Abstract
Four adenosine receptors have been cloned and characterized from several mammalian species. The receptors are named adenosine A1, A2A, A2B, and A3. The A2A and A2B receptors preferably interact with members of the Gs family of G proteins and the A1 and A3 receptors with Gi/o proteins. However, other G protein interactions have also been described. Adenosine is the preferred endogenous agonist at all these receptors, but inosine can also activate the A3 receptor. The levels of adenosine seen under basal conditions are sufficient to cause some activation of all the receptors, at least where they are abundantly expressed. Adenosine levels during, e.g., ischemia can activate all receptors even when expressed in low abundance. Accordingly, experiments with receptor antagonists and mice with targeted disruption of adenosine A1, A2A, and A3 expression reveal roles for these receptors under physiological and particularly pathophysiological conditions. There are pharmacological tools that can be used to classify A1, A2A, and A3 receptors but few drugs that interact selectively with A2B receptors. Testable models of the interaction of these drugs with their receptors have been generated by site-directed mutagenesis and homology-based modelling. Both agonists and antagonists are being developed as potential drugs.
I. Introduction
The nomenclature and classification of adenosine receptors has been covered in two publications by members of a previous NC-IUPHAR subcommittee, which was devoted to “purinoceptors” (Fredholm et al., 1994a, 1997). However, these two publications were progress reports and were not official documents of the NC-IUPHAR. They dealt with both adenosine receptors and P2 receptors. As a result of this work, separate subcommittees were set up for adenosine receptors, P2X receptors, and P2Y receptors. The present review will therefore cover adenosine receptors only. The previous publications contain the historical background and this will not be recapitulated.
The term adenosine receptor is used to denote this group of receptors. First, use of the name adenosine follows the recommendation of NC-IUPHAR that receptors be named after the preferred endogenous agonist. Second, and as discussed in the previous publications, the concept of adenosine receptors precedes the later concept of purinoceptors (P1 and P2) (Burnstock, 1978) by several years. The discovery by Drury and Szent-Györgyi (1929) that adenosine can influence several bodily functions inspired much research interest; the pronounced cardiovascular effects of adenosine were particularly well investigated. Several adenosine analogs were synthesized, and examination of the dose-response relationships suggested the presence of specific adenosine receptors (Cobbin et al., 1974). The essentially competitive nature of the antagonism by methylxanthines, including caffeine and theophylline, of adenosine effects in the heart (De Gubareff and Sleator, 1965) and in the brain (Sattin and Rall, 1970) also supported the idea of adenosine receptors. Finally, as the term P2 receptor has been superseded by the names P2X and P2Y there is little room for the term P1 except when referring specifically to older publications.
There are four different adenosine receptors, denoted A1, A2A, A2B, and A3 (Table 1). This terminology is well established and is coherent with the principles of receptor nomenclature adopted by NC-IUPHAR. Although the primary basis for adenosine receptor nomenclature is structural, historical reasons also played a role. As discussed previously (Fredholm et al., 1994a), careful pharmacological analysis first identified two subforms—A1 and A2 (van Calker et al., 1979; Londos et al., 1980a). Later pharmacological studies revealed that the A2 receptors, coupled to adenylyl cyclase, were heterogenous, necessitating subdivision into A2A and A2B.
TABLE 1.
Current adenosine receptor nomenclature
| Receptor | A1 | A2A | A2B | A3 |
|---|---|---|---|---|
| Receptor Code | 2.1:ADO: | 2.1:ADO: | 2.1:ADO: | 2.1:ADO: |
| Previous names | Ri | A2a, Rs | A2b, Rs | |
| Structural information | 7TM; human 326 aa, P30542, chr 1q32.1, rat 326 aa, P25099, mouse 326 aa | 7TM; human 410 aa, P29274, chr 22g11.2, rat 409 aa, P30543, mouse 409 aa, UO5672 | 7TM; human 328 aa, P29275, chr 17p11.2–12; rat 332 aa, P29276 mouse 332 aa, UO5673 | 7TM; human 318 aa, P33765, chr 1p21–13, rat 320 aa P28647 (see comments), mouse 320 aa, AF069778 |
| Selective agonists | CPA, CCPA, CHA | CGS 21680, HENECA, CV-1808, CV-1674, ATL146e | None | Cl-IB-MECA |
| Selective antagonists | DPCPXa 8-cyclopentyl-theophylline, WRC0571 | selective: SCH 58261b,c moderately selective: ZM241385,a KF 17387, CSC | MRS1754,d enprofylline, alloxazine (historical) | MRS 1220,e MRE 3008-F20,e MRS 1191; MRS 1523 |
| Tissue functions | Bradycardia; inhibition of lipolysis; reduced glomerular filtration; tubero-glomerular feedback, antinociception; reduction of sympathetic and parasympathetic activity; presynaptic inhibition; neuronal hyperpolarization; ischemic preconditioning | Regulation of sensorimotor integration in basal ganglia; inhibition of plateletaggregation and polymorphonuclear leukocytes; vasodilatation, protection against ischemic damage, stimulation of sensory nerve activity | Relaxation of smooth muscle in vasculature and intestine; inhibition of monocyte and macrophage function, stimulation of mast cell mediator release (some species) | Enhancement of mediator release from mast cells (some species). Preconditioning (some species) |
| Phenotypes | Tissue functions above confirmed in knockout mouse | Tissue functions above confirmed in knockout mouse | Tissue functions above confirmed in knockout mouse | |
| Comments | Also cloned from dog,f cow,g rabbiti,j | Also cloned from e.g. dog,h guinea pig.k Variations in structure among humans | Alternative splicing in rat can yield product with 337 aa.l Also cloned from sheepm 317 aa, rabbitj 320 aa |
DPCPX and ZM241385 also have nanomolar affinity for the adenosine A2B receptor.
II. Molecular Basis for Receptor Nomenclature
Once the adenosine A1 receptor was defined using binding assays, several attempts were made to purify the receptor. Despite considerable progress by several groups the receptor was never sufficiently pure to allow sequencing. Instead, the cloning of the first adenosine receptors was serendipitous. Four novel members of the G protein-coupled receptor family were cloned from a canine thyroid library (Libert et al., 1989). Of these, one turned out to be the adenosine A2A receptor (Maenhaut et al., 1990), and another the adenosine A1 receptor (Libert et al., 1991). Once these first sequences were obtained the same receptors were soon cloned from other mammals including human (Furlong et al., 1992; Libert et al., 1992; Townsend-Nicholson and Shine, 1992; Ren and Stiles, 1995; Deckert et al., 1996; Peterfreund et al., 1996). In addition, the adenosine A2B receptor was cloned (Stehle et al., 1992; Jacobson et al., 1995). More surprisingly, a fourth adenosine receptor, denoted A3, was cloned, first as an orphan (Meyerhof et al., 1991), later as a bona fide methylxanthine-insensitive adenosine receptor in rat (Zhou et al., 1992), a xanthine-sensitive receptor in sheep (Linden et al., 1993), and a partially xanthine-sensitive receptor in humans (Sajjadi and Firestein, 1993; Salvatore et al., 1993; Linden, 1994). Thus, a family of four adenosine receptors has been cloned from several mammalian and nonmammalian species (see below). The current nomenclature is summarized in Table 1.
III. Formation and Levels of the Endogenous Agonist Adenosine
Adenosine is the main agonist at this receptor class, and this is the reason for the name. In addition, the adenosine metabolite inosine can activate at least some of the receptors (Jin et al., 1997; Fredholm et al., 2001), and there may be circumstances under which inosine provides a larger activation than adenosine, but this remains to be proven.
When given in very high amounts, adenosine can affect intracellular nucleotide pools and even provide a source of metabolizable energy. In addition, it was reported very recently that the human growth hormone secretagogue receptor (GHS-R) also accepts adenosine as a highly potent endogenous agonist, in addition to the endogenous peptide GHS-R agonist, ghrelin (Smith et al., 2000; Tullin et al., 2000). However, most effects of adenosine are due to activation of adenosine receptors.
Before we describe the activation of adenosine receptors under physiological conditions and hence the actions of antagonists, the mechanisms regulating levels of extracellular adenosine must be briefly presented. Under normal conditions, adenosine is continuously formed intracellularly as well as extracellularly. The intracellular production is mediated either by an intracellular 5’-nucleotidase, which dephosphorylates AMP (Schubert et al., 1979; Zimmermann et al., 1998), or by hydrolysis of S-adenosyl-homocysteine (Broch and Ueland, 1980). Adenosine generated intracellularly is transported into the extracellular space mainly via specific bi-directional transporters through facilitated diffusion that efficiently evens out the intra- and extracellular levels of adenosine. In some tissues (e.g., kidney brush-border membranes) there is a concentrative nucleoside transport protein capable of maintaining high adenosine concentrations against a concentration gradient. These transport proteins have been cloned and were termed ENT1 and ENT2 (for the equilibrative transport proteins) and CNT1 and CNT2 (for the concentrative types) (e.g., Williams and Jarvis, 1991; Anderson et al., 1996; Baldwin et al., 1999). When the activity of transporters is decreased, e.g., by drugs or by reducing temperature, extracellular biologically active levels of adenosine increase (Dunwiddie and Diao, 2000). In view of the fact that several of the transporters are equilibrative, this might seem to be a paradox. However, as discussed previously (e.g., Fredholm et al., 1994b), it must be remembered that in tissue, some cells are net producers of adenosine, and in these, intracellular levels rise whereas most cells are net eliminators of the nucleoside.
The dephosphorylation of extracellular AMP to adenosine, mediated by ecto-5’-nucleotidase, is the last step in the enzymatic chain that catalyzes the breakdown of extracellular adenine nucleotides, such as ATP, to adenosine. Ectonucleotidases include ectonucleoside triphosphate diphosphohydrolases, including CD39, which can hydrolyze ATP or ADP, ectonucleotide pyrophosphatase/phosphodiesterases, alkaline phosphatases and 5’-nucleotidases such as CD73 (Zimmermann, 2000). These enzymes are essential for the nerve activity-dependent production of adenosine from released ATP under physiological conditions (Dunwiddie et al., 1997a; Zimmermann et al., 1998). The entire catalytic pathway is complete in a few hundred milliseconds, and the rate-limiting step seems to be the dephosphorylation of AMP to adenosine by ecto-5’-nucleotidase (Dunwiddie et al., 1997a). Recent data provide evidence for the presence of soluble 5’-nucleotidases of unknown structure that are released together with ATP from stimulated sympathetic nerve endings and participate in the extracellular hydrolysis of ATP to adenosine (Todorov et al., 1997). In striatum, local application of a 5’-nucleotidase inhibitor dose dependently decreases the normal levels of adenosine and thereby emphasizes the relevance of this enzyme in vivo (Delaney and Geiger, 1998). Nonetheless, there is good evidence that intracellular formation of adenosine is at least as important as adenosine formation from breakdown of extracellular ATP (Lloyd et al., 1993; Lloyd and Fredholm, 1995). Intracellular formation predominantly occurs as a consequence of activity of intracellular 5’-nucleotidases, of which two forms, cN-I and cN-II, have been cloned (Sala-Newby et al., 1999). These two enzymes may play different roles—cN-I breaking down AMP to adenosine and cN-II breaking down IMP and GMP to inosine and guanosine, respectively (Sala-Newby et al., 2000).
When adenosine levels in the extracellular space are high, adenosine is transported into cells by means of transporters. It is then phosphorylated to AMP by adenosine kinase (Km = approximately 100 nM; Spychala et al., 1996) or degraded to inosine by adenosine deaminase, a process with a severalfold lower affinity (Km = 20 –100 µM; Arch and Newsholme, 1978; Lloyd and Fredholm, 1995). In the heart, and probably also other tissues, hypoxia-induced inhibition of adenosine kinase amplifies small changes in free myocardial AMP, resulting in a major rise in adenosine (Decking et al., 1997). Adenosine deaminase, but not adenosine kinase, is also present in the extracellular space (Lloyd and Fredholm, 1995).
Adenosine can also be released into the extracellular space after application of specific neurotransmitter ligands. Glutamatergic agonists, such as NMDA or kainate, dose dependently increase adenosine levels (Carswell et al., 1997; Delaney et al., 1998). Activation of NMDA receptors seems to release adenosine itself rather than a precursor (Manzoni et al., 1994; Harvey and Lacey, 1997). Dopamine D1 receptors enhance adenosine release via an NMDA receptor-dependent increase in extracellular adenosine levels (Harvey and Lacey, 1997), but dopamine depletion causes no significant changes in the extracellular levels of striatal adenosine as measured by in vivo microdialysis (Ballarin et al., 1987). Thus, dopaminergic input may be important to transiently elevate adenosine but not so important in maintaining a basal level of the nucleoside. Nitric oxide can also control basal levels of endogenous adenosine in vivo (Fischer et al., 1995; Delaney et al., 1998) as well as in vitro (Fallahi et al., 1996).
Another potential source of extracellular adenosine is cAMP, which can be released from neurons and converted by extracellular phosphodiesterases into AMP and thereafter by an ecto-5’-nucleotidase to adenosine. Functional evidence for a relevant role of this pathway has been obtained in the ventral tegmental area and hippocampus (Bonci and Williams, 1996; Brundege et al., 1997; Dunwiddie et al., 1997a,b). However, to provide a physiologically important adenosine release, it seems that multiple cells must release cAMP over a prolonged period (Brundege et al., 1997).
Levels of adenosine in the rodent and cat brain have been determined by different methods including freeze-blowing (Winn et al., 1981), high-energy focused microwave irradiation (Delaney et al., 1998; Delaney and Geiger, 1998) and microdialysis (Zetterström et al., 1982; Porkka-Heiskanen et al., 1997) and have been estimated to be approximately 30 to 300 nM. These levels are sufficient to cause activation of adenosine A1 and A2A receptors.
The levels of adenosine, at least in the basal forebrain, striatum, hippocampus, and thalamus, are higher during wakefulness than sleep (Huston et al., 1996; Porkka-Heiskanen et al., 1997). The highest levels of adenosine in hippocampus were estimated during the hours before rats entered into a sleep-like behavior, suggesting that adenosine has sleep-promoting properties (Huston et al., 1996). Moreover, extracellular adenosine levels increased 2-fold in the basal forebrain of the cat after 4 h of handling to ensure prolonged wakefulness (Porkka-Heiskanen et al., 1997).
As is well known, levels of adenosine increase, up to 100-fold, as a result of oxidative stress and ischemia (Rudolphi et al., 1992a; Latini et al., 1999). Excitatory amino acid-mediated release of adenosine is certainly involved; however, of greater importance is probably the fact that whenever intracellular levels of adenine nucleotides fall as a result of excessive energy use, the intracellular levels of adenosine will rise dramatically (Rudolphi et al., 1992a). For example, following hypoxia (Zetterström et al., 1982), ischemia (Berne et al., 1974), or electrical stimulation (Pull and McIlwain, 1972), there is a decrease of intracellular ATP, accompanied by an accumulation of 5’-AMP and subsequently adenosine. The nucleoside is thereafter transported into the extracellular space via the above-mentioned transporters (Jonzon and Fredholm, 1985; Fredholm et al., 1994b). An elegant illustration of the capacity of these transporters to move adenosine from the intra- to the extracellular space was provided by loading a high concentration of adenosine into a single hippocampal CA 1 neuron and shortly thereafter, identifying in the same cell an inhibition of the excitatory postsynaptic potential mediated by extracellular adenosine (Brundege and Dunwiddie, 1996). Furthermore, when the intracellular level of adenosine is very high, adenosine simply diffuses out of cells. Direct release of intracellular adenine nucleotides, such as ATP, that is thereafter converted extracellularly by ecto-ATPase and ecto-ATP-diphosphohydrolase (ecto-apyrase) to AMP and dephosphorylated by ecto-5’-nucleotidase to adenosine, should also be considered (Rudolphi et al., 1992a; Zimmermann et al., 1998).
IV. Structure
By now all four adenosine receptors have been cloned from rat, mouse, and human (the structural information is available e.g., via GPCRDB www.gpcr.org/7tm/). In addition, A1 receptors are cloned from dog, cow, rabbit, guinea pig, and chick; the A2A receptor from dog and guinea pig; the A2B receptor from chick; and the A3 receptor from dog, sheep, rabbit, and chick. As seen from the dendrogram in Fig. 1, there is a close similarity between receptors of the same subtype, at least among mammals. The largest variability is seen for the A3 receptor for which there is almost a 30% difference at the amino acid level between human and rat. This difference is in fact larger than that between human and chick A1 receptors.
FIG. 1.
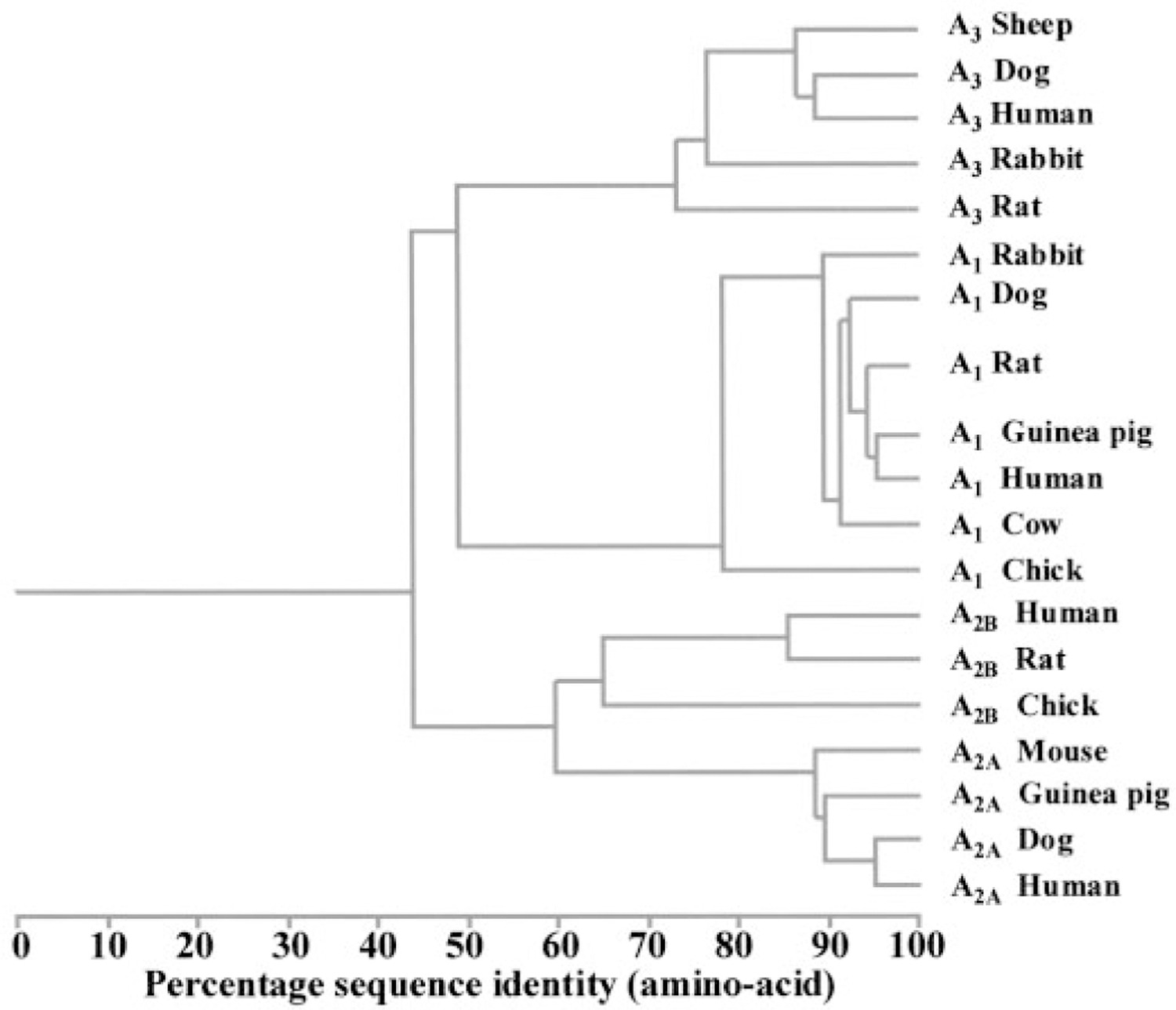
Dendrogram showing sequence similarity between cloned adenosine receptors. The figure is slightly redrawn from that available at http://www.gpcr.org/7tm/seq/007_001/001_007_001.TREE.html. This phylogenetic tree was automatically calculated by WHAT IF based on a neighbor-joining algorithm.
The four adenosine receptor subtypes are asparagine-linked glycoproteins and all but the A2A have sites for palmitoylation near the carboxyl terminus (Linden, 2001). Depalmitoylation of A3 (but not A1) receptors renders them susceptible to phosphorylation by G protein-coupled receptor kinases (GRKs), which in turn results in rapid phosphorylation and desensitization (Palmer and Stiles, 2000).
It has long been known that A1 and A3 receptors couple to Gi/o and that A2A and A2B receptors couple to Gs (see Section IX.). Experiments with chimeric A1/A2A receptors indicate that structural elements in both the third intracellular loop and the carboxyl terminus influence coupling of A1 receptors to Gi, whereas elements in the third intracellular loop but not the carboxyl terminus contribute to A2A receptor coupling to Gs (Tucker et al., 2000). Reconstitution experiments have revealed that the coupling of A1 receptors is influenced by the composition, prenylation state (Yasuda et al., 1996) and phosphorylation state (Yasuda et al., 1998) of G protein γ-subunits. A2A receptors vary in their affinity for Gs proteins containing various types of β-subunits and interact most avidly with G proteins containing β4 (McIntire et al., 2001).
V. Gene Structure
The genomic structure appears to be similar for all the human adenosine receptors. There is a single intron that interrupts the coding sequence in a region corresponding to the second intracellular loop (Ren and Stiles, 1994; Fredholm et al., 2000; Olah and Stiles, 2000). The best studied receptor is the A1 receptor. Already when the structure of the A1 receptor was first reported, the presence of two major transcripts was noted. It was originally thought that they might represent alternative splicing, and more recent data have yielded additional information (Ren and Stiles, 1994, 1995). Transcripts containing three exons, called exons 4, 5, and 6 were found in all tissues expressing the receptor, whereas transcripts containing exons 3, 5, and 6 are in addition found in tissues such as brain, testis, and kidney, which express high levels of the receptor. There are two promoters, a proximal one denoted promoter A, and a distal one denoted promoter B, which are about 600 base pairs apart. Promoter B and exon 1B are part of an intron when promoter A is active (Ren and Stiles, 1995). Both promoters were suggested to have nontraditional TATA boxes.
Reporter assay studies in DDT1 MF-2 cells show that 500 base pairs of promoter A contained essential elements for A1 receptor expression, and mice expressing promoter A driving the β-galactosidase reporter gene confirmed this (Rivkees et al., 1999b). Furthermore, this promoter contained binding sites for GATA and for Nkx2.5, which factors individually drive promoter activity and also act synergistically (Rivkees et al., 1999b). Promoter B has been shown to be activated by, among other things, glucocorticoids (Ren and Stiles, 1999). It is known that glucocorticoids can stimulate the expression of A1 receptors in DDT1 MF-2 cells (Gerwins and Fredholm, 1991) and in brain (Svenningsson and Fredholm, 1997). The exact reason for this is unknown, since neither promoter contains a canonical glucocorticoid response element (Ren and Stiles, 1999), but interactions with e.g., SRE-2 elements and AP-1 sites may be involved. The magnitude of the glucocorticoid effect that could be shown using reporter constructs was much higher when promoter B acted alone than when both promoters were present and active (Ren and Stiles, 1999). In DDT1 MF-2 cells and in brain, promoter A appears important (Rivkees et al., 1999b).
The cloning of much of chromosome 22, where the A2A receptor is located (MacCollin et al., 1994), suggested a two exon structure (Fredholm et al., 2000), which is similar to that reported for the rat A2A receptor (Chu et al., 1996; Peterfreund et al., 1996). By comparing the human sequence data with data from rodents, putative regulatory elements were identified, including AP-1, NF 1 and AP-4 elements (Fredholm et al., 2000). The A2A receptor shows one hybridizing transcript in most tissues examined (Maenhaut et al., 1990; Stehle et al., 1992; Peterfreund et al., 1996). However, examination of RNA isolated from PC12 cells suggested two different start sites (Chu et al., 1996). The expression of A2A receptor can be stimulated by protein kinase C (Peterfreund et al., 1997) and hypoxia (Kobayashi et al., 1998). We do not know the transcription factors involved in either case. It should also be mentioned that the human adenosine A2A receptor is polymorphic. In particular, a (silent) T1083C mutation occurs in various populations, more frequently in caucasians than in Asians (Deckert et al., 1996; Le et al., 1996; Soma et al., 1998).
Analysis of the A2B receptor gene, localized on chromosome 17 in man, reveals a similar overall structure as for the other adenosine receptors. The rat A2B receptor shows two hybridizing transcripts of 1.8 and 2.2 kb, where the latter is the dominant one (Stehle et al., 1992). This could, in analogy with the above, suggest the presence of multiple promoters, but so far this has not been studied to our knowledge.
The mouse A3 receptor appears to have two exons with coding sequences of 354 and 1135 base pairs separated by an intron of about 2.3 kb (Zhao et al., 1999). Several putative transcription factor-binding sites could be detected in the mouse gene (Zhao et al., 1999), but surprisingly, few of these are matched by similar elements in the human gene. This could mean that the truly important sites have not been identified, or else that the expression of the receptor is regulated very differently in the two species. The fact that the distribution of A3 receptors in humans and rodents is very different might indicate the latter. The human A3 receptor shows two transcripts: the most abundant is approximately 2 kb in size, and the much less abundant one is about 5 kb (Atkinson et al., 1997). There are several possible explanations for this, one of which being a similarity with the A1 receptor gene.
VI. Binding Sites As Revealed by Site-Directed Mutagenesis
Adenosine receptors, like the other G protein-coupled receptors (GPCR), are integral membrane proteins. Such macromolecules are not easily amenable to crystallization and, hence, to precise structure elucidation through X-ray diffraction. However, substantial progress has been made over the last decade or so in unraveling the three-dimensional architecture of two related membrane-bound proteins, i.e., bacteriorhodopsin and (mammalian) rhodopsin. The pivotal suggestion that the GPCR family bears structural homology to these two proteins has been an impetus to our current understanding of receptor structure. Bacteriorhodopsin, a proton pump present in the cell wall of Halobacterium halobium, and rhodopsin, itself a G protein-coupled receptor, are of similar size and share many characteristics between themselves and with other mammalian G protein-coupled receptors. They both have the typical seven-transmembrane α-helical architecture and bind retinal, their endogenous ligand, in the cavity formed by the barrel-like arrangement of the seven transmembrane domains. On the other hand there is little sequence (i.e., amino acid) homology between the two proteins and an almost total lack of homology between bacteriorhodopsin and G protein-coupled receptors. Hibert and coworkers were the first to realize and analyze in depth the opportunities and pitfalls of using the atomic coordinates of bacteriorhodopsin, at that time available at low resolution only (Henderson et al., 1990), and later, of rhodopsin, to construct putative receptor models (Hibert et al., 1991; Hoflack et al., 1994). In subsequent years ever greater resolution and accuracy were achieved (Kimura et al., 1997; Unger et al., 1997), eventually resulting in the elucidation of the structures of bacteriorhodopsin (Fig. 2A) and rhodopsin (Fig. 2B) at 1.55 and 2.8 Å, respectively (Luecke et al., 1999; Palczewski et al., 2000).
FIG. 2.
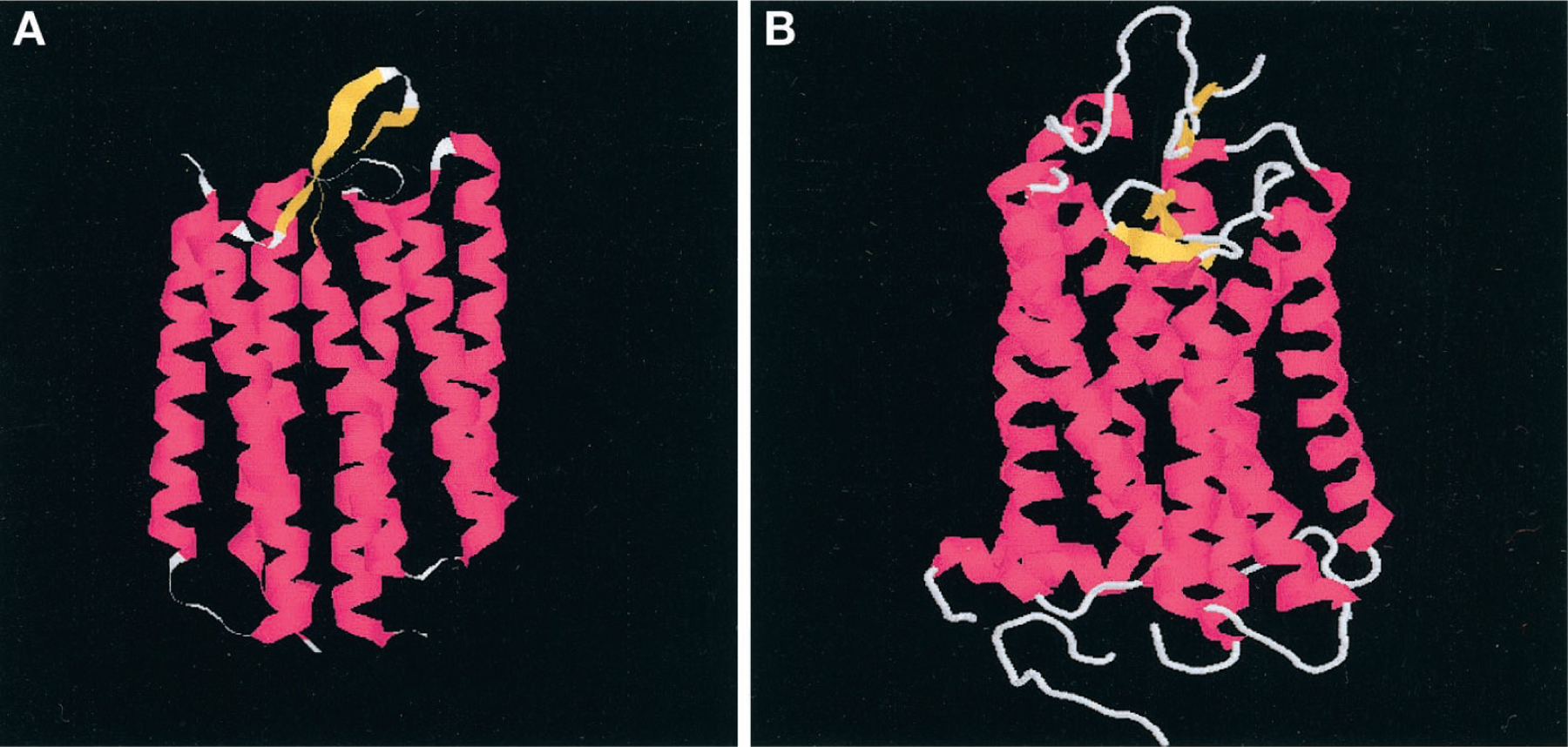
Protein backbone representations of the structures of bacteriorhodopsin (A) and rhodopsin (B) as retrieved from the Brookhaven Protein Data Bank (1C3W and 1F88, respectively).
It is obvious that so-called homology modeling, i.e., the construction of a three-dimensional model of a given protein (e.g., one of the adenosine receptor subtypes) on the basis of an experimentally determined structure of another related protein (e.g., bacteriorhodopsin or rhodopsin) can only generate highly speculative models. This is particularly true when it comes to apparent differences between the macromolecules under study, for instance in their ligand binding sites. Nevertheless, a number of receptor models have been developed on the basis of either bacteriorhodopsin or rhodopsin (at various degrees of resolution). Thus, Baldwin combined structural information on rhodopsin with a sequence analysis of other GPCRs to suggest a probable arrangement (including “borders”) of the seven α-helices (Baldwin et al., 1997). This template structure was used to provide models for all G protein-coupled receptors in an automated fashion, which can be easily retrieved from the internet (for information on G protein-coupled receptors, including these three-dimensional models, see the GPCR Database http://www.gpcr.org/7tm/). Although met with skepticism, such receptor models have been useful in clarifying the putative molecular basis of receptor-ligand recognition, in particular when combined with and adjusted to available pharmacological and structure-activity relationship data.
In the adenosine receptor field, the first receptor model targeted the adenosine A1 receptor (IJzerman et al., 1992) based on the sequence of the canine orphan receptor RDC7, later identified as an A1 receptor (Libert et al., 1989, 1991), and the low resolution structure of bacteriorhodopsin (Henderson et al., 1990). It was found that the pore formed by the seven amphipathic α-helices was characterized by a rather distinct partition between hydrophobic and hydrophilic regions. Chemical modification of histidine residues in the receptor, of which two are present in the transmembrane domains, one in helix VI and one in helix VII, strongly affects ligand binding. This provided the basis for docking the potent and A1-selective agonist N6-cyclopentyladenosine into this cavity. A similar model, again based on the bacteriorhodopsin structure and the two histidine residues, was developed for the rat adenosine A2A receptor (IJzerman et al., 1994).
Later, mutation studies indicated these and other amino acid residues as being important for either agonist or antagonist binding or both (see next four paragraphs). This led to further but similar models for instance for the human A2A receptor, based on the low resolution structure of rhodopsin (Kim et al., 1995). As can be inferred from Fig. 2, A and B, the two rhodopsin proteins are similar but certainly not identical in their transmembrane organization (at the time of the first receptor models, there was no structural information on the extracellular and intracellular domains). Studies of an increasing number of point mutations (often conceived and selected from the receptor models) led to further insight in the ligand binding site, giving rise to some refinement of the existing models. In Fig. 3 a snapshot of the most recently published adenosine A1 receptor model highlights the six residues in helices III and VII probably involved in agonist binding (Rivkees et al., 1999a). With the recent structure elucidation of rhodopsin (Fig. 2B), earlier results that were somewhat anomalous can be rationalized. For instance, Jacobson and coworkers identified glutamate residues in the second extracellular loop as being important for either direct or indirect ligand recognition (Kim et al., 1996). In rhodopsin, part of this domain folds deeply into the center of the macromolecule, the site where ligand binding in both rhodopsin and adenosine receptors is thought to take place. This arrangement causes the second extracellular loop to be in extensive contact with both the extracellular regions and the ligand binding site. The proteolytic analysis of A1 receptors photoaffinity-labeled with a xanthine antagonist indicates that the site of alkylation is in TM3 (Kennedy et al., 1996).
FIG. 3.
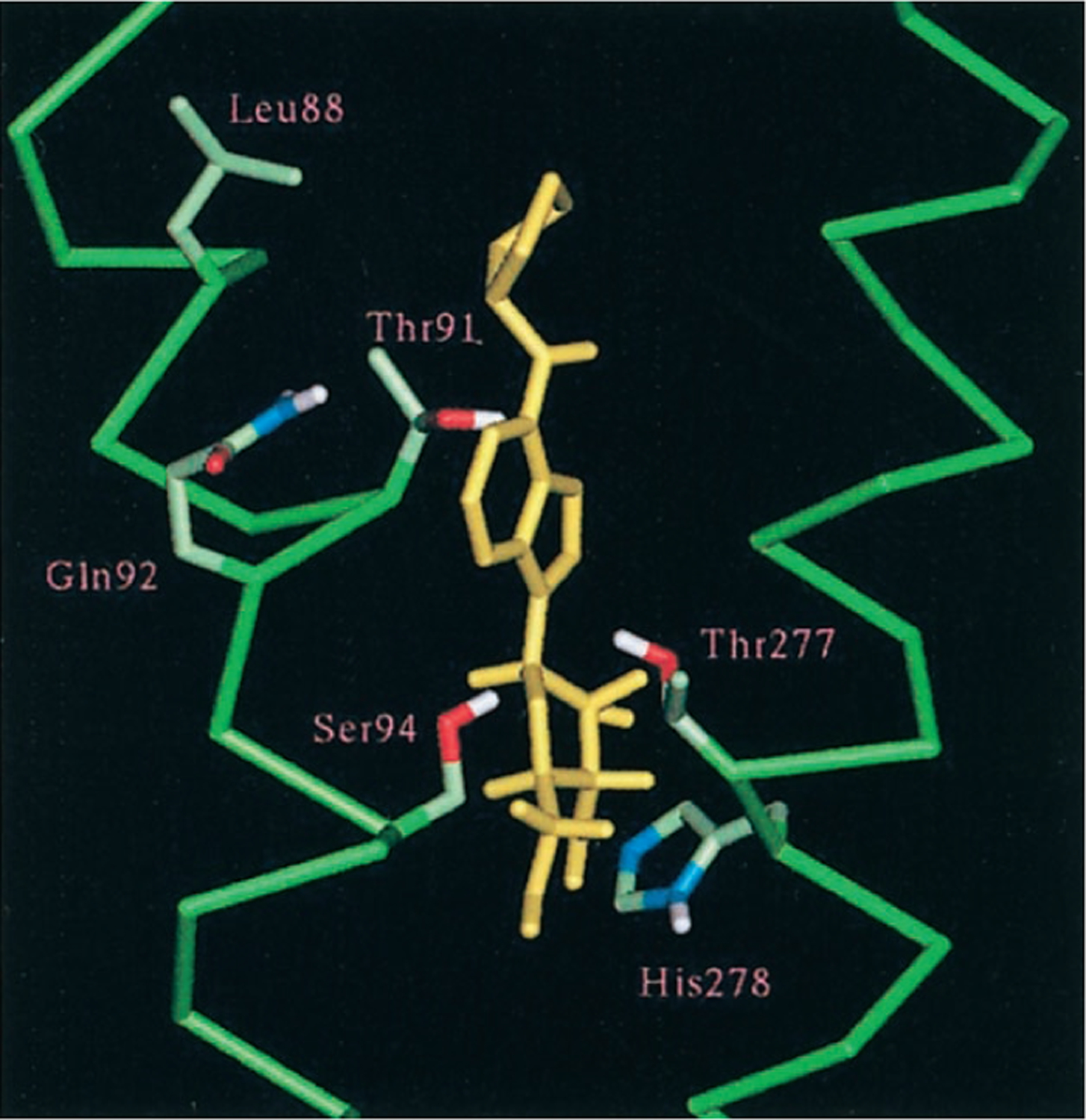
Computer modeling of CPA (yellow), human A1 adenosine receptor interactions. Only helices III (left) and VII (right) are shown (both in green) with relevant residues (half-bond colors).
Extensive mutagenesis, consisting of single amino acid replacement (typically Ala scanning), has been carried out for both A1 and A2A receptors (Tables 2 and 3), and to a lesser extent for A2B receptors (Table 4). The most essential interactions required for recognition of agonist and/or antagonist occur in TMs 3, 5, 6, and 7. Two His residues (6.52 and 7.43) are conserved among most of the adenosine receptor subtypes, with the exception of the A3 receptor, which lacks His at 6.52. These His residues are important for ligand recognition (Olah et al., 1992; Kim et al., 1995; Gao et al., 2000). Mutation of His at 6.52 to Leu in the A1 receptor selectively weakened binding of an antagonist, whereas at 7.43 the same mutation impaired both agonist and antagonist binding (Olah et al., 1992). When either of these residues in the A2A receptor was mutated to Ala, there was a dramatic loss of affinity for both agonist and antagonist (Kim et al., 1995). Some substitutions by amino acids having aromatic and other side chains partially restored function of the binding site. At the A2A receptor, substitution at 7.43 with Tyr selectively reduced agonist affinity (Gao et al., 2000). The hydrophilic residue at 7.42 (Thr in A1 and Ser in A2A receptors; also see Fig. 3) when mutated to Ala caused a significant loss of affinity for agonists while having little effect on antagonist binding (Townsend-Nicholson and Schofield, 1994; Kim et al., 1995). The hydrophobic residue at 7.35 in the A1 receptor (Ile in bovine and Met in canine) appeared to correlate with the species-related differences in agonist pharmacology (Tucker et al., 1994). Mutation of an Asn at 7.36 of the A2B receptor to Tyr, the homologous residue at the other subtypes, selectively enhanced the affinity of 2-substituted agonists (Beukers et al., 2000).
TABLE 2.
Mutational analysis of the adenosine A1 with respect to ligand binding
| Mutationa | Species | Helix/positionb | Effect on Ligand Binding and Signal Transduction | Reference |
|---|---|---|---|---|
| G14T | H | 1.37 | Increased agonist affinity | Rivkees et al., 1999a |
| E16A/Q | H | 1.39 | Agonist affinity reduced 4- to 40-fold; little change antagonist affinity | Barbhaiya et al., 1996 |
| P25L | H | 1.48 | Modest reduction of agonist affinity | Rivkees et al., 1999a |
| I31C | H | 1.54 | No changes in radioligand binding | Rivkees et al., 1999a |
| C46A/S | H | 2.41 | No changes in radioligand binding | Scholl and Wells, 2000 |
| S50A | H | 2.45 | No changes in radioligand binding | Barbhaiya et al., 1996 |
| D55A | H | 2.50 | Increase in agonist affinity with no change in antagonist affinity; disrupted regulation of agonist binding by sodium ions | Barbhaiya et al., 1996 |
| L65F | H | 2.60 | No changes in radioligand binding | Rivkees et al., 1999a |
| C80A/S | H | 3.25 | No detectable radioligand binding | Scholl and Wells, 2000 |
| M82F | H | 3.27 | No changes in radioligand binding | Rivkees et al., 1999a |
| C85A | H | 3.30 | No changes in radioligand binding | Scholl and Wells, 2000 |
| C85S | Agonist affinity reduced 4- to 13-fold; no change in antagonist affinity | Scholl and Wells, 2000 | ||
| P86F | H | 3.31 | Substantial reduction of agonist binding | Rivkees et al., 1999a |
| V87A | H | 3.32 | No change in ligand affinity | Rivkees et al., 1999a |
| L88A | H | 3.33 | Substantial reduction of agonist binding, but also of N-0840 antagonist binding | Rivkees et al., 1999a |
| T91A | H | 3.36 | Substantial reduction of agonist binding, but also of N-0840 antagonist binding | Rivkees et al., 1999a |
| Q92A | H | 3.37 | Substantial reduction of agonist binding, but also of N-0840 antagonist binding | Rivkees et al., 1999a |
| S93A | H | 3.38 | No changes in radioligand binding | Barbhaiya et al., 1996 |
| S94A | H | 3.39 | No detectable agonist or antagonist binding | Barbhaiya et al., 1996 |
| S94T | Minor changes in ligand binding | Barbhaiya et al., 1996 | ||
| A125K | H | 4.43 | No changes in radioligand binding | Rivkees et al., 1999a |
| C131A/S | H | 4.49 | No changes in radioligand binding | Scholl and Wells, 2000 |
| S135A | H | 4.53 | No changes in radioligand binding | Barbhaiya et al., 1996 |
| T141A | H | 4.59 | No changes in radioligand binding | Barbhaiya et al., 1996 |
| F144L | H | 4.62 | No changes in radioligand binding | Rivkees et al., 1999a |
| C169A/S | H | No detectable radioligand binding | Scholl and Wells, 2000 | |
| H251L | B | 6.52 | Antagonist affinity reduced 4-fold; no change in agonist affinity | Olah et al., 1992 |
| C255A/S | H | 6.56 | No changes in radioligand binding | Scholl and Wells, 2000 |
| C260A/S | H | No changes in radioligand binding | Scholl and Wells, 2000 | |
| C263A/S | H | No changes in radioligand binding | Scholl and Wells, 2000 | |
| I270M M270I | B, C | 7.35 | Amino acid in position 270 contributes to canine/bovine A1 AR binding selectivity | Tucker et al., 1994 |
| T277A | H | 7.42 | 400-fold decrease in affinity of NECA with modest changes in affinity for R-PIA and S-PIA (intact cells); substantial decrease in affinity of all agonists (membranes); no change in antagonist affinity | Townsend-Nicholson and Schofield, 1994; Dalpiaz et al., 1998 |
| T277S | Modest decrease in agonist affinity; no change in antagonist affinity; nature of residue in position 277 also involved in canine/bovine A1 AR binding specificity | Tucker et al., 1994; Townsend-Nicholson and Schofield, 1994 | ||
| H278L | B | 7.43 | Negligible agonist and antagonist binding | Olah et al., 1992 |
| C309A/S | H | No changes in radioligand binding | Scholl and Wells, 2000 |
The results were obtained from site-directed mutagenesis studies of the A1 AR (species; H, human; B, bovine; C, canine).
Amino acids are represented in single-letter code with position number shown. The first amino acid is that of the wild-type receptor, with the second residue being that used for substitution.
Position on helix, using notation of van Rhee and Jacobson (1996).
TABLE 3.
Mutational analysis of the human A2A AR with respect to ligand binding
| Mutationa | Helix/Positionb | Effect on Ligand Binding and Signal Transduction | Reference |
|---|---|---|---|
| E13Q | 1.39 | Slight reduction of agonist, but not antagonist, affinity | IJzerman et al., 1996 |
| T88A/S/R | 3.36 | Substantial decrease in agonist, but not antagonist, affinity | Jiang et al., 1996 |
| Q89A | 3.37 | Slight increase in agonist and antagonist affinity | Jiang et al., 1996 |
| Q89N/S/L | Marginal changes in ligand binding | ||
| Q89H/R | Antagonist binding affected | ||
| S90A | 3.38 | Marginal changes in ligand binding | Jiang et al., 1996 |
| S91A | 3.39 | Marginal changes in ligand binding | Jiang et al., 1996 |
| E151A/Q/D | Loss of agonist and antagonist radioligand binding | Jiang et al., 1996 | |
| E169A | ~1000-fold decrease in agonist potency | Kim et al., 1996 | |
| E169A | Gain in N6-substituted agonist affinity | Kim et al., 1996 | |
| D170K | No change in (radio)ligand binding | Kim et al., 1996 | |
| P173R | |||
| F180A | 5.41 | Minor changes in ligand binding | Kim et al., 1995 |
| N181S | 5.42 | Modest reduction of agonist binding | Kim et al., 1995 |
| F182A | 5.43 | Loss of agonist and antagonist radioligand binding | Kim et al., 1995 |
| F182Y | Modest reduction of agonist binding | Kim et al., 1995 | |
| F182W | Modest reduction of agonist binding | Kim et al., 1995 | |
| H250A | 6.52 | Loss of agonist and antagonist radioligand binding; no agonist activity in functional assays | Kim et al., 1995 |
| H250F/Y | Modest reduction of agonist binding; no effect on antagonist binding | Kim et al., 1995 | |
| N253A | 6.55 | Loss of agonist and antagonist radioligand binding | Kim et al., 1995 |
| C254A | 6.56 | Minor changes in ligand binding | Kim et al., 1995 |
| F257A | 6.59 | Loss of agonist and antagonist radioligand binding | Kim et al., 1995 |
| C262G | 6.64 | No change in radioligand binding | Kim et al., 1996 |
| I274A | 7.39 | Loss of agonist and antagonist radioligand binding; ~30-fold decrease in agonist potency | Kim et al., 1995 |
| S277A | 7.42 | Substantial decrease in agonist affinity and potency, but antagonist radioligand binding not altered | Kim et al., 1995 |
| S277T/C/N | Marginal changes in ligand binding | Jiang et al., 1996 | |
| H278A | 7.43 | Loss of agonist and antagonist radioligand binding; ~300-fold decrease in agonist potency | Kim et al., 1995 |
| H278Y | Modest reduction of agonist binding; no effect on antagonist binding | Gao et al., 2000 | |
| S281A | 7.46 | Loss of agonist and antagonist radioligand binding; no agonist activity in functional assays | Kim et al., 1995 |
| S281T | Enhanced affinity for most agonists | Kim et al., 1995 | |
| S281N | Marginal changes in ligand binding | Gao et al., 2000 |
Amino acids are represented in single-letter code with position number shown. The first amino acid is that of the wild-type receptor, with the second and further residues those used for substitution.
Position on helix, using notation of van Rhee and Jacobson (1996).
TABLE 4.
Mutational analysis of the human A2B AR with respect to ligand binding
| Mutationa | Helix/Positionb | Effect on Ligand Binding and Signal Transduction | Reference |
|---|---|---|---|
| V11I | 1.36 | No changes in (radio)ligand binding | Beukers et al., 2000 |
| A12T | 1.37 | No changes in (radio)ligand binding | Beukers et al., 2000 |
| L58V | 2.55 | No changes in (radio)ligand binding | Beukers et al., 2000 |
| F59L | 2.56 | No specific radioligand binding and no cAMP production | Beukers et al., 2000 |
| N273Y | 7.36 | Up to 60-fold increase in affinity of 2-substituted adenosines | Beukers et al., 2000 |
Amino acids are represented in single-letter code with position number shown. The first amino acid is that of the wild-type receptor, and the second is the one used for substitution, based on the corresponding amino acid in the human A2A receptor.
Position on helix, using notation of van Rhee and Jacobson (1996).
TM3 in the A2A receptor contains a sequence of four hydrophilic amino acids, of which the first two (Thr at 3.36 and Gln at 3.37) when mutated had major effects on ligand affinity (Jiang et al., 1996). An enhancement of affinity for N6-substituted adenosine derivatives (140-fold for IB-MECA) in the Gln to Ala mutant appeared to correlate inversely with size of the amino acid side chain. At the A1 receptor, mutation of the identical residues at 3.36 and 3.37 (Fig. 2) to Ala impaired agonist binding and caused a structure-dependent reduction of antagonist affinity (Rivkees et al., 1999a).
Modulatory residues have also been found in TM1, including a Glu residue (1.39) in both A1 and A2A receptors, which affects agonist binding selectively (Barbhaiya et al., 1996; IJzerman et al., 1996). A Gly to Thr mutation in TM1 (1.37) of the A1 receptor increases agonist affinity (Rivkees et al., 1999a). At the A2B receptor, mutation at the same position had no functional consequences (Beukers et al., 2000). A conserved Asp residue in TM2 (2.50) is the site of binding of Na+, which regulates agonist affinity (Barbhaiya et al., 1996). No mutations of TM4 in any of the adenosine receptors have yet been found to affect ligand binding.
Negatively charged residues in the second extracellular loop of the A2A receptor have been found to be required for binding of both agonist and antagonist (Kim et al., 1996). Among nine native Cys residues of the human A1 receptor, only a single pair, Cys80 and Cys169, were found to be essential for ligand binding (Scholl and Wells, 2000). This pair corresponds to Cys residues conserved among rhodopsin-like GPCRs, which form a disulfide bridge required for the structural integrity of the receptor.
VII. Distribution
It is important to study the distribution of receptors, because this will tell us where agonists and antagonists given to the intact organism can act. Furthermore, in general, the higher the number of receptors the more potent and/or efficacious will be the agonist. Thus, the rather low levels of endogenous adenosine present under basal physiological conditions have the potential of activating receptors where they are abundant, but not where they are sparse (Kenakin, 1993, 1995; Svenningsson et al., 1999c; Kull et al., 2000b; Fredholm et al., 2001).
There is much information on the distribution of the A1 and A2A receptors because good pharmacological tools including radioligands (see below) are available. There are also several studies that have used antibodies to localize adenosine A1 receptors in brain (Swanson et al., 1995; Saura et al., 1998; Middlekauff et al., 1998) and A2A receptors in striatum (Rosin et al., 1998; Hettinger et al., 2001), carotid body (Gauda et al., 2000), and T cells (Koshiba et al., 1999). In the case of the A2B and A3 receptors, the data are less impressive. Here one tends to rely on data on the expression of the corresponding mRNA. Some of this information is summarized in Table 5. The results presented there clearly show that there is much left to examine regarding the distribution of especially A2B and A3 receptors. Furthermore, it is likely that a better understanding of the transcriptional regulation (see above) will be of considerable help in understanding the spatio-temporal aspects of adenosine receptor distribution.
TABLE 5.
Summary of the distribution of adenosine receptors
Data where there is correspondence between mRNA distribution and receptor distribution are given in bold. Data based mainly or exclusively on distribution of mRNA are given in ordinary letters. Ambiguous data (including major species differences) are given in italics.
| A1 Receptor | A2A Receptor | A2B Receptor | A3 Receptor |
|---|---|---|---|
|
High expression Brain (cortex, cerebellum, hippocampus). Dorsal horn of spinal cord. Eye, adrenal gland, atria |
High expression Spleen, thymus, leukocytes (both lymphocytes and granulocytes), blood platelets. Striatopallidal GABAergic neurons (in caudate-putamen, nucleus accumbens, tuberculum olfactorium), olfactory bulb |
High expression Cecum, colon, bladder |
High expression Testis (rat), mast cells (rat) |
|
Intermediate levels Other brain regions. Skeletal muscle, liver, kidney, adipose tissue, salivary glands, esophagus, colon, antrum, testis |
Intermediate levels Heart, lung, blood vessels |
Intermediate levels Lung, blood vessels, eye, median eminence, mast cells |
Intermediate levels Cerebellum (human?), hippocampus (human?), lung, spleen (sheep), pineal |
|
Low levels Lung (but probably higher in bronchi), pancreas |
Low levels Other brain regions |
Low levels Adipose tissue, adrenal gland, brain, kidney, liver, ovary, pituitary gland |
Low levels Thyroid, most of brain, adrenal gland, spleen (human), liver, kidney, heart, intestine, testis (human) |
Receptor protein and the corresponding message are often colocalized but there are important differences. For example, in several regions of the central nervous system, receptor binding and expression of transcript do not exactly match (Johansson et al., 1993a), and the two are differently regulated by e.g., long term antagonist treatment (Johansson et al., 1993a) and during development (Ådén et al., 2000, 2001). Much of the differential distribution can probably be explained by the fact that a substantial number of adenosine A1 receptors are present at nerve terminals. A similar explanation probably underlies the observations that A2A receptors are present in globus pallidus, despite the fact that A2A receptor mRNA cannot be detected there (Svenningsson et al., 1997, 1999c). These receptors are probably located at the terminals of the striatopallidal GABAergic neurons (Rosin et al., 1998; Svenningsson et al., 1999b; Linden, 2001).
Besides regulation at the level of gene transcription, targeting of the receptor protein to different locations within the cell is crucial. This important aspect of receptor distribution is just starting to be explored in the case of adenosine receptors. Recently it was found that in MDCK cells (a canine kidney cell line) the adenosine A1 receptor is targeted to the apical surface, whereas the α-adrenoceptor, which is also Gi coupled, is directed to the basolateral surface (Saunders et al., 1996). The distribution is highly dependent on the third intracellular loop and/or the carboxyl-terminal segment of the receptor as judged by the distribution of receptor chimeras (Saunders et al., 1998). Interestingly, the apical distribution of adenosine A1 receptors was disrupted by agents that interfere with microtubules, whereas the basolateral distribution of α adrenergic receptors was not (Saunders and Limbird, 1997). Thus, G protein-coupled receptors appear to use several different targeting mechanisms. This is also borne out by the fact that distribution of these receptors in the kidney epithelial cell line does not predict distribution in neurons (Wozniak and Limbird, 1998).
VIII. Classification of Adenosine Receptors Using Pharmacological Tools
Since adenosine receptors have been studied for a long time there are several useful pharmacological tools (Table 6). The structures of some typical drugs used in receptor classification are shown in Fig. 4. Data on their binding to human and rat receptors are presented in Tables 7 and 8. These tables contain information on some of the most widely used compounds, but a large number of other compounds have also been examined over the years using more or less selective functional assay systems (see Section XI.). Here we will just comment on a few points of more general interest not contained in the tables.
TABLE 6.
Pharmacological tools used for classification of adenosine receptors
| Abbreviations | Chemical Names |
|---|---|
| AB-MECA | 4-aminobenzyl-5’-N-methylcarboxamidoadenosine |
| ATL146e | 4-{3-[6-amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]-prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester |
| BW-A1433 | 1,3-dipropyl-8-(4-acrylate)phenylxanthine |
| CGS 15943 | 5-amino-9-chloro-2-(2-furyl)-[1,2,4]-triazolo[1,5-c]quinazoline |
| CGS 21680 | 2-[p-(2-carbonyl-ethyl)-phenylethylamino]-5’-N-ethylcarboxamidoadenosine |
| CP 66713 | 4-amino-8-chloro-1-phenyl-[1,2,4]-triazolo-[4,3-a] quinoxaline |
| CPA | N6-cyclopentyladenosine |
| CPX | 8-cyclopentyl-1,3-dipropylxanthine (see also DPCPX) |
| CSC | 8-(3-chlorostyryl)caffeine |
| CV-1808 | 2-phenylaminoadenosine |
| DPCPX | 1,3-dipropyl-8-cyclopentylxanthine |
| HENECA | 2-hex-1-ynyl-5’-N-ethylcarboxamidoadenosine |
| I-ABA | N6-(4-amino-3-iodobenzyl)adenosine |
| I-ABOPX | 3-(3-iodo-4-aminobenzyl)-8-(4-oxyacetate)phenyl-1-propylxanthine |
| IB-MECA | N6-(3-iodobenzyl)adenosine-5’-N-methyluronamide |
| KF 17837 | 1,3-dipropyl-8-(3,4-dimethoxystyryl)-7-methylxanthine |
| KW 6002 | (E)-8-[2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-3,7-dihydro-7-methyl-1h-purine-2,6-dione |
| MRE 3008-F20 | 5-[[(4-methoxyphenyl)amino]carbonyl]amino-8-ethyl-2-(2-furyl)-pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine |
| MRS1191 | 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate |
| MRS1220 | 9-chloro-2-(2-furanyl)-5-[(phenylacetyl)amino][1,2,4]-triazolo[1,5-c]quinazoline |
| MRS1523 | 2,3-diethyl-4,5-dipropyl-6-phenylpyridine-3-thiocarboxylate-5-carboxylate |
| MRS1754 | N-(4-cyano-phenyl)-2-[4-(2,6-dioxo-1,3-dipropyl-2,3,4,5,6,7-hexahydro-1H-purin-8-yl)-phenoxy]acetamide |
| NECA | 5’-N-ethyl-carboxamidoadenosine |
| PAPA-APEC | 2-(4-[2-[(4-aminophenyl)methylcarbonyl]ethyl]phenyl)ethylamino-5’-N-ethylcarboxamidoadenosine |
| PD 81723 | (2-amino-4,5-dimethyl-3-thienyl)-[3-(trifluoromethyl)-phenyl]-methanone |
| R-PIA | R-N6-(phenylisopropyl)-adenosine |
| SCH 58261 | 5-amino-2-(2-furyl)-7-phenylethyl-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine |
| S-PIA | S-N6-(phenylisopropyl)-adenosine |
| VUF-8504 | 4-methoxy-N-[2-(2-pyridinyl)quinazolin-4-yl]benzamide |
| XAC | xanthine amine congener; 8-[4-[[[[(2-aminoethyl)amino]-carbonyl]methyl]oxy]phenyl]-1,3-dipropylxanthine |
| ZM241385 | 4-(2-[7-amino-2-[2-furyl]-[1,2,4]triazolo[2,3-a]{1,3,5}triazin-5-yl-amino]ethyl)phenol |
FIG. 4.
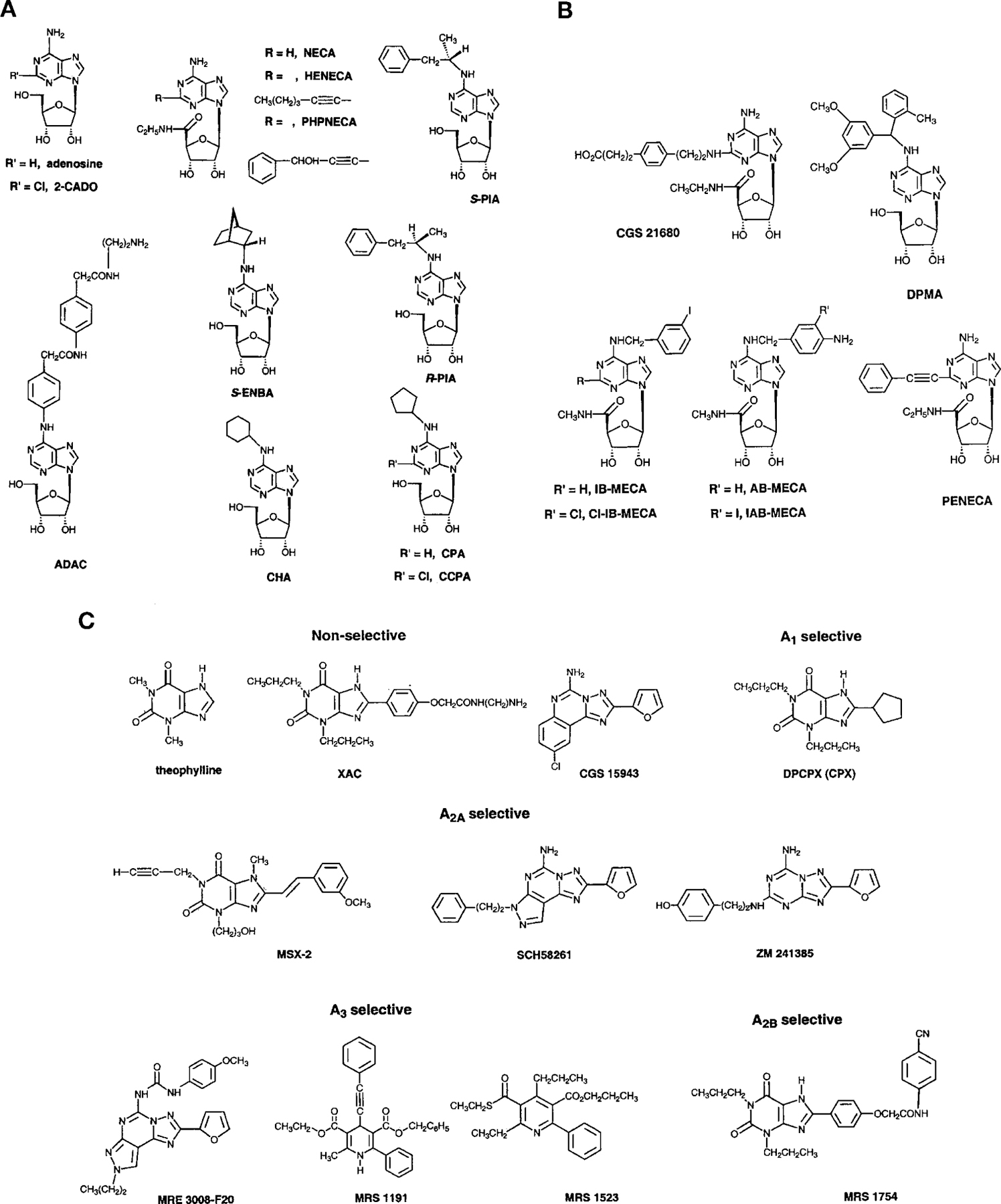
Structures of reference compounds used to classify adenosine receptors. Panel A, structure of some non-selective and A1 receptor-selective adenosine analogs; panel B, structure of adenosine analogs used to classify A2A and A3 receptors; panel C, structure of selected adenosine receptor antagonists.
TABLE 7.
Binding affinity of agonists and antagonists at human adenosine receptor subtypes (Ki values with 95% confidence intervals or ± S.E.M. in parentheses)
| A1 | A2A | A2B | A3 | Reference | |
|---|---|---|---|---|---|
| Agonists with unmodified ribose | |||||
| R-PIA | 2.0 (1–4.2) | 860 (480–1,540) | 16 (9.3–29) | —a | |
| 33,700 (±6,800) | —c | ||||
| 3,800 (±1,700) | —e | ||||
| S-PIA | 75 (42–134) | 7,800 (7,000–8,640) | 45 (33–61) | —a | |
| 62,800 (±21,600) | —c | ||||
| CPA | 2.3 (1.5–3.4) | 790 (470–1,360) | 43 (30–61) | —a | |
| 34,400 (±11,100) | —c | ||||
| 21,000 (±4,300) | —e | ||||
| CCPA | 0.8 (0.55–1.25) | 2,300 (2,000–2,700) | 42 (32–56) | —a | |
| 40,100 (±16,400) | —c | ||||
| 2-Cl-adenosine | 1.39 (1.28–1.51) | 180 (150–220) | 19 (14–28) | —i | |
| Agonists with 5’-modified ribose | |||||
| NECA | 14 (6.4–29) | 20* (12–35) | 6.2* (3.5–11) | —a | |
| 330 (±60) | —c | ||||
| 360 (±120) | —e | ||||
| CGS 21680 | 290 (230–360) | 27 (12–59) | 67 (50–90) | —a | |
| 361,000 (±21,000) | —c | ||||
| HENECA | 60 (50–72) | 6.4 (3.8–11) | 2.4 (2.0–2.9) | —b | |
| PENECA | 560 (480–650) | 620 (300–1,300) | 6.2 (5.1–7.5) | —b | |
| PHPNECA | 2.7 (1.7–7.1) | 3.1 (2.4–3.9) | 0.42 (0.17–1.0) | —b | |
| AB-MECA | 1,500 (1,300–1,800) | 3,600 (2,700–4,700) | 22 (20–24) | —a | |
| IB-MECA | 3.7 (1.8–7.7) | 2,500 (1,900–3,400) | 1.2 (0.96–1.5) | —a | |
| 54,000 (±5,600) | —c | ||||
| IAB-MECA | 8.5 (4.8–15) | 470 (300–740) | 0.64 (0.58–0.70) | —a | |
| Cl-IB-MECA | 115 (114–116) | 2,100 (1,700–2,500) | 11 (9.4–13) | —b | |
| Antagonists: xanthines | |||||
| DPCPX | 3.9* (3.5–4.2) | 129 (35–260) | 4,000 (2,600–6,000) | —a | |
| 50 (±3.7) | —c | ||||
| 51 (±6.1) | —e | ||||
| XAC | 29 (24–35) | 1 (0.58–1.7) | 92 (64–130) | —a | |
| 7.3 (±2.8) | —c | ||||
| 12 (±4.6) | —e | ||||
| MRS1754 | 400 (±190) | 500 (±11) | 2.0 (±0.31) | 570 (±180) | —f |
| MSX-2 | 2,500 (1,700–3,600) | 5.0 (±0.6) | >10,000 | —h | |
| Theophylline | 6,800 (4,100–11,000) | 1,700 (1,000–2,900) | 86,000 (74,000–101,000) | —a | |
| Antagonists: non-xanthines | |||||
| CGS 15943 | 3.5 (1.7–7.3) | 4.2 (2.6–6.6) | 51 (43–60) | —a | |
| 16 (±3.6) | —e | ||||
| SCH 58261 | 290 (210–410) | 0.6 (0.5–0.7) | >10,000 | —d | |
| ZM 241385 | 260 (190–390) | 0.8 (0.7–1.0) | >10,000 | —d | |
| 32 (±6) | —c | ||||
| MRE 3008F20 | 1,100 (±1,100) | 140 (±15) | 2,100 (±300) | 0.29 (±0.03) | —g |
Values are from saturation experiments.
Klotz et al., 1998, transfected CHO cells; radioligands [3H]CCPA (A1), [3H]NECA (A2A and A3).
Klotz et al., 1999, transfected CHO cells; radioligands [3H]CCPA (A1), [3H]NECA (A2A and A3).
Linden et al., 1999, transfected HEK 293 cells; radioligand 125I-ABOPX.
Ongini et al., 1999, transfected CHO cells (A1 and A2A) or HEK 293 cells (A2A and A3); radioligands [3H]DPCPX (A1), [3H]SCH 58261 (A2A), 125I-AB-MECA (A3).
Ji and Jacobson, 1999, transfected HEK 293 cells; radioligand [3H]ZM 241685.
Kim et al., 2000, transfected HEK 293 cells; radioligands 125I-ABA (A1), 125I-ZM 241685 (A2A), 125I-ABOPX (A2B), 125I-AB-MECA (A3).
Varani et al., 2000, transfected CHO cells; radioligands [3H]DPCPX (A1 and A2B), [3H]SCH 58261 (A2A), 125I-AB-MECA (A3).
Sauer et al., 2000, transfected CHO cells; radioligand [3H]CHA (A1), [3H]CGS 21680 (A2A), [3H]NECA (A3).
K.-N. Klotz and S. Kachler, unpublished, transfected CHO cells; radioligand [3H]NECA.
TABLE 8.
Binding affinity of agonists and antagonists at rat adenosine receptor subtypes (Ki values with 95% confidence intervals or ±S.E.M. in parentheses)
| A1 | A2A | A3 | Reference | |
|---|---|---|---|---|
| Agonists with unmodified ribose | ||||
| R-PIA | 1.2 (±0.16) | 124 (±9) | —a | |
| 1.3 (1.1–1.6) | 730 (690–770) | —b | ||
| 0.51 | —c | |||
| 220 (±43) | —d | |||
| 160 (±52) | —e | |||
| S-PIA | 49 (±2.4) | 1,800 (±380) | —a | |
| 29 | —f | |||
| 920 (±310) | —e | |||
| CPA | 0.59 (±0.02) | 460 (±15) | —a | |
| 0.8 (0.6–1.0) | 2,000 (1,400–2,900) | —b | ||
| 240 (±36) | —e | |||
| CCPA | 0.4 (0.2–0.7) | 3,900 (2,500–4,700) | —b | |
| 0.23 | —c | |||
| 240 (±71) | —e | |||
| ADAC | 0.85 (±0.35) | —w | ||
| 2-Cl-adenosine | 9.3 (±0.58) | 63 (±7.5) | —a | |
| 1,900 (±900) | —e | |||
| DPMA | 140 (±23) | 4.4 (±0.2) | —u | |
| 3,600 (±1,700) | —e | |||
| (S)-ENBA | 0.3 (±0.02) | 1,400 (±150) | —v | |
| Agonists with 5_-modified ribose | ||||
| NECA | 6.3 (±0.52) | 10 (±0.5) | —a | |
| 11 (7.0–17) | 22 (20–25) | —f | ||
| 3.7 | —c | |||
| 260 (±36) | —d | |||
| CGS 21680 | 3100 (±470) | 22 (±4.3) | —g | |
| 580 (±32) | —e | |||
| HENECA | 136 (95–195) | 3.8 (1.5–9.9) | —f | |
| PENECA | 698 (611–798) | 120 (112–128) | —h | |
| PHPNECA | 2.5 (2.2–2.9) | 0.9 (0.7–1.3) | —i | |
| AB-MECA | 430 (±45) | 1,600 (±180) | 14 (±3) | —j |
| IB-MECA | 54 (±5) | 56 (±8) | 1.1 (±0.3) | —j |
| IAB-MECA | 18 (±5) | 200 (±84) | 1.3 (±0.18) | —j |
| Cl-IB-MECA | 820 (±570) | 470 (±370) | 0.33 (±0.08) | —k |
| Antagonists: xanthines | ||||
| DPCPX | 0.3 | 340 | —l | |
| 0.18* (0.16–0.20) | —l | |||
| >10,000 | —e | |||
| XAC | 2.8 (1.9–4.2) | —m | ||
| 3.5 | 24 | —l | ||
| 4 | 50 | —n | ||
| >100,000 | —o | |||
| MRS1754 | 17 (±3.6) | 610 (±290) | —o | |
| MSX-2 | 900 (±10) | 8 (±3) | —p | |
| Theophylline | 11,000 | 32,000 | —b | |
| >100,000 | —o | |||
| Antagonists: non-xanthines | ||||
| CGS 15943 | 21 (±3.5) | 3.3 (±1.8) | —q | |
| 22 | 3 | —n | ||
| 6.4 (6.2–6.6) | 1.2 (1.1–1.3) | —r | ||
| >100,000 | —o | |||
| SCH 58261 | 120 (100–140) | 2.3 (2.0–2.7) | —r | |
| ZM 241385 | 2,000 (1,500–2,700) | 0.30 (0.10–0.95) | 150,000 (87,000–210,000) | —s |
| MRE 3008F20 | >10,000 | 2,000 (±220) | >10,000 | —t |
Data from saturation experiments; where confidence limits are missing, they are within 0.15 log units.
Bruns et al., 1986; brain membranes (A1), striatum (A2A); radioligands [3H]CHA (A1), [3H]NECA (A2A).
Lohse et al., 1988; brain membranes (A1), striatum (A2A); radioligands [3H]PIA (A1), [3H]NECA (A2A).
Klotz et al., 1991; brain membranes; radioligand [3H]DPCPX.
Olah et al., 1994; transfected CHO cells; radioligand 125I-AB-MECA.
van Galen et al., 1994; transfected CHO cells; radioligand 125I-APNEA.
Cristalli et al., 1992; brain membranes (A1), striatum (A2A); radioligands [3H]DPCPX (A1), [3H]NECA (A2A).
Hutchison et al., 1989; rat brain; radioligands [3H]CHA (A1), [3H]NECA (A2A). Given are IC50 values; based on the experimental conditions Ki values should be about 0.5– 0.7 and 0.9 times the IC50 values for A1 and A2A, respectively.
Cristalli et al., 1995; brain membranes (A1), striatum (A2A); radioligands [3H]CHA (A1), [3H]CGS 21680 (A2A).
Camaioni et al., 1997; brain membranes (A1), striatum (A2A); radioligands [3H]CHA (A1), [3H]CGS 21680 (A2A).
Gallo-Rodriguez et al., 1994; rat brain (A1), rat striatum (A2A), transfected CHO cells (A3); radioligand [3H]PIA (A1), [3H]CGS 21680 (A2A), 125I-AB-MECA (A3).
Kim et al., 1994; rat brain (A1), rat striatum (A2A), transfected CHO cells (A3); radioligand [3H]PIA (A1), [3H]CGS 21680 (A2A), 125I-AB-MECA (A3).
Lohse et al., 1987; brain membranes (A1), striatum (A2A); radioligands [3H]PIA (A1), [3H]NECA (A2A).
Jacobson et al., 1986; rat brain, radioligand [3H]XAC.
Jarvis et al., 1989; rat brain; radioligands [3H]CHA (A1), [3H]CGS 21680 (A2A).
Kim et al., 2000; transfected HEK 293 cells (A1), rat striatum (A2A); radioligands [3H]PIA (A1), [3H]CGS 21680 (A2A).
Sauer et al., 2000; brain cortex (A1), striatum (A2A); radioligand [3H]CHA (A1), [3H]CGS 21680 (A2A).
Williams et al., 1987; brain (A1), striatum (A2A); radioligands [3H]CHA (A1), [3H]NECA (A2A). Given are IC50 values; based on the experimental conditions Ki values should be about 0.5– 0.7 and 0.9 times the IC50 values for A1 and A2A, respectively.
Baraldi et al., 1994; rat brain (A1), rat striatum (A2A); radioligand [3H]CHA (A1), [3H]CGS 21680 (A2A).
Poucher et al., 1995; brain cortex (A1), PC 12 cells (A2A), transfected CHO cells (A3); radioligands [3H]PIA (A1), [3H]NECA (A2A) 125I-AB-MECA (A3). Given are IC50 values; based on the experimental conditions Ki values should be about 0.1, 0.2, and 0.5 times the IC50 values for A1, A2A, and A3, respectively.
Varani et al., 2000; brain cortex (A1), striatum (A2A), transfected CHO cells (A3); radioligands [3H]DPCPX (A1), [3H]SCH 58261 (A2A), 125I-AB-MECA (A3).
Bridges et al., 1988; brain (A1), striatum (A2A); radioligand [3H]CHA (A1), [3H]NECA (A2A); DPMA or PD 125944 is compound 16 in this paper.
Trivedi et al., 1989; brain (A1), striatum (A2A); radioligand [3H]CHA (A1), [3H]NECA (A2A).
Jacobson et al., 1987; brain (A1), radioligand [3H]PIA (A1).
Ideally, agonists and antagonists should differ in potency by at least two orders of magnitude at different receptors to be really useful in receptor classification. It is apparent from Tables 7 and 8 that this is rarely the case for any of the compounds often used in classifying adenosine receptors. Nevertheless, with a judicious use of agonists and antagonists at A1, A2A, and A3 receptors in in vitro experiments, strong conclusions can be drawn. The situation is less fortunate in vivo as the pharmacokinetics of these compounds have not been studied extensively. It has been found, however, that the A1-selective agonist CPA has a terminal half-life in conscious rats of 6 min in blood (Mathôt et al., 1993), whereas the half-life of the A3-selective agonist Cl-IB-MECA is significantly longer, i.e., 62 min (Van Schaick et al., 1996). The relatively short half-life of CPA may be due to its substantial uptake into erythrocytes (Pavan and IJzerman, 1998).
In the case of human, rat, and mouse A1 receptors the full agonist CCPA (and to a somewhat lesser extent CPA) and the antagonist DPCPX are quite useful. A minor problem with DPCPX is that it also interacts with appreciable affinity with A2B receptors. This does not appear to be a major problem, however, since binding of DPCPX is virtually eliminated in animals lacking the A1 receptor (Johansson et al., 2001). For the adenosine A1 receptor, partial agonists are available as well. Careful chemical manipulation of the CPA molecule yielded such compounds, for instance by substitution at the C8-position (Roelen et al., 1996) or by modification of the ribose 5’-substituent (van der Wenden et al., 1998). These compounds showed tissue selectivity in vivo, exploiting differences in receptor density, and in the efficiency of receptor coupling to further signal transduction (Mathot et al., 1995; van Schaick et al., 1998).
Another interesting class of compounds acting on adenosine A1 receptors are the so-called allosteric enhancers. The prototype here is PD81723 (Bruns and Fergus, 1990), which has been shown by various research groups to (allosterically) increase agonist binding and effect (e.g., Linden, 1997). In recent years, analogs of PD81723 have been synthesized that show similar effects (van der Klein et al., 1999; Baraldi et al., 2000b).
NECA was long considered to be a selective adenosine A2 receptor agonist, but as seen from the tables this view can no longer be upheld. Based on evidence that 2-substitution of NECA increased selectivity, CGS 21680 was developed as an A2A receptor-selective agonist (Hutchison et al., 1989). However, in humans it is less potent and less selective than in rats (Kull et al., 1999). Indeed, there are clear-cut differences in the order of potency of agonists, but not antagonists, between human and rat A2A receptors (Kull et al., 1999). There is an additional problem with CGS 21680 as a tool; it also binds to sites unrelated to A2A receptors (Johansson et al., 1993b; Johansson and Fredholm, 1995; Cunha et al., 1996; Lindström et al., 1996). This means that at least in organs or cells with few A2A receptors, effects of CGS 21680 must be viewed with skepticism. ATL146e was recently developed as a new A2A agonist that is over 50 times more potent than CGS 21680 at the human receptor (Rieger et al., 2001). It has strong in vivo effects on e.g., reperfusion injury in the rabbit lung (Ross et al., 1999) and the rat kidney (Okusa et al., 1999), and reduces expression of adhesion molecules on the reperfused vascular endothelium (Okusa et al., 2000). There are several useful A2A receptor antagonists. The most selective so far is SCH 58261. The structurally related ZM 241385 is more readily available (Poucher et al., 1995), but shows appreciable affinity to A2B receptors (Ongini et al., 1999).
The adenosine A2B receptor has low affinity for most agonists. For the other receptors, agonists with potency in the low nanomolar range are available, but in the case of A2B receptors the most potent agonists have affinities only marginally below 1 µM. Furthermore, selectivity is negligible. The situation is somewhat more favorable in the case of antagonists, where some potent and relatively selective antagonists have been found (Kim et al., 2000).
The most recently discovered adenosine receptor—the A3 receptor—is notably insensitive to several xanthines. Hence, most A3 antagonists have a nonxanthine structure, including dihydropyridines, pyridines, and flavonoids (Baraldi et al., 2000a). Isoquinoline and quinazoline derivatives constitute another class of highly selective human A3 receptor antagonists. VUF8504 (4-methoxy-N-[2-(2-pyridinyl)quinazolin-4-yl]benz-amide) was the first representative of this class, with a Ki value of 17 nM (van Muijlwijk-Koezen et al., 1998). Later, VUF5574 (N-(2-methoxyphenyl)-N-(2-(3-pyridyl)quinazolin-4-yl)urea) proved even more potent, with a Ki value of 4 nM, being at least 2500-fold selective versus A1 and A2A receptors (van Muijlwijk-Koezen et al., 2000). One of the most selective compounds (for human A3 receptors) is MRE-3008-F20, which is also a useful antagonist radioligand at human A3 receptors (Varani et al., 2000).
Species differences in the affinity of adenosine receptor ligands, especially antagonists, have been noted. For example, 8-phenylxanthines such as XAC that are selective for A1 receptors in the rat are less selective in the human, due to a decrease in the A1 affinity and a concomitant rise in the A2A receptor affinity. As expected from the structural data (see above), species differences in pharmacology are most marked for ligands at the A3 receptor. In general, the affinity at A3 receptors of most xanthines and other classes of antagonists is highly species-dependent, and the affinity at human receptors is typically >100-fold greater than that at rat receptors. While MRS 1523 is an A3 antagonist of broad applicability to various species, both MRS 1220 and MRE-3008-F20 are extremely potent in binding to the human but not rat A3 receptors and should be used cautiously in nonprimate species. In the rat, MRS 1220 is selective for the A2A receptor. In contrast, the affinity of the A3 agonist Cl-IB-MECA typically does not vary beyond an order of magnitude between species examined. Nevertheless, one must caution against receptor classification based on pharmacological tools alone, especially in nonmammalian species where the receptors have not been cloned and characterized. Despite the fact that there is much interest in mouse adenosine receptors owing to the development of several mouse strains with targeted deletions, the information on mouse adenosine receptor pharmacology is deficient. In one study potencies of selected agonists and antagonists were virtually identical at mouse and rat A1 receptors (Maemoto et al., 1997).
IX. Signaling
A. G Protein Coupling
The adenosine A1 and A2 receptors were initially subdivided on the basis of their inhibiting and stimulating adenylyl cyclase, respectively (van Calker et al., 1979; Londos et al., 1980a). Indeed, A1 and A2 receptors are coupled to Gi and Gs proteins, respectively (Table 9). The A3 receptor is also Gi coupled. In addition there is some evidence that the adenosine receptors may signal via other G proteins (Tables 1 and 9). However, much of the data on coupling to other G proteins are from transfection experiments and it is not known if such coupling is physiologically important. Recently, evidence was presented that the A2A receptor may be coupled to different G proteins in different areas (Kull et al., 2000a). In most peripheral tissues, the receptor subtype is coupled to Gs. However, in striatum, where the brain A2A receptors are enriched, Gs is very sparse. Here the dominant G protein of the class is instead Golf. Indeed, immunoprecipitation experiments show that A2A receptors and Golf are associated with each other in the medium-sized spiny neurons of striatum.
TABLE 9.
G protein coupling of the four adenosine receptor subtypes
| Adenosine Receptor Subtype | G Protein | Effects of G Protein Coupling | Cellular System | Reference |
|---|---|---|---|---|
| A1 | Gi1/2/3 | ↓ cAMP | General, CHO cellsa | Freissmuth et al., 1991; Akbar et al., 1994; Freund et al., 1994; Jockers et al., 1994; Gerwins and Fredholm, 1995a,b |
| ↑ IP3/DAG (PLC) | ||||
| ↑ Arachidonate (PLA2) | ||||
| ↑ PEtOH (PLD) | DDT1MF-2 | |||
| Go | Jockers et al., 1994; Matovcik et al., 1994 | |||
| A2A | Gs | ↑ cAMP | General | Olah, 1997 |
| Golf | ↑ cAMP | Kull et al., 2000a; Corvol et al., 2001 | ||
| G15/16 | ↑ IP3 | COS-7a | Offermanns and Simon, 1995 | |
| A2B | Gs | ↑ cAMP | General | Pierce et al., 1992 |
| Gq/11 | ↑ IP3/DAG (PLC) | HMC-1, HEK 293a | Gao et al., 1999; Linden et al., 1999b | |
| A3 | Gi2,3 | ↓ cAMP | General, CHO cellsa | Palmer et al., 1995 |
| Gq/11 | ↑ IP3/DAG (PLC) | CHO cellsa | Palmer et al., 1995 |
Receptor transfected cell system.
References propose Gq/11 coupling without direct evidence for Gq/11 activation.
One adenosine receptor may also be coupled to more than one G protein. This is common after transfection. Furthermore, endogenous A2B receptors of HEK 293 cells, human HMC-1 mast cells and canine BR mast cells are dually coupled to Gs and Gq (Auchampach et al., 1997; Linden et al., 1999).
B. Second Messengers and Signals
After activation of the G proteins, enzymes and ion channels are affected as can be predicted from what is known about G protein signaling (see Tables 1 and 9). Thus, A1 receptors mediate inhibition of adenylyl cyclase, activation of several types of K+-channels (probably via β,γ-subunits), inactivation of N-, P-, and Q-type Ca2+ channels, activation of phospholipase Cβ, etc. The same appears to be true for A3 receptors. In CHO cells transfected with the human A3 adenosine receptor both adenylyl cyclase inhibition and a Ca2+ signal are mediated via a Gi/o-dependent pathway (Klotz et al., 2000). Given that many of the steps in the signaling cascade involve signal amplification, it is not surprising that the position of the dose-response curve for agonists will depend on which particular effect is measured. For example, it was recently found that the so called receptor reserve in DDT1 MF-2 cells appears very different depending on whether G protein activation or cAMP accumulation is measured (Baker et al., 2000). Both A2A and A2B receptors stimulate the formation of cAMP, but other actions, including mobilization of intracellular calcium, have also been described. Actions of adenosine A2A receptors on neutrophil leukocytes are due in part to cAMP (Fredholm et al., 1996; Fredholm, 1997; Sullivan et al., 2001), but cAMP-independent effects of A2A receptor activation in these cells have also been suggested (Cronstein, 1994).
C. Adenosine Receptor-Mediated Changes in Cell Proliferation and in Mitogen-Activated Protein Kinase Activation
It was shown more than 15 years ago that adenosine A1 and A2B receptors could regulate proliferation and differentiation in vascular smooth muscle cells (Jonzon et al., 1985). Since then several examples of such effects have been described, and they may be related to changes in mitogen-activated protein kinases (MAPK), which play an essential role in processes such as cell differentiation, survival, proliferation, and death. The family of MAPK consists of the extracellular regulated kinases (ERK) such as ERK1/2, and the stress-activated protein kinases (SAPK), such as p38 and jun-N-terminal kinase (JNK). These kinases, usually activated via receptor tyrosine kinases (Seger and Krebs, 1995), have also been shown to be activated by G protein-coupled receptors.
Adenosine A1 receptors transiently expressed in COS-7 cells can activate ERK1/2 via β,γ-subunits released from pertussis toxin-sensitive G proteins Gi/o (Faure et al., 1994). Studies in CHO cells stably expressing the human A1 receptor later showed that the activation of ERK1/2 by A1 receptors is time- and dose-dependent (Schulte and Fredholm, 2000) and sensitive to the phosphoinositol-3-kinase inhibitors wortmannin and LY 294002 (Dickenson et al., 1998). Although speculative, this may imply a receptor tyrosine kinase transactivation as described for the epidermal growth factor (Daub et al., 1997).
Activation of A2A receptors also increases MAPK activity. Adenosine agonists exerting mitogenic effects on human endothelial cells via the adenosine A2A receptor activate ERK1/2 using the cAMP-ras-MEK1 pathway (Sexl et al., 1997). However, the signaling pathways used by the A2A receptor seem to vary with the cellular background and the signaling machinery that the cell possesses. Thus, the A2A receptor-mediated ERK1/2 activation in CHO cells is dependent on Gs-cAMP-PKA-rap1-p68 B-raf-MEK1. On the other hand, the A2A receptor-mediated activation in HEK 293 cells involves PKC, ras, and sos, but not Gs, cAMP, or PKA, even though cAMP levels do rise in a Gs-dependent manner (Seidel et al., 1999).
A2A receptor activation may not only stimulate, but also inhibit ERK phosphorylation. Activation of guinea pig A2A receptors expressed in CHO cells inhibited thrombin-induced ERK1/2 activation (Hirano et al., 1996). This inhibition was cAMP- and wortmannin-sensitive, implying that the nonselective adenosine analog NECA affects the two distinct pathways leading from the thrombin receptor to MAPK. In PC12 cells, activation of endogenously expressed A2A receptors inhibits nerve growth factor (NGF)-induced ERK1/2 phosphorylation (Arslan et al., 1997), even though activation of these receptors alone (i.e., in the absence of NGF) can lead to an activation (Arslan and Fredholm, 2000).
The adenosine A2B receptor is the only subtype that so far has been shown to activate not only ERK1/2 but also JNK and p38. In human mast cells (HMC), adenosine receptor activation leads to a time- and dose-dependent activation of ERK1/2 with a maximal degree of phosphorylation at 5 min, whereas p38 and JNK show a different kinetic profile with maximal phosphorylation at 1 and 10 to 15 min, respectively (Feoktistov et al., 1999). Adenosine A2B receptor-mediated activation of MAPK is relevant for IL-8 secretion and consequently for mast cell activation. In untransfected HEK 293 cells, a cascade depending on Gq/11, PLC, genistein-insensitive tyrosine kinases, ras, B-raf, and MEK1/2 has been delineated (Gao et al., 1999). NECA concentrations used in these studies of endogenous HEK 293 A2B receptors revealed the same potency in activating ERK and adenylyl cyclase (EC50 values in the micromolar range) whereas results from another study in transfected cells (Schulte and Fredholm, 2000) show a nearly 100-fold higher potency of both NECA and adenosine in inducing ERK1/2 phosphorylation than in inducing cAMP production. The EC50 value for ERK1/2 phosphorylation in transfected CHO lies in the nanomolar range, whereas cAMP production is half-maximally activated around 1 to 5 µM NECA. Thus, a G protein-coupled receptor can have substantially different potencies on different signaling pathways in the same cellular system.
It was recently reported that, in addition to binding adenosine and adenosine analogs, the A2B receptor in complex with another protein, DCC (deleted in colorectal cancer), may bind netrin-1, a protein that is involved in controlling axon elongation, and that netrin effects depend on the presence of the A2B receptor (Corset et al., 2000). These results have, however, recently been contested (Stein et al., 2001). Nevertheless, it seems possible that the A2B receptors play very important roles in cell proliferation and/or differentiation. These effects may not only be stimulatory, however. In vascular smooth muscle cells, activation of A2B receptors strongly decreases the mitogenic effects of different growth factors (Jonzon et al., 1985; Dubey et al., 1996, 1998), probably secondary to a blockade of MAP kinases (Dubey et al., 2000) stimulated by these growth factors.
The adenosine A3 receptor has been suggested to activate ERK1/2 in human fetal astrocytes (Neary et al., 1998). A recent study, indeed, shows a clear activation of ERK1/2 via the human A3 receptor expressed in CHO cells (Schulte and Fredholm, 2000). Both NECA and the endogenous agonist adenosine lead to a time- and dose-dependent increase in ERK1/2 phosphorylation already at concentrations as low as 10 to 30 nM. The A3 receptor agonists Cl-IB-MECA and IB-MECA have been reported to potently inhibit and less potently to activate apoptosis in various cells (Abbracchio et al., 1997). In RBL-2H3 mast-like cells, Cl-IB-MECA potently blocks UV irradiation-induced apoptosis by a process that correlates with protein kinase B phosphorylation and is blocked by pertussis toxin and wortmannin (Gao et al., 2001).
Thus, the adenosine receptor-mediated activation of MAPK is similar to that encountered in the remainder of the field of GPCR-mediated MAPK activation (Gutkind, 1998; Sugden and Clerk, 1998; Luttrell et al., 1999). The common feature of all adenosine receptors, however, is the positive coupling to ERK1/2 even though the classical cAMP/PKA pathway is both activated (A2) and inhibited (A1/3). Depending on the cellular background the required signaling elements vary widely, although activation of one of the small GTP-binding proteins p21ras and rap1 is essential.
D. Interactions with Other Receptor Systems
Adenosine is believed to play modulatory roles in a variety of tissues and physiological circumstances. Adenosine is, as discussed above, not primarily released in a transmitter- or hormone-like fashion, but instead it appears to be formed by groups of cells as part of a response, e.g., to challenges in energy metabolism. It can also be formed by breakdown of ATP released by cells either in a regulated fashion or in response to massive trauma. Adenosine is therefore likely to act in concert with several other messengers (transmitters, hormones, growth factors, autacoids). It is outside the scope of this type of review to delineate all such interactions and a few examples will have to suffice.
Adenosine can, as noted above, activate phospholipase C via adenosine A1 receptors and a Gi-dependent mechanism. Interestingly, adenosine acts synergistically with nucleotides, such as ATP or UTP (Gerwins and Fredholm, 1992a), histamine (Dickenson and Hill, 1993), or with bradykinin (Gerwins and Fredholm, 1992b). ATP, histamine, and bradykinin instead act via Gq/11. The interaction was believed to involve β,γ-subunits released from Gi proteins by A1 receptors, and αq/11-subunits derived via the other receptor, somehow acting synergistically at the level of PLC. The involvement of β,γ-subunits has been confirmed (Dickenson and Hill, 1998). More recently, good evidence was provided that the mechanism involved exchange of β,γ-subunits between the two G protein pathways (Quitterer and Lohse, 1999), but other mechanisms cannot be excluded. It was also recently shown that activation of PLC by Gi-coupled receptors requires that the enzyme is already activated (Chan et al., 2000), e.g., by ATP. Given that ATP is rapidly broken down to adenosine and that ATP and adenosine act synergistically, we may be dealing with a biologically quite important mechanism.
In the brain there is evidence of important interactions between adenosine A1, dopamine D1, and NMDA receptors. First, it is known that activation of NMDA receptors can increase the release of adenosine (Hoehn et al., 1990; Pedata et al., 1991; Chen et al., 1992; Manzoni et al., 1994; Delaney et al., 1998; Delaney and Geiger, 1998). Second, it is well known that adenosine, by activating presynaptic receptors, can decrease the release of glutamate, and thereby the activation of NMDA receptors (Fredholm and Hedqvist, 1980; Fredholm and Dunwiddie, 1988; Poli et al., 1991; Dunwiddie and Fredholm, 1997). In addition, activation of adenosine A1 receptors reduces the NMDA currents by a postsynaptic action (de Mendonca and Ribeiro, 1993; de Mendonca et al., 1995) and reduces toxicity (Finn et al., 1991). A third interaction is that dopamine D1 and NMDA receptors act synergistically in the brain, e.g., on the expression of immediate early genes (Berretta et al., 1992) and several long-term effects of dopamine D1 activation can be blocked by antagonizing NMDA receptors (Wolf et al., 1994; Konradi et al., 1996). However, this synergistic interaction differs between regions (Keefe and Gerfen, 1996). Part of this enhancement may depend on the ability of dopamine D1 agonists to phosphorylate DARPP-32, which in turn acts to decrease the dephosphorylation of NMDA receptors, leading to their remaining in an active conformation (Blank et al., 1997). Activation of NMDA receptors conversely leads to decreased DARPP-32 phosphorylation (Halpain et al., 1990). A fourth interaction is between dopamine D1 receptors and adenosine A1 receptors, which have been shown to act antagonistically (Ferré et al., 1994b, 1998; Popoli et al., 1996). All these interactions may be functionally connected (Harvey and Lacey, 1997). Thus, dopamine D1 activation has been shown to reduce release of glutamate by enhancing NMDA activity and thereby release of adenosine, which is the agent that acts at presynaptic A1 receptors.
Adenosine A2A receptors are highly enriched in the basal ganglia of several species including human. There they are present in highest abundance on a subset of the GABAergic output neurons, namely those that project to the globus pallidus (Schiffmann et al., 1991; Fink et al., 1992; Svenningsson et al., 1997; Rosin et al., 1998). These neurons also express dopamine D2 receptors and it is abundantly clear that the two receptors interact in binding assays (Ferré et al., 1991), on signal transduction (Kull et al., 1999), and behaviorally (Fink et al., 1992; Fredholm et al., 1999; Svenningsson et al., 1999b). It appears that one major role of dopamine in the striatum is to suppress signaling via A2A receptors (Svenningsson et al., 1998b, 1999a; Chen et al., 2001). Given that antagonists of D2 receptors are used in the treatment of schizophrenia, there are interesting implications for the pathophysiology of that disease. Adenosine A2A and dopamine D2 receptors also are colocalized on arterial chemoreceptors of the carotid body (Gauda et al., 2000). Carotid sinus nerve activity is augmented by adenosine binding to adenosine A2A receptors and attenuated by dopamine binding to dopamine D2 receptors.
Adenosine A2B receptors are present on many cells, although in low abundance. It has been repeatedly found that signaling via the A2B receptor is strongly influenced by concurrent signaling via other receptors that affect PLC-Ca2+-PKC. However, the type of interaction varies from cell to cell. In brain slices the cAMP accumulation mediated by activation of A2B receptors is markedly enhanced by drugs that stimulate PKC (Hollingsworth et al., 1985; Fredholm et al., 1987b; Nordstedt and Fredholm, 1987). Since cAMP accumulation in brain slices predominantly reflects events in glial cells, it seems likely that this is the target cell for the interaction. In glial cells the positive effect of PKC activation can, however, be counteracted by an inhibitory effect of strong increases in intracellular calcium (Altiok et al., 1992; Altiok and Fredholm, 1992, 1993; Balmforth et al., 1992). A similar effect is seen in lymphocytes (Fredholm et al., 1987a; Nordstedt et al., 1987, 1989; Kvanta et al., 1989). In these cells it could be shown that the stimulatory effect of PKC activation predominantly occurs not at the receptor itself but at a signaling step after the receptor.
Numerous other examples of interaction between adenosine receptors and other receptors have been reported, and such interactions are likely to be the rule rather than the exception. This has important consequences. For example, if two receptors act synergistically then blockade of either receptor will virtually abolish the response. This can easily be erroneously interpreted as evidence that the receptor that is blocked is solely responsible for the response studied. Another important case should also be mentioned. Sometimes more than one adenosine receptor is expressed on a single cell. The resultant response will therefore be a mixed one and consequently pharmacology may be quite atypical.
X. Receptor Regulation
Exposure of any GPCR to agonists for shorter or longer times generally leads to the attenuation of the agonist response (for recent reviews, see Krupnick and Benovic, 1998; Lefkowitz, 1998; Pitcher et al., 1998; Ferguson, 2001). Adenosine receptors are no exception (Olah and Stiles, 2000). However, the magnitude of this response and the mechanisms involved seem to vary between the adenosine receptor subtypes. For example, there are major differences between the two Gi/o coupled receptors, A1 and A3, when stably transfected into CHO cells (Palmer et al., 1996). Whereas the A1 receptor appears to desensitize slowly and incompletely, the A3 receptor desensitizes very rapidly. This difference may be due to differences in the ability of G protein receptor kinases to phosphorylate the two receptors. However, there may be additional factors to consider. Thus in DDT1 MF-2 cells, which constitutively express A1 receptors, desensitization is slow when cyclase responses are studied (Ramkumar et al., 1991), but have been reported to be rapid when, instead, phospholipase C activation is examined (Ciruela et al., 1997). In this case, phosphorylation mediated via other kinases might be involved. The receptor has also been reported to internalize within half an hour of agonist stimulation together with adenosine deaminase (Saura et al., 1996). However, many questions remain to be solved in the case of receptor regulation in DDT1 MF-2 cells, and not all reports are consistent (Olah and Stiles, 2000).
Recently, it was found that agonist treatment can rapidly translocate adenosine A1 receptors out of caveolae, providing another type of explanation for altered receptor signaling (Lasley et al., 2000). In addition, using still other types of cells or examining the situation in vivo, several groups have described a more or less rapid decrease in receptor number following agonist administration (Green, 1987; Longabaugh et al., 1989; Green et al., 1990; Fernandez et al., 1996; Hettinger et al., 1998). These changes are not accompanied by changes in the mRNA. Furthermore, antagonist treatment has long been known to up-regulate adenosine A1 receptors (Fredholm, 1982), even though other adenosine receptors are unaffected. Thus, we still do not understand the factors that are important in regulating adenosine A1 and A3 receptors under physiological and pathophysiological conditions.
Relatively little is known about desensitization of A2B receptors, except that desensitization does occur in different cell lines (Mundell and Kelly, 1998; Peters et al., 1998; Haynes et al., 1999). The mechanism may involve G protein receptor kinases. A2A receptors coupled to Gs proteins are believed to desensitize rapidly (Ramkumar et al., 1991; Chern et al., 1993, 1995; Palmer and Stiles, 1997). Several mechanisms, including phosphorylation of Thr298 via receptor kinases, and phosphorylation via cAMP-dependent kinases have been implicated. Nevertheless, there is good evidence that under in vivo conditions A2A receptors on leukocytes can be tonically activated by endogenous adenosine (Cronstein, 1994; Fredholm, 1997). Similarly, there is excellent evidence that A2A receptors on striatopallidal neurons are tonically activated by endogenous adenosine (Svenningsson et al., 1995, 1999a, 2000). Conversely, when tonic adenosine A2A receptor activation is unopposed by dopamine for a long period of time, the response to agonist stimulation decreases (Zahniser et al., 2000), even though significant effects remain (Chen et al., 2001). This is not due to any change in the receptor number, however. Thus, desensitization of adenosine A2A receptors, via whatever mechanism, may be important mainly under experimental or pathological situations.
XI. Assay Systems
Adenosine receptors and their ligands may be investigated in binding and functional assays. The first useful adenosine analogs R-PIA and NECA were developed using assays in intact animals. Specifically, blood pressure, heart rate, and body temperature were some of the readouts (Vapaatalo et al., 1975; Raberger, 1979). Studies using changes in cAMP in fat cells, liver, Leydig cells, brain homogenates, and cultured brain cells were instrumental in the definition of A1 (Ri) and A2 (Rs) receptors (van Calker et al., 1978, 1979; Londos et al., 1980b). Binding assays became available in the early 1980s, but functional assays continued to play an important role. For example, studies on coronary blood flow in dogs were used to screen and develop a large number of compounds acting at A2A receptors (Daly et al., 1986). Even today several functional assays remain very useful, not least because they examine receptors associated with the natural signaling pathways. For example, A1 receptor-mediated inhibition of adenylyl cyclase can be studied in fat cells (Fredholm, 1978; Londos et al., 1978; Longabaugh et al., 1989) whereas platelet membranes is a preferred system for stimulation of adenylyl cyclase via A2A adenosine receptors (Klotz et al., 1985). A2B receptors have been examined using fibroblasts (Bruns, 1980, 1981) and rat aorta (Collis and Brown, 1983; Poucher et al., 1995).
For a long time rat brain membranes were the standard system for examining binding to A1 (whole brain; Bruns et al., 1980; Schwabe and Trost, 1980) and A2A receptors (striatum; Bruns et al., 1986). Now cell lines stably transfected with the receptor subtype of interest have become the standard systems for the characterization of ligands, as they have several major advantages (Klotz et al., 1998). Transfected cells allow for the comparative characterization of receptor subtypes from different species including humans. With the expression of single receptor subtypes, cross-reaction of nonselective ligands with receptors not under investigation does not occur. In addition, receptors like the A3 subtype that occur naturally only at low expression levels become available for in vitro studies. Cellular models with transfected receptors may also be used for the characterization of effector coupling. All the typical second messenger responses for the adenosine receptor subtypes have been identified in CHO cells stably transfected with the human subtypes. However, these results may not represent the physiological situation because the receptor density and the availability of G proteins and other components necessary for signal generation may be different in artificial cellular models.
XII. Physiological Roles—Therapeutic Potential
Recently, genetically modified mice have been generated that provide insights into the physiology and pathophysiology of the different adenosine receptors. The adenosine A2A receptor was first knocked out. The group in Brussels that first cloned the adenosine receptors also generated the first knock-out mice, in which the first coding exon of the A2A receptor was targeted (Ledent et al., 1997). Later a group in Boston targeted the second exon (Chen et al., 1999). Using these mice has shown that A2A receptors play a role in mediating pain via peripheral sites, inhibiting platelet aggregation and regulating blood pressure (Ledent et al., 1997). A2A receptors are also critically important for the motor stimulant effects of caffeine (Ledent et al., 1997; El Yacoubi et al., 2000). It has also been demonstrated that A2A receptors contribute to ischemic brain damage in adult mice (Chen et al., 1999).
Mice with a targeted disruption of the A3 receptor show a decreased effect of adenosine analogs on mast cell degranulation (Salvatore et al., 2000) and a consequent decrease in vascular permeability (Tilley et al., 2000). These animals also, surprisingly, show increased cardiovascular effects of administered adenosine (Zhao et al., 2000). Given the species differences in A3 receptor distribution and pharmacology it is, however, not clear that the roles of this receptor are similar in humans.
Mice with a targeted disruption of A1 receptors have also been generated (Johansson et al., 2001). These mice show increased anxiety and are hyperalgesic, indicating a role for A1 receptors in mediating endogenous antinociception. In these animals, the effect of adenosine on excitatory neurotransmission is totally eliminated (Dunwiddie et al., 2000), and the neuronal response to hypoxia is markedly altered. They also lack tubuloglomerular feedback and have elevated renin levels (Brown et al., 2001; Sun et al., 2001). Interestingly, all these mice show essentially normal viability and fertility.
Adenosine protects tissues from ischemic damage in e.g., brain and heart through multiple receptor subtypes (Lasley et al., 1990; Marangos, 1990; Rudolphi et al., 1992a,b; Auchampach and Gross, 1993; Deckert and Gleiter, 1994; Fredholm, 1996). A1 and possibly A3 receptor activation produces preconditioning to protect the heart and other tissues from subsequent ischemic injury. Rapid preconditioning is mediated by a pathway including PKC and increased mitochondrial K-ATP channel activation (Sato et al., 2000). A late phase of preconditioning due to A1 receptor activation in the rabbit heart is mediated in part by the induction of manganese superoxide dismutase (Dana et al., 2000).
Fishman and coworkers demonstrated that low doses of adenosine cause a strong inhibition of lymphoma cell proliferation. A similar effect was seen with low doses of IB-MECA, a somewhat selective agonist for A3 receptors, whereas no such action was observed with the selective A1 receptor agonist CCPA (Fishman et al., 2000b). The same research group showed that adenosine also acts as a chemoprotective agent by stimulating granulocyte-colony-stimulating factor production. In this case, a combined action via both A1 and A3 receptors appeared causal (Fishman et al., 2000a).
Activation of A2A receptors protects tissues from injury by reducing inflammation during reperfusion following ischemia. CGS 21680 inhibits neutrophil accumulation and protects the heart from reperfusion injury (Jordan et al., 1997). However, similar cardiac protection has recently been attributed to A3 receptor activation (Jordan et al., 1999).
The localization of A2A receptors to the dopamine rich areas of the brain and the behavioral effects of methylxanthines suggested that antagonists at A2A receptors might be useful as adjuvants to dopaminergic drugs in Parkinson’s disease (Fredholm et al., 1976; Ongini and Fredholm, 1996; Ferré et al., 1997; Svenningsson et al., 1999b; Impagnatiello et al., 2000) as well as schizophrenia (Ferré et al., 1994a; Rimondini et al., 1997). This idea has gained additional support by the demonstration that the adenosine A2A receptor exerts effects that are at least to some extent independent of dopamine acting at D2 receptors (Svenningsson et al., 1995, 1998a, 1999a, 2000; Chen et al., 2001). The effect of an A2A receptor antagonist is synergistic with dopamine receptor agonists in primate models of Parkinson’s disease (Kanda et al., 2000). Rapid tolerance develops to the motor stimulant effects of caffeine, but no such tolerance is seen following long-term treatment with selective adenosine A2A receptor antagonists (Halldner et al., 2000; Popoli et al., 2000; Pinna et al., 2001).
There is evidence that IgE antibodies and mast cells play a central role in the symptoms and pathology of asthma. Aerosolized adenosine has the effect of causing mast cell-dependent bronchoconstriction in asthmatic subjects, but causes bronchodilation in nonasthmatics (Cushley et al., 1983; Vilsvik et al., 1990). Moreover, the nonselective adenosine receptor antagonist theophylline is widely used as an antiasthmatic drug although its mechanism of action is uncertain. A related xanthine, enprofylline (3-propylxanthine) (Robeva et al., 1996; Jacobson et al., 1999), is also therapeutically efficacious in the treatment of asthma, and was thought to act through a nonadenosine receptor-mediated mechanism due to its low affinity at A1 and A2A receptors.
Recently, attention has shifted to the A2B and A3 receptor subtypes found on mast cells that, when activated, facilitate antigen-mediated mast cell degranulation. The adenosine A3 receptor was initially implicated as the receptor subtype that triggers the degranulation of rat RBL 2H3 mast-like cells (Ramkumar et al., 1993) and perivascular mast cells of the hamster cheek pouch (Jin et al., 1997). There is also evidence of mast cell degranulation when agonists of A3 receptors are administered to rats or mice (Fozard et al., 1996; Tilley et al., 2000). In contrast, the A2B receptor has been implicated as the receptor subtype that facilitates the release of allergic mediators from canine and human HMC-1 mastocytoma cells (Feoktistov and Biaggioni, 1995; Auchampach et al., 1997). A role for A2B receptors in human asthma is also suggested by the efficacy of enprofylline, which at therapeutic concentrations of 20 to 50 µM, only blocks the A2B receptor subtype (Fredholm and Persson, 1982; Linden et al., 1999). In sum, the literature indicates that the release of allergic mediators from mast cells is mediated by A3 receptors and/or A2B receptors. This may result from tissue difference and/or species differences, with rodent (rat, guinea pig, and mouse) responding primarily to A3, and canine or human mast cells mainly to A2B adenosine receptor stimulation.
It is remarkable that despite intensive efforts, relatively few adenosine receptor ligands have made it into clinical trials. Adenosine itself is being marketed in several countries, for two purposes. Adenocard (i.v.) restores normal heart rhythm in patients with abnormally rapid heartbeats originating in the upper chambers of the heart, so-called paroxysmal supraventricular tachycardia. Adenoscan (i.v.) is indicated as an adjunct to thallium cardiac imaging in the evaluation of coronary artery disease in patients unable to exercise adequately. In the 1970s, metabolically stable adenosine receptor agonists were tested clinically as antihypertensives. In the last decade GR79236 (N-[(1S, trans)-2-hydroxycyclopentyl]adenosine) has been tested in human volunteers for various indications such as adjuvant therapy in insulin resistance (type II diabetes) and, more recently, in primary headache (P. P. A. Humphrey, personal communication).
With respect to antagonists, it can be argued that caffeine, the most widely used psychotropic substance, has its main action via adenosine receptors. The same might hold true for theophylline and enprofylline, both used in the treatment of asthma. Currently, selective adenosine A1 receptor antagonists are undergoing clinical trials. A recently concluded Phase II trial with BG9719 ((S)-1,3-dipropyl-8-[2-(5,6-epoxynorbornyl)] xanthine) was successful in treating acute renal failure in patients with congestive heart failure. This was seen as a critical factor for future development of a second-generation product, BG 9928. The A2A adenosine antagonist KW6002 (1,3-diethyl-8-(3,4-dimethoxystyryl)-7-ethylxanthine) is undergoing clinical trials as an anti-Parkinson agent (F. Suzuki, personal communication). Other compounds of this class are also under development. Thus, adenosine receptors remain an attractive target for drug development.
Contributor Information
BERTIL B. FREDHOLM, Department of Physiology and Pharmacology, Section of Molecular Neuropharmacology, Karolinska Institutet, Stockholm, Sweden.
ADRIAAN P. IJZERMAN, Leiden/Amsterdam Center for Drug Research, Gorlaeus Laboratories, Leiden, The Netherlands
KENNETH A. JACOBSON, Molecular Recognition Section, National Institutes of Health, Bethesda, Maryland
KARL-NORBERT KLOTZ, Institut für Pharmakologie und Toxikologie, Universität Würzburg, Würzburg, Germany.
JOEL LINDEN, Department of Physiology, Health Sciences Center, University of Virginia, Charlottesville, Virginia.
REFERENCES
- Abbracchio MP, Ceruti S, Brambilla R, Franceschi C, Malorni W, Jacobson KA, von Lubitz DK, and Cattabeni F (1997) Modulation of apoptosis by adenosine in the central nervous system: a possible role for the A3 receptor. Pathophysiological significance and therapeutic implications for neurodegenerative disorders. Ann NY Acad Sci 825:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ådén U, Leverin A-L, Hagberg H, and Fredholm BB (2001) Adenosine A1 receptor agonism in the immature rat brain and heart. Eur J Pharmacol 426:185–192. [DOI] [PubMed] [Google Scholar]
- Ådén U, Herlenius E, Tang L-Q, and Fredholm BB (2000) Maternal caffeine intake has minor effects on adenosine receptor ontogeny in the rat brain. Pediat Res 48:177–183. [DOI] [PubMed] [Google Scholar]
- Akbar M, Okajima F, Tomura H, Shimegi S, and Kondo Y (1994) A single species of A1 adenosine receptor expressed in Chinese hamster ovary cells not only inhibits cAMP accumulation but also stimulates phospholipase C and arachidonate release. Mol Pharmacol 45:1036–1042. [PubMed] [Google Scholar]
- Altiok N, Balmforth AJ, and Fredholm BB (1992) Adenosine receptor-induced cAMP changes in D384 astrocytoma cells and the effect of bradykinin thereon. Acta Physiol Scand 144:55–63. [DOI] [PubMed] [Google Scholar]
- Altiok N and Fredholm BB (1992) Bradykinin inhibition of cyclic AMP accumulation in D384 astrocytoma cells. Evidence against a role of cyclic GMP. Neurochem Int 21:209–213. [DOI] [PubMed] [Google Scholar]
- Altiok N and Fredholm BB (1993) Bradykinin inhibits cyclic AMP accumulation in D384-human astrocytoma cells via a calcium-dependent inhibition of adenylyl cyclase. Cell Signal 5:279–288. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Xiong W, Young JD, Cass CE, and Parkinson FE (1996) Demonstration of the existence of mRNAs encoding N1/cif and N2/cit sodium/nucleoside cotransporters in rat brain. Brain Res Mol Brain Res 42:358–361. [DOI] [PubMed] [Google Scholar]
- Arch JR and Newsholme EA (1978) The control of the metabolism and the hormonal role of adenosine. Essays Biochem 14:82–123. [PubMed] [Google Scholar]
- Arslan G and Fredholm BB (2000) Stimulatory and inhibitory effects of adenosine A2A receptors on nerve growth factor-induced phosphorylation of extracellular regulated kinases 1/2 in PC12 cells. Neurosci Lett 292:183–186. [DOI] [PubMed] [Google Scholar]
- Arslan G, Kontny E, and Fredholm BB (1997) Down-regulation of adenosine A2A receptors upon NGF-induced differentiation of PC12 cells. Neuropharmacology 36:1319–1326. [DOI] [PubMed] [Google Scholar]
- Atkinson MR, Townsend-Nicholson A, Nicholl JK, Sutherland GR, and Schofield PR (1997) Cloning, characterisation and chromosomal assignment of the human adenosine A3 receptor (ADORA3) gene. Neurosci Res 29:73–79. [DOI] [PubMed] [Google Scholar]
- Auchampach JA and Gross GJ (1993) Adenosine A1 receptors, KATP channels, and ischemic preconditioning in dogs. Am J Physiol 264:H1327–H1336. [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Jin X, Wan TC, Caughey GH, and Linden J (1997) Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol 52:846–860. [DOI] [PubMed] [Google Scholar]
- Baker SP, Scammells PJ, and Belardinelli L (2000) Differential A(1)-adenosine receptor reserve for inhibition of cyclic AMP accumulation and G-protein activation in DDT(1) MF-2 cells. Br J Pharmacol 130:1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JM, Schertler GF, and Unger VM (1997) An alpha-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J Mol Biol 272:144–164. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Mackey JR, Cass CE, and Young JD (1999) Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today 5:216–224. [DOI] [PubMed] [Google Scholar]
- Ballarin M, Herrera-Marschitz M, Casas M, and Ungerstedt U (1987) Striatal adenosine levels measured in vivo by microdialysis in rats with unilateral dopamine denervation. Neurosci Lett 83:338–344. [DOI] [PubMed] [Google Scholar]
- Balmforth AJ, Parkinson FE, Altiok N, and Fredholm BB (1992) Identification of a B2-bradykinin receptor linked to phospholipase C and inhibition of dopamine stimulated cyclic AMP accumulation in the human astrocytoma cell line D384. Naunyn-Schmiedeberg’s Arch Pharmacol 346:303–310. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Cacciari B, Romagnoli R, Merighi S, Varani K, Borea PA, and Spalluto G (2000a) A(3) adenosine receptor ligands: history and perspectives. Med Res Rev 20:103–128. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Manfredini S, Simoni D, Zappaterra L, Zocchi C, Dionisotti S, and Ongini E (1994) Synthesis of new pyrazolo [4–3-e]-1,2,4-triazolo[1,5-c] pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4 triazolo [1,5-c]pyrimidine displaying potent and selective activity as A2A adenosine receptor antagonists. Bioorg Med Chem Lett 4:2539–2544. [Google Scholar]
- Baraldi PG, Zaid AN, Lampronti I, Fruttarolo F, Pavani MG, Tabrizi MA, Shryock JC, Leung E, and Romagnoli R (2000b) Synthesis and biological effects of a new series of 2-amino-3-benzoylthiophenes as allosteric enhancers of A1-adenosine receptor. Bioorg Med Chem Lett 10:1953–1957. [DOI] [PubMed] [Google Scholar]
- Barbhaiya H, McClain R, Ijzerman A, and Rivkees SA (1996) Site-directed mutagenesis of the human A1 adenosine receptor: influences of acidic and hydroxy residues in the first four transmembrane domains on ligand binding. Mol Pharmacol 50:1635–1642. [PubMed] [Google Scholar]
- Berne RM, Rubio R, and Curnish RR (1974) Release of adenosine from ischemic brain. Circ Res 35:262–271. [Google Scholar]
- Berretta S, Robertson HA, and Graybiel AM (1992) Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J Neurophysiol 68:767–777. [DOI] [PubMed] [Google Scholar]
- Beukers MW, den Dulk H, van Tilburg EW, Brouwer J, and Ijzerman AP (2000) Why are A(2B) receptors low-affinity adenosine receptors? Mutation of Asn273 to Tyr increases affinity of human A(2B.) receptor for 2-(1-hexynyl)adenosine. Mol Pharmacol 58:1349–1356. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Dewitt DL, Burnatowska-Hledin M, Smith WL, and Spielman WS (1993) Cloning of an adenosine A1 receptor-encoding gene from rabbit. Gene (Amst) 128:285–288. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Teichert U, Kugler H, Behrsing H, Fienberg A, Greengard P, and Spiess J (1997) The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses. Proc Natl Acad Sci USA 94:14859–14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A and Williams JT (1996) A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron 16:631–639. [DOI] [PubMed] [Google Scholar]
- Bridges AJ, Bruns RF, Ortwine DF, Priebe SR, Szotek DL, and Trivedi BK (1988) N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]adenosine and its uronamide derivatives. Novel adenosine agonists with both high affinity and high selectivity for the adenosine A2 receptor. J Med Chem 31:1282–1285. [DOI] [PubMed] [Google Scholar]
- Broch OJ and Ueland PM (1980) Regional and subcellular distribution of S-adenosylhomocysteine hydrolase in the adult rat brain. J Neurochem 35:484–488. [DOI] [PubMed] [Google Scholar]
- Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, and Persson AE (2001) Abolished tubuloglomerular feedback and increased plasma renin in adenosine A(1) receptor-deficient mice. Am J Physiol 281:R1362–R1367. [DOI] [PubMed] [Google Scholar]
- Brundege JM, Diao L, Proctor WR, and Dunwiddie TV (1997) The role of cyclic AMP as a precursor of extracellular adenosine in the rat hippocampus. Neuropharmacology 36:1201–1210. [DOI] [PubMed] [Google Scholar]
- Brundege JM and Dunwiddie TV (1996) Modulation of excitatory synaptic transmission by adenosine released from single hippocampal pyramidal neurons. J Neurosci 16:5603–5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RF (1980) Adenosine receptor activation in human fibroblasts: nucleoside agonists and antagonists. Can J Physiol Pharmacol 58:673–691. [DOI] [PubMed] [Google Scholar]
- Bruns RF (1981) Adenosine antagonism by purines, pteridines and benzopteridines in human fibroblasts. Biochem Pharmacol 30:325–333 [DOI] [PubMed] [Google Scholar]
- Bruns RF, Daly JW, and Snyder SH (1980) Adenosine receptors in brain membranes: binding of N6-cyclohexyl-[3H]-adenosine and 1,3-diethyl-8-[3H]-phenylxanthine. Proc Natl Acad Sci USA 77:5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RF and Fergus JH (1990) Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Mol Pharmacol 38:939–949. [PubMed] [Google Scholar]
- Bruns RF, Lu GH, and Pugsley TA (1986) Characterization of the A2 adenosine receptor labeled by [3H]-NECA in rat striatal membranes. Mol Pharmacol 29:331–346. [PubMed] [Google Scholar]
- Burnstock G (1978) A basis for distinguishing two types of purinergic receptor, in Cell membrane receptors for drugs and hormones (Bolis L and Straub RW eds) pp 107–118, Raven Press, New York. [Google Scholar]
- Camaioni E, Di Francesco E, Vittori S, Volpini R, and Cristalli G (1997) Adenosine receptor agonists: synthesis and biological evaluation of the diastereoisomers of 2-(3-hydroxy-3-phenyl-1-propyn-1-yl)NECA. Bioorg Med Chem 5:2267–2275. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Graham DI, and Stone TW (1997) Kainate-evoked release of adenosine from the hippocampus of the anaesthetised rat: possible involvement of free radicals. J Neurochem 68:240–247. [DOI] [PubMed] [Google Scholar]
- Chan JS, Lee JW, Ho MK, and Wong YH (2000) Preactivation permits subsequent stimulation of phospholipase C by G(i)-coupled receptors. Mol Pharmacol 57:700–708. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, and Schwarzschild MA (1999) A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 19:9192–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hackett E, Fink JS, Low MJ, Ongini E and Schwarzschild MA (2001) The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proc Natl Acad Sci U S A 98:1970–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Graham DI, and Stone TW (1992) Release of endogenous adenosine and its metabolites by the activation of NMDA receptors in the rat hippocampus in vivo. Br J Pharmacol 106:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern Y, Chiou JY, Lai HL, and Tsai MH (1995) Regulation of adenylyl cyclase type VI activity during desensitization of the A2a adenosine receptor-mediated cyclic AMP response: role for protein phosphatase 2A. Mol Pharmacol 48:1–8. [PubMed] [Google Scholar]
- Chern Y, Lai HL, Fong JC, and Liang Y (1993) Multiple mechanisms for desensitization of A2a adenosine receptor-mediated cAMP elevation in rat pheochromocytoma PC12 cells. Mol Pharmacol 44:950–958. [PubMed] [Google Scholar]
- Chu YY, Tu KH, Lee YC, Kuo ZJ, Lai HL, and Chern Y (1996) Characterization of the rat A2a adenosine receptor gene. DNA Cell Biol 15:329–337. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Saura C, Canela EI, Mallol J, Lluis C, and Franco R (1997) Ligand-induced phosphorylation, clustering, and desensitization of A1 adenosine receptors. Mol Pharmacol 52:788–797. [DOI] [PubMed] [Google Scholar]
- Cobbin LB, Einstein R, and McGuire MH (1974) Studies on the coronary dilator actions of some adenosine analogues. Br J Pharmacol 50:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis MG and Brown CM (1983) Adenosine relaxes the aorta by interacting with an A2 receptor and an intracellular site. Eur J Pharmacol 96:61–69. [DOI] [PubMed] [Google Scholar]
- Corset V, Nguyen-Ba-Charvet KT, Forcet C, Moyse E, Chedotal A, and Mehlen P (2000) Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature (Lond) 407:747–750. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Studler JM, Schonn JS, Girault JA, and Herve D (2001) Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem 76:1585–1588. [DOI] [PubMed] [Google Scholar]
- Cristalli G, Camaioni E, Vittori S, Volpini R, Borea PA, Conti A, Dionisotti S, Ongini E, and Monopoli A (1995) 2-Aralkynyl and 2-heteroalkynyl derivatives of adeno- sine-5’-N-ethyluronamide as selective A2a adenosine receptor agonists. J Med Chem 38:1462–1472. [DOI] [PubMed] [Google Scholar]
- Cristalli G, Eleuteri A, Vittori S, Volpini R, Lohse MJ, and Klotz KN (1992) 2-Alkynyl derivatives of adenosine and adenosine-5’-N-ethyluronamide as selective agonists at A2 adenosine receptors. J Med Chem 35:2363–2368. [DOI] [PubMed] [Google Scholar]
- Cronstein BN (1994) Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol 76:5–13. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Johansson B, Constantino MD, Sebastião AM, and Fredholm BB (1996) Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn Schmiedebergs Arch Pharmacol 353:261–271. [DOI] [PubMed] [Google Scholar]
- Cushley MJ, Tattersfield AE, and Holgate ST (1983) Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br J Clin Pharmacol 15:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpiaz A, Townsend-Nicholson A, Beukers MW, Schofield PR and IJzerman AP (1998) Thermodynamics of full agonist, partial agonist, and antagonist binding to wild-type and mutant adenosine A1 receptors. Biochem Pharmacol 56:1437–1445. [DOI] [PubMed] [Google Scholar]
- Daly JW, Padgett W, Thompson RD, Kusachi S, Bugni WJ, and Olsson RA (1986) Structure-activity relationships for N6-substituted adenosines at a brain A1-adenosine receptor with a comparison to an A2-adenosine receptor regulating coronary blood flow. Biochem Pharmacol 35:2467–2481. [DOI] [PubMed] [Google Scholar]
- Dana A, Jonassen AK, Yamashita N, and Yellon DM (2000) Adenosine A(1) receptor activation induces delayed preconditioning in rats mediated by manganese superoxide dismutase. Circulation 101:2841–2848. [DOI] [PubMed] [Google Scholar]
- Daub H, Wallasch C, Lankenau A, Herrlich A, and Ullrich A (1997) Signal characteristics of G protein-transactivated EGF receptor. EMBO J 16:7032–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gubareff T and Sleator W Jr (1965) Effects of caffeine on mammalian atrial muscle, and its interaction with adenosine and calcium. J Pharmacol Exp Ther 148:202–214. [PubMed] [Google Scholar]
- de Mendonca A and Ribeiro JA (1993) Adenosine inhibits the NMDA receptor-mediated excitatory postsynaptic potential in the hippocampus. Brain Res 606: 351–356. [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Sebastiao AM, and Ribeiro JA (1995) Inhibition of NMDA receptor-mediated currents in isolated rat hippocampal neurones by adenosine A1 receptor activation. Neuroreport 6:1097–1100. [DOI] [PubMed] [Google Scholar]
- Deckert J and Gleiter CH (1994) Adenosine–an endogenous neuroprotective metabolite and neuromodulator. J Neural Transm Suppl 43:23–31. [PubMed] [Google Scholar]
- Deckert J, Nothen MM, Rietschel M, Wildenauer D, Bondy B, Ertl MA, Knapp M, Schofield PR, Albus M, Maier W, and Propping P (1996) Human adenosine A2a receptor (A2aAR) gene: systematic mutation screening in patients with schizophrenia. J Neural Transm 103:1447–1455. [DOI] [PubMed] [Google Scholar]
- Decking UK, Schlieper G, Kroll K, and Schrader J (1997) Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res 81:154–164. [DOI] [PubMed] [Google Scholar]
- Delaney SM and Geiger JD (1998) Levels of endogenous adenosine in rat striatum. II. Regulation of basal and N-methyl-D-aspartate-induced levels by inhibitors of adenosine transport and metabolism. J Pharmacol Exp Ther 285:568–572. [PubMed] [Google Scholar]
- Delaney SM, Shepel PN, and Geiger JD (1998) Levels of endogenous adenosine in rat striatum. I. Regulation by ionotropic glutamate receptors, nitric oxide and free radicals. J Pharmacol Exp Ther 285:561–567. [PubMed] [Google Scholar]
- Dickenson JM, Blank JL, and Hill SJ (1998) Human adenosine A1 receptor and P2Y2-purinoceptor-mediated activation of the mitogen-activated protein kinase cascade in transfected CHO cells. Br J Pharmacol 124:1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson JM and Hill SJ (1993) Homologous and heterologous desensitization of histamine H1- and ATP-receptors in the smooth muscle cell line, DDT1 MF-2: the role of protein kinase C. Br J Pharmacol 110:1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson JM and Hill SJ (1998) Involvement of G-protein betagamma subunits in coupling the adenosine A1 receptor to phospholipase C in transfected CHO cells. Eur J Pharmacol 355:85–93. [DOI] [PubMed] [Google Scholar]
- Dionisotti S, Ongini E, Zocchi C, Kull B, Arslan G, and Fredholm BB (1997) Characterization of human A2A adenosine receptors with the antagonist radioligand [2H]-SCH 58261. Br J Pharmacol 121:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury AN and Szent-György A (1929) The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol (Lond) 68:213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, and Jackson EK (1998) Adenosine inhibits growth of human aortic smooth muscle cells via A2B receptors. Hypertension 31:516–521. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Osaka K, Suzuki F, and Jackson EK (1996) Adenosine inhibits growth of rat aortic smooth muscle cells. Possible role of A2b receptor. Hypertension 27:786–793. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Shue H, and Jackson EK (2000) A(2B) receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension 35:267–272. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV and Diao L (2000) Regulation of extracellular adenosine in rat hippocampal slices is temperature dependent: role of adenosine transporters. Neuroscience 95:81–88. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, and Proctor WR (1997a) Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci 17:7673–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao LH, Kim HO, Jiang JL, and Jacobson KA (1997b) Activation of hippocampal adenosine A3 receptors produces a desensitization of A1 receptor-mediated responses in rat hippocampus. J Neurosci 17:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV and Fredholm BB (1997) Adenosine neuromodulation, in Purinergic Approaches in Experimental Therapeutics (Jacobson KA and Jarvis MF eds) pp 359–382, Wiley-Liss, Inc, New York. [Google Scholar]
- Dunwiddie TV, Masino SA, Poelchen W, Diao L, Johansson B, and Fredholm B (2000) Altered electrophysiological sensitivity to A1 but not GABAB agonists in the hippocampal CA1 region in adenosine A1 receptor knockout mice. Society for Neuroscience 26:816.18. [Google Scholar]
- El Yacoubi M, Ledent C, Menard JF, Parmentier M, Costentin J, and Vaugeois JM (2000) The stimulant effects of caffeine on locomotor behavior in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol 129:1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallahi N, Broad RM, Jin S, and Fredholm BB (1996) Release of adenosine from rat hippocampal slices by nitric oxide donors. J Neurochem 67:186–193. [DOI] [PubMed] [Google Scholar]
- Faure M, Voyno-Yasenetskaya TA, and Bourne HR (1994) cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem 269:7851–7854. [PubMed] [Google Scholar]
- Feoktistov I and Biaggioni I (1995) Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest 96:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistov I, Goldstein AE, and Biaggioni I (1999) Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol 55:726–734 [PubMed] [Google Scholar]
- Ferguson SS (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24. [PubMed] [Google Scholar]
- Fernandez M, Svenningsson P, and Fredholm BB (1996) Adaptive changes in adenosine receptors following long term treatment with the adenosine receptor agonist R-phenylisopropyl adenosine. Life Sci 58:769–776. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, and Fuxe K (1997) Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20:482–487. [DOI] [PubMed] [Google Scholar]
- Ferré S, O’Connor WT, Snaprud P, Ungerstedt U, and Fuxe K (1994a) Antagonistic interaction between adenosine A2A receptors and dopamine D2 receptors in the ventral striopallidal system. Implications for the treatment of schizophrenia. Neuroscience 63:765–773. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Gimenez-Llort L, Finnman UB, Martinez E, Scotti de Carolis A, and Fuxe K (1994b) Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport 6:73–76. [DOI] [PubMed] [Google Scholar]
- Ferré S, Torvinen M, Antoniou K, Irenius E, Civelli O, Arenas E, Fredholm BB, and Fuxe K (1998) Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. J Biol Chem 273:4718–4724. [DOI] [PubMed] [Google Scholar]
- Ferré S, von Euler G, Johansson B, Fredholm BB, and Fuxe K (1991) Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A 88:7238–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, and Reppert SM (1992) Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res 14:186–195. [DOI] [PubMed] [Google Scholar]
- Finn SF, Swartz KJ, and Beal MF (1991) 2-Chloroadenosine attenuates NMDA, kainate, and quisqualate toxicity. Neurosci Lett 126:191–194. [DOI] [PubMed] [Google Scholar]
- Fischer H, Prast H, and Philippu A (1995) Adenosine release in the ventral striatum of the rat is modulated by endogenous nitric oxide. Eur J Pharmacol 275:R5–R6. [DOI] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Farbstein T, Barer F, and Ohana G (2000a) Adenosine acts as a chemoprotective agent by stimulating G-CSF production: a role for A1 and A3 adenosine receptors. J Cell Physiol 183:393–398. [DOI] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Ohana G, Pathak S, Wasserman L, Barer F, and Multani AS (2000b) Adenosine acts as an inhibitor of lymphoma cell growth: a major role for the A3 adenosine receptor. Eur J Cancer 36:1452–1458. [DOI] [PubMed] [Google Scholar]
- Jr Fozard, Pfannkuche HJ and Schuurman HJ (1996) Mast cell degranulation following adenosine A3 receptor activation in rats. Eur J Pharmacol 298:293–297. [DOI] [PubMed] [Google Scholar]
- Fredholm BB (1978) Effect of adenosine, adenosine analogues and drugs inhibiting adenosine inactivation on lipolysis in rat fat cells. Acta Physiol Scand 102:191–198. [DOI] [PubMed] [Google Scholar]
- Fredholm BB (1982) Adenosine actions and adenosine receptors after 1 week treatment with caffeine. Acta Physiol Scand 115:283–286. [DOI] [PubMed] [Google Scholar]
- Fredholm BB (1996) Adenosine and neuroprotection, in Neuroprotective agents and cerebral ischemia (Green AR and Cross AJ eds) pp 259–280, Academic Press, London. [Google Scholar]
- Fredholm BB (1997) Purines and neutrophil leukocytes. Gen Pharmacol 28:345–350. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, and Williams M (1994a) Nomenclature and classification of purinoceptors. Pharmacol Rev 46:143–156. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, Schwabe U, and Williams M (1997) Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci 18:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, and Wasserman W (2000) Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg’s Arch Pharmacol 362:364–374. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, and Zvartau E (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51:83–153. [PubMed] [Google Scholar]
- Fredholm BB and Dunwiddie TV (1988) How does adenosine inhibit transmitter release? Trends Pharmacol Sci 9:130–134. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Fuxe K, and Agnati L (1976) Effect of some phosphodiesterase inhibitors on central dopamine mechanisms. Eur J Pharmacol 38:31–38. [DOI] [PubMed] [Google Scholar]
- Fredholm BB and Hedqvist P (1980) Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol 29:1635–1643. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, and Schulte G (2001) Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61:443–448. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Jondal M, and Nordstedt C (1987a) The adenosine receptor mediated accumulation of cyclic AMP in Jurkat cells is enhanced by a lectin and by phorbol esters. Biochem Biophys Res Commun 145:344–349. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Lindgren E, Lindström K, and Nordstedt C (1987b) ex-Adrenoceptor stimulation, but not muscarinic stimulation, increases cyclic AMP accumulation in brain slices due to protein kinase C mediated enhancement of adenosine receptor effects. Acta Physiol Scand 131:543–551. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Lindström K, and Wallman-Johansson A (1994b) Propentofylline and other adenosine transport inhibitors increase the efflux of adenosine following electrical or metabolic stimulation of rat hippocampal slices. J Neurochem 62:563–573. [DOI] [PubMed] [Google Scholar]
- Fredholm BB and Persson CGA (1982) Xanthine derivatives as adenosine receptor antagonists. Eur J Pharmacol 81:673–676. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Zhang Y, and van der Ploeg I (1996) Adenosine A2A receptors mediate the inhibitory effect of adenosine on formyl-Met-Leu-Phe-stimulated respiratory burst in neutrophil leukocytes. Naunyn Schmiedebergs Arch Pharmacol 354:262–267. [DOI] [PubMed] [Google Scholar]
- Freissmuth M, Selzer E, and Schütz W (1991) Interactions of purified bovine brain A1-adenosine receptors with G-proteins. Reciprocal modulation of agonist and antagonist binding. Biochem J 275:651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund S, Ungerer M, and Lohse MJ (1994) A1 adenosine receptors expressed in CHO-cells couple to adenylyl cyclase and to phospholipase C. Naunyn Schmiedebergs Arch Pharmacol 350:49–56. [DOI] [PubMed] [Google Scholar]
- Furlong TJ, Pierce KD, Selbie LA, and Shine J (1992) Molecular characterization of a human brain adenosine A2 receptor. Brain Res Mol Brain Res 15:62–66. [DOI] [PubMed] [Google Scholar]
- Gallo-Rodriguez C, Ji XD, Melman N, Siegman BD, Sanders LH, Orlina J, Fischer B, Pu Q, Olah ME, van Galen PJ, Stiles GL, and Jacobson KA (1994) Structure-activity relationships of N6-benzyladenosine-5’-uronamides as A3-selective adenosine agonists. J Med Chem 37:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Chen T, Weber MJ, and Linden J (1999) A2B adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells. Cross-talk between cyclic AMP and protein kinase C pathways. J Biol Chem 274:5972–5980. [DOI] [PubMed] [Google Scholar]
- Gao Z, Li BS, Day YJ, and Linden J (2001) A(3) adenosine receptor activation triggers phosphorylation of protein kinase B and protects rat basophilic leukemia 2H3 mast cells from apoptosis. Mol Pharmacol 59:76–82. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Jiang Q, Jacobson KA, and Ijzerman AP (2000) Site-directed mutagenesis studies of human A(2A) adenosine receptors: involvement of glu(13) and his(278) in ligand binding and sodium modulation. Biochem Pharmacol 60:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauda EB, Northington FJ, Linden J, and Rosin DL (2000) Differential expression of a(2a), A(1)-adenosine and D(2)-dopamine receptor genes in rat peripheral arterial chemoreceptors during postnatal development. Brain Res 872:1–10. [DOI] [PubMed] [Google Scholar]
- Gerwins P and Fredholm BB (1991) Glucocorticoid receptor activation leads to up-regulation of adenosine A1 receptors and down-regulation of adenosine A2 responses in DDT1 MF-2 smooth muscle cells. Mol Pharmacol 40:149–155. [PubMed] [Google Scholar]
- Gerwins P and Fredholm BB (1992a) ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J Biol Chem 267:16081–16087. [PubMed] [Google Scholar]
- Gerwins P and Fredholm BB (1992b) Stimulation of adenosine A1 receptors and bradykinin receptors, which act via different G-proteins, synergistically raises inositol 1,4,5-trisphosphate and intracellular free calcium in DDT1 MF-2 smooth muscle cells. Proc Natl Acad Sci U S A 89:7330–7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwins P and Fredholm BB (1995a) Activation of adenosine A1 and bradykinin receptors increases protein kinase C and phospholipase D activity in smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol 351:186–193. [DOI] [PubMed] [Google Scholar]
- Gerwins P and Fredholm BB (1995b) Activation of phospholipase C and phospholipase D by stimulation of adenosine A1, bradykinin or P2U receptors does not correlate well with protein kinase C activation. Naunyn Schmiedebergs Arch Pharmacol 351:194–201. [DOI] [PubMed] [Google Scholar]
- Green A (1987) Adenosine receptor down-regulation and insulin resistance following prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem 262:15702–15707. [PubMed] [Google Scholar]
- Green A, Johnson JL, and Milligan G (1990) Down-regulation of Gi sub-types by prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem 265:5206–5210. [PubMed] [Google Scholar]
- Gudermann T, Schoneberg T, and Schultz G (1997) Functional and structural complexity of signal transduction via G-protein-coupled receptors. Annu Rev Neurosci 20:399–427. [DOI] [PubMed] [Google Scholar]
- Gutkind JS (1998) The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem 273:1839–1842. [DOI] [PubMed] [Google Scholar]
- Halldner L, Lozza G, Lindström K, and Fredholm BB (2000) Lack of tolerance to motor stimulant effects of a selective adenosine A2A receptor antagonist. Eur J Pharmacol 406:345–354. [DOI] [PubMed] [Google Scholar]
- Halpain S, Girault JA, and Greengard P (1990) Activation of NMDA receptors induces dephosphorylation of DARPP-32 in rat striatal slices. Nature (Lond) 343:369–372. [DOI] [PubMed] [Google Scholar]
- Harvey J and Lacey MG (1997) A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci 17:5271–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J Jr, Obiako B, Babal P, and Stevens T (1999) 5-(N-ethylcarboxamido)adenosine desensitizes the A2b-adenosine receptor in lung circulation. Am J Physiol 276:H1877–H1883. [DOI] [PubMed] [Google Scholar]
- Henderson R, Baldwin JM, Ceska TA, Zemlin F, Beckmann E, and Downing KH (1990) Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol 213:899–929. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, and Rosin DL (2001) Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol 431:331–346. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Leid M, and Murray TF (1998) Cyclopentyladenosine-induced homologous down-regulation of A1 adenosine receptors (A1AR) in intact neurons is accompanied by receptor sequestration but not a reduction in A1AR mRNA expression or G protein alpha-subunit content. J Neurochem 71:221–230. [DOI] [PubMed] [Google Scholar]
- Hibert MF, Trumpp-Kallmeyer S, Bruinvels A, and Hoflack J (1991) Three-dimensional models of neurotransmitter G-binding protein-coupled receptors. Mol Pharmacol 40:8–15. [PubMed] [Google Scholar]
- Hill FJ, Oleynek JJ, Hoth CF, Kiron MA, Weng W, Wester RT, Tracey WR, Knight DR, Buchholz RA, and Kennedy SP (1997) Cloning, expression and pharmacological characterization of rabbit adenosine A1 and A3 receptors. J Pharmacol Exp Ther 280:122–128. [PubMed] [Google Scholar]
- Hirano D, Aoki Y, Ogasawara H, Kodama H, Waga I, Sakanaka C, Shimizu T, and Nakamura M (1996) Functional coupling of adenosine A2a receptor to inhibition of the mitogen-activated protein kinase cascade in Chinese hamster ovary cells. Biochem J 316:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn K, Craig CG, and White TD (1990) A comparison of N-methyl-D-aspartate-evoked release of adenosine and [3H]norepinephrine from rat cortical slices. J Pharmacol Exp Ther 255:174–181. [PubMed] [Google Scholar]
- Hoflack J, Trumpp-Kallmeyer S, and Hibert M (1994) Re-evaluation of bacteriorhodopsin as a model for G protein-coupled receptors. Trends Pharmacol Sci 15:7–9. [DOI] [PubMed] [Google Scholar]
- Hollingsworth EB, Sears EB, and Daly JW (1985) An activator of protein kinase C (phorbol-12-myristate-13-acetate) augments 2- chloroadenosine-elicited accumulation of cyclic AMP in guinea pig cerebral cortical particulate preparations. FEBS Lett 184:339–342. [DOI] [PubMed] [Google Scholar]
- Huston JP, Haas HL, Boix F, Pfister M, Decking U, Schrader J and Schwarting Rkw (1996) Extracellular adenosine levels in neostriatum and hippocampus druing rest and activity periods of rats. Neuroscience 73:99–107. [DOI] [PubMed] [Google Scholar]
- Hutchison AJ, Webb RL, Oei HH, Ghai GR, Zimmerman MB, and Williams M (1989) CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther 251:47–55. [PubMed] [Google Scholar]
- IJzerman AP, Van der Wenden EM, Van Galen PJM and Jacobson KA (1994) Molecular modeling of adenosine receptors. The ligand binding site on the rat adenosine A2A receptor. Eur J Pharmacol, Mol Pharmacol Sect 268:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJzerman AP, van Galen Pjm and Jacobson KA (1992) Molecular modeling of adenosine receptors. I. The ligand binding site of the A1 receptor. Drug Des Discover 9:49–67. [PMC free article] [PubMed] [Google Scholar]
- IJzerman AP, Von Frijtag Drabbe Kunzel JK, Kim J, Jiang Q and Jacobson KA (1996) Site-directed mutagenesis of the human adenosine A2A receptor. Critical involvement of Glu13 in agonist recognition. Eur J Pharmacol 310:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello F, Bastia E, Ongini E, and Monopoli A (2000) Adenosine receptors in neurological disorders. Emerging Therapeutic Targets 4:635–664. [Google Scholar]
- Jacobson KA, IJzerman A and Linden J (1999) 1,3-Dialkylxanthine derivatives having high potency as antagonists at human A2B adenosine receptors. Drug Dev Res 47:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Ukena D, Kirk KL, and Daly JW (1986) [3H]xanthine amine congener of 1,3-dipropyl-8-phenylxanthine: an antagonist radioligand for adenosine receptors. Proc Natl Acad Sci U S A 83:4089–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Zimmet J, Schulick R, Barone S, Daly JW, and Kirk KL (1987) Adenosine analogs with covalently attached lipids have enhanced potency at A1-adenosine receptors. FEBS Lett 225:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MA, Johnson RG, Luneau CJ, and Salvatore CA (1995) Cloning and chromosomal localization of the human A2b adenosine receptor gene (ADORA2B) and its pseudogene. Genomics 27:374–376. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, and Williams M (1989) [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther 251:888–893. [PubMed] [Google Scholar]
- Ji XD and Jacobson KA (1999) Use of the triazolotriazine [3H]ZM 241385 as a radioligand at recombinant human A2B adenosine receptors. Drug Des Discov 16:217–226. [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Van Rhee AM, Kim J, Yehle S, Wess J, and Jacobson KA (1996) Hydrophilic side chains in the third and seventh transmembrane helical domains of human A2A adenosine receptors are required for ligand recognition. Mol Pharmacol 50:512–521. [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shepherd RK, Duling BR, and Linden J (1997) Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest 100:2849–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers R, Linder ME, Hoehnegger M, Nanoff C, Bertin B, Strosberg AD, Marullo S, and Freissmuth M (1994) Species difference in the G protein selectivity of the human and bovine A1-adenosine receptor. J Biol Chem 269:32077–32084. [PubMed] [Google Scholar]
- Johansson B, Ahlberg S, van der Ploeg I, Brené S, Lindefors N, Persson H, and Fredholm BB (1993a) Effect of long term caffeine treatment on A1 and A2 adenosine receptor binding and on mRNA levels in rat brain. Naunyn Schmiedebergs Arch Pharmacol 347:407–414. [DOI] [PubMed] [Google Scholar]
- Johansson B and Fredholm BB (1995) Further characterization of the binding of the adenosine receptor agonist [3H]CGS 21680 to rat brain using autoradiography. Neuropharmacology 34:393–403. [DOI] [PubMed] [Google Scholar]
- Johansson B, Georgiev V, Parkinson FE, and Fredholm BB (1993b) The binding of the adenosine A2 receptor selective agonist [3H]CGS 21680 to rat cortex differs from its binding to rat striatum. Eur J Pharmacol Mol Pharmacol Sect 247:103–110. [DOI] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Giménez-Llort L, Escorihuela RM, Fernández-Teruel A, Wiesenfeld-Hallin Z, Xu X-J, Hårdemark A, Betsholtz C, Herlenius E and Fredholm BB (2001) Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA 98:9407–9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonzon B and Fredholm BB (1985) Release of purines, noradrenaline and GABA from rat hippocampal slices by field stimulation. J Neurochem 44:217–224. [DOI] [PubMed] [Google Scholar]
- Jonzon B, Nilsson J, and Fredholm BB (1985) Adenosine receptor-mediated changes in cyclic AMP production and DNA synthesis in cultured arterial smooth muscle cells. J Cell Physiol 124:451–456. [DOI] [PubMed] [Google Scholar]
- Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, and Vinten-Johansen J (1999) A(3) adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol 277:H1895–H1905. [DOI] [PubMed] [Google Scholar]
- Jordan JE, Zhao ZQ, Sato H, Taft S, and Vinten-Johansen J (1997) Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherence. J Pharmacol Exp Ther 280:301–309. [PubMed] [Google Scholar]
- Kanda T, Jackson MJ, Smith LA, Pearce RK, Nakamura J, Kase H, Kuwana Y, and Jenner P (2000) Combined use of the adenosine A(2A) antagonist KW-6002 with L-DOPA or with selective D1 or D2 dopamine agonists increases antiparkinsonian activity but not dyskinesia in MPTP-treated monkeys. Exp Neurol 162:321–327. [DOI] [PubMed] [Google Scholar]
- Keefe KA and Gerfen CR (1996) D1 dopamine receptor-mediated induction of zif268 and c-fos in the dopamine-depleted striatum: differential regulation and independence from NMDA receptors. J Comp Neurol 367:165–176. [DOI] [PubMed] [Google Scholar]
- Kenakin T (1993) Pharmacologic analysis of drug-receptor interaction, 2nd Edition. Raven Press, New York. [Google Scholar]
- Kenakin T (1995) Agonist-receptor efficacy I: mechanisms of efficacy and receptor promiscuity. Trends Pharmacol Sci 16:188–192. [DOI] [PubMed] [Google Scholar]
- Kennedy AP, Mangum KC, Linden J, and Wells JN (1996) Covalent modification of transmembrane span III of the A1 adenosine receptor with an antagonist photoaffinity probe. Mol Pharmacol 50:789–798. [PubMed] [Google Scholar]
- Kim HO, Ji XD, Siddiqi SM, Olah ME, Stiles GL, and Jacobson KA (1994) 2-Substitution of N6-benzyladenosine-5’-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem 37:3614–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jiang Q, Glashofer M, Yehle S, Wess J, and Jacobson KA (1996) Glutamate residues in the second extracellular loop of the human A2a adenosine receptor are required for ligand recognition. Mol Pharmacol 49:683–691. [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wess J, van Rhee AM, Schoneberg T, and Jacobson KA (1995) Site-directed mutagenesis identifies residues involved in ligand recognition in the human A2a adenosine receptor. J Biol Chem 270:13987–13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Ji X, Melman N, Linden J, and Jacobson KA (2000) Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem 43:1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Vassylyev DG, Miyazawa A, Kidera A, Matsushima M, Mitsuoka K, Murata K, Hirai T, and Fujiyoshi Y (1997) Surface of bacteriorhodopsin revealed by high-resolution electron crystallography. Nature (Lond) 389:206–211. [DOI] [PubMed] [Google Scholar]
- Klotz KN, Camaioni E, Volpini R, Kachler S, Vittori S, and Cristalli G (1999) 2-Substituted N-ethylcarboxamidoadenosine derivatives as high-affinity agonists at human A3 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 360:103–108. [DOI] [PubMed] [Google Scholar]
- Klotz KN, Cristalli G, Grifantini M, Vittori S, and Lohse MJ (1985) Photoaffinity labeling of A1-adenosine receptors. J Biol Chem 260:14659–14664. [PubMed] [Google Scholar]
- Klotz K-N, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, and Lohse MJ (1998) Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol 357:1–9. [DOI] [PubMed] [Google Scholar]
- Klotz KN, Quitterer U, and Englert M (2000) Effector coupling of human A3 adenosine receptors (Abstract). Drug Dev Res 50:80. [DOI] [PubMed] [Google Scholar]
- Klotz KN, Vogt H, and Tawfik-Schlieper H (1991) Comparison of A1 adenosine receptors in brain from different species by radioligand binding and photoaffinity labeling. Naunyn Schmiedebergs Arch Pharmacol 343:196–201. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Beitner-Johnson D, Conforti L, and Millhorn DE (1998) Chronic hypoxia reduces adenosine A2A receptor-mediated inhibition of calcium current in rat PC12 cells via downregulation of protein kinase A. J Physiol (Lond) 512:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, and Hyman SE (1996) Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci 16:4231–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba M, Rosin DL, Hayashi N, Linden J, and Sitkovsky MV (1999) Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol Pharmacol 55:614–624. [PubMed] [Google Scholar]
- Krupnick JG and Benovic JL (1998) The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol 38:289–319. [DOI] [PubMed] [Google Scholar]
- Kull B, Arslan G, Nilsson C, Owman C, Lorenzen A, Schwabe U, and Fredholm BB (1999) Differences in the order of potency for agonists, but not antagonists, at human and rat adenosine A2A receptors. Biochem Pharmacol 57:65–75. [DOI] [PubMed] [Google Scholar]
- Kull B, Svenningsson P, and Fredholm BB (2000a) Adenosine A2A receptors are co-localized with and activate Golf in rat striatum. Mol Pharmacol 58:771–777. [DOI] [PubMed] [Google Scholar]
- Kull B, Svenningsson P, Hall H, and Fredholm BB (2000b) GTP differentially affects antagonist radioligand binding to adenosine A1 and A2A receptors in human brain. Neuropharmacology 39:2374–2380. [DOI] [PubMed] [Google Scholar]
- Kvanta A, Nordstedt C, van der Ploeg I, Jondal M, and Fredholm BB (1989) CD3/T-cell receptor coupling to a pertussis and cholera toxin-insensitive G-protein. FEBS Lett 250:536–540. [DOI] [PubMed] [Google Scholar]
- Lasley RD, Narayan P, Uittenbogaard A, and Smart EJ (2000) Activated cardiac adenosine A(1) receptors translocate out of caveolae. J Biol Chem 275:4417–4421. [DOI] [PubMed] [Google Scholar]
- Lasley RD, Rhee JW, Van Wylen DG, and Mentzer RM Jr (1990) Adenosine A1 receptor mediated protection of the globally ischemic isolated rat heart. J Mol Cell Cardiol 22:39–47. [DOI] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Pedata F, and Corradetti R (1999) Extracellular adenosine concentrations during in vitro ischemia in rat hippocampal slices. Br J Pharmacol 127:729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le F, Townsend-Nicholson A, Baker E, Sutherland GR, and Schofield PR (1996) Characterization and chromosomal localization of the human A2a adenosine receptor gene: ADORA2A. Biochem Biophys Res Commun 223:461–467. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, and Parmentier M (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2A receptor. Nature (Lond) 388:674–678. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ (1998) G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem 273:s18677–18680. [DOI] [PubMed] [Google Scholar]
- Li AH, Moro S, Forsyth N, Melman N, Ji XD, and Jacobson KA (1999) Synthesis, CoMFA analysis, and receptor docking of 3,5-diacyl-2, 4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J Med Chem 42:706–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert F, Parmentier M, Lefort A, Dinsart C, Van Sande J, Maenhaut C, Simons MJ, Dumont JE, and Vassart G (1989) Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science (Wash DC) 244:569–572. [DOI] [PubMed] [Google Scholar]
- Libert F, Schiffmann SN, Lefort A, Parmentier M, Gerard C, Dumont JE, Vanderhaeghen JJ, and Vassart G (1991) The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J 10:1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert F, Van Sande J, Lefort A, Czernilofsky A, Dumont JE, Vassart G, Ensinger HA, and Mendla KD (1992) Cloning and functional characterization of a human A1 adenosine receptor. Biochem Biophys Res Commun 187:919–926 [DOI] [PubMed] [Google Scholar]
- Linden J (1994) Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci 15:298–306. [DOI] [PubMed] [Google Scholar]
- Linden J (1997) Allosteric enhancement of adenosine receptors, in Purinergic Approaches in Experimental Therapeutics (Jacobson KA and Jarvis MF eds) pp 85–97, Wiley-Liss, New York. [Google Scholar]
- Linden J (2001) Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 41:775–787. [DOI] [PubMed] [Google Scholar]
- Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, Rivkees SA, Fink JS, and Reppert SM (1993) Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol Pharmacol 44:524–532. [PubMed] [Google Scholar]
- Linden J, Thai T, Figler H, Jin X, and Robeva AS (1999) Characterization of human A(2B) adenosine receptors: radioligand binding, western blotting, and coupling to G(q) in human embryonic kidney 293 cells and HMC-1 mast cells. Mol Pharmacol 56:705–713. [PubMed] [Google Scholar]
- Lindström K, Ongini E, and Fredholm BB (1996) The selective adenosine A2A receptor antagonist SCH 58261 discriminates between two different binding sites for [3H]-CGS 21680 in the rat brain. Naunyn Schmiedebergs Arch Pharmacol 354:539–541. [DOI] [PubMed] [Google Scholar]
- Lloyd HGE and Fredholm BB (1995) Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int 26:387–395. [DOI] [PubMed] [Google Scholar]
- Lloyd HGE, Lindström K, and Fredholm BB (1993) Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation and energy depletion. Neurochem Int 23:173–185. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Klotz K-N, Lindenborn-Fotinos J, Reddington M, Schwabe U, and Olsson RA (1987) 8-cyclopentyl-1,3-dipropylxanthine (DPCPX)—a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn-Schmiedeberg’s Arch Pharmacol 336:204–210. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Klotz KN, Schwabe U, Cristalli G, Vittori S, and Grifantini M (1988) 2-Chloro-N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 337:687–689. [DOI] [PubMed] [Google Scholar]
- Londos C, Cooper DM, and Wolff J (1980a) Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A 77:2551–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C, Cooper DMF, Schlegel W, and Rodbell M (1978) Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci U S A 75:5362–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C, Cooper DMF, and Wolff J (1980b) Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A 77:2551–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longabaugh JP, Didsbury J, Spiegel A and Stiles GL (1989) Modification of the rat adipocyte A1 adenosine receptor-adenylate cyclase system during chronic exposure to an A1 adenosine receptor agonist: alterations in the quantity of Gs alpha and Gi alpha are not associated with changes in their mRNAs. Mol Pharmacol 36:681–688. [PubMed] [Google Scholar]
- Luecke H, Schobert B, Richter HT, Cartailler JP, and Lanyi JK (1999) Structure of bacteriorhodopsin at 1.55 A resolution. J Mol Biol 291:899–911. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, and Lefkowitz RJ (1999) Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 11:177–183. [DOI] [PubMed] [Google Scholar]
- MacCollin M, Peterfreund R, MacDonald M, Fink JS, and Gusella J (1994) Mapping of a human A2a adenosine receptor (ADORA2) to chromosome 22. Genomics 20:332–333. [DOI] [PubMed] [Google Scholar]
- Maemoto T, Finlayson K, Olverman HJ, Akahane A, Horton RW, and Butcher SP (1997) Species differences in brain adenosine A1 receptor pharmacology revealed by use of xanthine and pyrazolopyridine based antagonists. Br J Pharmacol 122:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenhaut C, Van Sande J, Libert F, Abramowicz M, Parmentier M, Vanderhaegen JJ, Dumont JE, Vassart G, and Schiffmann S (1990) RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem Biophys Res Commun 173:1169–1178. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Manabe T, and Nicoll RA (1994) Release of adenosine by activation of NMDA receptors in the hippocampus. Science (Wash DC) 265:2098–2101. [DOI] [PubMed] [Google Scholar]
- Marangos PJ (1990) Adenosinergic approaches to stroke therapeutics. Med Hypotheses 32:45–49. [DOI] [PubMed] [Google Scholar]
- Mathot RAA, Van der Wenden EM, Soudijn W, IJzerman AP and Danhof M (1995) Deoxyribose analogues of N6-cyclopentyladenosine (CPA): partial agonists at the adenosine A1 receptor in vivo. Br J Pharmacol 116:1957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathôt RA, Appel S, van Schaick EA, Soudijn W, IJzerman AP and Danhof M (1993) High-performance liquid chromatography of the adenosine A1 agonist N6-cyclopentyladenosine and the A1 antagonist 8-cyclopentyltheophylline and its application in a pharmacokinetic study in rats. J Chromatogr 620:113–120. [DOI] [PubMed] [Google Scholar]
- Matovcik LM, Karapetian O, Czernik AJ, Marino CR, Kinder BK, and Gorelick FS (1994) Antibodies to an epitope on synapsin I detect a protein associated with the endocytic compartment in non-neuronal cells. Eur J Cell Biol 65:327–340. [PubMed] [Google Scholar]
- McIntire WE, MacCleery G, and Garrison JC (2001) The G protein beta subunit is a determinant in the coupling of the Gs alpha subunit to the beta adrenergic and A2a adenosine receptors. J Biol Chem 276:15801–15809. [DOI] [PubMed] [Google Scholar]
- Meng F, Xie G, Chalmers D, Morgan C, Watson SJ, and Akil H (1994) Cloning and expression of the A2a adenosine receptor from guinea pig brain. Neurochem Res 19:613–621. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Müller-Brechlin R, and Richter D (1991) Molecular cloning of a novel putative G-protein coupled receptor expressed during rat spermiogenesis. FEBS Lett 284:155–160. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Rivkees SA, Raybould HE, Bitticaca M, Goldhaber JI, and Weiss JN (1998) Localization and functional effects of adenosine A1 receptors on cardiac vagal afferents in adult rats. Am J Physiol 274:H441–H447. [DOI] [PubMed] [Google Scholar]
- Mundell SJ and Kelly E (1998) Evidence for co-expression and desensitization of A2a and A2b adenosine receptors in NG108–15 cells. Biochem Pharmacol 55:595–603. [DOI] [PubMed] [Google Scholar]
- Neary JT, McCarthy M, Kang Y, and Zuniga S (1998) Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci Lett 242:159–162. [DOI] [PubMed] [Google Scholar]
- Nordstedt C and Fredholm BB (1987) Phorbol-12,13-dibutyrate enhances the cyclic AMP accumulation in rat hippocampal slices induced by adenosine analogues. Naunyn Schmiedebergs Arch Pharmacol 335:136–142. [DOI] [PubMed] [Google Scholar]
- Nordstedt C, Jondal M, and Fredholm BB (1987) Activation of protein kinase C inhibits prostaglandin- and potentiates adenosine receptor-stimulated accumulation of cyclic AMP in human T-cell leukemia line. FEBS Lett 220:57–60. [DOI] [PubMed] [Google Scholar]
- Nordstedt C, Kvanta A, van der Ploeg I, and Fredholm BB (1989) Dual effects of protein kinase-C on receptor-stimulated cAMP accumulation in a human T-cell leukemia line. Eur J Pharmacol Mol Pharmacol Sect 172:51–60. [DOI] [PubMed] [Google Scholar]
- Offermanns S and Simon MI (1995) G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem 270:15175–15180. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, and Huynh LP (2000) A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol 279:F809–F818. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Macdonald T, and Huang L (1999) Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol 277:F404–F412. [DOI] [PubMed] [Google Scholar]
- Olah ME (1997) Identification of A2a adenosine receptor domains involved in selective coupling to Gs. Analysis of chimeric A1/A2a adenosine receptors. J Biol Chem 272:337–344. [DOI] [PubMed] [Google Scholar]
- Olah ME, Gallo-Rodriguez C, Jacobson KA, and Stiles GL (1994) 125I-4-aminobenzyl-5’-N-methylcarboxamidoadenosine, a high affinity radioligand for the rat A3 adenosine receptor. Mol Pharmacol 45:978–982. [PMC free article] [PubMed] [Google Scholar]
- Olah ME, Ren H, Ostrowski J, Jacobson KA, and Stiles GL (1992) Cloning, expression, and characterization of the unique bovine A1 adenosine receptor. Studies on the ligand binding site by site-directed mutagenesis. J Biol Chem 267:10764–10770. [PMC free article] [PubMed] [Google Scholar]
- Olah ME and Stiles GL (2000) The role of receptor structure in determining adenosine receptor activity. Pharmacol Ther 85:55–75. [DOI] [PubMed] [Google Scholar]
- Ongini E, Dionisotti S, Gessi S, Irenius E, and Fredholm BB (1999) Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn-Schmiedeberg’s Arch Pharmacol 359:7–10. [DOI] [PubMed] [Google Scholar]
- Ongini E and Fredholm BB (1996) Pharmacology of adenosine A2A receptors. Trends Pharmacol Sci 17:364–372. [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, and Miyano M (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science (Wash DC) 289:739–745. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Benovic JL, and Stiles GL (1996) Molecular basis for subtype-specific desensitization of inhibitory adenosine receptors. Analysis of a chimeric A1-A3 adenosine receptor. J Biol Chem 271:15272–15278. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Gettys TW, and Stiles GL (1995) Differential interaction with and regulation of multiple G-proteins by the rat A3 adenosine receptor. J Biol Chem 270:16895–16902. [DOI] [PubMed] [Google Scholar]
- Palmer TM and Stiles GL (1997) Identification of an A2a adenosine receptor domain specifically responsible for mediating short-term desensitization. Biochemistry 36:832–838. [DOI] [PubMed] [Google Scholar]
- Palmer TM and Stiles GL (2000) Identification of threonine residues controlling the agonist-dependent phosphorylation and desensitization of the rat A(3) adenosine receptor. Mol Pharmacol 57:539–545. [PubMed] [Google Scholar]
- Pavan B and IJzerman AP (1998) Processing of adenosine receptor agonists in rat and human whole blood. Biochem Pharmacol 56:1625–1632 [DOI] [PubMed] [Google Scholar]
- Pedata F, Pazzagli M, and Pepeu G (1991) Endogenous adenosine release from hippocampal slices: excitatory amino acid agonists stimulate release, antagonists reduce the electrically-evoked release. Naunyn Schmiedebergs Arch Pharmacol 344:538–543. [DOI] [PubMed] [Google Scholar]
- Peterfreund RA, Gies EK, and Fink JS (1997) Protein kinase C regulates adenosine A2a receptor mRNA expression in SH-SY5Y cells. Eur J Pharmacol 336:71–80. [DOI] [PubMed] [Google Scholar]
- Peterfreund RA, MacCollin M, Gusella J, and Fink JS (1996) Characterization and expression of the human A2a adenosine receptor gene. J Neurochem 66:362–368. [DOI] [PubMed] [Google Scholar]
- Peters DM, Gies EK, Gelb CR, and Peterfreund RA (1998) Agonist-induced desensitization of A2B adenosine receptors. Biochem Pharmacol 55:873–882. [DOI] [PubMed] [Google Scholar]
- Pierce KD, Furlong TJ, Selbie LA, and Shine J (1992) Molecular cloning and expression of an adenosine A2b receptors from human brain. Biochem Biophys Res Commun 187:86–93. [DOI] [PubMed] [Google Scholar]
- Pinna A, Fenu S, and Morelli M (2001) Motor stimulant effects of the adenosine A2A receptor antagonist SCH 58261 do not develop tolerance after repeated treatments in 6-hydroxydopamine-lesioned rats. Synapse 39:233–238. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, and Lefkowitz RJ (1998) G protein-coupled receptor kinases. Annu Rev Biochem 67:653–692. [DOI] [PubMed] [Google Scholar]
- Poli A, Lucchi R, Vibio M, and Barnabei O (1991) Adenosine and glutamate modulate each other’s release from rat hippocampal synaptosomes. J Neurochem 57:298–306. [DOI] [PubMed] [Google Scholar]
- Popoli P, Ferre S, Pezzola A, Reggio R, Scotti de Carolis A, and Fuxe K (1996) Stimulation of adenosine A1 receptors prevents the EEG arousal due to dopamine D1 receptor activation in rabbits. Eur J Pharmacol 305:123–126. [DOI] [PubMed] [Google Scholar]
- Popoli P, Reggio R, and Pezzola A (2000) Effects of Sch 58261, an adenosine A(2A) receptor antagonist, on quinpirole-induced turning in 6-hydroxydopamine-lesioned rats. Lack of tolerance after chronic caffeine intake. Neuropsychopharmacology 22:522–529 [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, and McCarley RW (1997) Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science (Wash DC) 276:1265–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucher SM, Keddie Jr, Singh P, Stoggall SM, Caulkett PW, Jones G and Coll MG (1995) The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br J Pharmacol 115:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull I and McIlwain H (1972) Metabolism of [14C]-adenine and derivatives by cerebral tissues, superfused and electrically stimulated. Biochem J 126:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitterer U and Lohse MJ (1999) Crosstalk between Galpha(i)- and Galpha(q)- coupled receptors is mediated by Gbetagamma exchange. Proc Natl Acad Sci U S A 96:10626–10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raberger G (1979) Cardiovascular and metabolic actions of adenosine and adenosine analogs, in Physiological and Regulatory Functions of Adenosine and Adenine Nucleotides (Baer HP and Drummond GI eds) pp 155–166, Raven Press, New York. [Google Scholar]
- Ramkumar V, Olah ME, Jacobson KA, and Stiles GL (1991) Distinct pathways of desensitization of A1 and A2-adenosine receptors in DDT1 MF-2 cells. Mol Pharmacol 40:639–647. [PMC free article] [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, and Ali H (1993) The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem 268:16887–16890. [PubMed] [Google Scholar]
- Ren H and Stiles GL (1995) Separate promoters in the human A1 adenosine receptor gene direct the synthesis of distinct messenger RNAs that regulate receptor abundance. Mol Pharmacol 48:975–980. [PubMed] [Google Scholar]
- Ren H and Stiles GL (1999) Dexamethasone stimulates human A1 adenosine receptor (A1AR) gene expression through multiple regulatory sites in promoter B. Mol Pharmacol 55:309–316. [DOI] [PubMed] [Google Scholar]
- Ren J and Stiles G (1994) Characterization of the human A1 adenosine receptor gene. J Biol Chem 269:3104–3110. [PubMed] [Google Scholar]
- Rieger JM, Brown ML, Sullivan GW, Linden J and, MacDonald TL (2001) Design, synthesis, and evaluation of novel adenosine A2A receptor agonists. J Med Chem 44:531–539. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Ferré S, Ӧgren SO and Fuxe K (1997) Adenosine A2A agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacology 17:82–91. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Barbhaiya H and IJzerman AP (1999a) Identification of the adenine binding site of the human A1 adenosine receptor. J Biol Chem 274:3617–3621. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Chen M, Kulkarni J, Browne J, and Zhao Z (1999b) Characterization of the murine A1 adenosine receptor promoter, potent regulation by GATA-4 and Nkx2.5. J Biol Chem 274:14204–14209. [DOI] [PubMed] [Google Scholar]
- Robeva AS, Woodard R, Jin X, Gao Z, Bhattacharya S, Taylor HE, Rosin DL, and Linden J (1996) Molecular characterization of recombinant human adenosine receptors. Drug Dev Res 39:243–252. [Google Scholar]
- Roelen H, Veldman N, Spek AL, von Frijtag Drabbe Kunzel J, Mathot RA and IJzerman AP (1996) N6, C8-distributed adenosine derivatives as partial agonists for adenosine A1 receptors. J Med Chem 39:1463–1471. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, and Linden J (1998) Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol 401:163–186. [PubMed] [Google Scholar]
- Ross SD, Tribble CG, Linden J, Gangemi JJ, Lanpher BC, Wang AY, and Kron IL (1999) Selective adenosine-A2A activation reduces lung reperfusion injury following transplantation. J Heart Lung Transplant 18:994–1002. [DOI] [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, and Fredholm BB (1992a) Adenosine and brain ischemia. Cerebrovasc Brain Metab Rev 4:346–369. [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, and Fredholm BB (1992b) Neuroprotective role of adenosine in cerebral ischemia. Trends Pharmacol Sci 13:439–445. [DOI] [PubMed] [Google Scholar]
- Sajjadi FG, Boyle DL, Domingo RC, and Firestein GS (1996) cDNA cloning and characterization of A3i, an alternatively spliced rat A3 adenosine receptor variant. FEBS Lett 382:125–129. [DOI] [PubMed] [Google Scholar]
- Sajjadi FG and Firestein GS (1993) cDNA cloning and sequence analysis of the human A3 adenosine receptor. Biochim Biophys Acta 1179:105–107. [DOI] [PubMed] [Google Scholar]
- Sala-Newby GB, Freeman NV, Skladanowski AC, and Newby AC (2000) Distinct roles for recombinant cytosolic 5’-nucleotidase-I and -II in AMP and IMP catabolism in COS-7 and H9c2 rat myoblast cell lines. J Biol Chem 275:11666–11671. [DOI] [PubMed] [Google Scholar]
- Sala-Newby GB, Skladanowski AC, and Newby AC (1999) The mechanism of adenosine formation in cells. Cloning of cytosolic 5’-nucleotidase-I. J Biol Chem 274:17789–17793. [DOI] [PubMed] [Google Scholar]
- Salvatore CA, Jacobson MA, Taylor HE, Linden J, and Johnson RG (1993) Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci U S A 90:10365–10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, and Jacobson MA (2000) Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem 275:4429–4434. [DOI] [PubMed] [Google Scholar]
- Sato T, Sasaki N, O’Rourke B, and Marban E (2000) Adenosine primes the opening of mitochondrial ATP-sensitive potassium channels: a key step in ischemic preconditioning? Circulation 102:800–805. [DOI] [PubMed] [Google Scholar]
- Sattin A and Rall TW (1970) The effect of adenosine and adenine nucleotides on the cyclic adenosine 3’, 5’ phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol 6:13–23. [PubMed] [Google Scholar]
- Sauer R, Maurinsh J, Reith U, Fulle F, Klotz KN, and Muller CE (2000) Water-soluble phosphate prodrugs of 1-propargyl-8-styrylxanthine derivatives, A(2A)-selective adenosine receptor antagonists. J Med Chem 43:440–448. [DOI] [PubMed] [Google Scholar]
- Saunders C, Keefer Jr, Bonner CA and Limbird LE (1998) Targeting of G protein-coupled receptors to the basolateral surface of polarized renal epithelial cells involves multiple, non-contiguous structural signals. J Biol Chem 273:24196–24206. [DOI] [PubMed] [Google Scholar]
- Saunders C, Keefer Jr, Kennedy AP, Wells JN and Limbird LE (1996) Receptors coupled to pertussis toxin-sensitive G-proteins traffic to opposite surfaces in Madin-Darby canine kidney cells. A1 adenosine receptors achieve apical and alpha 2A adrenergic receptors achieve basolateral localization. J Biol Chem 271:995–1002. [DOI] [PubMed] [Google Scholar]
- Saunders C and Limbird LE (1997) Disruption of microtubules reveals two independent apical targeting mechanisms for G-protein-coupled receptors in polarized renal epithelial cells. J Biol Chem 272:19035–19045. [DOI] [PubMed] [Google Scholar]
- Saura C, Ciruela F, Casado V, Canela EI, Mallol J, Lluis C, and Franco R (1996) Adenosine deaminase interacts with A1 adenosine receptors in pig brain cortical membranes. J Neurochem 66:1675–1682. [DOI] [PubMed] [Google Scholar]
- Saura CA, Mallol J, Canela EI, Lluis C, and Franco R (1998) Adenosine deaminase and A1 adenosine receptors internalize together following agonist-induced receptor desensitization. J Biol Chem 273:17610–17617. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, and Vanderhaeghen JJ (1991) Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem 57:1062–1067. [DOI] [PubMed] [Google Scholar]
- Scholl DJ and Wells JN (2000) Serine and alanine mutagenesis of the nine native cysteine residues of the human A(1) adenosine receptor. Biochem Pharmacol 60:1647–1654. [DOI] [PubMed] [Google Scholar]
- Schubert P, Komp W, and Kreutzberg GW (1979) Correlation of 5’-nucleotidase activity and selective transneuronal transfer of adenosine in the hippocampus. Brain Res 168:419–424. [DOI] [PubMed] [Google Scholar]
- Schulte G and Fredholm BB (2000) Human adenosine A1, A2A, A2B, and A3 receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol 58:477–482. [PubMed] [Google Scholar]
- Schwabe U and Trost T (1980) Characterization of adenosine receptors in rat brain by (−)[3H]-N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol 313:179–187 [DOI] [PubMed] [Google Scholar]
- Seger R and Krebs EG (1995) The Mapk signaling cascade. FASEB J 9:726–735. [PubMed] [Google Scholar]
- Seidel MG, Klinger M, Freissmuth M, and Holler C (1999) Activation of mitogen-activated protein kinase by the A(2A)-adenosine receptor via a rap1-dependent and via a p21(ras)-dependent pathway. J Biol Chem 274:25833–25841. [DOI] [PubMed] [Google Scholar]
- Sexl V, Mancusi G, Holler C, Gloria-Maercker E, Schutz W, and Freissmuth M (1997) Stimulation of the mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. J Biol Chem 272:5792–5799. [DOI] [PubMed] [Google Scholar]
- Smith RG, Griffin PR, Xu Y, Smith AG, Liu K, Calacay J, Feighner SD, Pong C, Leong D, Pomes A, Cheng K, Van der Ploeg LH, Howard AD, Schaeffer J and Leonard RJ (2000) Adenosine A partial agonist of the growth hormone secretagogue receptor. Biochem Biophys Res Commun 276:1306–1313. [DOI] [PubMed] [Google Scholar]
- Soma M, Nakayama T, Satoh M, Uwabo J, Rahmutula D, Takahashi Y, Fukuda N, Watanabe Y, Izumi Y, and Kanmatsuse K (1998) A T1083C polymorphism in the human adenosine A2a receptor gene is not associated with essential hypertension. Am J Hypertens 11:1492–1494. [DOI] [PubMed] [Google Scholar]
- Spychala J, Datta NS, Takabayashi K, Datta M, Fox IH, Gribbin T, and Mitchell BS (1996) Cloning of human adenosine kinase cDNA: sequence similarity to microbial ribokinases and fructokinases. Proc Natl Acad Sci U S A 93:1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, and Reppert SM (1992) Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol Endocrinol 6:384–393. [DOI] [PubMed] [Google Scholar]
- Stein E, Zou Y, Poo M, and Tessier-Lavigne M (2001) Binding of Dcc by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science (Wash DC) 291:1976–1982. [DOI] [PubMed] [Google Scholar]
- Sugden PH and Clerk A (1998) Regulation of mitogen-activated protein kinase cascades in the heart. Adv Enzyme Regul 38:87–98. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Rieger JM, Scheld WM, MacDonald TL, and Linden J (2001) Cyclic Amp-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A2A receptor agonists. Br J Pharmacol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, and Schnermann J (2001) Meditation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Nat Acad Sci USA 98: 9983–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Fourreau L, Bloch B, Fredholm BB, Gonon F, and Le Moine C (1999a) Opposite tonic modulation of dopamine and adenosine on c-fos mRNA expression in striatopallidal neurons. Neuroscience 89:827–837. [DOI] [PubMed] [Google Scholar]
- Svenningsson P and Fredholm BB (1997) Glucocorticoids regulate the expression of adenosine A1 but not A2A receptors in rat brain. J Pharmacol Exp Ther 280:1094–1101. [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, and Fredholm BB (1999b) Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol 59:355–396. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, and Fredholm BB (1997) Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience 80:1171–1185. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm BB, and Fisone G (2000) Regulation of the phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa in vivo by dopamine D1, dopamine D2 and adenosine A2A receptors. Proc Natl Acad Sci U S A 97:1856–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Rognoni F, Fredholm BB, Greengard P, and Fisone G (1998a) Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience 84:223–228. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nergårdh R, and Fredholm BB (1998b) Regional differences in the ability of caffeine to affect haloperidol-induced striatal c-fos mRNA expression in the rat. Neuropharmacology 37:331–337. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, and Fredholm BB (1995) Biphasic changes in locomotor behavior and in expression of mRNA for NGFI-A and NGFI-B in rat striatum following acute caffeine administration. J Neurosci 15:7612–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, and Fredholm BB (1999c) The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J Neurosci 19:4011–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson TH, Drazba JA, and Rivkees SA (1995) Adenosine A1 receptors are located predominantly on axons in the rat hippocampal formation. J Comp Neurol 363:517–531. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, and Koller BH (2000) Adenosine and inosine increase cutaneous vasopermeability by activating A(3) receptors on mast cells. J Clin Invest 105:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA, and Westfall DP (1997) Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature (Lond) 387:76–79. [DOI] [PubMed] [Google Scholar]
- Townsend-Nicholson A and Schofield PR (1994) A threonine residue in the seventh transmembrane domain of the human A1 adenosine receptor mediates specific agonist binding. J Biol Chem 269:2373–2376. [PubMed] [Google Scholar]
- Townsend-Nicholson A and Shine J (1992) Molecular cloning and characterization of a human brain A1 adenosine receptor cDNA. Brain Res Mol Brain Res 16:365–370. [DOI] [PubMed] [Google Scholar]
- Trivedi BK, Bridges AJ, Patt WC, Priebe SR, and Bruns RF (1989) N6-bicycloalkyladenosines with unusually high potency and selectivity for the adenosine A1 receptor. J Med Chem 32:8–11. [DOI] [PubMed] [Google Scholar]
- Tucker AL, Jia L, Holeton D, Taylor AJ, and Linden J (2000) Dominance of Gs in doubly Gs/Gi-coupled chimeric A1/A2A adenosine receptors in HEK-293 cells. Biochem J 352(Pt 1):203–210. [PMC free article] [PubMed] [Google Scholar]
- Tucker AL, Linden J, Robeva AS, D’Angelo DD, and Lynch KR (1992) Cloning and expression of a bovine adenosine A1 receptor cDNA. FEBS Lett 297:107–111. [DOI] [PubMed] [Google Scholar]
- Tucker AL, Robeva AS, Taylor HE, Holeton D, Bockner M, Lynch KR, and Linden J (1994) A1 adenosine receptors. Two amino acids are responsible for species differences in ligand recognition. J Biol Chem 269:27900–27906. [PubMed] [Google Scholar]
- Tullin S, Hansen BS, Ankersen M, Moller J, Von Cappelen KA, and Thim L (2000) Adenosine is an agonist of the growth hormone secretagogue receptor. Endocrinology 141:3397–3402. [DOI] [PubMed] [Google Scholar]
- Unger VM, Hargrave PA, Baldwin JM, and Schertler GF (1997) Arrangement of rhodopsin transmembrane alpha-helices. Nature (Lond) 389:203–206. [DOI] [PubMed] [Google Scholar]
- van Calker D, Muller M, and Hamprecht B (1978) Adenosine inhibits the accumulation of cyclic AMP in cultured brain cells. Nature (Lond) 276:839–841. [DOI] [PubMed] [Google Scholar]
- van Calker D, Muller M, and Hamprecht B (1979) Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem 33:999–1005. [DOI] [PubMed] [Google Scholar]
- van der Klein PA, Kourounakis AP and IJzerman AP (1999) Allosteric modulation of the adenosine A(1) receptor. Synthesis and biological evaluation of novel 2-amino-3-benzoylthiophenes as allosteric enhancers of agonist binding. J Med Chem 42:3629–3635. [DOI] [PubMed] [Google Scholar]
- van der Wenden EM, Carnielli M, Roelen HC, Lorenzen A, von Frijtag Drabbe Kunzel JK and IJzerman AP (1998) 5’-substituted adenosine analogs as new high-affinity partial agonists for the adenosine A1 receptor. J Med Chem 41:102–108. [DOI] [PubMed] [Google Scholar]
- van Galen Pjm, Van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL and Jacobson KA (1994) A binding site model and structure-activity relationships for the rat A2 adenosine receptor. Mol Pharmacol 45:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- van Muijlwijk-Koezen JE, Timmerman H, Link R, van der Goot H, and Ijzerman AP (1998) A novel class of adenosine A3 receptor ligands. 2. Structure affinity profile of a series of isoquinoline and quinazoline compounds. J Med Chem 41:3994–4000. [DOI] [PubMed] [Google Scholar]
- van Muijlwijk-Koezen JE, Timmerman H, van der Goot H, Menge WM, Frijtag Von Drabbe Kunzel J, de Groote M and IJzerman AP (2000) Isoquinoline and quinazoline urea analogues as antagonists for the human adenosine A(3) receptor. J Med Chem 43:2227–2238. [DOI] [PubMed] [Google Scholar]
- van Rhee AM and Jacobson KA (1996) Molecular architecture of G protein-coupled receptors. Drug Dev Res 37:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaick EA, Jacobson KA, Kim HO, IJzerman AP and Danhof M (1996) Hemodynamic effects and histamine release elicited by the selective adenosine A3 receptor agonist 2-Cl-IB-MECA in conscious rats. Eur J Pharmacol 308:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaick EA, Tukker HE, Roelen HC, IJzerman AP and Danhof M (1998) Selectivity of action of 8-alkylamino analogues of N6-cyclopentyladenosine in vivo: hemodynamic versus anti-lipolytic responses in rats. Br J Pharmacol 124:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapaatalo H, Onken D, Neuvonen PJ, and Westermann E (1975) Stereospecificity in some central and circulatory effects of phenylisopropyl-adenosine (PIA). Arzneimittelforschung 25:407–410. [PubMed] [Google Scholar]
- Varani K, Merighi S, Gessi S, Klotz KN, Leung E, Baraldi PG, Cacciari B, Romagnoli R, Spalluto G, and Borea PA (2000) [(3)H]MRE 3008F20 a novel antagonist radioligand for the pharmacological and biochemical characterization of human A(3) adenosine receptors. Mol Pharmacol 57:968–975. [PubMed] [Google Scholar]
- Vilsvik JS, Persson CG, Amundsen T, Brenna E, Naustdal T, Syvertsen U, Storstein L, Kállén AG, Eriksson G, and Holte S (1990) Comparison between theophylline and an adenosine non-blocking xanthine in acute asthma. Eur Respir J 3:27–32. [PubMed] [Google Scholar]
- Williams M, Francis J, Ghai G, Braunwalder A, Psychoyos S, Stone GA, and Cash WD (1987) Biochemical characterization of the triazoloquinazoline, CGS 15943, a novel, non-xanthine adenosine antagonist. J Pharmacol Exp Ther 241:415–420. [PubMed] [Google Scholar]
- Williams TC and Jarvis SM (1991) Multiple sodium-dependent nucleoside transport systems in bovine renal brush-border membrane vesicles. Biochem J 274:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn HR, Rubio R, and Berne RM (1981) Brain adenosine concentration during hypoxia in rat. Am J Physiol 241:H235–H242. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, and Hu XT (1994) MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J Neurosci 14:1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak M and Limbird LE (1998) Trafficking itineraries of G protein-coupled receptors in epithelial cells do not predict receptor localization in neurons. Brain Res 780:311–322. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Lindorfer MA, Myung CS, and Garrison JC (1998) Phosphorylation of the G protein gamma12 subunit regulates effector specificity. J Biol Chem 273:21958–21965. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Lindorfer MA, Woodfork KA, Fletcher JE, and Garrison JC (1996) Role of the prenyl group on the G protein gamma subunit in coupling trimeric G proteins to A1 adenosine receptors. J Biol Chem 271:18588–18595. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Simosky JK, Mayfield RD, Negri CA, Hanania T, Larson GA, Kelly MA, Grandy DK, Rubinstein M, Low MJ, and Fredholm BB (2000) Functional uncoupling of adenosine A2A receptors and reduced response to caffeine in mice lacking dopamine D2 receptors. J Neurosci 20:5949–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterström T, Vernet L, Ungerstedt U, Tossman U, Jonzon B, and Fredholm BB (1982) Purine levels in the intact rat brain. Studies with an implanted perfused hollow fiber. Neurosci Lett 29:111–115. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Francis C, and Ravid K (1999) Characterization of the mouse A3 adenosine receptor gene: exon/intron organization and promoter activity. Genomics 57:152–155. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Makaritsis K, Francis CE, Gavras H, and Ravid K (2000) A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: studies in knock-out mice. Biochim Biophys Acta 1500:280–290. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, and Civelli O (1992) Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci U S A 89:7432–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362:299–309. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Braun N, Kegel B, and Heine P (1998) New insights into molecular structure and function of ectonucleotidases in the nervous system. Neurochem Int 32:421–425. [DOI] [PubMed] [Google Scholar]


