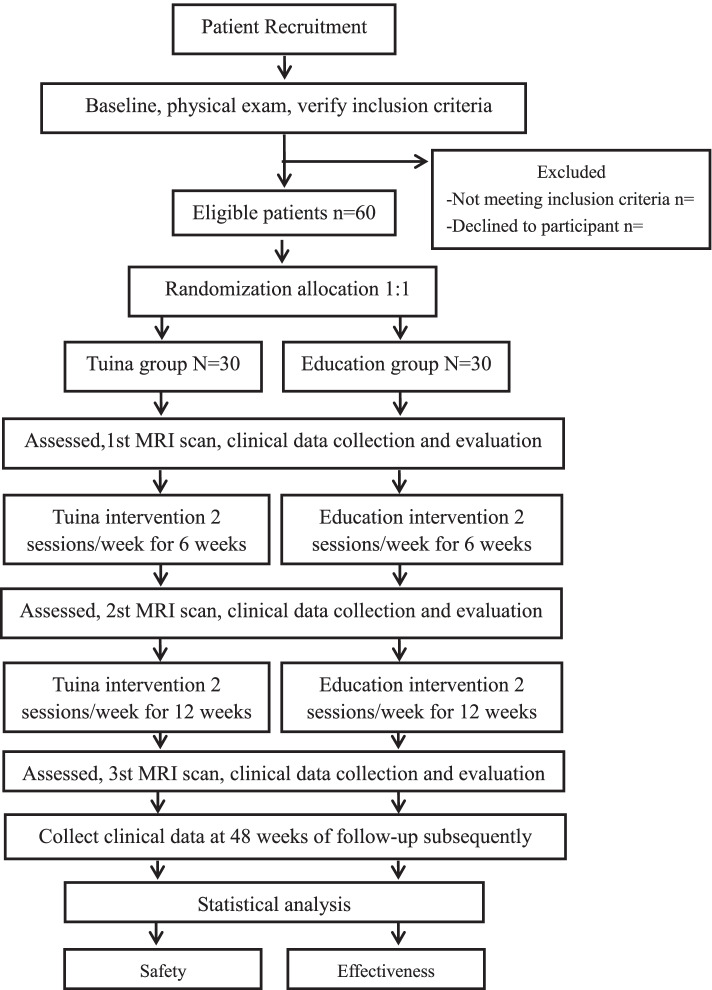
Fig. 1.
Flow chart of the trial. The present study is a randomized controlled trial using multimodal MRI. Sixty KOA patients will be included and randomized equally to two groups, a Tuina group and an Education group. For 30 patients in each group, this trial will include a 12-week treatment period. During the treatment, patients in the Tuina group will receive 24 sessions of Tuina treatments, while the Education group will receive health care education. Both the outcome assessments and MRI scan will be performed at 3 time points, namely the baseline, the end of the treatments at 6 weeks and 12 weeks. Only the clinical outcomes will be assessed during 48 weeks of follow-up subsequently. The central mechanism of Tuina in the treatment of KOA will be analyzed after data collection