Abstract
Electrosynthesis of hydrogen peroxide (H2O2) through oxygen reduction reaction (ORR) is an environment-friendly and sustainable route for obtaining a fundamental product in the chemical industry. Co–N4 single-atom catalysts (SAC) have sparkled attention for being highly active in both 2e– ORR, leading to H2O2 and 4e– ORR, in which H2O is the main product. However, there is still a lack of fundamental insights into the structure–function relationship between CoN4 and the ORR mechanism over this family of catalysts. Here, by combining theoretical simulation and experiments, we unveil that pyrrole-type CoN4 (Co–N SACDp) is mainly responsible for the 2e– ORR, while pyridine-type CoN4 catalyzes the 4e– ORR. Indeed, Co–N SACDp exhibits a remarkable H2O2 selectivity of 94% and a superb H2O2 yield of 2032 mg for 90 h in a flow cell, outperforming most reported catalysts in acid media. Theoretical analysis and experimental investigations confirm that Co–N SACDp—with weakening O2/HOO* interaction—boosts the H2O2 production.
Introduction
Hydrogen peroxide (H2O2) is one of the most important chemicals, playing an essential role in chemical production, environmental treatment, paper and textile industry, and medical disinfection.1−4 The demand for H2O2 is growing year by year, especially resulting from global public health safety troubles. The traditional industrial anthraquinone process for H2O2 production suffers from more and more serious challenges such as intensive energy consumption, large amounts of organic waste generation, and safety issues from the instability of H2O2 in transport and storage.5−7 Recently, electroreduction of O2 to H2O2 through a two-electron (2e–) oxygen reduction reaction (ORR) has emerged as a promising alternative to the traditional anthraquinone process because of the environment-friendly merits and on-site production ability.8−13 The key to realizing the electrosynthesis of H2O2 from 2e– ORR is to develop low-cost and high-performance catalysts. Currently, the development of high-performance 2e– ORR catalysts in alkaline systems has advanced satisfactorily.14−20 However, the H2O2 production in acidic media possesses prominent advantages relative to the alkaline condition such as higher H2O2 stability (H2O2 in alkaline conditions is prone to self-decomposition). Most recently, the solid-state electrolytes were used to produce pure water–H2O2 solutions, which has gained special attention.1,21,22 Nevertheless, the acidic H2O2 production is suitable for most actual application scenarios,23,24 but state-of-the-art electrocatalysts for 2e– ORR in acid are presently lacking.
In recent years, there has been an incremental interest in searching for highly efficient and stable 2e– ORR catalysts in acid environments, including noble-based materials,23,25−28 transitional metal compounds,29−33 single-atom catalysts (SACs),24,34−38 and carbon materials.39−41 Among these, the carbon-based transition metal SAC has received special attention due to its high atom unitization, high electrical conductivity, and adjustable coordination environment.37 For instance, both Liu et al.(42) and Strasser et al.(43) have screened different carbon-based transition metal SACs (such as Fe, Co, Ni, Cu, and Mn). They found that the Co SAC had the most 2e– ORR activity and the CoN4 coordination structure was identified as the most active site. Recently, the Co SAC has emerged as the starred catalyst for 2e– ORR.4,6,11,24,34 On the other hand, it is worth noting that the CoN4 coordination structure has been demonstrated as the highly active site for 4e– ORR in quite a few previous studies.44−49 For instance, Li et al.(45) have prepared the single-atom CoN4 in carbon matrix and demonstrated it as the outstanding 4e– ORR catalyst with the high half potential of 0.773 V versus reversible hydrogen electrode (RHE) and low H2O2 selectivity of 0.76% at 0.8 V versus RHE in 0.5 M H2SO4. Summarizing present studies, the structure–function relationship between CoN4 coordination structure and ORR pathway (2e– or 4e– ORR) is highly controversial. The limited understanding is unfavorable for the development of high-performance catalysts for 2e– ORR in the oxygen reduction community. Therefore, identification of the most active CoN4 coordination structure for 2e– ORR is greatly desirable, especially for the development of highly active and selective catalysts in acidic media.
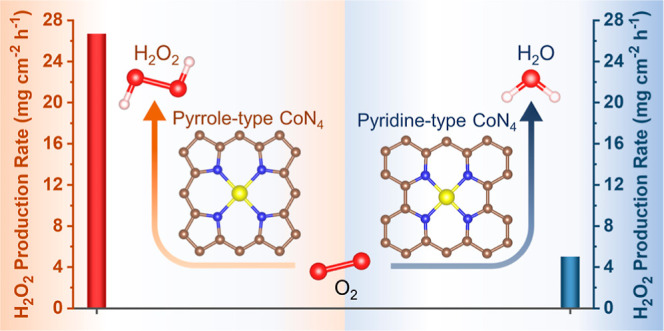
Herein, we focus on the identification of the highly active CoN4 coordination structure for 2e– ORR among a series of prepared Co–N SAC to develop the high-performance catalyst for H2O2 production in acidic media. Theoretically, screened from a series of Co–N motifs, the pyrrole-type CoN4 is found to show the optimal HOO* adsorption strength and highest 2e– ORR activity. Experimentally, the three types of Co–N SACs (Co–N SACDp, Co–N SACPc, and Co–N SACMm) are prepared using the pyrolysis strategy. Here, the Co–N SACDp and Co–N SACMm are obtained by using the nitrogen precursor of 4-dimethylaminopyridine and 2-methylimidazole, respectively, and the Co–N SACPc involves in the pyrolysis of cobalt phthalocyanine (CoPc) during the synthesis process. The results of catalyst characterization and performance evaluation confirm that Co–N SACDp (pyrrole-type CoN4) formation occurs in the dominant 2e– ORR pathway, while the Co–N SACMm formation with pyridine-type CoN4 occurs in the 4e– ORR. Impressively, the Co–N SACDp shows a remarkable mass activity of 14.4 A gcat–1 (0.5 V vs RHE) and H2O2 selectivity of 94% in 0.1 M HClO4. Furthermore, the Co–N SACDp has been demonstrated to have a prominent H2O2 production rate of 26.7 mg cm–2 h–1 and H2O2 yield of up to 2032 mg for 90 h in the flow cell, leading to, for example, a practical electro-Fenton degradation of carbamazepine (CBZ). This work affords essential insights into the ORR mechanism based on SAC catalysts and the development of efficient catalysts for H2O2 production.
Results and Discussion
Theoretical Calculation and ORR Mechanism
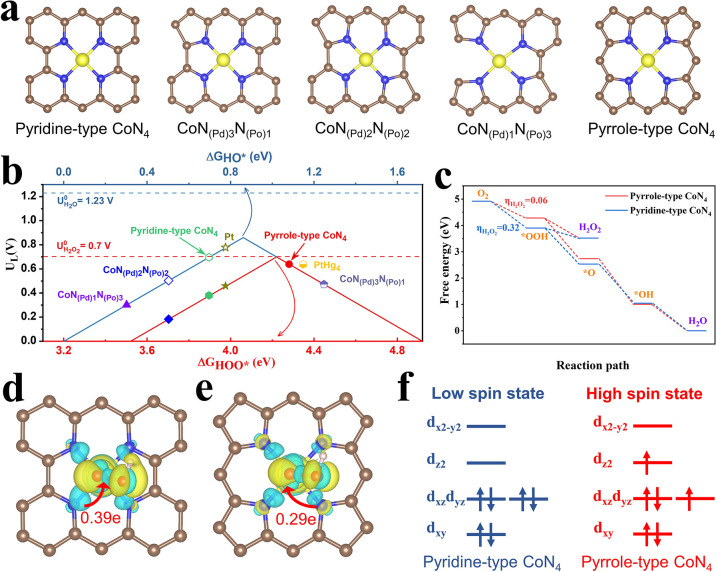
Among the most reported CoN4 active sites,3,42−45 the unsaturated pyridinic-N and pyrrolic-N are considered to be coordinated with the Co atom. Herein, we focus on varying the type of coordination nitrogen species in CoN4 sites, which has less been investigated previously. A series of Co–N coordination structures with different amounts of pyridinic-N or pyrrolic-N are constructed (Figure 1a). Also, most models show a thermodynamically favorable formation energy (Table S1). The pyridinic-N and pyrrolic-N are abbreviated as N(Pd) and N(Po), respectively. The HOO* and HO* are the crucial intermediates to determine if the reaction occurs through the 2e– or 4e– ORR pathway, and the Gibbs free energy of them (ΔHO* and ΔHOO*) for different Co–N coordination structures are computed. As shown in Figure 1b, ΔHO* is related to 4e– ORR, while ΔHOO* governs H2O2 production through 2e– ORR. According to the previous reports,25,50 there is an approximately linear relationship between the ΔHO* and ΔHOO* with a constant value of 3.2 ± 0.2 eV. Besides, the strong adsorption of HOO* or HO* is located in the left region, and the weak adsorption corresponds to the right downhill in the volcano plot. The pyridine-type CoN4, CoN(Pd)1(Po)3, and CoN(Pd)2(Po)2 with strong adsorption of HOO* prefer to break O–O bond, proceeding the 4e– ORR pathway. More interestingly, the pyridine-type CoN4 approaches the Pt implying the excellent 4e– ORR activity. In sharp contrast, the pyrrole-type CoN4 shows ΔHOO* of 4.28 eV, which is close to the optimal HOO* adsorption energy of 4.22 eV (corresponding to limiting the potential of 0.7 V), meaning the main 2e– ORR process. Impressively, the pyrrole-type CoN4 exhibits better 2e– ORR performances than other Co–N coordination structures, considering the small overpotential, which is comparable to the prominent PtHg4 catalyst25 (Figures 1b and S1). These results indicate that the coordination nitrogen type may govern the ORR pathway for CoN4, and pyrrole-type CoN4 is identified as the most active motif for H2O2 production. These results also can address the contradictory phenomenon that the CoN4 sites are highly active for both 2e– ORR and 4e– ORR reported in previous reports.42−49
Figure 1.
(a) Simulated different CoN4 coordination structures. (b) Volcano plot depicting the Gibbs free energy of reaction intermediates (ΔHO* and ΔHOO*) on different Co–N coordination structures. The Pt and PtHg4 were obtained from ref (25). (c) Free energy diagram of ORR on the pyridine-type and pyrrole-type CoN4. (d) Differential charge distribution on pyridine-type CoN4 with adsorption of HOO*. (e) Differential charge distribution on pyrrole-type CoN4 with adsorption of HOO*. (f) 3d electron configuration of pyridine-type and pyrrole-type CoN4 with adsorption of HOO*.
Next, we focus on the investigation of distinctly different ORR processes on the pyrrole-type CoN4 and pyridine-type CoN4. The general 2e– and 4e– ORR pathway can be depicted as follow (* indicates the catalytically-active site):
| 1 |
| 1a |
| 1b |
| 2 |
| 2a |
| 2b |
| 2c |
| 2d |
For O2 reduction to H2O2 through 2e– ORR, the pyrrole-type CoN4 shows a lower thermodynamic overpotential (η) of 0.06 eV (Figure 1c) relative to the pyridine-type CoN4 (ηH2O2 = 0.32 eV). For 4e– ORR to produce H2O, the pyridine-type CoN4 (ηH2O = 0.21 eV) exhibits a smaller thermodynamic overpotential than that of pyrrole-type CoN4 (ηH2O = 0.59 eV). These results reveal that pyrrole-type CoN4 prefers the 2e– ORR (Figure S2), while the pyridine-type CoN4 tends to proceed via 4e– ORR (Figure S3), in accordance with the results from the volcano plot (Figure 1b). Importantly, the kinetic analyses also confirm the kinetic favorable HOO* protonation process rather than HOO* dissociation on pyrrole-type CoN4 (Figure S4-5). Furthermore, we investigate the interplay between the CoN4 sites and the important HOO* intermediate, which largely determines the ORR pathway. As shown in Figure 1d,e, the pyridine-type CoN4 shows a more prominent electron transfer (0.39 e) from the CoN4 site to HOO* intermediate as compared to the pyrrole-type CoN4 (0.29 e) determined by Bader charge analysis. This result indicates the strong electron interaction between the pyridine-type CoN4 and HOO*. Moreover, the density of states (DOS) of Co in pyrrole-type and pyridine-type CoN4 with adsorption of HOO* were calculated (Figure S6), and the Co orbital occupation of electrons are shown in Figure 1f. After adsorption of HOO*, there are two single electrons in the dyzdxz and dz2 orbits for Co of pyrrole-type CoN4, while the electrons are paired in Co of pyridine-type CoN4. It is reported that the high spin state is associated to the weaker adsorption of the HOO* intermediate relative to the low spin state.51,52 Therefore, after adsorption of HOO*, the pyrrole-type CoN4 showing a high spin state facilitate the HOO* desorption to generate H2O2. However, the pyridine-type CoN4 with the low spin state tends to dissociate the O–O bond in HOO* and, lastly, form H2O. These results elucidate that the different ORR pathways for pyrrole-type and pyridine-type CoN4 may come from the electron interaction with the important reaction intermediate (such as HOO*) and the accompanying spin state difference.
Synthesis and Characterization of Co–N SAC
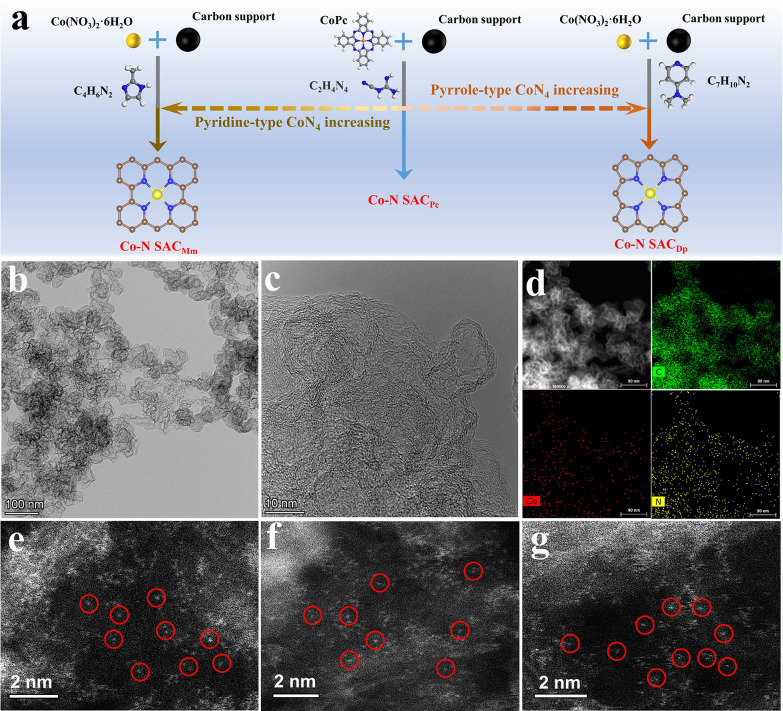
Inspired by the density functional theory (DFT) results, we prepared three different Co–N SACs through a pyrolysis strategy (synthesis details see the Experimental Section in Supporting Information). To exclude the effect of the carbon support, the Ketjen Black (ECP600JD) is used as the support for the three Co–N SACs (Figure 2a). Thereinto, the Co–N SACDp represents the sample derived from the 4-dimethylaminopyridine and cobaltous nitrate hexahydrate. The Co–N SACPc comes from pyrolysis of CoPc and dicyandiamide. For Co–N SACMm, the 2-methylimidazole and cobaltous nitrate hexahydrate are employed as the nitrogen and Co sources, respectively. Herein, considering the different transformation processes of the nitrogen precursor and coordination strength with the Co53,54 (Figure S7), altering the nitrogen precursor may result in a different coordination structure for Co–N SAC samples.
Figure 2.
(a) Schematic diagram of the synthesis route for the three SAC catalysts. (b,c) TEM images, and (d) element mapping images of the Co–N SACDp. AC-HAADF-STEM image of (e) Co–N SACDp, (f) Co–N SACPc, and (g) Co–N SACMm.
The structure and morphology of the catalysts are characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and aberration-corrected high-angle annular dark-field scanning TEM (AC-HAADF-STEM). As shown in Figure S8, the three Co–N SAC samples show a similar nanoparticle morphology for the initial carbon black. As shown in TEM images (Figures 2b,c, S9a,b, and S10a,b), there are only wrinkled carbon nanoparticles without obvious metal particles. The X-ray diffraction (XRD) patterns (Figure S11) show the only (002) peak for graphite carbon at about 26° and the absence of metal nanoparticles. The HAADF-TEM and element mapping images (Figures 2d, S9c–f, and S10c–f) reveal that the Co and nitrogen are homogeneously dispersed in the carbon matrix, indicating the atomical dispersion of Co for the three Co–N SAC samples. The AC-HAADF-STEM measurements are carried out to investigate the Co single-atom in the three samples. As displayed in Figure 2e–g, the bright and isolated metal atoms can be observed, confirming the successful preparation of the three Co–N SAC samples.
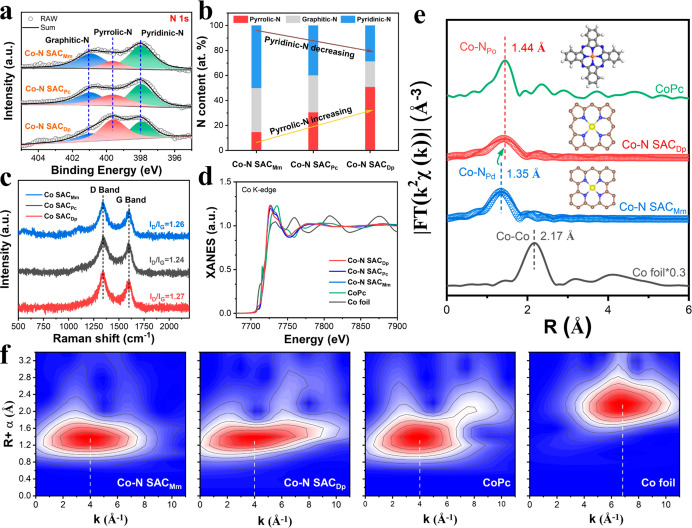
To ascertain the chemical state and coordination structure, the X-ray photoelectron spectroscopy (XPS) and X-ray absorption fine structure (XAFS) were performed. Deconvolution of N 1s XPS spectra (Figure 3a) confirms that all the three Co–N SAC samples contain the pyridinic-N (398.0 eV), pyrrolic-N (399.6 eV), and graphitic-N (400.9 eV) species.55−57 However, the proportion of the three N species has a great difference (Figure 3b and Table S2), suggesting the different coordination environments. Previous studies demonstrated that the transition metals tended to coordinate with the N species to form the MN4 structure, in which the pyridinic-N and pyrrolic-N species were generally considered.3,43−45 In our prepared three Co–N SAC samples, the Co–N SACDp shows the highest content of pyrrolic-N species (50.9%) while the Co–N SACMm exhibits the highest content of pyridinic-N species (50.0%, Figure 3b, and Table S2). That is to say, the Co–N SACDp may dominate the pyrrole-type CoN4 sites while the Co–N SACMm mainly contains the pyridine-type CoN4 sites. The Fourier transform infrared (FT-IR) spectra (Figure S12) show that all the three Co–N SAC samples exhibit a peak at 803 cm–1 which is related to the Co–N bonds.57 Especially, the Co–N SACDp emerges the characteristic stretch peak of pyrrole-type metal–N at 853 cm–1, which has been identified in Fe phthalocyanine and pyrrole-type FeN4 in the previous reports.57,58 According to the N K-edge X-ray absorption near edge structure (XANES) spectra (Figure S13), the more distinct pyrrolic-N species can be identified for the Co–N SACDp relative to the Co–N SACMm. Moreover, the splitting pyrrolic-N peak (pyrrolic-N′) for the Co–N SACDp may be related to the metal–pyrrolic N site.57 Although no oxygen-containing chemicals have been used in the preparation process, the oxygen signals have been detected in XPS for the three Co–N SACs and a similar phenomenon also has occurred in previously reported SAC materials.42,43,46,48 All the three Co–N SAC samples show similar oxygen functional groups and contents (Figure S14 and Table S3), which is expected to have less impact on ORR performances.
Figure 3.
(a) N 1s XPS spectra, (b) proportion of different N species, and (c) Raman spectra of the three samples. (d) Co K-edge XANES spectra and (e) FT k2-weighted and fitting extended XAFS (EXAFS) spectra of the Co–N SACDp, Co–N SACMm, and reference samples, inset: actual or fitting model. (f) Wavelet transform (WT) k2-weighted EXAFS contour plots of the Co–N SACDp, Co–N SACMm, and the reference samples.
It is shown in Co K-edge XANES spectra (Figure 3d) that the Co–N SACDp and Co–N SACMm exhibit higher pre-edge absorption energy than the Co foil but is comparable to the CoPc, indicating the positive valency of Co. The Fourier-transformed EXAFS spectra (Figure 3e) prove the absence of Co–Co bonds (2.17 Å) in the Co–N SACDp and Co–N SACMm further verifying the single-atom dispersion of Co, which agrees well with the AC-HAADF-STEM results. Moreover, the main peaks in 1.35 Å for the Co–N SACMm and 1.44 Å for the Co–N SACDp are ascribed to the Co–N coordination structure referring to the CoPc and the reported SACs.35,42,45,48 Noting that the slightly shifted Co–N peak between the Co–N SACMm and Co–N SACDp may come from the different Co–N coordination structures. Interestingly, the position of the Co–N peak in the Co–N SACDp is equivalent to that in CoPc, suggesting the Co–pyrrolic-N (Co–NPo) coordination structure in the Co–N SACDp.
Furthermore, the quantitative least-squares fitting of EXAFS spectra confirms (Figures 3e and S15, S16) that the Co atom is coordinated with four pyrrolic-N in the Co–N SACDp, while the four coordinated nitrogen species are pyridinic-N for the Co–N SACMm screened from different theoretical models (Figures S17, S18, Tables S4, and S5). Besides, the Co–N SACPc matches well with the CoN(Pd)3(Po)1 model where the Co atom is coordinated with three pyridinic-N and one pyrrolic-N (Figure S19). The coordination number for the Co–N SACDp and Co–N SACMm is 3.86 and 3.88 (Table S6), respectively. The bond length of Co−NPo (2.02 Å) in the Co−N SACDp is longer than that of Co−NPd (1.90 Å) in the Co−N SACMm, which is also corroborated by the proposed DFT model (Figure S20). As shown in the WT-EXAFS contour plots (Figure 3f), the three samples (Co–N SACDp, Co–N SACMm, and CoPc) with the Co–N coordination structure exhibit an intensity maximum of about 4 Å–1, which is different from the 6.8 Å–1 of Co–Co bond in Co foil. Summarizing the abovementioned results, we have experimentally confirmed the pyridine-type CoN4 (Co–N SACDp) and pyrrole-type CoN4 (Co–N SACMm) as illustrated in DFT simulation.
The physicochemical property has been further studied by Brunauer–Emmett–Teller (BET) specific surface area and Raman spectroscopy. The three Co–N SAC samples show the typical mesopore structure (Figure S21) and a comparable BET-specific surface area (Table S7). The characteristic D band and G band can be observed (Figure 3c) for carbon-based materials. Also, the intensity ratio of ID/IG for the Co–N SACDp, Co–N SACPc, and Co–N SACMm is comparable, indicating a similar defect degree after N-doping. Thus, the three Co–N SAC samples can be used to study the structure–function relationship between the Co–N coordination structure and the ORR pathway, due to the similar physicochemical parameters and similar element contents (Table S8) but different local coordination structures.
ORR Performances and Experimental Investigation
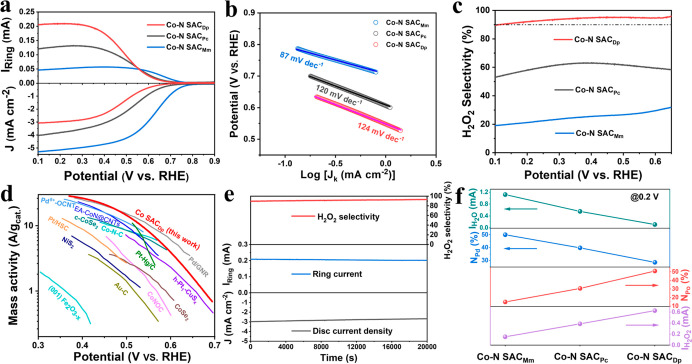
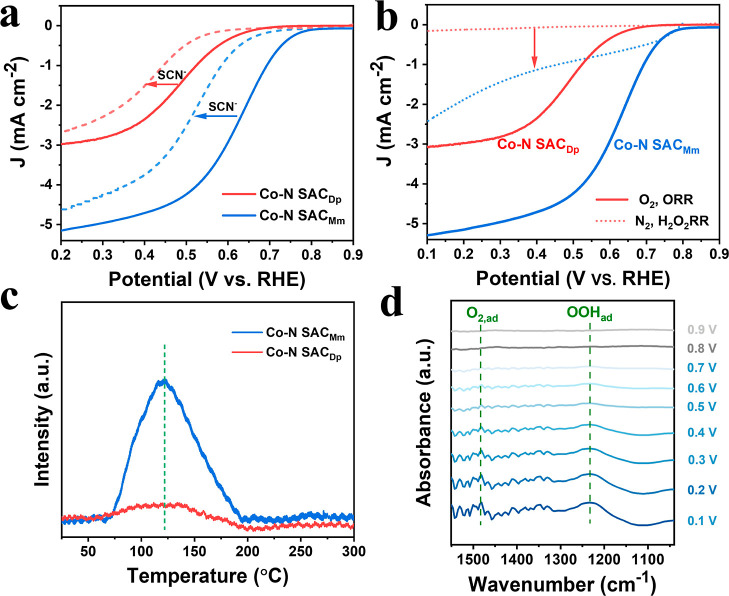
Now, we turn to the investigation of ORR performances. The ORR measurement is performed on the three-electrode system with the rotating ring-disk electrode (RRDE) used as the working electrode. Prior to the ORR performance evaluation, the collection efficiency (N) of the RRDE has been determined (Figure S22). Also, the ORR polarization curves are collected on RRDE at 1600 rpm in O2-saturated 0.1 M HClO4. As shown in Figure 4a, the ORR activity follows the order: Co–N SACMm > Co–N SACPc > Co–N SACDp in terms of the E–0.2 (The potential corresponds to the current density of −0.2 mA cm–2) and limiting current density (JL). Thereinto, the Co–N SACMm exhibits a high E–0.2 of 0.767 V versus RHE (Table S9) and large JL of 5.47 mA cm–2, in correspondence with the reported typical 4e– ORR polarization curves.45,46,48 Besides, the calculated electrochemical surface area (ECSA) based on the CV curves at different sweep rates accords well with the order of ORR activity (Figure S23 and Table S10). Using Koutecky–Levich (K–L) diffusion equation, the Tafel slopes of the Co–N SACMm, Co–N SACPc, and Co–N SACDp are calculated to be 87, 120, and 124 mV dec–1 (Figure 4b), respectively, implying the different reaction kinetics and rate-determining step (RDS).59,60 Thereinto, the RDS of Co–N SACMm may relate to the HOO* dissociation,20 while the first electron transfer process (* + O2 + e– → *O2–) is ascribed to the RDS for the Co–N SACPc and Co–N SACDp.37,42 The Co–N SACMm and Co–N SACDp show the transfer electron number (n) of 3.6–3.5 and 2.3–2.2 in the potential range of 0.6–0.2 V versus RHE, respectively, indicating the dominant 4e– ORR and 2e– ORR, respectively (Figure S24). Furthermore, according to the ORR polarization curves at different rotate rates and K–L diffusion equation, the n of the Co–N SACMm and Co–N SACDp is determined to be 3.7 and 2.2 (Figures S25 and S26), respectively. This result is consistent with the RRDE results. To investigate the relationship between the coordination structure and ORR pathway, the correlation between the content of nitrogen species and IH2O and IH2O2 (0.2 V vs RHE) is concluded in Figure 4f. The IH2O2 increases with the incremental content of pyrrolic-N (NPo), while the IH2O decreases with the reduced content of pyridinic-N (NPd). Also, this result implies that pyrrole-type CoN4 (Co–N SACDp) contributes to 2e– ORR for H2O2 generation, while the pyridine-type CoN4 (Co–N SACMm) accounts for the 4e– ORR to form H2O through an associative mechanism.37 These analyses from catalytic performances agree well with the DFT calculation results.
Figure 4.
(a) ORR polarization curves of RRDE at 1600 rpm in 0.1 M HClO4. (b) Calculated Tafel plots. (c) H2O2 selectivity. (d) Mass activity of the Co–N SACDp and recently reported catalysts (the detailed information about these reference catalysts can see in Table S11). (e) Chronoamperometry measurement of the Co–N SACDp for 20,000 s at 0.25 V (vs RHE). (f) Correlation between IH2O and IH2O2 current at 0.2 V (vs RHE) and the proportion of nitrogen species for the three Co–N SACs.
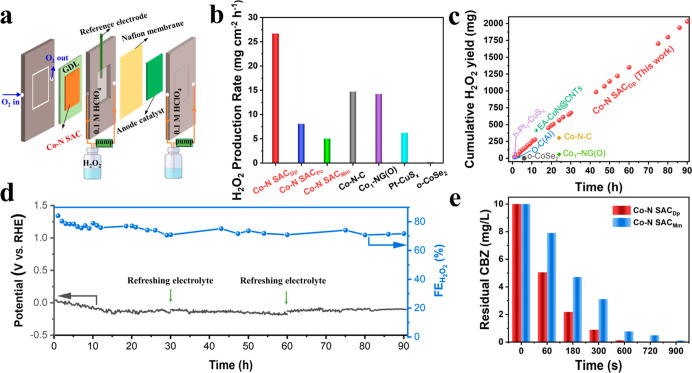
Considering the dominant 2e– ORR pathway on the Co–N SACDp, we focus on its performance for H2O2 production. Screened from the pyrolysis temperature of 700 to 900 °C (Figure S27), the Co–N SACDp pyrolyzed at 800 °C is found to show the highest 2e– ORR performance. The optimal Co–N SACDp exhibits a remarkable H2O2 selectivity of 94% at 0.3 V versus RHE (Figure 4c), outperforming that of the Co–N SACPc (62%) and Co–N SACMm (23%). Impressively, the Co–N SACDp reveals a superior mass activity of 14.4 A gcat.–1 (0.5 V vs RHE), prominent Co mass activity (Figure S28), and a wide potential range (0.65–0.1 V vs RHE) of >90% selectivity for H2O2 production. The 2e– ORR of the Co–N SACDp excels most reported non-noble metal catalysts and is even comparable to advanced noble metal catalysts in acidic media (Figure 4d). These outstanding 2e– ORR performances make the Co–N SACDp rank as one of the top catalysts for H2O2 production in acidic media (Table S11). It is shown in Figure 4e that the chronoamperometry measurement is performed to assess the catalytic stability of the Co–N SACDp on RRDE. The Co–N SACDp shows almost unchanged current signals and maintained H2O2 selectivity of >90% for 20,000 s, verifying its robust catalytic durability for 2e– ORR in acidic media. The ORR performances of the three Co–N SACs also have been evaluated in the different electrolytes. As displayed in Figures S29 and S30, the ORR activity shows a similar trend to that in acidic media, that is, Co–N SACMm > Co–N SACPc > Co–N SACDp. Also, the Co–N SACDp exhibits a higher H2O2 selectivity than that of the Co–N SACPc and Co–N SACMm both in 0.1 M KOH and 0.1 M phosphate-buffered saline (PBS). Especially, the Co–N SACDp possesses the maximal H2O2 selectivity of 86.1% in 0.1 M KOH (pH = 13) and 88.5% in 0.1 M PBS (pH = 7.2), respectively, demonstrating its greatly promising potential for H2O2 production in different environments.
Next, we perform further experimental investigations on the typical 4e– ORR catalyst of the Co–N SACMm and 2e– ORR catalyst of the Co–N SACDp to probe the origin of different ORR pathways. The SCN– poisoning experiments (Figure 5a) verify that the Co metal center is the actual active site in the two Co–N SAC. Furthermore, the prepared nitrogen-doped carbon (CN) catalyst without adding Co exhibits much inferior 2e– ORR activity to the Co–N SACDp (Figure S31), further confirming the substantial ORR contribution of the Co center. In the N2-saturated 0.1 M HClO4 containing 10 mM H2O2, Co–N SACDp shows negligible H2O2 reduction reaction (H2O2RR) activity while the Co–N SACMm exhibits apparent H2O2RR current (Figure 5b). That is to say, when the 2e– ORR proceeds, the generated H2O2 will be maintained on Co–N SACDp resulting in high H2O2 selectivity. However, the generated H2O2 on Co–N SACMm will be reduced to H2O leading to the dominated 4e– ORR. The O2 temperature-programed desorption (O2-TPD) curves show that the Co–N SACMm exhibits stronger O2 adsorption than that of Co–N SACDp (Figure 5c), which is in line with the facile O2 adsorption and activation for pyridine-type CoN4 (Co–N SACMm) in DFT calculation. However, the strong O2 adsorption for the Co–N SACMm will hamper the desorption of HOO* intermediates resulting in 4e– ORR and lowered H2O2 selectivity. However, the weak O2 adsorption may favor the facile HOO* desorption and ensure the high H2O2 selectivity for the Co–N SACDp. Furthermore, the in situ attenuated total reflectance surface–enhanced infrared absorption spectroscopy (ATR–SEIRAS) measurements are performed to detect the reaction intermediate (Figure S32). As shown in Figure 5d, there emerge two absorbance peaks at 1482 and 1231 cm–1 which can be attributed to the adsorption of O2 (O2,ad) and HOO* (OOHad), respectively, according to previous reports.34,61 A similar OOHad peak with increasing strength can be observed for Co–N SACMm (Figure S33) relative to Co–N SACDp. The relatively strong OOHad peak on the Co–N SACMm may be related to the higher coverage of HOO* considering the similar measurement procedure of in situ ATR–SEIRAS.62−64 Summarizing the abovementioned results, we use the experimental evidence to verify the DFT calculation results that the Co–N SACDp with pyrrole-type CoN4 favors the H2O2 production as compared to the Co–N SACMm (pyridine-type CoN4).
Figure 5.
(a) ORR polarization curves in 0.1 M HClO4 before and after the addition of 1 mM SCN–. (b) ORR polarization curves in 0.1 M HClO4 and H2O2RR polarization curves in 0.1 M HClO4 containing 10 mM H2O2. (c) O2-TPD curves for the Co–N SACDp and Co–N SACMm. (d) In situ ATR–SEIRAS spectra for the Co–N SACDp at potential range of 0.9–0.1 V.
Furthermore, based on the control experiment and DFT calculation results (Figures S34–S36), we deduce that the oxygen functional groups cannot account for the totally different ORR selectivity for the three Co–N SAC samples and have negligible influence on the H2O2 selectivity in the present pyrrole-type CoN4 system. Noting that the previously reported Co SAC4,24,34 usually used pyridine-type CoN4 as the theoretical model and the introduction of the C–O–C group in pyridine-type CoN4 could enhance the H2O2 selectivity. However, according to the theoretical results (Figure S34), the pyrrole-type CoN4 initially shows high H2O2 selectivity, and introducing C–O–C results in lower H2O2 selectivity.
H2O2 Production Ability and Electro-Fenton Application
Inspired by the outstanding 2e– ORR performances, the H2O2 production ability in the amplifying device of the flow cell has been assessed in 0.1 M HClO4. As shown in Figures 6a and S37, the Co–N SAC catalysts have been assembled into the cathode for ORR. Moreover, the IrO2 coating on the titanium sheet is used as the anode for oxygen evolution reaction, which is separated by the Nafion membrane. The Co–N SACDp in the flow cell can reach a large ORR current of −75 mA (Figure S38). As shown in Figure 6b, the Co–N SACDp shows a superior H2O2 production rate (26.7 mg cm–2 h–1) to the Co–N SACPc (8.1 mg cm–2 h–1) and Co–N SACMm (5.0 mg cm–2 h–1), consistent with the 2e– ORR activity order on RRDE. Impressively, the H2O2 production rate of Co–N SACDp surpasses most previously reported catalysts (Figure 6b and Table S11). Furthermore, the longstanding and stable H2O2 production has been performed at a fixed current of −50 mA, and the generated H2O2 is determined by the Ce4+ titration method (Figure S39). It is shown in Figure 6d that the Co–N SACDp shows a high FEH2O2 of 84% in the initial 1 h and maintains the FEH2O2 of >70% in the continuous H2O2 production operation up to 90 h (the electrolyte is periodically refreshed every 30 h). Importantly, the produced H2O2 amount linearly increases with the operating time and the cumulative H2O2 yield reaches up to 2032 mg for 90 h (Figure 6c), which is one of the highest H2O2 production yields in acidic media, to the best of our knowledge (Table S11). Moreover, the Co–N SACDp after the stability test shows no aggregated Co nanoparticles and maintains the atomical dispersion of Co species according to the XRD analysis (Figure S40), TEM, and AC-HAADF-STEM results (Figure S41), confirming its robust stability.
Figure 6.
(a) Schematic diagram of the flow cell for H2O2 production. (b) H2O2 production rate for different catalysts (the catalysts in this work are highlighted in red color and the other catalysts referred to the previous reports in Table S11). (c) Accumulatively produced H2O2 for Co–N SACDp and previously reported catalysts (detailed information in Table S11). (d) Chronopotentiometry curve at the fixed current of −50 mA and the corresponding FEH2O2 in the flow cell for Co–N SACDp. (e) Residual CBZ concentration at a different time in the electro-Fenton process.
In a preliminary application, the collected electrolyte after 30 h is used to decompose 200 ppm of methylene blue (MB, pH = 1, containing 10 mmol Fe2+). After adding the electrolyte to the MB solution, the MB can be totally removed (Figure S42), indicative of the promising potential for pollutant degradation. Furthermore, we also have compared the electro-Fenton activity of the Co–N SACDp and Co–N SACMm, and the recalcitrant CBZ is selected as the targeted organic pollutant65 (Figures S43 and S44). As shown in Figure 6e, the Co–N SACDp shows a superior degradation ability to the Co–N SACMm in which the 10 ppm CBZ can be completely removed within 12 min (Figure S45). Therefore, the remarkable H2O2 productivity and outstanding degradation ability make the Co–N SACDp a promising and ready catalyst for the actual application.
Conclusions
In summary, by combining DFT calculations and experiments, we addressed key issues about the structure–function relationship for a series of Co–N4 catalysts with different coordination structures and the ORR pathway over this family of SACs. We disclose that the pyrrole-type CoN4 mainly accounts for the 2e– ORR for producing H2O2, while the pyridine-motif promotes the 4e– ORR. This striking difference may originate from the electron interaction with the important HOO* intermediate and the accompanying spin state difference between the different coordinations of the active center. Experimentally, a series of Co–N SAC catalysts with different coordination structures were prepared. The Co–N SACDp with pyrrole-type of CoN4 and the Co–N SACMm with pyridine-type of CoN4 showed selectivity toward 2e– ORR and 4e– ORR, respectively. Impressively, the Co–N SACDp exhibits superior 2e– ORR performances than most previously reported catalysts in acidic media, with a mass activity of 14.4 A gcat.–1 at 0.5 V versus RHE and H2O2 selectivity of 94% at 0.3 V versus RHE. Our experimental results indicate that the Co–N SACDp (pyrrole-type CoN4) with weaker intermediate interaction favors the H2O2 production compared to the Co–N SACMm (pyridine-type CoN4). This is also in agreement with our DFT calculation results over these intermediates. Importantly, the Co–N SACDp has been tested in a flow cell, accumulating a H2O2 yield of 2032 mg for 90 h. As such, this work identifies the pyrrole-type CoN4 as the highly active Co–N coordination motif for electrosynthesis of H2O2, and provides fundamental insights into the ORR mechanism on SAC catalysts and beyond.
Acknowledgments
This study was financially supported by the Natural Science Foundation of China (grant nos. 21872174, 22002189, and U1932148), International Science and Technology Cooperation Program (grant no. 2017YFE0127800), China Postdoctoral Science Foundation (2021M701415 and 2022T150265), Hunan Provincial key research and development program (2020WK2002), the Hunan Provincial Natural Science Foundation of China (2020JJ2041 and 2020JJ5691), Hunan Provincial Science and Technology Program (2017XK2026), Guangdong Basic and Applied Basic Research Foundation (nos. 2020B1515020038 and 2021A1515110907), Shenzhen Science and Technology Innovation Project (grant no. JCYJ20180307151313532), Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2089/1-390776260, the Bavarian program Solar Energies Go Hybrid (SolTech), the Center for NanoScience (CeNS), and the European Commission through the ERC Starting Grant CATALIGHT (802989). M.Z. acknowledges the support of the Pearl River Talent Recruitment Program of Guangdong Province (2019QN01L148). The authors gratefully thank the National Synchrotron Radiation Research Center (NSRRC, the TLS 01C1 and TLS 16A1 beamlines, Taiwan) for XAFS measurement and BL10B in NSRL for soft XAS characterizations by Synchrotron Radiation. We are grateful for resources from the High Performance Computing Center of Central South University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c01194.
Catalyst synthesis, characterization, ORR measurement, kinetic barrier, DOS analysis, SEM images, TEM images, XRD patterns, FT-IR spectra, XANES spectra, O 1s XPS spectra, additional X-ray absorption spectroscopy (XAS) fitting data, N2 adsorption/desorption isotherms and BET data, ECSA data, additional ORR performance data, in situ ATR–SEIRAS setup and spectra, catalyst characterization after stability test, CBZ degradation data, and performance comparison with the reported catalysts (PDF)
Author Contributions
# S.C. and T.L. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Xia C.; Xia Y.; Zhu P.; Fan L.; Wang H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 2019, 366, 226–231. 10.1126/science.aay1844. [DOI] [PubMed] [Google Scholar]
- Bu Y.; Wang Y.; Han G.-F.; Zhao Y.; Ge X.; Li F.; Zhang Z.; Zhong Q.; Baek J.-B. Carbon-Based Electrocatalysts for Efficient Hydrogen Peroxide Production. Adv. Mater. 2021, 33, 2103266. 10.1002/adma.202103266. [DOI] [PubMed] [Google Scholar]
- Jiang K.; Back S.; Akey A. J.; Xia C.; Hu Y.; Liang W.; Schaak D.; Stavitski E.; Nørskov J. K.; Siahrostami S.; Wang H. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 2019, 10, 3997. 10.1038/s41467-019-11992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E.; Shin H.; Lee B.-H.; Efremov V.; Lee S.; Lee H. S.; Kim J.; Hooch Antink W.; Park S.; Lee K.-S.; Cho S.-P.; Yoo J. S.; Sung Y.-E.; Hyeon T. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 2020, 19, 436–442. 10.1038/s41563-019-0571-5. [DOI] [PubMed] [Google Scholar]
- Gao R.; Pan L.; Li Z.; Shi C.; Yao Y.; Zhang X.; Zou J.-J. Engineering Facets and Oxygen Vacancies over Hematite Single Crystal for Intensified Electrocatalytic H2O2 Production. Adv. Funct. Mater. 2020, 30, 1910539. 10.1002/adfm.201910539. [DOI] [Google Scholar]
- Li B.-Q.; Zhao C.-X.; Liu J.-N.; Zhang Q. Electrosynthesis of Hydrogen Peroxide Synergistically Catalyzed by Atomic Co–Nx–C Sites and Oxygen Functional Groups in Noble-Metal-Free Electrocatalysts. Adv. Mater. 2019, 31, 1808173. 10.1002/adma.201808173. [DOI] [PubMed] [Google Scholar]
- Jia Y.; Xue Z.; Yang J.; Liu Q.; Xian J.; Zhong Y.; Sun Y.; Zhang X.; Liu Q.; Yao D.; Li G. Tailoring the Electronic Structure of an Atomically Dispersed Zinc Electrocatalyst: Coordination Environment Regulation for High Selectivity Oxygen Reduction. Angew. Chem., Int. Ed. 2022, 61, e202110838 10.1002/anie.202110838. [DOI] [PubMed] [Google Scholar]
- Choi C. H.; Kim M.; Kwon H. C.; Cho S. J.; Yun S.; Kim H.-T.; Mayrhofer K. J. J.; Kim H.; Choi M. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun. 2016, 7, 10922. 10.1038/ncomms10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia F.; Li B.; Liu Y.; Liu Y.; Gao S.; Lu K.; Kaelin J.; Wang R.; Marks T. J.; Cheng Y. Carbon Free and Noble Metal Free Ni2Mo6S8 Electrocatalyst for Selective Electrosynthesis of H2O2. Adv. Funct. Mater. 2021, 31, 2104716. 10.1002/adfm.202104716. [DOI] [Google Scholar]
- Wang Y.; Shi R.; Shang L.; Waterhouse G. I. N.; Zhao J.; Zhang Q.; Gu L.; Zhang T. High-Efficiency Oxygen Reduction to Hydrogen Peroxide Catalyzed by Nickel Single-Atom Catalysts with Tetradentate N2O2 Coordination in a Three-Phase Flow Cell. Angew. Chem., Int. Ed. 2020, 59, 13057–13062. 10.1002/anie.202004841. [DOI] [PubMed] [Google Scholar]
- Liu W.; Feng J.; Yin R.; Ni Y.; Zheng D.; Que W.; Niu X.; Dai X.; Shi W.; Wu F.; Yang J.; Cao X. Tailoring oxygenated groups of monolithic cobalt-nitrogen-carbon frameworks for highly efficient hydrogen peroxide production in acidic media. Chem. Eng. J. 2022, 430, 132990. 10.1016/j.cej.2021.132990. [DOI] [Google Scholar]
- Tang C.; Jiao Y.; Shi B.; Liu J.-N.; Xie Z.; Chen X.; Zhang Q.; Qiao S.-Z. Coordination Tunes Selectivity: Two-Electron Oxygen Reduction on High-Loading Molybdenum Single-Atom Catalysts. Angew. Chem., Int. Ed. 2020, 59, 9171–9176. 10.1002/anie.202003842. [DOI] [PubMed] [Google Scholar]
- Li L.; Tang C.; Zheng Y.; Xia B.; Zhou X.; Xu H.; Qiao S.-Z. Tailoring Selectivity of Electrochemical Hydrogen Peroxide Generation by Tunable Pyrrolic-Nitrogen-Carbon. Adv. Energy Mater. 2020, 10, 2000789. 10.1002/aenm.202000789. [DOI] [Google Scholar]
- Dong K.; Lei Y.; Zhao H.; Liang J.; Ding P.; Liu Q.; Xu Z.; Lu S.; Li Q.; Sun X. Noble-metal-free electrocatalysts toward H2O2 production. J. Mater. Chem. A 2020, 8, 23123–23141. 10.1039/d0ta08894c. [DOI] [Google Scholar]
- Wang Y.; Waterhouse G. I. N.; Shang L.; Zhang T. Electrocatalytic Oxygen Reduction to Hydrogen Peroxide: From Homogeneous to Heterogeneous Electrocatalysis. Adv. Energy Mater. 2021, 11, 2003323. 10.1002/aenm.202003323. [DOI] [Google Scholar]
- Wang M.; Zhang N.; Feng Y.; Hu Z.; Shao Q.; Huang X. Partially Pyrolyzed Binary Metal-Organic Framework Nanosheets for Efficient Electrochemical Hydrogen Peroxide Synthesis. Angew. Chem., Int. Ed. 2020, 59, 14373–14377. 10.1002/anie.202006422. [DOI] [PubMed] [Google Scholar]
- Chen S.; Luo T.; Chen K.; Lin Y.; Fu J.; Liu K.; Cai C.; Wang Q.; Li H.; Li X.; Hu J.; Li H.; Zhu M.; Liu M. Chemical Identification of Catalytically Active Sites on Oxygen-doped Carbon Nanosheet to Decipher the High Activity for Electro-synthesis Hydrogen Peroxide. Angew. Chem., Int. Ed. 2021, 60, 16607–16614. 10.1002/anie.202104480. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Xu W.; Gong S.; Zheng G.; Tian Z.; Wen Y.; Peng L.; Zhang L.; Lu Z.; Chen L. Atomically dispersed Lewis acid sites boost 2-electron oxygen reduction activity of carbon-based catalysts. Nat. Commun. 2020, 11, 5478. 10.1038/s41467-020-19309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Liang J.; Wang Y.; Dong K.; Shi X.; Liu Q.; Luo Y.; Li T.; Jia Y.; Asiri A. M.; Feng Z.; Wang Y.; Ma D.; Sun X. Enhanced Electrochemical H2O2 Production via Two-Electron Oxygen Reduction Enabled by Surface-Derived Amorphous Oxygen-Deficient TiO2–x. ACS Appl. Mater. Interfaces 2021, 13, 33182–33187. 10.1021/acsami.1c09871. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Zhu Y.; Tang C.; Chen Y.; Qian B.; Hu Z.; Chang Y.-C.; Pao C.-W.; Lin Q.; Kazemi S. A.; Wang Y.; Zhang L.; Zhang X.; Wang H. High-Efficiency Electrosynthesis of Hydrogen Peroxide from Oxygen Reduction Enabled by a Tungsten Single Atom Catalyst with Unique Terdentate N1O2 Coordination. Adv. Funct. Mater. 2021, 32, 2110224. 10.1002/adfm.202110224. [DOI] [Google Scholar]
- Xia Y.; Zhao X.; Xia C.; Wu Z.-Y.; Zhu P.; Kim J. Y.; Bai X.; Gao G.; Hu Y.; Zhong J.; Liu Y.; Wang H. Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates. Nat. Commun. 2021, 12, 4225. 10.1038/s41467-021-24329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Xia Y.; Xia C.; Wang H. Insights into Practical-Scale Electrochemical H2O2 Synthesis. Trends Chem. 2020, 2, 942–953. 10.1016/j.trechm.2020.07.007. [DOI] [Google Scholar]
- Chang Q.; Zhang P.; Mostaghimi A. H. B.; Zhao X.; Denny S. R.; Lee J. H.; Gao H.; Zhang Y.; Xin H. L.; Siahrostami S.; Chen J. G.; Chen Z. Promoting H2O2 production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon. Nat. Commun. 2020, 11, 2178. 10.1038/s41467-020-15843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Tan X.; Bedford N. M.; Han Z.; Thomsen L.; Smith S.; Amal R.; Lu X. Direct insights into the role of epoxy groups on cobalt sites for acidic H2O2 production. Nat. Commun. 2020, 11, 4181. 10.1038/s41467-020-17782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siahrostami S.; Verdaguer-Casadevall A.; Karamad M.; Deiana D.; Malacrida P.; Wickman B.; Escudero-Escribano M.; Paoli E. A.; Frydendal R.; Hansen T. W.; Chorkendorff I.; Stephens I. E. L.; Rossmeisl J. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. 10.1038/nmat3795. [DOI] [PubMed] [Google Scholar]
- Verdaguer-Casadevall A.; Deiana D.; Karamad M.; Siahrostami S.; Malacrida P.; Hansen T. W.; Rossmeisl J.; Chorkendorff I.; Stephens I. E. L. Trends in the Electrochemical Synthesis of H2O2: Enhancing Activity and Selectivity by Electrocatalytic Site Engineering. Nano Lett. 2014, 14, 1603–1608. 10.1021/nl500037x. [DOI] [PubMed] [Google Scholar]
- Fortunato G. V.; Pizzutilo E.; Mingers A. M.; Kasian O.; Cherevko S.; Cardoso E. S. F.; Mayrhofer K. J. J.; Maia G.; Ledendecker M. Impact of Palladium Loading and Interparticle Distance on the Selectivity for the Oxygen Reduction Reaction toward Hydrogen Peroxide. J. Phys. Chem. C 2018, 122, 15878–15885. 10.1021/acs.jpcc.8b04262. [DOI] [Google Scholar]
- Jirkovský J. S.; Halasa M.; Schiffrin D. J. Kinetics of electrocatalytic reduction of oxygen and hydrogen peroxide on dispersed gold nanoparticles. Phys. Chem. Chem. Phys. 2010, 12, 8042–8053. 10.1039/c002416c. [DOI] [PubMed] [Google Scholar]
- Sheng H.; Janes A. N.; Ross R. D.; Kaiman D.; Huang J.; Song B.; Schmidt J. R.; Jin S. Stable and selective electrosynthesis of hydrogen peroxide and the electro-Fenton process on CoSe2 polymorph catalysts. Energy Environ. Sci. 2020, 13, 4189–4203. 10.1039/d0ee01925a. [DOI] [Google Scholar]
- Sheng H.; Hermes E. D.; Yang X.; Ying D.; Janes A. N.; Li W.; Schmidt J. R.; Jin S. Electrocatalytic Production of H2O2 by Selective Oxygen Reduction Using Earth-Abundant Cobalt Pyrite (CoS2). ACS Catal. 2019, 9, 8433–8442. 10.1021/acscatal.9b02546. [DOI] [Google Scholar]
- Zhang X.-L.; Su X.; Zheng Y.-R.; Hu S.-J.; Shi L.; Gao F.-Y.; Yang P.-P.; Niu Z.-Z.; Wu Z.-Z.; Qin S.; Wu R.; Duan Y.; Gu C.; Zheng X.-S.; Zhu J.-F.; Gao M.-R. Strongly Coupled Cobalt Diselenide Monolayers for Selective Electrocatalytic Oxygen Reduction to H2O2 under Acidic Conditions. Angew. Chem., Int. Ed. 2021, 60, 26922–26931. 10.1002/anie.202111075. [DOI] [PubMed] [Google Scholar]
- Liang J.; Wang Y.; Liu Q.; Luo Y.; Li T.; Zhao H.; Lu S.; Zhang F.; Asiri A. M.; Liu F.; Ma D.; Sun X. Electrocatalytic hydrogen peroxide production in acidic media enabled by NiS2 nanosheets. J. Mater. Chem. A 2021, 9, 6117–6122. 10.1039/d0ta12008a. [DOI] [Google Scholar]
- Ross R. D.; Sheng H.; Parihar A.; Huang J.; Jin S. Compositionally Tuned Trimetallic Thiospinel Catalysts for Enhanced Electrosynthesis of Hydrogen Peroxide and Built-In Hydroxyl Radical Generation. ACS Catal. 2021, 11, 12643–12650. 10.1021/acscatal.1c03349. [DOI] [Google Scholar]
- Tang C.; Chen L.; Li H.; Li L.; Jiao Y.; Zheng Y.; Xu H.; Davey K.; Qiao S.-Z. Tailoring Acidic Oxygen Reduction Selectivity on Single-Atom Catalysts via Modification of First and Second Coordination Spheres. J. Am. Chem. Soc. 2021, 143, 7819–7827. 10.1021/jacs.1c03135. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Wang Y.; Lai W.-H.; Xiao F.; Lyu Y.; Liao C.; Shao M. Approaching a high-rate and sustainable production of hydrogen peroxide: oxygen reduction on Co-N-C single-atom electrocatalysts in simulated seawater. Energy Environ. Sci. 2021, 14, 5444–5456. 10.1039/d1ee00878a. [DOI] [Google Scholar]
- Shen R.; Chen W.; Peng Q.; Lu S.; Zheng L.; Cao X.; Wang Y.; Zhu W.; Zhang J.; Zhuang Z.; Chen C.; Wang D.; Li Y. High-Concentration Single Atomic Pt Sites on Hollow CuSx for Selective O2 Reduction to H2O2 in Acid Solution. Chem 2019, 5, 2099–2110. 10.1016/j.chempr.2019.04.024. [DOI] [Google Scholar]
- Gao J.; Liu B. Progress of Electrochemical Hydrogen Peroxide Synthesis over Single Atom Catalysts. ACS Mater. Lett. 2020, 2, 1008–1024. 10.1021/acsmaterialslett.0c00189. [DOI] [Google Scholar]
- Zhu Y.; Li J.; Chen Y.; Zou J.; Cheng Q.; Chen C.; Hu W.; Zou L.; Zou Z.; Yang B.; Yang H. Switching the Oxygen Reduction Reaction Pathway via Tailoring the Electronic Structure of FeN4/C Catalysts. ACS Catal. 2021, 11, 13020–13027. 10.1021/acscatal.1c03728. [DOI] [Google Scholar]
- Park J.; Nabae Y.; Hayakawa T.; Kakimoto M.-a. Highly Selective Two-Electron Oxygen Reduction Catalyzed by Mesoporous Nitrogen-Doped Carbon. ACS Catal. 2014, 4, 3749–3754. 10.1021/cs5008206. [DOI] [Google Scholar]
- Pang Y.; Wang K.; Xie H.; Sun Y.; Titirici M.-M.; Chai G.-L. Mesoporous Carbon Hollow Spheres as Efficient Electrocatalysts for Oxygen Reduction to Hydrogen Peroxide in Neutral Electrolytes. ACS Catal. 2020, 10, 7434–7442. 10.1021/acscatal.0c00584. [DOI] [Google Scholar]
- Iglesias D.; Giuliani A.; Melchionna M.; Marchesan S.; Criado A.; Nasi L.; Bevilacqua M.; Tavagnacco C.; Vizza F.; Prato M.; Fornasiero P. N-Doped Graphitized Carbon Nanohorns as a Forefront Electrocatalyst in Highly Selective O2 Reduction to H2O2. Chem 2018, 4, 106–123. 10.1016/j.chempr.2017.10.013. [DOI] [Google Scholar]
- Gao J.; Yang H. b.; Huang X.; Hung S.-F.; Cai W.; Jia C.; Miao S.; Chen H. M.; Yang X.; Huang Y.; Zhang T.; Liu B. Enabling Direct H2O2 Production in Acidic Media through Rational Design of Transition Metal Single Atom Catalyst. Chem 2020, 6, 658–674. 10.1016/j.chempr.2019.12.008. [DOI] [Google Scholar]
- Sun Y.; Silvioli L.; Sahraie N. R.; Ju W.; Li J.; Zitolo A.; Li S.; Bagger A.; Arnarson L.; Wang X.; Moeller T.; Bernsmeier D.; Rossmeisl J.; Jaouen F.; Strasser P. Activity-Selectivity Trends in the Electrochemical Production of Hydrogen Peroxide over Single-Site Metal-Nitrogen-Carbon Catalysts. J. Am. Chem. Soc. 2019, 141, 12372–12381. 10.1021/jacs.9b05576. [DOI] [PubMed] [Google Scholar]
- Wu F.; Pan C.; He C.-T.; Han Y.; Ma W.; Wei H.; Ji W.; Chen W.; Mao J.; Yu P.; Wang D.; Mao L.; Li Y. Single-Atom Co–N4 Electrocatalyst Enabling Four-Electron Oxygen Reduction with Enhanced Hydrogen Peroxide Tolerance for Selective Sensing. J. Am. Chem. Soc. 2020, 142, 16861–16867. 10.1021/jacs.0c07790. [DOI] [PubMed] [Google Scholar]
- Han Y.; Wang Y.-G.; Chen W.; Xu R.; Zheng L.; Zhang J.; Luo J.; Shen R.-A.; Zhu Y.; Cheong W.-C.; Chen C.; Peng Q.; Wang D.; Li Y. Hollow N-Doped Carbon Spheres with Isolated Cobalt Single Atomic Sites: Superior Electrocatalysts for Oxygen Reduction. J. Am. Chem. Soc. 2017, 139, 17269–17272. 10.1021/jacs.7b10194. [DOI] [PubMed] [Google Scholar]
- He Y.; Shi Q.; Shan W.; Li X.; Kropf A. J.; Wegener E. C.; Wright J.; Karakalos S.; Su D.; Cullen D. A.; Wang G.; Myers D. J.; Wu G. Dynamically Unveiling Metal–Nitrogen Coordination during Thermal Activation to Design High-Efficient Atomically Dispersed CoN4 Active Sites. Angew. Chem., Int. Ed. 2021, 60, 9516–9526. 10.1002/anie.202017288. [DOI] [PubMed] [Google Scholar]
- Wang X. X.; Cullen D. A.; Pan Y.-T.; Hwang S.; Wang M.; Feng Z.; Wang J.; Engelhard M. H.; Zhang H.; He Y.; Shao Y.; Su D.; More K. L.; Spendelow J. S.; Wu G. Nitrogen-Coordinated Single Cobalt Atom Catalysts for Oxygen Reduction in Proton Exchange Membrane Fuel Cells. Adv. Mater. 2018, 30, 1706758. 10.1002/adma.201706758. [DOI] [PubMed] [Google Scholar]
- He Y.; Guo H.; Hwang S.; Yang X.; He Z.; Braaten J.; Karakalos S.; Shan W.; Wang M.; Zhou H.; Feng Z.; More K. L.; Wang G.; Su D.; Cullen D. A.; Fei L.; Litster S.; Wu G. Single Cobalt Sites Dispersed in Hierarchically Porous Nanofiber Networks for Durable and High-Power PGM-Free Cathodes in Fuel Cells. Adv. Mater. 2020, 32, 2003577. 10.1002/adma.202003577. [DOI] [PubMed] [Google Scholar]
- Wu D.; Hu J.; Zhu C.; Zhang J.; Jing H.; Hao C.; Shi Y. Salt melt synthesis of Chlorella-derived nitrogen-doped porous carbon with atomically dispersed CoN4 sites for efficient oxygen reduction reaction. J. Colloid Interface Sci. 2021, 586, 498–504. 10.1016/j.jcis.2020.10.115. [DOI] [PubMed] [Google Scholar]
- Kulkarni A.; Siahrostami S.; Patel A.; Nørskov J. K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. 10.1021/acs.chemrev.7b00488. [DOI] [PubMed] [Google Scholar]
- Duan Z.; Henkelman G. Surface Charge and Electrostatic Spin Crossover Effects in CoN4 Electrocatalysts. ACS Catal. 2020, 10, 12148–12155. 10.1021/acscatal.0c02458. [DOI] [Google Scholar]
- Chen Z.; Niu H.; Ding J.; Liu H.; Chen P.-H.; Lu Y.-H.; Lu Y.-R.; Zuo W.; Han L.; Guo Y.; Hung S.-F.; Zhai Y. Unraveling the Origin of Sulfur-Doped Fe-N-C Single-Atom Catalyst for Enhanced Oxygen Reduction Activity: Effect of Iron Spin-State Tuning. Angew. Chem., Int. Ed. 2021, 60, 25404–25410. 10.1002/anie.202110243. [DOI] [PubMed] [Google Scholar]
- Menga D.; Low J. L.; Li Y.-S.; Arčon I.; Koyutürk B.; Wagner F.; Ruiz-Zepeda F.; Gaberšček M.; Paulus B.; Fellinger T.-P. Resolving the Dilemma of Fe-N-C Catalysts by the Selective Synthesis of Tetrapyrrolic Active Sites via an Imprinting Strategy. J. Am. Chem. Soc. 2021, 143, 18010–18019. 10.1021/jacs.1c04884. [DOI] [PubMed] [Google Scholar]
- Wang H.; Shao Y.; Mei S.; Lu Y.; Zhang M.; Sun J.-k.; Matyjaszewski K.; Antonietti M.; Yuan J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. 10.1021/acs.chemrev.0c00080. [DOI] [PubMed] [Google Scholar]
- Chen S.; Bi F.; Xiang K.; Zhang Y.; Hao P.; Li M.; Zhao B.; Guo X. Reactive Template-Derived CoFe/N-Doped Carbon Nanosheets as Highly Efficient Electrocatalysts toward Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. ACS Sustainable Chem. Eng. 2019, 7, 15278–15288. 10.1021/acssuschemeng.9b02426. [DOI] [Google Scholar]
- Chen S.; Yan Y.; Hao P.; Li M.; Liang J.; Guo J.; Zhang Y.; Chen S.; Ding W.; Guo X. Iron Nanoparticles Encapsulated in S,N-Codoped Carbon: Sulfur Doping Enriches Surface Electron Density and Enhances Electrocatalytic Activity toward Oxygen Reduction. ACS Appl. Mater. Interfaces 2020, 12, 12686–12695. 10.1021/acsami.9b20007. [DOI] [PubMed] [Google Scholar]
- Zhang N.; Zhou T.; Chen M.; Feng H.; Yuan R.; Zhong C. a.; Yan W.; Tian Y.; Wu X.; Chu W.; Wu C.; Xie Y. High-purity pyrrole-type FeN4 sites as a superior oxygen reduction electrocatalyst. Energy Environ. Sci. 2020, 13, 111–118. 10.1039/c9ee03027a. [DOI] [Google Scholar]
- Zhang Z.; Dou M.; Ji J.; Wang F. Phthalocyanine tethered iron phthalocyanine on graphitized carbon black as superior electrocatalyst for oxygen reduction reaction. Nano Energy 2017, 34, 338–343. 10.1016/j.nanoen.2017.02.042. [DOI] [Google Scholar]
- Lin Y.; Liu K.; Chen K.; Xu Y.; Li H.; Hu J.; Lu Y.-R.; Chan T.-S.; Qiu X.; Fu J.; Liu M. Tuning Charge Distribution of FeN4 via External N for Enhanced Oxygen Reduction Reaction. ACS Catal. 2021, 11, 6304–6315. 10.1021/acscatal.0c04966. [DOI] [Google Scholar]
- Chen K.; Liu K.; An P.; Li H.; Lin Y.; Hu J.; Jia C.; Fu J.; Li H.; Liu H.; Lin Z.; Li W.; Li J.; Lu Y.-R.; Chan T.-S.; Zhang N.; Liu M. Iron phthalocyanine with coordination induced electronic localization to boost oxygen reduction reaction. Nat. Commun. 2020, 11, 4173. 10.1038/s41467-020-18062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S.; McPherson I. J.; Vincent K. A. Adsorbed Intermediates in Oxygen Reduction on Platinum Nanoparticles Observed by In Situ IR Spectroscopy. Angew. Chem., Int. Ed. 2018, 57, 12855–12858. 10.1002/anie.201804978. [DOI] [PubMed] [Google Scholar]
- Wang T.; Zhang Y.; Huang B.; Cai B.; Rao R. R.; Giordano L.; Sun S.-G.; Shao-Horn Y. Enhancing oxygen reduction electrocatalysis by tuning interfacial hydrogen bonds. Nat. Catal. 2021, 4, 753–762. 10.1038/s41929-021-00668-0. [DOI] [Google Scholar]
- Ohta N.; Nomura K.; Yagi I. Adsorption and Electroreduction of Oxygen on Gold in Acidic Media: In Situ Spectroscopic Identification of Adsorbed Molecular Oxygen and Hydrogen Superoxide. J. Phys. Chem. C 2012, 116, 14390–14400. 10.1021/jp302857q. [DOI] [Google Scholar]
- Zhou P.; Li L.; Mosali V. S. S.; Chen Y.; Luan P.; Gu Q.; Turner D. R.; Huang L.; Zhang J. Electrochemical Hydrogenation of Furfural in Aqueous Acetic Acid Media with Enhanced 2-Methylfuran Selectivity Using CuPd Bimetallic Catalysts. Angew. Chem., Int. Ed. 2022, 61, e202117809 10.1002/anie.202117809. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhao X.; Niu C.; Tang N.; Guo H.; Wen X.; Liang C.; Zeng G. Enhanced activation of peroxymonosulfate by magnetic Co3MnFeO6 nanoparticles for removal of carbamazepine: Efficiency, synergetic mechanism and stability. Chem. Eng. J. 2019, 362, 851–864. 10.1016/j.cej.2019.01.078. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.