Key Points
Question
Is there an association of cabazitaxel treatment with progression-free survival in patients with advanced dedifferentiated liposarcoma (DDLPS)?
Findings
In this international, multicenter phase 2 nonrandomized clinical trial of cabazitaxel, median progression-free survival was 6.5 months, with 55% of patients progression free at 12 weeks. These results, according to the study primary end point, exceeded what had been foreseen to declare the trial as successful.
Meanings
A further phase 3 study of cabazitaxel is strongly recommended to assess the efficacy of this drug in DDLPS, hopefully improving the armamentarium of the medical oncologist and outcomes for these patients.
This phase 2 nonrandomized clinical trial examines the association of cabazitaxel treatment for patients with advanced dedifferentiated liposarcoma with progression-free survival.
Abstract
Importance
Treatment options for patients with unresectable and/or metastatic dedifferentiated liposarcoma (DDLPS) are limited. New drugs are required.
Objective
To assess whether cabazitaxel demonstrated sufficient antitumor activity in patients with metastatic or inoperable locally advanced DDLPS to justify further investigation in a phase 3 setting.
Design, Setting, and Participants
This international multicenter, open-label single-arm phase 2 trial was conducted at 10 institutions in 4 European countries from March 2015 to March 2019. Eligible patients had to have metastatic or locally advanced histologically proven DDLPS with evidence of disease progression within the past 6 months and had to have received no more than 1 previous line of chemotherapy.
Interventions
After mandatory central review of tumor blocks, if the DDLPS diagnosis was confirmed, patients started treatment within 72 hours after registration. Cabazitaxel was administered at a dose of 25 mg/m2 IV infusion over 1 hour every 21 days until intolerance, progression, or withdrawal of consent.
Main Outcomes and Measures
The primary end point was progression-free survival (PFS) rate at 12 weeks per RECIST 1.1. Based on a Simon 2-stage design, at least 4 of 17 (stage 1) and 11 of 37 (stage 2) eligible and evaluable patients who were progression free at 12 weeks were needed. The final analysis report was completed on November 17, 2021.
Results
Forty patients were registered, with 2 patients being ineligible. The number of cycles ranged from 1 to 30, with a median of 5; 26 patients (65%) received at least 4 cycles of cabazitaxel. Progression-free survival at 12 weeks was 55%, achieving the primary study end point. At a median follow-up of 21.6 months, median PFS was 6 months and median OS 21 months. Response rate (RR) was 8% with 1 clinical response (CR) and 2 partial responses (PR). Twenty-three (60.5%) patients had a stable disease (SD). Disease control (PR+SD) was achieved in 26 patients (68%).
Conclusions and Relevance
This nonrandomized phase 2 clinical trial met its primary end point, with 21 of 38 patients (55%) being progression free at 12 weeks. These results suggest important activity of cabazitaxel in patients with metastatic or inoperable locally advanced DDLPS. The drug is worth being further studied in these tumors in a phase 3 setting.
Introduction
Liposarcoma (LPS) represents the most common histologic subtype of soft-tissue sarcoma in adults. Based on molecular and clinical characteristics, LPS can be classified into different subtypes that include well-differentiated/dedifferentiated (WD/DD) LPS, myxoid/round cell LPS, and pleomorphic LPS. Well-differentiated/DDLPS represent the most common subtypes.1 These subgroups have distinct clinical behaviors and sensitivity to systemic agents.2 In particular WD/DDLPS are less sensitive to the classic first-line therapy for soft-tissue sarcomas with doxorubicin and ifosfamide compared with myxoid round cell and pleomorphic liposarcoma.3,4 After failure of first-line agents, other options with limited activity include trabectedin,5 high-dose continous infusion of ifosfamide,6,7 and eribulin.8
In particular, for DDLPS the median progression-free survival (PFS) with these drugs is very limited, and when disease progresses no other active agents are available. Indeed, better understanding of the genetic and molecular profile of LPS has recently led to the development of new targeted agents such as selinexor,9,10 MDM2 inhibitors,11 and CDK4 inhibitors.12,13 However, none of these agents are currently approved. Although immunotherapy has shown exiciting results in some solid cancers,14 it has had a limited effect in advanced soft-tissue sarcomas, including DDLPS.15 In this scenario, other effective and tolerable treatment options are strongly needed.
Cabazitaxel, like other taxanes, exerts its effect through inhibition of microtubular disassembly, and is approved for patients with metastatic hormone refractory prostate cancer, and shown to be a relatively safe, effective, and well-tolerated antimicrotubular agent.16 Before this phase 2 study, direct experience of cabazitaxel in WD/DDLPS was limited, but, notably, in a previous phase 1 study on cabazitaxel, a patient with DDLPS had been successfully treated with dose-reduced cabazitaxel continuously for more than 4 years without progression. Moreover, since then, another antimicrotubular agent, eribulin, also demonstrated some activity in soft-tissue sarcomas and specifically in DDLPS, it was reasonable to explore cabazitaxel. Thus, the European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group embarked on this phase 2 study, with PFS at 3 months as its end point.
Methods
Patients
In this international, multicenter, open-label phase 2 clinical trial, eligible patients had to have metastatic or locally advanced histologically proven DDLPS with evidence of disease progression within the past 6 months; World Health Organization (WHO) performance status of 0 or 1; aged 18 to 75 years; and adequate renal, liver, and bone marrow function (absolute neutrophil count >1 .5 × 109 cells/L; platelet count >100 × 109 cells/L; bilirubin ≤1.0 times the upper limit of normal [1.0 × ULN] of institutional limits, ALT and/or AST≤1.5 × ULN, and serum creatinine clearance >30 mL/min). Eligible patients had to have received no more than 1 previous line of chemotherapy and have no preexisting toxic effects higher than grade 1 (as assessed by National Cancer Institute Common Toxicity Criteria [NCI-CTC, version 3.0]). The protocol was approved by the institutional review board at each participating center, and the study was performed in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent prior to any trial procedures. The complete trial protocol is available in Supplement 1.
History of the Study
This study was originally designed to evaluate 2 different chemotherapy regimes in second-line treatment, in 2 independent Simon 2-stage phase 2 trials run in parallel, 1 addressing cabazitaxel, the other addressing prolonged infusional ifosfamide. Recruitment issues led to an amendment and on October 26, 2016, the EORTC Board decided to move on with a phase 2 design with single-agent cabazitaxel, in the expectation that the amended protocol would attract more patients. The protocol (current version 3.0) as well as the patient information sheets were amended to remove the ifosfamide arm. There were 2 patients taking ifosfamide prior to the amendment to protocol version 3.0. These patients are not part of the scope of this article.
Procedures
Central review of tumor blocks to confirm the histologic diagnosis before the final confirmation of eligibility was mandatory. If the DDLPS diagnosis was confirmed, patients started treatment within 72 hours after registration. Cabazitaxel was administered at a dose of 25 mg/m2 IV infusion over 1 hour every 21 days until intolerance, progression, or withdrawal of consent. Treatment was delayed until recovery if hematologic (absolute neutrophil count ≥1.5 × 109/L) or nonhematologic (any > grade 2 except inadequately treated nausea or vomiting) adverse events were recorded on day 21. If recovery occurred before day 21, treatment was resumed at the same dose at the first occurrence, and at a lower dose in case of subsequent epidoses. Only 1 dose reduction was permitted; then, if further episodes occurred despite prior dose reduction, or if treatment was delayed for more then 2 weeks the patient was withdrawn.
Tumor assessments were done at baseline and every 12 weeks during treatment by computed tomographic (CT) or magnetic resonance imaging (MRI) and were based on the Response Evaluation Criteria for Solid Tumors (RECIST) version 1.1,17 defining as target lesions those with solid or higher density elements of the disease, which usually correlate pathologically with the DD element. A Blinded Independent Central Review (BICR) reviewed all scans. Centers were requested to submit all scan results, which were collected during the course of the study. The BICR performed a central reading of all scan results of all patients retrospectively.
Outcomes
The primary end point of the trial was PFS at 12 weeks after start of therapy, measured as a binary variable. Treatment was regarded as a “success” if this assessment indicated stable disease, partial response (PR), or complete response (CR) as evalauted by local investigators per RECIST (version 1.1).17 All other outcomes (including patients who progressed, symptomatically deteriorated, or died before the 12-week evaluation, patients with an unknown progression status at 12 weeks, or treatment of patients who started new antitumor therapy in the absence of progressive disease) was considered “failure.” Secondary end points included overall survival (OS), PFS, time to progression (TTP), response to therapy, time to onset and duration of response, and incidence and severity of adverse events (AE) rated using Common Terminology Criteria for Adverse Events (version 4.0). No end points on the potential molecular mechanisms of activity were reported.
Statistical Analysis
The study was based on a Simon optimal 2-stage design, where the success rates under the null (P0) and alternative (P1) hypotheses were set as 20% and 40%, respectively. The P0 and P1 were based on a retrospective analysis of the EORTC STBSG database of patients treated with second-line therapy.18 Type 1 error and type 2 error were set at 10%. Based on these design parameters, at least 4 of 17 (stage 1) and 11 of 37 (stage 2) eligible and evaluable patients who were progression free at 12 weeks were needed to declare the trial as successful.
Because patients were only evaluable for the primary end point 12 weeks after start of treatment, it was planned to temporarily stop accrual after the inclusion of 19 patients, to allow for 10% of untreated or ineligible patients. Up to 4 patients could be added to the total recruitment. Furthermore, to account for screening failure at central review, up to 50 patients could be enrolled. Note that sufficient information was observed within the first 17 patients (stage 1), allowing the trial to continue to stage 2 without stopping. This study was registered with ClinicalTrials.gov (NCT01913652).
The main analysis of all efficacy end points was performed in the per-protocol population. The binary indicator for PFS at 12 weeks after start of treatment was reported as a proportion with a conditional 95% confidence intervals (CIs).19 The decision rules were evaluated in the interim analysis population (stage 1) and final decision rule population, corresponding respectively to the first 17 and 37 eligible patients for cabazitaxel. Time to progression, PFS, and OS were summarized using the Kaplan-Meier method. The respective full survival curves, estimates at 3-month intervals, and medians were provided along with 95% CIs. The rate of objective tumor response (CR+PR) was computed with 95% CIs (from the exact binomial distribution). Patients in response categories of progression, early death, or unknown were considered as failures. Time to onset of response and duration of response were reported as median and range in months in responding patients.
Role of the Funding Source
The EORTC STBSG developed the trial design, collected, analyzed, and interpreted the data. Only EORTC headquarters staff had access to the raw study data when the actual statistical analysis was done. The final analysis report was completed on November 17, 2021. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Forty patients were recruited between March 2015 and March 2019, at 10 institutions in 4 European countries (Italy, United Kingdom, the Netherlands, France). Two of the 40 patients were ineligible after medical review (1 diagnosis of DDLPS not confirmed and the other with an unstable medical condition) and were only considered in the safety analysis (data summarized in eFigure A in Supplement 2). The clinical cutoff date for the primary analysis was August 31, 2020. Database lock was performed on February 2, 2021. One of 38 eligible patients was still receiving treatment at the time of the clinical cutoff date. For this patient, only data with dates before or at the clinical cutoff date were included in the analysis. Patient characteristics were evaluated in the per-protocol population (Table 1). The median (range) age of enrolled patients was 65 (31-74) years. There were slightly more men (20 of 38 patients [53%]) and patients with performance status 0 (21 of 38 patients [55%]). Thirty-six patients (89.5%) had poorly or undifferentiated tumors and 32 (84.2%) had grade 2 disease. Overall, 29 participants (76.3%) were reported to have other medical conditions at trial entry.
Table 1. Patient Characteristics.
| Characteristic | Cabazitaxel, No. (%) |
|---|---|
| No. | 38 |
| Age (randomization), y | |
| <65 | 18 (47.4) |
| ≥65 | 20 (52.6) |
| Median (range) | 65 (31-74) |
| Sex | |
| Male | 20 (52.6) |
| Female | 18 (47.4) |
| World Health Organization performance status | |
| 0 | 21 (55.3) |
| 1 | 17 (44.7) |
| Grade | |
| Grade 2 | 32 (84.2) |
| Grade 3 | 6 (15.8) |
| Tumor differentiation | |
| Moderate | 2 (5.3) |
| Poor/undifferentiated | 36 (94.7) |
| Concomitant nonmalignant disease | |
| No | 8 (21.1) |
| Yes | 30 (78.9) |
Exposure to Treatment
Of the 40 patients treated with cabazitaxel, 26 (65%) received at least 4 cycles of cabazitaxel. The median (range) number of cycles for all patients was 5 (1-30). Twenty-three patients (57.5%) experienced a schedule modification, whereas 13 patients (32.5%) had a dose modification. Reason for dose modification was hematologic adverse events in 4 cases and nonhematologic adverse events in 9 cases. The main reason for stopping treatment in this trial was disease progression (22 patients [55%]). There were 9 cases (22.5%) of stopping protocol treatment owing to toxic effects.
Treatment Activity
The results presented in this article are based on a median (IQR) follow-up of 21.6 (5.3-21.5) months.
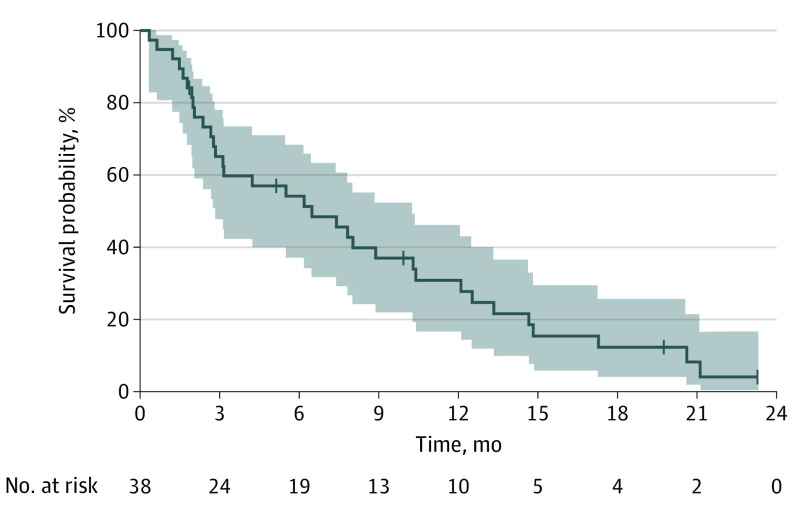
The proportion of successes for PFS at 12 weeks after start of cabazitaxel treatment was 55%, achieving the primary study end point (21 successes were observed of the 37 eligible patients, which also exceeded the minimum number of successes needed to declare the trial successful). The median PFS was 6.5 months (95% CI, 2.8-10.3) (Figure 1), median TTP was 7.4 (95% CI, 2.8-12.1) months (eFigure 1 in Supplement 2) and median OS was 21.1 (95% CI, 14.8-33.5) months (eFigure 2 in Supplement 2). The rate of objective tumor response (CR+PR) was 8% with 1 CR and 2 PR. The median (range) time to onset of response was 8 (5.1-8.3) months. Twenty-three (60.5%) patients had an SD. Disease control (PR+SD) was achieved in 26 patients (68%). Radiologic responses per RECIST, together with other main efficacy end points are summarized in eTable 1 in Supplement 2.
Figure 1. Progression-Free Survival.
The shaded areas indicate the 95% CIs.
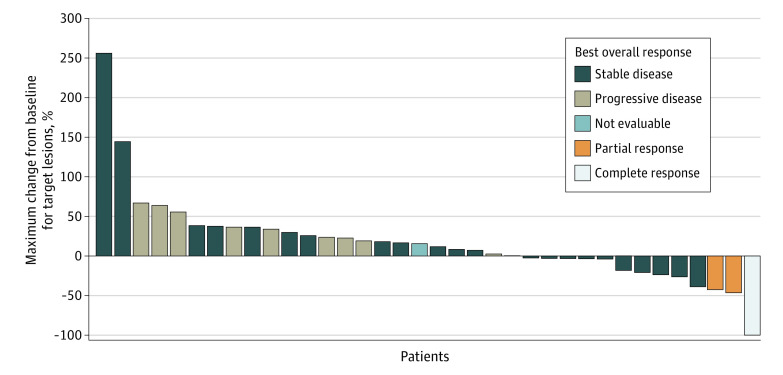
Figure 2 shows the waterfall plot, presenting the maximum percent change from baseline for target lesions by patient. Only 1 of 3 eligible patients who experienced objective response had progressed at the time of clinical cutoff for this analysis.
Figure 2. Waterfall Plot.
Safety
The most common cabazitaxel-related adverse events, irrespective of grade, were decreased neutrophil count in 21 of 40 patients (52.5%), diarrhea in 17 (42.5%), fatigue in 16 (40%), anorexia in 13 (32.5%), and anemia in 12 (30%) (eTable 2 in Supplement 2).
The most frequent grade 3 to 4 adverse events, regardless of their relationship to cabazitaxel, included decreased neutrophil count in 24 of 40 patients (50%), WBC count in 17 (42.5%), febrile neutropenia in 10 (25%), fatigue in 5 (12.5%), and anemia in 4 (10%). All grade 3 to 4 adverse events occurring in at least 2 patients (5%) are summarized in Table 2. In general, there were 27 serious adverse events and 20 serious adverse reactions. No deaths associated with toxic effects were reported.
Table 2. Occurrence of Grade 3 to 4 Adverse Events.
| Grade 3 to 4 events | Total, No. (%) |
|---|---|
| No. | 40 |
| Neutropenia | 24 (60.0) |
| White blood cell count | 17 (42.5) |
| Febrile neutropenia | 10 (25.0) |
| Fatigue | 5 (12.5) |
| Anemia | 4 (10.0) |
| Hypoalbuminemia | 3 (7.5) |
| Diarrhea | 3 (7.5) |
| White blood cell count decreased | 3 (7.5) |
| Back pain | 2 (5.0) |
| Cystitis noninfective | 2 (5.0) |
Discussion
In this international, multicenter phase 2 clinical trial of cabazitaxel in advanced pretreated DDLPS, median PFS was 6.5 months, with 55% of patients progression-free at 12 weeks and an RR of 8%. Disease control (PR+SD) was achieved in 26 patients (68%). These results, according to the study primary end point, exceeded what had been foreseen in order to declare the trial as successful.
In essence, the activity of cabazitaxel as assessed in this phase 2 study compares favorably with the other drugs currently used in second- or further-line therapy for DDLPS. Notably, a subgroup of patients, 11 of 38 (30%), received more than 10 cycles of cabazitaxel, with 1 patient recieving 30 cycles. This suggests not only a prolonged benefit of the drug in a subgroup of patients, but even a good tolerability of the treatment. Among other available agents, trabectedin, the second most frequent drug used in DDLPS after failure of anthracycline, showed a PFS of 2.2 months in the randomized phase 3 study5 vs dacarbazine, which led to the approval of the drug by the US Food and Drug Administration. However, retrospective studies20 suggest an increased activity of this drug in a subgroup of DDLPS, with a particular benefit in low-grade DDLPS. Ifosfamide, administered as high-dose continous infusion,6,7, represents another option in second- and further-line therapy in patients with DDLPS. Indeed, to our knowledge, there are no published prospective studies on high-dose ifosfamide selectively focusing on WD/DDLPS, but a small restrospective case series7 reporting a PFS of 6 months with a 30% of PR, all observed in patients with high-grade dedifferentiated component. Eribulin was approved specifically in the liposarcoma subtype among soft-tissue sarcomas. Of note, this is the first drug approved in later-line therapy with an improvement in OS without any advantage in PFS, which was assessed at 3 months.8 After trabectedin and eribulin, no other licensed options are available in DDLPS, except in clinical studies. Recently, the results of an international randomized clinical trial10 of the nuclear export inhibitor selinexor vs placebo have been presented specifically in DDLPS. In this study, the median PFS reported in patients treated with selinexor was 2.8 months vs 2 months in the placebo arm, thus with an improvement of only 3 weeks in the arm with the active drug, where, on the other hand, the toxic effects were not negligible. In the landscape of clinical studies of new targeted therapies in patients with DDLPS, the results of a phase 2 study of anti-CDK4 amebaciclib were reported, showing a PFS of around 6 months,12 whereas new anti-MDM2 molecules are still under investigation.11 Overall, treatment options for patients with advanced DDLPS remain limited, with a strong need for more effective and tolerable treatment options. Of note, none of the target agents tested in patients with DDLPS in clinical studies reported, until now, a better outcome than cabazitaxel.
Limitations
The main limitation of this study is its limited number of patients, the absence of a control arm, and the absence of quality of life as a secondary end point.
Conclusions
Given the positive findings of this open-label single-arm phase 2 trial, a further phase 3 study of cabazitaxel is strongly recommended to assess the efficacy of this drug in patients with DDLPS, hopefully improving the armamentarium of the medical oncologist and outcomes for these patients.
Trial Protocol
eFigure A. Patient disposition for the Phase II trial of cabazitaxel
eFigure 1.Time to progression
eFigure 2. Overall survival
eTable 1. Summary of main clinical efficacy endpoints
eTable 2. Cabazitaxel-related clinical AEs (all grades) occurring in ≥ 10% of patients in the safety population
References
- 1.WHO Classification of Tumours Editorial Board . Soft tissue and bone tumours. Lyon (France): International Agency for Research on Cancer; 2020. (WHO classification of tumours series, 5th ed.; vol. 3). [Google Scholar]
- 2.Gronchi A, Miah AB, Dei Tos AP, et al. ; ESMO Guidelines Committee, EURACAN and GENTURIS. Electronic address: clinicalguidelines@esmo.org . Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021;32(11):1348-1365. doi: 10.1016/j.annonc.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 3.Jones RL, Fisher C, Al-Muderis O, Judson IR. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer. 2005;41(18):2853-2860. doi: 10.1016/j.ejca.2005.07.023 [DOI] [PubMed] [Google Scholar]
- 4.Livingston JA, Bugano D, Barbo A, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep. 2017;7(1):11836. doi: 10.1038/s41598-017-12132-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34(8):786-793. doi: 10.1200/JCO.2015.62.4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Liberal J, Alam S, Constantinidou A, et al. Clinical activity and tolerability of a 14-day infusional Ifosfamide schedule in soft-tissue sarcoma. Sarcoma. 2013;2013:868973. doi: 10.1155/2013/868973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanfilippo R, Bertulli R, Marrari A, et al. High-dose continuous-infusion ifosfamide in advanced well-differentiated/dedifferentiated liposarcoma. Clin Sarcoma Res. 2014;4(1):16. doi: 10.1186/2045-3329-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629-1637. doi: 10.1016/S0140-6736(15)01283-0 [DOI] [PubMed] [Google Scholar]
- 9.Zuco V, Pasquali S, Tortoreto M, et al. Selinexor versus doxorubicin in dedifferentiated liposarcoma PDXs: evidence of greater activity and apoptotic response dependent on p53 nuclear accumulation and survivin down-regulation. J Exp Clin Cancer Res. 2021;40(1):83. doi: 10.1186/s13046-021-01886-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gounder MM, Razak AA, Somaiah N, et al. Selinexor in advanced, metastatic dedifferentiated liposarcoma: a multinational, randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2022;JCO2101829:JCO2101829; Epub ahead of print. doi: 10.1200/JCO.21.01829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer TM, Gounder MM, Amy M.Weise AMet al. A phase 1 study of MDM2 inhibitor DS-3032b in patients with well/de-differentiated liposarcoma (WD/DD LPS), solid tumors (ST) and lymphomas (L). J Clin Oncol. 2018;36(15_suppl):11514-11514. doi: 10.1200/JCO.2018.36.15_suppl.11514 [DOI] [Google Scholar]
- 12.Dickson MA, Tap WD, Keohan ML, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31(16):2024-2028. doi: 10.1200/JCO.2012.46.5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson MA, Koff A, D’Angelo SP, et al. Phase 2 study of the CDK4 inhibitor abemaciclib in dedifferentiated liposarcoma. J Clin Oncol. 2019;37(15)(suppl):11004. doi: 10.1200/JCO.2019.37.15_suppl.11004 [DOI] [Google Scholar]
- 14.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J Clin Oncol. 2021;39(15)(suppl):9506. doi: 10.1200/JCO.2021.39.15_suppl.9506 [DOI] [Google Scholar]
- 15.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493-1501. doi: 10.1016/S1470-2045(17)30624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bono JS, Oudard S, Ozguroglu M, et al. ; TROPIC Investigators . Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154. doi: 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 18.Van Glabbeke M, Verweij J, Judson I, Nielsen OS; EORTC Soft Tissue and Bone Sarcoma Group . Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38(4):543-549. doi: 10.1016/S0959-8049(01)00398-7 [DOI] [PubMed] [Google Scholar]
- 19.Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med. 2008;27(16):3145-3154. doi: 10.1002/sim.3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabbroni C, Fucà G, Ligorio F, et al. Impact of pathological stratification on the clinical outcomes of advanced well-differentiated/dedifferentiated liposarcoma treated with trabectedin. Cancers (Basel). 2021;13(6):1453. doi: 10.3390/cancers13061453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure A. Patient disposition for the Phase II trial of cabazitaxel
eFigure 1.Time to progression
eFigure 2. Overall survival
eTable 1. Summary of main clinical efficacy endpoints
eTable 2. Cabazitaxel-related clinical AEs (all grades) occurring in ≥ 10% of patients in the safety population