Abstract
Background
In critically ill patients requiring extracorporeal membrane oxygenation (ECMO) therapy, early initiation of continuous renal replacement therapy (CRRT) and beta-blockade of catecholamine-induced inotropic effects may improve outcomes.
Methods
A 2 × 2 partial factorial randomized controlled trial in eligible ECMO patients without a clear indication or contraindication to either intervention is centrally randomly assigned to (A) early or conventional-indicated CRRT and/or (B) beta-blocker or usual care. The primary outcome is all-cause mortality at 30 days for both arms. A total of 496 participants provides 80% power to determine a 20% risk reduction in mortality at 30 days with 5% type I error.
Discussion
This trial will help define the role of early CRRT and beta-blockade in ECMO patients. There have been 89 patients enrolled at 10 hospitals in study A and is ongoing. However, study B was stopped in August 2019 in the absence of any patients being enrolled.
Trial registration
ClinicalTrials.govNCT03549923. Registered on 8 June 2018. World Health Organization International Clinical Trials Registry Platform (WHO ICTEP) network. The Ethics Committee of Beijing Anzhen Hospital Approval ID is 2018013.
Keywords: Cardiogenic shock, Extracorporeal membrane oxygenation, Acute kidney injury, Renal replacement therapy, Mortality, Randomization
Background
Introduction
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) provides short-term circulatory support for critically ill patients with severe cardiac shock, but mortality remains high (60 to 75%) [1] and acute kidney injury (AKI) and fluid overload (FO) are frequent complications [2, 3] that are managed with continuous renal replacement therapy (CRRT) [3–6]. However, the optimal timing of CRRT is uncertain: the positive results of the early vs late initiation of renal replacement therapy in critically ill patients with acute kidney injury (the ELAIN) trial [7] were not confirmed in another study, the Artificial Kidney Initiation in Kidney Injury (AKIKI) [8]. As critically ill patients have excessive sympathetic activation and autonomic dysfunction from high circulating catecholamines that may compromise cardiac function [9–14], beta-blockade could improve outcomes by improving myocardial oxygen consumption, ventricular remodeling, and left ventricular function [15, 16]. A randomized, open, single-center study in patients with septic shock requiring norepinephrine to maintain mean arterial pressure showed a benefit of esmolol on 28-day mortality [17]. The circulatory support offered by ECMO may avoid the negative inotropic effect of beta-blockers in the setting of cardiogenic shock [18]. We designed the EvaLuation of early CRRT and beta-blocker InTervention in patients with ECMO (ELITE) trial to determine the effects of early CRRT versus conventionally indicated CRRT and/or beta-blockade on top of routine care.
Objective
The objective is to determine whether (A) early CRRT compared to conventional-indicated CRRT and (B) beta-blockade with esmolol compared to standard treatment will reduce 30-day mortality in ECMO patients.
Methods
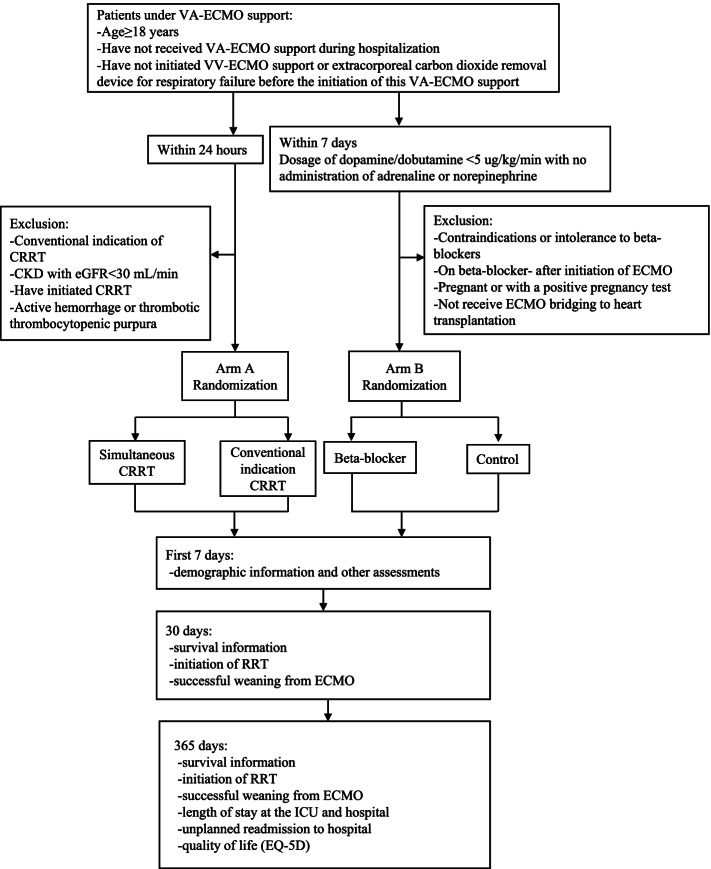
Study design (Fig. 1)
Fig. 1.
Study design. CKD, chronic kidney disease; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; VA-ECMO, veno-arterial extracorporeal membrane oxygenation
ELITE is a prospective, multi-center, open-label, randomized controlled trial, with a 2 × 2 partial factorial design to evaluate whether early CRRT support and beta-blockade will reduce 30-day mortality in a broad range of ECMO patients admitted to hospitals in China from July 2018. In arm A, patients are randomized to early CRRT within 24 h of initiation of ECMO or to control, regardless of whether there is a conventional indication, or to the control where patients only receive CRRT according to a conventional indication. In arm B, patients are randomized to the intravenous esmolol group or usual care. The trial design and protocol adhere to the Recommendations for Interventional Trials (SPIRIT) criteria [19] (Table 1) and checklist in Table 5 in Appendix. The study is planned to enroll patients from 10 centers in China (see Table 5 in Appendix for the hospital list).
Table 1.
Study period
| CRRT screening | CRRT randomization | Beta-blocker screening | Beta-blocker randomization | Days post-randomization | ||||
|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 7 | Day 30 | Day 365 | |||||
| Window for time for evaluation | ± 5 days | ± 35 days | ||||||
| Visit number | 1 | 1 | 2 | 3 | 4 | 5 | ||
| Informed consent | × | × | ||||||
| Demographic data | × | × | ||||||
| Physical measures | × | × | × | × | ||||
| Medical history | × | × | ||||||
| Concomitant medications | × | × | × | × | × | × | × | × |
| Physical examinationsa | × | × | ||||||
| Vital signsb | × | × | × | × | ||||
| Complete blood countc | × | × | × | × | ||||
| Arterial blood gasd | × | × | × | × | ||||
| Biochemistry itemse | × | × | × | × | ||||
| Coagulation indexesf | × | × | × | × | ||||
| UCGg | × | × | × | × | ||||
| Intake and output volume in 24 h | × | |||||||
| Medication, type, and doseh | × | × | × | × | × | × | ||
| Mechanical ventilation and mode | × | × | × | × | × | |||
| Left ventricular unloading method | × | × | × | |||||
| RRT | × | × | × | × | × | |||
| APACHE II score | × | × | ||||||
| SOFA score | × | × | ||||||
| EQ-5D score | × | |||||||
| SAEs | × | × | ×: | × | ||||
aPhysical examinations: height (cm) and weight (kg)
bVital signs: blood pressure (mmHg), pulse (beats/min), heart rate (beats/min), temperature (°C), and respiratory (/min)
cComplete blood count: white blood cell count (103/mm3), hemoglobin (g/dL), and platelet count (109/L)
dArterial blood gas: PH, PaO2 (mmHg), PaCO2 (mmHg), K+ (mmol/L), Na+ (mmol/L), hematocrit (%), and lactic acid (mmol/L)
eBiochemistry items: creatine (μmol/L) and total bilirubin (μmol/L)
fCoagulation indexes: PT (s) and APTT (s)
gUCG: left ventricular ejection fraction (%), left ventricular end-diastolic diameter (cm), moderate and above pulmonary hypertension, and moderate and above tricuspid regurgitation
hMedication: dopamine, dobutamine, epinephrine, norepinephrine, vasopressin, pituitrin, milrinone, and beta blockers
Eligibility
The broad inclusion criteria are used for both arms, whereby adult patients who have received ECMO for any reason within 24 h and 7 days, for arms A and arm B, respectively, are eligible (Tables 2 and 3). Patients are excluded from arms A and B if they had a definite indication or contraindication to either CRRT or beta-blockers, respectively. As ECMO is often initiated by a specialist team to rescue and transfer patients to larger hospitals in China, a timeframe of 24 h after ECMO implantation was used for early CRRT in arm A, while a relatively stable hemodynamic status (dopamine/dobutamine < 5 μg/kg/min, with no administration of adrenaline or norepinephrine) and within 7 days after initiation of ECMO was required for eligibility into arm B. Identification of potentially eligible patients is the responsibility of the ECMO team in participating centers. After ECMO implantation or when the patient is transferred to the participating center, eligibility for study arm A will be assessed. Prior to 7 days after ECMO implantation, when patients are stable on ECMO, eligibility for study arm B will be assessed.
Table 2.
Inclusion and exclusion criteria for study A
| Inclusion criteria | |
| Patients receiving VA-ECMO for any reason within 24 h | |
| Provision of informed consent | |
| Exclusion criteria | |
| Age < 18 years | |
| Receiving ECMO bridging to heart transplantation | |
| With convention indication of CRRT: AKI prior to enrollment caused by any reason, at least one of the following criteria is met: | |
| Severe hyperkalemia (> 6.5 mmol/L) | |
| Metabolic acidosis (pH < 7.2) | |
| Pulmonary edema | |
| Blood urea nitrogen level > 112 mg/dL | |
| Oliguria (urine output < 200 mL/12 h) for more than 72 h | |
| CKD, with estimated GFR<30 mL/min | |
| Have already initiated CRRT | |
| Active hemorrhage/thrombotic thrombocytopenic purpura | |
| Receiving ECMO again during hospitalization or respiratory failure has already initiated VV-ECMO or extracorporeal carbon dioxide removal device before the initiation of VA-ECMO of this time |
AKI chronic kidney disease, CKD chronic kidney disease, CRRT continuous renal replacement therapy, ECMO extracorporeal membrane oxygenation, GFR glomerular filtration rate, VA-ECMO veno-arterial extracorporeal membrane oxygenation, VV-ECMO veno-venous extracorporeal membrane oxygenation
Table 3.
Inclusion and exclusion criteria for study B
| Inclusion criteria | |
| Patients receiving VA-ECMO for any reason | |
| Dopamine/dobutamine < 5 μg/kg/min, no administration of adrenaline or norepinephrine | |
| Within 7 days after initiation of VA-ECMO | |
| Exclusion criteria | |
| Age < 18 years | |
| Receiving ECMO bridging to heart transplantation | |
| Contraindications or intolerance to beta-blockers | |
| Moderate or severe bronchial asthma attack or history of bronchial asthma | |
| Sinus bradycardia (heart rate < 60 bpm) | |
| Type II second-degree or third-degree AVB | |
| Allergy to esmolol | |
| Receiving ECMO again during hospitalization or respiratory failure has already initiated VV-ECMO or extracorporeal carbon dioxide removal device before the initiation of VA-ECMO of this time | |
| Have been on beta-blocker treatment after initiation of ECMO | |
| Women at child-bearing age, pregnant or positive pregnancy test |
ECMO extracorporeal membrane oxygenation, AVB atrioventricular block, VA-ECMO veno-arterial extracorporeal membrane oxygenation, VV-ECMO veno-venous extracorporeal membrane oxygenation
Randomization
All eligible patients are centrally randomized in a 1:1 ratio to either early CRRT or conventional indication CRRT, and/or beta-blocker group or usual care (control) groups, via a computer-generated randomization schedule, stratified by age (< 65 vs. ≥ 65 years) and implantation of ECMO for extracorporeal cardiopulmonary resuscitation (yes vs no). The sequence of randomization is generated by an independent statistician, using SAS version 9.4. Investigators use a cellphone application to confirm eligibility and obtain the randomized treatment allocation.
Interventions
Patients assigned to early CRRT should have it applied concurrently with ECMO treatment and continued for ≥ 12 h. Those assigned to standard therapy should receive CRRT according to the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI stage 3 and have at least one of the following criteria met: severe hyperkalemia (> 6.5 mmol/L), metabolic acidosis (pH < 7.2), pulmonary edema unresponsive to diuretic therapy, requiring oxygen flow rate of > 5L/min to maintain an oxygen saturation (SpO2) of > 95% or requiring a forced inspired oxygen concentration (FiO2) of > 50% on ventilation, blood urea nitrogen level > 112 mg/dL, or oliguria (urine output < 200 mL per 12 h) for > 72 h. CRRT can be initiated or discontinued according to the attending clinician’s decision in managing the patient.
In arm B, patients assigned to the intervention group are to receive a continuous infusion of esmolol on top of conventional management, commencing at 25 mg/h and increasing by 25 mg/h every 20 min until the heart rate is reduced to 75 ± 5 bpm or an upper dose limit of 2000 mg/h is reached. Esmolol infusion should be continued to maintain the heart rate threshold or according to the clinician’s discretion until discharge from the intensive care unit (ICU) (or death, if sooner). Oral beta-blockers should be introduced before esmolol is withdrawn. Esmolol can be transiently stopped or completely withdrawn should a patient develops third-degree atrial-ventricular block, bradycardia (< 60 bpm), extreme left ventricular systolic dysfunction, aortic valve dysfunction, or severe cardiogenic pulmonary edema. Patients in the control group will not receive any type of beta-blockers, unless the clinician considers there is a strong indication. Concomitant diseases will be treated following the current guidelines.
Outcomes
The proportion of all-cause mortality at 30 days will be compared between the intervention and control groups. Survival data will be collected through telephone follow-up. Secondary outcomes are all-cause mortality at 365 days; use of long-term RRT; success in weaning from ECMO, defined as survival after 24 h from weaning; health-related quality of life according to scores on the EuroQoL (EQ-5D-5L) questionnaire at 365 days; length of stay at ICU and hospital; unplanned hospital readmission; and separately on cardiac and non-cardiac death.
Severe adverse events (SAEs), such as major bleeding, cardiac arrhythmias, ventilator-associated pneumonia, bloodstream infection, surgical site infection, limb ischemia from any cause, and ischemic or hematologic stroke will be collected systemically. Any other SAEs determined by the physicians through spontaneous reporting (Table 4 outlines SAE definitions). The publication will detail each documented SAE. All the SAEs will be coded through MedDRA.
Table 4.
Definition of SAEs
| The SAEs include but are not limited to | |
| Major bleeding | |
| Bleeding requires transfusion for > 2 units | |
| Severe arrhythmias | |
| Type II second-degree AVB or third-degree AVB | |
| Sustained ventricular tachycardia (> 30 s) | |
| Ventricular fibrillation | |
| Ventilator associated pneumonia, which needs to meet all the criteria below: | |
| At least 48 h after endotracheal intubation | |
| Chest X-ray showing sustained or worsening shadowing (infiltrates or consolidations) | |
| Signs of pulmonary consolidation and/or crackles on auscultation, at least meet one of the following criteria: | |
| a) White blood cell count > 10 × 109/L or < 4 × 109/L | |
| b) Temperature > 37.5 °C, purulent secretions | |
| c) Positive cultures obtained directly from bronchial secretions | |
| Bloodstream infection | |
| Positive cultures obtained from the peripheral blood | |
| SSI | |
| Purulent drainage in the operated region with pain or tenderness, localized swelling, redness, and heat or fever, requiring operation, including superficial incisional wound SSI, deep incisional wound SSI, and organ/space SSI | |
| Limb ischemia from any cause | |
| Physical examination demonstrating pain, pallor, pulseless, cold, and motor or sensor deficit | |
| Requiring decannulation of ECMO catheter or surgical intervention | |
| Stroke | |
| New onset abnormal neurological signs and symptoms last for at least 24 h with radiographic evidence | |
| Any other severe adverse events determined by the physicians |
AVB atrioventricular block, ECMO extracorporeal membrane oxygenation, SAE serious adverse events, SSI surgical site infection
Data management
Data are collected on patient demography, medical history, concomitant therapy, duration of CRRT, and dose and duration of esmolol. Vital and health status are assessed at the time of weaning from ECMO, discharged from ICU and hospital, unplanned hospital readmission, EQ-5D scores, and SAEs, during follow-up via telephone, face-to-face, or remote medical consultation over 365 days. To improve monitoring adherence, the clinician will take time to explain about the need for follow-up surveillance and encourage the participants to undergo routine examinations. Data are entered into a secure password-protected electronic data capture system and checked for quality by research staff. All queries are listed for content and raised and resolved dates. All study records required by the coordinating center at the Heart Health Research Center (HHRC) and applicable regulatory bodies are maintained for 15 years. The confidentiality of all participant information must be protected at the clinical sites and the coordinating center. Paper records and computer files must be appropriately safeguarded from unauthorized access.
Sample size
Sample size calculation is performed using the software PASS 15.0.5 based on the following assumption: a 70% 30-day mortality in the control group and a 20% relative risk reduction for each intervention (early CRRT and beta-blocker). The estimate of the intervention’s effect size was based on clinical expertise and the clinical significance of an intervention, no loss of follow-up or crossover, and 5% type I error and 20% type II error. We assume no interaction between interventions, as the overlap of the time of interventions would be limited and no carry-on effect expected. A total of 496 patients (248 per group) are required for each arm.
To achieve adequate participant enrolment to reach the target sample size, the study will be conducted at 10 high-volume centers in China. A smartphone application has been developed to facilitate randomization at the emergency room.
Statistical analysis
All analyses will be conducted according to the intention-to-treat principle by blinded statisticians. All patients will be analyzed in accordance with the results of randomization, regardless of whether they received the prescribed therapy. Baseline characteristics between the groups will be reported as frequencies and percentages for categorical variables and as means and standard deviations (SD) or medians and interquartile ranges (IQR) for continuous variables. The primary outcome will be compared between the groups in a log-rank test. Other efficacy and safety endpoints will be reported with the t test or Wilcoxon test, and the chi-square test for continuous and categorial variables, respectively, as appropriate. All tests are two-sided, and the nominal level of α will be 5%.
In the subgroup analysis, age and ECPR will be defined by the presence or absence of a pre-randomization variable and the primary outcome as in the main analysis. The main analysis for each subgroup will be an unadjusted test of interaction in a logistic model to determine whether the effect of treatment differs significantly across categories. The missing values will not be imputed unless substantial. The number of observations used in the analysis will be reported.
Monitoring
The data monitoring committee (DMC) consists of a critical care physician, nephrologist, and cardiovascular expert, all experienced in clinical research. The principal investigator will report the process of the trial, quality of data collected, adverse events, and protocol deviation and violation to the DMC. The DMC will meet every 6 months during the conduction of the trial. No interim analysis for efficiency is planned as the assumptions in sample size calculation are less likely to be changed.
An investigator meeting is held annually to communicate the progress, quality control, and approaches to improve recruitment and address any quality of data issues.
Trial status
There have been 89 patients enrolled at 10 hospitals in study A, and the enrollment is still ongoing. However, study B was stopped in August 2019 in the absence of any patients being enrolled. Although we aimed to test the hypothesis that beta-blockers would protect the myocardium in ECMO patients, we found this too challenging to undertake as these patients have low BP, which is a contraindication to such treatment and raised concerns of harm among investigators. Moreover, the short time window for enrollment was another barrier to recruitment, and by the time a patient is stable and without vasopressors, it is near the time to wean them off ECMO. Consequently, we decided to close recruitment into arm B. During the COVID pandemic, patient enrollment has become more challenging. A review of the feasibility of the trial will be made by the Steering Committee at the end of 2022.
Discussion
Being one of the most seriously ill groups in clinical practice, with high mortality and requiring heavy use of critical care resources, there are considerable challenges to generate reliable evidence to guide the management of ECMO patients. Our ELITE trial attempts to address two important clinical questions with strong pathophysiological mechanisms.
A multicenter retrospective cohort study has shown that FO is common in a pediatric population (peak FO ≥ 10% in 84.8%, ≥ 20% in 67.2%, and ≥ 50% in 29%) [20] where FO has consistently been shown to be associated with adverse outcomes [21–23], while a meta-analysis of observational studies has shown lower mortality in those who receive CRRT [24]. These data suggest that early initiation of CRRT to avoid FO in the context of ECMO may be beneficial. The Kidney Interventions During Membrane Oxygenation (KIDMO) study showed that CRRT use was 43%, 16%, and 35% of patients for FO, for FO prevention, and for AKI, respectively, after ECMO [25]. However, there has not been a randomized trial to support these approaches. So, we designed this ELITE trial. As the closing of arm B, the question of whether beta-blocker is efficient in patients with ECMO remains unaddressed, and more effects should be made.
Other limitations of our trial include the lack of blinding, and for practical reasons, recruitment has been slow due to the small number of patients receiving ECMO despite the expectation of a least 10 cases treated annually across our network of 30 ICUs in China. We did not stratify our randomization by ECMO centers but will perform a subgroup analysis to explore the effect between small and large centers.
In summary, the ELITE trial is the first randomized controlled trial of critically ill patients receiving ECMO support, powered to test the effects of early CRRT versus conventional timing of CRRT on 30-day mortality. The results should inform the management of this important patient group.
Acknowledgements
We would like to thank all the participant centers in the ELITE trial.
Ancillary and post-trial care
The trial insurance to cover for non-negligent harm associated with the protocol. This will include cover for additional health care, compensation, or damages.
Dissemination
The results of the study will be disseminated through international peer-reviewed journals and medical conferences. There is no intention to use the services of professional writers. Authorship will be determined in accordance with the ICMJE guidelines, and other contributors will be acknowledged.
Appendix
Please insert Table 5 here
Table 5.
ELITE study centers
| Centers | Location |
|---|---|
| The Secondary Affiliated Hospital of Zhengzhou University | Zhengzhou, Henan province |
| The First Affiliated Hospital of Zhengzhou University | Zhengzhou, Henan province |
| The Second Hospital of Jilin University | Changchun, Jilin province |
| The People’s Hospital of Guangxi Zhuang Autonomous Region | Nanning, Guangxi Zhuang Autonomous Region |
| Chinese PLA General Hospital | Beijing |
| Beijing Anzhen Hospital | Beijing |
| Zhongshan City People’s Hospital | Zhongshan, Guangdong province |
| Sichuan Provincial People’s Hospital | Chengdu, Sichuan province |
| Taizhou Hospital of Zhejiang Province | Taizhou, Zhejiang province |
| Zhongshan Hospital Affiliated to Fudan University | Shanghai |
Authors’ contributions
Author contributions are consistent with the International Committee of Medical Journal Editors (ICMJE) Recommendations. XW, HW, CL, and JZ participated in the intervention of the study. CA and XD participated in the study design. XW, XD, and ZW drafted and revised this manuscript. XH and JD are the principal investigators and conceived the study. The authors read and approved the final manuscript.
Authors’ information
Organization:
Coordinating center: Heart Health Research Center (HHRC)
Steering committee: Jianzeng Dong, Xiaotong Hou, Craig Anderson, Xin Du, Hong Wang, and Simon Finfer
Endpoint adjudication committee: We would not adjudicate the primary or secondary endpoints.
International advisor committee: Anushka Patel, Kazem Rahimi, Daniel Brodie, Roberto Lorusso, and Alain Combes
Data monitoring committee (DMC): Bruce Neal, Jicheng Lv, and Xiang Guo
Data management: Heart Health Research Center (HHRC)
Funding
The funding for the trial was provided by the National Thirteenth Five-year Key Technology Research and Development Program, sponsored by the Ministry of Science & Technology, China (2016YFC1301000, to J Dong), and the Capital Characteristic Clinic Project, sponsored by the Beijing Municipal Science and Technology Commission, China (No. Z161100000516017 to X Hou). The sponsor and funder are responsible for the development and dissemination of the project.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
This trial complied with the Declaration of Helsinki regarding the investigation of humans. Its ethical clearance, protocol (version 3.0 on September 25, 2019), and associated documents were approved by the Ethics Committee of Beijing Anzhen hospital (Approval ID: 2018013). Any significant modifications to the protocol will need approval from the ethics committee before implementation.
Informed consent and written consent forms of patients are mandatory before study participation. After the participant has been assessed as eligible, they or their guardians will be invited by the treating physicians to discuss the details and sign the informed consent. Additional consent provisions for use of participant data in ancillary studies are required. This trial does not involve collecting biological specimens for storage. Participants’ data is stored using a participant identification number, and the key to the identification code list is only available to the research team.
Consent for publication
Written informed consent was obtained from the participants to publish this manuscript and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaotong Hou, Email: xt.hou@ccmu.edu.cn.
Jianzeng Dong, Email: jz_dong@126.com.
References
- 1.Aso S, Matsui H, Fushimi K, Yasunaga H. In-hospital mortality and successful weaning from venoarterial extracorporeal membrane oxygenation: analysis of 5,263 patients using a national inpatient database in Japan. Crit Care. 2016;20:80. doi: 10.1186/s13054-016-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YC, Tsai FC, Fang JT, Yang CW. Acute kidney injury in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc. 2014;113(11):778–785. doi: 10.1016/j.jfma.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Selewski DT, Wille KM. Continuous renal replacement therapy in patients treated with extracorporeal membrane oxygenation. Semin Dial. 2021. 10.1111/sdi.12965. [DOI] [PMC free article] [PubMed]
- 4.Schmidt M, Bailey M, Kelly J, Hodgson C, Cooper DJ, Scheinkestel C, et al. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 2014;40(9):1256–1266. doi: 10.1007/s00134-014-3360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein SL, Somers MJ, Baum MA, Symons JM, Brophy PD, Blowey D, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 6.Vanmassenhove J, Kielstein J, Jorres A, Biesen WV. Management of patients at risk of acute kidney injury. Lancet. 2017;389(10084):2139–2151. doi: 10.1016/S0140-6736(17)31329-6. [DOI] [PubMed] [Google Scholar]
- 7.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstadt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 8.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 9.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 10.Alonso de Vega JM, Diaz J, Serrano E, Carbonell LF. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit Care Med. 2002;30(8):1782–1786. doi: 10.1097/00003246-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Rudiger A. Beta-block the septic heart. Crit Care Med. 2010;38(10 Suppl):S608–S612. doi: 10.1097/CCM.0b013e3181f204ca. [DOI] [PubMed] [Google Scholar]
- 12.Werdan K, Muller U, Reithmann C, Pfeifer A, Hallstrom S, Koidl B, et al. Mechanisms in acute septic cardiomyopathy: evidence from isolated myocytes. Basic Res Cardiol. 1991;86(5):411–421. doi: 10.1007/BF02190709. [DOI] [PubMed] [Google Scholar]
- 13.Andreis DT, Singer M. Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med. 2016;42(9):1387–1397. doi: 10.1007/s00134-016-4249-z. [DOI] [PubMed] [Google Scholar]
- 14.Schmittinger CA, Torgersen C, Luckner G, Schroder DC, Lorenz I, Dunser MW. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med. 2012;38(6):950–958. doi: 10.1007/s00134-012-2531-2. [DOI] [PubMed] [Google Scholar]
- 15.Bohm M, Link A, Cai D, Nieminen MS, Filippatos GS, Salem R, et al. Beneficial association of beta-blocker therapy on recovery from severe acute heart failure treatment: data from the survival of patients with acute heart failure in need of intravenous inotropic support trial. Crit Care Med. 2011;39(5):940–944. doi: 10.1097/CCM.0b013e31820a91ed. [DOI] [PubMed] [Google Scholar]
- 16.Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of beta-blocker withdrawal in acute decompensated heart failure: a systematic review and meta-analysis. JACC Heart Fail. 2015;3(8):647–653. doi: 10.1016/j.jchf.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683–1691. doi: 10.1001/jama.2013.278477. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 19.Chan A-W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin J, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selewski DT, Askenazi DJ, Bridges BC, Cooper DS, Fleming GM, Paden ML, et al. The impact of fluid overload on outcomes in children treated with extracorporeal membrane oxygenation: a multicenter retrospective cohort study. Pediatr Crit Care Med. 2017;18(12):1126–1135. doi: 10.1097/PCC.0000000000001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong KM, Au SY, Ng GWY, Leung AKH. Positive fluid balance and mortality in adult patients treated with extracorporeal membrane oxygenation: a retrospective study. J Intensive Care Soc. 2020;21(3):210–220. doi: 10.1177/1751143719862240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besnier E, Boubèche S, Clavier T, Popoff B, Dureuil B, Doguet F, et al. Early positive fluid balance is associated with mortality in patients treated with veno-arterial extra corporeal membrane oxygenation for cardiogenic shock: a retrospective cohort study. Shock. 2020;53(4):426–433. doi: 10.1097/SHK.0000000000001381. [DOI] [PubMed] [Google Scholar]
- 23.McCanny P, Smith MW, O’Brien SG, Buscher H, Carton EG. Fluid balance and recov- ery of native lung function in adult patients supported by venovenous extracorporeal membrane oxygenation and continuous renal replacement therapy. ASAIO J. 2019;65(6):614–619. doi: 10.1097/MAT.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 24.Han S-S, Kim HJ, Lee SJ, Kim WJ, Hong Y, Lee HY, et al. Effects of renal replacement therapy in patients receiving extracorporeal membrane oxygenation: a meta-analysis. Ann Thorac Surg. 2015;100(4):1485–1495. doi: 10.1016/j.athoracsur.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Fleming GM, Askenazi DJ, Bridges BC, et al. A multicenter international survey of renal supportive therapy during ECMO: the kidney intervention during extracorporeal membrane oxygenation (KIDMO) group. ASAIO J. 2012;58(4):407–414. doi: 10.1097/MAT.0b013e3182579218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan X, Jia S, Meng X, Dong P, Jia M, Wan J, et al. Acute kidney injury in adult postcardiotomy patients with extracorporeal membrane oxygenation: evaluation of the RIFLE classification and the acute kidney injury network criteria. Eur J Cardiothorac Surg. 2010;37(2):334–338. doi: 10.1016/j.ejcts.2009.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.