Abstract
Background:
Major depression and inadequate self-care are common in patients with heart failure (HF). Little is known about how to intervene when both problems are present. This study examined the efficacy of a sequential approach to treating these problems.
Methods:
Stepped Care for Depression in Heart Failure was a single-site, single-blind, randomized controlled trial of cognitive behavior therapy (CBT) versus usual care (UC) for major depression in patients with HF. The intensive phase of the CBT intervention lasted between 8 and 16 weeks, depending upon the rate of improvement in depression. All participants received a tailored HF self-care intervention that began 8 weeks after randomization. The intensive phase of the self-care intervention ended at 16 weeks post-randomization. The coprimary outcome measures were the Beck Depression Inventory (BDI-II) and the Maintenance scale of the Self-Care of Heart Failure Index (SCHFI v6.2) at Week 16.
Results:
139 patients with HF and major depression were enrolled; 70 were randomized to UC and 69 to CBT. At Week 16, the patients in the CBT arm scored 4.0 points (95% C.I., −7.3 to −0.8; p=.02) lower on the BDI-II than those in the UC arm. Mean scores on the SCHFI Maintenance scale were not significantly different between the groups (95% C.I., −6.5 to 1.5; p=.22).
Conclusions:
CBT is more effective than usual care for major depression in patients with HF. However, initiating CBT before starting a tailored HF self-care intervention does not increase the benefit of the self-care intervention.
Keywords: Cognitive Therapy, Depressive Disorder, Heart Failure, Self-Care, Self-Management
The prevalence of major depression in patients hospitalized with heart failure (HF) increased from 6.2% in 2008 to 9.1% in 2017 according to a recent National Inpatient Sample study1, and it was even higher (23.5%) in a recent study in which standardized interviews were administered to diagnose major depression.2 Depression is a robust predictor of poor quality of life3, rehospitalization4, and mortality5–7 in HF, and it is associated with inadequate HF self-care.8
Both major depression and inadequate self-care are difficult to treat in patients with HF, and little is known about how to intervene when both problems are present. Self-care interventions for patients with HF rarely address depression or other psychiatric comorbidities9, and conversely, interventions for depression in patients with HF rarely address self-care deficits.10
In a previous randomized clinical trial (RCT), we tested an integrated cognitive-behavioral intervention that simultaneously targeted major depression and inadequate self-care in 158 patients in New York Heart Association (NYHA) Class I-III HF. Compared to usual care (UC), the intervention was efficacious for depression but not for HF self-care.11 We thus decided to test a sequential approach in which therapy for depression is initiated before HF self-care is addressed. We hypothesized that both depression and HF self-care outcomes could be improved by initiating therapy for depression before initiating an intervention for inadequate HF self-care. The rationale for this hypothesis was depression-related symptoms such as poor concentration, fatigue, and hopelessness interfere with self-care and that consequently, improvements in these symptoms should facilitate self-care education and engagement in appropriate HF self-care behaviors.
METHODS
Transparency and Openness Promotion
This study was preregistered on ClinicalTrials.gov (NCT02997865). The data, analytic methods, and other materials that support the findings of the study are available from the corresponding author upon reasonable request.
Study Population
This two-arm, randomized, controlled, parallel groups clinical trial was approved by the Institutional Review Board of Washington University School of Medicine in St. Louis and preregistered on clinicaltrials.gov (NCT02997865). Patients with a clinical diagnosis of NYHA Class I-III heart failure who received medical care at Washington University Medical Center were screened for study eligibility between February 2017 and January 2021. Patients who were younger than 25 years of age or who were too ill or cognitively impaired to participate were excluded. Eligible patients who provided written informed consent were enrolled in the trial.
Interventions
All participants in both arms of the trial continued to receive usual care for HF and other medical conditions while participating in the study. They were also allowed to obtain antidepressant medications from their own physician, but they were asked to refrain from engaging in any nonstudy psychotherapeutic interventions for depression during their participation in the trial.
Patients who were randomly assigned to the cognitive behavior therapy (CBT) arm were seen for an initial clinical evaluation and for intervention sessions by a licensed clinical social worker or licensed clinical psychologist with CBT training and experience. The interventionists adhered to a standard CBT protocol.12 The sessions were held weekly for the first 8 weeks. If the patient’s depression was in remission by Week 8, session frequency was reduced during Weeks 8–16. Regardless of remission status, the frequency was reduced in most cases to 1–2 per month between Weeks 16 and 32. The sessions were held in person when possible and via telephone when necessary for logistical, medical, or other reasons.
Patients completed the Patient Health Questionnaire (PHQ-9)13 and the Generalized Anxiety Disorder (GAD-7)14 at each CBT session to track progress toward remission. Weekly progress targets were defined in terms of percentage improvement on the PHQ-9. Adaptive cognitive-behavioral strategies were implemented if the targets were not met, and the patients were also asked to contact their physician to discuss whether an antidepressant medication was indicated. Thus, patients who did not show a rapid improvement in depression had more frequent sessions and more intensive intervention than patients who did improve rapidly. Statistics on the delivery of CBT are provided in Supplementary Table S1.
Participants in both the CBT and UC arms received a Tailored Self-Care (TSC) intervention starting approximately 8 weeks after randomization. The intervention was based on HF self-care guidelines15 and included components that had been developed for previous trials of HF self-care interventions.16,17 It was provided by an experienced cardiac research nurse and focused on HF self-care education, motivational interviewing, overcoming barriers to self-care, setting individualized self-care goals, and tracking self-care behaviors. It included an initial self-care evaluation session 8 weeks after randomization, weekly intervention sessions through Week 15, and less frequent maintenance sessions through Week 32. Data on the delivery of TSC are provided in Supplementary Table S2. All participants in both the CBT and UC arms also continued to receive their usual medical care for HF and comorbidities throughout their participation in the trial.
Randomization and Blinding
Participants were randomly assigned to the CBT or UC arm in a 1:1 allocation ratio within permuted blocks of 2, 4, or 6 pairs immediately after completion of the baseline assessments. Participants in the intervention arm received CBT from a study therapist in addition to their usual nonstudy medical care; those in the UC arm received their usual nonstudy medical care but not CBT or any other psychotherapy for depression. Randomization was also stratified by the presence or absence of nonstudy antidepressant use at baseline. The allocations were generated by the study statistician and stored and concealed on the Research Electronic Data Capture (REDCap) platform until disclosed after baseline to the study coordinator and the participant. The outcome assessors were blinded to the assignments. An analysis of the adequacy of blinding is presented in the Supplementary materials.
Outcome Measures
Outcome measures were obtained at baseline and at 8, 16, and 32 weeks after randomization. When possible, the assessments were conducted in-person at Washington University Medical Center. If an in-person visit was not feasible, the data were collected by mail or telephone. The primary outcome measure was the Beck Depression Inventory (BDI-II)18–20 at 16 weeks, and the co-primary outcome measure was the Maintenance scale of the Self-Care of Heart Failure Index (SCHFI v6.2)21 at 16 weeks. The BDI-II is a widely-used, 21-item self-report measure of depression with total scores ranging from 0 (not at all depressed) to 63 (severely depressed) and a screening cutoff score of ≥14. We used a conservative threshold (<10) to define remission on the BDI-II to account for nonspecific symptoms such as fatigue that might be attributable at least partially to medical illness. The BDI-II is often used as an outcome measure in trials of CBT for depression.22,23
The SCHFI is also a widely-used self-report questionnaire. It assesses routine HF self-care maintenance behaviors such as following a low salt diet and performing weight checks; management of worsening dyspnea, peripheral edema, or other symptoms, such as by taking a diuretic; and the patient’s confidence in his or her HF self-care skills. Adequate self-care on each scale is defined by a cutoff score of ≥70.24 An a priori interpretation rule stipulated that the sequential intervention strategy would be considered efficacious only if the treatment effects were statistically and clinically significant for both of the co-primary outcomes.
Secondary outcome measures included the SCHFI Management and Confidence scales, the Beck Anxiety Inventory (BAI)25, and the Kansas City Cardiomyopathy Questionnaire (KCCQ).26 A difference of ≥5 points on the KCCQ is considered to be clinically significant.27 The Hamilton Rating Scale for Depression (HAMD-17)28 was obtained at baseline and 16 weeks.
Statistical Analysis
Chi-square and one-way analysis of variance tests were used to compare the groups at baseline. Consistent with the intention-to-treat principle, data that were plausibly missing at random were imputed by a model that included the variables that would be used in the planned analyses, as well as auxiliary variables that correlated at least moderately (r≥0.30) with missing outcome data. Missing data that were attributable to the death of the participant were not imputed. Twenty-five imputed datasets were generated, and parameter estimates from each statistical model were combined across the datasets to strengthen valid statistical inference.
The imputed data were fitted to a series of linear mixed-effect models to test whether the treatment groups differed on the outcomes. Each outcome measure was regressed on fixed factors for treatment, time, treatment by time interaction, antidepressant use (stratification factor), and the baseline value of the outcome measure. Random factors for subject and intercept were included in the model, and a spatial covariance structure was used to account for the unequally-spaced measurements (baseline, 8, 16, and 32 weeks.) Standard diagnostics were used to identify violations of the assumptions or goodness-of-fit of each model. Remission rates at 16 weeks (BDI-II<10, HAM-D-17 ≤ 7) were compared between groups by chi-square tests. Tests of potential moderators of treatment effects, including age, race, severity of depression at baseline, and antidepressant use at baseline, were specified a priori. Statistical significance for all analyses was set at α = 0.05. The statistical significance criterion for the coprimary outcomes was Bonferroni corrected to hold the family-wise error rate to α = 0.05. SAS 9.4 software (SAS Institute, Inc.) was used for all analyses.
Power Analysis
A between-group difference (treatment effect, |ΔT|) of ≥5 points on the BDI-II at 16 weeks was defined a priori as the minimum clinically important difference in depression, a value that was slightly larger than the 4.5-point difference observed in our earlier trial.11 The rationale was that a relatively large between-group difference in depression was needed for a strong test of the hypothesis that treatment of depression can improve HF self-care outcomes. The power analysis assumed a between-group difference of ≥5 points on the BDI-II, a pooled standard deviation of 10.4, a Type I error rate of .05, 90% power, and ≤18% attrition, and it produced a target sample size of 180 patients. For the actual sample of 139 patients and under the same assumptions, 80.1% power was available for the trial to detect a difference in treatment means (|ΔT| = CBT - UC) greater than 5 points on the BDI-II. The clinical significance of the effect of treatment on HF self-care was evaluated in terms of the proportions of patients within each group who scored ≥70 on the SCHFI Maintenance scale at 16 weeks.
RESULTS
Participants
A total of 139 patients (77% of the target sample size) met the eligibility criteria and were enrolled in the trial. The under-recruitment primarily occurred during the first year of the COVID-19 pandemic, at a time when fewer HF patients were available for screening and the research team was required to transition to remote procedures. Figure 1 displays a CONSORT diagram of the sample, and Table 1 displays their demographic, medical, and psychosocial characteristics. Fourteen percent of the participants in the CBT arm discontinued treatment by Week 16, and 19% discontinued by Week 32, figures that are within the typical range for trials of CBT for depression.29–31 Nineteen (14%) of the participants discontinued study participation prior to the end of the study. Reasons for premature study termination are shown in Supplementary Table S3.
Figure 1. Participants evaluated, excluded, randomized, and analyzed in the Stepped Care for Depression in Heart Failure trial.
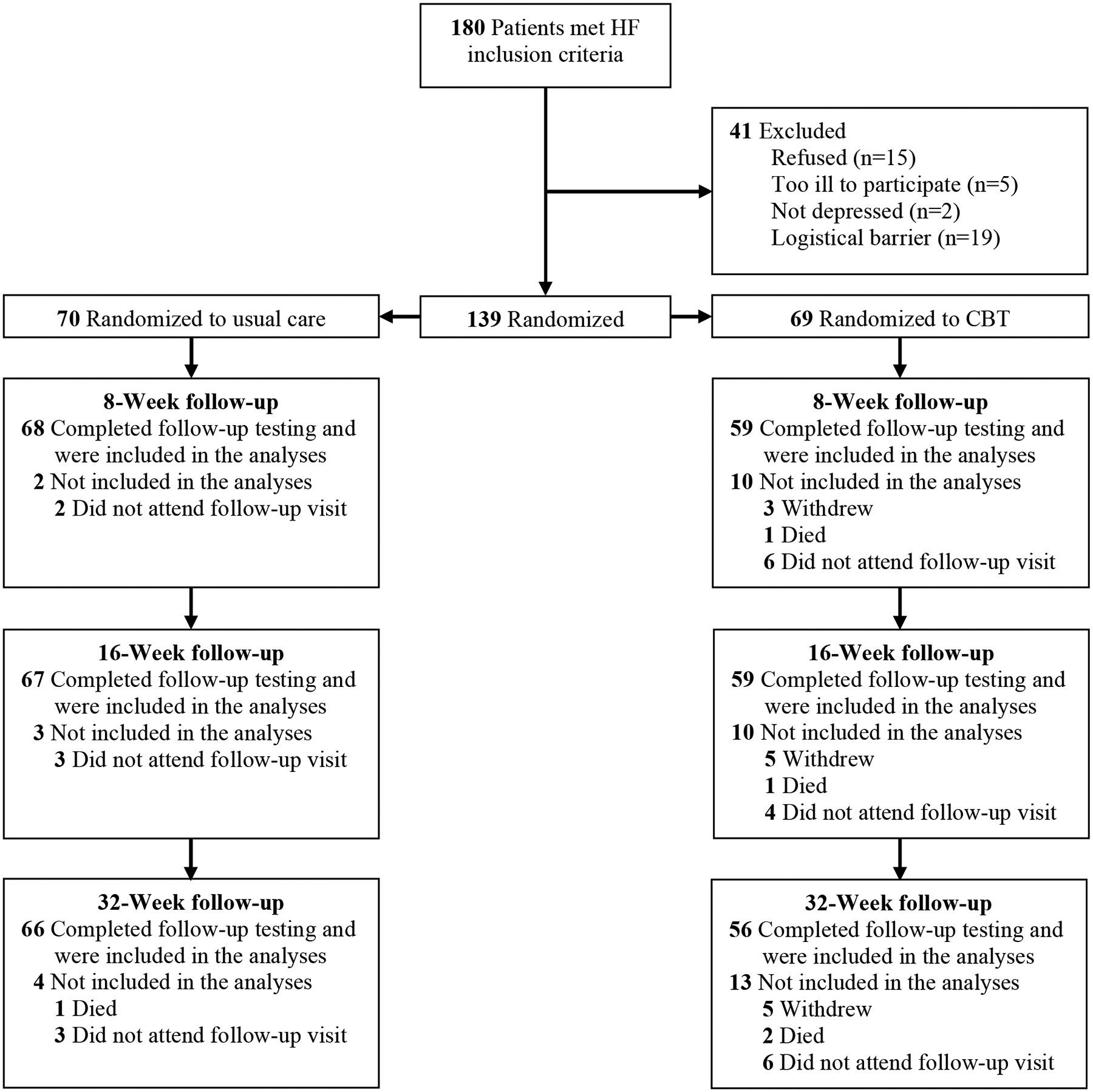
CBT, cognitive behavior therapy.
Table 1.
Baseline characteristics.
| Characteristic | Total Sample (n = 139) |
UC (n = 70) |
CBT (n = 69) |
P |
|---|---|---|---|---|
| Demographics | ||||
| Age (y) | 58.2 ± 11.8 | 58.3 ± 12.2 | 58.0 ± 11.5 | .89 |
| Gender (female) | 68 (48.9) | 35 (50.0) | 33 (47.8) | .80 |
| Race (white) | 66 (47.5) | 34 (48.6) | 32 (46.4) | .80 |
| Education (≤ 12 years) | 31 (22.3) | 14 (20.0) | 17 (24.6) | .51 |
| Income (<$30,000/year) | 65 (46.8) | 30 (42.9) | 35 (50.7) | .31 |
| Married or partnered | 53 (38.1) | 27 (38.6) | 26 (37.7) | .91 |
| Medical status | ||||
| Never smoked | 58 (41.7) | 27 (38.6) | 31 (44.9) | .45 |
| Hypertension | 117 (84.2) | 61 (87.1) | 56 (81.2) | .33 |
| Diabetes | 63 (45.3) | 35 (50.0) | 28 (40.6) | .26 |
| Chronic obstructive pulmonary disease | 35 (25.2) | 21 (30.0) | 14 (20.3) | .20 |
| Ischemic heart disease | 72 (51.8) | 29 (41.4) | 43 (62.3) | .01 |
| Heart failure diagnosis, past year | 21 (15.1) | 11 (15.7) | 10 (14.5) | .84 |
| History of cardiomyopathy | 115 (82.7) | 57 (81.4) | 58 (84.1) | .69 |
| History of atrial fibrillation | 51 (36.7) | 28 (40.0) | 23 (33.3) | .41 |
| Sleep apnea | 76 (54.7) | 39 (55.7) | 37 (53.6) | .84 |
| History of renal disease | 33 (23.7) | 12 (17.1) | 21 (30.4) | .07 |
| History of coronary disease | 67 (48.2) | 28 (40.0) | 39 (56.5) | .06 |
| History of peripheral arterial disease | 29 (20.9) | 13 (18.6) | 16 (23.2) | .50 |
| History of myocardial infarction | 49 (35.3) | 21 (30.0) | 28 (40.6) | .17 |
| History of coronary revascularization | 45 (32.4) | 17 (24.3) | 28 (40.6) | .04 |
| 48 (34.5) | 24 (34.3) | 24 (34.8) | .95 | |
| <45% | 77 (55.4) | 41 (58.6) | 36 (52.2) | .45 |
| I-II | 91 (65.5) | 47 (67.1) | 44 (63.8) | .68 |
| Medications | ||||
| Aspirin | 84 (60.4) | 40 (57.1) | 44 (63.8) | .42 |
| Beta blocker | 125 (89.9) | 61 (87.1) | 64 (92.8) | .27 |
| Statin | 95 (68.3) | 44 (62.9) | 51 (73.9) | .16 |
| ACE Inhibitor or ARB | 108 (77.7) | 52 (74.3) | 56 (81.2) | .33 |
| Aldosterone receptor antagonist | 68 (48.9) | 39 (55.7) | 29 (42.0) | .11 |
| Diuretic | 108 (77.7) | 57 (81.4) | 51 (73.9) | .29 |
| Anti-arrhythmic | 11 (7.9) | 6 (8.6) | 5 (7.3) | .77 |
| Antidiabetic | 56 (40.3) | 29 (41.4) | 27 (39.1) | .78 |
| Characteristic | Total Sample (n = 139) |
UC (n = 70) |
CBT (n = 69) |
P |
|---|---|---|---|---|
| Laboratory values | ||||
| BNP* | 690 (1403) | 620 (1339) | 760 (1415_ | .40 |
| NT-proBNP* | 3794 (9388) | 3317 (8975) | 4219 (9768) | .39 |
| Blood urea nitrogen | 19.7 ± 11.1 | 18.8 ± 10.5 | 20.6 ± 11.7 | .34 |
| Creatinine | 1.2 ± 0.5 | 1.15 ± 0.64 | 1.23 ± 0.39 | .41 |
| <60 (abnormal) | 43 (30.9) | 16 (22.9) | 27 (39.1) | .04 |
| Hemoglobin | 12.7 ± 1.9 | 12.7 ± 1.9 | 12.7 ± 2.0 | .90 |
| Depression | ||||
| Antidepressant (stratification) | 62 (44.6) | 31 (44.3) | 31 (44.9) | .94 |
| History of depression | 103 (74.1) | 54 (77.1) | 49 (71.0) | .41 |
| History of depression treatment | 64 (46.0) | 35 (50.0) | 29 (42.0) | .35 |
| Beck Depression Inventory-II | 32.3 ± 8.8 | 32.1 ± 9.2 | 32.6 ± 8.3 | .70 |
| Patient Health Questionnaire-9 | 16.5 ± 5.0 | 16.2 ± 5.3 | 16.9 ± 4.7 | .47 |
| Hamilton Depression Scale-17 | 22.6 ± 5.5 | 22.6 ± 5.4 | 22.5 ± 5.6 | .93 |
| HF Self-Care | ||||
| SCHFI Maintenance score | 58.1 ± 16.1 | 55.5 ± 16.2 | 60.7 ± 15.7 | .055 |
| SCHFI Management score | 52.0 ± 22.0 | 52.4 ± 20.6 | 55.7 ± 22.2 | .37 |
| SCHFI Confidence score | 54.8 ± 23.7 | 51.0 ± 23.3 | 58.8 ± 23.7 | .052 |
| Psychosocial | ||||
| Beck Anxiety Inventory | 22.7 ± 12.7 | 22.1 ± 13.2 | 23.2 ± 12.3 | .61 |
| Generalized Anxiety Inventory-7 | 13.0 ± 5.5 | 12.5 ± 5.6 | 13.6 ± 5.4 | .24 |
| Clinical summary score | 51.4 ± 21.7 | 52.2 ± 20.2 | 50.6 ± 23.2 | .66 |
Estimates are reported as mean ± SD for interval-scaled variables and number (%) for categorical variables.
BNP and NT-proBNP estimates are reported as median (IQR).
ACE, angiotensin-converting enzyme; AICD, automatic implantable cardioverter defibrillator; ARB, angiotensin II receptor blocker; BNP, Brain natriuretic peptide; CBT, cognitive behavior therapy; GFR, Glomerular Filtration Rate; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SCHFI, Self-Care of Heart Failure Index; UC, usual care.
Safety and Efficacy Outcomes
There were no study-related serious adverse events. As shown in Table 2 and Figure 2, mean BDI-II depression scores were statistically lower by 4.0 points in the CBT (mean, 15.6; 95% C.I., 13.3 to 17.4) than the UC arm (mean, 19.6; 95% C.I., 17.9 to 21.8) at 16 weeks ([ΔT = −4.0; 95% C.I., −7.3 to −0.8; p=.02). SCHFI Maintenance scores averaged 70.4 (95% C.I., 67.4 to 73.4) in the CBT arm and 73.0 (95% C.I., 70.9 to 76.4) in the UC arm at 16 weeks (ΔT = −2.5; 95% C.I., −6.5 to 1.5; p=.22). Group mean scores on the HAM-D-17 differed statistically between the CBT arm (mean, 12.5; 95% C.I., 11.1 to 13.8) and the UC arm (mean, 15.0; 95% C.I., 13.9 to 16.2) at 16 weeks ([ΔT = −2.6; 95% C.I., −4.3 to −0.7; p=.004). None of the other secondary outcome measures showed a benefit of CBT at 16 weeks. By 32 weeks, in contrast, BDI-II depression scores were statistically lower in the CBT arm (mean, 13.7; 95% C.I., 11.3 to 16.1) than the UC arm (mean, 19.3; 95% C.I., 17.0 to 21.5; ΔT = −5.6; 95% C.I., −8.9 to −2.3; p=.001). There were statistically significant treatment effects at 32 weeks on the BAI anxiety scale (CBT mean, 12.5; 95% C.I., 10.1 to 14.4; UC mean, 16.5; 95% C.I., 14.8 to 18.7; ΔT = −4.1; 95% C.I., −7.2 to −0.9; p=.01) and on the KCCQ overall quality of life scale (CBT mean, 66.9; 95% C.I., 62.5 to 71.3; UC mean, 57.0; 95% C.I., 53.2 to 60.9; ΔT = 9.8; 95% C.I., 4.1 to 15.5; p=.001).
Table 2.
Intention-to-Treat (ITT) estimates of the treatment effect for the trial’s co-primary and secondary outcomes.
| Outcome | Least-Squares Mean ± SD |
Treatment Effect (ΔT = CBT – UC) Estimate (95% CI) |
Cohen’s d | P | |
|---|---|---|---|---|---|
| UC (n = 70) |
CBT (n = 69) |
||||
| Beck Depression Inventory-II | |||||
| Baseline | 32.4 ± 9.2 | 32.6 ± 9.2 | 0.2 (−2.8, 3.3) | .02 | .88 |
| 8 Weeks | 24.4 ± 9.4 | 22.1 ± 10.2 | −2.4 (−5.7, 0.9) | .24 | .16 |
| 16 Weeks | 19.6 ± 9.4 | 15.6 ± 9.7 | −4.0 (−7.3, −0.8) | .42 | .015 |
| 32 Weeks | 19.3 ± 9.4 | 13.7 ± 10.0 | −5.6 (−8.9, −2.3) | .58 | .001 |
| SCHFI-Maintenance | |||||
| Baseline | 57.2 ± 11.1 | 58.9 ± 11.1 | 1.8 (−2.0, 5.5) | .16 | .35 |
| 8 Weeks | 61.9 ± 11.5 | 62.1 ± 12.0 | 0.3 (−3.6, 4.2) | .02 | .90 |
| 16 Weeks | 73.0 ± 11.6 | 70.4 ± 12.5 | −2.5 (−6.5, 1.5) | .21 | .22 |
| 32 Weeks | 73.6 ± 11.7 | 71.8 ± 12.4 | −1.8 (−5.9, 2.2) | .15 | .37 |
| SCHFI-Management | |||||
| Baseline | 52.7 ± 20.2 | 54.8 ± 19.3 | 2.1 (−4.3, 8.4) | .11 | .52 |
| 8 Weeks | 57.1 ± 21.9 | 55.9 ± 22.0 | −1.2 (−8.4, 5.9) | .06 | .74 |
| 16 Weeks | 63.2 ± 23.7 | 64.0 ± 22.8 | 0.8 (−6.6, 8.1) | .03 | .83 |
| 32 Weeks | 65.9 ± 23.2 | 64.3 ± 24.2 | −1.7 (−9.9, 6.6) | .07 | .69 |
| SCHFI-Confidence | |||||
| Baseline | 53.0 ± 18.1 | 56.6 ± 18.1 | 3.6 (−2.5, 9.7) | .20 | .24 |
| 8 Weeks | 52.7 ± 18.4 | 54.0 ± 19.9 | 1.3 (−5.2, 7.7) | .07 | .70 |
| 16 Weeks | 62.1 ± 18.9 | 66.6 ± 20.1 | 4.5 (−2.1, 11.0) | .23 | .18 |
| 32 Weeks | 65.9 ± 18.6 | 68.8 ± 19.3 | 2.8 (−3.4, 9.1) | .15 | .37 |
| Beck Anxiety Inventory | |||||
| Baseline | 22.6 ± 8.7 | 23.0 ± 8.7 | 0.4 (−2.5, 3.3) | .05 | .77 |
| 8 Weeks | 19.8 ± 8.8 | 19.1 ± 9.2 | −0.8 (−3.8, 2.2) | .09 | .61 |
| 16 Weeks | 15.1 ± 8.9 | 12.5 ± 9.3 | −2.6 (−5.6, 0.5) | .28 | .10 |
| 32 Weeks | 16.5 ± 9.1 | 12.5 ± 9.8 | −4.1 (−7.2, −0.9) | .43 | .01 |
| KCCQ Overall Summary | |||||
| Baseline | 46.0 ± 15.6 | 45.9 ± 15.6 | −0.1 (−5.4, 5.1) | .01 | .96 |
| 8 Weeks | 49.8 ± 16.1 | 54.6 ± 18.0 | 4.8 (−1.1, 10.6) | .28 | .11 |
| 16 Weeks | 61.2 ± 16.0 | 63.7 ± 16.9 | 2.5 (−3.0, 8.0) | .15 | .38 |
| 32 Weeks | 57.0 ± 16.1 | 66.9 ± 18.4 | 9.8 (4.1, 15.5) | .57 | .001 |
| KCCQ Clinical Summary | |||||
| Baseline | 51.4 ± 15.9 | 50.9 ± 15.9 | −0.4 (−5.8, 4.9) | .03 | .87 |
| 8 Weeks | 55.2 ± 16.4 | 57.9 ± 18.0 | 2.7 (−3.0, 8.5) | .16 | .35 |
| 16 Weeks | 66.0 ± 16.3 | 66.1 ± 17.9 | 0.1 (−5.6, 5.9) | .10 | .97 |
| 32 Weeks | 61.1 ± 16.6 | 69.3 ± 18.2 | 8.2 (2.4, 14.1) | .48 | .006 |
| HAMD-17 | |||||
| Baseline | 22.6 ± 4.8 | 22.6 ± 4.8 | −0.1 (−1.6, 1.6) | .01 | .98 |
| 16 Weeks | 15.0 ± 4.9 | 12.5 ± 5.7 | −2.6 (−4.3, −0.8) | .48 | .004 |
CBT, cognitive behavior therapy; HAMD-17, Hamilton Rating Scale for Depression; KCCQ, Kansas City Cardiomyopathy Questionnaire; SCHFI, Self-Care of Heart Failure Index; UC, usual care.
Figure 2. Scores on the Beck Depression Inventory (BDI-II) at randomization and the 8-, 16-, and 32-Week follow-up visits.
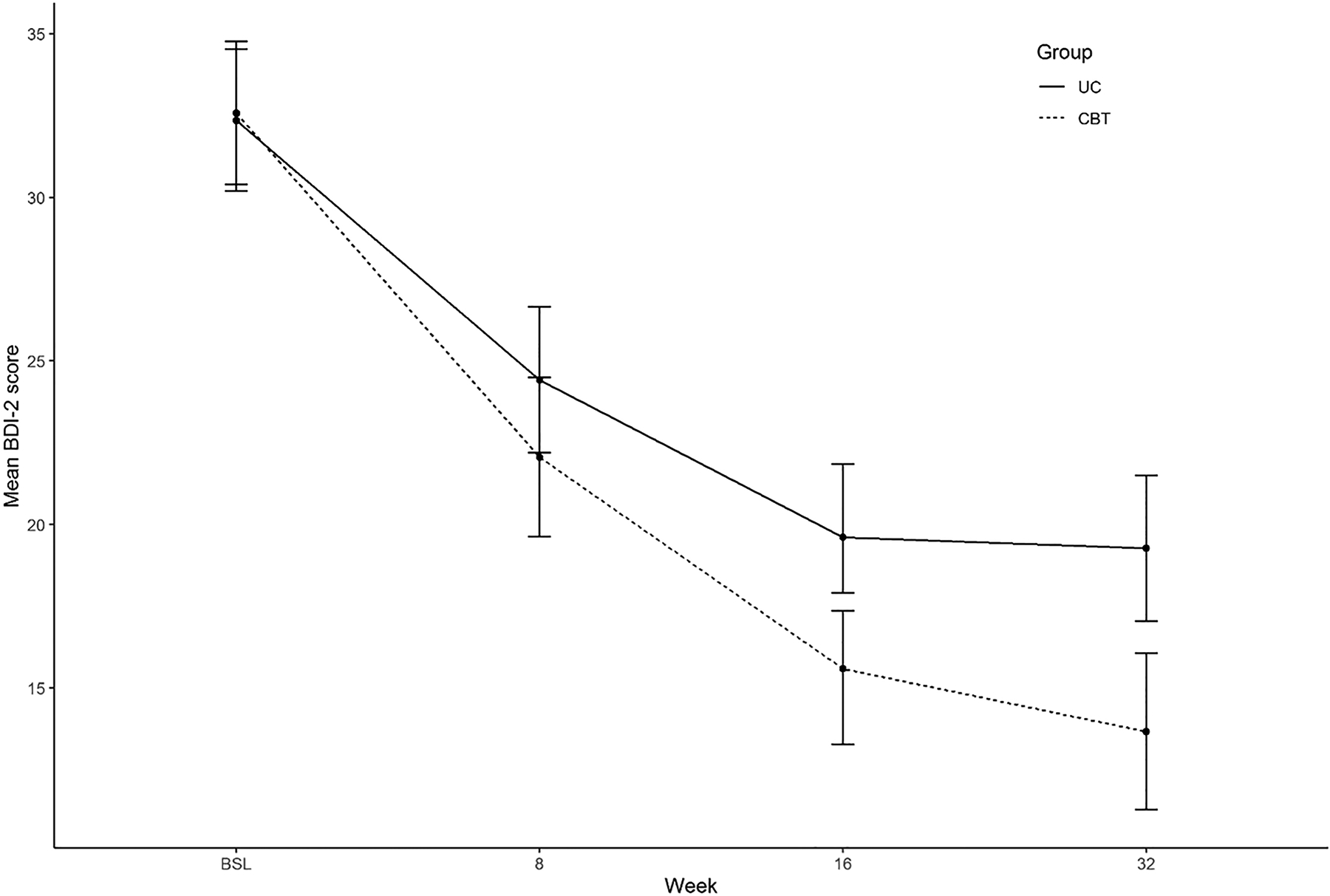
Standard score ranges on the BDI-II are 14 to 19 for mild depression, 20 to 28 for moderate depression, and 29 to 63 for severe depression. BDI-II, Beck Depression Inventory; CBT, cognitive behavior therapy; UC, usual care.
As shown in Table 3, the proportions of patients who were taking antidepressant, anxiolytic, or other psychiatric medicines did not statistically differ between the CBT and UC arms at any point during the trial. Ancillary analyses of depression remission, treatment effect moderators, and cointerventions are presented in the Supplementary materials. The groups did not differ with respect to remission of depression by 16 weeks. No treatment moderation effects were found for age, race, baseline (i.e., pre-randomization) severity of depression, or baseline antidepressant use. The percentage of patients reporting receipt of nonstudy medical or psychiatric care between baseline and 16 weeks was slightly higher in the CBT arm (74%) than in the UC arm (65%).
Table 3.
Patients who were taking nonstudy psychiatric medications during the trial.
| Medication | Total Sample (n = 139) |
UC (n = 70) |
CBT (n = 69) |
*P |
|---|---|---|---|---|
| Antidepressant | ||||
| Baseline | 62 (44.6) | 31 (44.3) | 31 (44.9) | .99 |
| Week 8 | 62 (44.6) | 29 (41.4) | 33 (47.8) | .50 |
| Week 16 | 69 (49.6) | 30 (42.9) | 39 (56.5) | .13 |
| Week 32 | 71 (51.1) | 30 (42.9) | 41 (59.4) | .06 |
| Week 40 | 72 (51.8) | 31 (44.3) | 41 (59.4) | .09 |
| Week 52 | 71 (51.1) | 30 (42.9) | 41 (59.4) | .06 |
| Anxiety | ||||
| Baseline | 22 (15.8) | 10 (14.3) | 12 (17.4) | .65 |
| Week 8 | 20 (14.4) | 11 (15.7) | 9 (13.0) | .81 |
| Week 16 | 26 (18.7) | 11 (15.7) | 15 (21.7) | .39 |
| Week 32 | 26 (18.7) | 12 (17.1) | 14 (20.3) | .67 |
| Week 40 | 26 (18.7) | 11 (15.7) | 15 (21.7) | .39 |
| Week 52 | 24 (17.3) | 10 (14.3) | 14 (20.3) | .38 |
| † Other psychiatric | ||||
| Baseline | 4 (2.9) | 1 (1.4) | 3 (4.4) | .37 |
| Week 8 | 7 (5.0) | 2 (2.9) | 5 (7.3) | .27 |
| Week 16 | 7 (5.0) | 2 (2.9) | 5 (7.3) | .27 |
| Week 32 | 8 (5.8) | 2 (2.9) | 6 (8.7) | .17 |
| Week 40 | 8 (5.8) | 2 (2.9) | 6 (8.7) | .17 |
| Week 52 | 9 (6.5) | 3 (4.3) | 6 (8.7) | .33 |
Table values are reported as number (column-wise percentage) of patients.
P-values are based on Fisher’s exact test.
Other psychiatric medications include antipsychotics and mood stabilizers.
CBT, cognitive behavior therapy; UC, usual care.
DISCUSSION
Good HF self-care requires initiative, active engagement, and the ability to organize daily activities. Symptoms of depression such as fatigue, hopelessness, and poor concentration interfere with these capabilities and thereby create barriers to effective self-care. For this reason, we expected successful treatment of depression to improve patients’ ability to benefit from an individualized HF self-care intervention.
This single-center trial confirmed that CBT is effective for major depression in patients with HF, and that it also decreases anxiety and improves HF-related quality of life. The sample was diverse with respect to sex, race, and socioeconomic status, suggesting that the findings are generalizable to a wide range of patients with HF. However, the results do not support a sequential approach to intervening in depression and HF self-care. We employed an adaptive intervention for depression that included intensification of CBT and referral to nonstudy primary care physicians for antidepressant medications for patients who did not achieve rapid progress toward remission of depression. Nevertheless, on average, their depression did not improve rapidly enough to enable them to benefit more from a tailored HF self-care intervention that began 8 weeks after initiation of CBT, compared to the patients who received usual care for depression. HF self-care improved in both groups over the course of the tailored self-care intervention, but the extent of improvement did not differ between the CBT and UC arms, and it did not depend on the severity of depression during the self-care intervention.
Many of the patients needed more than 8 weeks of treatment to reach remission of major depression; in fact, many of them needed more than 16 weeks. Consequently, a large percentage of the patients in the intervention arm were involved in two different active interventions (i.e., CBT and tailored self-care) starting 8 weeks after randomization. The pattern of 16-week outcomes suggests that it may have been difficult for some patients to improve their HF self-care behaviors while continuing to work on overcoming their depression, but that other patients were able to address both problems concurrently.
Our previous trial tested a cognitive-behavioral intervention that integrated depression and HF self-care goals, and the present trial tested a sequential approach in which CBT for depression was initiated antecedent to a separate tailored self-care intervention. Both approaches produced favorable depression outcomes, but neither was more effective than usual care for improving the benefit of self-care education and support. This raises the question of whether there are better alternatives for intervening in HF self-care for patients who have major depression.
In a recent study, we found that 25% of a cohort of 400 patients with HF were taking antidepressant medications.2 Antidepressant monotherapy may be less burdensome than CBT, and it may be more feasible for patients to initiate and continue while also working on their HF self-care goals. Unfortunately, the two largest trials of antidepressant medications for patients with HF and comorbid major depression produced no evidence of efficacy for depression.32,33 Thus, antidepressant augmentation of HF self-care interventions may not seem to be a very promising alternative. On the other hand, there have not been any large trials in patients with HF of non-SSRI antidepressants or of other types of treatment for depression, such as transcranial magnetic stimulation. Further research is needed to determine whether these treatments are efficacious for comorbid major depression in HF. The potential benefits of early treatment for depression for patients with include not only improvement in depression but also improvements in anxiety and health-related quality of life. Large trials are also needed to determine whether treatment of depression can reduce hospital readmissions and improve survival in patients with HF.
An even more personalized strategy is probably needed for patients with major depression and HF self-care deficits. In this approach, patients would be able to work on their HF self-care goals as soon as they are ready to do so, regardless of their depression status. This would empower patients to address their HF self-care goals at personally opportune times in the course of HF, rather than on a schedule that is tied to treatment for depression. Trials should be conducted to test this approach and to determine whether HF self-care interventions can help to improve symptoms of depression. Further research is also needed to develop additional strategies to address other barriers to HF self-care such as health literacy deficits, limited comprehension of patient-provider communications including self-care instructions, and social determinants of health.34
The most significant limitation of this study is that it was a single-center trial and that it was underpowered, due primarily to recruitment challenges during the COVID-19 pandemic. However, patients in the intervention arm had slightly worse, not better, self-care maintenance scores at 16 weeks compared to the patients in the usual care arm. Thus, it seems unlikely that a larger sample would have yielded positive results on both co-primary outcomes (i.e., depression and self-care maintenance).
Conclusions
Cognitive behavior therapy is an effective treatment for major depression in patients with HF, and depressed patients can also benefit from an individualized self-care intervention. However, neither the concurrent (integrated) nor the sequential strategies for treating major depression and HF self-care deficits that we have tested are optimal. Future trials should test personalized strategies in which depressed patients can address their self-care goals at opportune times in the course of their heart failure, regardless of the current status of their depression.
Supplementary Material
WHAT IS NEW?
A sequential approach to intervening in major depression and inadequate heart failure self-care improves depression but does not increase the benefit of HF self-care education and support.
Cognitive behavior therapy also decreases anxiety and improves quality of life in patients with heart failure and comorbid major depression.
WHAT ARE THE CLINICAL IMPLICATIONS?
Major depression is a common comorbidity that impedes HF self-care, increases the risks of rehospitalization and mortality, and is treatable with cognitive behavior therapy.
Patients can benefit from an intervention to improve HF self-care even if they are depressed and while they are being treated for depression.
The optimal time(s) to intervene in self-care depend more on the course of HF and the patient’s preferences than on the presence or severity of depression.
Source of Funding
This study was funded by grant number 5R01HL131524 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland. There are no relationships with industry.
Non-standard Abbreviations and Acronyms
- BDI-II
Beck Depression Inventory, version 2
- CBT
Cognitive behavior therapy
- GAD-7
Generalized Anxiety Disorder Questionnaire
- HAMD-17
Hamilton Rating Scale for Depression
- HF
Heart failure
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- NYHA
New York Heart Association
- PHQ-9
Patient Health Questionnaire
- REDCap
Research Electronic Data Capture
- RCT
Randomized clinical trial
- SCHFI
Self-Care of Heart Failure Index
- TSC
Tailored self-care intervention
- UC
Usual care
Footnotes
Trial Registration: ClinicalTrials.gov Identifier NCT02997865
Disclosures
None.
REFERENCES
- 1.Sreenivasan J, Khan MS, Ochani RK, Bhinder J, Usman MS, Anker SD, Fonarow GC, Butler J. Major depression and anxiety among patients hospitalized with heart failure. Am J Cardiol. 2021;142:153–155. doi: 10.1016/j.amjcard.2020.12.053 [DOI] [PubMed] [Google Scholar]
- 2.Freedland KE, Steinmeyer BC, Carney RM, Skala JA, Rich MW. Antidepressant use in patients with heart failure. Gen Hosp Psychiatry. 2020;65:1–8. doi: 10.1016/j.genhosppsych.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedland KE, Rich MW, Carney RM. Improving quality of life in heart failure. Current Cardiology Reports. 2021;23:159. doi: 10.1007/s11886-021-01588-y [DOI] [PubMed] [Google Scholar]
- 4.Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Skala JA, Davila-Roman VG. Depression and multiple rehospitalizations in patients with heart failure. Clin Cardiol. 2016;39:257–262. doi: 10.1002/clc.22520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedland KE, Hesseler MJ, Carney RM, Steinmeyer BC, Skala JA, Davila-Roman VG, Rich MW. Major depression and long-term survival of patients with heart failure. Psychosom Med. 2016;78:896–903. doi: 10.1097/psy.0000000000000346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adelborg K, Schmidt M, Sundboll J, Pedersen L, Videbech P, Botker HE, Egstrup K, Sorensen HT. Mortality risk among heart failure patients with depression: a nationwide population-based cohort study. J Am Heart Assoc. 2016;5:e004137. doi: 10.1161/jaha.116.004137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gathright EC, Goldstein CM, Josephson RA, Hughes JW. Depression increases the risk of mortality in patients with heart failure: A meta-analysis. J Psychosom Res. 2017;94:82–89. doi: 10.1016/j.jpsychores.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedland KE, Skala JA, Steinmeyer BC, Carney RM, Rich MW. Effects of depression on heart failure self-care. J Card Fail. 2021;27:522–532. doi: 10.1016/j.cardfail.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 9.Riegel B, Westland H, Iovino P, Barelds I, Bruins Slot J, Stawnychy MA, Osokpo O, Tarbi E, Trappenburg JCA, Vellone E, et al. Characteristics of self-care interventions for patients with a chronic condition: A scoping review. International journal of nursing studies. 2021;116:103713. doi: 10.1016/j.ijnurstu.2020.103713 [DOI] [PubMed] [Google Scholar]
- 10.van Eck van der Sluijs JF, Castelijns H, Eijsbroek V, Rijnders CAT, van Marwijk HWJ, van der Feltz-Cornelis CM. Illness burden and physical outcomes associated with collaborative care in patients with comorbid depressive disorder in chronic medical conditions: A systematic review and meta-analysis. Gen Hosp Psychiatry. 2018;50:1–14. doi: 10.1016/j.genhosppsych.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 11.Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive behavior therapy for depression and self-care in heart failure patients: A randomized clinical trial. JAMA Intern Med. 2015;175:1773–1782. doi: 10.1001/jamainternmed.2015.5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck JS. Cognitive behavior therapy : basics and beyond. Third edition. ed. New York: The Guilford Press; 2021. [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 15.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, Gurvitz MZ, Havranek EP, Lee CS, Lindenfeld J, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/circulationaha.109.192628 [DOI] [PubMed] [Google Scholar]
- 16.Dracup K, Moser DK, Pelter MM, Nesbitt TS, Southard J, Paul SM, Robinson S, Cooper LS. Randomized, controlled trial to improve self-care in patients with heart failure living in rural areas. Circulation. 2014;130:256–264. doi: 10.1161/circulationaha.113.003542 [DOI] [PubMed] [Google Scholar]
- 17.Masterson Creber R, Patey M, Lee CS, Kuan A, Jurgens C, Riegel B. Motivational interviewing to improve self-care for patients with chronic heart failure: MITI-HF randomized controlled trial. Patient education and counseling. 2016;99:256–264. doi: 10.1016/j.pec.2015.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Brown GK. BDI-II Manual. San Antonio, Texax: The Psychological Corporation; 1996. [Google Scholar]
- 19.Freedland KE, Steinmeyer BC, Carney RM, Rubin EH, Rich MW. Use of the PROMIS(R) Depression scale and the Beck Depression Inventory in patients with heart failure. Health Psychol. 2019;38:369–375. doi: 10.1037/hea0000682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammash MH, Hall LA, Lennie TA, Heo S, Chung ML, Lee KS, Moser DK. Psychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure. European journal of cardiovascular nursing : journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology. 2013;12:446–453. doi: 10.1177/1474515112468068 [DOI] [PubMed] [Google Scholar]
- 21.Vellone E, Riegel B, Cocchieri A, Barbaranelli C, D’Agostino F, Antonetti G, Glaser D, Alvaro R. Psychometric testing of the Self-Care of Heart Failure Index Version 6.2. Research in nursing & health. 2013;36:500–511. doi: 10.1002/nur.21554 [DOI] [PubMed] [Google Scholar]
- 22.Serfaty M, King M, Nazareth I, Moorey S, Aspden T, Mannix K, Davis S, Wood J, Jones L. Effectiveness of cognitive-behavioural therapy for depression in advanced cancer: CanTalk randomised controlled trial. Br J Psychiatry. 2020;216:213–221. doi: 10.1192/bjp.2019.207 [DOI] [PubMed] [Google Scholar]
- 23.Lemmens LH, Arntz A, Peeters F, Hollon SD, Roefs A, Huibers MJ. Clinical effectiveness of cognitive therapy v. interpersonal psychotherapy for depression: results of a randomized controlled trial. Psychological medicine. 2015;45:2095–2110. doi: 10.1017/s0033291715000033 [DOI] [PubMed] [Google Scholar]
- 24.Riegel B, Lee CS, Dickson VV, Carlson B. An update on the self-care of heart failure index. J Cardiovasc Nurs. 2009;24:485–497. doi: 10.1097/JCN.0b013e3181b4baa0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893 [DOI] [PubMed] [Google Scholar]
- 26.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 27.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 28.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohr DC, Ho J, Duffecy J, Reifler D, Sokol L, Burns MN, Jin L, Siddique J. Effect of telephone-administered vs face-to-face cognitive behavioral therapy on adherence to therapy and depression outcomes among primary care patients: a randomized trial. JAMA. 2012;307:2278–2285. doi: 10.1001/jama.2012.5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruijniks SJE, Lemmens L, Hollon SD, Peeters F, Cuijpers P, Arntz A, Dingemanse P, Willems L, van Oppen P, Twisk JWR, et al. The effects of once- versus twice-weekly sessions on psychotherapy outcomes in depressed patients. Br J Psychiatry. 2020;216:222–230. doi: 10.1192/bjp.2019.265 [DOI] [PubMed] [Google Scholar]
- 31.Vittengl JR, Jarrett RB, Weitz E, Hollon SD, Twisk J, Cristea I, David D, DeRubeis RJ, Dimidjian S, Dunlop BW, et al. Divergent outcomes in cognitive-behavioral therapy and pharmacotherapy for adult depression. Am J Psychiatry. 2016;173:481–490. doi: 10.1176/appi.ajp.2015.15040492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angermann CE, Gelbrich G, Stork S, Gunold H, Edelmann F, Wachter R, Schunkert H, Graf T, Kindermann I, Haass M, et al. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683–2693. doi: 10.1001/jama.2016.7635 [DOI] [PubMed] [Google Scholar]
- 33.O’Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, Zakhary B, Stough WG, Arias RM, Rivelli SK, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692–699. doi: 10.1016/j.jacc.2010.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaarsma T, Hill L, Bayes-Genis A, La Rocca HB, Castiello T, Čelutkienė J, Marques-Sule E, Plymen CM, Piper SE, Riegel B, et al. Self-care of heart failure patients: practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23:157–174. doi: 10.1002/ejhf.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


