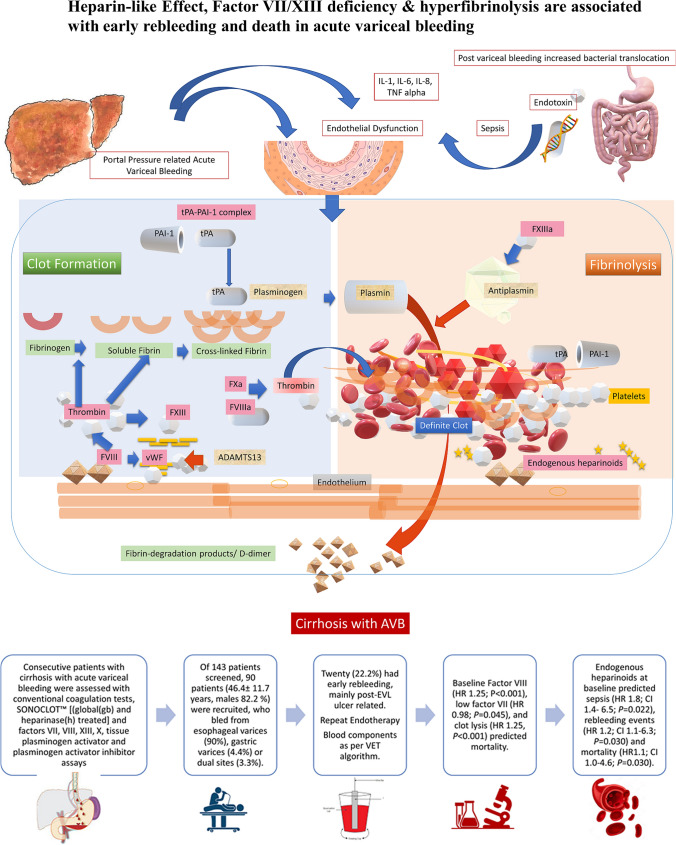
Abstract
Background
Hyperfibrinolysis and coagulation dysfunction may occur in cirrhotic patients with acute variceal bleed (AVB) despite successful endotherapy.
Aims
To prospectively study the association of endogenous heparinoids and coagulation dysfunction with variceal rebleeding and outcome in cirrhosis.
Methods
Consecutive patients were assessed with conventional coagulation tests, SONOCLOT™ [(global(gb) and heparinase(h) treated] and factors VII, VIII, XIII, X, tissue plasminogen activator, and plasminogen activator inhibitor ELISA assays in a university hospital. Heparin-like-effect (HLE) was defined as ≥ 20% difference in paired gb/h-SONOCLOT™ traces for activated clotting time (ACT).
Results
Of 143 patients screened, 90 (46.4 ± 11.7 years, males 82.2%, ethanol-related 58.8%) were recruited, who bled from esophageal varices (81,90.0%), gastric varices (6,6.6%), or esophageal varices with portal hypertensive gastropathy (3,3.3%). Twenty (21.7%) had early rebleeding, mainly post-variceal ligation ulcer related (70%). Patients who rebled had low Factor XIII [1.6 (1.2–2.1) vs 2.4 ng/ml (2.0–2.8) P = 0.035] and Factor VII (94.1 ± 46.9 vs. 124.0 ± 50.4, P = 0.023). On receiver operating curve analysis, the gbACT > 252 s (sensitivity 86.8%, specificity 76.9%, P < 0.001), hACT > 215 s (sensitivity 71.1%, specificity 70.3%, P < 0.001), and HLE > 50% (sensitivity 69.5%, specificity 70.3%, P = 0.006) predicted rebleeding. Baseline Factor VIII (HR 1.26; 95% CI 1.17–1.34, P < 0.001), low factor VII (HR 0.89; 95% CI 0.76–0.98, P = 0.035), and lysis (HR 1.25, 95% CI 1.17–1.33, P < 0.001) predicted mortality. Endogenous heparinoids at baseline predicted sepsis (HR 1.8; 95% CI 1.4–6.5; P = 0.022), rebleeding events (HR 1.2; 95% CI 1.1–6.3; P = 0.030), and mortality (HR 1.1; 95% CI 1.0–4.6; P = 0.030).
Conclusions
Hyperfibrinolysis, Factor VII/XIII deficiency, and HLE are associated with rebleeding after AVB.
Trial Registration NCT04111120 available from https://clinicaltrials.gov/ct2/show/NCT04111120.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-022-07656-9.
Keywords: Endogenous heparinoids, Portal hypertension, Sonoclot, Transfusion algorithms, Acute-on-chronic liver failure
Introduction
Patients with cirrhosis and acute-on-chronic liver failure (ACLF) have complex dynamic coagulation dysfunction [1]. In patients with cirrhosis who present with acute variceal bleeding (AVB), early appropriate endoscopic intervention, use of portal pressure reducing vasoconstrictor agents, and antibiotic prophylaxis are the main methods to reduce mortality. However, changes in anti- and procoagulant factors, hyperfibrinolysis, and endothelial activation can lead to early rebleeding or even thromboses [2]. Endothelial activation due to systemic inflammation or sepsis in cirrhosis results in an increase in Factor VIII and von Willebrand factor (vWF). This causes glycosaminoglycans (heparin sulfate, heparin, and dermatan sulfate) to be released from the endothelium into the circulation, which cause a ‘heparin-like effect’ (HLE) [3]. This anticoagulant effect cannot be detected by standard coagulation tests (SCTs) like prothrombin time (PT), international normalized ratio (INR), D-dimer, or activated partial thromboplastin time (aPTT) but can be demonstrated on heparinase-treated viscoelastic tests [4]. Bacterial sepsis is a known risk factor for rebleeding due to increased portal pressures, splanchnic arterial vasodilation, and coagulation failure [5].
The rationale for evaluating complex coagulation dysfunction in patients with AVB is predicting the patients at higher risk of rebleeding, sepsis, and decompensation events. It is also unclear if coagulation failure contributes to rebleeding, as portal hypertensive bleeding is directly linked to higher portal pressures and severity of underlying disease. The role of specific coagulation factors like VII, VIII, XIII, and X, vWF, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), tissue plasminogen activator (tPA), and plasminogen activator inhibitor-1 (PAI-1) in the process of clot formation and fibrinolysis in AVB and thromboses needs further evaluation [4, 6–11].
Point-of-care (POC) viscoelastic tests like thromboelastography, Sonoclot, or thromboelastometry can detect hyperfibrinolysis, and help guide use of blood component therapy in patients with diffuse mucosal bleeding, disseminated intravascular coagulation, or bleeding from multiple sites [1, 2, 4, 12]. Assessing the role of specific components like Factor VII concentrate or multifactor concentrate like prothrombin complex concentrate (PCC) has been assessed in a few studies [13–15]. Use of these viscoelastic tests can minimize blood transfusions as an adjunct to the main goal of timely endoscopic interventions and use of drugs to reduce portal pressure like terlipressin and octreotide.
In this prospective study, we evaluated patients with cirrhosis who presented with AVB to our center, assessed them with SCTs and glass bead (gb) and heparinase(h)-treated Sonoclot assays, clot lysis, specific coagulation factor levels (including factors VII, VIII, X, and XIII), vWF, tPA, PAI-1, and ADAMTS13 and determined the role of endogenous heparinoids, viscoelastic tests, coagulation parameters that were associated with fibrinolysis and early rebleeding, sepsis, and outcomes at 30 and 90 days. We aimed to assess the association of endothelial dysfunction and fibrinolysis as a predictor of rebleeding.
Methods
Consecutive patients with cirrhosis with acute variceal bleeding (AVB), aged between 18 and 65 years, either gender who presented to the Department of Hepatology, Postgraduate Institute of Medical Education and Research (PGIMER) Chandigarh were recruited with written informed consent between November 2019 and July 2021. The exclusion criteria were recent blood or blood component transfusion in the last 2 weeks, HIV infection, antiplatelet, anticoagulant or antifibrinolytic therapy, dialysis, pregnancy, portal vein thrombosis or recent deep vein thromboses in the last 1 year, transjugular intrahepatic portosystemic shunt (TIPS) in situ, active malignancy in the last 5 years, chronic heart failure, and/or chronic pulmonary or end-stage renal disease. Patients who tested positive for COVID-19 infection were excluded from analysis as they would also have confounding coagulation abnormalities [16]. The protocol was performed in accordance with the Declaration of Helsinki and adhered to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Definitions
Cirrhosis was defined based on clinical and imaging criteria with endoscopic presence of varices. Early rebleeding was defined as: (1) recurrent hematemesis, and/or melena, and/or bloody fluid drained by nasogastric tube, occurring 6 to 42 days after the first endoscopy [17]. The presence of infection was assessed by blood, urine, sputum, ascitic fluid, and tracheal aspirate cultures, C-reactive protein, and procalcitonin or new infiltrates on chest radiographs. Major bleeding was defined as patients with fatal bleeding, symptomatic bleeding in a critical area and/or causing a fall in hemoglobin level of ≥ 2 g/dL or leading to transfusion of ≥ 2 units of packed red cells [18]. Sepsis (life-threatening organ dysfunction caused by a dysregulated host response to infection) was defined as per the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [19].
Standard Treatment
Interventions included the use of fluid resuscitation, prophylactic antibiotics, terlipressin, or octreotide as necessary. All patients underwent endoscopic management of AVB as per standard guidelines [17, 20]. Patients with esophageal variceal bleed were managed with endoscopic variceal ligation (EVL). Patients with gastric variceal bleed received endotherapy with N-butyl acrylate glue. Terlipressin or octreotide infusion for 5 days with use of proton pump inhibitors was as per standard guidelines. Standard medical therapy included nutritional intervention, albumin, diuretics, antivirals for hepatitis B and hepatitis C, and vitamin supplements. The standard antibiotic regimen was a cephalosporin, or a beta-lactam, which was changed as per cultures. Administration of blood components was based on our POC viscoelastic test based algorithm which ensures a restrictive transfusion strategy to improve ACT, platelet function or fibrinogen defects following primary endoscopic management where additional coagulation related bleeding was suspected (Supplementary information) [21, 22]. No prophylactic transfusions were given to correct abnormal INR or platelet count, and blood component therapy was as per standard guidelines [20].
COVID-19 Related Changes in Healthcare Delivery During the Study
One of the factors affecting the protocol was that the study was conducted during the COVID-19 pandemic in India. The intensive care, hepatology inpatient wards and routine endoscopy units were diverted to the COVID-19 care for more than a year in our center, and out patient clinics were restricted to admit only a few persons. We were offering emergency services including endoscopy for inpatients, and critically ill patients with AVB, cholangitis etc. Only such patients were admitted in our medical wards. Thus, all patients enrolled in the study were managed as per standard of care during the primary admission, when primary management, coagulation tests and endoscopic interventions were done. After discharge, they were called for outpatient follow ups or teleconsultation. However due to limited slots for surveillance endoscopy at our center, many patients would seek treatment at secondary healthcare centers or accessible endoscopy units. Hence patients with decompensation events were often managed at peripheral medical units and follow up data was completed using the telemedical records.
Outcome Measures
The primary outcomes were to assess the viscoelastic test results, endogenous heparinoids, association of fibrinolysis, and coagulation factor levels with rebleeding and outcomes like sepsis/death at 30 days and 90 days. Predictors of mortality were based on SCTs, Sonoclot tests and coagulation factor assays.
Assessment of Coagulation Parameters
Blood samples were taken at admission to the liver intensive care unit. The complete blood count was performed on a hematology autoanalyzer (Coulter Hmx Hematology Analyser; Beckman Coulter Inc., Brea, California, USA). Coagulation parameters measured included PT, aPTT, INR, D-dimer, and fibrinogen. The individual coagulation factor assays performed were Factor VIII (EL-H3641), vWF (EL-H2168), Factor XIII, PAI (EL-H2104) and tPA (EL-H2106), Factor X (EL-H0761) and ADAMTS13(EL-H1552) using commercially available ELISA kits from Elabscience; Houston, Texas, USA. Cytokine levels, IL-1β and IL-6 (Assaypro;St. Charles, MO 63301, USA) and serum endotoxin levels were tested using ELISA kits (Pierce Chromogenic Endotoxin Quant kit; A39552) by Thermo Scientific. [1, 4]
SONOCLOT™ Analysis
Global and heparinase-treated coagulation analysis was done on the analyser (SONOCLOT Coagulation and Platelet Function Analyzer, Sienco Inc., Arvada, CO, USA). HLE was calculated with the percentage correction of the ACT time on SONOCLOT with the addition of heparinase (Supplementary Fig. 1).
HLE was diagnosed when the correction of the ACT time is greater than 20%, and severe HLE was defined as a value greater than 50% [1]. In addition to the AVB cohort, we also performed coagulation factor assays in chronic hepatitis C related cirrhosis (N = 20) and healthy controls (N = 20) to get population reference ranges for these tests (Supplementary Table 1).
Statistical Analysis
Descriptive statistics are expressed as mean with standard deviation (SD) for parametric data and median with interquartile range (IQR) for non-parametric continuous data. Categorical data are expressed as numbers (n, %). We used Student’s t-test to compare continuous data and Pearson’s Chi-square test or Fisher’s exact test (as appropriate) to compare categorical data among the two groups. The main outcomes are reported as estimated effect sizes along with precision (95% confidence intervals [CIs]). Cox proportional hazard analysis was performed for the predictors of 90-day mortality or rebleeding events using models incorporating coagulation factor assays or Sonoclot components for hyperfibrinolysis or HLE. Kaplan Meier survival analysis was also carried out to assess the incidence of rebleeding and its effect on mortality at days 30 and 90. Statistical significance was set at P < 0.05. All statistical tests were performed using SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). Sample size was estimated assuming the incidence of HLE in cirrhosis with AVB to be 10–30% as per prior published data [4, 11, 12]. It was estimated that a total sample size of 75 patients with cirrhosis would be required, with an effect size of 0.5, alpha 0.05, and power 0.85. Due to the onset of the COVID-19 pandemic, we recruited an additional 20% cases to account for attrition.
Results
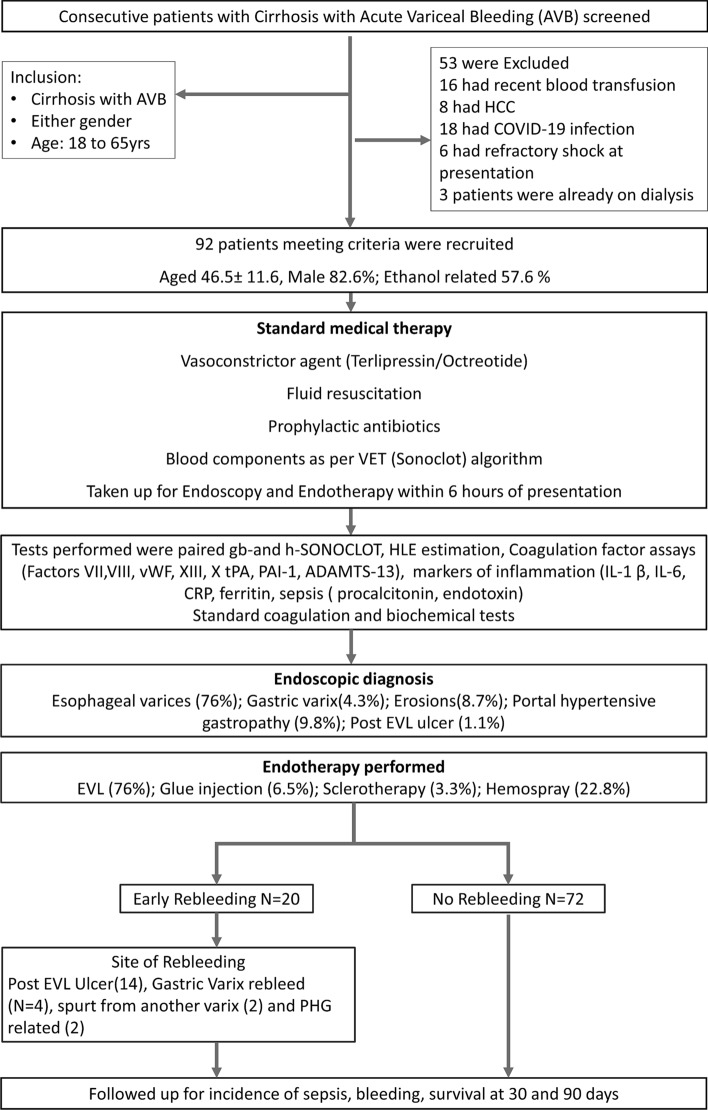
A total of 143 patients with cirrhosis with AVB were assessed between December 1, 2019, and July 31, 2021, of whom 90 were enrolled in the study. Fifty-three patients were excluded due to recent blood transfusions (16,30.1%), presence of hepatocellular carcinoma (8,15.0%), COVID-19 infection (18,33.9%), refractory septic shock (6,11.3%) or were on dialysis (3,5.6%). Two (3.7%) patients were excluded from analysis as they only had portal hypertensive gastropathy (PHG) related bleeding (Fig. 1). Finally, 90 patients (age-46.4 ± 11.7 years, males 82.2%, alcohol 58.8%) were recruited, and followed up for rebleeding, sepsis, and transplant-free survival at 30 and 90 days (Fig. 1).
Fig. 1.
Enrollment flowchart of the study
The baseline characteristics of the patients are shown in Table 1. The site of bleeding was due to esophageal varices (81, 90%), gastric varices (6,6.6%), or from both esophageal varices and portal hypertensive gastropathy (PHG; 3, 3.3%). The primary modalities of management were EVL (75,83.3%), glue injection (6,6.6%), EVL & glue injection (6,6.6%) EST (3, 3.3%), and hemospray (21,22.8%) as monotherapy or in combination. Overall, rebleeding was noted in 20 (21.7%). The main causes of rebleeding were post-EVL ulcers (14,70%), rebleed from gastric varix (4,20%), spurt from another varix (1,5%), diffuse PHG related bleed (1,5%). They were managed with one or more endotherapy techniques, including banding below the ulcers (4,20%), hemospray (8,40%), repeat glue injection (5,25%), SX-Ella Danis stent (2,10%), and 1 (5%) patient underwent rescue TIPS successfully. No significant differences were noted in baseline hemoglobin, platelet count, INR, albumin, MELDNa (Model for End-stage Liver Disease) or Child score between those who did or did not rebleed. However, IL1β (P = 0.010), aPTT (P = 0.045), D-dimer (P = 0.013) were significantly different between the two groups. Overall mortality was 39(43.3%), with 90-day mortality of 15(75%) and 24 (34.2%) in patients with and without rebleeding, respectively.
Table 1.
Baseline characteristics, endoscopic management, investigations, and blood transfusion details of patients with cirrhosis with acute variceal bleeding
| Parameter | Total patients | Bleeding | No rebleeding | P value |
|---|---|---|---|---|
| N = 90 | N = 20 | N = 70 | ||
| Age (years) | 46.4 ± 11.7 | 46.4 ± 12.1 | 46.5 ± 11.7 | 0.994 |
| Sex (male) | 74 (82.2%) | 17 (85.0%) | 57 (81.4%) | 0.502 |
| BMI (kg/m2) | 24.2 ± 6.3 | 21.8 ± 4.1 | 24.9 ± 6.7 | 0.079 |
| Height (cm) | 164.4 ± 21.1 | 167.0 ± 8.9 | 163.6 ± 23.7 | 0.568 |
| Weight (kg) | 68.8 ± 18.6 | 64.1 ± 15.8 | 70.4 ± 19.2 | 0.218 |
| DM-2 | 20 (22.2%) | 7 (35.0%) | 13 (18.5%) | 0.370 |
| HTN | 21 (23.3%) | 5 (25.0%) | 16 (22.8%) | 0.848 |
| Etiology of liver disease | ||||
| • NASH | 21 (23.3%) | 4 (20.0%) | 17 (24.2%) | 0.988 |
| • Ethanol | 53 (58.8%) | 14 (70.0%) | 39 (55.7%) | 0.967 |
| • AIH | 2 (2.2%) | 0 | 2 (2.8%) | 0.841 |
| • Viral HBV/HCV | 16 (17.7%) | 2 (10%) | 14 (20.0%) | 0.724 |
| CTP* | 9 (8–12) | 9 (8–14) | 9 (8–11) | 0.887 |
| MELDNa* | 18.5 (14.8–21.1) | 20.1 (15.6–24.4) | 17.4 (13.8–22.2) | 0.074 |
| Hemoglobin (g/dl) | 8.1 ± 2.3 | 7.7 ± 1.6 | 8.1 ± 2.4 | 0.498 |
| TLC (× 109/l)* | 11.2 (9.2–13.2) | 9.7 (6.0–13.3) | 11.8 (9.3–14.2) | 0.376 |
| Platelet Count (× 109/l)* | 94.8 (80.2–109.5) | 103.7 (57.5–150) | 92.0 (78.5–105.5) | 0.497 |
| Urea (mg/dl)* | 69.6 (59.3–79.9) | 75.0 (55.9–94.0) | 67.8 (55.4–80.3) | 0.558 |
| Creatinine(mg/dl)* | 1.9 (0.9–2.6) | 1.8 (1.2–2.4) | 2.0 (1.6–2.4) | 0.627 |
| AST (U/L)* | 133.9 (88.5–179.4) | 189.3 (18.6–360.5) | 116.2 (85–147.4) | 0.170 |
| ALT (U/L)* | 68.9 (41.6–96.1) | 68.8 (23.8–113.8) | 68.9 (35.1–102.7) | 0.998 |
| LDH (U/L) | 357.5 ± 55.1 | 303.6 ± 62.5 | 365 ± 76.1 | 0.538 |
| Total bilirubin (mg/dl) | 2.8 ± 0.9 | 3.2 ± 0.5 | 2.1 ± 0.8 | 0.289 |
| Total protein (g/dl) | 5.9 ± 1.0 | 5.8 ± 1.1 | 6.0 ± 0.9 | 0.584 |
| Albumin (g/dl) | 2.7 ± 0.5 | 2.6 ± 0.5 | 2.7 ± 0.5 | 0.543 |
| Lactate (mmol/l) | 3.0 (2.5–3.1) | 3.1 (2.2–3.9) | 3.0 (2.4–3.6) | 0.809 |
| IL-1β (ng/ml) | 14.4 ± 5.4 | 17.2 ± 6.8 | 13.6 ± 4.7 | 0.010 |
| IL-6 (ng/ml) | 13.2 (10.6–15.7) | 15.2 (10.3–19.9) | 12.7 (9.8–15.6) | 0.414 |
| CRP (mg/l)* | 46.8 (21.3–58.6) | 50.1 (18.6–70.3) | 45.6 (18.2–60.1) | 0.075 |
| Procalcitonin (ng/ml)* | 1.1 (0.04–46.2) | 1.9 (0.12–61.9) | 0.9 (0.04–29.4) | 0.129 |
| Ferritin (ng/ml) | 186.5 ± 43.5 | 165.7 ± 67.3 | 194.2 ± 32.5 | 0.083 |
| NLR* | 10.4 (8.7–12.1) | 13.4 (9.1–17.6) | 9.5 (7.7–11.3) | 0.057 |
| Serum endotoxin level (EU/mL) | 17.8 ± 5.2 | 19.7 ± 6.2 | 16.8 ± 4.7 | 0.065 |
| Endoscopic diagnosis (active bleeding from one or more sites) | ||||
| Esophageal varices | 81 (90.0%) | 16 (75.0%) | 65 (92.8%) | 0.069 |
| Gastric varices | 4 (4.4%) | 1 (5.0%) | 3 (4.2%) | 0.784 |
| Esophageal varices with severe portal hypertensive gastropathy | 3 (3.3%) | 2 (10.0%) | 1 (1.4%) | 0.782 |
| Post-EVL ulcer | 2 (2.2%) | 1 (5.0%) | 1 (1.4%) | |
| Endotherapy (monotherapy or combination of interventions used) | ||||
| Endoscopic sclerotherapy | 3 (3.3%) | 1 (5.0%) | 2 (2.8%) | 0.065 |
| Endoscopic variceal ligation (EVL) | 75 (83.3%) | 15 (75.0%) | 50 (71.4%) | 0.422 |
| EVL + glue injection | 6 (6.5%) | 0 | 6 (8.3%) | 0.632 |
| Glue injection | 6 (6.6%) | 1 (5.0%) | 5 (6.9%) | 0.280 |
| Hemospray | 21 (23.3%) | 5 (25.0%) | 16 (22.2%) | 0.089 |
| New thrombosis at any site | 8 (8.8%) | 3 (15.0%) | 5 (7.1%) | 0.056 |
| Blood/component received (N, %) | 55 (61.1%) | 11 (55.0%) | 44 (62.8%) | 0.043 |
| PRBC* | 2.1 (1.5–2.7) | 2.3 (1.1–3.7) | 2.1 (1.4–2.8) | 0.864 |
| FFP* | 2.2 (2.1–4.2) | 2.3 (1.9–3.9) | 1.5 (1.3–2.4) | 0.746 |
| SDPC* | 0.7 (0.3–1.2) | 1.1 (0.8–2.4) | 0.5 (0.3–1.1) | 0.056 |
| Cryoprecipitate* | 3.4 (1.3–3.5) | 4 (1.4–5.1) | 3.1 (1.2–3.9) | 0.032 |
| RDPC* | 2.2 (1.2–4.1) | 2.5 (1.4–4.8) | 2.9 (1.1–4.2) | 0.055 |
AIH autoimmune hepatitis, AST aspartate transaminase, ALT alanine transaminase, BMI body mass index, CTP child turcotte pugh, CRP high-sensitivity C-reactive protein, DM-2 Diabetes mellitus, EVL endoscopic variceal ligation, EST endoscopic sclerotherapy, TN hypertension, HBV hepatitis B virus, HCV hepatitis C virus, LDH lactate dehydrogenase, MELD model for end-stage liver disease, NLR neutrophil lymphocyte ratio, PRPC packed red blood cells, FFP fresh frozen plasma, SDPC single donor platelet concentrate, RDPC random donor platelet concentrate, TLC total leucocyte count
*Data are presented as mean with 95% confidence intervals
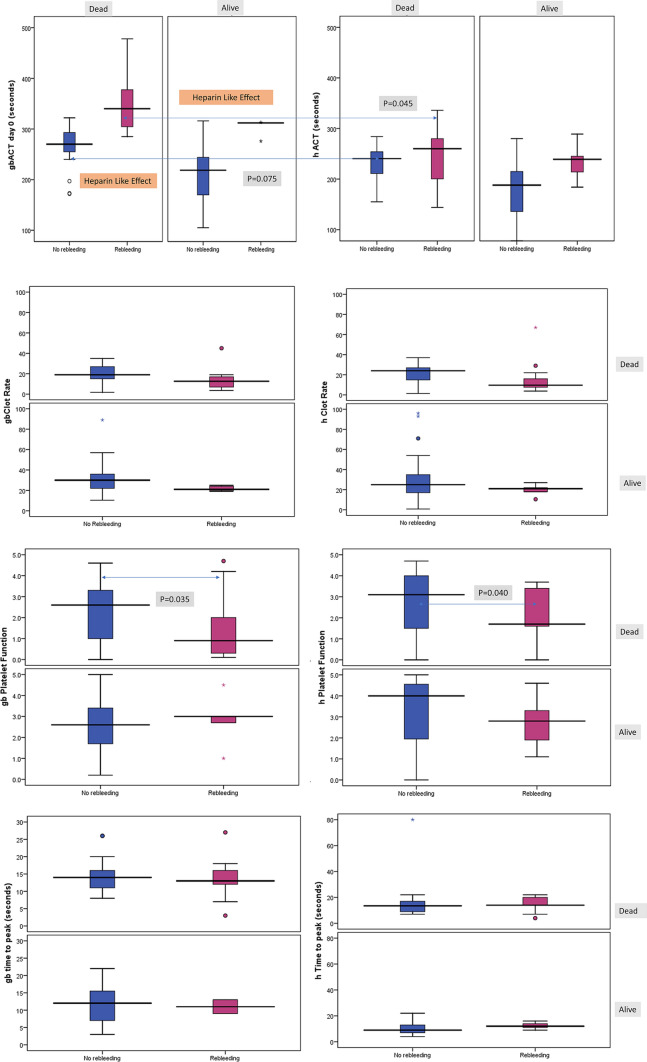
Table 2 lists the results of SCTs and paired gb and heparinase-treated Sonoclot assays. There was significantly higher gb-ACT (343.1 ± 60.3 vs 230.8 ± 53.8, P < 0.001), h ACT (241.3 ± 55.4 vs 198.7 ± 50.6, P = 0.001) and lower gb-clot rate (15.9 ± 9.5 vs 27.3 ± 11.6, P < 0.001) in rebleeders vs non-rebleeders, respectively. Also, the hSonoclot assay showed that those without rebleeding had a higher peak amplitude (P = 0.006), and lower clot Lysis (P = 0.041). Figure 2 shows the presence of the HLE in paired Sonoclot tests showing partial correction of the prolonged gbACT and gbCR on the corresponding hACT and hCR curves.
Table 2.
Conventional coagulation parameters and Sonoclot results of the rebleeding study participants
| Parameter | Total patients N = 90 |
Early rebleeding N = 20 |
No rebleeding N = 70 |
P value |
|---|---|---|---|---|
| INR | 2.0 (1.5–2.2) | 2.2 (1.4–2.9) | 1.9 (1.5–2.1) | 0.190 |
| aPTT (s) | 49.5 ± 22.8 | 58.9 ± 31.1 | 46.4 ± 18.6 | 0.037 |
| D-Dimer (ng/ml) | 523.8 (213.4–632.2) | 586.4 (275.4–674.5) | 475.4 (259.4–765.4) | 0.015 |
| Fibrinogen (g/L) | 2.2 (1.6–2.5) | 2.3 (1.7–2.4) | 2.2 (1.7–2.5) | 0.945 |
| Sonoclot variables | ||||
| gbACT (s) | 256.9 ± 72.5 | 343.1 ± 60.3 | 230.8 ± 53.3 | 0.000 |
| gbCR (units/min) | 24.7 ± 11.2 | 15.9 ± 9.5 | 27.3 ± 11.6 | 0.000 |
| gbPF (units) | 2.3 (2.0–2.6) | 1.8 (1.1–2.6) | 2.5 (2.1–2.8) | 0.102 |
| gbPeak Amplitude (units) | 78.8 ± 25.2 | 75.1 ± 28.5 | 79.8 ± 24.4 | 0.494 |
| gbTime to peak (min) | 12.7 ± 5.2 | 12.8 ± 4.9 | 12.7 ± 5.3 | 0.931 |
| hACT (s) | 208.3 ± 54.5 | 241.3 ± 55.7 | 198.5 ± 50.6 | 0.002 |
| hCR (units/min) | 23.6 (20.1–27.8) | 16.7 (9.8–23.7) | 25.6 (21.7–29.6) | 0.029 |
| hPF (units) | 2.9 (2.5–3.2) | 2.2 (1.7–2.8) | 3.1 (2.6–3.5) | 0.045 |
| hPeak-Amplitude (units) | 89.4 ± 22.9 | 76.7 ± 26.3 | 92.9 ± 20.7 | 0.006 |
| hTime to peak (min) | 11.5 (4.8–42.5) | 14.3 (4.8–46.1) | 12.3 (5.3–38.8) | 0.408 |
| Clot lysis % | 8.9 (7.7–10.1) | 10.4 (7.5–13.2) | 7.2 (6.0–9.7) | 0.041 |
| Heparin-like effect (HLE) | ||||
| No HLE | 44 (48.9%) | 6 (30%) | 38 (54.3%) | 0.060 |
| Mild HLE | 33 (36.7%) | 10 (50.0%) | 23 (32.9%) | 0.044 |
| Severe HLE | 13 (14.4%) | 4 (20%) | 9 (12.9%) | 0.030 |
| Coagulation factor assays | ||||
| Factor XIII (ng/ml) | 2.4 (1.9–2.5) | 1.6 (1.2–2.1) | 2.4 (2.0–2.8) | 0.035 |
| Factor VII (ng/ml) | 117.3 ± 51.0 | 94.1 ± 46.9 | 124.0 ± 50.4 | 0.023 |
| Factor X (μg/ml) | 6.0 ± 2.4 | 5.2 ± 2.4 | 6.2 ± 2.4 | 0.141 |
| VWF:Ag (ng/ml) | 24.8 ± 14.5 | 27.8 ± 15.4 | 23.9 ± 14.2 | 0.316 |
| Factor VIII:Ag (IU/ml) | 5.6 (4.6–6.9) | 7.6 (5.4–9.9) | 5.0 (3.9–6.1) | 0.026 |
| TPA (ng/ml) | 22.1 ± 11.0 | 22.2 ± 10.0 | 22.0 ± 10.8 | 0.954 |
| PAI-1 (ng/ml) | 6.9 (6.1–7.8) | 7.9 (6.6–9.3) | 6.8 (5.6–7.7) | 0.211 |
| ADAMTS13 (ng/ml) | 670.5 ± 108.5 | 512.3 ± 138.7 | 688.5 ± 120.5 | 0.040 |
ACT activated coagulation time, aPTT activated partial thromboplastin time, CR clot rate on glass bead (gbCR) and heparinase-treated(hCR) curve, HLE heparin-like effect, ADAMTS13 a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13, INR international normalized ratio, NLR neutrophil lymphocyte ratio, MELDNa model for end-stage liver disease, PF platelet function on glass bead (gbPF) and heparinase-treated (hPF) curve, vWF:Ag von Willebrand Factor Antigen, tPA tissue plasminogen activator, PAI-1 plasminogen activator inhibitor
*Data are presented as Mean with 95% confidence intervals
Fig. 2.
Glass bead (gb) and heparinase-treated (h)Sonoclot tests in patients with AVB with calculation of heparin-like effect (HLE) as a predictor of rebleeding and mortality
Endogenous Heparinoids and Risk of Rebleeding
Endogenous heparinoids were identified in 46 (51.1%) patients at enrollment. HLE was present in 14(70%) and 32 (44%) of patients with and without rebleeding, respectively (P = 0.038). The presence of HLE indicates the presence of an unstable clot with lysis of 12.9% (5–20%) as compared to 4.7% (1.7–8%) in rebleeders vs. non-rebleeders, respectively (P < 0.001). The presence of HLE was also associated with increased cryoprecipitate requirements (P = 0.027), lower fibrinogen level (P = 0.021), and high NLR (P < 0.001). However, IL-6, procalcitonin, endotoxin, lactate, or ferritin levels did not differentiate the risk of rebleeding. Patients with rebleeding showed reduced Factor XIII [1.6 (1.2–2.1) vs. 2.4(2.0–2.8) ng/ml, P = 0.035], Factor VII (94.1 ± 46.9 vs. 124.0 ± 50.4 ng/ml, P = 0.023), and ADAMTS13 (512.3 ± 138.7 vs 688.5 ± 120.5 ng/ml, P = 0.040). Supplementary Table 1 provides the reference ranges for the factor assays in cirrhotics and healthy controls.
Supplementary Figure 2 shows the results of individual coagulation factor assays in patients with and without rebleeding stratified by outcome. There were reductions in Factors VII, X, & XIII, ADAMTS13, and PAI-1 in patients with rebleeding, while there was an increased Factor VIIIAg and tPA level (Table 2). The presence of HLE > 50% at day 0 predicted new episode of sepsis (HR 1.8; CI 1.4–6.5, P = 0.022), rebleeding events (HR 1.2; CI 1.1–6.3, P = 0.030), and 90-mortality risk (HR 1.1; CI 1.0–4.6, P = 0.030).
Incidence of Sepsis
At 30 days, 31.1% (28/90) of patients from the entire cohort developed evidence of sepsis, including 10 (50%) of those with rebleeding. The most common sites of sepsis were spontaneous bacterial peritonitis (16,57.1%), pneumonia (4,14.2%), systemic sepsis (6, 21.4%), and urinary tract infection (2,7.1%). Cultures were positive in 50% (14/28) of the patients, with multidrug-resistant Klebsiella pneumonia, Escherichia coli, and Acinetobacter baumannii being the common isolates. Table 3 shows that patients who developed sepsis were more likely to have rebleeding (P = 0.033) and had increased mortality (P = 0.000). Patients with sepsis showed significant difference in ACT on both gb and h-SONOCLOT traces, with increased clot lysis and endotoxin levels, but both groups did not differ on severity of HLE. Patients with sepsis had higher Factor VIIIAg, high tPA, and lower Factor VII and Factor X. Plasma ADAMTS13 levels (547.8 ± 187.6 vs. 653.5 ± 105.6, ng/ml; P = 0.037) were significantly lower in patients with endotoxin levels ≥ 18 EU/mL, as were Factor VII levels (95.6 ± 35.6 vs. 120.5 ± 57.2 ng/ml; P = 0.043). All patients who developed sepsis in the post-rebleeding did not survive as compared with only 17.7% mortality in those who did not develop sepsis (P < 0.001).
Table 3.
Clinical and coagulation parameters in patients who developed sepsis (N = 28) and those who did not (N = 62)
| Parameter | Total patients N = 90 |
Sepsis N = 28 |
No Sepsis N = 62 |
P value |
|---|---|---|---|---|
| Age (years) | 46.4 ± 11.7 | 46.0 ± 10.9 | 48.6 ± 12.1 | 0.816 |
| Incidence of rebleeding | 20 (22.2%) | 10 (35.7%) | 10 (16.1%) | 0.039 |
| Mortality | 39 (43.3%) | 28 (100%) | 11 (17.7%) | 0.000 |
| MELDNa | 16.6 (14.5–22.3) | 17.5 (15.6–23.4) | 16.5 (13–21.5) | 0.377 |
| INR | 1.9 (1.4–2.1) | 2.0 (1.4–2.4) | 1.7 (1.6–1.9) | 0.584 |
| Platelet count × 109/l | 94.8 (80.2–107.5) | 89.7 (75.3–104.5) | 105.2 (70.0–131.1) | 0.310 |
| aPTT (s) | 49.5 ± 22.8 | 55.5 ± 23.3 | 46.8 ± 19.9 | 0.119 |
| D-Dimer (ng/ml) | 481.4 (213.4–632.2) | 555.6 (435.6–1256.7) | 468.0 (355.6–894.6) | 0.166 |
| Fibrinogen (g/L) | 2.2 (1.6–2.8) | 1.8 (1.2–2.7) | 2.4 (1.6–3.3) | 0.341 |
| Procalcitonin (ng/ml) | 4.3 (0.7–7.8) | 6.9 (3.7–17.7) | 3.0 (0.7–5.3) | 0.302 |
| IL1β (ng/ml) | 14.4 ± 5.4 | 18.7 ± 4.1 | 12.3 ± 4.6 | 0.000 |
| IL-6 (ng/ml) | 13.2 (10.8–15.7) | 15.8 (11.1–20.6) | 12.0 (9.2–14.8) | 0.146 |
| NLR | 10.3 ± 3.4 | 11.6 ± 2.3 | 9.8 ± 3.4 | 0.291 |
| Serum endotoxin level (EU/mL) | 15.6 ± 6.5 | 20.1 ± 8.4 | 14.6 ± 7.6 | 0.042 |
| Presence of HLE | 0.938 | |||
| No HLE | 44 (48.9%) | 13 (46.4%) | 31 (50.0%) | |
| Mild HLE | 33 (36.7%) | 11 (39.3%) | 22 (35.5%) | |
| Severe HLE | 13 (14.4%) | 4 (14.3%) | 9 (14.3%) | |
| Sonoclot parameters | ||||
| gbACT (s) | 255.2 ± 72.9 | 290.6 ± 51.8 | 239.4 ± 75.6 | 0.002 |
| hACT (s) | 207.3 ± 54.7 | 242.7 ± 41.0 | 191.9 ± 53 | 0.000 |
| Lysis (%) | 8.8 (4.5–12.8) | 10.8 (4.5–14.5) | 6.9 (3.2–9.8) | 0.043 |
| Coagulation factor assays | ||||
| Factor VIII (IU/ml) | 5.5 (2.5–8.7) | 9.1 (3.7–11.2) | 3.8 (2.1–6.4) | 0.000 |
| vWF (ng/ml) | 25.1 (18.4–35.8) | 27.1 (18.5–35.6) | 23.4 (15.6–28.5) | 0.227 |
| Factor VII (ng/ml) | 116.5 ± 51.2 | 91.5 ± 42.4 | 128.5 ± 51.1 | 0.001 |
| tPA (ng/ml) | 22.0 ± 9.1 | 25.8 ± 11.1 | 20.2 ± 9.8 | 0.027 |
| PAI-1 (ng/ml) | 6.8 (5.1–11.8) | 7.3 (3.1–9.8) | 6.8 (3.9–10.1) | 0.580 |
| Factor X (μg/ml) | 6.0 ± 2.4 | 5.1 ± 1.5 | 6.4 ± 2.6 | 0.014 |
| Factor XIII (ng/ml) | 2.3 (1.9–2.6) | 2.5 (1.9–3.1) | 2.1 (1.7–2.8) | 0.276 |
| ADAMTS13 (ng/ml) | 648.5 ± 108.5 | 485.8 ± 88.7 | 523.5 ± 10.3 | 0.065 |
ACT activated coagulation time, aPTT activated partial thromboplastin time, CR clot rate, HLE heparin-like effect, ADAMTS13 a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13, INR international normalized ratio, NLR neutrophil lymphocyte ratio, MELDNa model for end-stage liver disease, vWF:Ag von Willebrand Factor Antigen, tPA tissue plasminogen activator, PAI-1 plasminogen activator inhibitor
Predictors of Rebleeding and Thrombotic Events
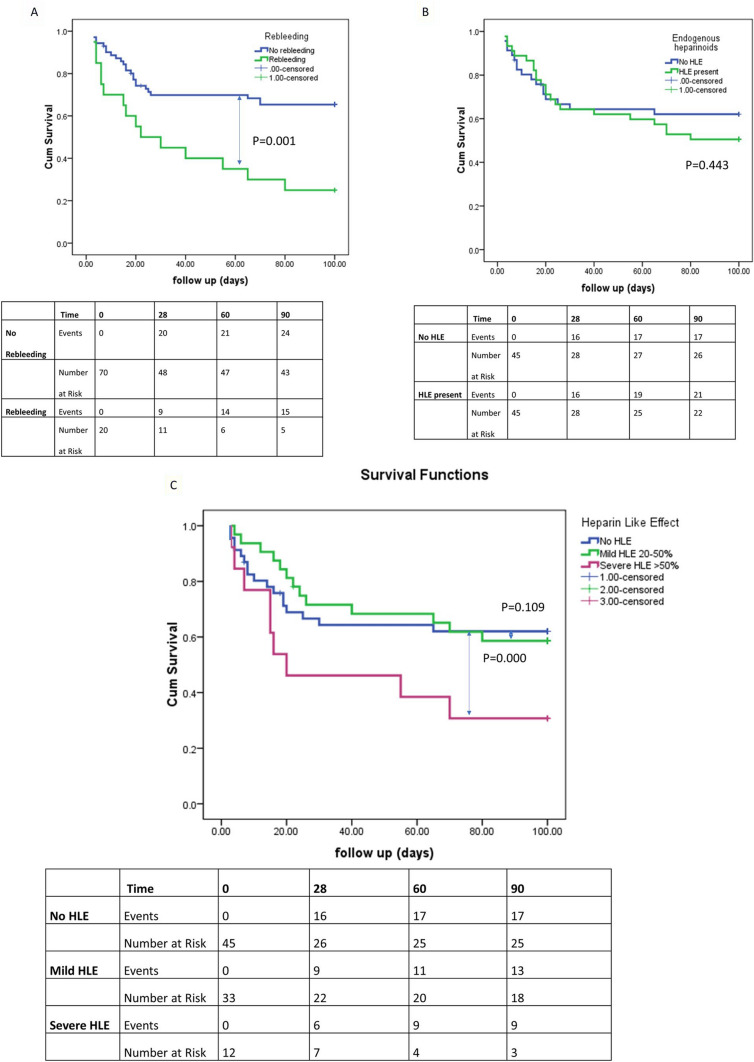
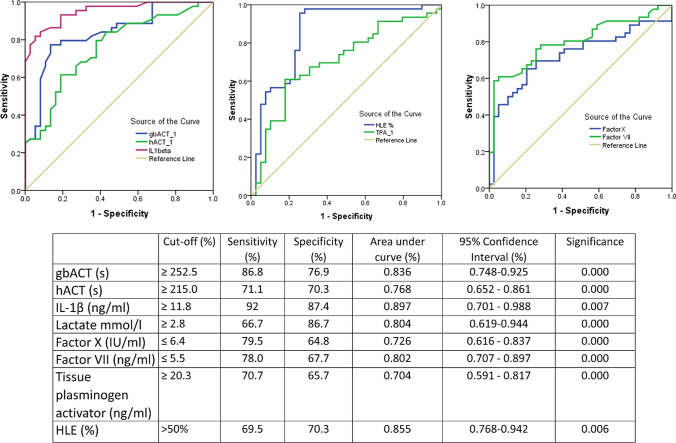
On Kaplan–Meier analysis, the overall incidence of rebleeding at day 30 was found to be 17.8% (5/28, 95% CI 8.7–30.9, P = 0.061) in patients without HLE, 13.6.0% (3/22, 95% CI 7.8–28.3, P = 0.075) in those with mild HLE, and 50% (3/6, 95% CI 24.5–46.7, P = 0.012) in those with severe HLE (Fig. 3). On ROC curve analysis, the following parameters were predictive of rebleeding; gbACT > 252 s (sensitivity 86.8%, specificity 76.9%, P < 0.001), hACT > 215 s (sensitivity 71.1%, specificity 70.3%, P < 0.001), and HLE > 50% (sensitivity 69.5%, specificity 70.3%, P = 0.006) (Fig. 4). Eight (8.8%) patients also demonstrated new thrombosis, with the sites being clotting of central lines (5,62.5%), partial portal vein thrombosis (1,12.5%), and superior mesenteric vein thrombosis (1,12.5%).
Fig. 3.
Kaplan–Meier (KM) survival analysis for overall transplant-free survival in patients with and without rebleeding (panel A), risk of mortality in presence of heparin-like effect (HLE) (panel B), and outcomes based on severity of HLE (panel C)
Fig. 4.
Receiver operating Characteristics (ROC) curves for predictors of mortality in patients with cirrhosis and acute variceal bleeding
Predictors of Mortality
The overall mortality in the cohort of patients was 39 (43.3%) with 15 (75%) and 24 (34.2%) mortality in those with and without rebleeding, respectively (P = 0.001). Most of the patients were managed successfully for rebleeding (14/20, 70%) but later succumbed to sepsis or progressive liver failure as causes of death, or following another decompensation event. Age, gender, etiology of liver disease, conventional tests like, INR, PT, aPTT, creatinine, bilirubin, and D-dimer were not found to be predictive of mortality on logistic regression. MELDNa (OR 1.2, 95% CI 1.1–2.1, P = 0.046) showed a trend to increased mortality on univariable analysis, but not on multivariable analysis. We tested the use of blood component therapy, packed red cell transfusion, or use of terlipressin or octreotide, but it had no bearing on mortality in this cohort. On Cox proportional hazards analysis, predictors of mortality at day 0 were Factor VIII (HR 1.26; 95% CI 1.17–1.34, P < 0.001), low factor VII (HR 0.98; 95% CI 0.88–0.94, P < 0.001), and lysis (HR 1.25, 95% CI 1.17–1.33, P < 0.001). In multivariate analysis on Sonoclot tests (Model 1), gbCR (HR 0.93; 0.89–0.98, P = 0.008), lysis (HR 1.21, 95% CI 1.17–1.31, P = 0.005), and presence of HLE (HR 1.1, 95% CI 1.0–4.6, P = 0.030) were significant predictors of outcome. In model 2 based on multivariate analysis of coagulation factors, Factor VIIIAg (HR 1.21; CI 1.14–1.31, P < 0.001) & Factor VII (HR 0.98, 95% CI 0.98–0.99, P = 0.006) remained significant for predicting mortality (Table 4). When adjusted for baseline MELD, prolonged gbACT HR 1.009 (P = 0.003), reduced gbCR (HR 0.839, P = 0.004), increased lysis (HR 1.129, P = 0.015), and FVIII levels (HR 1.120, P = 0.040) remained predictive of mortality. (Supplementary Table 2).
Table 4.
Results of cox proportional hazards analysis for mortality and rebleeding using relevant parameters, and two predictive models based on Sonoclot results and coagulation factor assays
| Univariable analysis | Multivariate analysis | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors of mortality | ||||||||||||||||||||
| Parameter | HR | 95% CI | P value | Parameter | HR | 95% CI | P value | Parameter | HR | 95% CI | P value | |||||||||
| Model 1 | Model 2 | |||||||||||||||||||
| gbACT (s) | 1.010 | 1.009–1.018 | 0.000 | gbACT | 1.009 | 1.003–1.015 | 0.003 | Factor VIII | 1.217 | 1.142–1.319 | 0.000 | |||||||||
| gbCR (units/min) | 0.915 | 0.882–0.949 | 0.000 | gbCR | 0.939 | 0.891–0.983 | 0.008 | Factor VII | 0.989 | 0.982–0.997 | 0.006 | |||||||||
| hACT (s) | 1.02 | 1.010–1.025 | 0.000 | Lysis | 1.215 | 1.174–1.312 | 0.005 | |||||||||||||
| hCR (units/min | 0.969 | 0.943–0.996 | 0.024 | Presence of HLE | 1.1 | 1.0–4.6 | 0.030 | |||||||||||||
| Lysis (%) | 1.25 | 1.173–1.332 | 0.000 | |||||||||||||||||
| Factor VIII (ng/ml) | 1.26 | 1.175–1.349 | 0.000 | |||||||||||||||||
| Factor VII (ng/ml) | 0.98 | 0.979–0.992 | 0.000 | |||||||||||||||||
| Factor X (μg/ml) | 0.79 | 0.693–0.908 | 0.001 | |||||||||||||||||
| Presence of HLE > 50% | 1.3 | 1.1–6.8 | 0.015 | |||||||||||||||||
| Predictors of rebleeding | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | HR | 95% CI | P value | Parameter | HR | 95% CI | P value | ||||
| Factor XIII | 0.81 | 0.78–0.95 | 0.008 | Factor XIII | 0.90 | 0.87–0.96 | 0.030 | ||||
| Factor VII | 0.89 | 0.76–0.98 | 0.035 | Factor VII | 0.92 | 0.84–0.98 | 0.043 | ||||
| Presence of HLE > 50% | 1.50 | 1.1–5.6 | 0.044 | Presence of HLE | 1.2 | 1.1–6.3 | 0.030 | ||||
| Predictors of sepsis event | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | HR | 95% CI | P value | Parameter | HR | 95% CI | P value | ||||
| Presence of HLE > 50% | 1.9 | 1.3–7.3 | 0.018 | Presence of HLE > 50% | 1.8 | 1.4–6.5 | 0.022 | ||||
| IL1β | 1.2 | 1.1–4.5 | 0.045 | ||||||||
CI confidence interval, HLE heparin-like effect, HR hazard ratio, ACT activated clotting time, gbACT glass bead-activated ACT, h ACT heparinase-modified ACT, CR clot rate, gb GR glass bead-activated CR, hCR heparinase-treated CR
Discussion
Our study demonstrates the association of factor XIII and factor VII deficiency with viscoelastic measurements of clot lysis and endogenous heparinoids in cirrhosis with AVB, which are associated with early rebleeding and mortality. The primary variceal bleeding event is due to high portal pressure, and early appropriate endoscopic intervention and use of portal pressure reducing vasoconstrictor agents are the main therapeutic strategies. Rebleeding from post-EVL ulcers, erosions, PHG, or post-glue ulcers should be addressed by reducing portal pressure, endoscopic interventions, use of TIPS, and appropriate measures to assess coagulation dysfunction. We have shown that the ‘HLE’ was associated with altered tPA–PAI-1 interaction, increased vWF/ADAMTS13 ratio, elevated plasma endotoxin, increased cytokines (IL-1β, IL-6), and hyperfibrinolysis. Patients with rebleeding showed elevated D-dimer, reduced factor XIII (fibrin stabilizing factor), and factor VII, which is not uncommon in cirrhosis [4, 11, 15]
In normal hemostasis, tPA and PAI-1 act reciprocally to control fibrinolysis. tPA is a natural fibrinolytic agent released from the endothelium and works specifically at the fibrin clot surface. An increase in tPA, results in increased plasmin, which lyses the clot. PAI-1 inhibits tPA, and increase in PAI-1 is associated with delayed bleeding [6]. Ferguson et al. had shown that high tPA, with reduced PAI-1 was the hallmark of hyperfibrinolysis in alcohol-associated cirrhosis due to failure to clear tPA from the circulation [23]. However, we found that both tPA and PAI are increased in cirrhosis with AVB (Supplementary Fig. 4). Elevation of D-dimer observed in our study could be due to increased fibrinolysis or after rebleeding. There is poor correlation between hyperfibrinolysis and D-Dimer level with Sonoclot signature [24]. High D-Dimer observed in patients with cirrhosis may be due to reduced hepatic clearance and not solely due to increased fibrinolysis [25]. The study by Saxena et al. [24] points out that although D-Dimer was very high in 8 patients, hyperfibrinolysis on Sonoclot was seen in only one patient.
ADAMTS13, also called vWF cleaving protease, is synthesized in the hepatic stellate cells and cleaves multimeric VWF. Deficiency of ADAMTS13 predisposes to further liver injury due to increased large multimeric vWF, causing microthrombosis and changes in capillary circulation in the setting of sepsis [7, 8]. Takaya et al. reported similar increased vWF:Ag and reduced ADAMTS13 with increasing cytokine concentrations (IL-6 and IL-8) in patients with acute liver failure with endotoxemia [26]. Reduced activity of ADAMTS13 in the setting of SIRS in cirrhosis increases the prothrombotic function of vWF [27].
In this cohort, patients with post-bleeding sepsis had higher IL1β and serum endotoxin levels, increased ACT and clot lysis, increased factor VIII:Ag and tPA, and reduced factor VII levels. Increased Factor VIII/vWF and reduced ADAMTS13 can explain the thromboses that occurred in 8 (8.8%) patients in this cohort due to complex coagulation dysfunction [28].
Supplementary Fig. 5 provides a schematic diagram explaining the coagulation dysfunction associated with sepsis, HLE, and rebleeding. In our study, multiple mechanisms could have contributed to the increased endogenous heparinoids, including (i) failure of the liver to clear the circulating GAGs following AVB, (ii) sepsis or bacterial translocation with endotoxemia causing neutrophil-mediated injury of the hepatocytes that can release heparan sulfate, and (iii) the direct release of GAGs from the endothelial surface following AVB, which was associated with the characteristic Sonoclot signature. On Kaplan–Meier analysis, the overall incidence of rebleeding at day 30 was found to be 17.8% (P = 0.061) in patients without HLE, and 50% (P = 0.012) in those with severe HLE.
The typical coagulation defect was seen in the Sonoclot signature as prolonged ACT, reduced CR, and increased clot lysis. Therefore, these POC tests can be used as a quick bedside tool to assess the hemostatic defect and hyperfibrinolysis, which can be an indicator of rebleeding risk [29].
We followed a restrictive transfusion strategy based on POC tests, which is in accordance with the new BAVENO VII consensus guidelines [30]. We reiterate that AVB is due to increased portal pressures, and therapy should be focused on the management of portal hypertension, timely endotherapy, and preventing rebleeding. There is no role of prophylactic correction of coagulation abnormalities in portal hypertension related AVB, as it may cause more harm with limited benefit as portal pressure remains the primary cause of bleeding [31].
Use of tranexamic acid for hyperfibrinolysis or substitution of factor VII or 4 factor prothrombin complex concentrate (PCC) containing Factors II, VII, IX, and X has been described. However, more data from randomized trials are required to support use of such drugs as PCC has also been associated with increased risk of thromboembolism [13–15].
A major limitation of our study is that recruitment commenced just before the COVID-19 wave started in India. The overall mortality in the patients was 39 (43.3%) in patients who presented with AVB, which is quite high compared to our center’s data in the pre-COVID-19 era [21]. These patients were successfully managed for the AVB episode but failed to report for follow-up after their discharge. This is due to late presentation of patients due to COVID-19 restrictions, limited beds in non-COVID intensive care units, delays in COVID-19 testing, and impaired endoscopic surveillance of stable patients with cirrhosis during the pandemic. Patients were unable to report to tertiary care units following acute decompensation or report for follow-up endoscopy due to conversion of inpatient wards, endoscopy units, and intensive care units for COVID-19 care. Access to speciality procedures like TIPS or liver transplantation was also limited. Many cases were managed using telemedicine at peripheral sites or were not referred to our center due to significant restriction of access to specialist care [32]. Thus, our data highlight the collateral damage due to COVID-19 in specialist care units such as ours, with increased mortality due to changes in healthcare delivery and diversion of health resources for COVID-19 care. Nonetheless, the principles of usage of POC tests, and interaction between sepsis, endothelial activation, endogenous heparinoids, and hyperfibrinolysis leading to rebleeding and mortality in cirrhosis and AVB remain pertinent. Another limitation of our study was that we measured only platelet count and Sonoclot as a marker of platelet function. We did not measure tests of platelet aggregation and secretion to assess platelet function. Patients with decompensated cirrhosis and AKI have reduced platelet aggregation and secretion measured by whole blood lumiaggregometry which improved to baseline after resolution of AKI [33]. Similarly, another study demonstrated reduced platelet aggregation in patients with decompensated cirrhosis, which reduced further in those with bacterial infection. However, this reduction in platelet aggregation persisted even after resolution of infection [34]. Further studies assessing role of platelet aggregation in early rebleeding after AVB may help in bridging the knowledge gap.
Although viscoelastic tests are considered better than standard coagulation tests, they are not free of limitations. These are in vitro and do not assess contribution of injured endothelium to clot formation, and hence cannot be used to predict risk of bleeding. However, these tests may provide a guide to specific hemostatic abnormality in a clinically bleeding patient. Also, contribution of platelet and fibrinogen defect to abnormal clot rate is difficult to assess by means of Sonoclot alone and functional fibrinogen assays should be performed.
Conclusion
Our results demonstrate the presence of a HLE and a possible association of Factor VII & XIII deficiency, with increased fibrinolysis with early rebleeding in patients with AVB. The main focus in such patients should be on the management of portal hypertension with drugs like terlipressin, timely endoscopy, referral for TIPS or transplantation, and follow-up to prevent further acute decompensation or sepsis. A restrictive transfusion strategy is preferred, and use of specific blood component therapies as supportive care requires further research. Patients with sepsis and SIRS have worsening coagulation function, with increased Factor VIII/vWF, endogenous heparinoids, and endotoxin levels. Our data argue for the need for careful evaluation of sepsis and secondary coagulation anomalies in cirrhosis following an episode of AVB.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AST
Aspartate transaminase
- ALT
Alanine transaminase
- ACT
Activated clotting time
- ACLF
Acute-on-chronic liver failure
- aPTT
Activated partial thromboplastin time
- ATIII
Antithrombin III
- ADAMTS13
A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13
- CI
Confidence interval
- BMI
Body mass index
- CTP
Child Turcotte Pugh
- CR
Clot rate
- CRP
High-sensitivity C-reactive protein
- EVL
Endoscopic variceal ligation
- EST
Endoscopic sclerotherapy
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- LDH
Lactate dehydrogenase
- HLE
Heparin-like effect
- HR
Hazard ratio
- INR
International normalized ratio
- Lys30 and 60
Lysis at 30 minutes and 60 minutes
- MA
Maximum amplitude
- MELD
Model for end-stage liver disease
- NLR
Neutrophil lymphocyte ratio
- PRBC
Packed red blood cells,
- FFP
Fresh frozen plasma
- PAI-1
Plasminogen activator inhibitor type 1
- SDPC
Single donor platelet concentrate
- RDPC
Random donor platelet concentrate
- SCT
Standard coagulation tests
- SIRS
Systemic inflammatory response syndrome
- TLC
Total leucocyte count
- TEG
Thromboelastography
- tPA
Tissue plasminogen activator
- vWF
Von Willebrand factor
Author's contribution
MP, AVK, AR, and RM were all involved in the manuscript preparation. The study design and execution were done by MP and KK. NV, AD, VS, ST, and AD were the attending consultants and were involved in data curation and clinical management of cases. JA, NK, and SD were involved in coagulation assessment and interpretation of tests. AG, KK, HK, and HK performed the point-of-care tests. All the authors have read and approved the manuscript.
Funding
Supported by an intramural research Grant No. RGC-6701 awarded to MP by the Research Grant Cell, PGIMER, Chandigarh.
Declarations
Conflict of interest
None of the authors have potential conflicts (financial, professional, or personal) which are relevant to this manuscript.
Ethical approval
Ethical clearance was obtained from the Institutional Review Board (INT/IEC/2019/002239 dated 15/10/2019) and the study was registered at NCT04111120 available from https://clinicaltrials.gov/ct2/show/NCT04111120.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Premkumar M, Bihari C, Saxena P, et al. Heparin-like Effect Associated With Risk of Bleeding, Sepsis, and Death in Patients With Severe Alcohol-Associated Hepatitis. Clin Gastroenterol Hepatol. 2020;18:486–495.e3. doi: 10.1016/j.cgh.2019.04.057. [DOI] [PubMed] [Google Scholar]
- 2.Premkumar M, Sarin SK. Current Concepts in Coagulation Profile in Cirrhosis and Acute-on-Chronic Liver Failure. Clin Liver Dis (Hoboken). 2020;16:158–167. doi: 10.1002/cld.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thalheimer U, Triantos C, Samonakis D, et al. Endogenous heparinoids in acute variceal bleeding. Gut. 2005;54:310–311. doi: 10.1136/gut.2004.051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Premkumar M, Saxena P, Rangegowda D, et al. Coagulation failure is associated with bleeding events and clinical outcome during systemic inflammatory response and sepsis in acute-on-chronic liver failure: An observational cohort study. Liver Int. 2019;39:694–704. doi: 10.1111/liv.14034. [DOI] [PubMed] [Google Scholar]
- 5.Bernard B, Cadranel JF, Valla D, et al. Prognostic significance of bacterial infection in bleeding cirrhotic patients: a prospective study. Gastroenterology. 1995;108:1828–1834. doi: 10.1016/0016-5085(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 6.Mehta R, Shapiro AD. Plasminogen activator inhibitor type 1 deficiency. Haemophilia. 2008;14:1255–1260. doi: 10.1111/j.1365-2516.2008.01834.x. [DOI] [PubMed] [Google Scholar]
- 7.Uemura M, Fujimura Y, Ko S, et al. Pivotal role of ADAMTS13 function in liver diseases. Int J Hematol. 2010;91:20–29. doi: 10.1007/s12185-009-0481-4. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa M, Uemura M, Matsuyama T, et al. Potential role of enhanced cytokinemia and plasma inhibitor on the decreased activity of plasma ADAMTS13 in patients with alcoholic hepatitis: relationship to endotoxemia. Alcohol Clin Exp Res. 2010;34:S25–33. doi: 10.1111/j.1530-0277.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 9.de Laat-Kremers RMW, Yan Q, Ninivaggi M, et al. Deciphering the coagulation profile through the dynamics of thrombin activity. Sci Rep. 2020;10:12544. doi: 10.1038/s41598-020-69415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuenschwander PF, Jesty J. Thrombin-activated and factor Xa-activated human factor VIII: differences in cofactor activity and decay rate. Arch Biochem Biophys. 1992;296:426–434. doi: 10.1016/0003-9861(92)90593-L. [DOI] [PubMed] [Google Scholar]
- 11.Bedreli S, Sowa JP, Malek S. Rotational thromboelastometry can detect factor XIII deficiency and bleeding diathesis in patients with cirrhosis. Liver Int. 2017;37:562–568. doi: 10.1111/liv.13254. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal S, Senzolo M, Melikian C, et al. The prevalence of a heparin-like effect shown on the thromboelastograph in patients undergoing liver transplantation. Liver Transpl. 2008;14:855–860. doi: 10.1002/lt.21437. [DOI] [PubMed] [Google Scholar]
- 13.Small C, Attridge RL, Franco-Martinez C, et al. Prothrombin Complex Concentrate Use in Intracranial Hemorrhage Patients With Cirrhosis Not on Prior Anticoagulation. J Intensive Care Med. 2021 doi: 10.1177/08850666211012650. [DOI] [PubMed] [Google Scholar]
- 14.Tischendorf M, Fuchs A, Zeuzem S, Lange CM. Use of prothrombin complex concentrates in patients with decompensated liver cirrhosis is associated with thromboembolic events. J Hepatol. 2019;70:800–801. doi: 10.1016/j.jhep.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Tsochatzis E, Papatheodoridis GV, et al. Prophylactic and therapeutic use of recombinant activated factor VII in patients with cirrhosis and coagulation impairment. Dig Liver Dis. 2007;39:490–494. doi: 10.1016/j.dld.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Premkumar M, Loganathan S, Kajal K, et al. COVID-19-related dynamic coagulation disturbances and anticoagulation strategies using conventional D-dimer and point-of-care Sonoclot tests: a prospective cohort study. BMJ Open. 2022;12:e051971. doi: 10.1136/bmjopen-2021-051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarin SK, Kumar A, Angus PW, et al. Asian Pacific Association for the Study of the Liver (APASL) Working Party on Portal Hypertension. Diagnosis and management of acute variceal bleeding: Asian Pacific Association for Study of the Liver recommendations. Hepatol Int. 2011;5:607–624. doi: 10.1007/s12072-010-9236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 19.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology. 2019;157:34–43.e1. doi: 10.1053/j.gastro.2019.03.070. [DOI] [PubMed] [Google Scholar]
- 21.Premkumar M, Mehtani R, Divyaveer S, et al. Clinical Validation of Global Coagulation Tests to Guide Blood Component Transfusions in Cirrhosis and ACLF. J Clin Transl Hepatol. 2021;9:210–219. doi: 10.14218/JCTH.2020.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Premkumar M, Kulkarni AV, Kajal K, et al. Principles, Interpretation, and Evidence-Based Role of Viscoelastic Point-of-Care Coagulation Assays in Cirrhosis and Liver Failure. Journal of Clinical and Experimental Hepatology. 2021 doi: 10.1016/j.jceh.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson JW, Helmy A, Ludlam C, Webb DJ, Hayes PC, Newby DC. Hyperfibrinolysis in alcoholic cirrhosis: relative plasminogen activator inhibitor type 1 deficiency. Thromb Res. 2008;121:675–680. doi: 10.1016/j.thromres.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Saxena P, Bihari C, Rastogi A, Agarwal S, Anand L, Sarin SK. Sonoclot Signature Analysis in Patients with Liver Disease and Its Correlation with Conventional Coagulation Studies. Advances in Hematology. 2013 doi: 10.1155/2013/237351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Violi F, Ferro D, Basili S, et al. Hyperfibrinolysis resulting from clotting activation in patients with different degrees of cirrhosis. Hepatology. 1993;17:78–83. doi: 10.1002/hep.1840170115. [DOI] [PubMed] [Google Scholar]
- 26.Takaya H, Yoshiji H, Kawaratani H, et al. Decreased activity of plasma ADAMTS13 are related to enhanced cytokinemia and endotoxemia in patients with acute liver failure. Biomed Rep. 2017;7:277–285. doi: 10.3892/br.2017.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuken PA, Kussmann A, Kiehntopf M, et al. Imbalance of von Willebrand factor and its cleaving protease ADAMTS13 during systemic inflammation superimposed on advanced cirrhosis. Liver Int. 2015;35:37–45. doi: 10.1111/liv.12657. [DOI] [PubMed] [Google Scholar]
- 28.Carnevale R, Raparelli V, Nocella C, et al. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J Hepatol. 2017;67:950–956. doi: 10.1016/j.jhep.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Kumar M, Ahmad J, Maiwall R, et al. Thromboelastography-Guided Blood Component Use in Patients With Cirrhosis With Nonvariceal Bleeding: A Randomized Controlled Trial. Hepatology. 2020;71:235–246. doi: 10.1002/hep.30794. [DOI] [PubMed] [Google Scholar]
- 30.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C. Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohanty A, Kapuria D, Canakis A, et al. Fresh frozen plasma transfusion in acute variceal haemorrhage: Results from a multicentre cohort study. Liver Int. 2021;41:1901–1908. doi: 10.1111/liv.14936. [DOI] [PubMed] [Google Scholar]
- 32.Mahmud N, Hubbard RA, Kaplan DE, Serper M. Declining Cirrhosis Hospitalizations in the Wake of the COVID-19 Pandemic: A National Cohort Study. Gastroenterology. 2020;159:1134–1136.e3. doi: 10.1053/j.gastro.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanetto A, Rinder HM, Campello E, et al. Acute Kidney Injury in Decompensated Cirrhosis Is Associated With Both Hypo-coagulable and Hyper-coagulable Features. Hepatology. 2020;72:1327–1340. doi: 10.1002/hep.31443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanetto A, Campello E, Bulato C, et al. Global hemostatic profiling in patients with decompensated cirrhosis and bacterial infections. JHEP Rep. 2022;4:100493. doi: 10.1016/j.jhepr.2022.100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.