Abstract
Abstract
The harmful effects of mechanical ventilation (MV) on the preterm lung are well established. Avoiding MV at birth and stabilization on continuous positive airway pressure (CPAP) decreases the composite outcome of death or bronchopulmonary dysplasia. Although preterm infants are increasingly being admitted to the neonatal intensive care unit on CPAP, centers differ in the ability to manage infants primarily on CPAP. Over the last decade, less invasive surfactant administration (LISA), a method of administering surfactant with a thin catheter, has been devised and has been shown to decrease the need for MV and improve outcomes compared to surfactant administration via an endotracheal tube following intubation. While LISA has been widely adopted in Europe and other countries, its use is not widespread in the United States. This article provides a summary of the existing evidence on LISA, and practical guidance for US units choosing to implement a change of practice incorporating optimization of CPAP and LISA.
Impact
The accumulated body of evidence for less invasive surfactant administration (LISA), a widespread practice in other countries, justifies its use as an alternative to intubation and surfactant administration in US neonatal units.
This article summarizes the current evidence for LISA, identifies gaps in knowledge, and offers practical tips for the implementation of LISA as part of a comprehensive non-invasive respiratory support strategy.
This article will help neonatal units in the US develop guidelines for LISA, provide optimal respiratory support for infants with respiratory distress syndrome, improve short- and long-term outcomes of preterm infants, and potentially decrease costs of NICU care.
Introduction
The benefits of surfactant therapy in decreasing mortality and air leak are well established.1 Over the last decade, surfactant administration using a thin catheter, known as less invasive surfactant administration (LISA), minimally invasive surfactant therapy (MIST), and surfactant without ETT intubation (SURE) have emerged and become widely adopted in Europe,2,3 Canada,4 and Australia.5,6 This method avoids endotracheal intubation and PPV during surfactant administration. It is an evolution of earlier INSURE (Intubation, SURfactant and Extubation) strategy developed by Verder et al. to limit exposure to MV.7,8
In the management of neonatal respiratory distress syndrome (RDS), an earlier aggressive approach of intubation and early or prophylactic surfactant administration has been replaced, as new evidence emerged, with a less invasive approach that relies on early initiation of continuous positive airway pressure (CPAP) to establish functional residual capacity (FRC) and early rescue surfactant therapy.1,9–17 However, around 40–50% of preterm infants worsen despite CPAP within the first few days of life, primarily due to surfactant deficiency, and require exogenous surfactant and mechanical ventilation (MV).18–21 These infants who experience CPAP failure have worse outcomes than those in whom it is successful.22–26 Over 80% of infants born <27 weeks GA are still exposed to MV during the NICU stay.16,17 As the incidence of BPD is increasing,17 it is important to combine the benefits of early CPAP and surfactant therapy to minimize exposure to PPV, which can be accomplished by using the LISA method.
As a large body of evidence has accumulated about LISA, clinicians in many US NICUs are now faced with the decision of whether to adopt LISA or to wait for more evidence to accumulate about its benefits and harms.27 In this article we summarize the existing evidence for LISA and describe the practical aspects of introducing LISA into a unit as part of an overall strategy to optimize non-invasive respiratory support.
The LISA procedure
The first report of surfactant administration using a feeding tube in preterm infants with RDS maintained on CPAP was by Verder et al.7 Subsequently, three LISA techniques have been described in randomized controlled trials.28–31 Common to all three is the administration of surfactant through a thin catheter too small to be used for PPV. The thin catheter is inserted into the trachea under visualization with a laryngoscope blade, while the infant is maintained on CPAP. Surfactant instilled into this catheter moves down the airways with the infant’s own respiratory effort. In the Cologne method, first described by Kribs et al., the thin catheter was a 4 French feeding tube inserted 1.5 cm below the vocal cords under direct laryngoscopy with the aid of Magill forceps. Atropine 0.025 mg/kg–1 was given prior to the procedure.32 In the Take Care method, described by Kanmaz et al., the thin catheter was a shortened 5 Fr feeding tube, inserted without using Magill forceps or premedication to a depth of 1 cm in infants 25–26 weeks, 1.5 cm in infants 27–28 weeks, and 2 cm in infants 29–32 weeks GA.30 In the MIST method (also known as the Hobart method) Dargaville et al. administered surfactant without premedication through a 16 gauge vascular catheter (Angiocath, BD, Sandy, Utah) inserted to the same depth as in the Take Care method.33
There are no prospective clinical trials comparing the safety and effectiveness of flexible versus stiff catheters. However, an in-vitro study comparing different catheters in a mannequin model showed that stiff catheter insertion is quicker and easier compared to flexible catheters.34 A single-center retrospective study reported no difference between these two types of catheters in the rate of procedural success.35 The stiffer and straight catheter is an attractive option for centers where oral intubation is the preferred practice and use of Magill forceps is uncommon.
The success rate reported in randomized control trials (RCT) for insertion of the catheter on the first attempt is between 72 and 95%.28,29,31 The adverse events associated with thin catheter insertion include coughing, gagging, desaturation, bradycardia, and surfactant reflux (Table 1). The reported frequency of desaturation episodes with LISA ranges from 12 to 100% and the frequency of bradycardia ranges from 6 to 32%. Similarly, the need for rescue PPV for these events ranged between 6 and 14%. However, the definition of events and criteria for intervention varied across the studies. In the OPTIMIST-A trial that used stiff catheters, the frequency of LISA-related events requiring PPV (14%) or emergent intubation (0.4%) was relatively uncommon.31
Table 1.
Adverse events during LISA vs ETT surfactant.
| Study and technique | Success rate and adverse events LISA vs ETT surfactant (%, p value) |
|---|---|
| Göpel et al.29 | Adverse events during ETT intubation not reported |
| 4 French feeding tube, Cologne methoda | First attempt success rate: 95% in the LISA group |
| Optional sedative, analgesic, and atropine | Bradycardia (HR <100): 6% in the LISA group |
| Kribs, 201528 | ↔ First attempt success rate between LISA and ETT surfactant (73% in both) |
| 4 French feeding tube, Cologne method | ↑ Bradycardia (HR <100) in the LISA group (11 vs 3%, p = 0.029)d |
| No sedative or analgesic | ↑ Desaturation (SpO2 <80) in the LISA group (56 vs 26%, p = <0.001) |
| Kanmaz et al., 201330 | ↔ First attempt success rate between groups (82 vs 90%, p = 0.07) |
| 5 French feeding tube shortened to 33 cm at hub | ↑ Coughing, gagging in the LISA group (11 vs 0%) |
| Take Care methodb | ↔Bradycardia and desaturation between groups (18 vs 17%) |
| No premedication | ↑ Surfactant reflux in the LISA group (21 vs 10%, p = 0.02) |
| ↓ Need for face mask PPV in the LISA group (12 vs 100%) | |
| Mohammadizadeh, 201549 | ↑ First attempt in the LISA group (88 vs 72%, p = 0.08) |
| 4 French feeding tube, Cologne method | ↓ Adverse events with LISA (11 vs 31%, p = 0.049) |
| Premedication with atropine 25 mcg/kg to LISA group | |
| Bao, 2015, MIST, LISA vs rescue50 | ↔ First attempt success rate between groups (89 vs 86%) |
| Hobart methodc | |
| No premedication | |
| Choupani et al., 201854 | ↔ First attempt success rate (96 vs 90%) |
| Hobart method | ↓ Desaturation (SpO2 <80) in the LISA group (12 vs 29%, p = 0.028) |
| No premedication | ↔ Cough (0 vs 4%) |
| Yang et al., 201959 | ↔ First attempt success rate between two groups (94 vs 98%) |
| 6 French feeding tube, Cologne method | ↔ Surfactant reflux (13 vs 6%) |
| No premedication | |
| Olivier et al., 201752 | ↔ Need for laryngoscopy attempts (2.3 ± 1.9 vs 2.3 ± 1.2) |
| 5 French feeding tube, Cologne method | ↑ Surfactant reflux in the LISA group (66%) |
| Premedication with atropine 20 mcg/kg and fentanyl 1 mcg/kg | ↑ Moderate desaturation (SpO2 60–80) in the LISA group (58 vs 16%, p ≤ 0.01) |
| ↔ Severe desaturation (SpO2 <60) in the control group (42 vs 58%, p = 0.03) | |
| 2 infants had to be intubated prior to LISA due to chest wall rigidity | |
| Dargaville, 202131 | Adverse events with ETT intubation not reported |
| Hobart method | First attempt success rate 76% with LISA |
| Atropine, sucrose optional | Hypoxemia (SpO2 <80 for 30 s) 42% with LISA |
| No sedatives or opioids | Bradycardia (HR <100 for >10 s) 32% with LISA |
GA gestational age, SpO2 oxygen saturation, HR heart rate, PPV positive pressure ventilation.
aCologne method: insertion of a flexible catheter (i.e., feeding tube) below the vocal cords with direct laryngoscopy using Magill forceps.
bTake Care method: insertion of a flexible catheter (i.e., feeding tube) below the vocal cords with direct laryngoscopy without using Magill forceps.
cHobart method: insertion of a stiff catheter (i.e., 16 G 5.25” vascular catheter) below the vocal cords with direct laryngoscopy.
dFirst % value refers to LISA group and second value refers to control group.
↑ higher, ↓ lower, ↔Not significantly different.
Two custom-made stiff catheters specifically designed for LISA (with centimeter markings for determining insertion depth) are currently not available in the US—a straight catheter similar to the Hobart catheter (LISACath, Chiesi Farmaceutici S.p.A, Parma, Italy), and a straight catheter with an angulated tip (Surfcath, Vygon, Swindon, UK). A multicenter RCT to evaluate the LISAcath,36 is no longer recruiting patients due to manufacturing issues related to the catheter. Another plastic application device with a soft tip, with an angle similar to Magill forceps (Neofact, Lyomark Pharma, Germany) has been evaluated in a feasibility trial.37,38
A large body of evidence (summarized below) has accumulated demonstrating the benefits of LISA. However, several questions still remain. LISA requires direct laryngoscopy or video laryngoscopy, which are associated with adverse events such as increased intracranial pressure,39–42 higher systemic blood pressure,39 prolonged hypoxemia,39 bradycardia,43 and pain.44 CPAP transmission might decrease during direct laryngoscopy. The LISA catheter may occupy over 50% of airway diameter of extremely low GA neonates, potentially causing hypoxia and poor ventilation.45,46 There are no reliable methods of confirming the placement of thin catheter into the airway, and therefore there is a risk of inadvertent esophageal administration of surfactant if the catheter is misplaced. With respect to the type of laryngoscopy for LISA, thus far no studies have evaluated the use of video laryngoscopy during LISA. Finally, there are concerns about respiratory depression with the use of medications for analgesia and sedation with LISA.46 This poses challenges in balancing the pain and discomfort from laryngoscopy against the harmful effects of intubation and MV that might result from use of sedation.47
The evidence for LISA
Thus far LISA has been evaluated in 20 trials in preterm infants,28–31,48–62 of which 17 are summarized in this review. Three are excluded—one that evaluated CPAP and NIPPV, and two that evaluated the role of sedation during LISA.51,63 Of the 17 trials, only 2 were powered for the primary outcome of death or BPD28,31 and only 2 trials included infants born <25 weeks GA.28,30 Details of these trials are provided in Tables 2 and 3, with key findings summarized below.
Table 2.
Randomized controlled trials evaluating LISA vs surfactant via endotracheal tube and continued mechanical ventilation.
| Author/year/design | LISA group threshold/method | Control group threshold/method | Results LISA vs ETT surfactant (%, p value), aRD (95% CI) |
|---|---|---|---|
| LISA vs surfactant administration via ETT at similar FiO2 threshold as LISA arm and continued MV after surfactant administration | |||
|
Kribs et al., 201528 13 centers 23–26 weeks GA Enrolled ≤2 h of life Poractant alfa,100 mg/kg No sedatives/analgesics |
FiO2 >0.3, CPAP 5–8 Silverman score ≥5 4 Fr feeding tube, Cologne methoda Intubation criteria: FiO2 ≥0.45 for ≥2 h |
FiO2 >0.3, CPAP ≥5–8 cm H2O Silverman score ≥5 Surfactant via ETT |
n = 211 ↔ Survival without BPDc (67 vs 59%, p = 0.02d), 8.6 (–5 to 22) ↑ Survival without major complications (51 vs 36%, p = 0.02), 15 (1.4 to 28) ↓ MV during NICU stay, (75 vs 99%, p ≤ 0.001), 24 (16 to 34) ↓Pneumothorax (5 vs 12.6%, p = 0.04) ↓Grade 3–4 IVH (10 vs 22%, p = 0.02) |
| LISA vs continued CPAP, selective ETT intubation using either a physician-dependent criteria or criteria established a priori and continued MV | |||
|
Dargaville et al., 202131 33 centers 25–28 weeks GA Enrolled ≤6 h of life Double blind Poractant alfa 200 mg/kg No sedatives and analgesics Optional atropine/sucrose |
FiO2 ≥0.3, CPAP 5–8, or NIV Hobart methodb Repeat LISA not allowed Intubation criteria: FiO2 ≥0.45, persistent apnea Decision to give repeat surfactant after intubation was per physician discretion |
FiO2 ≥0.3, CPAP 5–8 cm H2O or NIV Sham procedure: only gentle repositioning Intubation criteria: FiO2 ≥0.45, persistent apnea. Decision to give surfactant after intubation was per physician discretion |
n = 485 ↔ Death or BPD, (44 vs 50%, p = 0.10), −6.3 (−14.2 to 1.6) ↔ Mortality (12 vs 8%, p = 0.30), 2.1 (–3.6 to −7.8) ↓ BPD (37 vs 45%, p = 0.03), −7.8 (−15 to −0.7) ↓ Pneumothorax (4.6 vs 10%, p = 0.005), −5.8 (−10.2 to −1.4) ↓ PDA medical treatment (35 vs 45%), −10.5 (−20.2 to −0.9) ↓ Intubation within 72 h of life (37 vs 72%, p ≤ 0.001), −36 (−47 to −24) ↓ Intubation during NICU stay (55 vs 81%) −27 (−40 to −13.5) |
|
Gopel et al., 201129 12 centers 26–28 weeks GA BWT <1.5 kg, Enrolled ≤12 h of life Surfactant: not specified Optional atropine, sedatives, and analgesics |
FiO2 >0.3, CPAP ≥4 cm H2O 4 Fr Feeding tube, Cologne method |
Physician-dependent threshold for intubation. Surfactant per physician discretion Surfactant via ETT |
n = 220 ↓ Need for MV (or if not intubated PaCO2 >65 or FiO2 >60% for ≥2 h) between 25 and 72 hc (28 vs 46%, p = 0.008), −0.18 (−0.30 to −0.05) ↓ MV during NICU stay (33 vs 73%), −0.40 (−0.52 to −0.27) ↔Air leak, BPD and mortality |
|
Olivier et al., 201752 3 centers 32–36 weeks GA Beractant, 100 mg/kg Atropine 20 mcg/kg + fentanyl 1 mcg/kg |
FiO2 ≥0.35 at CPAP 6 cm H2O 5 Fr feeding tube, Cologne method |
Physician-dependent threshold for intubation. Surfactant per physician discretion Surfactant via ETT |
n = 45 ↓ MV/pneumothorax requiring chest tube within 72 h of lifec (33 vs 90%, p ≤ 0.001), 0.57 (95% CI 0.54 to 0.6) ↔ Average laryngoscopy attempts (mean ± SD) 2.3 ± 1.2 vs 2.3 ± 1.9 |
BWT birth weight, GA gestational age, MV mechanical ventilation, PDA hemodynamically significant PDA, OR odds ratio, aRD absolute risk difference.
aCologne method: insertion of a flexible catheter (i.e., feeding tube) below the vocal cords with direct laryngoscopy using Magill forceps.
bHobart method: insertion of a stiff catheter (i.e., 16 G 5.25” vascular catheter) below the vocal cords with direct laryngoscopy.
cPrimary outcome defined a priori.
dFirst % value refers to LISA group and second value refers to control group.
↑ higher, ↓ lower, ↔Not significantly different.
Table 3.
Randomized controlled trials evaluating LISA vs INSURE.
| Study design | LISA vs INSURE threshold, method | Results |
|---|---|---|
|
Kanmaz et al., 201230 Single center <32 weeks GA Poractant alfa, 100 mg/kg |
FiO2 ≥40% within 2 HOL, CPAP 5–7 cm H2O LISA—only by experienced neonatologists 5 Fr Feeding tube, Take Care methoda No premedication |
n = 200 ↓ Need for MV within 72 HOLd in the LISA group ↓ Duration of MV and CPAP in the LISA group ↔ BPD |
|
3 centers 27–32 weeks GA Poractant alfa, 100 mg/kg |
FiO2 >0.3, CPAP 8–10 cm H2O 5 Fr feeding tube, Take Care method Premedication with atropine 5 mcg/kg |
n = 136 ↔ Need for MV within 72 HOL in the LISA group ↓ Mortality and NEC in the LISA group |
|
Mohammadizadeh et al., 201549 2 centers <34 weeks GA 1000–1800 g Poractant alfa, 200 mg/kg |
FiO2 >0.4, CPAP 6 cm H2O 4 Fr Feeding tube, Cologne methodb Premedication with atropine 25 mcg/kg |
n = 38 ↔ Need for MV within 72 HOLd between groups |
|
Bao et al., 201550 Single center, pilot 28–32 weeks GA Poractant alfa, 200 mg/kg |
FiO2 ≥0.3 for 28–29 weeks GA, ≥0.35 for 30–32 weeks GA, CPAP ≥7 cm H2O Hobart methodc No premedication |
n = 100 ↔ Need for MV within 72 HOL between groups ↔ Duration of MV between groups |
|
Mosayebi et al., 201853 Single center 28–34 weeks GA Poractant alfa 200 mg/kg |
FiO2 >0.4, CPAP 5–8 cm H2O 5 Fr feeding tube, Take Care method No premedication |
n = 53 ↔MV within 72 HOLd between groups |
|
Choupani et al., 201854 Single center No GA or weight criteria, Poractant alfa, 200 mg/kg |
FiO2 >0.4, CPAP 6 cm H2O Hobart method No premedication |
n = 104 ↔ MV within 72 HOLd between groups |
|
Halim, 201955 Single center ≤34 weeks GA Beractant, 100 mg/kg |
FiO2 ≥0.3, CPAP 5–7 cm H2O 6 Fr feeding tube, Take Care method No premedication or sedation |
n = 100 ↓ Need for MV in the LISA group ↓ Duration of MV in the LISA group |
|
Boskabadi et al., 201957 Single center <32 weeks GA Poractant alfa, 200 mg/kg |
FiO2 >0.4, CPAP 5–8 cm H2O 5 Fr feeding tube, Take Care method No premedication |
n = 40 ↓ MV within 72 HOL in the LISA group |
|
Jena et al., 201958 3 centers ≤34 weeks GA Bovine lung extract, 135 mg/kg |
FiO2 >0.3, CPAP 6 cm H2O Hobart method or 6 Fr feeding tube without Magill forceps No premedication |
n = 350 ↓ MV within 72 HOLd in the LISA group ↓ BPD in the LISA group ↓ NEC in the LISA group |
|
Yang et al., 202059 Single center 32–36 weeks GA Poractant alfa 200 mg/kg |
FiO2 >0.4, CPAP 6 cm H2O, 6 Fr feeding tube, Cologne method (insertion depth: 2 cm for 32–34 weeks, 2.5 cm for 34–35 weeks GA) No premedication |
n = 97 ↔ Procedural adverse events between two groups ↔ Need for MV or pneumothorax between groups |
|
Han, 202060 8 centers 25–31 weeks GA Calf pulmonary surfactant, 70–100 mg/kg |
FiO2 >0.4, CPAP 5-6 cm H2O 5 Fr feeding tube with ophthalmic forceps No premedication |
n = 298 ↔ BPDd between the groups ↓ hsPDA in the LISA group |
|
Gupta et al., 202061 Single center 28–34 weeks GA Poractant alfa 200 mg/kg |
FiO2 >0.3, NIPPV PEEP 5–6 cm H2O 5 Fr feeding tube, Cologne method No premedication |
n = 58 ↔MV within 72 HOLd between groups |
|
Single center 28–36 weeks GA Unspecified surfactant 100 mg/kg |
NIPPV, Silverman Score ≥4, FiO2 >0.3 5 Fr Feeding tube, ± Magill forceps No premedication |
n = 40 ↔ MV within 72 HOLd between two groups ↔ Difference adverse events between two groups |
BPD bronchopulmonary dysplasia, HOL hours of life, hsPDA hemodynamically significant patent ductus arteriosus, NEC necrotizing enterocolitis, NIPPV non-invasive positive pressure ventilation.
aTake Care method: insertion of a flexible catheter (i.e., feeding tube) below the vocal cords with direct laryngoscopy without using Magill forceps.
bCologne method: insertion of a flexible catheter (i.e., feeding tube) below the vocal cords with direct laryngoscopy using Magill forceps.
cHobart method: insertion of a stiff catheter (i.e., 16 G 5.25” vascular catheter) below the vocal cords with direct laryngoscopy.
dPrimary outcome defined a priori.
↑ Higher, ↓ Lower, ↔ Not significantly different.
In these trials, LISA was compared against three different strategies.
Comparison 1. LISA vs surfactant administration through an ETT at a similar FiO2 threshold as the LISA arm and continued MV without attempting to extubate the infant soon after surfactant administration
The multicenter study by Kribs et al.28 is the only study in this category and the only one to evaluate LISA in 23–24 weeks GA infants. The primary outcome (death or BPD) was not different between the two groups. However, a higher proportion of infants in the LISA group survived without major morbidity (Table 2). Among infants in the LISA group, 53% escaped MV during the first 72 h of life (HOL). The need for MV during NICU stay was also lower with the LISA group - this effect was mainly seen in 25–26 weeks GA infants. Of infants 23–24 weeks GA, 93% required MV.
Comparison 2. LISA vs selective ETT intubation and continued MV based either on physician judgment or criteria established a priori, with surfactant administration based on physician judgment
In the first RCT evaluating LISA,29 26–28 weeks GA infants were enrolled in 12 centers within the German Neonatal Network (GNN). The primary outcome (the need for MV or not being ventilated but having PaCO2 >65 or FiO2 >0.6 for >2 h between 25 and 72 HOL) was lower in the LISA group compared to control group. There were no differences between the two groups in important clinical outcomes. A large blinded multicenter trial (OPTIMIST-A trial), of 25–28 weeks GA infants involving 33 international centers31 had a planned enrollment of 606 infants. After 10 years, with 485 infants enrolled, it was terminated due to impaired enrollment, a consequence of the Covid-19 pandemic. Infants were randomized if they required FiO2 0.3 at CPAP 5–8 cm H2O. The control group of infants underwent a sham procedure that included only repositioning but mimicked LISA procedure in team interaction and duration. Infants in both groups were intubated at threshold of FiO2 0.4–0.45, and received surfactant based on clinicians’ judgment. Overall, there were no differences in baseline demographics between the two groups. Infants in the 25–26 weeks GA stratum had a higher frequency of incomplete or absent antenatal steroid use, multiple births, and male sex. There was no difference between the groups in the composite primary outcome of the study—death or BPD (43.6% in LISA group vs 49.6 and in controls, RR 0.87, 95% CI 0.74–1.03). However, compared to the control group, infants in the LISA group had a lower incidence of BPD, and pneumothorax, and a decreased need for MV and PDA treatment. An exploratory subgroup analysis showed higher mortality in the LISA group for 25–26 infants GA compared to control group infants (15.6 vs 6.90%, RR 1.95, 95% CI 0.9–4.23).
A third RCT, the only one to evaluate LISA in 32–36 weeks GA infants52 used premedication in both groups as part of the protocol. The primary outcome of the study—incidence of air leak or need for MV within 72 HOL—was lower in the LISA arm.
Comparison 3. Surfactant administration through an ETT at a similar FiO2 threshold as the LISA arm, followed by rapid extubation (INSURE)
Of the 13 trials comparing these two methods, 7 trials included infants born >32 weeks GA, and only 1 included <25 weeks GA infants,30 None of these trials were powered for the composite outcome of death or BPD, or for other important clinical outcomes. These studies are summarized in Table 2. A meta-analysis64 of these trials showed that compared to INSURE, LISA decreases death or BPD, need for MV within 72 h, and mortality.
Systematic reviews and meta-analyses of comparisons of LISA vs surfactant via ETT
Several systematic reviews with meta-analyses have addressed trials comparing LISA with ETT surfactant (Table 4).64–73
Table 4.
Meta-analyses of studies comparing LISA with surfactant administration via ETT.
| Author | Results | Certainty of evidence according to GRADE |
|---|---|---|
|
Abdel-Latif et al., 202173 16 RCTs n = 1324 for death of BPD n = 1422 for MV <72 HOL n = 857 for severe IVH |
Compared to ETT surfactant, LISA lead to • ↓ Death or BPD (RR 0.59 (95% CI 0.48–0.73), NNB 9 (95% CI 7–16) • ↓ BPD (RR 0.57, 95% CI 0.45–0.74), NNB 13 (95% CI 9–24) • ↓ MV within 72 h (RR 0.63, 95% CI 0.54–0.74), NNB 8 (95% CI 6–12) • ↓ Severe IVH (RR 0.63, 95% CI 0.42–0.96), NNB 22 (95% CI 12–193) • ↓ Mortality (RR 0.63, 95% CI 0.47–0.84), NNB 20 (95% CI 12–58) Compared to INSURE, LISA lead to • ↓ Death or BPD, (RR 0.52, 95% CI 0.4–0.68) NNB 9 (95% CI 6–15) • ↓ BPD (RR 0.57, 95% CI 0.44–0.75), NNB 14 (95% CI 9–28) • ↓ Mortality (RR 0.60, 95% CI 0.44–0.82), NNB 19 (95% CI 11–52) • ↓ MV within 72 h (RR 0.61, 95% CI 0.50–0.75), NNB 8 (95% CI 6–14) |
Moderate Moderate Moderate Low Low |
|
Barkhuff et al., 201964 7 RCTs n = 940 for pneumothorax n = 895 for all other outcomes |
Compared to ETT surfactant ± MV, LISA lead to • ↓ MV within 72 HOL, RR 0.74 (0.65–0.85) • ↓ Death or BPD, RR 0.74 (0.59–0.94), NNB: 14 • ↓ Pneumothorax 0.61 (0.37–1) Compared to INSURE, LISA lead to • ↓ MV within 72 h, RR 0.72 (0.53–0.97) • ↓ Death or BPD, RR 0.66 (0.46–0.93), NNB 11 (6–50) |
– |
|
Rigo et al., 201667 6 RCTs n = 895 Subgroup: INSURE vs LISA-4 RCTs, n = 464 |
Compared with ETT surfactant ± MV, LISA lead to • ↓ Early CPAP failure, (RR 0.67, 95% CI 0.53–0.84) • ↓ MV requirements during NICU stay (RR 0.65, 95% CI 0.45–0.95) • ↓ Death/BPD (RR 0.74, 95% CI 0.58–0.94), NNB 15 Compared to INSURE, LISA lead to • ↓ Need for MV within 72 h (RR 0.71, 95% CI 0.53–0.96) • ↓ Death of BPD (RR 0.63, 95% CI 0.44–0.92) |
– |
|
Aldana-Aguirre et al., 201768 6 RCTs n = 895 |
Compared to ETT surfactant ±MV, LISA lead to • ↓ Death/BPD with LISA; RR = 0.75 (0.59–0.94) • ↑ Surfactant reflux with LISA; RR = 2.52 (1.47–4.31) |
– |
|
Isayama, 201666 Network meta-analysis 30 RCTs Total n = 5598 LISA studies n = 637 |
Compared with MV, LISA group had • ↓ Death/BPD (OR 0.49, 95% CI 0.30–0.79) • ↓ BPD (OR 0.53, 95% CI 0.27–0.96) • ↓ Severe IVH (OR, 0.44, 95% CI 0.19–0.99) Compared with CPAP alone, LISA group had • ↓ Death/BPD (OR 0.58, 95% CI 0.35–0.93) • ↓ Air leak (OR 0.24, 95% CI 0.05–0.96) |
Moderate Moderate Moderate Moderate Very low |
CI confidence interval, NNB number needed for treatment benefit, OR odds ratio, RR typical risk ratio.
A recent Cochrane systematic review included 16 RCTs comparing LISA with ETT surfactant.73 It does not include data from the OPTIMIST-A trial described above. Compared to ETT surfactant, LISA decreased death or BPD (RR 0.59, 95% CI 0.48–0.73), BPD (RR 0.57, 95% CI 0.45–0.74, number need to treat for benefit [NNTB] 13, 95% CI 9–24) and the need for MV within 72 HOL (RR 0.63, 95% CI 0.54–0.74, NNTB 8, 95% CI 6–12). LISA also decreased mortality and severe intracranial hemorrhage (RR 0.63, 95% CI 0.42–0.96, NNTB 22, 95% CI 12–193), and in-hospital mortality (RR 0.63, 95% CI 0.47–0.84 NNTB 20, 95% CI 12–58). The authors of this review noted significant methodological weaknesses among included studies that decreased the certainty of evidence, with high risk of bias in eight studies related to randomization, blinding of outcome assessment, and incomplete or selective outcome reporting. However, a sensitivity analysis excluding these 8 studies showed results similar to those of the overall meta-analysis. Meta-analyses of a subgroup of studies comparing LISA with INSURE also showed a decrease in death or BPD, BPD, mortality, and need for MV within 72 HOL with LISA (Table 4).
Other methods of surfactant administration
Several alternative methods of surfactant administration without intubation have been or are being investigated, including pharyngeal instillation,74,75 nebulization (aerosolization),76,77 and laryngeal mask airway (LMA).78–80 None of these methods has been compared against LISA. Therefore, their potential superiority over LISA requires investigation. One ongoing trial is comparing LISA (MIST) versus surfactant administration through an LMA.81
Long-term neurodevelopmental outcome of infants managed with LISA
Among infants enrolled in the Göpel et al.29 study, the neurodevelopmental outcome was assessed at 18–36 months in 86% of eligible infants.82 Compared to control infants, infants managed with LISA did not differ in the Bayley II mental development index (98.5 ± 16.6% vs 92 ± 24, p = 0.07) and psychomotor development index (89 ± 19 vs 88 ± 23, p = 0.75). Among infants enrolled in the Kribs et al. trial,28 the neurodevelopmental outcome at 2 years was assessed in 86% of survivors.83 Compared to the LISA group, a higher proportion of control infants had low (<70) Bayley II psychomotor development score (22 vs 42%, p = 0.012) and low mental developmental index (<70) in the 25–26 weeks GA stratum (4 vs 21%, p = 0.008). These two studies suggest that neurodevelopmental outcomes are not worse, and possibly better with LISA compared to surfactant administration by ETT and continued MV. Of note, there are no studies comparing neurodevelopmental outcomes of infants treated with LISA vs INSURE.
Premedication for LISA
The role of premedication with LISA is not well evaluated. Of the 17 trials described above, only one included fentanyl (1 mcg/kg) as part of the protocol (Tables 1–3). Two infants in the LISA arm of this study required intubation and MV due to chest wall rigidity.52 A recent unblinded RCT (n = 34) showed better pain scores in infants receiving fentanyl (1 mcg/kg) during LISA.63 Several observational studies from Europe have reported the use of sedatives such as propofol, ketamine, and midazolam with LISA.84–88 In one RCT of 78 infants, compared to infants receiving no sedation prior to LISA, those receiving propofol had lower pain scores but a higher incidence of hypoxia requiring PPV.84 A systematic review that included both observational studies and RCTs found no significant impact on the duration of procedure or procedure success rates from sedation but a higher risk of desaturation, apnea, and need for non-invasive PPV, albeit with a low certainty of evidence. Currently, there is insufficient evidence to make strong recommendations about the routine use of sedatives/opioids for LISA. Further studies are urgently needed to address this issue. Until we have more evidence, it is reasonable to consider an individualized approach based on an infant’s GA, respiratory drive, and bedside assessment of pain and distress to guide the use of opioids and sedatives.47 If a decision is made to administer medication for pain, slow infusion of low dose fentanyl (0.7 mcg/kg) as recommended by a consensus guideline from the United Kingdom is prudent.3 In addition, non-pharmacological measures such as swaddling, oral/buccal sucrose or breast milk may be used for infants receiving LISA.3
Although atropine is recommended as part of premedication for non-emergent endotracheal intubation, only three RCTs comparing LISA and ETT surfactant included atropine as part of the protocol. A prospective observational study of LISA procedure from two tertiary centers in Australia reported that the use of atropine decreased the incidence of bradycardia associated with LISA procedure.6 Further studies are necessary before routine use of atropine can be recommended for LISA.
Optimum threshold and timing of LISA
Early surfactant therapy (≤2 HOL) improves outcomes compared to delayed therapy in preterm infants mechanically ventilated at birth.1 In studies evaluating early CPAP selective surfactant therapy was generally administered after ET intubation at a threshold of 0.4–0.6 FiO2 and CPAP level 5–8 cm H2O.18–21 None of these studies reported an association between timing of surfactant administration and outcome. Interestingly, nearly 50% of infants ≤28 weeks GA infants in these studies did not require surfactant therapy and escaped MV. In one large RCT, infants in the CPAP arm who received CPAP 8 cm H2O at birth with a threshold FiO2 of 0.6 for surfactant therapy had a higher incidence of pneumothorax.18 Subsequently, several observational studies have consistently shown that infants requiring FiO2 greater than 0.3 within the first 2 HOL (while using CPAP up to 7–8 cm H2O) have a higher need for MV within 72 HOL.23,24,26,89 Studies comparing LISA and ETT surfactants have used a threshold of FiO2 ≥0.3 and CPAP level 5–8 cm H2O in the LISA arm (Tables 2 and 3). The 2019 European consensus guideline suggests a threshold of 0.3 FiO2 with CPAP of at least 6 cm H2O. Based on the threshold criteria used in the RCTs and observational studies described above, if there is no reduction in FiO2 after initially escalating CPAP, it seems reasonable to use a threshold of FiO2 ≥0.3 at CPAP 7 cm H2O for LISA.90
Observational studies describing experience with LISA
A large body of observational evidence on LISA has been generated from Europe and Australia. In a study of 22–31 weeks GA infants born between 2009 and 2012 Göpel et al.91 compared the outcomes of infants receiving LISA (n = 1103) against matched control infants receiving selective surfactant therapy and MV (n = 1103). The LISA group had a decreased need for MV (41 vs 62%, p < 0.001), a lower BPD rate (12 vs 18%, p < 0.001), and a lower rate of death or BPD (14 vs 21%, p < 0.001). In a GNN study of 22–29 weeks GA infants born between 2009 and 2016 (n = 7533), Hartel et al.92 compared the outcomes of infants receiving no surfactant, LISA, and surfactant through ETT. The use of LISA increased during the study period (29% in 2009 to 50% in 2016). Infants treated with LISA (n = 2624) had a lower rate of mortality, BPD, severe IVH, PVL, PDA, and ROP compared to those treated with ETT surfactant (n = 3695). However, the subgroup of infants born at less than 26 weeks GA had a higher rate of spontaneous intestinal perforation (SIP) in the LISA group compared to the ETT surfactant group (10 vs 7.4%, p = 0.029). A multivariate analysis identified LISA as an independent risk factor for SIP [OR 1.42 (95% CI 1.06–1.89) among <26 weeks GA infants.
In a subsequent report, LISA had become the preferred method of surfactant administration within the GNN.93 Interestingly, this trend was also associated with an increase in the use of surfactant in ≤30 weeks GA infants across the network. Importantly, use of LISA was associated with decreased need for MV at every GA during the first 72 HOL, with a majority of infants ≥26 weeks GA completely escaping MV during their hospital stay. Among infants ≤25 weeks GA, although the need for MV in the first 72 HOL was lower, the need for MV during the overall hospital stay was high.93 Variables associated with CPAP failure among LISA-treated infants include lower birth weight and GA,28,94 frequent apnea, absence of maternal antenatal steroid therapy, and lower surfactant dose (<200 mg/kg).95
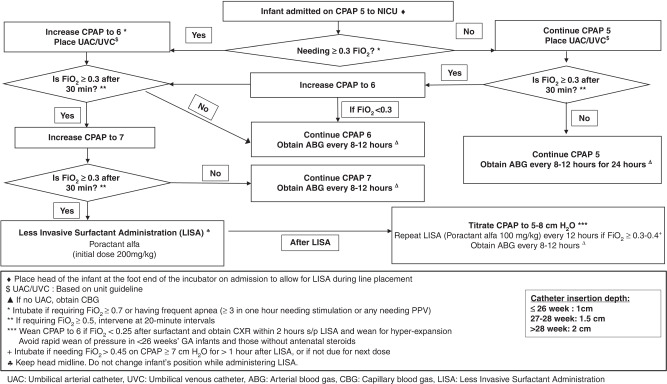
Our center introduced LISA as part of a quality improvement project (named OPTISURF) to minimize lung injury, which included optimization of CPAP and use of LISA in ≤29 weeks GA infants admitted to NICU on CPAP.90 The CPAP level was increased stepwise from 5 to 7 cm H2O if the FiO2 was ≥0.3, followed by LISA.90 Compared to historical controls, infants managed with the new algorithm (Fig. 1) received CPAP 7 cm H2O within 4 HOL more often, and had a lower rate of MV (within the first 72 HOL as well as during overall hospital stay), pneumothorax, and patent ductus arteriosus treatment. The lower need for MV within 72 HOL was seen in both infants 23–26 weeks GA, and 27 to 29 weeks GA. Of the LISA procedures, 39% required more than one attempt, and 55% were associated with desaturation and bradycardia. Most bradycardia and desaturation events were either self-resolving or required mild tactile stimulation, and/or supplemental oxygen.
Fig. 1. Algorithm for Management of Infants with RDS Using CPAP and Less Invasive Surfactant Administration.
Optimization of CPAP and less invasive surfactant administration (OPTISURF) guideline.
Implementing LISA
Based on the above evidence, many neonatal units in the US may choose to implement the practice of LISA, while others may choose to wait for more evidence to accumulate before adopting LISA. For units that wish to implement LISA, we provide the below guidance, based on our experience with implementing it in two large neonatal units. LISA should be used as part of a comprehensive strategy to optimize non-invasive support to maintain adequate FRC with interventions such as a bubble CPAP system,96 an optimum CPAP level,97 an optimal interface,98,99 airway clearance, positioning, and nursing care.100 CPAP failure rate varies between centers based on the proficiency of unit personnel with the use of non-invasive support,22,24,26,101 and all attempts should be made to ensure optimal CPAP delivery before going down the path of LISA. Adequate time should be provided for CPAP to exert its beneficial effects before declaring CPAP failure.
Every unit implementing LISA should create a local clinical practice guideline based on evidence and consensus that includes stabilization of preterm infants after admission to NICU, optimization of non-invasive support, threshold and timing of LISA, administration of caffeine, eligibility and exclusion criteria for LISA, use of premedication (if any),3 non-pharmacological interventions to reduce pain and discomfort,3 the exact steps to be followed for the procedure, minimum competencies required for the personnel performing the procedure, and post-procedure management. All relevant unit personnel should be educated about the guideline, preferably using simulation,3,102 and readily available instructional videos.90 It is important to establish role clarity for individual team members of multidisciplinary teams during these simulation training sessions. The training of nurses and respiratory therapists is best conducted by discipline-specific team champions prior to practice change. An algorithm detailing criteria for escalation of CPAP and LISA should be kept at the bedside. The formulation of surfactant selected for use within the unit, and the standard surfactant dose and regimen should be used for LISA. The following material can be used and modified by individual units to create a local practice guideline.
Selection of cases for LISA
Infants born ≤32 weeks gestation with respiratory distress requiring CPAP are eligible to receive LISA if they require FiO2 >0.3 after ensuring optimal CPAP therapy.
Although a chest radiograph may help in confirming the diagnosis of RDS and excluding other causes of respiratory distress, i.e., pneumothorax, it should not delay the administration of surfactant in eligible infants.
An arterial or capillary blood gas is recommended for infants requiring increasing oxygen requirement to identify severe respiratory acidosis.
- Contraindications for LISA:
-
○Infants with rapidly worsening respiratory disease (e.g., persistently requiring FiO2 ≥0.70, PCO2 >70 mm Hg, pH <7.1) despite optimal non-invasive support.
-
○Infants with frequent apnea (≥3 requiring stimulation in 1 h or any apnea requiring PPV) should be intubated and mechanically ventilated.
-
○
LISA procedure steps and safety
LISA should be performed by clinicians proficient with intubation skills, who have undergone simulation-based training about LISA, and viewed the procedure video.
A respiratory therapist should ensure optimum CPAP setup and level, infant positioning, and equipment before starting the LISA procedure. The CPAP level should be optimized to achieve and maintain optimal FRC during the procedure. Avoid discontinuation of CPAP during the procedure.
Assemble the team including a nurse, respiratory therapist, a clinician proficient in intubation and LISA, and a supporting clinician.
Gather supplies: 5 ml syringe, LISA catheter, sterile marker pen, sterile tape measure, sterile gloves, and drape. A LISA kit can be prepared that contains all these supplies. Also have an appropriate-sized face mask and a readily available functioning bag (or a T-piece resuscitator) for positive pressure ventilation.
A nurse will draw up surfactant and 1 ml of air into a syringe.
The first clinician will create a sterile field with drapes, wear sterile gloves, and will mark the insertion depth on the catheter with the pen (1 cm for ≤26 weeks GA, 1.5 cm for 27–28 weeks GA, 2 cm for ≥29 weeks GA).
A nurse will monitor the vital signs during the procedure and an audible beat-to-beat monitor is activated for the procedure to alert the provider about bradycardia.
The first clinician, using an appropriate size laryngoscope, will pass the catheter to the insertion depth, hold it in place at the lips, and withdraw the laryngoscope blade. Each attempt to place a thin catheter should last for a maximum of 30 s. The attempt should be abandoned if there is severe bradycardia or desaturation, or if >2 attempts are required. At that point, the infant should be intubated with an ETT.
A second clinician will connect the syringe and instill surfactant in 4 aliquots over 1–2 min followed by 1 ml of air.
The catheter is removed, and CPAP continued.
An orogastric tube is placed and the stomach is aspirated to check for surfactant reflux.
The nurse and RT will continuously monitor the infant during the procedure, and provide tactile stimulation and titration of FiO2 during the procedure. The infant should be continuously monitored as described above. The nurse should alert the clinicians about bradycardia.
Persistent bradycardia or desaturation events may require face mask PPV during or after the procedure.
Improvement in oxygenation will be seen within minutes after surfactant administration, confirming successful administration.
Each procedure should be documented in a customized audit sheet (Supplementary File 1) that captures information about the number of attempts, duration of the procedure, duration and severity of desaturation and bradycardia during the procedure, other adverse events, surfactant reflux, and the interventions required to mitigate such events.
Post-procedure monitoring after LISA
Infants developing respiratory failure on CPAP should be identified early by closely monitoring clinical status and blood gas values.
The CPAP level should be titrated based on the need for supplemental oxygen and clinical status. Infants ≤26 weeks GA are at a higher risk of failing CPAP after LISA. Therefore, caution should be exercised while decreasing the CPAP level.
Repeat doses of LISA should be considered at a FiO2 threshold of 0.30–0.4 at the standard time intervals. Repeat dose should also be considered if >50% of the dose of surfactant is aspirated from the stomach AND there is no significant improvement in the oxygenation (using either LISA or INSURE method).
Conclusions
The current body of evidence suggests that, in preterm infants who have worsening RDS after initial stabilization on nasal CPAP, use of LISA when compared to the INSURE or to continued MV has potential benefits, and might lead to a lower need for MV, decrease in BPD and composite death or BPD. The available long-term outcome studies show promise for improved neurodevelopmental outcomes with LISA. US-based clinicians are justified if, based on the available body of evidence they decide to routinely use LISA in preterm infants with RDS as an alternative to intubation and surfactant administration. A unit guideline based on evidence and consensus for LISA should be developed that specifies all aspects of the procedure. In the absence of high-quality evidence, decisions about the exact catheter type and insertion method, and the use of pharmacologic and non-pharmacologic interventions to minimize pain and discomfort should be made based on the expertise and skill level at the individual center, and on clinician consensus. All team members involved in the LISA procedure should undergo extensive education and training in the technique of LISA and their specific roles. When LISA is implemented, details of every LISA procedure should be documented and individual outcomes followed. LISA should be part of an extensive strategy to promote non-invasive respiratory support in the NICU.
Supplementary Information
Acknowledgement
Dr. Kakkilaya acknowledges the support from Parkland Community Health Plan.
Author contributions
V.K. conceptualized and designed the study, analyzed and interpreted the data, drafted the initial manuscript, and reviewed and revised the manuscript. K.S.G. conceptualized and designed the study, analyzed and interpreted the data, provided the original structure and organization of the manuscript, and reviewed and revised the manuscript. ACKNOWLEDGEMENT: Dr. Kakkilaya acknowledges the support from Parkland Community Health Plan
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-022-02265-8.
References
- 1.Suresh GK, Soll RF. Overview of surfactant replacement trials. J. Perinatol. 2005;25:S40–S44. doi: 10.1038/sj.jp.7211320. [DOI] [PubMed] [Google Scholar]
- 2.Sweet DG, et al. European Consensus Guidelines on the management of respiratory distress syndrome – 2019 update. Neonatology. 2019;115:432–450. doi: 10.1159/000499361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds P, et al. Less-invasive surfactant administration for neonatal respiratory distress syndrome: a consensus guideline. Neonatology. 2021;118:586–592. doi: 10.1159/000518396. [DOI] [PubMed] [Google Scholar]
- 4.Ng EH, Shah V. Guidelines for surfactant replacement therapy in neonates. Paediatr. Child Health. 2021;26:35–49. doi: 10.1093/pch/pxaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vento M, Bohlin K, Herting E, Roehr CC, Dargaville PA. Surfactant administration via thin catheter: a practical guide. Neonatology. 2019;116:211–226. doi: 10.1159/000502610. [DOI] [PubMed] [Google Scholar]
- 6.Roberts CT, et al. Outcomes after introduction of minimally invasive surfactant therapy in two Australian tertiary neonatal units. J. Pediatr. 2021;229:141–146. doi: 10.1016/j.jpeds.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Verder, H. et al. Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study. Ugeskr Laeger. 154, 2136–2139 (1992). [PubMed]
- 8.Verder H, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N. Engl. J. Med. 1994;331:1051–1055. doi: 10.1056/NEJM199410203311603. [DOI] [PubMed] [Google Scholar]
- 9.Rojas-Reyes, M. X., Morley, C. J. & Soll, R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. CD000510 (2012). [DOI] [PubMed]
- 10.Schmölzer GM, et al. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347:f5980. doi: 10.1136/bmj.f5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramaniam, P., Ho, J. J. & Davis, P. G. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst. Rev. CD001243 (2016). [DOI] [PubMed]
- 12.Committee on Fetus and Newborn; American Academy of Pediatrics. Respiratory support in preterm infants at birth. Pediatrics. 133, 171–174 (2014). [DOI] [PubMed]
- 13.LeVan JM, et al. Change in care among nonenrolled patients during and after a randomized trial. Pediatrics. 2013;132:e960–e970. doi: 10.1542/peds.2013-1595. [DOI] [PubMed] [Google Scholar]
- 14.Weydig H, Ali N, Kakkilaya V. Noninvasive ventilation in the delivery room for the preterm infant. NeoReviews. 2019;20:e489–e499. doi: 10.1542/neo.20-9-e489. [DOI] [PubMed] [Google Scholar]
- 15.Kakkilaya, V. et al. Quality improvement project to decrease delivery room intubations in preterm infants. Pediatrics143, e20180201 (2019). [DOI] [PMC free article] [PubMed]
- 16.Hatch LD, III, et al. Changes in use of respiratory support for preterm infants in the US, 2008-2018. JAMA Pediatr. 2021;175:1017–1024. doi: 10.1001/jamapediatrics.2021.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell EF, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 2022;327:248–263. doi: 10.1001/jama.2021.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morley, C. J. et al. Nasal CPAP or intubation at birth for very preterm infants. N. Engl. J. Med. 358, 700–708 (2008). [DOI] [PubMed]
- 19.Finer NN, et al. Early CPAP versus surfactant in extremely preterm infants. N. Engl. J. Med. 2010;362:1970–1979. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandri F, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics. 2010;125:e1402–e1409. doi: 10.1542/peds.2009-2131. [DOI] [PubMed] [Google Scholar]
- 21.Dunn, M. S., Kaempf, J. & Klerk, A. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics128, e1069–e1076 (2011). [DOI] [PubMed]
- 22.Ammari, A., Suri, M. & Milisavljevic, V. Variables associated with the early failure of nasal CPAP in very low birth weight infants. J. Pediatr. 147, 341–347 (2005). [DOI] [PubMed]
- 23.Fuchs, H., Lindner, W., Leiprecht, A., Mendler, M. R. & Hummler, H. D. Predictors of early nasal CPAP failure and effects of various intubation criteria on the rate of mechanical ventilation in preterm infants of <29 weeks gestational age. Arch. Dis. Child Fetal Neonatal Ed.96, F343–F347 (2011). [DOI] [PubMed]
- 24.Dargaville, P. A., Aiyappan, A. & Paoli A. G. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology104, 8–14 (2013). [DOI] [PubMed]
- 25.Dargaville, P. A. et al. Incidence and outcome of CPAP failure in preterm infants. Pediatrics138, e20153985 (2016). [DOI] [PubMed]
- 26.Kakkilaya V, et al. Early predictors of continuous positive airway pressure failure in preterm neonates. J. Perinatol. 2019;39:1081–1088. doi: 10.1038/s41372-019-0392-z. [DOI] [PubMed] [Google Scholar]
- 27.Kurepa D, Perveen S, Lipener Y, Kakkilaya V. The use of less invasive surfactant administration (LISA) in the United States with review of the literature. J. Perinatol. 2019;39:426–432. doi: 10.1038/s41372-018-0302-9. [DOI] [PubMed] [Google Scholar]
- 28.Kribs A, et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2015;169:723–730. doi: 10.1001/jamapediatrics.2015.0504. [DOI] [PubMed] [Google Scholar]
- 29.Göpel W, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378:1627–1634. doi: 10.1016/S0140-6736(11)60986-0. [DOI] [PubMed] [Google Scholar]
- 30.Kanmaz, H. G., Erdeve, O., Canpolat, F. E., Mutlu, B. & Dilmen, U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics131, e502–e509 (2013). [DOI] [PubMed]
- 31.Dargaville, P. A. et al. Effect of minimally invasive surfactant therapy vs sham treatment on death or bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome: the OPTIMIST-a randomized clinical trial. JAMA. 326, 2478–2487 (2021). [DOI] [PMC free article] [PubMed]
- 32.Kribs, A., Pillekamp, F., Hünseler, C., Vierzig, A. & Roth, B. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age</=27 weeks). Paediatr. Anaesth. 17, 364–369 (2007). [DOI] [PubMed]
- 33.Dargaville PA, et al. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch. Dis. Child. Fetal Neonatal Ed. 2011;96:F243–F248. doi: 10.1136/adc.2010.192518. [DOI] [PubMed] [Google Scholar]
- 34.Rigo V, Debauche C, Maton P, Broux I, Van Laere D. Rigid catheters reduced duration of less invasive surfactant therapy procedures in manikins. Acta Paediatr. 2017;106:1091–1096. doi: 10.1111/apa.13850. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharya, S., Read, B., Miller, M. & da Silva, O. Impact of catheter choice on procedural success of minimally invasive surfactant therapy. Am. J. Perinatol. 2021. 10.1055/s-0041-1733956 [Epub ahead of print]. [DOI] [PubMed]
- 36.NCT. A Study in Preterm Neonates With RDS to Compare CUROSURF® Administration Through LISA and Conventional Administration LISPAP. https://clinicaltrials.gov/show/NCT02772081 (2016).
- 37.Maiwald CA, Neuberger P, Vochem M, Poets C. QuickSF: a new technique in surfactant administration. Neonatology. 2017;111:211–213. doi: 10.1159/000450823. [DOI] [PubMed] [Google Scholar]
- 38.Maiwald, C. A. et al. Clinical evaluation of an application aid for less-invasive surfactant administration (LISA). Arch. Dis. Childhood Fetal Neonatal Ed. 106, 211–214 (2021). [DOI] [PMC free article] [PubMed]
- 39.Marshall TA, Deeder R, Pai S, Berkowitz GP, Austin TL. Physiologic changes associated with endotracheal intubation in preterm infants. Crit. Care Med. 1984;12:501–503. doi: 10.1097/00003246-198406000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Stow PJ, McLeod ME, Burrows FA, Creighton RE. Anterior fontanelle pressure responses to tracheal intubation in the awake and anaesthetized infant. Br. J. Anaesth. 1988;60:167–170. doi: 10.1093/bja/60.2.167. [DOI] [PubMed] [Google Scholar]
- 41.Friesen RH, Honda AT, Thieme RE. Changes in anterior fontanel pressure in preterm neonates during tracheal intubation. Anesth. Analg. 1987;66:874–878. [PubMed] [Google Scholar]
- 42.Raju TN, Vidyasagar D, Torres C, Grundy D, Bennett EJ. Intracranial pressure during intubation and anesthesia in infants. J. Pediatr. 1980;96:860–862. doi: 10.1016/s0022-3476(80)80558-0. [DOI] [PubMed] [Google Scholar]
- 43.Kelly MA, Finer NN. Nasotracheal intubation in the neonate: physiologic responses and effects of atropine and pancuronium. J. Pediatr. 1984;105:303–309. doi: 10.1016/s0022-3476(84)80137-7. [DOI] [PubMed] [Google Scholar]
- 44.Caldwell CD, Watterberg KL. Effect of premedication regimen on infant pain and stress response to endotracheal intubation. J. Perinatol. 2015;35:415–418. doi: 10.1038/jp.2014.227. [DOI] [PubMed] [Google Scholar]
- 45.Jourdain G, De Tersant M, Dell’Orto V, Conti G, De Luca D. Continuous positive airway pressure delivery during less invasive surfactant administration: a physiologic study. J. Perinatol. 2018;38:271–277. doi: 10.1038/s41372-017-0009-3. [DOI] [PubMed] [Google Scholar]
- 46.De Luca D, et al. Less invasive surfactant administration: a word of caution. Lancet Child Adolesc. health. 2020;4:331–340. doi: 10.1016/S2352-4642(19)30405-5. [DOI] [PubMed] [Google Scholar]
- 47.Peterson J, den Boer MC, Roehr CC. To sedate or not to sedate for less invasive surfactant administration: an ethical approach. Neonatology. 2021;118:639–646. doi: 10.1159/000519283. [DOI] [PubMed] [Google Scholar]
- 48.Mirnia K, et al. Comparison outcome of surfactant administration via tracheal catheterization during spontaneous breathing with InSurE. Med. J. Islamic World Acad. Sci. 2013;109:1–6. [Google Scholar]
- 49.Mohammadizadeh, M., Ardestani, A. G. & Sadeghnia, A. R. Early administration of surfactant via a thin intratracheal catheter in preterm infants with respiratory distress syndrome: feasibility and outcome. J. Res. Pharm. Pract. 4, 31–36 (2015). [DOI] [PMC free article] [PubMed]
- 50.Bao Y, Zhang G, Wu M, Ma L, Zhu J. A pilot study of less invasive surfactant administration in very preterm infants in a Chinese tertiary center. BMC Pediatr. 2015;15:21. doi: 10.1186/s12887-015-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oncel MY, et al. Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: a randomised controlled trial. Arch. Dis. Child. Fetal neonatal Ed. 2016;101:F323–F328. doi: 10.1136/archdischild-2015-308204. [DOI] [PubMed] [Google Scholar]
- 52.Olivier F, et al. Efficacy of minimally invasive surfactant therapy in moderate and late preterm infants: a multicentre randomized control trial. Paediatr. Child Health. 2017;22:120–124. doi: 10.1093/pch/pxx033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosayebi Z, et al. A randomized trial comparing surfactant administration using InSurE technique and the minimally invasive surfactant therapy in preterm infants (28 to 34 weeks of gestation) with respiratory distress syndrome. J. Compr. Ped. 2017;8:e60724. [Google Scholar]
- 54.Choupani R, Mashayekhy G, Hmidi M, Kheiri S, Dehkordi MK. A comparative study of the efficacy of surfactant administration through a thin intratracheal catheter and its administration via an endotracheal tube in neonatal respiratory distress syndrome. Iran. J. Neonatol. IJN. 2018;9:33–40. [Google Scholar]
- 55.Halim A, Shirazi H, Riaz S, Gul SS, Ali W. Less invasive surfactant administration in preterm infants with respiratory distress syndrome. J. Coll. Physicians Surg. Pak. 2019;29:226–330. doi: 10.29271/jcpsp.2019.03.226. [DOI] [PubMed] [Google Scholar]
- 56.Dekker J, et al. Sedation during minimal invasive surfactant therapy: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2019;104:F378–F383. doi: 10.1136/archdischild-2018-315015. [DOI] [PubMed] [Google Scholar]
- 57.Boskabadi H, Maamouri G, Gharaei Jomeh R, Zakerihamidi M. Comparative study of the effect of the administration of surfactant through a thin endotracheal catheter into trachea during spontaneous breathing with intubation (intubation-surfactant-extubation method) J. Clin. Neonatol. 2019;8:227–231. [Google Scholar]
- 58.Jena SR, et al. Surfactant therapy in premature babies: SurE or InSurE. Pediatr. Pulmonol. 2019;54:1747–1752. doi: 10.1002/ppul.24479. [DOI] [PubMed] [Google Scholar]
- 59.Yang G, et al. Effects of less invasive surfactant administration (LISA) via a gastric tube on the treatment of respiratory distress syndrome in premature infants aged 32 to 36 weeks. Medicine. 2020;99:e19216. doi: 10.1097/MD.0000000000019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han T, et al. Minimally invasive surfactant administration for the treatment of neonatal respiratory distress syndrome: a multicenter randomized study in China. Front. Pediatr. 2020;8:182. doi: 10.3389/fped.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta BK, Saha AK, Mukherjee S, Saha B. Minimally invasive surfactant therapy versus InSurE in preterm neonates of 28 to 34 weeks with respiratory distress syndrome on non-invasive positive pressure ventilation-a randomized controlled trial. Eur. J. Pediatr. 2020;179:1287–1293. doi: 10.1007/s00431-020-03682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pareek, P. et al. Less invasive surfactant administration (LISA) vs. intubation surfactant extubation (InSurE) in preterm infants with respiratory distress syndrome: a pilot randomized controlled trial. J. Trop. Pediatr. 67, fmab086 (2021). [DOI] [PubMed]
- 63.Sk, H., Saha, B., Mukherjee, S. & Hazra, A. Premedication with fentanyl for less invasive surfactant application (LISA): a randomized controlled trial. J. Trop. Pediatr. 68, fmac019 (2022). [DOI] [PubMed]
- 64.Barkhuff WD, Soll RF. Novel surfactant administration techniques: will they change outcome? Neonatology. 2019;115:411–422. doi: 10.1159/000497328. [DOI] [PubMed] [Google Scholar]
- 65.More K, Sakhuja P, Shah PS. Minimally invasive surfactant administration in preterm infants: a meta-narrative review. JAMA Pediatr. 2014;168:901–908. doi: 10.1001/jamapediatrics.2014.1148. [DOI] [PubMed] [Google Scholar]
- 66.Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA. 2016;316:611–624. doi: 10.1001/jama.2016.10708. [DOI] [PubMed] [Google Scholar]
- 67.Rigo V, Lefebvre C, Broux I. Surfactant instillation in spontaneously breathing preterm infants: a systematic review and meta-analysis. Eur. J. Pediatr. 2016;175:1933–1942. doi: 10.1007/s00431-016-2789-4. [DOI] [PubMed] [Google Scholar]
- 68.Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2017;102:F17–F23. doi: 10.1136/archdischild-2015-310299. [DOI] [PubMed] [Google Scholar]
- 69.Lau CSM, Chamberlain RS, Sun S. Less invasive surfactant administration reduces the need for mechanical ventilation in preterm infants: a meta-analysis. Glob. Pediatr. Health. 2017;4:2333794x17696683. doi: 10.1177/2333794X17696683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao ZL, et al. Less invasive surfactant administration in preterm infants with respiratory distress syndrome-an updated meta-analysis. J. Chin. Med. Assoc. 2020;83:170–179. doi: 10.1097/JCMA.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 71.Panza R, Laforgia N, Bellos I, Pandita A. Systematic review found that using thin catheters to deliver surfactant to preterm neonates was associated with reduced bronchopulmonary dysplasia and mechanical ventilation. Acta Paediatr. 2020;109:2219–2225. doi: 10.1111/apa.15374. [DOI] [PubMed] [Google Scholar]
- 72.Bellos, I., Fitrou, G., Panza, R. & Pandita, A. Comparative efficacy of methods for surfactant administration: a network meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 106, 474–487 (2021). [DOI] [PubMed]
- 73.Abdel-Latif ME, Davis PG, Wheeler KI, De Paoli AG, Dargaville PA. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst. Rev. 2021;5:Cd011672. doi: 10.1002/14651858.CD011672.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ten Centre Study G. Ten centre trial of artificial surfactant (artificial lung expanding compound) in very premature babies. Br. Med. J. (Clin. Res. Ed.) 1987;294:991–996. doi: 10.1136/bmj.294.6578.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murphy MC, et al. Study protocol for the POPART study-prophylactic oropharyngeal surfactant for preterm infants: a randomised trial. BMJ Open. 2020;10:e035994. doi: 10.1136/bmjopen-2019-035994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jorch G, et al. Surfactant aerosol treatment of respiratory distress syndrome in spontaneously breathing premature infants. Pediatr. Pulmonol. 1997;24:222–224. doi: 10.1002/(sici)1099-0496(199709)24:3<222::aid-ppul9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 77.Cummings, J. J. et al. Aerosolized calfactant for newborns with respiratory distress: a randomized trial. Pediatrics146, e20193967 (2020). [DOI] [PubMed]
- 78.Brimacombe, J. et al. The laryngeal mask airway for administration of surfactant in two neonates with respiratory distress syndrome. Paediatr. Anaesth. 14, 188–190 (2004). [DOI] [PubMed]
- 79.Pinheiro, J. M., Santana-Rivas, Q. & Pezzano, C. Randomized trial of laryngeal mask airway versus endotracheal intubation for surfactant delivery. J. Perinatol. 36, 196–201 (2016). [DOI] [PubMed]
- 80.Roberts KD, et al. Laryngeal mask airway for surfactant administration in neonates: a randomized, controlled trial. J. Pediatr. 2018;193:40–46.e41. doi: 10.1016/j.jpeds.2017.09.068. [DOI] [PubMed] [Google Scholar]
- 81.ANZCTR. Surfactant Administration by Supraglottic Airway for Preterm Infants with Respiratory Distress Syndrome: The SURFSUP 1 Trial. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12620001184965 (2020).
- 82.Herting E, et al. Two-year outcome data suggest that less invasive surfactant administration (LISA) is safe. Results from the follow-up of the randomized controlled AMV (avoid mechanical ventilation) study. Eur. J. Pediatr. 2020;179:1309–1313. doi: 10.1007/s00431-020-03572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mehler, K. et al. Outcome of extremely low gestational age newborns after introduction of a revised protocol to assist preterm infants in their transition to extrauterine life. Acta Paediatr.101, 1232–1239 (2012). [DOI] [PubMed]
- 84.Dekker J, et al. Sedation during minimal invasive surfactant therapy in preterm infants. Neonatology. 2016;109:308–313. doi: 10.1159/000443823. [DOI] [PubMed] [Google Scholar]
- 85.Bourgoin L, et al. Administering atropine and ketamine before less invasive surfactant administration resulted in low pain scores in a prospective study of premature neonates. Acta Paediatr. 2018;107:1184–1190. doi: 10.1111/apa.14317. [DOI] [PubMed] [Google Scholar]
- 86.Krajewski, P., Szpecht, D. & Hożejowski, R. Premedication practices for less invasive surfactant administration – results from a nationwide cohort study. J. Matern. Fetal Neonatal Med. 1–5 (2020). [DOI] [PubMed]
- 87.Descamps CS, et al. Propofol for sedation during less invasive surfactant administration in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2017;102:F465. doi: 10.1136/archdischild-2017-312791. [DOI] [PubMed] [Google Scholar]
- 88.Brotelande C, et al. Premedication with ketamine or propofol for less invasive surfactant administration (LISA): observational study in the delivery room. Eur. J. Pediatr. 2021;180:3053–3058. doi: 10.1007/s00431-021-04103-1. [DOI] [PubMed] [Google Scholar]
- 89.Gulczyńska E, Szczapa T, Hożejowski R, Borszewska-Kornacka MK, Rutkowska M. Fraction of inspired oxygen as a predictor of CPAP failure in preterm infants with respiratory distress syndrome: a prospective multicenter study. Neonatology. 2019;116:171–178. doi: 10.1159/000499674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kakkilaya, V. B. et al. Decreasing continuous positive airway pressure failure in preterm infants. Pediatrics148, e2020014191 (2021). [DOI] [PubMed]
- 91.Göpel W, et al. Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr. 2015;104:241–246. doi: 10.1111/apa.12883. [DOI] [PubMed] [Google Scholar]
- 92.Härtel C, et al. Less invasive surfactant administration and complications of preterm birth. Sci. Rep. 2018;8:8333. doi: 10.1038/s41598-018-26437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herting E, Härtel C, Göpel W. Less invasive surfactant administration (LISA): chances and limitations. Arch. Dis. Child. Fetal Neonatal Ed. 2019;104:F655–F659. doi: 10.1136/archdischild-2018-316557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Langhammer K, et al. Treatment and outcome data of very low birth weight infants treated with less invasive surfactant administration in comparison to intubation and mechanical ventilation in the clinical setting of a cross-sectional observational multicenter study. Eur. J. Pediatr. 2018;177:1207–1217. doi: 10.1007/s00431-018-3179-x. [DOI] [PubMed] [Google Scholar]
- 95.Janssen LC, et al. Minimally invasive surfactant therapy failure: risk factors and outcome. Arch. Dis. Child. Fetal Neonatal Ed. 2019;104:F636–f642. doi: 10.1136/archdischild-2018-316258. [DOI] [PubMed] [Google Scholar]
- 96.Wung JT, Driscoll JM, Jr., Epstein RA, Hyman AI. A new device for CPAP by nasal route. Crit. Care Med. 1975;3:76–78. doi: 10.1097/00003246-197503000-00006. [DOI] [PubMed] [Google Scholar]
- 97.Morley C. Continuous distending pressure. Arch. Dis. Child. Fetal Neonatal Ed. 1999;81:F152–F156. doi: 10.1136/fn.81.2.f152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Paoli AG, Davis PG, Faber B, Morley CJ. Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database Syst. Rev. 2008;2008:Cd002977. doi: 10.1002/14651858.CD002977. [DOI] [PubMed] [Google Scholar]
- 99.Jasani B, Ismail A, Rao S, Patole S. Effectiveness and safety of nasal mask versus binasal prongs for providing continuous positive airway pressure in preterm infants–a systematic review and meta-analysis. Pediatr. Pulmonol. 2018;53:987–992. doi: 10.1002/ppul.24014. [DOI] [PubMed] [Google Scholar]
- 100.Bonner, K. M. & Mainous, R. O. The nursing care of the infant receiving bubble CPAP therapy. Adv. Neonatal Care. 8, 78–95 (2008). [DOI] [PubMed]
- 101.Aly H, Mohamed MA. An experience with a bubble CPAP bundle: is chronic lung disease preventable? Pediatr. Res. 2020;88:444–450. doi: 10.1038/s41390-020-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sawyer T, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Academic Med. 2015;90:1025–1033. doi: 10.1097/ACM.0000000000000734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.