the survival of Mtb and is a promising target for the discovery of novel anti-TB drugs. The crystal structure of MtTrpAB with the inhibitor GSK2 ((1-[2-fluorobenzoyl]-N-methyl-2,3-dihydro-1H-indole-5-sulfonamide) has been deposited in the Protein Data Bank under accession code 6U6C (Michalska et al., 2020). A fragment-growing and fragment linking strategy has been used to design pentothenate synthase inhibitors. The atomic coordinates and structure factors for enzyme-ligand complexes have been deposited in the Protein Data Bank under accession codes3IMC, 3ISJ, 3IUB, 3IUE, 3IME, 3IVC, 3IVG, 3IVX, and 3IMG (Hung et al., 2009). Several novel binding chemotypes have been discovered through a fragment-based approach on GlcB from Mtb. The novel interaction observed between the indole-containing fragments and GlcB in the existing potent phenyl-diketo acid (PDKA) series of inhibitors has been found to be 100 times potent than that of the parent PDKA. All determined structures have been deposited in the Protein Data Bank (Huang et al., 2016). The fragment-based approach has been used to identify hits against enzymes involved in the pathways, ArgB, ArgC, ArgD, and ArgF. The crystal structure of ArgB in complex with 1H-indole-3-carbonitrile has also been deposited in the Protein Data Bank (Gupta et al., 2021). In Mtb CYP121, the indole-heme stacking interaction closely contacts the distal face of the heme group (Fonvielle et al., 2013).
5. Conclusion
Indole is one of the prime scaffolds used to synthesize analogs for various biological applications, including TB treatment. This scaffold is found in various bioactive molecules of natural and synthetic origin. Several mechanistic and computational studies of novel indole derivatives have been performed against mycobacteria to understand the modes of action of the compounds. Some novel synthesized indole bearing compounds have also been reported to have potent cell wall inhibition activity, including 2-hydroxy-4-(4-nitro-1,3-dioxoisoindolin-2-yl) benzoic acid (IDDB40), H1L and N-(2-hydroxyethyl)-1-((6-methoxy-5-methylpyrimidin-4-yl) methyl)-6-methyl-1H pyrrolo[3,2-b] pyridine-3-carboxamide, with IC50 values of 0.39 μg/ml, 1.6 μg/ml, and 0.032 μM, respectively. Designing indole-bearing cationic amphiphiles selectively targeting cell membrane permeabilization and depolarization has yielded potent anti-mycobacterial agents. Indole-based MmpL3 inhibitors such as indole-2-carboxamides, N-cyclooctyl-6-trifluoromethylindol-2-ylmethylamine, and N-(1-(adamantane-1-yl) ethyl)-4,6-dichloro-1H-indole-2-carboxamide have been reported to have favorable inhibitory activity. The success of the novel non-covalent DprE1 inhibitor 1,4-azaindole, which is currently undergoing clinical trials for the treatment of Mtb, has been notable. Another DG167 indazole sulfonamide drug candidate with potent anti-tubercular activity may enter clinical trials, given the favorable results of early-stage development in preclinical studies. The role of the structure-based drug design approach expanded after the seminal publication of the Mtb genome in 1998. The availability of crystal structures of Mtb proteins complexed with indole-based compounds in the Protein Data Bank indicates the growth of the in silico drug design approach. Efforts to design and develop indole-based compounds may lead to the identification of potential anti-tubercular drugs effective against drug-resistant TB.
6. Future perspectives
Several indole-containing drug molecules are available on the market to treat various disease conditions. The development of anti-tubercular drugs with novel mechanistic properties can be accelerated by incorporating promising moieties such as indoles into molecules. The potential results of a diverse range of functionalized indole derivatives acting on multiple targets are highly encouraging for the discovery and development of indole-based anti-tubercular agents or drugs. The emergence of new synthesis methods and advances in computational drug design tools have provided tremendous opportunities for designing indole derivatives. Newer in silico approaches, such as fragment-based drug design and advances in virtual screening, will be instrumental in developing potent new compounds. In addition, the recent progress in structural biology methods has played a crucial role in target-based drug discovery (de Souza Neto et al., 2020). The potential molecules 1,4-azaindoles and DG167 indazole sulfonamide are currently under clinical development and may enter the market in the future to treat Mtb. Therefore, the discovery and development of indole-based therapeutics is an attractive potential approach for identifying potent and promising anti-tubercular drugs.
CRediT authorship contribution statement
Nilesh Gajanan Bajad: Conceptualization, Methodology, Writing – original draft. Sudhir Kumar Singh: Validation, Writing – review & editing. Sushil Kumar Singh: Supervision, Validation. Tryambak Deo Singh: Supervision, Investigation. Meenakshi Singh: Supervision, Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by Institute of Eminence, Banaras Hindu University, Varanasi, India. SKS is grateful to the Institute of Eminence, Banaras Hindu University, Varanasi, India, for providing the Malaviya Postdoctoral Fellowship.
References
- Alsayed S.S., Lun S., Bailey A.W., Suri A., Huang C.-C., Mocerino M., et al. Design, synthesis and evaluation of novel indole-2-carboxamides for growth inhibition of Mycobacterium tuberculosis and paediatric brain tumour cells. RSC Adv. 2021;11(26):15497–15511. doi: 10.1039/d0ra10728j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok D., Gundu S., Aamate V.K., Devulapally M.G. Conventional and microwave-assisted synthesis of new indole-tethered benzimidazole-based 1, 2, 3-triazoles and evaluation of their antimycobacterial, antioxidant and antimicrobial activities. Mol. Divers. 2018;22(4):769–778. doi: 10.1007/s11030-018-9828-1. [DOI] [PubMed] [Google Scholar]
- Baeyer A., Knop C. Mittheilungen aus dem organischen Laboratorium der Gewerbe-Academie in Berlin. I. Untersuchungen über die Gruppe des Indigblau's. Justus Liebigs Ann. Chem. 1866;140(1):1–38. [Google Scholar]
- Bajad N.G., Swetha R., Gutti G., Singh M., Kumar A., Singh S.K. A systematic review of carbohydrate-based bioactive molecules for Alzheimer's disease. Future Med. Chem. 2021;13(19):1695–1711. doi: 10.4155/fmc-2021-0109. [DOI] [PubMed] [Google Scholar]
- Bajad N.G., Rayala S., Gutti G., Sharma A., Singh M., Kumar A., et al. Systematic review on role of structure based drug design (SBDD) in the identification of anti-viral leads against SARS-Cov-2. Curr. Pharmacol. Drug Discover. 2021;2 doi: 10.1016/j.crphar.2021.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakhar K.A., Sureja D.K., Dhameliya T.M. Synthetic account of indoles in search of potential anti-mycobacterial agents: a review and future insights. J. Mol. Struct. 2022;1248 [Google Scholar]
- Biswal S., Sahoo U., Sethy S., Kumar H., Banerjee M. Indole: the molecule of diverse biological activities. Asian J. Pharmaceut. Clin. Res. 2012;5(1):1–6. [Google Scholar]
- Bolla J.R. Targeting MmpL3 for anti-tuberculosis drug development. Biochem. Soc. Trans. 2020;48(4):1463–1472. doi: 10.1042/BST20190950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borad M.A., Bhoi M.N., Rathwa S.K., Vasava M.S., Patel H.D., Patel C.N., et al. Microwave-assisted ZrSiO 2 catalysed synthesis, characterization and computational study of novel spiro [indole-thiazolidines] derivatives as anti-tubercular agents. Interdiscipl. Sci. Comput. Life Sci. 2018;10(2):411–418. doi: 10.1007/s12539-016-0195-2. [DOI] [PubMed] [Google Scholar]
- Bruch E.M., Petrella S., Bellinzoni M. Structure-based drug design for tuberculosis: challenges still ahead. Appl. Sci. 2020;10(12):4248. [Google Scholar]
- Bugaenko D.I., Karchava A.V., Yurovskaya M.A. Synthesis of indoles: recent advances. Russ. Chem. Rev. 2019;88(2):99. [Google Scholar]
- Bugg T.D., Braddick D., Dowson C.G., Roper D.I. Bacterial cell wall assembly: still an attractive antibacterial target. Trends Biotechnol. 2011;29(4):167–173. doi: 10.1016/j.tibtech.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Chatterji M., Shandil R., Manjunatha M., Solapure S., Ramachandran V., Kumar N., et al. 1, 4-Azaindole, a potential drug candidate for treatment of tuberculosis. Antimicrobiol. Agents Chemother. 2014;58(9):5325–5331. doi: 10.1128/AAC.03233-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Nyantakyi S.A., Li M., Gopal P., Aziz D.B., Yang T., et al. The mycobacterial membrane: a novel target space for anti-tubercular drugs. Front. Microbiol. 2018;9:1627. doi: 10.3389/fmicb.2018.01627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty S., Ramesh M., Singh-Pillay A., Soliman M.E. Recent advancements in the development of anti-tuberculosis drugs. Bioorg. Med. Chem. Lett. 2017;27(3):370–386. doi: 10.1016/j.bmcl.2016.11.084. [DOI] [PubMed] [Google Scholar]
- Chikhale R.V., Barmade M.A., Murumkar P.R., Yadav M.R. Overview of the development of DprE1 inhibitors for combating the menace of tuberculosis. J. Med. Chem. 2018;61(19):8563–8593. doi: 10.1021/acs.jmedchem.8b00281. [DOI] [PubMed] [Google Scholar]
- Danne A.B., Choudhari A.S., Chakraborty S., Sarkar D., Khedkar V.M., Shingate B.B. Triazole–diindolylmethane conjugates as new antitubercular agents: synthesis, bioevaluation, and molecular docking. MedChemComm. 2018;9(7):1114–1130. doi: 10.1039/c8md00055g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sa Alves F.R., Barreiro E.J., Manssour Fraga C.A. From nature to drug discovery: the indole scaffold as a ‘privileged structure’. Mini Rev. Med. Chem. 2009;9(7):782–793. doi: 10.2174/138955709788452649. [DOI] [PubMed] [Google Scholar]
- de Souza Neto L.R., Moreira-Filho J.T., Neves B.J., Maidana R.L.B.R., Guimarães A.C.R., Furnham N., et al. In silico strategies to support fragment-to-lead optimization in drug discovery. Front. Chem. 2020;8:93. doi: 10.3389/fchem.2020.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N., Somani H., Trivedi A., Bhatt K., Nawale L., Khedkar V.M., et al. Synthesis, biological evaluation and molecular docking study of some novel indole and pyridine based 1, 3, 4-oxadiazole derivatives as potential antitubercular agents. Bioorg. Med. Chem. Lett. 2016;26(7):1776–1783. doi: 10.1016/j.bmcl.2016.02.043. [DOI] [PubMed] [Google Scholar]
- Dewangan R.P., Singh M., Ilic S., Tam B., Akabayov B. Cell-penetrating peptide conjugates of indole-3-acetic acid-based DNA primase/Gyrase inhibitors as potent anti-tubercular agents against planktonic and biofilm culture of Mycobacterium smegmatis. Chem. Biol. Drug Des. 2021;98(5):722–732. doi: 10.1111/cbdd.13925. [DOI] [PubMed] [Google Scholar]
- Dey P., Kundu A., Kumar A., Gupta M., Lee B.M., Bhakta T., et al. Recent Advances in Natural Products Analysis. Elsevier; 2020. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids) pp. 505–567. [Google Scholar]
- Dighe S.N., Collet T.A. Recent advances in DNA gyrase-targeted antimicrobial agents. Eur. J. Med. Chem. 2020;199 doi: 10.1016/j.ejmech.2020.112326. [DOI] [PubMed] [Google Scholar]
- Ditse Z., Lamers M.H., Warner D.F. DNA replication in Mycobacterium tuberculosis. Microbiol. Spectr. 2017;5(2):5–2. 20. doi: 10.1128/microbiolspec.TBTB2-0027-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonvielle M., Le Du M.-H., Lequin O., Lecoq A., Jacquet M., Thai R., et al. Substrate and reaction specificity of Mycobacterium tuberculosis cytochrome P450 CYP121: insights from biochemical studies and crystal structures. J. Biol. Chem. 2013;288(24):17347–17359. doi: 10.1074/jbc.M112.443853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz N.D., Belardinelli J.M., Kaminski M.A., Dunn L.C., de Moura V.C.N., Blaha M.A., et al. Design, synthesis and evaluation of indole-2-carboxamides with pan anti-mycobacterial activity. Bioorg. Med. Chem. 2017;25(14):3746–3755. doi: 10.1016/j.bmc.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman P.G., Van Bergen T., Gilbert D.P., Cue B.W., Jr. General method for the synthesis of indoles. J. Am. Chem. Soc. 1974;96(17):5495–5508. [Google Scholar]
- Gupta P., Thomas S.E., Zaidan S.A., Pasillas M.A., Cory-Wright J., Sebastián-Pérez V., et al. A fragment-based approach to assess the ligandability of ArgB, ArgC, ArgD and ArgF in the L-arginine biosynthetic pathway of Mycobacterium tuberculosis. Comput. Struct. Biotechnol. J. 2021;19:3491–3506. doi: 10.1016/j.csbj.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid H.A., Ramli A.N., Yusoff M.M. Indole alkaloids from plants as potential leads for antidepressant drugs: a mini review. Front. Pharmacol. 2017;8:96. doi: 10.3389/fphar.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hett E.C., Rubin E.J. Bacterial growth and cell division: a mycobacterial perspective. Microbiol. Mol. Biol. Rev. 2008;72(1):126–156. doi: 10.1128/MMBR.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-L., Krieger I.V., Parai M.K., Gawandi V.B., Sacchettini J.C. Mycobacterium tuberculosis malate synthase structures with fragments reveal a portal for substrate/product exchange. J. Biol. Chem. 2016;291(53):27421–27432. doi: 10.1074/jbc.M116.750877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey G.R., Kuethe J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006;106(7):2875–2911. doi: 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]
- Hung A.W., Silvestre H.L., Wen S., Ciulli A., Blundell T.L., Abell C. Application of fragment growing and fragment linking to the discovery of inhibitors of Mycobacterium tuberculosis pantothenate synthetase. Angew. Chem. Int. Ed. 2009;48(45):8452–8456. doi: 10.1002/anie.200903821. [DOI] [PubMed] [Google Scholar]
- Inoyama D., Awasthi D., Capodagli G.C., Tsotetsi K., Sukheja P., Zimmerman M., et al. A preclinical candidate targeting Mycobacterium tuberculosis KasA. Cell. Chem. Biol. 2020;27(5):560–570. doi: 10.1016/j.chembiol.2020.02.007. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.I., Seo H., Kim S., Sadu V.S., Lee K.-I., Song H.-Y. Antimicrobial activity of IDD-B40 against drug-resistant Mycobacterium tuberculosis. Sci. Rep. 2021;11(1):1–9. doi: 10.1038/s41598-020-80227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankute M., Cox J.A., Harrison J., Besra G.S. Assembly of the mycobacterial cell wall. Annu. Rev. Microbiol. 2015;69:405–423. doi: 10.1146/annurev-micro-091014-104121. [DOI] [PubMed] [Google Scholar]
- Jeffrey North E., Jackson M., E Lee R. New approaches to target the mycolic acid biosynthesis pathway for the development of tuberculosis therapeutics. Curr. Pharmaceut. Des. 2014;20(27):4357–4378. doi: 10.2174/1381612819666131118203641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap A., Singh P.K., Silakari O. In silico designing of domain B selective gyrase inhibitors for effective treatment of resistant tuberculosis. Tuberculosis. 2018;112:83–88. doi: 10.1016/j.tube.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Khan A., Jasinski J.P., Smolenski V.A., Hotchkiss E.P., Kelley P.T., Shalit Z.A., et al. Enhancement in anti-tubercular activity of indole based thiosemicarbazones on complexation with copper (I) and silver (I) halides: structure elucidation, evaluation and molecular modelling. Bioorg. Chem. 2018;80:303–318. doi: 10.1016/j.bioorg.2018.06.027. [DOI] [PubMed] [Google Scholar]
- Khanapur M., Alvala M., Prabhakar M., Kumar K.S., Edwin R., Saranya P.S., et al. Mycobacterium tuberculosis chorismate mutase: a potential target for TB. Bioorg. Med. Chem. 2017;25(6):1725–1736. doi: 10.1016/j.bmc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Khawbung J.L., Nath D., Chakraborty S. Drug resistant tuberculosis: a review. Comp. Immunol. Microbiol. Infect. Dis. 2021;74 doi: 10.1016/j.cimid.2020.101574. [DOI] [PubMed] [Google Scholar]
- Kleinhans K, Brandl C, Brandl L, Ebert G, Jirschele R, Maala N, Neumeyer P, Schneider G, Schuh L, Weyker E, Olson A. Inhibiting Dihydrofolate Reductase as a Treatment for Tuberculosis. FASEB J. 2010 Apr;24:lb118. [Google Scholar]
- Kochanowska-Karamyan A.J., Hamann M.T. Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010;110(8):4489–4497. doi: 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontsevaya I., Lange C., Comella-del-Barrio P., Coarfa C., DiNardo A.R., Gillespie S.H., et al. Perspectives for systems biology in the management of tuberculosis. Eur. Respir. Rev. 2021;30(160) doi: 10.1183/16000617.0377-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K.S., Rambabu D., Sandra S., Kapavarapu R., Krishna G.R., Rao M.B., et al. AlCl3 induced (hetero) arylation of 2, 3-dichloroquinoxaline: a one-pot synthesis of mono/disubstituted quinoxalines as potential antitubercular agents. Bioorg. Med. Chem. 2012;20(5):1711–1722. doi: 10.1016/j.bmc.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Kumar P., Capodagli G.C., Awasthi D., Shrestha R., Maharaja K., Sukheja P., et al. Synergistic lethality of a binary inhibitor of Mycobacterium tuberculosis KasA. mBio. 2018;9(6):e02101–e02117. doi: 10.1128/mBio.02101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Karkara B.B., Panda G. Novel candidates in the clinical development pipeline for TB drug development and their synthetic approaches. Chem. Biol. Drug Des. 2021;98(5):787–827. doi: 10.1111/cbdd.13934. [DOI] [PubMed] [Google Scholar]
- Kumari A., Singh R.K. Medicinal chemistry of indole derivatives: current to future therapeutic prospectives. Bioorg. Chem. 2019;89 doi: 10.1016/j.bioorg.2019.103021. [DOI] [PubMed] [Google Scholar]
- Kuo M.R., Morbidoni H.R., Alland D., Sneddon S.F., Gourlie B.B., Staveski M.M., et al. Targeting tuberculosis and malaria through inhibition of enoyl reductase: compound activity and structural data. J. Biol. Chem. 2003;278(23):20851–20859. doi: 10.1074/jbc.M211968200. [DOI] [PubMed] [Google Scholar]
- Li M., Nyantakyi S.A., Gopal P., Aziz D.B., Dick T., Go M.-L. Indolylalkyltriphenylphosphonium analogues are membrane-depolarizing mycobactericidal agents. ACS Med. Chem. Lett. 2017;8(11):1165–1170. doi: 10.1021/acsmedchemlett.7b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libardo M.D.J., Boshoff H.I., Barry C.E., III The present state of the tuberculosis drug development pipeline. Curr. Opin. Pharmacol. 2018;42:81–94. doi: 10.1016/j.coph.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun S., Guo H., Onajole O.K., Pieroni M., Gunosewoyo H., Chen G., et al. Indoleamides are active against drug-resistant Mycobacterium tuberculosis. Nat. Commun. 2013;4(1):1–8. doi: 10.1038/ncomms3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi V., Haj Mohammad Ebrahim Tehrani K., Azerang P., Sardari S., Kobarfard F. Synthesis, antimycobacterial and anticancer activity of novel indole-based thiosemicarbazones. Arch Pharm. Res. (Seoul) 2021;44(8):1–13. doi: 10.1007/s12272-013-0242-z. [DOI] [PubMed] [Google Scholar]
- Matviiuk T., Rodriguez F., Saffon N., Mallet-Ladeira S., Gorichko M., Ribeiro ALdJL., et al. Design, chemical synthesis of 3-(9H-fluoren-9-yl) pyrrolidine-2, 5-dione derivatives and biological activity against enoyl-ACP reductase (InhA) and Mycobacterium tuberculosis. Eur. J. Med. Chem. 2013;70:37–48. doi: 10.1016/j.ejmech.2013.09.041. [DOI] [PubMed] [Google Scholar]
- Michalska K., Chang C., Maltseva N.I., Jedrzejczak R., Robertson G.T., Gusovsky F., et al. Allosteric inhibitors of Mycobacterium tuberculosis tryptophan synthase. Protein Sci. 2020;29(3):779–788. doi: 10.1002/pro.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison D.A., Davies G.R. Assessment of the efficacy of new anti-tuberculosis drugs. Open Infect. Dis. J. 2008;2:59. doi: 10.2174/1874279300802010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhi A., Prasad B., Reddy U., Rao R.M., Sandra S., Kapavarapu R., et al. A new route to indoles via in situ desilylation–Sonogashira strategy: identification of novel small molecules as potential anti-tuberculosis agents. MedChemComm. 2011;2(10):1006–1010. [Google Scholar]
- Negatu D.A., Liu J.J., Zimmerman M., Kaya F., Dartois V., Aldrich C.C., et al. Whole-cell screen of fragment library identifies gut microbiota metabolite indole propionic acid as antitubercular. Antimicrobiol. Agents Chemother. 2018;62(3) doi: 10.1128/AAC.01571-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto J.S.S., Zeni G. Recent advances in the synthesis of indoles from alkynes and nitrogen sources. Org. Chem. Front. 2020;7(1):155–210. doi: 10.1039/d0ob00670j. [DOI] [PubMed] [Google Scholar]
- Prasad B., Adepu R., Sandra S., Rambabu D., Krishna G.R., Reddy C.M., et al. AlCl 3 mediated unexpected migration of sulfonyl groups: regioselective synthesis of 7-sulfonyl indoles of potential pharmacological interest. Chem. Commun. 2012;48(84):10434–10436. doi: 10.1039/c2cc35757g. [DOI] [PubMed] [Google Scholar]
- Ramesh D., Joji A., Vijayakumar B.G., Sethumadhavan A., Mani M., Kannan T. Indole chalcones: design, synthesis, in vitro and in silico evaluation against Mycobacterium tuberculosis. Eur. J. Med. Chem. 2020;198 doi: 10.1016/j.ejmech.2020.112358. [DOI] [PubMed] [Google Scholar]
- Rathod A.S., Godipurge S.S., Biradar J.S. Synthesis of indole, coumarinyl and pyridinyl derivatives of isoniazid as potent antitubercular and antimicrobial agents and their molecular docking studies. Int. J. Pharm. Pharmaceut. Sci. 2017;9:233–240. [Google Scholar]
- Rayasam G.V. MmpL3 a potential new target for development of novel anti-tuberculosis drugs. Expert Opin. Ther. Targets. 2014;18(3):247–256. doi: 10.1517/14728222.2014.859677. [DOI] [PubMed] [Google Scholar]
- Reddy G.S., Snehalatha A.V., Edwin R.K., Hossain K.A., Giliyaru V.B., Hariharapura R.C., et al. Synthesis of 3-indolylmethyl substituted (pyrazolo/benzo) triazinone derivatives under Pd/Cu-catalysis: identification of potent inhibitors of chorismate mutase (CM) Bioorg. Chem. 2019;91 doi: 10.1016/j.bioorg.2019.103155. [DOI] [PubMed] [Google Scholar]
- Reddy G.S., Hossain K.A., Kumar J.S., Thirupataiah B., Edwin R.K., Giliyaru V.B., et al. Novel isatin–indole derivatives as potential inhibitors of chorismate mutase (CM): their synthesis along with unexpected formation of 2-indolylmethylamino benzoate ester under Pd–Cu catalysis. RSC Adv. 2020;10(1):289–297. doi: 10.1039/c9ra09236f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiche M.A., Warner D.F., Mizrahi V. Targeting DNA replication and repair for the development of novel therapeutics against tuberculosis. Front. Mol. Biosci. 2017;4:75. doi: 10.3389/fmolb.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers E.C., Mancera R.L. New anti-tuberculosis drugs in clinical trials with novel mechanisms of action. Drug Discov. Today. 2008;13(23–24):1090–1098. doi: 10.1016/j.drudis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Robertson G.T., Ramey M.E., Massoudi L.M., Carter C.L., Zimmerman M., Kaya F., et al. Comparative analysis of pharmacodynamics in the C3HeB/FeJ mouse tuberculosis model for DprE1 inhibitors TBA-7371, PBTZ169, and OPC-167832. Antimicrobiol. Agents Chemother. 2021;65(11) doi: 10.1128/AAC.00583-21. e00583-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales P.F., Bordin G.S., Gower A.E., Moura S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia. 2020;143 doi: 10.1016/j.fitote.2020.104558. [DOI] [PubMed] [Google Scholar]
- Rožman K., Sosič I., Fernandez R., Young R.J., Mendoza A., Gobec S., et al. A new ‘golden age’ for the antitubercular target InhA. Drug Discov. Today. 2017;22(3):492–502. doi: 10.1016/j.drudis.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Sanna G., Madeddu S., Giliberti G., Piras S., Struga M., Wrzosek M., et al. Synthesis and biological evaluation of novel indole-derived thioureas. Molecules. 2018;23(10):2554. doi: 10.3390/molecules23102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K., Tanwar O., Sharma S., Ali S., Alam M., Zaman M., et al. Structural comparison of Mtb-DHFR and h-DHFR for design, synthesis and evaluation of selective non-pteridine analogues as antitubercular agents. Bioorg. Chem. 2018;80:319–333. doi: 10.1016/j.bioorg.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Sharma K., Tanwar O., Deora G.S., Ali S., Alam M., Zaman M., et al. Expansion of a novel lead targeting M. tuberculosis DHFR as antitubercular agents. Bioorg. Med. Chem. 2019;27(7):1421–1429. doi: 10.1016/j.bmc.2019.02.053. [DOI] [PubMed] [Google Scholar]
- Shirude P.S., Shandil R., Sadler C., Naik M., Hosagrahara V., Hameed S., et al. Azaindoles: noncovalent DprE1 inhibitors from scaffold morphing efforts, kill Mycobacterium tuberculosis and are efficacious in vivo. J. Med. Chem. 2013;56(23):9701–9708. doi: 10.1021/jm401382v. [DOI] [PubMed] [Google Scholar]
- Singh M., Ilic S., Tam B., Ben-Ishay Y., Sherf D., Pappo D., et al. Dual-acting small-molecule inhibitors targeting mycobacterial DNA replication. Chem.--Eur. J. 2020;26(47):10849–10860. doi: 10.1002/chem.202001725. [DOI] [PubMed] [Google Scholar]
- Tan Y.J., Li M., Gunawan G.A., Nyantakyi S.A., Dick T., Go M.-L., et al. Amide–amine replacement in indole-2-carboxamides yields potent mycobactericidal agents with improved water solubility. ACS Med. Chem. Lett. 2020;12(5):704–712. doi: 10.1021/acsmedchemlett.0c00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velezheva V., Brennan P., Ivanov P., Kornienko A., Lyubimov S., Kazarian K., et al. Synthesis and antituberculosis activity of indole–pyridine derived hydrazides, hydrazide–hydrazones, and thiosemicarbazones. Bioorg. Med. Chem. Lett. 2016;26(3):978–985. doi: 10.1016/j.bmcl.2015.12.049. [DOI] [PubMed] [Google Scholar]
- Yadav N., Khanam T., Shukla A., Rai N., Hajela K., Ramachandran R. Tricyclic dihydrobenzoxazepine and tetracyclic indole derivatives can specifically target bacterial DNA ligases and can distinguish them from human DNA ligase I. Org. Biomol. Chem. 2015;13(19):5475–5487. doi: 10.1039/c5ob00439j. [DOI] [PubMed] [Google Scholar]
- Yang T., Moreira W., Nyantakyi S.A., Chen H., Aziz D.B., Go M.-L., et al. Amphiphilic indole derivatives as antimycobacterial agents: structure–activity relationships and membrane targeting properties. J. Med. Chem. 2017;60(7):2745–2763. doi: 10.1021/acs.jmedchem.6b01530. [DOI] [PubMed] [Google Scholar]
- Yang X., Hu T., Yang X., Xu W., Yang H., Guddat L.W., et al. Structural basis for the inhibition of mycobacterial MmpL3 by NITD-349 and SPIRO. J. Mol. Biol. 2020;432(16):4426–4434. doi: 10.1016/j.jmb.2020.05.019. [DOI] [PubMed] [Google Scholar]