Abstract
Issues.
Alcohol use has been shown to impact on various forms of liver disease, not restricted to alcoholic liver disease.
Approach.
We developed a conceptual framework based on a narrative review of the literature to identify causal associations between alcohol use and various forms of liver disease including the complex interactions of alcohol with other major risk factors. Based on this framework, we estimate the identified relations for 2017 for the USA.
Key Findings.
The following pathways were identified and modelled for the USA for the year 2017. Alcohol use caused 35 200 (95% uncertainty interval 32 800–37 800) incident cases of alcoholic liver cirrhosis. There were 1700 (uncertainty interval 1100–2500) acute hepatitis B and C virus (HBV and HCV) infections attributable to heavy-drinking occasions, and 14 000 (uncertainty interval 5900–19 500) chronic HBV and 1700 (uncertainty interval 700–2400) chronic HCV infections due to heavy alcohol use interfering with spontaneous clearance. Alcohol use and its interactions with other risk factors (HBV, HCV, obesity) led to 54 500 (uncertainty interval 50 900–58 400) new cases of liver cirrhosis. In addition, alcohol use caused 6600 (uncertainty interval 4200–9300) liver cancer deaths and 40 700 (uncertainty interval 36 600–44 600) liver cirrhosis deaths.
Implications.
Alcohol use causes a substantial number of incident cases and deaths from chronic liver disease, often in interaction with other risk factors.
Conclusion.
This additional disease burden is not reflected in the current alcoholic liver disease categories. Clinical work and prevention policies need to take this into consideration.
Keywords: alcohol, liver disease, hepatitis C virus, metabolic dysfunction, mortality incidence
Introduction
The causal impact of alcohol use on the formation and progression of liver disease in general, and for liver cirrhosis in particular, has long been known. As early as the late 18th century, Benjamin Rush, in his famous An Inquiry into the Effects of Ardent Spirits upon the Human Body and Mind, listed obstruction of the liver as the second ‘usual’ disease consequence of habitual spirits use and compared its effects on that organ with the preying on Prometheus’ liver by a vulture [1]. While the causal impact of alcohol use is not disputed, and liver cirrhosis has been established as the prime somatic disorder resulting from alcohol use [2,3], to our knowledge there has never been an integrated review of the role of alcohol use on the different stages of liver disease, including the complex interactions with other major risk factors. Thus, this contribution:
establishes a conceptual model to determine the various impacts of alcohol use on the aetiology and course of different liver diseases;
summarises the epidemiological findings;
gives a first estimate for the magnitude of the effects of alcohol use on different liver diseases for the USA in 2017.
A conceptual model on the aetiology and course of liver disease
Figure 1 gives an overview of the aetiology and course of liver disease and its major stages. It is based on current research on establishing causal relations between alcohol use and various kinds of liver disease in the tradition of the Global Burden of Disease studies [4] and the World Health Organization Global Status Reports on Alcohol and Health ([5]; for an overview on risk relations between level and patterns of alcohol and liver disease: [6]; for biological pathways: [7]).
Figure 1.
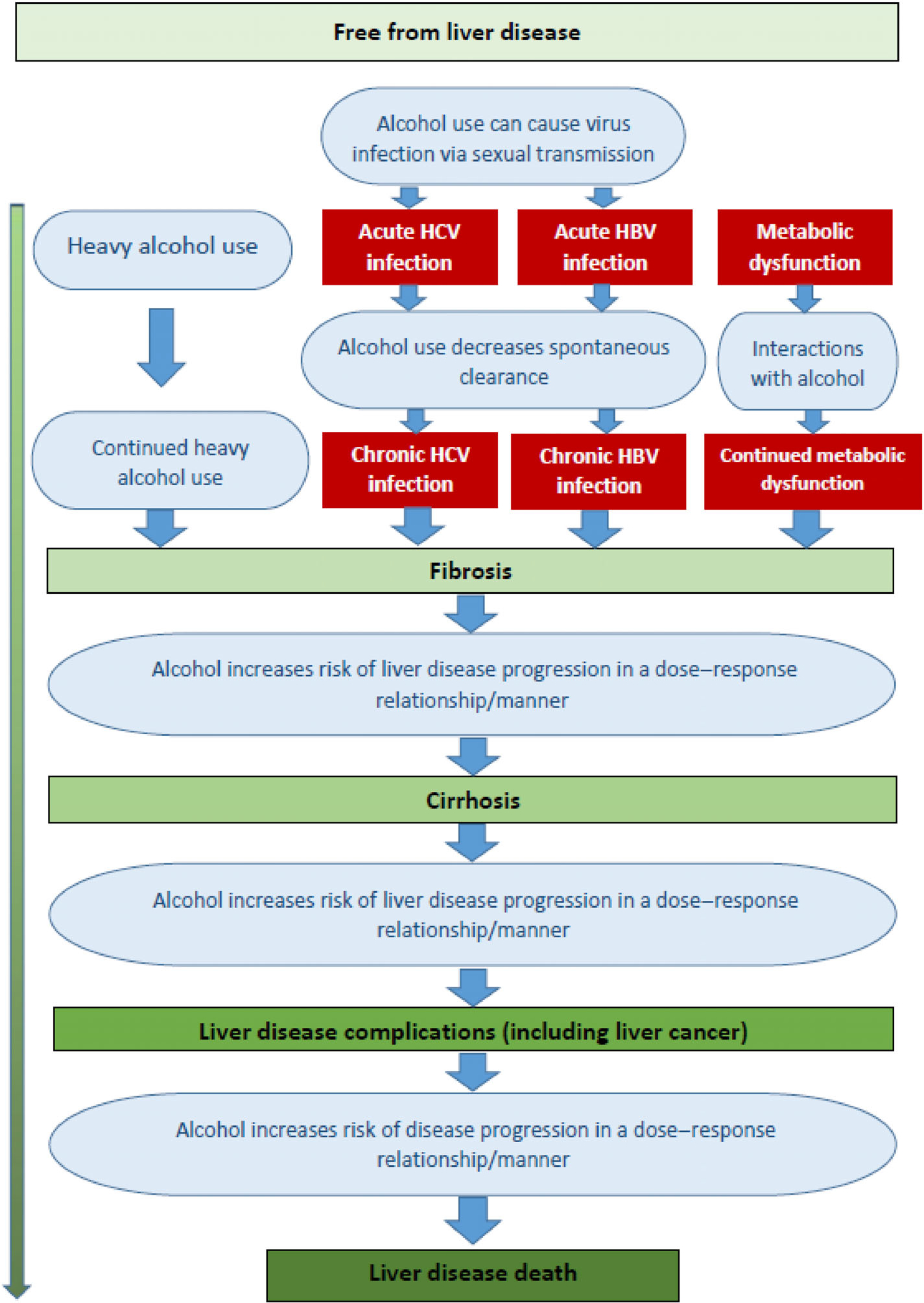
The role of alcohol use in the aetiology and course of liver disease. HBV, hepatitis B virus; HCV, hepatitis C virus.
Liver disease is usually considered in stages that range from relatively mild and reversible inflammation, to fibrosis and sclerosis of the liver, to severe and irreversible stages, such as liver cirrhosis and hepatic failure, and liver cancers, and, finally, to liver death [8]. Most scientific articles on the natural history focus on one type of liver disease based on aetiology, such as alcoholic liver disease [9], liver disease due to hepatitis B or C virus (HBV or HCV) infection [10,11], or liver disease due to metabolic dysfunction [12] (for an early exception, see [13]). However, these limited perspectives are problematic, as there are marked interactions between disease types and underlying risk factors. For instance, in a recent study of all hospitalisations in mainland France, more than 70% of decompensated liver cirrhosis in people with chronic HCV infection was attributable to alcohol [14]. In fact, alcohol use plays a role in all types of liver disease, including in metabolic dysfunction [15], and even in so-called non-alcoholic liver disease [16,17].
Consequently, we have included all major types of liver cirrhosis in a unified conceptual model. As indicated above, we will provide specific examples of the impact of alcohol use on the incidence of the respective disease categories for the USA in 2017.
Alcohol use and the aetiology of liver disease
For the aetiology of liver disease, we will need to restrict ourselves to the aetiology of acute and chronic HBC and HCV infections and of liver cirrhosis, as the epidemiological data on the national level for inflammation or for fibrosis are usually scarce and unreliable. In the aetiology of liver cirrhosis [18], alcohol use is involved:
in the aetiology of alcoholic liver cirrhosis as the main factor;
in the aetiology of acute and chronic HBV and HCV infections, which may lead to cirrhosis;
in the aetiology of liver cirrhosis due to HBV and HCV; and
in the aetiology of liver cirrhosis due to metabolic dysfunction, as all of these risk factors interact with alcohol use in the advancement of liver disease (see Figure 1 and sections below).
Alcoholic liver cirrhosis.
Alcohol is a necessary and sufficient cause for alcoholic liver cirrhosis by definition (for a review, see [9]). Two dimensions of alcohol use determine the risk for liver cirrhosis [19,20]: overall cumulative lifetime consumption [21] and recent level of drinking in grams of pure alcohol (ethanol) per day [22].
For overall cumulative lifetime consumption, a threshold of 100 kg intake of pure alcohol has been found in the Dionysos study [13,23,24], indicating that the usual dose–response curves are only broad estimates. Thus, the meta-analyses for risk of liver cirrhosis based on level of alcohol use in cohort studies [22,25] assume that the level of alcohol use has stayed more or less constant over time since the baseline measurement (see sidebar in [26]).
However, this does not suggest that fluctuations in the current level of alcohol use play no role. The effects of these fluctuations have been seen in natural experiments, which occurred whenever there was a sudden decline in the availability of alcohol due to environmental circumstances and the rates of liver cirrhosis fell abruptly (e.g. Prohibition [27]; German invasion of Paris in World War II [28,29]; Gorbachev Reform [30], see also [31]).
Aetiology of HBC and HCV infections.
Alcohol use is a cause for acute HBV and HCV infections contracted through sexual transmission (the same mechanism involved in the transmission of HIV [32,33]) and due to weakening of the immune system (see the different contributions in the special volume on alcohol and the immune system entitled ‘Alcohol Research: Current Reviews’ [34]). Both effects seem to be particularly associated with episodic and chronic heavy drinking.
Alcohol use is also a cause of chronic HBV and HCV infections as it interferes with the clearance of the virus after acute hepatitis infections [35]. Based on the meta-analysis of Aisyah and colleagues [35], approximately 36% of people with acute HCV infections spontaneously cleared the virus within 12 months (point estimate: 36.1%; 95% confidence interval 23.5–50.9), and individuals who had not spontaneously cleared the virus within 12 months were unlikely to do so. ‘Excess alcohol use’ was related to a lower clearance rate: odds ratio 0.67 (95% confidence interval 0.47–0.95) and, thus, heavy alcohol use interferes with the clearance of acute HBV and HCV.
Liver cirrhosis due to HBV and HCV infection.
Alcohol use is an important contributory cause of liver cirrhosis in people with chronic HCV and HBV infection, based on the risk relations of the meta-analysis of Llamosas-Falcón and colleagues [36]. This meta-analysis showed clear dose–response relationships between levels of alcohol use and the risk of liver cirrhosis and further liver complications, such as decompensated liver cirrhosis or liver death. There is no meta-analysis that specifically considers the contribution of alcohol use to liver cirrhosis due to HBV infection, but the biological pathways may be assumed to be similar.
Liver cirrhosis due to metabolic dysfunction.
Alcohol use is a potentially interactive factor for metabolic liver disease [37]. Based on the most recent review of studies that used body mass index as the indicator, four longitudinal cohorts showed a potential interaction in predicting liver hospitalisations or mortality [16,38–40], all with large sample sizes (up to more than 1.2 million participants in the Million Women Study [38]). All studies found increases in risk for liver cirrhosis for a high body mass index, either defined with a cut-off of 25 or 30, starting at 1.3 and going as high as 2.8 [40]. The interactions between body mass index and alcohol use varied more markedly, as the thresholds for alcohol use varied considerably across studies.
The impact of alcohol use on liver complications and liver cirrhosis deaths
Current evidence on risk relations also allows us to estimate liver complications, such as decompensated liver cirrhosis, which includes jaundice, ascites, hepatic encephalopathy, hepatorenal syndrome and/or variceal haemorrhage [41,42], and other complications, such as liver transplants or liver cancer. Liver transplants are the most difficult to model, as there are ethical controversies surrounding such transplants being performed in people with complications arising from alcoholic liver cirrhosis (for the USA, see [43]) and, depending on the country, periods of abstinence for 6 or more months are necessary before a patient is eligible for a transplant [44]. Fatal endpoints, such as liver cirrhosis deaths, seem to be more closely linked to the level of alcohol consumption than non-fatal (morbidity) outcomes [25]; alcohol-attributable liver deaths can be modelled based on the meta-analysis by Rehm and colleagues [25], which separated mortality from other outcomes.
Methods
While the above gives an epidemiological overview on the impact of alcohol use on aetiology and various stages of different categories of liver disease using a lifetime perspective, the empirical part of this contribution will be restricted to exemplarily estimating the impact of alcohol use on liver disease in the USA in 2017 (Table 1). For this snapshot, the USA was selected as it is one of the countries with the most data available of relatively good quality (i.e. cause of death and hospitalisations based on routine data-collection systems within the medical system, instead of relying on estimates via verbal autopsies [54], or statistical models based on data from other countries [55]). In the following, we give an overview of methodologies and data sources. For further detail, see the Data S1 (Supporting Information).
Table 1.
Sources and assumptions for modelling the impact of alcohol on incidence and course of liver disease
| Mechanism | Consequence |
Exposure on (data based on [45]) | Risk relations | Further assumptions |
|---|---|---|---|---|
| All data from [46] | ||||
|
| ||||
| Heavy drinking over time on alcoholic liver cirrhosis | Alcoholic liver cirrhosisa incidence | Not applicable; alcoholic liver cirrhosis was estimated directly | Not applicable | An underestimate as it is a stigmatised disease (see text) |
| Heavy-drinking occasion on sexual transmission of HBV and HCV | Acute HBV and HCV incidence | Heavy-drinking occasions (defined as >48 g pure alcohol/day in women; >60 g/day in men; [32]) | Rehm et al., 2017 [32] | Alcohol’s impact on sexual transmission applies to all sexually transmitted diseases |
| Alcohol use is linked to decreased clearance of acute HBV and HCV leading to increased chronic HBV and HCV cases | Chronic HBV incidence; chronic HCV incidence | All populations with HBV or HCV infections -> higher average drinking level (for distribution based on mean, see [36,47]); effect on clearance only by chronic heavy drinkers [48] | Aisyah et al., 2018 [35] | Similar impact of alcohol on clearance of HBV and HCV |
| Alcohol use interaction with chronic HBV and HCV in the course of liver disease progression | Cirrhosis due to HBV; cirrhosis due to HCVa | All populations with HBV or HCV infections -> higher average drinking level (for distribution based on mean see [36,47]) | Llamosas-Falcón et al., 2020 [36] | Similar impact of alcohol on disease progression of HBV and HCV infections |
| Alcohol use interaction with obesity on liver cirrhosis | Liver cirrhosis incidencea | Chronic heavy drinkers (defined as >40 g pure alcohol/day in women; >60 g/day in men) | Patra et al., 2020 [49] | |
| Alcohol use on liver disease complications, for example liver cancer | Liver cancer incidence Liver cancer death |
Population distribution of consumption plus effect of former drinkers [50] | For drinkers: Turati et al., 2015 [51]; for former drinkers [52] | Average lag time of 10 years between exposure and outcome [53] |
| Continued alcohol consumption on chronic liver disease death (excluding liver cancer) | Liver cirrhosis deatha (as per the broader GBD definition) | Rehm et al., 2010 [25]; for former drinkers [50] | Assumption of impact of alcohol on mortality within a year (comparative risk assessment methodology) | |
Liver cirrhosis is defined broadly as comprising other chronic liver disease: B18-B18.9, I85-I85.9, I98.2, K70-K70.9, K71.3-K71.51, K71.7, K72.1-K74.69, K74.9, K75.8-K76.0, K76.6-K76.7, K76.9. HBV, hepatitis B virus; HCV, hepatitis C virus.
The overall estimation methodology followed the approach of the comparative risk assessment for alcohol developed for the Global Burden of Disease and Injury Study [56], comparing the impact of current drinking against a counterfactual scenario involving no use of alcohol. For this approach, data on exposure and risk relations are necessary to estimate alcohol-attributable fractions, which are then applied to the outcomes.
Exposure data were taken from the most recent review by Manthey and colleagues [45]. These estimates are based on triangulation between adult alcohol per capita and survey data. Such a triangulation is necessary as survey data alone markedly underestimate real consumption [56]. Details on the triangulation method can be found elsewhere [57,58].
Risk relations were taken from published meta-analyses. Data on exposure and risk relations were combined using the attributable-fraction approach, originally developed by Levin [59]. Data on prevalence and incidence of various types of liver disease were taken from the 2017 Global Burden of Disease and Injury Study [46]. This study uses a broad definition for liver cirrhosis, which includes almost all chronic liver disease and comprises the following International Statistical Classification of Diseases and Related Health Problems, 10th revision, codes: B18-B18.9, I85-I85.9, I98.2, K70-K70.9, K71.3-K71.51, K71.7, K72.1-K74.69, K74.9, K75.8-K76.0, K76.6-K76.7, K76.9. Population data were taken from the same source.
Results
Alcohol exposure in the USA in 2017
In 2017, the annual per capita consumption of pure alcohol per adult (defined as 15 years and older) was estimated at 9.8 L. This value includes recorded and unrecorded consumption and is corrected for by tourist consumption [45,60]. In Table 2, the annual per capita consumption is divided into groups based on sex and age. Only 73% of the US adult population had at least one alcoholic drink in that year, and alcohol consumption differed considerably by sex and age. Across all age groups, female drinkers had on average one standard drink per day (14 g/day), while male drinkers had on average between two and three standard drinks per day (33 to 40 g/day). The vast majority of drinkers fall into the lower-risk drinking category, defined as less than 40 g/day. However, every third male drinker and every 12th female drinker aged 35–64 drank hazardously, that is more than 40 g of pure alcohol per day.
Table 2.
Estimates of alcohol exposure in the USA in the year 2017 (based on [45])
| Sex | Age, years | Mean daily alcohol intake among drinkersa | Prevalence of |
|||||
|---|---|---|---|---|---|---|---|---|
| Lifetime abstinence | Past-year abstinence | Less than 40 g/day | 40–60 g/day | 60–100 g/day | At least 100 g/day | |||
|
| ||||||||
| Women | 15–34 | 14.3 (13.6–15.1) | 14.4% (12.5–17.1) | 16.6% (14.7–19.3) | 63.2% (62.4–63.8) | 3.7% (3.3–4.1) | 1.8% (1.6–2.2) | 0.3% (0.2–0.5) |
| 35–49 | 14.0 (13.4–14.6) | 10.3% (9–12) | 16.2% (15–17.9) | 67.5% (66.9–68.1) | 3.8% (3.4–4.2) | 1.9% (1.6–2.2) | 0.3% (0.2–0.4) | |
| 50–64 | 13.2 (12.5–13.9) | 13.7% (11.9–15.7) | 19.6% (17.8–21.6) | 61.9% (61.3–62.4) | 3.1% (2.8–3.5) | 1.4% (1.2–1.7) | 0.2% (0.1–0.3) | |
| 65+ | 12.0 (11.2–13.0) | 24.5% (21.5–27.6) | 25.1% (22.2–28.3) | 47.5% (46.9–48) | 2% (1.7–2.4) | 0.8% (0.6–1.1) | 0.1% (0–0.2) | |
| Men | 15–34 | 32.9 (30.9–34.7) | 8.6% (7.1–10.2) | 10.8% (9.3–12.4) | 57.5% (56.1–59) | 9.3% (8.8–9.8) | 8.7% (8–9.3) | 5.2% (4.3–5.9) |
| 35–49 | 40.0 (37.1–42.6) | 4.5% (3.8–5.2) | 10.2% (9.5–11) | 55.8% (54–57.7) | 10.3% (9.8–10.9) | 10.8% (10–11.5) | 8.4% (7.3–9.6) | |
| 50–64 | 39.1 (36.6–41.7) | 5.5% (4.8–6.5) | 12.1% (11.4–13.1) | 54.4% (52.7–56.1) | 9.9% (9.4–10.5) | 10.2% (9.5–10.9) | 7.8% (6.7–8.8) | |
| 65+ | 33.4 (31.5–35.5) | 8.7% (7.3–10.2) | 19% (17.7–20.6) | 51.2% (49.9–52.6) | 8.4% (7.9–8.8) | 7.9% (7.3–8.5) | 4.8% (4.1–5.6) | |
In grams pure alcohol per day. Numbers in parentheses denote 95% uncertainty interval.
Overview of effects of alcohol consumption on liver disease in the USA in 2017
Table 3 gives an overview of the results of the impact of alcohol use on various pathways involved in the aetiology and course of different liver diseases. The most striking results concern the impact of alcohol on the aetiology of alcoholic liver cirrhosis (broadly defined; see above). A total of 54 500 (95% uncertainty interval 50 900–58 400) new cases were caused by alcohol use, and alcohol-attributable liver disease deaths, including liver cancer deaths, were estimated at 47300 (95% uncertainty interval 42 500–52 000).
Table 3.
Estimates of the effects of alcohol consumption on liver disease in the USA in 2017
| Mechanism | Consequence | Women | Men | Total |
|---|---|---|---|---|
|
| ||||
| Heavy drinking over time on alcoholic liver cirrhosis | Alcoholic liver cirrhosis incidence | 13 000 (12 100–14 000) | 22 100 (20 700–23 700) | 35 200 (32 800–37 800) |
| Heavy-drinking occasions on sexual transmission of HBV and HCV | Acute HBV incidence | 10 (0–30) | 210 (140–320) | 220 (150–340) |
| Acute HCV incidence | 100 (0–250) | 1300 (900–2000) | 1400 (900–2100) | |
| Acute HBV and HCV incidence | 100 (0–250) | 1600 (1000–2400) | 1700 (1100–2500) | |
| Alcohol use linked-decrease in clearance of acute HBV and HCV leading-increased chronic HBV and HCV cases | Chronic HBV incidence | 3100 (2200–4000) | 10 900 (3800–15 500) | 14 000 (5900–19 500) |
| Chronic HCV incidence | 350 (250–460) | 1350 (470–1900) | 1700 (700–2400) | |
| Alcohol use interaction with chronic HBV and HCV in the course of liver disease progression | Cirrhosis due—HBV | 170 (50–300) | 1000 (700–1400) | 1200 (900–1500) |
| Cirrhosis due—HCV | 1300 (390–2200) | 6400 (4300–8500) | 7700 (6000–9400) | |
| Alcohol use interaction with obesity on liver cirrhosis | Liver cirrhosis incidence | 2600 (1600–3800) | 7800 (5300–10 800) | 10 400 (8400–12 800) |
| Alcohol use on liver disease, for example liver cancer | Liver cancer incidence | 2800 (1200–4300) | 5500 (3000–8400) | 8300 (5300–11 600) |
| Liver cancer death | 3600 (1200–4100) | 4100 (2200–6300) | 6600 (4200–9300) | |
| Continued alcohol consumption on chronic liver disease death (excluding liver cancer) | Liver cirrhosis death | 14 000 (11 700–16 800) | 26 600 (23 100–29 500) | 40 700 (36 600–44 600) |
Data were rounded—to the next 10 for numbers below 1000; and to the next 100 for numbers above 1000. Numbers in parentheses denote the 95% uncertainty interval. See Methods and Materials and Data S1 (Supporting Information) for details on calculation. HBV, hepatitis B virus; HCV, hepatitis C virus.
For both alcohol-attributable chronic liver disease and liver cancer, the number of incident cases in 2017 was larger than the number of deaths. As expected, given the differences in exposure, there was higher alcohol-attributable incidence and mortality in men than in women in all outcome categories.
Discussion
Limitations
With our current approach, we have modelled the impact of alcohol consumption at various timepoints over the drinker’s lifetime, and the aetiology and the progression of the liver disease. This methodology covers most liver diseases, since even in their earliest stages there are relatively small proportions of such diseases outside of the categories included in our study. However, a number of limitations in our approach need to be mentioned. First, the analyses of alcohol consumption were restricted to average levels of consumption over time. While the largest impact of alcohol use on the liver certainly is captured by this approach, there may be additional impacts from the pattern of drinking involved ([61]; for liver diseases: [62,63]). For instance, Simpson and colleagues [64], in the Million Women Study, observed higher risks when the same amount of alcohol was consumed without a meal rather than with a meal or was consumed on a daily compared to a non-daily basis; the latter confirming some earlier results. It has also been suggested that wine may be associated with a lower risk for liver cirrhosis than other alcoholic beverages [e.g. 65] and spirits with more risk [e.g. 66], but beverage-specificity of effects often cannot be distinguished from other behavioural and sociodemographic characteristics associated with drinking various beverages in different societies [67]. Unfortunately, there is not enough systematic research to summarise patterns of drinking while controlling for potential confounders [62]. For example, heavy episodic drinking has been suggested as a potential risk factor for the progression of non-alcoholic fatty liver disease, but the evidence is still insufficient [37]. As a result, meta-analyses on alcohol use and liver disease, while trying to estimate the effects of heavy episodic drinking, have restricted themselves to more narrative reviews of the literature [22,25].
Another area where there is insufficient data concerns the interactions of alcohol use with HBV infection. There are no meta-analyses on the role of alcohol in the spontaneous clearance of acute HBV infections, or on the disease progression of chronic HBV infections. Such data would be especially necessary to estimate the role of alcohol in low- and middle-income countries with high HBV infection and liver cirrhosis rates [50,68], but given the high prevalence and incidence of HBV infections in high-income countries like the USA, such data also would improve estimates for the USA.
Obviously, any statistical modelling is only as good as its assumptions. However, we took great care to explain all assumptions for our results in the text and the Data S1 (Supporting Information) and to only use very conservative assumptions. For example, we assumed that around 50% of patients with HBV or HCV infection will abstain after being infected, and we did not model the impact of former drinking on the course of these diseases. As well, we used the estimates of alcoholic liver cirrhosis and chronic liver disease from the Global Burden of Disease Study; these estimates are based on death records, which grossly underestimate the real magnitude of this cause of death. In a landmark study in 12 cities in 10 countries [69], after triangulating data on death certificates with data from hospital records and interviews of attending physicians or family members, the number of deaths due to ‘alcoholic liver cirrhosis’ more than doubled, with the majority of new cases being recoded using corresponding categories of cirrhosis, which made no mention of alcohol. This underreporting of alcoholic liver cirrhosis has persisted to this day [70,71]; for a short summary and discussion, see [72,73].
Another limitation concerns the fact that the impact of modern therapies on HCV—that is direct-acting antiviral medication—has not yet been fully integrated into these models. Obviously, the epidemiological data about incidence and prevalence do reflect these medications, but the estimates of the impact of alcohol use have mainly been based on studies before these medications were introduced. Better studies are needed on the potential interaction of alcohol use with these medications in the course of treatment (e.g. role of alcohol use in treatment uptake as well as treatment compliance, which includes compliance with regular intake of medications).
A final limitation to be mentioned here concerns the fact that our calculations only give a cross-sectional snapshot for 1 year, and from a real life-course perspective, some of the pathways occur very early in the development of liver disease and others only at the end. It would be more informative to observe a large cohort over several years, or to at least model these developments in a long-term perspective study, for example using agent-based modelling [74,75]. In addition, reviews and calculations, such as the current one, should regularly be updated to continuously increase the evidence base on alcohol epidemiology.
Clinical and preventive perspectives
The evidence presented above suggests that alcohol consumption plays a role in all types of liver disease. Thus, no matter how a liver disease is classified, care must be taken to assess alcohol use and, in the presence of alcohol use, at least a reduction in its level or, where possible, abstinence must be encouraged [76]. This recommendation can also be based on the most rigorous meta-analysis available for all types of liver cirrhosis, where categorical analysis showed an elevated risk for liver cirrhosis [22]. In any case, while most dose–response relationships are exponential, indicating most harm is linked to heavy drinking, there are no apparent thresholds for the various impacts of alcohol consumption. And, clearly, the most severe consequences, such as decompensated liver cirrhosis or death, can be triggered by even a small amount of drinking.
In terms of implications for interventions to reduce alcohol consumption in people with liver cirrhosis, a distinction needs to be made between individual and societal-level interventions. While abstinence from alcohol is best for people with liver disease, any reduction in the level of alcohol use is beneficial, especially for those drinking at higher levels [77]. This can be achieved by so-called brief interventions, or by alcohol therapy for people with alcohol use disorders; both forms of intervention have shown effectiveness in reducing levels of alcohol consumption and cost-effectiveness (brief interventions: [78]; therapy: [79]; cost-effectiveness: [80,81]). To initiate brief interventions or referral to treatment, current alcohol consumption should be screened for regularly, similar to the regular monitoring of blood pressure [82]. Such a routine medical screening could also help de-stigmatise heavy alcohol consumption and alcohol use disorders [83].
Brief interventions for prevention may also potentially have positive population health consequences if carried out systematically in the primary health-care setting [80]. But, overall, population health effects in reducing alcohol-attributable liver disease could best be achieved by alcohol control policies using the so-called best buys of the World Health Organization: increases in alcohol taxation, limitations on the availability of alcohol and a ban on its marketing and advertisement [84]. As indicated above, natural experiments with reductions of alcohol consumption, such as Prohibition, the German invasion of France, or the Gorbachev Reform, all showed marked reductions in the incidence of liver cirrhosis in the affected countries. The World Health Organization’s best buys have all been shown to reduce alcohol consumption [80,84] and will also be effective in reducing the incidence and mortality of liver cirrhosis.
However, while population-based alcohol control policies will be effective, regular monitoring of consumption and alcohol-attributable harm should be initiated to initiate implementation. Monitoring alcohol-attributable liver harm is important in this context as recent experiences in North America have shown that liver disease in general and liver cirrhosis in particular may serve as indicators for overall health problems, especially in persons of lower socioeconomic status [85].
Conclusion
Alcohol use causes a substantial number of incident cases and deaths from chronic liver disease, over and above alcoholic liver disease, often in interaction with other risk factors. This additional disease burden is not reflected in the current estimates of alcoholic liver disease categories. Clinical work and prevention policies need to take this into consideration.
Supplementary Material
Acknowledgements
The authors thank Astrid Otto for her referencing and English copy editing. Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (Award Number R01AA024443). This research was conducted as part of the Calibrated Agent Simulations for Combined Analysis of Drinking Etiologies (CASCADE) project and the authors would like to thank the whole CASCADE team for their input to wider discussions in generating the research reported in this paper. Content is the responsibility of the authors and does not reflect official positions of National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health. This contribution was also supported by funding from the Canadian Institutes of Health Research, Institute of Neurosciences, and Mental Health and Addiction (CRISM Ontario Node grant no. SMN-13950) to the first author.
Footnotes
Conflict of Interest
The authors have no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
Data S1. Supplemental data.
References
- [1].Rush B An inquiry into the effects of ardent spirits upon the human body and mind: with an account of the means of preventing, and of the remedies for curing them, 6th edn. New York: Cornelius Davis, 1811. (originally published 1785). [Google Scholar]
- [2].Rehm J, Shield KD. Global burden of alcohol use disorders and alcohol liver disease. Biomedicine 2019;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013;59:160–8. [DOI] [PubMed] [Google Scholar]
- [4].GBD 2017 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2017: a systematic analysis for the global burden of disease study 2017 [published correction appears in Lancet 2019;393:e44]. Lancet 2018;392:1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization. Global status report on alcohol and health 2018. Geneva: World Health Organization, 2018. Available at: https://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed 20 May 2019). [Google Scholar]
- [6].Rehm J, Gmel GE Sr, Gmel G et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 2017;112:968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 2015;12:231–42. [DOI] [PubMed] [Google Scholar]
- [8].American Liver Foundation. The progression of liver disease. 2020. Available at: https://liverfoundation.org/for-patients/about-the-liver/the-progression-of-liver-disease/ (accessed 16 February 2020).
- [9].Parker R, Aithal GP, Becker U et al. Natural history of histologically proven alcohol-related liver disease: a systematic review. J Hepatol 2019; 71:586–93. [DOI] [PubMed] [Google Scholar]
- [10].Likhitsup A, Lok AS. Understanding the natural history of hepatitis B virus infection and the new definitions of cure and the endpoints of clinical trials. Clin Liver Dis 2019;23:401–16. [DOI] [PubMed] [Google Scholar]
- [11].Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin North Am 2015;44:717–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lindenmeyer CC, McCullough AJ. The natural history of nonalcoholic fatty liver disease-an evolving view. Clin Liver Dis 2018;22:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bellentani S, Tiribelli C, Saccoccio G et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos study. Hepatology 1994;20:1442–9. [DOI] [PubMed] [Google Scholar]
- [14].Schwarzinger M, Thiébaut SP, Baillot S, Mallet V, Rehm J. Alcohol use disorders and associated chronic disease - a national retrospective cohort study from France. BMC Public Health 2017;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lieber CS. Alcohol and malnutrition in the pathogenesis of liver disease. JAMA 1975;233:1077–82. [PubMed] [Google Scholar]
- [16].Åberg F, Helenius-Hietala J, Puukka P, Färkkilä M, Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology 2018;67:2141–9. [DOI] [PubMed] [Google Scholar]
- [17].Roerecke M, Nanau R, Rehm J, Neuman M. Ethnicity matters: a systematic review and meta-analysis of the non-linear relationship between alcohol consumption and prevalence and incidence of hepatic steatosis. EBioMedicine 2016;8:317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371:838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Skog OJ. The risk function for liver cirrhosis from lifetime alcohol consumption. J Stud Alcohol 1984;45:199–208. [DOI] [PubMed] [Google Scholar]
- [20].Day C Alcoholic liver disease: dose and threshold—new thoughts on an old topic. Gut 1997;41:857–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lelbach WK. Cirrhosis in the alcoholic and its relation to the volume of alcohol abuse. Ann N Y Acad Sci 1975;252:85–105. [DOI] [PubMed] [Google Scholar]
- [22].Roerecke M, Vafaei A, Hasan OSM et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol 2019;114:1574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bellentani S, Saccoccio G, Costa G et al. Drinking habits as cofactors of risk for alcohol induced liver damage. Gut 1997;41:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bellentani S, Tiribelli C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J Hepatol 2001;35:531–7. [DOI] [PubMed] [Google Scholar]
- [25].Rehm J, Taylor B, Mohapatra S et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev 2010;29:437–45. [DOI] [PubMed] [Google Scholar]
- [26].Rehm J, Gmel G, Sempos CT, Trevisan M. Alcohol-related morbidity and mortality. Alcohol Res Health 2003;27:39–51. [PMC free article] [PubMed] [Google Scholar]
- [27].Dills AK, Miron JA. Alcohol prohibition and cirrhosis. Am Law Econ Rev 2004;6:285–318. [Google Scholar]
- [28].Ledermann S Alcool, alcoolism, alcoolisation. Paris: Presse Universitaire de France, 1956. [Google Scholar]
- [29].Fillmore KM, Roizen R, Farrell M, Kerr W, Lemmens P. Wartime Paris, cirrhosis mortality, and the ceteris paribus assumption. J Stud Alcohol 2002;63:436–46. [DOI] [PubMed] [Google Scholar]
- [30].Leon D, Chenet L, Shkolnikov V et al. Huge variation in Russian mortality rates 1984–1994: artefact, alcohol, or what? Lancet 1997;350:383–8. [DOI] [PubMed] [Google Scholar]
- [31].Zatoński WA, Sulkowska U, Mańczuk M et al. Liver cirrhosis mortality in Europe, with special attention to central and Eastern Europe. Eur Addict Res 2010;16:193–201. [DOI] [PubMed] [Google Scholar]
- [32].Rehm J, Probst C, Shield KD, Shuper PA. Does alcohol use have a causal effect on HIV incidence and disease progression? A review of the literature and a modeling strategy for quantifying the effect. Popul Health Metr 2017;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N, Rehm J. Causal considerations on alcohol and HIV/AIDS – a systematic review. Alcohol Alcohol 2010;45:159–66. [DOI] [PubMed] [Google Scholar]
- [34].Sarkar D, Jung MK, Wang HJ. Alcohol and the immune system. Alcohol Res 2015;37:153–5. [Google Scholar]
- [35].Aisyah DN, Shallcross L, Hully AJ, O’Brien A, Hayward A. Assessing hepatitis C spontaneous clearance and understanding associated factors—a systematic review and meta-analysis. J Viral Hepat 2018;25:680–98. [DOI] [PubMed] [Google Scholar]
- [36].Llamosas-Falcón L, Shield KD, Gelovany M, Manthey J, Rehm J. Alcohol use disorders and the risk of progression of liver disease in people with hepatitis C virus infection – a systematic review. Subst Abuse Treat Prev Policy 2020;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Åberg F, Färkkilä M, Männistö V. Interaction between alcohol use and metabolic risk factors for liver disease: a critical review of epidemiological studies. Alcohol Clin Exp Res 2020;44:384–403. [DOI] [PubMed] [Google Scholar]
- [38].Liu B, Balkwill A, Reeves G, Beral V, Million Women Study C. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ 2010;340:c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey SG. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yi S-W, Hong J-S, Yi J-J, Ohrr H Impact of alcohol consumption and body mass index on mortality from nonneoplastic liver diseases, upper aerodigestive tract cancers, and alcohol use disorders in Korean older middle-aged men: prospective cohort study. Medicine (Baltimore) 2016;95:e4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mansour D, McPherson S. Management of decompensated cirrhosis. Clin Med (Lond) 2018;18:s60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60. [DOI] [PubMed] [Google Scholar]
- [43].DRUGABUSE.COM. Should People Who Abuse Alcohol Qualify for Liver Transplants? 2020. Available at: https://drugabuse.com/should-people-who-abuse-alcohol-qualify-for-liver-transplants/ (accessed 17 February 2020).
- [44].Marroni CA, Fleck AM Jr, Fernandes SA et al. Liver transplantation and alcoholic liver disease: history, controversies, and considerations. World J Gastroenterol 2018;24:2785–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 2019;393:2493–502. [DOI] [PubMed] [Google Scholar]
- [46].Global Health Data Exchange (GHDx). GBD Results Tool. 2020 for results of the GBD 2017 Study. Seattle, DC: Institute for Health Metrics and Evaluation, 2019. Available at: http://ghdx.healthdata.org/gbd-results-tool (accessed 17 February 2020). [Google Scholar]
- [47].Rovira P, Rehm J. Population alcohol use distribution among drinkers based on a known mean consumption. Barcelona: Agència de Salut Pública de Catalunya, 2020. (March). Available at: http://drogues.gencat.cat/web/.content/minisite/drogues/professionals/health-metrics/Population-alcohol-use-distribution-among-drinkers-based-on-a-known-mean-consumption_ok.pdf (accessed 30 March 2020). [Google Scholar]
- [48].European Medicines Agency. Guideline on the development of medicinal products for the treatment of alcohol dependence; 2010. Available at: https://www.ema.europa.eu/documents/scientific-guideline/guideline-development-medicinal-products-treatment-alcohol-dependence_en.pdf (accessed 16 February 2019).
- [49].Patra J, Buckley C, Kerr W, Brennan A, Pursehouse R, Rehm J. Impact of body mass index and alcohol consumption on all-cause and liver mortality in 240,000 adults in the United States. Toronto, ON, Canada; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shield K, Manthey J, Rylett M et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health 2020;5:e51–61. [DOI] [PubMed] [Google Scholar]
- [51].Turati F, Galeone C, Rota M et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol 2014;25:1526–35. [DOI] [PubMed] [Google Scholar]
- [52].World Cancer Research Fund International, American Institute for Cancer Research. Continuous update project report: food, nutrition, physical activity, and the prevention of liver cancer. London: World Cancer Research Fund International, 2015. [Google Scholar]
- [53].Grundy A, Poirier AE, Khandwala F, McFadden A, Friedenreich CM, Brenner DR. Cancer incidence attributable to alcohol consumption in Alberta in 2012. CMAJ Open 2016;4:E507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Leitao J, Chandramohan D, Byass P et al. Revising the WHO verbal autopsy instrument to facilitate routine cause-of-death monitoring. Glob Health Action 2013;6:21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].PyPI – Python Community. dismod-mr 1.1.1 Project description (Released 6 November 2019); 2019. Available at: https://pypi.org/project/dismod-mr/ (accessed 16 February 2020).
- [56].Rehm J, Klotsche J, Patra J. Comparative quantification of alcohol exposure as risk factor for global burden of disease. Int J Methods Psychiatr Res 2007;16:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kehoe T, Gmel G, Shield KD, Gmel G, Rehm J. Determining the best population-level alcohol consumption model and its impact on estimates of alcohol-attributable harms. Popul Health Metr 2012;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rehm J, Kehoe T, Gmel G, Stinson F, Grant B, Gmel G. Statistical modeling of volume of alcohol exposure for epidemiological studies of population health: the US example. Popul Health Metr 2010;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum 1953;9:531–41. [PubMed] [Google Scholar]
- [60].Poznyak V, Fleischmann A, Rekve D, Rylett M, Rehm J, Gmel G. The World Health Organization’s global monitoring system on alcohol and health. Alcohol Res 2013;35:244–9. [PMC free article] [PubMed] [Google Scholar]
- [61].Rehm J, Ashley MJ, Room R et al. On the emerging paradigm of drinking patterns and their social and health consequences. Addiction 1996;91:1615–21. [PubMed] [Google Scholar]
- [62].Rehm J, Roerecke M. Patterns of drinking and liver cirrhosis - what do we know and where do we go? J Hepatol 2015;62:1000–1. [DOI] [PubMed] [Google Scholar]
- [63].Rehm J, Greenfield TK, Kerr W. Patterns of drinking and mortality from different diseases—an overview. Contemp Drug Probl 2006;33:205–35. [Google Scholar]
- [64].Simpson RF, Hermon C, Liu B et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UKMillion Women Study. Lancet Public Health 2019;4:e41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Askgaard G, Gronbaek M, Kjaer MS, Tjonneland A, Tolstrup JS. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol 2015;62:1061–7. [DOI] [PubMed] [Google Scholar]
- [66].Kerr WC, Fillmore KM, Marvy P. Beverage-specific alcohol consumption and cirrhosis mortality in a group of English-speaking beer-drinking countries. Addiction 2000;95:339–46. [DOI] [PubMed] [Google Scholar]
- [67].Rehm J, Hasan OSM. Is burden of disease differentially linked to spirits? A systematic scoping review and implications for alcohol policy. Alcohol 2019;82:1–10. [DOI] [PubMed] [Google Scholar]
- [68].World Health Organization. Fact Sheet: Hepatitis B. 2018. Available at: http://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed 13 March 2018).
- [69].Puffer R, Griffith G. Patterns of urban mortality, scientific publication no. 151. Washington, DC: Pan American Health Organization, 1967. [Google Scholar]
- [70].Andreev E, Zbarskaja I. Alkogol’ kak prichina smerti. [Alcohol as cause of death]. Demoskop Weekly; 2010;425/426:1. [Google Scholar]
- [71].Tuusov J, Lang K, Väli M et al. Prevalence of alcohol-related pathologies at autopsy: Estonian forensic study of alcohol and premature death. Addiction 2014;109:2018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rehm J, Hasan OSM, Imtiaz S, Neufeld M. Quantifying the contribution of alcohol to cardiomyopathy: a systematic review. Alcohol 2017;61:9–15. [DOI] [PubMed] [Google Scholar]
- [73].Lange S, Roerecke M, Rehm J. For most fully alcohol-attributable diagnoses in the ICD, the etiological specification should be removed. Adicciones 2020;32:90–3. [DOI] [PubMed] [Google Scholar]
- [74].Badham J, Chattoe-Brown E, Gilbert N, Chalabi Z, Kee F, Hunter RF. Developing agent-based models of complex health behaviour. Health Place 2018;54:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nianogo RA, Arah OA. Agent-based modeling of noncommunicable diseases: a systematic review. Am J Public Health 2015;105:e20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].European Association for the Study of the Liver (EASL) & European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- [77].Rehm J, Roerecke M. Reduction of drinking in problem drinkers and all-cause mortality. Alcohol Alcohol 2013;48:509–13. [DOI] [PubMed] [Google Scholar]
- [78].McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev 2011;8:CD005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rehm J, Shield KD, Gmel G, Rehm MX, Frick U. Modeling the impact of alcohol dependence on mortality burden and the effect of available treatment interventions in the European Union. Eur Neuropsychopharmacol 2013;23:89–97. [DOI] [PubMed] [Google Scholar]
- [80].Chisholm D, Moro D, Bertram M et al. Are the “best buys” for alcohol control still valid? An update on the comparative cost-effectiveness of alcohol control strategies at the global level. J Stud Alcohol Drugs 2018;79:514–22. [PubMed] [Google Scholar]
- [81].Rehm J, Barbosa C. The cost-effectiveness of therapies to treat alcohol use disorders. Expert Rev Pharmacoecon Outcomes Res 2018;18:43–9. [DOI] [PubMed] [Google Scholar]
- [82].Nutt DJ, Rehm J. Doing it by numbers: a simple approach to reducing the harms of alcohol. J Psychopharmacol 2014;28:3–7. [DOI] [PubMed] [Google Scholar]
- [83].Rehm J, Marmet S, Anderson P et al. Defining substance use disorders: do we really need more than heavy use? Alcohol Alcohol 2013;48:633–40. [DOI] [PubMed] [Google Scholar]
- [84].Babor TF, Caetano R, Casswell S et al. Alcohol: no ordinary commodity. Research and public policy, 2nd edn. Oxford: Oxford University Press, 2010. [Google Scholar]
- [85].Case A, Deaton A. Deaths of despair and the future of capitalism. Princeton, NJ: Princeton University Press, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


