Abstract
Aims
Adults with pelvic floor disorders commonly present with overlapping bladder and bowel symptoms; however, the relationship between urinary and defecatory dysfunction is not well understood. Our aim was to compare and determine if overlapping brain regions are activated during bladder filling and rectal distention in healthy adults.
Methods
We conducted separate Pubmed searches for neuroimaging studies investigating the effects of rectal distention and bladder filling on brain activation in healthy subjects. Coordinates of activated regions were extracted with cluster level threshold p<0.05 and compared using the activation likelihood estimate (ALE) approach. Results from the various studies were pooled and a contrast analysis was performed to identify any common areas of activation between bladder filling and rectal distension.
Results
We identified 96 foci of activation from 14 neuroimaging studies on bladder filling and 182 foci from 17 studies on rectal distension in healthy adults. Regions activated during bladder filling included right insula, right and left thalamus, and right periaqueductal grey. Regions activated during rectal distention included right and left insula, right and left thalamus, left postcentral gyrus, and right inferior parietal lobule. Contrast analysis revealed common activation of the right insula with both rectal distention and bladder filling.
Conclusion
Bladder filling and rectal distention activate several separate areas of the brain involved in sensory processing in healthy adults. The common activation of the insula, the region responsible for interoception, in these two conditions may offer an explanation for the coexistence of bladder and defecatory symptoms in pelvic floor disorders.
Keywords: bladder filling, rectal distention, neuroimaging, insula, interoception, pelvic floor disorders
Introduction
Subjects with pelvic floor disorders commonly present with overlapping bladder and bowel symptoms. Prior studies suggest that reported rates of overlap in adults with pelvic floor disorders is as high as 60% [1]. For example, urinary symptoms such as urgency, frequency, urinary incontinence, and sense of incomplete emptying are common in both men and women with constipation and fecal incontinence [1]. Urinary symptoms such as frequency, incontinence, and nocturia are also common in patients with irritable bowel syndrome (IBS) [2]. Similarly, bowel symptoms such as constipation and fecal incontinence are common in adults with urinary incontinence [3, 4]. However, the mechanism for why urinary and defecatory symptoms coexist in adults with pelvic floor disorders is not well understood. Proposed mechanisms include the physical proximity of the bladder and rectum, resulting in mechanical obstruction of one organ by the distention of the other; however, this model only explains coexistence of symptoms in conditions such as constipation. Some authors have suggested a potential neurologic basis for the coexistence of bowel and bladder symptoms, however, the precise mechanism has not been elucidated [1].
Sensory input from the bowel and bladder are processed in specific regions of the brain. A potential mechanism for overlapping urinary and bowel symptoms may be that afferent input from both rectal distension and bladder filling activates similar regions of the brain. An understanding of brain response to the processes of urine storage and rectal distention in healthy adults could advance our understanding of mechanisms of disease in patients suffering from pelvic floor disorders.
Various neuroimaging techniques have identified brain regions activated during bladder filling or rectal distention both in healthy subjects and in subjects with pathologic conditions such as irritable bowel syndrome and overactive bladder [5, 6]. In order to analyze neuroimaging data, the activation likelihood estimate (ALE) meta-analytic approach is often used. This method incorporates the brain coordinates from individual studies to determine the statistical probability of a brain region being activated during a specific task [7]. Prior studies using ALE approach have reported on brain regions activated in several conditions such as sleep disorders [8], depression [9], and irritable bowel syndrome [6]. Using ALE meta-analysis, we have previously reported on regions of the brain activated during bladder filling in healthy adults [10]. However, a contrast analysis in which regions of the brain activated during bladder and rectal filling are compared has not been performed.
The aim of our study was to identify and compare brain regions activated during bladder filling and rectal distention in healthy adults. We hypothesized that there would be areas of overlap between bladder filling and rectal distention that could account for the common occurrence of bowel and bladder symptomatology.
Materials and Methods
We conducted two separate searches in the PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/) from years 1990 to 2016 for neuroimaging studies using functional magnetic resonance imaging (fMRI), positron emission tomography (PET), or single photon emission computed tomography (SPECT) scan that investigated the effects of rectal distention and bladder filling on brain activation in healthy subjects. In order to identify functional neuroimaging studies of rectal distention, the keywords “rectal distention”, “rectal filling”, “rectal stimulation”, “defecation” and “brain”, “brain activity”, and “cerebral activation” were combined with “MRI”, “PET, or “brain imaging”. For bladder filling, the keywords “bladder filling”, “micturition”, “brain”, and “cerebral activation” were combined with “MRI”, “PET”, and “SPECT”. Abstracts of the retrieved results were reviewed for relevance, and bibliographies were reviewed to ensure all pertinent studies were included. Inclusion criteria were studies with 1) healthy human subjects age >18 years that were imaged during rectal distention and bladder filling; 2) coordinates differentiating between empty bladder and full bladder and empty rectum and full rectum reported in a standard reference space (i.e., Talairach or Montreal Neurological Institute). Neuroimaging studies in disease (such as irritable bowel syndrome or interstitial cystitis) were only included if brain coordinates were reported separately for the healthy (control) group. Our exclusion criteria were 1) case reports, review articles, book chapters, letters to the editor, and studies published in non-English languages 2) studies that did not report the numerical coordinates of the activated brain regions and 3) studies in which bladder or rectal filling was associated with an additional task (such as concurrent auditory stimulation or injection of an intravenous medication).
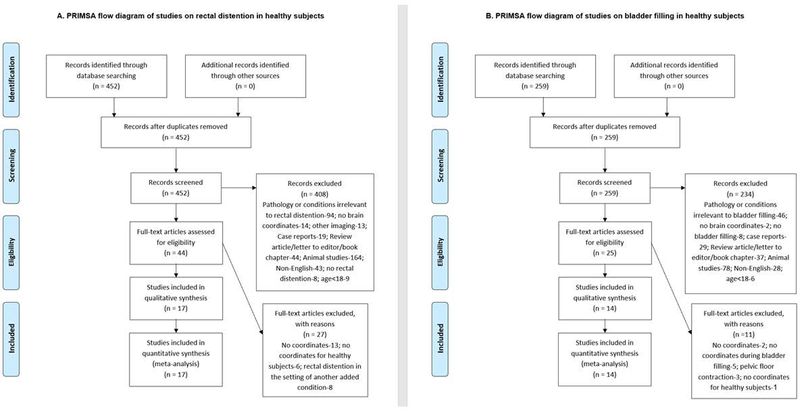
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram for studies on bladder filling is outlined in Figure 1 and for rectal distension in Figure 2. In the included studies of bladder filling, bladder filling was conducted via a transurethral catheter in 9 studies [11–19], oral intake in 4 studies [20–23], and diuretic injection in 1 study [24]. In studies involving rectal filling, the rectum was distended using a rectal balloon on the end of a catheter that was inflated via pressure-controlled barostat in 14 studies [25–38], mechanical air balloon inflation in 1 study [39], or manual inflation in 2 studies [40, 41].
Figure 1:
PRISMA flow diagram of studies on rectal distention in healthy subjects (A) and PRISMA flow diagram of studies on bladder filling in healthy subjects
Figure 2:
Brain regions activated during bladder filling (A) and rectal distention (B)
In neuroimaging studies, brain coordinates are reported using x, y, z coordinates in standard stereotaxic space in millimeters for the maximally significantly activated voxel where x is the lateral displacement from the midline, y is the anterior-posterior displacement relative to the anterior commissure, z is the vertical displacement relative to the anterior-posterior commissure line. In brief, Activation Likelihood Estimation (ALE) is a method of meta-analysis that accumulates coordinates from separate neuroimaging studies and synthesizes them in order to establish the statistical probability of activation of a particular brain region during a specific task [7]. An individual brain focus is regarded as a spatial probability distribution centered about a peak at the reported coordinate, instead of being treated as a single point. At each voxel, the ALE statistic is calculated, which denotes the probability that at least one of the activation foci is contained within a particular voxel. Performing this calculation at each voxel within the brain then generates an ALE map. To test the null hypothesis that the foci are uniformly distributed throughout the brain (i.e. the random clustering of foci is rejected), a nonparametric permutation test was performed [42].
We extracted coordinates of activated regions, represented in three dimensional space as x,y,z coordinates, with cluster level threshold set at p<0.05. We then conducted the meta-analyses via separate ALE analyses for bladder filling and rectal distention coordinates. The current version of the GingerALE version 2.3.6 (http://brainmap.org/ale, Research Imaging Institute of the University of Texas Health Science Center, San Antonio, Texas) was used to evaluate the coordinates for topographic convergence in the MNI reference space. Prior to conducting the ALE analysis, coordinates reported in Talariach space were converted to MNI reference space with the use of the Lancaster transformation, which is integrated into the GingerALE program [43]. Results from the various studies were pooled and a contrast analysis was performed to identify any common areas of activation between bladder filling and rectal distension using GingerALE. In the contrast analysis, the coordinates were randomly divided and paired. The pairings were then subtracted 10,000 times in order to ascertain the differences between subjects during rectal distention and subjects during bladder filling at a 1/4 0.05 with a significance threshold was set at P < 0.05. A cluster-size threshold was used to correct for multiple comparisons with 1,000 permutations for the calculations.
Results
We identified 14 studies examining areas of the brain activated during bladder filling and 17 studies investigating rectal distention that met the inclusion criteria (Table 1A and 1B, respectively). Of studies investigating bladder filling, 7 used fMRI, 6 used PET, and one used SPECT. These studies involved 197 subjects and yielded 96 foci of activation during bladder filling. All studies investigating rectal distention utilized fMRI. These studies provided 182 foci of activation from 284 subjects.
Table 1A:
Studies included in the meta-analysis of brain activation during rectal distention
| Author | Year | Imaging Modality | Subjects (number, sex) | Method of Rectal Distention | Subjects’ age range (mean or median, years) |
|---|---|---|---|---|---|
| Lotze | 2001 | fMRI | 8 females | Manual air inflation of balloon | 28–54 (37.3) |
| Hobday | 2001 | fMRI | 8 males | Mechanical air balloon inflation | 21–39 |
| Berman | 2006 | fMRI | 6 females, 7 males | Pressure-controlled barostat | 33.1 ± 10.9 (males); 34.8 +/− 7.9 (females) |
| Eickhoff | 2006 | fMRI | 4 females, 4 males | Manual air inflation of balloon | 37.3 |
| Song | 2006 | fMRI | 12 females | Pressure-controlled barostat | 23 |
| Rosenberger | 2009 | fMRI | 14 females | Pressure-controlled barostat | 32.78 ± 8.38 |
| Moisset | 2010 | fMRI | 11 females | Pressure-controlled barostat | 38.4 ±3.1 |
| Hall | 2010 | fMRI | 6 females | Pressure-controlled barostat | 30–40 |
| Elsenbruch | 2010 | fMRI | 12 females | Pressure-controlled barostat | 31.4 |
| Benson | 2012 | fMRI | 15 females, 15 males | Pressure-controlled barostat | 26.1 ± 8.3 (females); 25.4±3.8 (males) |
| Smith | 2011 | fMRI | 14 females | Pressure-controlled barostat | 19–53 (29) |
| Kotsis | 2012 | fMRI | 18 females, 18 males | Pressure-controlled barostat | 25.2 and 27.1 |
| Schmid | 2013 | fMRI | 18 females, 18 males | Pressure-controlled barostat | 35.3 and 24.1 |
| Bouhassira | 2013 | fMRI | 11 females | Pressure-controlled barostat | 41.5±12.8 |
| Rubio | 2015 | fMRI | 9 females, 6 males | Pressure-controlled barostat | 21–43 (24) |
| Schmid | 2015 | fMRI | 14 females and 14 males | Pressure-controlled barostat | 24 |
| Benson | 2015 | fMRI | 26 males | Pressure-controlled barostat | 26.3 ± 0.7 |
Table 1B:
Studies included in the meta-analysis of brain activation during bladder filling
| Author | Year | Imaging Modality | Subjects (number, sex) | Method of Bladder Filling | Subjects’ age range (mean, years) |
|---|---|---|---|---|---|
| Blok | 1997 | PET | 10 males | Oral intake | 21–50 (32) |
| Blok | 1998 | PET | 18 females | Oral intake | 20–51 (27) |
| Nour | 2000 | PET | 12 males | Catheter | 22–25 (23.4) |
| Athwal | 2001 | PET | 11 males | Catheter | 19–54 |
| Matsuura | 2002 | PET | 17 males | Catheter | 24–41 (30) |
| Yin | 2006 | SPECT | 15 males | Diuretic injection | 24–45 (32.7) |
| Mehnert | 2008 | fMRI | 8 females | Catheter | 21–27 (24.3) |
| Takao | 2008 | PET | 6 males | Oral intake | 31–40 |
| Tadic | 2008 | fMRI | 10 females | Catheter infusion-withdrawal cycles | 60–71 (65) |
| Griffiths | 2009 | fMRI | 10 females | Catheter infusion-withdrawal cycles | 30–79 |
| Tadic | 2013 | fMRI | 11 females | Catheter infusion-withdrawal cycles | 60–71 (65) |
| Krhut | 2014 | fMRI | 19 females | Catheter infusion-withdrawal cycles | 20–68 |
| Nardos | 2014 | fMRI | 20 females | Catheter infusion-withdrawal cycles | 40–64 |
| Gao | 2015 | fMRI | 22 females, 8 males | Oral intake | 24–49 (29.8) |
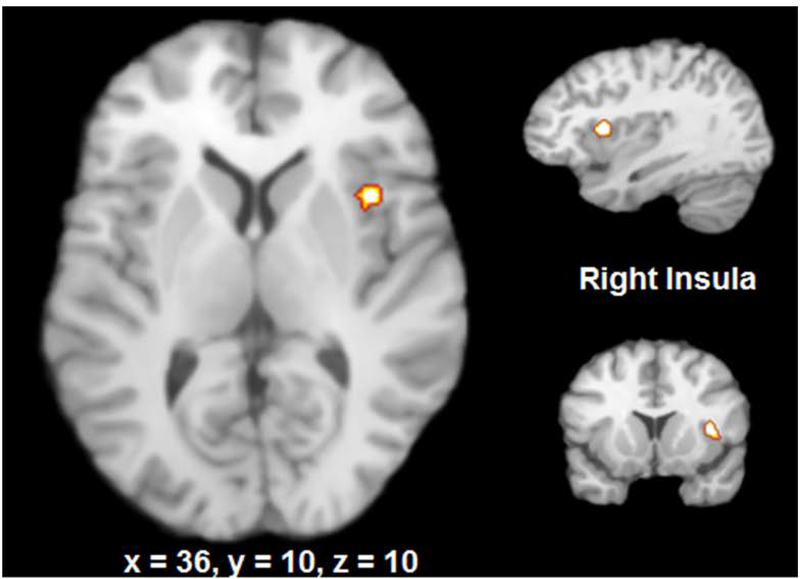
ALE analysis demonstrated the following regions of activation in response to bladder filling: right insula, right and left thalamus, and right periaqueductal grey (Figure 3A). The specific coordinates for these regions are reported in Table 2. In response to rectal distention, the activated clusters were in the right and left insula, right and left thalamus, left postcentral gyrus, right inferior parietal lobule, inferior frontal gyrus, and amygdala (Figure 3B and Table 2).
Figure 3:
Common brain regions activated in bladder filling and rectal distention
Table 2:
Brain Regions Activated During Bladder Filling and Rectal Filling
| BLADDER FILLING | Brain Region | Volume (mm3) | x | y | z | ALE value |
| Bilateral thalamus | 968 | 4 | −6 | 12 | 0.013750 | |
| −4 | −8 | 12 | 0.012373 | |||
| Right anterior insula | 896 | 38 | 10 | 10 | 0.014993 | |
| Right periaqueductal gray (midbrain) | 704 | 10 | −22 | −10 | 0.012906 | |
| Rostral pons | 704 | −2 | −20 | −16 | 0.009082 | |
| RECTAL DISTENTION | Brain Region | Volume (mm3) | x | y | z | ALE value |
| Right anterior insula | 4728 | 34 | 28 | 0 | 0.013759 | |
| Left insula | 1872 | −38 | 14 | 0 | 0.029081 | |
| Bilateral thalamus | 1848 | −4 | −24 | 0 | 0.016269 | |
| 2 | −16 | 2 | 0.012333 | |||
| Left postcentral gyrus | 1264 | −62 | −24 | 20 | 0.021144 | |
| Right postcentral gyrus | 696 | 50 | −42 | 54 | 0.018775 | |
| Right parietal lobule | 688 | 60 | −30 | 30 | 0.013953 | |
| 58 | −34 | 38 | 0.013788 |
Contrast analysis revealed common activation of the right insula in rectal distention and bladder filling.
Discussion
Our study demonstrates that experimental bladder filling and rectal distension activate several distinct regions of the brain; however, both tasks result in activation of the insula. This common activation of the insula during bladder and rectal distension may offer an explanation for the overlap in bowel and bladder symptoms in adults with pelvic floor disorders.
The insula, an area of the cerebral cortex that lies deep to the lateral sulcus, plays a vital role in interoception, the sense of the physiologic state of the body. Afferent homeostatic information from the viscera, such as heart rate, respiration, digestive processes, and micturition are mapped to the insula such that the insula has been called the “sensory cortex of the autonomic nervous system” [44, 45]. Functional MRI studies in healthy women have shown that the degree of activation of the anterior insula increases as the volume of bladder filling increases and the desire to void became more intense [5, 19]. Similarly, the insula processes the awareness of rectal filling and desire to defecate in healthy adults [6]. Our study shows that afferent sensation from the bladder and rectum are registered in the ventroanterior insula, a region that has also been implicated in the experience of disgust, a negative reaction that likely prompts evacuation of the bladder and/or rectum [46]. Co-activation of the insula by bladder and rectal filling may also explain why defecation is frequently associated with micturition in humans.
Our findings help to explain the common coexistence of bowel and bladder symptoms in adults with pelvic floor disorders. Greater activation of the insula has been reported with bladder filling in women with overactive bladder than healthy controls [5, 44]. Similarly, greater activation of the insula with rectal distension has been reported in adults with irritable bowel syndrome [6]. Increased activation of the insula in adults with pelvic floor disorders may represent abnormal perception of physiologic happenings within the body resulting in pathologic symptoms [44]. Examples of such abnormalities include a heightened sense of urgency in adults with overactive bladder and an awareness of gastric motility or peristaltic activity in subjects with irritable bowel syndrome [5, 44]. Since the insula is also part of the limbic system or the emotional arousal circuit, recent treatments for functional bladder and bowel syndromes have focused on the reduction of anxiety associated with urinary and gastrointestinal symptoms [44]. Our findings that overlapping regions of the insula are involved in the sensory processing of bladder and bowel symptoms suggests that functional bowel and bladder disorders may have a common underlying mechanism and treatments that reduce anxiety associated with bladder symptoms could also benefit co-existing bowel symptoms.
Our study also reveals important differences in the processing of afferent sensations from the bladder and the rectum. Though the thalamus was activated during both processes, bladder filling was associated with activation of the mediodorsal nuclei while the ventral anterior nuclei were activated during rectal distension. The thalamus serves as a “relay center” for sensory information and determines which signals from the bladder or the rectum should reach the insula and somatosensory cortex for processing. As a key component of the attention network, the thalamus does not allow afferent sensations of bladder or the rectum to reach the cortex until an adequate degree of bladder or rectal filling has been achieved [47]. The ventral anterior thalamic nuclei that are activated during rectal distension integrate sensory information from the limbic system and viscera. The mediodorsal nucleus that is activated during bladder filling contributes to “adaptive decision-making” and associative learning. Given that hypofunction of the dorsomedial nuclei results in “cognitive inflexibility”, a condition where individuals are unable to appropriately use stimuli within their surroundings to adjust their behavior, hypofunction of these nuclei could potentially contribute to symptoms such as “key in the door” in adults with overactive bladder [48]. Greater insight into the differential role of thalamic nuclei in the processing of afferent sensations from the bladder and rectum may provide greater insight into pelvic floor disorders such as urinary or fecal urgency.
We also identified other important differences between regions of the brain activated during rectal distension and bladder filling. In the case of rectal distension, we identified several regions known to be involved in rectal filling such as amygdala, postcentral gyrus and the parietal lobule. The postcentral gyrus in the somatosensory cortex receives afferent information from the anal canal via the thalamus, such as the duration and strength of noxious stimuli [49]. The primary somatosensory cortex is disproportionately activated during rectal distention in female subjects compared to males, possibly accounting for female predominance of irritable bowel syndrome [49]. The inferior parietal lobule receives afferent input from the limbic system as well as the viscera and serves a significant function in the processing of sensory attention [50]. As a part of the emotional arousal network, the amygdala helps to integrate the emotional experience into visceral responses, such as anxiety or fear of a certain sensation [6]. Activation of the amygdala has been reported with bladder filling in patients with irritable bowel syndrome and bladder pain syndrome. Our meta-analysis identified activation of the amygdala with rectal filling but not with bladder filling in healthy adults. This may be because the amygdala is a deep subcortical structure that is difficult to visualize. Alternatively, it is possible that rectal distension is more likely to arouse fear and anxiety than bladder filling. Studies that compare brain activations during bladder and rectal filling in the same subject are required to determine differences in afferent signaling from the bladder and rectum.
Our study has several limitations. These include heterogeneity of neuroimaging techniques and differences in protocols for bladder and rectal filling. The method of bladder filling and rectal distention as well as the extent of filling or distention (first desire vs. normal or strong desire and varying thresholds of rectal distention) was not standardized across studies; this could have resulted in variation in reported activated areas. Similarly, differences in neuroimaging techniques could have limited our ability to identify common regions involved in sensory processing from the bladder and the rectum. In spite of these limitations, our meta-analysis allowed us to integrate the findings of existing studies and identify key regions of the brain that could potentially be targeted in the treatment of dysfunctional voiding.
Conclusions
The common activation of the insula during bladder filling and rectal distention may offer an explanation for the coexistence of bladder and defecatory symptoms in pelvic floor disorders. This area of the brain may be a target for future treatment of bowel and bladder dysfunction.
Acknowledgments
Conflicts of Interest and Source of Funding:
UU Andy: No conflicts of interest, Financial Disclaimer: Dr. Andy is supported by a grant from the National Institute of Aging (R03-AG-053277-01, PI: Andy).
H Rao: UU Andy: No conflicts of interest, Financial Disclaimer: Dr. Rao is supported by a grant from the National Institutes of Health - National Heart, Lung, and Blood Institute (R01-HL102119). The remaining authors report no conflicts of interest.
References
- 1.Wyndaele M, D.W.B., Pelckmans PA, De Wachter S, Van Outryve M, Wyndaele JJ., Exploring associations between lower urinary tract symptoms (LUTS) and gastrointestinal (GI) problems in women: a study in women with urological and GI problems vs a control population. BJU Int., 2015. Jun. 115(6):958–67.. [DOI] [PubMed] [Google Scholar]
- 2.Guo YJ, H.C., Chen SC, Yang SS, Chiu HM, Huang KH., Lower urinary tract symptoms in women with irritable bowel syndrome. Int J Urol, 2010. 17(2):175–81. [DOI] [PubMed] [Google Scholar]
- 3.Jelovsek JE, B.M., Paraiso MF, Walters MD., Functional bowel and anorectal disorders in patients with pelvic organ prolapse and incontinence. Am J Obstet Gynecol, 2005. 193(6):2105–11. [DOI] [PubMed] [Google Scholar]
- 4.Helfand BT, S.A., Lai HH, Yang CC, Gore JL, Erickson BA, Kreder KJ, Cameron AP, Weinfurt KP, Griffith JW, Lentz A, Talaty P, Andreev VP, Kirkali Z; LURN, Prevalence and Characteristics of Urinary Incontinence in a Treatment Seeking Male Prospective Cohort: Results from the LURN Study. J Urol, 2018. 200(2):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths D, D.S., Stenger A, et al. , Brain control of normal and overactive bladder. J Urol 2005. 174:1862–7. [DOI] [PubMed] [Google Scholar]
- 6.Tillisch K, M.E., Labus JS., Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome.. Gastroenterology, 2011. 140:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eickhoff SB, L.A., Grefkes C, et al. , Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp, 2009. 30:2907–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma N, D.D., Basner M, Rao H, How acute total sleep loss affects the attending brain: a meta-analysis of neuroimaging studies. Sleep, 2015. 38:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald PB, L.A., Maller J, Daskalakis ZJ, A meta-analytic study of changes in brain activation in depression [Erratum]. Hum Brain Mapp 2008. 29(6):736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arya NG, W.S., Xu S, Rao H, Brain activation in response to bladder filling in healthy adults: An activation likelihood estimation meta-analysis of neuroimaging studies. Neurourol Urodyn, 2017. 2;36(4):960–965. [DOI] [PubMed] [Google Scholar]
- 11.Athwal BS, B.K., Hussain I, et al. , Brain responses to changes in bladder volume and urge to void in healthy men. Brain, 2001. 124:369–77. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths DJ, T.S., Schaefer W, Resnick NM., Cerebral control of the lower urinary tract: how age-related changes might predispose to urge incontinence. Neuroimage., 2009. 7(3):981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krhut J, H.P., Tintera J, et al. , Brain activity during bladder filling and pelvic floor muscle contractions: A study using functional magnetic resonance imaging and synchronous urodynamics. Int J Urol, 2014. 21:169–74. [DOI] [PubMed] [Google Scholar]
- 14.Matsuura S, K.H., Mitsui T, et al. , Human brain region response to distention or cold stimulation of the bladder: A positron emission tomography study. J Urol, 2001. 168:2035–9. [DOI] [PubMed] [Google Scholar]
- 15.Nardos R, G.W., Krisky C, et al. Examining mechanisms of brain control of bladder function with resting state functional connectivity MRI, Examining mechanisms of brain control of bladder function with resting state functional connectivity MRI. Neurourol Urodyn 2014. 33:493–501. [DOI] [PubMed] [Google Scholar]
- 16.Nour S, S.C., Kristensen JK, et al. , Cerebral activation during micturition in normal men.. Brain, 2000. 123:781–9. [DOI] [PubMed] [Google Scholar]
- 17.Tadic SD, T.C., Resnick NM, et al. , Brain responses to bladder filling in older women without urgency incontinence. Neurourol Urodyn, 2013. 32:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehnert U, B.S., Svensson J, et al. , Brain activation in response to bladder filling and simultaneous stimulation of the dorsal clitoral nerve—an fMRI study in healthy women. Neuroimage, 2008. 41:682–9. [DOI] [PubMed] [Google Scholar]
- 19.Tadic SD, G.D., Schaefer W, et al. , Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. Neuroimage, 2008. 39:1647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blok B, S.L., Holstege G, Brain activation during micturition in women. Brain, 1998. 121:2033–42. 36.. [DOI] [PubMed] [Google Scholar]
- 21.Blok B, W.A., Holstege G, A PET study on brain control of micturition in humans. Brain, 1997. 120:111–21. 35. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, L.L., Blok BF, A resting-state functional MRI study on central control of storage: Brain response provoked by strong desire to void. Int Urol Nephrol, 2015. 47:927–35. [DOI] [PubMed] [Google Scholar]
- 23.Takao T, T.A., Miyagawa Y, et al. , Brain responses during the first desire to void: A positron emission tomography study.. Int J Urol, 2008. 15:724–8. [DOI] [PubMed] [Google Scholar]
- 24.Yin Y, S.N., Okizaki A, et al. , Cerebral activation during withholding urine with full bladder in healthy men using 99mTc-HMPAO SPECT. J Nucl Med, 2006. 47:1093–8. [PubMed] [Google Scholar]
- 25.Benson S, K.V., Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER, Elsenbruch S, Behavioural and neural correlates of visceral pain sensitivity in healthy men and women: does sex matter? Eur J Pain., 2012. 16(3):349–58. [DOI] [PubMed] [Google Scholar]
- 26.Benson S, R.L., Wegner A, Kleine-Borgmann J, Engler H, Schlamann M, Forsting M, Schedlowski M, Elsenbruch S., Neural circuitry mediating inflammation-induced central pain amplification in human experimental endotoxemia. Brain Behav Immun., 2015. 48:222–31. [DOI] [PubMed] [Google Scholar]
- 27.Berman SM,, N.B., Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA., Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol., 2006. 291(2):R268–76. [DOI] [PubMed] [Google Scholar]
- 28.Elsenbruch S, R.C., Bingel U, Forsting M, Schedlowski M, Gizewski ER., Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology., 2010. 139(4):1310–9. [DOI] [PubMed] [Google Scholar]
- 29.Hall GB, K.M., Collins S, Ganguli S, Spaziani R, Miranda KL, Bayati A, Bienenstock J., Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterol Motil., 2010. 22(3):276–e80.. [DOI] [PubMed] [Google Scholar]
- 30.Kotsis V, B.S., Bingel U, Forsting M, Schedlowski M, Gizewski ER, Elsenbruch S., Neurogastroenterol Motil. Perceived treatment group affects behavioral and neural responses to visceral pain in a deceptive placebo study., 2012. 24(10):935–e462. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberger C, E.S., Scholle A, de Greiff A, Schedlowski M, Forsting M, Gizewski ER., Effects of psychological stress on the cerebral processing of visceral stimuli in healthy women. Neurogastroenterol Motil, 2009. 21(7):740–e45. [DOI] [PubMed] [Google Scholar]
- 32.Rubio A, V.O.L., Pellissier S, Ly HG, Dupont P, Lafaye de Micheaux H, Tack J, Dantzer C, Delon-Martin C, Bonaz B., Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system--a fMRI study in healthy volunteers. Neuroimage., 2015. 107:10–22. [DOI] [PubMed] [Google Scholar]
- 33.Schmid J, B.U., Ritter C, Benson S, Schedlowski M, Gramsch C, Forsting M, Elsenbruch S., Neural underpinnings of nocebo hyperalgesia in visceral pain: A fMRI study in healthy volunteers. Neuroimage, 2015. 15;120:114–22. [DOI] [PubMed] [Google Scholar]
- 34.Schmid J, T.N., Gaß F, Benson S, Gramsch C, Forsting M, Gizewski ER, Elsenbruch S, Neural mechanisms mediating positive and negative treatment expectations in visceral pain: a functional magnetic resonance imaging study on placebo and nocebo effects in healthy volunteers. Pain, 2013. 154(11):2372–80. [DOI] [PubMed] [Google Scholar]
- 35.Smith JK, H.D., Head KE, Bush D, White TP, Stevenson CM, Brookes MJ, Marciani L, Spiller RC, Gowland PA, Francis ST., fMRI and MEG analysis of visceral pain in healthy volunteers. Neurogastroenterol Motil, 2011. 23(7):648–e260. [DOI] [PubMed] [Google Scholar]
- 36.Song GH, V.V., Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH., Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain, 2006. 15;126(1–3):79–90. [DOI] [PubMed] [Google Scholar]
- 37.Bouhassira D, M.X., Jouet P, Duboc H, Coffin B, Sabate JM., Changes in the modulation of spinal pain processing are related to severity in irritable bowel syndrome. Neurogastroenterol Motil., 2013. 25(7):623–e468. [DOI] [PubMed] [Google Scholar]
- 38.Moisset X, B.D., Denis D, Dominique G, Benoit C, Sabaté JM., Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur J Pain, 2010. 14(2):142–8. [DOI] [PubMed] [Google Scholar]
- 39.Hobday DI, A.Q., Thacker N, Hollander I, Jackson A, Thompson DG., A study of the cortical processing of ano-rectal sensation using functional MRI. Brain, 2001. 124(Pt 2):361–8. [DOI] [PubMed] [Google Scholar]
- 40.Eickhoff SB, L.M., Wietek B, Amunts K, Enck P, Zilles K., Segregation of visceral and somatosensory afferents: an fMRI and cytoarchitectonic mapping study. Neuroimage, 2006. 1;31(3):1004–14. [DOI] [PubMed] [Google Scholar]
- 41.Lotze M, W.B., Birbaumer N, Ehrhardt J, Grodd W, Enck P., Cerebral activation during anal and rectal stimulation. Neuroimage., 2001. 14(5):1027–34. [DOI] [PubMed] [Google Scholar]
- 42.Nichols TE, H.A., Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp, 2002. 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lancaster JL, T.-G.D., Martinez M, et al. , Bias between MNI and talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp, 2007. 28:1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komesu YM, K.L., Mayer AR, et al. , Functional MRI of the brain in women with overactive bladder: Brain activation during urinary urgency. Female Pelvic Med Reconstr Surg, 2011. 17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craig AD, Interoception: The sense of the physiological condition of the body. Curr Opin Neurobio, 2003. 13:500–5. [DOI] [PubMed] [Google Scholar]
- 46.Woolley JD, S.E., Sturm VE, et al. , Impaired Recognition and Regulation of Disgust Is Associated with Distinct but Partially Overlapping Patterns of Decreased Gray Matter Volume in the Ventroanterior Insula. Biol Psychiatry, 2015. 1;78(7):505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fowler CJ, G.D., de Groat WC, The neural control of micturition. Nat Rev Neurosci, 2008. 9:453–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parnaudeau S, T.K., Bolkan SS, Ward RD, Balsam PD, Kellendonk C, Mediodorsal thalamus hypofunction impairs flexible goal-directed behavior. Biol Psychiatry, 2015. 1;77(5):445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derbyshire SW, A systematic review of neuroimaging data during visceral stimulation. Am J Gastroenterol, 2003. 98(1):12–20. [DOI] [PubMed] [Google Scholar]
- 50.Rizzo G, M.D., Bertino S, et al. , The Limbic and Sensorimotor Pathways of the Human Amygdala: A Structural Connectivity Study. Neuroscience, 2018. 10;385:166–180. [DOI] [PubMed] [Google Scholar]