Abstract
Temperate Myxococcus xanthus phage Mx8 integrates into the attB locus of the M. xanthus genome. The phage attachment site, attP, is required in cis for integration and lies within the int (integrase) coding sequence. Site-specific integration of Mx8 alters the 3′ end of int to generate the modified intX gene, which encodes a less active form of integrase with a different C terminus. The phage-encoded (Int) form of integrase promotes attP × attB recombination more efficiently than attR × attB, attL × attB, or attB × attB recombination. The attP and attB sites share a common core. Sequences flanking both sides of the attP core within the int gene are necessary for attP function. This information shows that the directionality of the integration reaction depends on arm sequences flanking both sides of the attP core. Expression of the uoi gene immediately upstream of int inhibits integrative (attP × attB) recombination, supporting the idea that uoi encodes the Mx8 excisionase. Integrase catalyzes a reaction that alters the primary sequence of its gene; the change in the primary amino acid sequence of Mx8 integrase resulting from the reaction that it catalyzes is a novel mechanism by which the reversible, covalent modification of an enzyme is used to regulate its specific activity. The lower specific activity of the prophage-encoded IntX integrase acts to limit excisive site-specific recombination in lysogens carrying a single Mx8 prophage, which are less immune to superinfection than lysogens carrying multiple, tandem prophages. Thus, this mechanism serves to regulate Mx8 site-specific recombination and superinfection immunity coordinately and thereby to preserve the integrity of the lysogenic state.
Like other temperate bacteriophages, phage Mx8 of Myxococcus xanthus integrates into a preferred chromosomal locus, attB, to generate a linear prophage. Maintenance of the Mx8 prophage depends not only on the stability of its integrated state but also on the repression of phage lytic genes. Repression of the phage lytic genes confers a selective advantage upon the lysogenic host by conferring immunity to superinfection.
Surprisingly, lysogens with single Mx8 prophages maintain a lower level of immunity than lysogens with tandem prophages. A wild-type stock of Mx8 plates with efficiencies of 10−5 on lysogens with a single integrated copy of the Mx8 genome and <10−8 on lysogens with two or more tandem copies of the Mx8 genome. Thus, the relative level of superinfection immunity conferred upon a lysogen is dependent upon the number of integrated Mx8 prophages and presumably the dosage of the imm gene, which encodes the primary Mx8 repressor (29).
In the accompanying paper (19), we show that, unlike the case for most other temperate phages, the integration of Mx8 is unusual for two reasons. First, the Mx8 bacterial attachment locus, attB, contains two nearby attachment sites, attB1 and attB2. Plasmids with the Mx8 int-attP genes prefer to integrate into the attB1 site much more than into the attB2 site, and plasmid integration often is accompanied by a deletion between the attB1 and attB2 sites. Second, the Mx8 phage attachment site, attP, lies within the int coding sequence (34). Thus, integration of the Mx8 prophage results in a change in the primary structure of the int gene and generates a recombinant intX gene predicted to encode a product with a new C terminus.
To explain these unusual features of Mx8 site-specific recombination, we have explored the ability of integrase to mediate site-specific recombination between different pairs of attachment sites. We find that Mx8 Int can promote a variety of recombination events between different att sites with different efficiencies. We also show that the substitution of a new 3′ end of int upon the integration of Mx8 does not result in the inactivation of integrase but rather in a reduction in its specific activity. This novel genetic switch allows M. xanthus lysogens carrying single or multiple Mx8 prophages to coordinate the rate of prophage excision with the level of superinfection immunity.
MATERIALS AND METHODS
Bacterial strains.
The multiple-mutant strain M. xanthus DZ1 (5) is the preferred host for the growth of phage Mx8 and was used to assay plasmid integration. Escherichia coli JM107 (38) was used for the construction of plasmids and the preparation of plasmid DNA. Plasmids were constructed by standard cloning procedures (30) and introduced into E. coli (33) or M. xanthus (13) hosts by electroporation. Derivatives of M. xanthus DZ1 with integrated plasmids were grown in CTPM medium (36) with kanamycin (40 μg/ml) and/or the combination of spectinomycin (800 μg/ml) and streptomycin sulfate (1 mg/ml). Derivatives of E. coli with plasmids were grown in Luria-Bertani medium supplemented with ampicillin (100 μg/ml), kanamycin (40 μg/ml), or spectinomycin and streptomycin (50 μg/ml each). Antibiotics were from Sigma Chemical Co. Oligonucleotides used for plasmid construction and mutagenesis were made by Biosource Inc. Methods for the PCR amplification of M. xanthus genomic DNA with the attP, attB, attL, and attR primer pairs are described in the accompanying paper (19). Methods for the growth and assay of phage Mx8 have been described previously (20, 29).
Simple derivatives of plasmid pAY721.
Plasmids with portions of the Mx8 genome were derived from DNA isolated directly from the wild-type strain of Mx8 (21), from Kmr int-attP plasmid pAY721 (29), which is a 2.2-kilobase-pair (kb) subclone of Mx8 DNA in plasmid pBGS18 (31), or from amplified products of M. xanthus DZ1 chromosomal DNA and are listed in Table 1. Plasmid pAY952 (18) is an Spr Smr, integration-proficient derivative of plasmid pGB2 (6) with the same insert of Mx8 DNA as that in pAY721. Plasmid pAY980 is a derivative of pAY721 with a substitution of the 86-bp StuI-PstI fragment of pLITMUS28 for the StuI-PstI fragment internal to int (bp 5403 to 5851) (19). Plasmid pAY994, a Δint attP+ derivative of pAY721, contains the PstI-HindIII fragment representing the 959 bp at the 3′ end of int (bp 5851 to 6809). pAY994 was made by subcloning this fragment from pAY721 into pBGS18. The attP core sequence (bp 6447 to 6469) is located approximately in the middle of this fragment. pAY999 is the attR+ derivative of plasmid pAY721 with the intX gene in place of int (in which the int coding sequences represented by the P′ arm of attP, POP′, were replaced with those represented by the B′ arm of attB, BOB′). To construct pAY999, genomic DNA isolated from a DZ1 lysogen carrying the Mx8 prophage was amplified with 5′ primers having the sequences GGGAAGCTTGAATTCATAAAAGCCCGCCTCACCGAA and TCAGCGCTTCAGGTCCGGGACTGGGAC; the product containing attR was cleaved with NcoI and HindIII and ligated in place of the NcoI-HindIII fragment of pAY721 to replace the 3′ end of int. Plasmid pAY1131 was made by subcloning the PstI-HindIII fragment with attR from pAY999 into pBGS18. Plasmid pAY1300 is an otherwise isogenic derivative of pAY721 with the attR5 mutation. Primer pairs with the sequences CGGCTCTAGACCGGTACTCCTCGCCACCCCTGCCCCAGCAAGTT and GCGGTGCGCATCGGGGAGGCGT and the sequences CCGGTCTAGACGCGGTACTCCTGGGCTCCTTCTA and CCCAAGCTTCCTAGGTAGCGGAAGGGCTCTC were used to amplify plasmid pAY721, yielding products of 646 and 355 bp, respectively. The 646-bp product was cleaved with PstI and XbaI, and the 355-bp product was cleaved with XbaI and HindIII. The cleaved products were ligated to the PstI-HindIII backbone of pLITMUS28 to make plasmid pAY1299, and the smaller PstI-HindIII fragment of pAY1299 was ligated to the larger PstI-HindIII fragment of pAY721 to make pAY1300.
TABLE 1.
Plasmids
| Plasmid | bpa | Vectorb | Relevant genotype | Source or reference |
|---|---|---|---|---|
| pBGS18 | Kmr | 31 | ||
| pAY1099 | Spr Smr | 18 | ||
| pLITMUS28 | Apr | New England Biolabs | ||
| pLITMUS29 | Apr | New England Biolabs | ||
| pAY721 | 4585–6809 | pBGS18/Kmr | uoi+ int+ attP+ | This study |
| pAY952 | 4585–6809 | pGB2/Spr Smr | uoi+ int+ attP+ | This study |
| pAY980 | 4585–6809 | pBGS18/Kmr | uoi+ Δint-5403/5851 attP+ | 19 |
| pAY994 | 5851–6809 | pBGS18/Kmr | attP+ | This study |
| pAY999 | 4585–6469 | pBGS18/Kmr | uoi+ intX+ attR+ | This study |
| pAY1311 | 5851–6469 | pBGS18/Kmr | attR+ | This study |
| pAY1299 | 5851–6809 | pLITMUS28/Apr | attP5 | This study |
| pAY1300 | 4585–6809 | pBGS18/Kmr | attP5 | This study |
| pAY971 | 5073–6809 | pAY703/Kmr | int+-5085 | This study |
| pAY972 | 5208–6809 | pAY703/Kmr | int+-5208 | This study |
| pAY973 | 4979–6809 | pAY703/Kmr | uoi+ int+ | This study |
| pAY1301 | 5073–6809 | pAY1099/Spr Smr | int+-5085 | This study |
| pAY1302 | 5208–6809 | pAY1099/Spr Smr | int+-5208 | This study |
| pAY1303 | 4979–6809 | pAY1099/Spr Smr | uoi+ int+ | This study |
| pAY1304 | 5073–6809 | pAY1099/Spr Smr | int+-5085 attP5 | This study |
| pAY1305 | 5208–6809 | pAY1099/Spr Smr | int+-5208 attP5 | This study |
| pAY1306 | 4979–6809 | pAY1099/Spr Smr | uoi+ int attP5 | This study |
| pAY1307 | 5851–6469 | pLITMUS28/Apr | attR5 | This study |
| pAY1308 | 5073–6469 | pAY1099/Spr Smr | intX+-5085 attR5 | This study |
| pAY1309 | 5208–6469 | pAY1099/Spr Smr | intX+-5208 attR5 | This study |
| pAY1310 | 4979–6469 | pAY1099/Spr Smr | uoi+ intX+ attR5 | This study |
| pAY1048 | 5851–6469 | pBGS18/Kmr | ΔattP-1048 | This study |
| pAY1044 | 6398–6469 | pBGS18/Kmr | ΔattP-1044 | This study |
| pAY1046 | 6398–6809 | pBGS18/Kmr | ΔattP-1046 | This study |
| pAY1042 | 6447–6809 | pBGS18/Kmr | ΔattP-1042 | This study |
| pAY1041 | 6447–6469 | pBGS18/Kmr | ΔattP-1041 | This study |
| pAY1039 | pBGS18/Kmr | attB1+ attB2+ | This study | |
| pAY1037 | pLITMUS28/Apr | attL1+ | This study | |
| pAY1038 | pBGS18/Kmr | attL1+ | This study |
Coordinates of Mx8 DNA are those of GenBank accession no. U64984.
Antibiotic resistance determinants retained from the vector are indicated.
Plasmids that express int or intX (attP5) from the mgl promoter.
Plasmids pAY971, pAY972, and pAY973 express int from the constitutive mglBA promoter and are described in the accompanying paper (19). Spr Smr derivatives of these plasmids with the attP5 mutation were constructed in two steps. First, plasmids pAY971, pAY972, and pAY973 were cleaved with EcoRI and XbaI to yield DNA fragments of 2.9, 2.8, and 3.4 kb; these were ligated to the EcoRI-XbaI backbone of Spr Smr plasmid vector pAY1099 (18) to make pAY1301, pAY1302, and pAY1303, respectively. Second, primers with the sequences GCGGTGCGCATCGGGGAGGCGT and CCCGAATTCTCAGGTAGCGGAAGGGCTCT were used to amplify plasmid template pAY1300 (attP5). After treatment with PstI and EcoRI, the 1-kb fragment with the attP5 mutation was ligated to the larger PstI-EcoRI fragments of plasmids pAY1301, pAY1302, and pAY1303 to replace the attP+ allele and to yield pAY1304, pAY1305, and pAY1306, respectively.
An otherwise isogenic set of plasmids that express intX from the mgl promoter and carry the attP5 mutation was constructed in two additional steps. First, primer pairs with the sequences CGGCTCTAGACCGGTACTCCTCGCCACCCCTGCCCCAGCAAGTT and GCGGTGCGCATCGGGGAGGCGT and the sequences CCGGTCTAGAGCCGGTGGCCTCCGGCGTGACAGGCCGGCGTTCTAA and GGGAAGCTTGAATTCATAAAAGCCCGCCTCACCGAA were used to amplify plasmid templates pAY721 and pAY999, respectively. The 646-bp product of the pAY721 amplification was treated with PstI and XbaI, and the 366-bp product of the pAY999 amplification was treated with XbaI and HindIII. The pair of cleaved fragments was ligated to the PstI-HindIII backbone of pLITMUS28 to generate the construction intermediate, pAY1307, with the attP5 mutation in the attR core. Second, the smaller PstI-EcoRI fragment of pAY1307 was ligated to the larger PstI-EcoRI fragments of plasmids pAY1301, pAY1302, and pAY1303 to make plasmids pAY1308, pAY1309, and pAY1310, respectively.
Plasmids with deletions extending into attP.
To localize the elements required for attP function in reactions with attB, we constructed derivatives of plasmid pAY994 with different portions of the 3′ end of int, including the attP core. Primers with the sequences AGCGGATAACAATTTCACACAGGA and CCCCCAAGCTTACGGGTTCAAGTCCCGTA were used to amplify template plasmid pAY994. After cleavage of the PCR product with PstI and HindIII, the resulting 637-bp fragment was ligated to the PstI and HindIII sites of pBGS18 to make pAY1048 (bp 5851 to 6469), with the left arm of attP and the attP core. Primers with the sequences AAAAAACTGCAGAAAAGAAAAACCCCAGC AAGTCCGAGAACTTGCTGGGGCAGGGGTGGCGAGGAGTACGGGA CTTGAA and CCCCCAAGCTTACGGGTTCAAGTCCCGTA were used in a similar way to make pAY1044 (bp 6398 to 6469), with the attP core and the Mx8 terminator for trnD. Primers with the sequences AAAAAACTGCAGAAAAG AAAAACCCCAGCAAGTCCGAGAACTTGCTGGGGCAGGGGTGGCGA GGAGTACGGGACTTGAA and CCCAAGCTTAGGTAGCGGAAGGGCTCTC were used to make pAY1046 (bp 6398 to 6809), with the Mx8 terminator, the attP core, and the right arm of attP. pAY1042 (bp 6447 to 6809) and pAY1041 (bp 6447 to 6469) are smaller versions of pAY1046 and pAY1044 without the terminator and were made in a similar way with primer pairs having the sequences AAAAAACTGCAGAGGAGTACGGGACTTGAA and CCCAAGCTTAGGTAGCGGAAGGGCTCTC and the sequences AAAAAACTGCAGAGGAGTACGGGACTTGAA and CCCCCAAGCTTACGGGTTCAAGTCCCGTA, respectively.
Plasmids carrying the Mx8 prophage attL (attL1) junction site.
Chromosomal DNA isolated from the Kmr electroporant DZ1(1308) was amplified by PCR with attL primers having the sequences GGGGGAATTCGTCGACTGCGCAGGTCCGCGGAGGA and CCCAAGCTTCCTAGGTAGCGGAAGGGCTCTC. After treatment with HindIII and EcoRI, the 526-bp PCR product was ligated to pLITMUS29 to make plasmid pAY1037. The smaller Acc65I-XhoI fragment of pAY1037 was ligated to the larger Acc65I-SalI fragment of pBGS18 to make the Kmr attL plasmid pAY1038. Plasmid pAY1039 was made by subcloning the EcoRI-HindIII fragment made by amplification of M. xanthus DZ1 DNA with attB primers (19) into plasmid pBGS18.
Construction and phenotypic analysis of lysogens of DZ1 carrying single and multiple, tandem Mx8 prophages.
To construct lysogens of DZ1, wild-type Mx8 (107 phage/ml) was spotted on a lawn of host DZ1, and plates were incubated at 32°C for 96 h. Single colonies were purified from the zone of phage clearing. Lysogens of Mx8 carry either a single Mx8 prophage or multiple, tandem prophages (24). A lysogen with a single Mx8 prophage (“low yielders”) was distinguished from a lysogen with multiple, tandem Mx8 prophages (“high-yielders”) by amplification of chromosomal DNAs isolated from candidate lysogens with the attR, attL, and attP primer pairs. Whereas DNA isolated from the multiple, tandem-prophage lysogen yielded products in all three amplification reactions, DNA isolated from the single-prophage lysogen did not yield an abundant product in the attP reaction.
To measure phage released spontaneously from single- and tandem-prophage lysogens, cultures inoculated with single colonies of a lysogen were grown to an exponential density of 4 × 108/ml in CTPM medium at 32°C, cells were pelleted by low-speed centrifugation, and culture supernatants were treated with chloroform. Numbers of plaques formed by serial dilutions of the supernatants on host DZ1 were scored after incubation at 32°C for 48 h. Reported titers are the averages for three independent determinations.
To show that phage released spontaneously from lysogens have an intact int gene, high-titer phage stocks were prepared from single plaques. DNA prepared from these stocks was cleaved separately with restriction endonucleases EcoRI (which cleaves at sites flanking int) and MluI (which cleaves within int), as well as several additional endonucleases. In all (20 of 20) cases, the restriction patterns of DNA from phage released by spontaneous induction were identical to those of wild-type Mx8 DNA.
Nucleotide sequences of the int and uoi genes and the attB locus and their accession numbers.
The sequence of a region of the Mx8 genome including uoi and int has been assigned GenBank accession no. U64984 (29). The sequence of the attB locus, determined by Tojo et al. (34), has been assigned GenBank accession no. D26557.
RESULTS
Site-specific integration results in a change in the C terminus of integrase that decreases its activity in promoting integration (attP × attB recombination).
When a plasmid with the functional int-attP genes integrates into the attB locus, the int gene acquires a new 3′ end because attP lies within the int coding sequence (see Fig. 1 in accompanying paper [19]). We designate this altered int gene, with a new coding sequence for the C terminus of its product, intX. To test whether the intX gene is functional, we amplified its 3′ coding region from the DNA of strain DZ1(pAY721) by using primers specific for the amplification of attR. This 3′ end was subcloned in place of the phage-encoded 3′ end of int on plasmid pAY721 to make the otherwise isogenic plasmid pAY999.
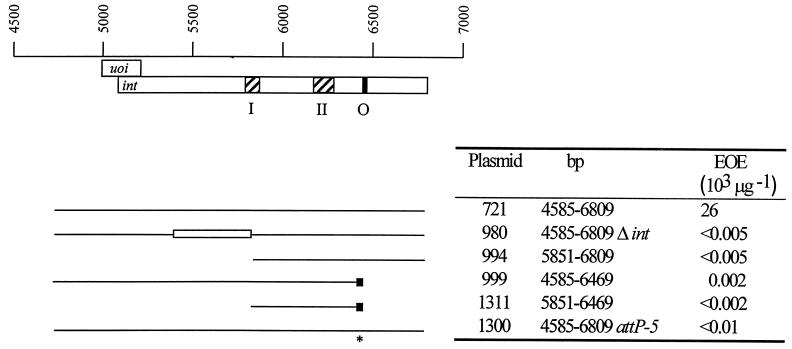
FIG. 1.
Plasmids with the intX and attP5 mutations cannot integrate efficiently. Coordinates of Mx8 DNA inserts (base pairs), based on GenBank accession no. U64984 (29), are shown above the open boxes representing the uoi and int coding sequences (top) which, like the coliphage λ xis and int genes (10), overlap. The hatched regions in the int gene correspond to conserved domains I and II of integrases (2, 17); conserved domain II includes the active-site tyrosine residue (26). The filled region within int, O, is the attP common core. The extents of Mx8 DNA inserts present in each plasmid are shown as horizontal thin lines (bottom left). Mutant plasmids are derived from pAY721, which contains a 2.2-kb region of the Mx8 genome with the functional int-attP genes. pAY980 is a derivative of pAY721 with a deletion, Δint-5403/5851 (open box), that removes bp 5403 to 5851 internal to int, including a portion of a portion of domain I (19). pAY999 contains the attR site and encodes the recombinant IntX integrase, with an altered C terminus (small black box). pAY994 and pAY1131 contain smaller regions of int and intX with minimal, functional attP and attR sites, respectively. pAY1300 is an otherwise isogenic version of pAY721 in which the attP site has been inactivated by the attP5 mutation (asterisk) (see Fig. 2). These plasmids were used in experiments to measure the relative abilities of Int and IntX to mediate a subset of site-specific recombination reactions involving different combinations of att sites (see Fig. 3). The efficiencies of electroporation (EOE) of the plasmids are the numbers of Kmr recombinants arising per microgram of DNA electroporated into host DZ1 and are the averages of at least three independent determinations (see Materials and Methods). attP-5 is attP5.
When electroporated into DZ1, the intX-attR+ plasmid pAY999 gives rise to Kmr recombinants at a very low but detectable efficiency. Unlike int, the intX gene has an internal attR site, not an internal attP site. Thus, when we measure the ability of pAY999 to integrate, we are assaying the ability of intX to promote attR × attB recombination. Consequently, the results show only that the pAY999-encoded IntX integrase can catalyze attR × attB recombination inefficiently at best.
To determine whether intX can promote attP × attB recombination, we examined whether the IntX integrase made from one plasmid (pAY999) can catalyze the integration of a second plasmid with an inactive int gene but an active attP site. As shown in Fig. 1, the electroporation of the host M. xanthus DZ1 with plasmid pAY721, carrying the functional int-attP genes, results in Kmr recombinants at a high efficiency. In contrast, the otherwise isogenic deletion derivative pAY980, missing a central region of int, does not result in Kmr recombinants. However, when Kmr plasmid pAY980 and Spr Smr int-attP+ plasmid pAY952 are coelectroporated into DZ1, Kmr Sps Sms recombinants arise at a high frequency. This result shows that pAY980 retains an active attP site that can be complemented in trans for integration by integrase made from plasmid pAY952 (19). Similarly, plasmid pAY994, which retains 959 bp at the 3′ end of int, including the attP core, is also complemented efficiently by pAY952 upon coelectroporation, indicating that it also retains a functional attP site (data not shown).
When Kmr plasmid pAY999 is coelectroporated with Kmr attP+ plasmids pAY980 and pAY994 (Table 2), Kmr electroporants are obtained at low but measurable efficiencies, showing that IntX can promote attP × attB recombination. In addition, eight of eight Kmr electroporants resulting from the coelectroporation of host DZ1 with pAY999 (intX+ attR+) and pAY980 (intΔ5403/5851 attP+) carry a single copy of pAY980, and not pAY999, integrated into attB (Fig. 2). Clearly, however, IntX promotes attP × attB recombination at a much lower efficiency than does Int.
TABLE 2.
The IntX integrase can promote integrative (attP × attB) recombinationa
| Plasmid(s) [relevant genotype(s)] | EOE (103 μg−1) |
|---|---|
| pAY980 (Δint-5403/5851 attP+) | <0.005 |
| pAY994 (attP+) | <0.005 |
| pAY999 (intX+ attR+) | 0.002 |
| pAY999 (intX+ attR+) + pAY980 (Δint-5403/5851 attP+) | 0.07 |
| pAY999 (intX+ attR+) + pAY994 (attP+) | 0.17 |
The efficiency of electroporation (EOE) of each plasmid alone or in coelectroporations was measured as the numbers of Kmr recombinants of host DZ1 as described elsewhere (19).
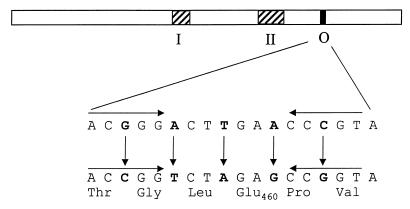
FIG. 2.
attP5 mutation. Within the int coding sequence, the attP common core (O) is comprised of an asymmetric 7-bp sequence flanked by two 5-bp dyad symmetry sequences (horizontal arrows). To inactivate attP and create attP5, we introduced the five single-base-pair substitutions shown in bold; these mutations change five codons within int to synonymous codons.
Separating the int and attP functions: construction of attP5, a mutation that inactivates attP but not int.
Because the Mx8 attP site lies within int and the attR site lies within intX, it is difficult to measure the relative activities of the Int and IntX integrases in vivo, because plasmids that produce these proteins are also potential substrates for the reactions that these integrases catalyze. To separate the trans-acting int function from the cis-acting attP function, we constructed a mutation within the int gene that inactivates attP but has no effect on int.
As shown in Fig. 2, the Mx8 attP site shares a 17-bp core sequence with the two bacterial attachment sites, attB1 and attB2, within which site-specific recombination occurs (19). The core is comprised of an asymmetric 7-bp sequence flanked by two 5-bp dyad symmetry sequences. Using PCR, we introduced into the common core five different single-base-pair substitutions each of which changes a base pair in the third positions of the int codons. Because these changes replace wild-type int codons with synonymous codons, they should not change the amino acid sequence of integrase. As expected, an integrated plasmid that expresses a mutant int gene with these changes produces active integrase that functions in trans (see below).
Because the attP5 mutation alters the common core sequence, however, it should inactivate the attP site. The integrases in the λ Int family, to which Mx8 Int belongs, catalyze four-strand exchange events between sites with a similar structure, a central asymmetric sequence flanked by short inverted repeats. For three integrases in this family, phage λ Int (7), the yeast 2μm FLP recombinase (1), and phage HK022 Int (14), cleavage of these sites is known to occur on opposite single strands of DNA at the junctions between the central asymmetric sequence and its flanking repeats. Efficient completion of the four-strand exchange reaction catalyzed by these recombinases will occur inefficiently if the two central asymmetric sequences of a pair of sites are not identical, because homology in this region is required for the proper resolution of the Holliday junction formed during the recombination event (3, 23). This information predicts that, because the attP5 mutation alters the sequence of the attP core in the predicted region of branch migration between recombining attP5 and attB sites, this mutation should abolish attP × attB (integrative) recombination. Consistent with this prediction, pAY1300, an otherwise isogenic derivative of pAY721 with the attP5 mutation, cannot integrate into the M. xanthus genome (Fig. 1).
Expression of int or intX from the mgl locus promotes integrative (attP × attB) recombination in trans.
To show that the attP5 mutation does not inactivate int, we constructed a plasmid that expresses the mutant int (attP5) gene from the constitutive mgl promoter, integrated this plasmid at the mgl locus by homologous recombination, and assayed the ability of Int made from this plasmid to promote the integration of int-attP+ plasmid pAY994 into the attB locus.
Plasmid pAY703 has the mglBA genes and can integrate into the mgl locus of DZ1 by homologous recombination to give rise to Kmr recombinants after electroporation of this host (20). We have shown that when the phage Mx8 mox gene (20), the M. xanthus sglK gene (36), or the E. coli glk gene (unpublished results) is subcloned into pAY703 and the subclones are integrated by homologous recombination into the M. xanthus mgl locus, the genes are expressed constitutively as part of the mglBA operon. Also, in the accompanying paper (19), we show that when we add functional int-attP genes to pAY703, we give this plasmid the option of integrating either at the mgl locus by homologous recombination or at the attB locus by site-specific recombination. Site-specific recombination prevails over homologous recombination, and the majority of Kmr electroporants carrying a plasmid with both the mgl locus and functional int-attP genes carry the plasmid integrated at the attB locus, not at the mgl locus (19).
As described in Materials and Methods, we constructed plasmids pAY1305 and pAY1306, which carry the int (attP5) gene immediately downstream of the mglA gene (Fig. 3). These plasmids should express int from the constitutive mgl promoter and couple the termination of mglA translation with the initiation of int or uoi translation, beginning at either the second start codon of int (GTG-5208 [GTG at bp 5208]; pAY1305) or the start codon of the upstream uoi gene (GTG-4991; pAY1306). When these plasmids are electroporated into host DZ1, Spr Smr recombinants are found to carry these plasmids integrated at the mgl locus, not at the attB locus. Thus, when chromosomal DNA is isolated from these recombinants and amplified, reactions with the attB primer pair yield a 591-bp product, indicating that these recombinants retain an intact attB locus (19). This information shows that int+-attP5 plasmids pAY1304 and pAY1306, unlike their int-attP+ counterparts without the attP5 mutation, pAY971 and pAY973 (19), are defective in site-specific integration.
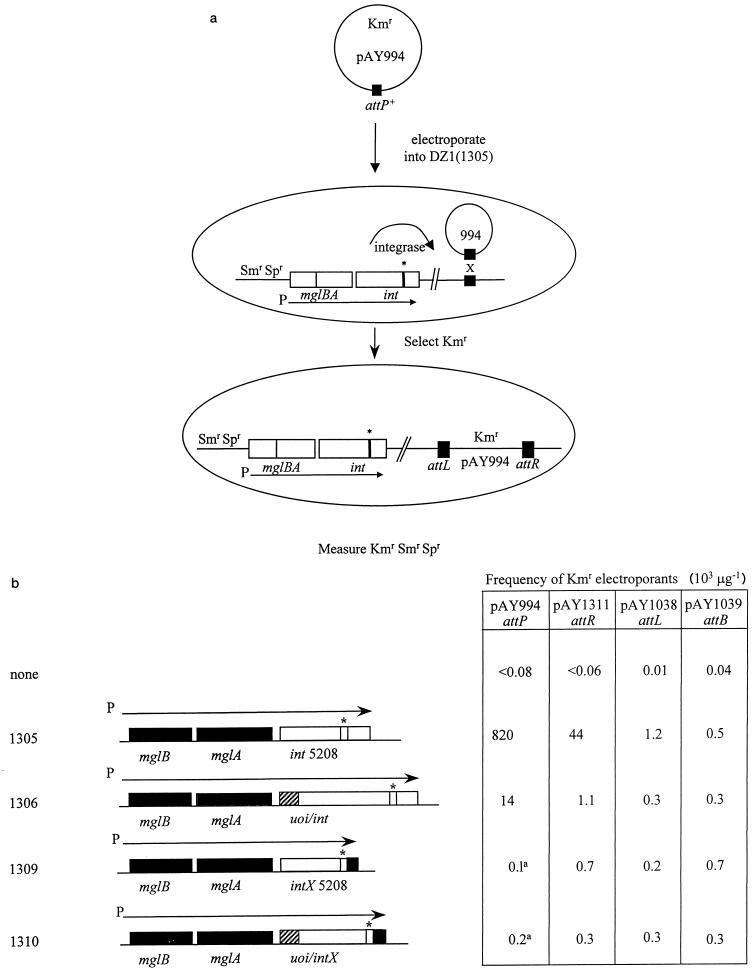
FIG. 3.
Expression of the mutant int (attP5) gene promotes site-specific recombination in trans. (a) When Spr Smr plasmid pAY1305 is integrated into the mgl locus of host DZ1 by homologous recombination, it expresses the int (attP5) gene from the constitutive mgl promoter (P). To show that the product of this mutant int gene functions in trans, strain DZ1::pAY1305 was electroporated with Kmr plasmid pAY994, and Kmr electroporants were selected. These recombinants arise upon site-specific recombination between the attP site of pAY994 and the attB locus to yield strains in which the linear, integrated plasmid pAY994 sequences are flanked by attL and attR sites. (b) DZ1 and Spr Smr derivatives of DZ1 carrying plasmids pAY1305, pAY1306, pAY1309, and pAY1310 were electroporated with plasmids pAY994 (attP), pAY1311 (attR), pAY1038 (attL), and pAY1039 (attB). The structures of the first four plasmids, which express int (attP5) or intX (attR5) from the mgl promoter, without and with uoi, are shown on the left; the attP5 (attR5) mutation is indicated by asterisks. On the right are given the efficiencies of electroporation, expressed as the numbers of Kmr electroporants arising per microgram of plasmid DNA and representing the average determinations from at least three independent experiments.
To show that these plasmids express active integrase in trans, we measured the efficiency with which Spr Smr derivatives of strain DZ1 with these plasmids integrated at the mgl locus could complement Kmr Δint-attP+ plasmid pAY994 for integration. As shown in Fig. 3, electroporation of pAY994 into strain DZ1::pAY1304, which expresses the int+ (attP5) gene from the mgl promoter, results in about 8 × 105 Kmr electroporants μg−1. This efficiency is significantly (>20-fold) higher than that for the integration of control int+-attP+ plasmid pAY721, which expresses int in cis from the int promoter (Fig. 1). This efficiency is also much higher than that for a deletion derivative of pAY721, pAY759, which should be missing the uoi promoter (19). Similar results are observed when pAY994 is electroporated into host DZ1::pAY1304, which expresses the version of the int (attP5) gene that initiates translation at the first int start codon (data not shown). The simplest interpretation of these results is that Int activity is expressed at higher levels from the constitutive mgl promoter than from its own promoter.
The data in Fig. 3 also reveal that strain DZ1::pAY1306, in which both uoi and int are expressed as part of the mgl operon, promotes the integration of plasmid pAY994 much less (<50-fold) efficiently than does strain DZ1::pAY1305. This result suggests that the expression of uoi inhibits the ability of Int to mediate the attP × attB integration reaction in trans, supporting the ideas that the uoi gene encodes Mx8 excisionase and that excisionase inhibits integrative recombination.
We also constructed plasmids pAY1309 and pAY1310, which express intX (attP5) from the mgl promoter, without and with uoi, respectively. Because the attP5 mutation affects the common core, it changes the attR site internal to intX in the same way that it changes the attP site internal to int. This mutation also should prevent the attR5 site from participating in productive site-specific recombination reactions with substrates that have the wild-type common core. When these plasmids are electroporated into host DZ1, again, Spr Smr recombinants are found to carry these plasmids integrated at the mgl locus. When pAY994 is electroporated into hosts with these plasmids, Kmr recombinants arise at very low but detectable frequencies (Fig. 3). Analysis of these Kmr recombinants by PCR shows that they carry pAY994 integrated at attB, because these recombinants have acquired an attL site (data not shown). These results confirm independently that the product of the prophage intX gene can promote attP × attB recombination but at an efficiency much lower than can the product of the phage int gene. They also show that the presence of the uoi gene has little effect on the ability of IntX to mediate attP × attB recombination, in contrast to its pronounced inhibition of the ability of Int to mediate the same reaction. Again, strain DZ1::pAY1308, which expresses intX from its first start codon, yielded results similar to those obtained with strain DZ1::pAY1309 (data not shown).
Int promotes attP × attB recombination more efficiently than attR × attB, attL × attB, or attB × attB recombination.
Prophage integration depends on a recombination reaction that is catalyzed by integrase and that proceeds in the highly favored, forward direction, attP + attB → attR + attL. For phages λ, P22, ϕ80 (17), and HK022 (37), this directionality of the recombination reaction depends on the difference between the structures of the attP and attB sites. Whereas the function of the attB site (BOB′) depends almost solely on the common core, O, the function of the attP site (POP′) depends on the common core, O, and both flanking P and P′ arm sequences. This is because the P and P′ arms have stronger binding sites for integrase and weaker binding sites for the integrative host factor required for the efficient catalysis of the integration reaction, whereas the B and B′ arms lack such sites (8, 11, 27, 28). Thus, the directionality of the integration reaction depends on this fundamental, functional asymmetry between the attP and attB sites, which facilitates the assembly of the catalytically active, asymmetric intasome complex (4).
Consequently, for phage λ, Int promotes site-specific recombination between the attP and attB (POP′ × BOB′) sites much more efficiently than between the attR (POB′), attL (BOP′), or attB (BOB′) site and an attB (BOB′) partner site, because only the attP × attB combination of substrates facilitates the proper assembly of the intasome. As shown in Fig. 3, when Mx8 integrase is expressed constitutively from the mgl promoter in strain DZ1::pAY1305, it can stimulate the integration of plasmids pAY1311 (attR), pAY1038 (attL), and pAY1039 (attB), but at lower efficiencies than that of plasmid pAY994 (attP). These results suggest that, as is the case for many other temperate phage attP sites, both the P and the P′ arms of the Mx8 attP site contribute significantly to attP function in the integrative recombination reaction.
Two secondary conclusions may be drawn from the additional data presented in Fig. 3. First, the expression of uoi together with int in host strain DZ1::pAY1306 inhibits not only attP × attB recombination but also the other three site-specific recombination reactions involving attR, attL, or attB and an attB partner. Second, IntX made in DZ1::pAY1309 or the combination of IntX and Uoi made in DZ1::pAY1310 promotes these other recombination reactions less efficiently than Int.
Both arm sequences flanking the attP core contribute significantly to attP function.
To confirm that both the P and the P′ arms of the attP site are important for attP function, we constructed a variety of deletion derivatives of Kmr attP+ plasmid pAY994, electroporated these derivatives into host strain DZ1::pAY1305, which produces integrase constitutively from the mgl promoter, and measured the frequencies of Kmr electroporants. As shown in Fig. 4, plasmid pAY1048, missing the right (P′) arm of the attP site, integrates into this host to give rise to Kmr recombinants with a >10-fold lower efficiency than does plasmid pAY994. This result is consistent with the finding that attR plasmid pAY1311, with the prophage attR (POB′) site, also gives rise to fewer recombinants than does plasmid pAY994. Plasmid pAY1042, missing the left (P) arm of the attP site, also is impaired in its ability to be complemented in trans for integration. Plasmid pAY1041, with deletions of both attP arms, can be complemented for integration only very poorly, at a very low efficiency comparable to that observed for plasmid pAY1039, carrying the attB (BOB′) sequence (Fig. 4).
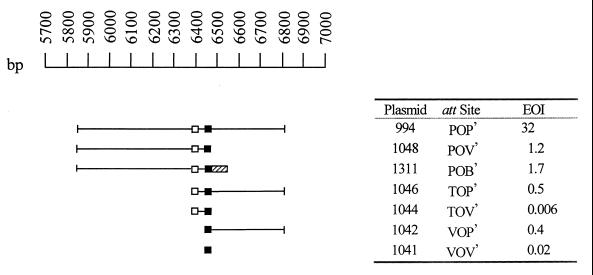
FIG. 4.
Both arms of the attP site contribute to attP function in the attP × attB integrative recombination reaction. Plasmids carrying various portions of attP or attR were electroporated into host strain DZ1::pAY1305, and the efficiencies with which they gave rise to Kmr recombinants were determined. The coordinates of Mx8 DNA inserts in Kmr plasmids with portions of attP (or attR) are shown on the top left, numbered as in GenBank accession no. U64984 (29). The extents of DNA inserts in the plasmids are represented as horizontal lines. All of the plasmids include the common core (filled boxes), and all but two of the plasmids carry the tMx8 terminator (open boxes). Attachment sites in these plasmids carry various combinations of the left (P) and right (P′) arms of attP, a deletion of the left arm of attP that retains the tMx8 terminator (T), the right (B′) arm of attB1 (hatched box), and/or vector sequences (V or V′) flanking the common core. Efficiencies of integration (EOI) are the average frequencies of Kmr Spr Smr recombinants recovered per microgram of plasmid DNA divided by the average frequencies of Kmr recombinants obtained from parallel electroporations of int-attP+ plasmid pAY721 and represent the average determinations from at least three independent experiments.
In the accompanying paper (19), we show that when a plasmid with the Mx8 attP site integrates into the attB locus, the natural terminators for the trnD genes are replaced by a phage-encoded terminator embedded within the int coding sequence. To explore whether this Mx8-encoded terminator is important for attP function, we constructed two additional deletion derivatives of plasmid pAY994, pAY1046 and pAY1044, retaining this terminator but missing left-arm sequences upstream of the terminator. The data in Fig. 4 show that this terminator is not sufficient for P-arm function and may contribute little to attP function, because pAY1046 integrates at the same lower efficiency in this assay as does otherwise isogenic plasmid pAY1042, which lacks the terminator. Similarly, plasmid pAY1044 is as defective for integration as plasmid pAY1041. We obtained similar results from experiments in which we measured the efficiencies of formation of Sps Sms Kmr recombinants after coelectroporation of each of these plasmids with Spr Smr plasmid pAY952 (data not shown).
DISCUSSION
Unlike the situation for most other integrative elements, Mx8 site-specific recombination involves an attP site located within the int gene. In this study, we have shown that the attP site is required in cis for integration. Also, two lines of evidence show that sequences flanking both sides of the attP core are required for efficient Mx8 integration, as for the integration of other temperate phages. When an int gene with an inactive, internal attP site is expressed from the constitutive mgl promoter, it promotes attP × attB recombination between a second plasmid and the chromosome more efficiently than attR × attB, attL × attB, or attB × attB recombination (Fig. 3). When int is expressed from the mgl locus, it promotes recombination between a plasmid with attP and the attB locus more efficiently than between plasmids with deletions of one or both arms flanking the attP core and the attB locus (Fig. 4).
The attB locus on the M. xanthus genome is also unusual, because it has two attB sites. Integrated plasmids with the Mx8 attP-int genes are found at the attB1 site much more frequently than at the attB2 site, and integration often is accompanied by a deletion between the attB1 and attB2 sites. The result that Mx8 integrase can stimulate attR × attB recombination at a reasonably high efficiency (Fig. 3) may account in part for the spectrum of events that we observe when plasmids with the Mx8 int-attP genes integrate into the bipartite attB locus. For example, after integration into the attB2 site, a subsequent attR × attB1 recombination event would generate an integrated plasmid accompanied by a deletion between the two sites. However, this factor cannot be the only one that accounts for this bias, because simple integration events into the attB1 site are more frequent than the sum of integration events into the attB2 site and integration events accompanied by deletion. Therefore, we must conclude that differences in the sequences flanking the attB1 and attB2 cores also contribute to this site preference and that the attB arms may influence the assembly of the phage Mx8 intasome more than they influence such assembly in other phage site-specific recombination systems. Consistent with this idea, we found that int, when expressed in trans from the mgl locus, can mediate attB × attB recombination events at a considerable efficiency (Fig. 3).
It comes as no surprise that the expression of the uoi gene together with int inhibits attP × attB recombination. The uoi gene is predicted to encode an excisionase that resembles phage P22 excisionase in sequence (17) and likely changes the directionality of site-specific recombination.
Upon the integration of Mx8, the attP × attB integration reaction per se changes the structure of the int gene and thereby reduces the specific activity of the enzyme that catalyzes this reaction. Thus, Mx8 integrase is among the class of enzymes with specific activities regulated by reversible covalent modification. Most often the reversible covalent modification of an enzyme involves the modification of a single or only a few amino acid side chains, usually by a different enzyme. For example, the regulation of the specific activity of E. coli isocitrate dehydrogenase involves the phosphorylation and dephosphorylation of a serine residue by the AceK kinase-phosphatase (15, 16). The reversible modification of Mx8 integrase is unusual in two respects. This modification involves not only the activity of integrase, the specific activity of which it itself modifies, but also a dramatic change in the primary amino acid sequence of the integrase protein.
Several other phage integrases result in the reversible covalent modification of the coding sequence of an enzyme. The lambdoid coliphages e14 (9) and 21 (35) integrate into an attB site located within the 3′ end of the E. coli K-12 icd gene, which encodes isocitrate dehydrogenase. Integration of these phages results in the replacement of an alternative 165-bp 3′ end of icd. However, unlike the change in Mx8 int caused by integration, the change in icd is conservative and is predicted to have little effect on the specific activity of isocitrate dehydrogenase. Thus, the new 165-bp 3′ end is predicted to encode a new enzyme C terminus differing in amino acid sequence by only two conservative changes (9).
The purpose of this novel mechanism of reversible covalent modification is even more intriguing and provides us with a new example of how temperate phages have coadapted with their hosts in elegant ways to maintain the integrity of the lysogenic state. The purpose of this modification is to coordinate the different levels of superinfection immunity exhibited by Mx8 lysogens with the different levels of (potentially virulent) phage that are released spontaneously from lysogens as a result of excisive recombination.
M. xanthus lysogens are of two classes, low yielders and high yielders (24), which carry single and multiple, tandem prophages, respectively. Lysogens with single Mx8 prophages maintain a lower level of immunity than do those with tandem prophages. A wild-type stock of Mx8 plates with efficiencies of 10−5 on low yielders, with a single integrated copy of the Mx8 genome, and 10−9 on high yielders, with two or more tandem copies of the Mx8 genome. Thus, the relative level of superinfection immunity conferred upon a lysogen is dependent upon the number of integrated Mx8 prophages and presumably the dosage of the imm gene, which encodes the primary Mx8 repressor (29).
Low-yielder lysogens of DZ1 with a single Mx8 prophage express the intX gene. When such lysogens are grown to an exponential density of 4 × 108 cells/ml and treated with chloroform, a titer of 102 phage/ml is found in the cell-free supernatant. In contrast, high-yielder lysogens of DZ1 with multiple, tandem Mx8 prophages release 106 phage/ml (24 and data not shown). These high yielders express both the less active, IntX form of integrase and the more active, Int form of integrase because, in addition to intX, they can carry an intact int gene at the (attP) junction of the two tandem prophage genomes. These results again suggest that the IntX integrase has a lower specific activity than the Int integrase, in this case for promoting excisional (attL × attR) recombination of the Mx8 prophage.
In lysogens with either single or multiple prophage genomes, the titer of spontaneous phage released by each type of lysogen is lower than the titer of wild-type phage required to form plaques on each type of lysogen. The rare derivatives of wild-type Mx8 that form plaques on a lysogen are virulent mutant phages, which can lyse an exponential culture of a lysogen in short order. The simple correlation between the rate of spontaneous phage release and the level of superinfection immunity of single- and multiple-prophage lysogens explains why the attP site is located within the Mx8 int gene. Lysogens of M. xanthus with Mx8 prophages can coordinate the rate of excisional prophage recombination with the relative level of immunity conferred by the Mx8 prophages, which is dependent upon the copy number of integrated prophage genomes. Such a mechanism limits the rate at which virulent Mx8 mutants, which threaten the life of a lysogen, are released from the lysogen. It is likely that this amazing mechanism of phage-host adaptation is not a unique feature of Mx8 and M. xanthus. Because Sulfolobus shibatae virus SSV1 is the only other integrative element known to have an attP site within its int gene (22, 25), it is inviting to speculate that SSV1 also enjoys the same adaptation. Other myxophages may also share this mechanism. Many wild-type strains of M. xanthus carry long arrays of tandem repeats of an 80-kb sequence called Mxα, which gives rise to particles capable of mediating generalized transduction (32). These strains include M. xanthus FB, which appears to carry five tandem copies of the Mxα genome. Our wild-type strain DK1622, which was derived from FB by the use of UV mutagenesis and generalized transduction with Mx8 (12), carries only a single copy of the Mxα element (32). Recently, we have found that lysates of a strain derived from FB include phages that form plaques on host DZ1 (which carries a single Mxα prophage), suggesting that phage Mxα may share this mechanism (unpublished results). If this is the case, then the Mxα int gene should also promote the site-specific recombination of a plasmid and carry an attP site within its coding sequence, predictions that we are now testing.
ACKNOWLEDGMENT
This work was supported by a grant from the National Institute of General Medical Sciences (GM53392) to P.Y.
Footnotes
This work is dedicated to the memory of Hatch Echols, teacher and friend.
REFERENCES
- 1.Andrews B J, Proteau G A, Beatty L G, Sadowski P D. The FLP recombinase of the 2 micron circle DNA of yeast: interaction with its target sequences. Cell. 1985;40:795–803. doi: 10.1016/0092-8674(85)90339-3. [DOI] [PubMed] [Google Scholar]
- 2.Argos P, Landy A, Abremski K, Egan J B, Haggard-Ljungquist E, Hoess R H, Kahn M L, Kalionis B, Narayana S V, Pierson L S. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986;5:433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azaro M A, Landy A. The isomeric preference of Holliday junctions influences resolution bias by lambda integrase. EMBO J. 1997;16:3744–3755. doi: 10.1093/emboj/16.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Better M, Lu C, Williams R C, Echols H. Site-specific DNA condensation and pairing mediated by the int protein of bacteriophage lambda. Proc Natl Acad Sci USA. 1982;79:5837–5841. doi: 10.1073/pnas.79.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos J M, Geisselsoder J, Zusman D R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- 6.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 7.Craig N L, Nash H A. The mechanism of phage lambda site-specific recombination: site-specific breakage of DNA by Int topoisomerase. Cell. 1983;35:795–803. doi: 10.1016/0092-8674(83)90112-5. [DOI] [PubMed] [Google Scholar]
- 8.Craig N L, Nash H A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984;39:707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- 9.Hill C W, Gray J A, Brody H. Use of the isocitrate dehydrogenase structural gene for attachment of e14 in Escherichia coli K-12. J Bacteriol. 1989;171:4083–4084. doi: 10.1128/jb.171.7.4083-4084.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoess R H, Foeller C, Bidwell K, Landy A. Site-specific recombination functions of bacteriophage lambda: DNA sequence of regulatory regions and overlapping structural genes for Int and Xis. Proc Natl Acad Sci USA. 1980;77:2482–2486. doi: 10.1073/pnas.77.5.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu P L, Ross W, Landy A. The lambda phage att site: functional limits and interaction with Int protein. Nature. 1980;285:85–91. doi: 10.1038/285085a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashefi K, Hartzell P L. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF− defect. Mol Microbiol. 1995;15:483–494. doi: 10.1111/j.1365-2958.1995.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 14.Kolot M, Yagil E. Position and direction of strand exchange in bacteriophage HK022 integration. Mol Gen Genet. 1994;245:623–627. doi: 10.1007/BF00282225. [DOI] [PubMed] [Google Scholar]
- 15.LaPorte D C, Chung T. A single gene codes for the kinase and phosphatase which regulate isocitrate dehydrogenase. J Biol Chem. 1985;260:15291–15297. [PubMed] [Google Scholar]
- 16.LaPorte D C, Koshland D E J. Phosphorylation of isocitrate dehydrogenase as a demonstration of enhanced sensitivity in covalent regulation. Nature. 1983;305:286–290. doi: 10.1038/305286a0. [DOI] [PubMed] [Google Scholar]
- 17.Leong J M, Nunes-Duby S E, Oser A B, Lesser C F, Youderian P, Susskind M M, Landy A. Structural and regulatory divergence among site-specific recombination genes of lambdoid phage. J Mol Biol. 1986;189:603–616. doi: 10.1016/0022-2836(86)90491-2. [DOI] [PubMed] [Google Scholar]
- 18.Magrini V, Creighton C, White D, Hartzell P L, Youderian P. The aadA gene of plasmid R100 confers resistance to spectinomycin and streptomycin in Myxococcus xanthus. J Bacteriol. 1998;180:6757–6760. doi: 10.1128/jb.180.24.6757-6760.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magrini V, Creighton C, Youderian P. Site-specific recombination of temperate Myxococcus xanthus phage Mx8: genetic elements required for integration. J Bacteriol. 1999;181:4050–4061. doi: 10.1128/jb.181.13.4050-4061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magrini V, Salmi D, Thomas D, Herbert S K, Hartzell P L, Youderian P. Temperate Myxococcus xanthus phage Mx8 encodes a DNA adenine methylase, Mox. J Bacteriol. 1997;179:4254–4263. doi: 10.1128/jb.179.13.4254-4263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin S, Sodergren E, Masuda T, Kaiser A D. Systematic isolation of transducing phages for Myxococcus xanthus. Virology. 1978;88:44–53. doi: 10.1016/0042-6822(78)90108-3. [DOI] [PubMed] [Google Scholar]
- 22.Muskhelishvili G, Palm P, Zillig W. SSV1-encoded site-specific recombination system in Sulfolobus shibatae. Mol Gen Genet. 1993;237:334–342. doi: 10.1007/BF00279436. [DOI] [PubMed] [Google Scholar]
- 23.Nunes-Duby S E, Azaro M A, Landy A. Swapping DNA strands and sensing homology without branch migration in lambda site-specific recombination. Curr Biol. 1995;5:139–148. doi: 10.1016/s0960-9822(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 24.Orndorff P, Stellwag E, Starich T, Dworkin M, Zissler J. Genetic and physical characterization of lysogeny by bacteriophage Mx8 in Myxococcus xanthus. J Bacteriol. 1983;154:772–779. doi: 10.1128/jb.154.2.772-779.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palm P, Schleper C, Grampp B, Yeats S, McWilliam P, Reiter W D, Zillig W. Complete nucleotide sequence of the virus SSV1 of the archaebacterium Sulfolobus shibatae. Virology. 1991;185:242–250. doi: 10.1016/0042-6822(91)90771-3. [DOI] [PubMed] [Google Scholar]
- 26.Pargellis C A, Nunes-Duby S E, de Vargas L M, Landy A. Suicide recombination substrates yield covalent lambda integrase-DNA complexes and lead to identification of the active site tyrosine. J Biol Chem. 1988;263:7678–7685. [PubMed] [Google Scholar]
- 27.Ross W, Landy A. Bacteriophage lambda int protein recognizes two classes of sequence in the phage att site: characterization of arm-type sites. Proc Natl Acad Sci USA. 1982;79:7724–7728. doi: 10.1073/pnas.79.24.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross W, Landy A. Patterns of lambda Int recognition in the regions of strand exchange. Cell. 1983;33:261–272. doi: 10.1016/0092-8674(83)90355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salmi D, Magrini V, Hartzell P L, Youderian P. Genetic determinants of immunity and integration of temperate Myxococcus xanthus phage Mx8. J Bacteriol. 1998;180:614–621. doi: 10.1128/jb.180.3.614-621.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Spratt B G, Hedge P J, Te H S, Edelman A, Broome-Smith J K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41:337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- 32.Starich T, Zissler J. Movement of multiple DNA units between Myxococcus xanthus cells. J Bacteriol. 1989;171:2323–2336. doi: 10.1128/jb.171.5.2323-2336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taketo A. DNA transfection of Escherichia coli by electroporation. Biochim Biophys Acta. 1988;949:318–324. doi: 10.1016/0167-4781(88)90158-3. [DOI] [PubMed] [Google Scholar]
- 34.Tojo N, Sanmiya K, Sugawara H, Inouye S, Komano T. Integration of bacteriophage Mx8 into the Myxococcus xanthus chromosome causes a structural alteration at the C-terminal region of the IntP protein. J Bacteriol. 1996;178:4004–4011. doi: 10.1128/jb.178.14.4004-4011.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Yang C H, Lee G, Chang F, Wilson H, del Campillo-Campbell A, Campbell A. Integration specificities of two lambdoid phages (21 and e14) that insert at the same attB site. J Bacteriol. 1997;179:5705–5711. doi: 10.1128/jb.179.18.5705-5711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weimer R M, Creighton C, Stassinopoulos A, Youderian P, Hartzell P L. A chaperone in the HSP70 family controls production of extracellular fibrils in Myxococcus xanthus. J Bacteriol. 1998;180:5357–5368. doi: 10.1128/jb.180.20.5357-5368.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yagil E, Dolev S, Oberto J, Kislev N, Ramaiah N, Weisberg R A. Determinants of site-specific recombination in the lambdoid coliphage HK022. An evolutionary change in specificity. J Mol Biol. 1989;207:695–717. doi: 10.1016/0022-2836(89)90238-6. [DOI] [PubMed] [Google Scholar]
- 38.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]