Abstract
Background
Checkpoint inhibitor immunotherapy has not proven clinically effective in glioblastoma. This lack of effectiveness may be partially attributable to the frequent administration of dexamethasone in glioblastoma patients. In this systematic review, we assess whether dexamethasone (1) affects the glioblastoma microenvironment and (2) interferes with checkpoint inhibitor immunotherapy efficacy in the treatment of glioblastoma.
Methods
PubMed and Embase were systematically searched for eligible articles published up to September 15, 2021. Both in vitro and in vivo preclinical studies, as well as clinical studies were selected. The following information was extracted from each study: tumor model, corticosteroid treatment, and effects on individual immune components or checkpoint inhibitor immunotherapy.
Results
Twenty-one preclinical studies in cellular glioma models (n = 10), animal glioma models (n = 6), and glioblastoma patient samples (n = 7), and 3 clinical studies were included. Preclinical studies show that dexamethasone decreases the presence of microglia and other macrophages as well as the number of T lymphocytes in both tumor tissue and periphery. Dexamethasone abrogates the antitumor effects of checkpoint inhibitors on T lymphocytes in preclinical studies. Although randomized studies directly addressing our research question are lacking, clinical studies suggest a negative association between corticosteroids and survival outcomes in glioblastoma patients receiving checkpoint inhibitors after adjustment for relevant prognostic factors.
Conclusions
Preclinical research shows that dexamethasone inhibits the antitumor immune response in glioma, thereby promoting a protumorigenic microenvironment. The efficacy of checkpoint inhibitor immunotherapy in glioblastoma patients may therefore be negatively affected by the use of dexamethasone. Future research could investigate the potential of edema-reducing alternatives to dexamethasone.
Keywords: checkpoint inhibitors, corticosteroids, dexamethasone, glioblastoma, immunotherapy
Key Points.
Dexamethasone decreases number of macrophages and T cells in GBM microenvironment.
Dexamethasone abolishes checkpoint inhibitor-induced antitumor effects in GBM.
Dexamethasone is negatively associated with survival in GBM patients on checkpoint inhibitors.
Importance of the Study.
The lack of efficacy of checkpoint inhibitors in glioblastoma trials is often suggested to be partially attributable to the common use of dexamethasone, but systematic evidence is lacking. This systematic review addresses the effects of dexamethasone on checkpoint inhibitor immunotherapy in glioblastoma in preclinical models and clinical studies. We show that in preclinical studies, dexamethasone decreases the presence of myeloid and lymphoid immune cell populations in the glioblastoma microenvironment. This results in an inhibition of the checkpoint inhibitor-induced antitumor immune response in preclinical models of glioblastoma. In clinical studies, baseline corticosteroid use is associated with shorter survival in glioblastoma patients on checkpoint inhibitors, even after adjustment for other prognostic factors, but data are limited. This is important, as it indicates that future studies assessing survival benefit of checkpoint inhibitors in glioblastoma should consider excluding patients on higher dose dexamethasone treatment. Future research should investigate the potential of edema-reducing alternatives to dexamethasone.
Glioblastomas are malignant brain tumors associated with a very poor life expectancy of patients.1 Standard treatment consists of a combination of maximal safe surgical resection, radiotherapy, and alkylating agent chemotherapy.2 However, there is an evident need for novel therapeutic approaches.
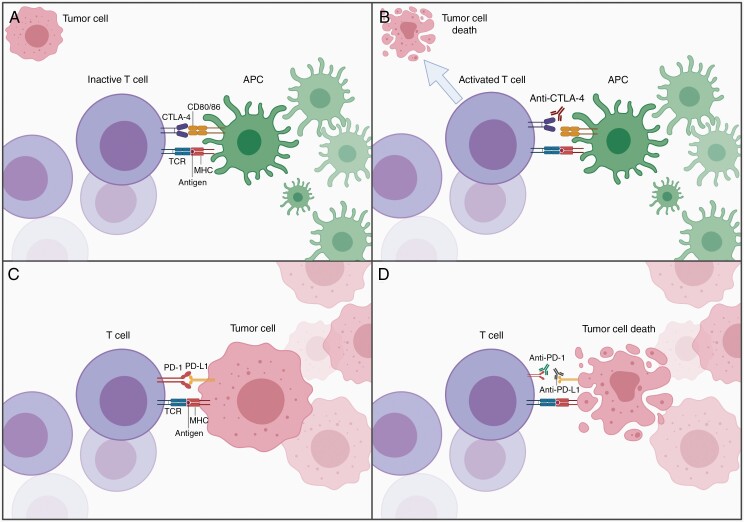
Antibody-mediated checkpoint inhibitor immunotherapy is a groundbreaking therapeutic approach in the field of oncology. The checkpoint inhibitors currently used in clinical practice target the inhibitory checkpoint molecules cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PD-L1). All of these molecules are implicated in regulating T lymphocyte activation. T cell activation is inhibited by the expression of CTLA-4, which binds to CD80 or CD86, or PD-1, which binds to its inhibitory ligand PD-L1 (Figure 1A and C).3,4 Antibodies directed against CTLA-4, PD-1, and PD-L1 thus prevent these molecules from suppressing the immune response, thereby enabling cytotoxic T lymphocytes to destroy tumor cells (Figure 1B and D).
Figure 1.
Checkpoint inhibitor immunotherapy. (A) Suppression of T cell activation by CTLA-4. (B) Working mechanism of anti-CTLA-4 antibody. (C) Suppression of T cell by PD-1 and PD-L1. (D) Working mechanism of anti-PD-1 and anti-PD-L1 antibodies. APC, antigen-presenting cell; CD, cluster of differentiation; CTLA-4, cytotoxic T lymphocyte antigen 4; MHC, major histocompatibility complex; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TCR, T cell receptor. Adapted from “Cancer Lymphocyte Interaction,” by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates.
These checkpoint inhibitors have shown tremendous clinical benefit in various tumor types including melanoma, non-small cell lung cancer, and urothelial carcinoma.3 Preclinical glioblastoma research has yielded promising results as well. Treatment of GL261 glioblastoma-bearing mice with antibodies targeting CTLA-4, PD-1, PD-L1, or a combination of those was shown to increase the number of CD8+ T cells and activated natural killer (NK) cells, while decreasing the presence of regulatory T cells (Tregs) in the tumor tissue and tumor-draining lymph nodes.5 Most importantly, all of these checkpoint inhibitors significantly improved the survival of these mice.
In addition to the promising results in preclinical glioblastoma models, early phase clinical trials observed that neoadjuvant treatment with the PD-1 inhibitor nivolumab increased immune cell infiltration in the glioblastoma microenvironment6 and neoadjuvant treatment with pembrolizumab, another PD-1 inhibitor, significantly extended overall survival (OS) of recurrent glioblastoma patients.7 Unfortunately, checkpoint inhibitors have failed to prove effective in the 3 phase III clinical trials conducted in patients with either newly diagnosed or recurrent glioblastoma.8,9 In the CheckMate 143 trial (NCT02017717), recurrent glioblastoma patients were randomized to the PD-1 inhibitor nivolumab or the vascular endothelial growth factor (VEGF) inhibitor bevacizumab.10 In the CheckMate 498 trial (NCT02617589), newly diagnosed glioblastoma patients with an unmethylated O6-methylguanine-DNA methyltransferase (MGMT) gene promoter received standard care in combination with a placebo or nivolumab.11 The CheckMate 548 trial (NCT02667587) investigated the addition of a placebo or nivolumab to standard care in newly diagnosed glioblastoma patients with a methylated MGMT gene promotor.12 None of these trials detected a survival benefit of checkpoint inhibitor immunotherapy.10–12
This lack of efficacy may be attributable to several factors. First of all, the immunogenicity of glioblastomas is relatively low. Second, the number of T cells infiltrating the glioblastoma microenvironment is small compared to other tumor types. Third, glioblastoma cells actively create an immunosuppressive tumor microenvironment by interacting with various immune components.8,13 Myeloid cells, predominantly resident microglia and peripheral macrophages, form the primary immune component of the glioblastoma microenvironment.13,14 The relatively small lymphoid component mainly comprises T and NK cells.13,15 Inflammatory molecules, which include inflammatory cytokines, colony-stimulating factors (CSFs), chemokines, and immune-related enzymes, can be produced by both glioblastoma and immune cells and are able to modulate the antitumor immune response.
Dexamethasone is a synthetic glucocorticoid that is widely used in order to alleviate cerebral edema and the associated symptoms in glioblastoma patients. Tumor cells induce vasogenic edema via the release of VEGF, which promotes increased vascular permeability in the tumor microenvironment. Dexamethasone counteracts this process by acting on glucocorticoid receptors, thereby reducing both VEGF expression by the tumor cells and VEGF sensitivity of the endothelial target cells.16
However, dexamethasone is also reported to suppress the immune system.17,18 Glucocorticoids are known to downregulate the expression of proinflammatory cytokines and inhibit the activity of dendritic cells (DCs) and T cells.17 As such, there are reports suggesting that dexamethasone reduces the efficacy of various immunotherapeutic interventions,19–23 but systematic evidence on the effect on checkpoint inhibitor immunotherapy is lacking. In this systematic review, we therefore assess whether dexamethasone (1) affects the glioblastoma microenvironment and (2) interferes with checkpoint inhibitor immunotherapy efficacy in the treatment of glioblastoma.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.24
Literature Search
A systematic literature search was conducted in PubMed and Embase to retrieve all eligible articles published up to September 15, 2021. The search terms corticosteroid, immune, immunotherapy, and glioblastoma as well as synonyms of these terms were used. The complete search strings for PubMed and Embase are reported in Supplementary Information. Additional studies were identified by consulting our own literature database and searching reference lists of relevant review articles.
Eligibility Criteria
Original research articles regarding the effect of dexamethasone or other corticosteroids on (1) individual immune components or (2) checkpoint inhibitor immunotherapy efficacy in glioma were selected. Both in vitro and in vivo preclinical studies in mice, rat, and human, as well as clinical studies were considered eligible. Publications in languages other than English, with no full text available or lacking a control condition were excluded.
Study Selection and Data Extraction
Eligibility assessment was performed by 1 reviewer (K.X.S.) in a standardized manner by subsequently screening the titles, abstracts, and full-text articles. The following information was extracted from each included study by 1 reviewer (K.X.S.): tumor model, type of corticosteroid, corticosteroid dosage, method of administration, treatment duration, group size, and outcome(s). In case of doubt, regarding inclusion or exclusion of studies as well as data extraction, a second reviewer (M.G.) was consulted.
Risk of Bias Assessment
The risk of bias in individual studies was assessed by 1 reviewer (K.X.S.) according to the Cochrane Risk of Bias Tool.25
Results
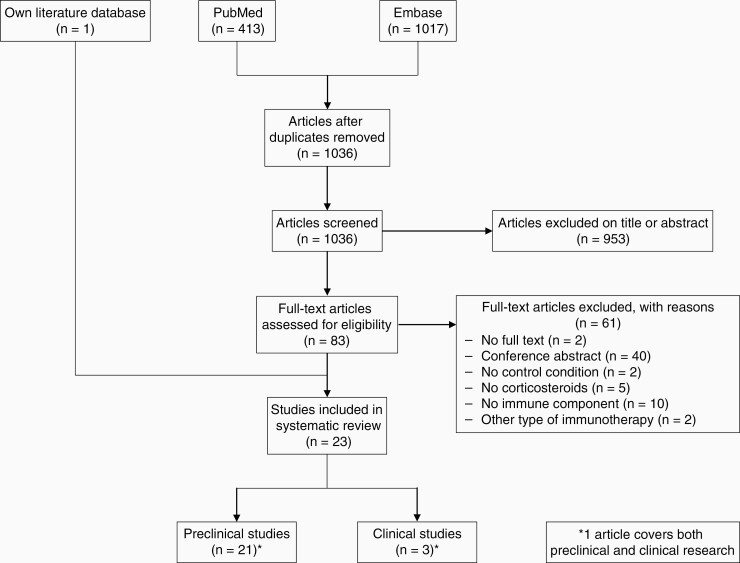
The primary literature search resulted in 1036 unique articles. After screening the titles and abstracts of these articles, 83 articles were subjected to full-text assessment. Subsequent exclusion of 61 articles led to 22 remaining eligible studies (Figure 2). Moreover, 1 article fulfilling the selection criteria was identified by consulting our own literature database. This article was not retrieved by the literature search because the search term corticosteroid or synonyms of this term were not mentioned in its title or abstract, but only in the main text. Ultimately, 23 studies, comprising 21 preclinical and 3 clinical studies, were selected for inclusion in this systematic review (Figure 2).
Figure 2.
Flow chart of the systematic literature search.
Risk of Bias
The risk of bias assessment in individual studies is provided in Supplementary Table S1. Since randomization was lacking in most human preclinical and clinical studies, there is a high risk of selection bias. The 40 conference abstracts without a peer-reviewed publication counterpart suggest that there is a considerable risk of publication and selective reporting bias. Since only 3 clinical studies were included, the risk of bias across studies could not be quantified.
Preclinical Research
Twenty-one preclinical studies investigated corticosteroid effects on the immunological component of the glioma microenvironment and checkpoint inhibitor immunotherapy in the treatment of glioma. These studies were conducted in cellular glioma models (n = 10), animal glioma models (n = 6), and glioblastoma patient samples (n = 7). Human preclinical studies comprise the examination of patient samples as opposed to clinical parameters such as survival. The main findings of the preclinical studies are listed in Table 1.
Table 1.
Overview of included preclinical studies
| Author | Tumor type | Model | Treatment (dosage) | Duration | Group size | Outcome(s) |
|---|---|---|---|---|---|---|
| Cellular studies | ||||||
| Curran et al26 | Glioblastoma | Cells | Dexamethasone (0.01–0.1 µM) | 24 h | N/A | = basal GM-CSF secretion |
| ↓ TNF-α-induced GM-CSF secretion | ||||||
| Gottschall et al27 | Glioblastoma | Cells | Dexamethasone (0.01–0.1 µM) | 6 or 15 h | N/A | ↓ IL-1β-induced IL-6 secretion |
| Herting et al28 | Glioblastoma | Co-cultured macrophages and tumor slices | Dexamethasone (5 µM) | 26 h | N/A | ↓ IL-1α and IL-1β expression by macrophages and microglia |
| Hong et al29 | Glioblastoma | Co-cultured macrophages and tumor cells | Dexamethasone (1–10 µM) | 24 h | N/A | ↓ CXCL8 expression by tumor cells |
| Mukaida et al30 | Glioblastoma | Cells | Dexamethasone (0.00001–1 µM) | 3 or 24 h | N/A | ↓ IL-1α-induced CXCL8 expression and secretion |
| Ott et al31 | Glioblastoma | Cells | Dexamethasone (0.1 µM) | 120 h | N/A | ↓ TDO expression |
| Rieger et al32 | Glioma | Cells | Dexamethasone (0.1 µM) | 24 h | N/A | ↓ LPS- and cytokine-induced nitrite secretion |
| Shinoda et al33 | Glioma | Cells | Dexamethasone (0.002–2 µM) | 6–72 h | N/A | ↓ LPS-induced nitrite secretion |
| ↓ LPS-induced iNOS expression | ||||||
| Wielgat et al34 | Glioma | Co-cultured microglia and tumor cells | Dexamethasone (10 µM) | 24 h | N/A | ↓ IL-1β production by microglia |
| ↑ IL-10 production by microglia | ||||||
| Wielgat et al35 | Glioblastoma | Co-cultured microglia or monocytes and tumor cells | Dexamethasone (10 µM) | 24 h | N/A | ↓ IL-1β, IL-6, IL-8, IL-12, and TNF-α production by microglia and monocytes |
| ↑ IL-10 production by microglia | ||||||
| = IL-10 production by monocytes | ||||||
| Animal studies | ||||||
| Badie et al36 | Glioma | Rats | Dexamethasone (0.1 or 1 mg/kg/day i.p.) | 7 days | 7–10 | ↓ microglia in tumor |
| = macrophages in tumor | ||||||
| ↓ total lymphocytes and T cells in tumor | ||||||
| Giles et al37 | Glioma | Mice | Dexamethasone (0.5–2.5 mg/kg/day p.o.) | 1 h and ≥17 days | 8–10 | = CD4+ T cells, CD8+ T cells, and Tregs in tumor |
| ↓ CD4+ T cells, CD8+ T cells, and Tregs in tumor-draining lymph nodes | ||||||
| ↑ CTLA-4 expression by CD4 + and CD8+ T cells in blood | ||||||
| = or ↑ survival after anti-CTLA-4 | ||||||
| = CD4+ T cells, CD8+ T cells, and Tregs in tumor after anti-CTLA-4 | ||||||
| ↓ CD4+ T cells, CD8+ T cells, and Tregs in tumor-draining lymph nodes after anti-CTLA-4 | ||||||
| Herting et al28 | Glioblastoma | Mice | Dexamethasone (10 mg/kg/day i.p.) | 5 days | 9–15 | ↓ IL-1α and IL-1β expression in tumor |
| ↓ total myeloid cells and macrophages in tumor | ||||||
| = microglia in tumor | ||||||
| ↑ monocytes in blood | ||||||
| = neutrophils in blood | ||||||
| ↓ total lymphocytes in tumor and blood | ||||||
| Iorgulescu et al38 | Glioma | Mice | Dexamethasone (1–10 mg/kg/day i.p.) | 11–22 days | 4–42 | = total myeloid cells, microglia, macrophages, monocytes, DCs, and CD8 + T cells in tumor |
| ↓ T cells, CD4+ T cells, and NK cells in tumor | ||||||
| ↓ total myeloid cells, macrophages, T cells, CD4+ T cells, CD8+ T cells, and NK cells in tumor-draining lymph nodes | ||||||
| = monocytes and DCs in tumor-draining lymph nodes | ||||||
| ↓ total myeloid cells, macrophages, monocytes, DCs, T cells, CD4+ T cells, CD8+ T cells, and NK cells in spleen | ||||||
| ↓ T cells, CD4+ T cells, and CD8+ T cells in thymus ↑ tumor growth after anti-PD-1 |
||||||
| ↓ or = survival after anti-PD-1 | ||||||
| ↓ total myeloid cells, macrophages, monocytes, DCs, CD4+ T cells, and NK cells in tumor after anti-PD-1 | ||||||
| = microglia, T cells, and CD8+ T cells in tumor after anti-PD-1 | ||||||
| ↓ total myeloid cells, macrophages, monocytes, DCs, T cells, CD4+ T cells, CD8+ T cells, and NK cells in tumor-draining lymph nodes after anti-PD-1 | ||||||
| ↓ total myeloid cells, macrophages, monocytes, DCs, T cells, CD4+ T cells, CD8+ T cells, and NK cells in spleen after anti-PD-1 | ||||||
| ↓ T cells, CD4+ T cells, and CD8+ T cells in thymus after anti-PD-1 | ||||||
| Maxwell et al39 | Glioma | Mice | Dexamethasone (10 mg/kg/day i.p.) | 5–28 days | 10 | ↓ CD4+ and CD8+ T cells in tumor-draining lymph nodes |
| ↓ CD4+ T cells, CD8+ T cells, and Tregs in blood | ||||||
| = survival after anti-PD-1 | ||||||
| = CD4+ and CD8+ T cells in tumor-draining lymph nodes after anti-PD-1 | ||||||
| ↓ CD4+ T cells, CD8+ T cells, and Tregs in blood after anti-PD-1 | ||||||
| Ott et al31 | Glioblastoma | Mice | Dexamethasone (1 mg/kg/day i.p.) | 3 days | 2 | ↓ TDO expression in tumor |
| Human studies | ||||||
| Adhikaree et al40 | Glioblastoma | Patients | Dexamethasone (0.5–5 mg/day N/A) | N/A | 5–11 | ↓ CDc1+, CD141+, CD303+, and Slan+ DCs in blood |
| Chitadze et al41 | Glioblastoma | Patients | Dexamethasone (N/A) | N/A | 13–22 | ↑ monocytes and neutrophils in blood |
| ↓ total lymphocytes, CD4+ T cells, CD8+ T cells, and Tregs in blood | ||||||
| = B cells and NK cells in blood | ||||||
| Fries et al42 | Glioblastoma | Patients | Dexamethasone (3–12 mg/day p.o.) | N/A | 6–7 | = IL-1β secretion by monocytes |
| Gustafson et al43 | Glioblastoma | Patients | Dexamethasone (N/A) | N/A | 5–14 | = monocytes in blood |
| ↑ neutrophils in blood | ||||||
| ↓ total lymphocytes, T cells, CD4+ T cells, and Tregs in blood | ||||||
| = CD8+ T cells, B cells, and NK cells in blood | ||||||
| Moyes et al44 | Glioblastoma | Patients | Dexamethasone (N/A) | N/A | 8–17 | ↓ CCL21, CCL22, and CXCL10 in blood |
| ↑ CCL23 in blood | ||||||
| = CCL2, CCL27, and CXCL12 in blood | ||||||
| Otvos et al45 | Glioblastoma | Patients | Dexamethasone (N/A) | N/A | 34–61 | ↓ total lymphocytes in blood |
| = monocytes in blood | ||||||
| Quillien et al46 | Glioblastoma | Patients | Corticosteroids (N/A) | N/A | 18–43 | ↑ neutrophils in blood |
CD, cluster of differentiation; CTLA-4, cytotoxic T lymphocyte antigen 4; DC, dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; N/A, not available or not applicable; NK cell, natural killer cell; PD-1, programmed cell death protein 1; Slan, 6-sulfo LacNAc; TDO, tryptophan 2,3-dioxygenase; TNF, tumor necrosis factor; Treg, regulatory T cell.
Cellular Research
Inflammatory molecules
—Seven studies examined the effect of dexamethasone on inflammatory molecules in glioma cell cultures. Overall, dexamethasone generally inhibits the expression and secretion of various inflammatory molecules. Dexamethasone was shown to dose-dependently inhibit interleukin (IL)-1β-induced secretion of the proinflammatory cytokine IL-6 by human U87MG glioblastoma cells.27 Similarly, although baseline granulocyte-macrophage (GM)-CSF levels were unaffected in the human A172 and U87MG glioblastoma cell lines, tumor necrosis factor (TNF)-α-induced GM-CSF secretion, which promotes the proliferation and maturation of eosinophils and macrophages, was reduced by dexamethasone in a dose-dependent fashion.26 Furthermore, in human GBM8401 and T98G glioblastoma cells stimulated by co-culture with macrophages or IL-1α administration, dexamethasone was demonstrated to dose-dependently decrease the expression and secretion of the chemokine CXCL8, which acts as a chemoattractant for neutrophils.29,30
Dexamethasone was also shown to downregulate immune-related enzymes. Two studies established that dexamethasone dose-dependently inhibits lipopolysaccharide- or cytokine-induced nitrite release into the culture supernatant of rat C6 glioma cells.32,33 This is indicative for the enzyme activity of inducible nitric oxide synthase (iNOS), which is typically increased in inflammatory conditions. Moreover, the iNOS mRNA level was temporarily reduced after incubation with dexamethasone.33 Treatment of U87MG cells with dexamethasone also significantly suppressed the expression of tryptophan 2,3-dioxygenase (TDO), an enzyme involved in the degradation of the essential amino acid tryptophan that thereby exerts an immunosuppressive effect.31,47,48
Immune cells
—Three co-culture studies showed that dexamethasone decreases the expression of proinflammatory cytokines by myeloid cells, while promoting the production of anti-inflammatory cytokines. In co-cultures of microglia or bone marrow-derived macrophages with murine platelet-derived growth factor subunit B (PDGFB)-overexpressing glioblastoma slices, dexamethasone-treated immune cells expressed the proinflammatory cytokines IL-1α and IL-1β to a lesser extent than vehicle-treated microglia or macrophages.28 Correspondingly, another study co-culturing microglia and murine GL261 glioma cells demonstrated that dexamethasone administration reduced IL-1β production in microglia, whereas the anti-inflammatory cytokine IL-10 was upregulated.34 The same research group recently showed that dexamethasone also lowered the production of IL-1β, IL-6, IL-8, IL-12, and TNF-α by both microglia and monocytes in co-cultures of microglia or monocytes with human A172 or U87MG glioblastoma cells. Furthermore, dexamethasone enhanced the microglial production of IL-10, but did not affect IL-10 production by monocytes.35
Animal Research
Inflammatory molecules
—Two studies in animal models for glioma confirmed some of the effects of dexamethasone that were observed in glioma cell cultures: tumor tissue of either immunocompromised U87MG or immunocompetent PDGFB-overexpressing glioblastoma-bearing mice that had received dexamethasone treatment contained lower mRNA levels of both IL-1α and IL-1β as well as TDO in comparison with control-treated mice.28,31
Immune cells
—Five studies in animal models established how dexamethasone affects the presence of different immune cell populations in the glioma microenvironment as well as in different body compartments.
In PDGFB-overexpressing glioblastoma-bearing mice, dexamethasone was shown to exert a negative effect on the total myeloid cell content in the tumor tissue.28 However, a study in immunocompetent GL261 glioma mice revealed that the total number of myeloid cells was unaffected by dexamethasone in the intracranial tumor but reduced in tumor-draining lymph nodes and the spleen.38
In the PDGFB-overexpressing glioblastoma-bearing mice, dexamethasone administration was shown to reduce the number of peripheral macrophages in the tumor tissue without altering the microglial number.28 In immunogenic C6 glioma-bearing rats on the other hand, dexamethasone decreased microglial presence in the tumor tissue, whereas the number of peripheral macrophages invading the tumor was unaffected.36 Finally, a study in GL261 glioma mice found no differences in either microglial or macrophage number in the tumor after dexamethasone treatment but found considerably fewer macrophages in tumor-draining lymph nodes and the spleen of dexamethasone-treated animals.38 In general, the total number of macrophages thus appears to be reduced in animal models for glioma following dexamethasone treatment, although there are conflicting results on whether microglia or peripheral macrophages are responsible.
Two of these studies also investigated the influence of dexamethasone on other myeloid cell populations in glioma mouse models. One group found that dexamethasone increased the number of circulating monocytes in PDGFB-overexpressing glioblastoma-bearing mice.28 The second study reported that the number of monocytes and DCs was unaltered in the tumor or tumor-draining lymph nodes and even reduced in the spleen of GL261 glioma mice.38 Finally, it was reported that dexamethasone did not influence the presence of neutrophils in peripheral blood of PDGFB-overexpressing glioblastoma-bearing mice.28
In C6 glioma-bearing rats, dexamethasone was shown to dose-dependently decrease the total number of tumor-infiltrating lymphocytes.36 This effect on total lymphocyte counts was also observed in both tumor tissue and blood samples of PDGFB-overexpressing glioblastoma mice.28
Similar to lymphocytes in general, dexamethasone reduces the number of T lymphocytes in glioma animal models. The study in C6 glioma rats detected that dexamethasone dose-dependently inhibited T cell infiltration in tumor tissue.36 Other research groups investigated the effects of dexamethasone on specific T cell subsets in GL261 murine glioma models. Examination of tumor tissue yielded varying results. One study reported that the numbers of all T cell subsets, comprising the effector CD4+ and CD8+ T lymphocytes as well as the immunosuppressive Tregs, were unaltered by dexamethasone.37 Another study detected no effect on the number of CD8+ T cells, but reported that dexamethasone administration resulted in fewer total and CD4+ tumor-infiltrating T cells.38 More conclusive findings were generated when investigating effects of dexamethasone in other body compartments. In tumor-draining lymph nodes of dexamethasone-treated mice, cell counts of total T cells, CD4+ T cells, CD8+ T cells, and Tregs were significantly decreased.37–39 Dexamethasone also reduced the number of CD4+ T cells, CD8+ T cells, and Tregs in peripheral blood39 as well as the number of total, CD4+ and CD8+ T cells in both the spleen and thymus.38 Administration of dexamethasone also resulted in fewer NK cells in the tumor tissue, tumor-draining lymph nodes and spleen.38 In conclusion, several studies demonstrate that dexamethasone negatively affects T cell numbers in glioma models, with several of them showing a downregulation within the tumor. Overall, dexamethasone seems to downregulate the presence of the most important myeloid and lymphoid immune cell populations.
Checkpoint inhibitor immunotherapy
—Three of the previously described studies also examined the influence of dexamethasone on survival and immune cell populations in glioma mice receiving checkpoint inhibitor immunotherapy.37–39 A study by Giles et al investigated the effect of dexamethasone on CTLA-4 immunotherapy in GL261 glioma-bearing mice.37 First, it was demonstrated that dexamethasone dose-dependently increased the percentage of circulating CD4+ and CD8+ T cells expressing CTLA-4. Despite this increase, the survival of mice that had received dexamethasone concurrent with the CTLA-4 inhibitor ipilimumab was increased (median survival of 49 vs 39 days; P = .013) in comparison to mice treated with anti-CTLA-4 monotherapy. Of note, a subsequent experiment in which dexamethasone treatment was already initiated prior to tumor implantation found no significant effect of dexamethasone on the survival of these mice.37 Follow-up experiments revealed that dexamethasone did not alter the number of tumor-infiltrating CD4+ T cells, CD8+ T cells, or Tregs, but decreased the numbers of these cell populations in tumor-draining lymph nodes of ipilimumab-treated mice.37 CTLA-4 immunotherapy on its own had no effect on any of these T cell subsets in either compartment.
The influence of dexamethasone on the efficacy of PD-1 immunotherapy was investigated by 2 research groups.38,39 In a study by Maxwell et al, GL261 glioma mice received PD-1 inhibitors alone or in combination with 1 of 3 dexamethasone treatment regimens that differed in onset and duration. Anti-PD-1 monotherapy increased survival of the mice and this was unaffected by any of the 3 dexamethasone treatment regimens.39 Furthermore, dexamethasone did not clearly affect the presence of CD4+ and CD8+ T lymphocytes in tumor-draining lymph nodes of mice treated with anti-PD-1 antibodies. On the other hand, all 3 dexamethasone treatment regimens decreased the number of circulating CD4+ T cells, CD8+ T cells, and Tregs.39
In another study by Iorgulescu et al, GL261 glioma-bearing mice were treated with PD-1 inhibitors alone or in combination with dexamethasone. Administration of dexamethasone solely prior to checkpoint inhibitor immunotherapy did not significantly alter the survival of the mice. However, administration during checkpoint inhibitor immunotherapy was shown to dose-dependently increase tumor growth and decrease survival, thereby abrogating the positive effects of the checkpoint inhibitor immunotherapy.38 Additionally, the combination of anti-PD-1 therapy and dexamethasone clearly reduced the presence of both myeloid and lymphoid cell populations in different tissues in comparison to anti-PD-1 monotherapy. The numbers of total myeloid cells, macrophages, monocytes, and DCs were all downregulated in tumor tissue, tumor-draining lymph nodes and spleen of dexamethasone-treated mice. Only microglial counts in the tumor were unaffected by dexamethasone. Dexamethasone also did not influence the number of T lymphocytes and CD8+ T cells in the tumor tissue. However, CD4+ T cells in the tumor as well as the number of T lymphocytes, CD4+ T cells, and CD8+ T cells in the tumor-draining lymph nodes, spleen, and thymus were reduced by dexamethasone. Finally, dexamethasone lowered the number of NK cells in tumor tissue, lymph nodes, and spleen.38
In summary, 2 out of 3 independent preclinical studies did not observe a negative effect of dexamethasone on the survival of glioma-bearing mice receiving checkpoint inhibitor immunotherapy. In the third study, dexamethasone seemed to exert a negative effect when given during checkpoint inhibitor immunotherapy, but this effect was not observed when administered prior to checkpoint inhibitor immunotherapy. It is difficult to unify the results of the 3 studies, since each study used different dexamethasone treatment regimens. Nonetheless, while it differed per body compartment, all studies reported dexamethasone to reduce immune cell numbers, especially T cell subsets.
Human Research
Inflammatory molecules
—One study investigated the effect of dexamethasone on various chemokines in glioblastoma patients. Members of the CC chemokine subfamily are mainly involved in the recruitment of monocytes, whereas CXC chemokines generally induce migration of neutrophils or lymphocytes. In dexamethasone-treated patients, the chemokines CCL21, CCL22, and CXCL10 were downregulated in peripheral blood, while the chemokine CCL23 was upregulated in comparison to steroid-naive patients. The serum levels of CCL2, CCL27, and CXCL12 were not significantly altered in dexamethasone-treated subjects.44
Immune cells
—The effects of dexamethasone on immune cell populations have not been studied as extensively in glioma patients as in animal models. Data on immune cell populations in the glioma microenvironment itself are lacking.
There are 6 studies that investigated circulating myeloid cells in glioblastoma patients. In all of these studies, blood samples were compared between patients that did and did not receive corticosteroids. One study detected higher monocyte numbers in the blood of dexamethasone-treated patients,41 while 2 other studies observed no effect on the number of circulating monocytes.43,45 Another study demonstrated that IL-1β release by circulating monocytes, which is indicative for their activation state, was unaltered by dexamethasone.42 Dexamethasone was shown to negatively affect various DC subsets in peripheral blood, as dexamethasone dose-dependently decreased the number of the myeloid CDc1+, CD141+, and 6-sulfo LacNAc (Slan)+ DC subsets as well as the plasmacytoid CD303+ DC count.40 Finally, 3 studies demonstrated that both corticosteroids in general as well as dexamethasone specifically increased neutrophil cell counts in peripheral blood samples.41,43,46
Three studies also investigated lymphocyte numbers in glioblastoma patients. One study demonstrated that dexamethasone reduced the presence of circulating lymphocytes in general.45 Another research group also measured significantly lower blood counts of total lymphocytes as well as of CD4+ T cells, CD8+ T cells, and Tregs in dexamethasone-treated patients.41 A final study confirmed that dexamethasone resulted in fewer total lymphocytes, total T cells, CD4+ T cells, and Tregs in peripheral blood samples, but detected no difference in the CD8+ T cell subset.43 Furthermore, 2 of these research groups showed that the numbers of circulating B and NK cells were unaffected by dexamethasone administration.41,43
Clinical Research
Three clinical studies investigated corticosteroid effects on the efficacy of checkpoint inhibitor immunotherapy in the treatment of glioblastoma. The main findings of these studies, as well as their effect sizes, are listed in Table 2. A retrospective study assessed the OS in 181 patients with both newly diagnosed and recurrent isocitrate dehydrogenase (IDH) wild-type glioblastoma that were treated with PD-1 or PD-L1 inhibitors.38 In this study, dexamethasone usage at treatment initiation was shown to be negatively correlated with OS, which was 8.1 and 6.3 months in patients on <2 and ≥2 mg/day dexamethasone, respectively, compared to 13.1 months in patients not on baseline dexamethasone (P < .001 and P = .001, respectively). Multivariable analysis revealed that this negative effect of dexamethasone persisted after adjustment for relevant prognostic factors, including disease setting, patient age, MGMT promoter methylation status, Karnofsky performance scale, tumor volume at anti-PD(L)1 initiation and extent of resection (<2 mg/day hazard ratio [HR]: 2.16; 95% confidence interval [CI]: 1.30–3.60 and ≥2 mg/day HR: 1.97; 95% CI: 1.23–3.16).38 In a phase II randomized clinical trial (RCT), 80 recurrent glioblastoma patients received either pembrolizumab monotherapy (200 mg IV) every 3 weeks or pembrolizumab in combination with bevacizumab (10 mg/kg IV) every 2 weeks. In the small cohort of 30 subjects receiving pembrolizumab monotherapy, there was no significant association between baseline dexamethasone and OS. In the other 50 patients that received pembrolizumab and bevacizumab combined, univariate analysis showed that baseline dexamethasone use was significantly associated with a worsened OS (HR: 3.27; 95% CI: 1.61–6.65).49 However, it was not investigated whether this association persisted in multivariable analysis adjusted for prognostic factors, but sample size may have been a limiting factor for multivariable analysis. Finally, in the CheckMate 143 phase III RCT, 369 recurrent glioblastoma patients were randomized to nivolumab (3 mg/kg) or bevacizumab (10 mg/kg) every 2 weeks. The OS of patients did not significantly differ between the 2 treatment groups. Post hoc multivariate analysis adjusted for age, sex, MGMT promotor methylation status, Karnofsky performance scale, tumor load, and time from glioblastoma diagnosis to recurrence, revealed that among the 184 nivolumab-treated subjects, baseline corticosteroid use was significantly associated with shorter OS (HR: 1.69; 95% CI: 1.05–2.69). This association was absent in the control group receiving bevacizumab.10 In summary, 1 retrospective and 1 randomized clinical study demonstrated a negative association between baseline corticosteroid use and OS of glioblastoma patients receiving checkpoint inhibitors after correction for relevant prognostic factors. In the third study, this association was absent in a fairly small patient group treated with pembrolizumab monotherapy but present in patients receiving both pembrolizumab and bevacizumab.
Table 2.
Overview of included clinical studies
| Author | Type of study | Tumor type | Checkpoint inhibitor(s) | Corticosteroid treatment (dosage) | N of patients (N on steroids) | Outcome(s) |
|---|---|---|---|---|---|---|
| Iorgulescu et al38 | Retrospective cohort | Newly diagnosed and recurrent glioblastoma | PD-1 or PD-L1 inhibitors | Baseline dexamethasone (N/A) | 181 (64) | ↓ OS (<2 mg/day HR: 2.16; 95% CI: 1.30–3.60 and ≥2 mg/day HR: 1.97; 95% CI: 1.23–3.16) |
| Nayak et al49 | Phase II RCT | Recurrent glioblastoma | Pembrolizumab | Baseline dexamethasone (≤4 mg/day) | 30 (7) | = OS |
| Nayak et al49 | Phase II RCT | Recurrent glioblastoma | Pembrolizumab + bevacizumab | Baseline dexamethasone (≤4 mg/day) | 50 (12) | ↓ OS (HR: 3.27; 95% CI: 1.61–6.65) |
| Reardon et al10 | Phase III RCT | Recurrent glioblastoma | Nivolumab | Baseline corticosteroids (prednisone-equivalent dose ≤10 mg/day) | 184 (73) | ↓ OS (HR: 1.69; 95% CI: 1.05–2.69) |
CI, confidence interval; HR, hazard ratio; N/A, not available; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; RCT, randomized clinical trial.
Discussion
Main Findings
The effect of dexamethasone on the immunological component of the glioblastoma microenvironment has been investigated in various preclinical studies. However, only a few clinical studies have examined corticosteroid effects on checkpoint inhibitor immunotherapy efficacy in glioblastoma patients.
In general, preclinical glioma research demonstrated that dexamethasone decreases the presence of microglia and other macrophages as well as the number of T lymphocytes in the tumor, tumor-draining lymph nodes, blood, spleen, and/or thymus. Nevertheless, it remains difficult to assess the overall effect of dexamethasone on the antitumor immune response. Since the majority of macrophages in the glioblastoma microenvironment induces immunosuppression, a decrease in macrophage numbers might actually be beneficial.50,51 Furthermore, several studies observe a decrease in all T lymphocyte subsets, but if dexamethasone affects both effector and regulatory T cells, it is difficult to state how this will influence the overall T cell-mediated immune response. Remarkably, dexamethasone seems to decrease the presence of CD4+ T cells more consistently than CD8+ T cell numbers, but we could not find an explanation for this phenomenon. Additionally, while dexamethasone seems to downregulate various inflammatory molecules, decreasing the release of proinflammatory cytokines acts immunosuppressive, whereas downregulating TDO is suggested to stimulate the immune response.47,48
Preclinical studies on checkpoint inhibitor immunotherapy demonstrated that dexamethasone abolishes the positive effects of checkpoint inhibitors on the presence of immune cells, especially T lymphocytes. The effect of dexamethasone on survival, on the other hand, remains elusive, as studies have yielded conflicting results. Moreover, it is important to note that this has only been investigated by 3 different research groups.
Clinical studies in glioblastoma patients have revealed a negative association between baseline corticosteroid use and survival outcomes in glioblastoma patients on checkpoint inhibitors. However, corticosteroid use has also been shown to be a poor prognostic indicator in glioblastoma patients that do not receive checkpoint inhibitors.52,53 In the previously described clinical studies, the use of corticosteroids was not a randomization factor. Not all studies accounted for the fact that as a rule glioblastoma patients receive corticosteroids for symptom control, resulting in the need for corticosteroid treatment to be an important confounder in itself. Additionally, research on this specific topic in glioblastoma patients is very scarce.
In conclusion, corticosteroids negatively affect the most important cellular and molecular immune mediators and may thereby affect the efficacy of checkpoint inhibitor immunotherapy in glioblastoma models and patients. Since other types of immunotherapy are designed to generate the same results as checkpoint inhibitors, a similar effect would be expected on their efficacy. However, although preclinical and early clinical studies have suggested that checkpoint inhibitors are effective in improving survival outcomes, this finding has not been replicated in phase III clinical trials. If this indicates a lack of efficacy of checkpoint inhibitors, no clear effect of dexamethasone would be expected.
An important consideration with regard to the preclinical studies is the relevance of the investigated model systems, especially with regard to the tumor microenvironment. It is important to acknowledge that the various cell lines not only resemble the histological characteristics of human glioblastoma to a different extent, but also lack the ability to resemble the interactions between the tumor and its microenvironment. Similarly, mouse models bear little resemble with human glioblastomas. Tumor generation using xenograft transplantation, as in U87MG glioblastoma-bearing mice, requires immunocompromised animals to prevent rejection of the tumor cells, which makes these models unsuitable for studies focusing on the immune system. Transplantation of carcinogen-induced tumor cells in syngeneic hosts or viral tumor induction, as in GL261 and PDGFB-overexpressing glioblastoma-bearing mice, respectively, is performed in immunocompetent mice, enabling more reliable study of the role of the immune system.54–56 The conflicting results of preclinical studies in this review could very well be a reflection of the flaws of the models. Because of the lack of high-level evidence from preclinical studies, the presented results have to be interpreted with the greatest caution.
Future clinical trials on checkpoint inhibitors in glioblastoma patients should either only include patients with no or very low dosage steroid therapy, use the administration of dexamethasone as a stratification factor at randomization or properly adjust for prognostic factors. Possible non-corticosteroid alternatives for patients requiring edema control include VEGF inhibitors and cyclooxygenase-2 inhibitors.57,58 Bevacizumab, a monoclonal antibody targeting VEGF does not improve survival outcomes,59 but has been demonstrated to alleviate vasogenic edema and diminish the need for corticosteroids in glioblastoma patients.60,61 In addition, it has been assumed that bevacizumab acts synergistically with checkpoint inhibitors.62 Therefore, bevacizumab might be an alternative to dexamethasone in future trials assessing checkpoint inhibitor immunotherapy in glioblastoma patients.
Strengths and Limitations
This is the first systematic review regarding corticosteroid effects on checkpoint inhibitor immunotherapy that specifically focuses on glioblastoma. The major strength of this review is its translational approach. However, this study also has its limitations. The most important limitations are the small number of relevant articles and the fact that some of them report conflicting data. The results that are reported in this review may have also been skewed by publication bias. Moreover, randomization was lacking in the great majority of the studies, resulting in a high risk of selection bias. In addition, various articles failed to provide specific information about the type of corticosteroid, dosage, and treatment duration, which could be of great importance for the observed effects on the immune response. Finally, preclinical studies in glioma tumor models did often not report the specific glioma subtype or WHO grade, although this has a substantial impact on the prognosis and possibly on the effect of corticosteroids.
Future Perspectives
The most important challenge is to establish whether checkpoint inhibitor immunotherapy may contribute to improving clinical outcomes in glioblastoma patients. Current strategies combine checkpoint inhibitors with, for instance, radiotherapy, chemotherapy, and other types of immunotherapy such as vaccination or oncolytic virus immunotherapy, which are thought to sensitize the tumor to checkpoint inhibitor immunotherapy.8,13,63 Several studies have already examined corticosteroid effects in glioma models receiving vaccination, oncolytic virus, T cell, IL-2 or IL-12 immunotherapy, but further research is required to make well-founded recommendations regarding the combination of dexamethasone and different immunotherapies.19–23,64–69 In addition, the precise molecular mechanisms by which glucocorticoids impair the immune response remain to be elucidated. Finally, alternatives to dexamethasone in the treatment of edema in glioblastoma patients should be investigated.
Supplementary Material
Contributor Information
Kyra X Swildens, Department of Neurology, Brain Tumor Center, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Peter A E Sillevis Smitt, Department of Neurology, Brain Tumor Center, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Martin J van den Bent, Department of Neurology, Brain Tumor Center, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Pim J French, Department of Neurology, Brain Tumor Center, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Marjolein Geurts, Department of Neurology, Brain Tumor Center, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Funding
No funding was utilized in the writing of this manuscript.
Conflict of interest statement. None declared.
Authorship Statement
Literature search, study selection, and data extraction: K.X.S. and M.G.; writing the draft manuscript: K.X.S.; writing manuscript at revision stage: K.X.S., P.A.E.S.S., M.J.B., P.J.F., and M.G.
References
- 1. Ostrom QT, Gittleman H, Stetson L, Virk SM, Barnholtz-Sloan JS. Epidemiology of gliomas. Cancer Treat Res. 2015;163:1–14. [DOI] [PubMed] [Google Scholar]
- 2. Bush NAO, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2017;40(1):1–14. [DOI] [PubMed] [Google Scholar]
- 3. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. [DOI] [PubMed] [Google Scholar]
- 4. Wilcox JA, Ramakrishna R, Magge R. Immunotherapy in glioblastoma. World Neurosurg. 2018;116:518–528. [DOI] [PubMed] [Google Scholar]
- 5. Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4(2):124–135. [DOI] [PubMed] [Google Scholar]
- 6. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. [DOI] [PubMed] [Google Scholar]
- 7. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weenink B, French PJ, Sillevis Smitt PAE, Debets R, Geurts M. Immunotherapy in glioblastoma: current shortcomings and future perspectives. Cancers (Basel). 2020;12(3):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woroniecka K, Fecci PE. Immuno-synergy? Neoantigen vaccines and checkpoint blockade in glioblastoma. Neuro Oncol. 2020;22(9):1233–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sampson JH, Omuro AMP, Preusser M, et al. A randomized, phase 3, open-label study of nivolumab versus temozolomide (TMZ) in combination with radiotherapy (RT) in adult patients (pts) with newly diagnosed, O-6-methylguanine DNA methyltransferase (MGMT)-unmethylated glioblastoma (GBM): CheckMate-498. J Clin Oncol. 2016;34(15 suppl):TPS2079. [Google Scholar]
- 12. Weller M, Lim M, Idbaih A, et al. CTIM-25. A randomized phase 3 study of nivolumab or placebo combined with radiotherapy plus temozolomide in patients with newly diagnosed glioblastoma with methylated MGMT promotor: CheckMate 548. Neuro Oncol. 2021;23(suppl 6):vi55–vi56. [Google Scholar]
- 13. Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–442. [DOI] [PubMed] [Google Scholar]
- 14. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Investig. 2017;97(5):498–518. [DOI] [PubMed] [Google Scholar]
- 16. Cenciarini M, Valentino M, Belia S, et al. Dexamethasone in glioblastoma multiforme therapy: mechanisms and controversies. Front Mol Neurosci. 2019;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiocca EA, Yu JS, Lukas RV, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med. 2019;11(505):eaaw5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleijn A, Kloezeman J, Treffers-Westerlaken E, et al. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS One. 2014;9(5):e97495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mueller S, Taitt JM, Villanueva-Meyer JE, et al. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J Clin Invest. 2020;130(12):6325–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lesniak MS, Gabikian P, Tyler BM, Pardoll DM, Brem H. Dexamethasone mediated inhibition of local IL-2 immunotherapy is dose dependent in experimental brain tumors. J Neurooncol. 2004;70(1):23–28. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Curran CS, Evans MD, Bertics PJ. GM-CSF production by glioblastoma cells has a functional role in eosinophil survival, activation, and growth factor production for enhanced tumor cell proliferation. J Immunol. 2011;187(3):1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gottschall PE, Tatsuno I, Arimura A. Increased sensitivity of glioblastoma cells to interleukin 1 after long-term incubation with dexamethasone. Mol Cell Neurosci. 1992;3(1):49–55. [DOI] [PubMed] [Google Scholar]
- 28. Herting CJ, Chen Z, Maximov V, et al. Tumour-associated macrophage-derived interleukin-1 mediates glioblastoma-associated cerebral oedema. Brain. 2019;142(12):3834–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hong TM, Teng LJ, Shun CT, Peng MC, Tsai JC. Induced interleukin-8 expression in gliomas by tumor-associated macrophages. J Neurooncol. 2009;93(3):289–301. [DOI] [PubMed] [Google Scholar]
- 30. Mukaida N, Morita M, Ishikawa Y, et al. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-κB is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269(18):13289–13295. [PubMed] [Google Scholar]
- 31. Ott M, Litzenburger UM, Rauschenbach KJ, et al. Suppression of TDO-mediated tryptophan catabolism in glioblastoma cells by a steroid-responsive FKBP52-dependent pathway. Glia. 2015;63(1):78–90. [DOI] [PubMed] [Google Scholar]
- 32. Rieger J, Ständer M, Löschmann PA, et al. Synthesis and biological effects of NO in malignant glioma cells: modulation by cytokines including CD95L and TGF-β, dexamethasone, and p53 gene transfer. Oncogene. 1998;17(18):2323–2332. [DOI] [PubMed] [Google Scholar]
- 33. Shinoda J, McLaughlin KE, Bell HS, et al. Molecular mechanisms underlying dexamethasone inhibition of iNOS expression and activity in C6 glioma cells. Glia. 2003;42(1):68–76. [DOI] [PubMed] [Google Scholar]
- 34. Wielgat P, Czarnomysy R, Trofimiuk E, Car H. The sialoglycan-Siglec-E checkpoint axis in dexamethasone-induced immune subversion in glioma-microglia transwell co-culture system. Immunol Res. 2019;67(4–5):348–357. [DOI] [PubMed] [Google Scholar]
- 35. Wielgat P, Wawrusiewicz-Kurylonek N, Czarnomysy R, et al. The paired Siglecs in brain tumours therapy: the immunomodulatory effect of dexamethasone and temozolomide in human glioma in vitro model. Int J Mol Sci. 2021;22(4):1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Badie B, Schartner JM, Paul J, et al. Dexamethasone-induced abolition of the inflammatory response in an experimental glioma model: a flow cytometry study. J Neurosurg. 2000;93(4):634–639. [DOI] [PubMed] [Google Scholar]
- 37. Giles AJ, Hutchinson MKND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iorgulescu JB, Gokhale PC, Speranza MC, et al. Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin Cancer Res. 2021;27(1):276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maxwell R, Luksik AS, Garzon-Muvdi T, et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology. 2018;7(12):e1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adhikaree J, Franks HA, Televantos C, et al. Impaired circulating myeloid CD1c+ dendritic cell function in human glioblastoma is restored by p38 inhibition—implications for the next generation of DC vaccines. Oncoimmunology. 2019;8(7):e1593803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chitadze G, Flüh C, Quabius ES, et al. In-depth immunophenotyping of patients with glioblastoma multiforme: impact of steroid treatment. Oncoimmunology. 2017;6(11):e1358839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fries G, Perneczky A, Kempski O. Enhanced interleukin-1 beta release and longevity of glioma-associated peripheral blood monocytes in vitro. Neurosurgery. 1994;35(2):264–271. [DOI] [PubMed] [Google Scholar]
- 43. Gustafson MP, Lin Y, New KC, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moyes KW, Davis A, Hoglund V, et al. Effects of tumor grade and dexamethasone on myeloid cells in patients with glioma. Oncoimmunology. 2018;7(11):e1507668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Otvos B, Alban TJ, Grabowski MM, et al. Preclinical modeling of surgery and steroid therapy for glioblastoma reveals changes in immunophenotype that are associated with tumor growth and outcome. Clin Cancer Res. 2021;27(7):2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quillien V, Carpentier AF, Gey A, et al. Absolute numbers of regulatory T cells and neutrophils in corticosteroid-free patients are predictive for response to bevacizumab in recurrent glioblastoma patients. Cancer Immunol Immunother. 2019;68(6):871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Platten M, von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2015;5:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu CP, Song YL, Zhu ZM, et al. Targeting TDO in cancer immunotherapy. Med Oncol. 2017;34(5):73. [DOI] [PubMed] [Google Scholar]
- 49. Nayak L, Molinaro AM, Peters K, et al. Randomized phase II and biomarker study of pembrolizumab plus bevacizumab versus pembrolizumab alone for patients with recurrent glioblastoma. Clin Cancer Res. 2021;27(4):1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tamura R, Tanaka T, Yamamoto Y, Akasaki Y, Sasaki H. Dual role of macrophage in tumor immunity. Immunotherapy. 2018;10(10):899–909. [DOI] [PubMed] [Google Scholar]
- 51. De Leo A, Ugolini A, Veglia F. Myeloid cells in glioblastoma microenvironment. Cells. 2020;10(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gorlia T, Stupp R, Brandes AA, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48(8):1176–1184. [DOI] [PubMed] [Google Scholar]
- 53. Zhou L, Shen Y, Huang T, et al. The prognostic effect of dexamethasone on patients with glioblastoma: a systematic review and meta-analysis. Front Pharmacol. 2021;12:727707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robertson FL, Marqués-Torrejón MA, Morrison GM, Pollard SM. Experimental models and tools to tackle glioblastoma. Dis Model Mech. 2019;12(9):dmm040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu P, Griffiths S, Veljanoski D, et al. Preclinical models of glioblastoma: limitations of current models and the promise of new developments. Expert Rev Mol Med. 2021;23:e20. [DOI] [PubMed] [Google Scholar]
- 56. Miyai M, Tomita H, Soeda A, et al. Current trends in mouse models of glioblastoma. J Neurooncol. 2017;135(3):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dasararaju R, Mehta A. Current advances in understanding and managing secondary brain metastasis. CNS Oncol. 2013;2(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dixit KS, Kumthekar PU. Optimal management of corticosteroids in patients with intracranial malignancies. Curr Treat Options Oncol. 2020;21(9):77. [DOI] [PubMed] [Google Scholar]
- 59. Taal W, Oosterkamp HM, Walenkamp AME, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 60. Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7(12):1152–1160. [DOI] [PubMed] [Google Scholar]
- 61. Thomas AA, Omuro A. Current role of anti-angiogenic strategies for glioblastoma. Curr Treat Options Oncol. 2014;15(4):551–566. [DOI] [PubMed] [Google Scholar]
- 62. Gao X, McDermott DF. Combinations of bevacizumab with immune checkpoint inhibitors in renal cell carcinoma. Cancer J. 2018;24(4):171–179. [DOI] [PubMed] [Google Scholar]
- 63. Montoya ML, Kasahara N, Okada H. Introduction to immunotherapy for brain tumor patients: challenges and future perspectives. Neurooncol Pract. 2020;7(5):465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lillehei KO, Mitchell DH, Johnson SD, McCleary EL, Kruse CA. Long-term follow-up of patients with recurrent malignant gliomas treated with adjuvant adoptive immunotherapy. Neurosurgery. 1991;28(1):16–23. [DOI] [PubMed] [Google Scholar]
- 65. Kruse CA, Visonneau S, Kleinschmidt-DeMasters BK, et al. The human leukemic T-cell line, TALL-104, is cytotoxic to human malignant brain tumors and traffics through brain tissue: implications for local adoptive immunotherapy. Cancer Res. 2000;60(20):5731–5739. [PubMed] [Google Scholar]
- 66. Rampling R, Peoples S, Mulholland PJ, et al. A cancer research UK first time in human phase I trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin Cancer Res. 2016;22(19):4776–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Read SB, Kulprathipanja NV, Gomez GG, et al. Human alloreactive CTL interactions with gliomas and with those having upregulated HLA expression from exogenous IFN-γ or IFN-γ gene modification. J Interf Cytokine Res. 2003;23(7):379–393. [DOI] [PubMed] [Google Scholar]
- 68. Todo T, Rabkin SD, Chahlavi A, Martuza RL. Corticosteroid administration does not affect viral oncolytic activity, but inhibits antitumor immunity in replication-competent herpes simplex virus tumor therapy. Hum Gene Ther. 1999;10(17):2869–2878. [DOI] [PubMed] [Google Scholar]
- 69. Brown CE, Aguilar B, Starr R, et al. Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther. 2018;26(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.