ABSTRACT
The purpose of this study was to perform an initial, prospective evaluation of imaging findings and outcomes after open surgical repair of gluteus medius tendon tears with bioinductive collagen patch augmentation. A prospective study was performed of patients with clinical and magnetic resonance imaging (MRI) evidence of symptomatic gluteus medius tears who underwent open, double-row suture anchor repair with bioinductive bovine collagen patch augmentation. Preoperative and 6-month postoperative MRIs were reviewed by a fellowship-trained musculoskeletal radiologist, and outcome scores were recorded preoperatively and 6 months postoperatively [Hip Outcome Score (HOS) Sport; HOS Activities of Daily Living (HOS ADL); Modified Harris Hip Score (mHHS) and International Hip Outcomes Tool (iHOT-33)]. Nine patients, four high-grade tears (≥50% tendon thickness) and five low-grade tears (<50% thickness) underwent surgical repair. At 6 months, 7/9 (77.8%) of tendons were qualitatively classified as completely healed on MRI, with no complications. Mean tendon thickness increased significantly: mediolateral dimension by 5.8 mm (P < 0.001), anteroposterior dimension by 4.1 mm (P = 0.02) and cross-sectional area (CSA) by 48.4 mm2 (P = 0.001). Gluteus medius and minimus CSA did not change significantly (P > 0.05). Patients demonstrated improvements in mean scores for HOS ADL, mHHS and iHOT that met defined minimum clinically important differences (P < 0.05). Open surgical repair of gluteus medius tendon tears with bioinductive collagen patch augmentation is safe and associated with increased tendon thickness on postoperative MRI. Early outcome scores are encouraging and should be evaluated after patients have completed postoperative rehabilitation to measure the whole effect of treatment.
INTRODUCTION
It is estimated that the prevalence of greater trochanteric pain syndrome is around 10–25% of the population, with a predominance in women [1, 2]. Historically, patients presenting with peritrochanteric pain are often misdiagnosed with primary greater trochanteric bursitis and typically offered physical therapy or corticosteroid injections but frequently experienced persistent debilitation and pain [3, 4]. It was only within the last two decades, paralleling the development of hip arthroscopy and a deeper understanding of pathology of the athletic hip, that clinicians began to understand the previously missed underlying structural pathology in these patients [5, 6]. Abductor tendon tears are caused by attritional tendinopathy and are analogous to rotator cuff tears in the shoulder, causing debilitating weakness, a trendelenburg gait and dysfunction. In his 1999 study ‘Rotator Cuff Tears of the Hip’, Kagan first illustrated that many patients thought to have refractory greater trochanteric bursitis actually had partial-thickness undersurface gluteus medius and/or minimus tears that were identified by intraoperative palpation and repaired with good results [5]. Recent imaging-based research has shown that the incidence of partial gluteus medius and/or minimus tears in patients imaged for any reason is nearly 9% and confirmed that isolated trochanteric bursitis is rare, with nearly 90% of patients diagnosed with trochanteric bursitis actually having intrinsic, structural and degenerative pathology of the gluteus medius and/or minimus tendons [7]. Considered together, these studies suggest that the prevalence of abductor pathology may be as high as 9–23% of the general population and that the number of people with partial gluteus medius and/or minimus tears at any time may be as high as 3.3–7.5 million in the United States alone [1, 2, 7].
In recent years, surgeons have elucidated the precise anatomy of the gluteus medius tendon and its insertion in addition to another peritrochanteric anatomy [6], progress that has enhanced the clinician’s ability to properly diagnose and surgically treat tears that are refractory to nonoperative interventions. Several studies of both open and endoscopic hip abductor repair have demonstrated postoperative improvement in pain levels and patient-reported outcome (PRO) scores for most patients [8–14]. Despite these encouraging clinical results, there remains an estimated 5–25% of tears that do not heal [8–10], leaving room for potential improvement or augmentation of surgical techniques. In an effort to address the analogous clinical problem of healing limitations of the rotator cuff of the shoulder, Thon et al. described their case series of patients undergoing arthroscopic repair of large or massive rotator cuff tendon tears augmented with a bioinductive collagen patch [15]. Thon et al. demonstrated a greater than 95% healing rate, no complications related to the patch and final tendon thickness on postoperative imaging that would be expected of a healthy rotator cuff tendon. Despite its widespread use in rotator cuff tears, the use of this bioinductive patch for augmented repair of analogous hip abductor mechanism tears has been proposed [16]; however, no studies to date have described the safety, clinical or imaging outcomes related to the use of this implant in gluteus medius repairs. The present study aims to evaluate the safety and postoperative imaging findings after open repair of partial gluteus medius tendon tears with bioinductive collagen patch augmentation.
MATERIALS AND METHODS
A prospective study at a large musculoskeletal specialty hospital was performed. Patients who had clinical and magnetic resonance imaging (MRI) evidence of a partial-thickness, symptomatic gluteus medius tear who underwent open repair with bioinductive bovine collagen patch augmentation (Regeneten® Bioinductive Implant, Smith & Nephew, Andover, MA, USA) by a single surgeon from June 2018 to October 2020 were included. Exclusion criteria were arthroscopic procedures, age <18 years or a history of previous hip surgery.
The surgical technique involved a double-row repair [17] and was consistent for all patients. In the lateral position, an 8-cm skin incision centered over the tip of the greater trochanter was used, the fascia lata was incised along the anterior border of the gluteus maximus and retracted and the underlying bursa was debrided of hypertrophic tissue. The gluteus medius tendon was inspected and palpated to localize the typical undersurface tear and then incised longitudinally over the palpated undersurface defect. Through the longitudinal incision, the underlying insertional footprint was identified and debrided back to healthy, bleeding bone. A double-row repair was carried out by first placing a medial row using one or two (depending on defect size) triple-loaded 2.8-mm all-suture anchors or one or two polyether-ether-ketone (PEEK) open-architecture anchors (HEALICOIL®, Smith & Nephew, Andover, MA, USA). The medial row was then tied to repair the medial tendon back down to the prepared greater trochanter using a tension-slide technique. One free suture limb from each tied medial row knot was then affixed using a more lateral and posteriorly placed 5.5-mm PEEK suture anchor; the remaining limb from each tied medial row knot was similarly affixed to an additional anterolateral 5.5-mm PEEK anchor. The bioabsorbable collagen patch was then sutured over the repaired tendon using 2-0 nonabsorbable braided sutures in each corner and additional absorbable braided sutures along the edges (Fig. 1). Postoperatively, patients were admitted overnight for analgesia and given restrictions of 30% partial weight-bearing with a brace locked at 15° abduction and 0°–60° flexion allowed for the first 6 weeks. At 6 weeks, patients were then advanced out of the brace and progressed to full weight-bearing and range of motion of the hip. Patients were placed on 325-mg aspirin twice daily for 6 weeks for deep vein thrombosis prophylaxis. Active strengthening was not allowed until at least 3 months postoperatively.
Fig. 1.
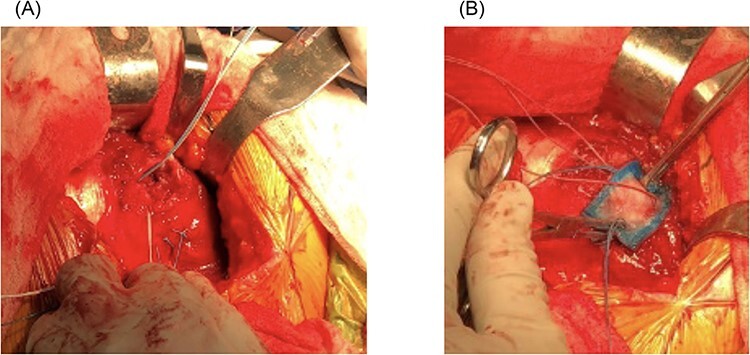
(A, B) Gluteus medius repair augmented with bioinductive collagen patch.
Preoperative and 6-month postoperative MRIs (1.5T) were reviewed by a fellowship-trained musculoskeletal radiologist who was blinded to procedure details, and the following imaging variables were assessed (Fig. 2): preoperative tear classification (low-grade [<50%], high-grade [≥50% but <100%]); fatty infiltration (FI) (reported as modified Goutallier grade 0–4 with 0 = normal muscle, 1 = minimal fatty streaks with mostly normal muscle, 2 = some FI with more muscle than fat, 3 = equivalent fat and muscle, 4 = more fat than muscle) [18]; tendon anteroposterior (AP) width; tendon mediolateral (ML) width; gluteus medius tendon cross-sectional area (CSA); gluteus medius muscle CSA; gluteus minimus CSA, which were measured at the tendon footprint using the coronal small field-of-view sequence; and qualitative classification of healed (no tear recurrence), partially healed (defined as incomplete healing) or not healed (defined as recurrence of tear). PRO scores were recorded preoperatively and 6 months postoperatively for the following measures: Hip Outcome Score Sport (HOS Sport); HOS Activities of Daily Living (HOS ADL); Modified Harris Hip Score (mHHS) and International Hip Outcomes Tool (iHOT). The Student’s t-test or Wilcoxon signed-rank test were used where appropriate to calculate significance, defined as P ≤ 0.05.
Fig. 2.
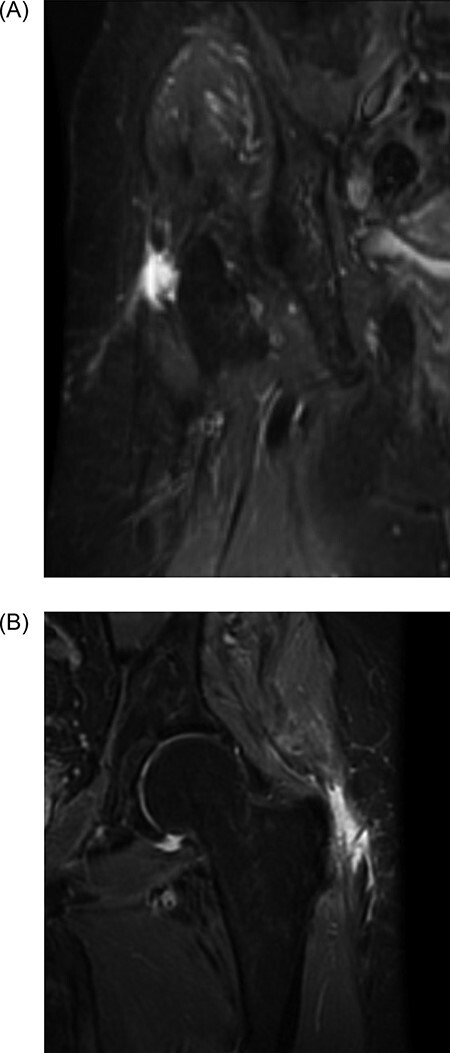
(A, B) Sagittal and coronal magnetic resonance images demonstrating high-grade abductor tear.
RESULTS
Ten patients were enrolled, and nine patients, four with high-grade tears (≥50% tendon thickness) and five with low-grade tears (<50% thickness), who underwent surgical repair with bioinductive patch augmentation, met inclusion and exclusion criteria and had both pre- and postoperative MRIs available for imaging analysis. Eight patients (89%) had complete baseline and 6-month PROs, as one patient was lost to follow-up. Demographic data included 88.9% of females with a mean age of 60.3 ± 9.3 years and a mean body mass index (BMI) of 29.4 ± 6.3 kg/m2 (Table I).
Table I.
Demographics
| Variable | n | % | Mean | SD |
|---|---|---|---|---|
| Patients | 9 | 100.0 | – | – |
| Gender | ||||
| Female | 8 | 88.9 | – | – |
| Male | 1 | 11.1 | – | – |
| Laterality | ||||
| Right | 3 | 33.3 | – | – |
| Left | 6 | 66.7 | – | – |
| Age (years) | – | – | 60.3 | 9.3 |
| BMI (kg/m2) | – | – | 29.4 | 6.3 |
Imaging characteristics are reported in Table II and in Fig. 3A and C. At 6 months postoperative, 7/9 (77.8%) of tendons were qualitatively classified as completely healed on 6-month postoperative MRI. There was a statistically significant average increase in mean tendon ML thickness by 5.8 mm (5.9 ± 2.1 mm preoperatively to 11.7 ± 4.3 mm postoperatively), P < 0.001. In the AP dimension, mean tendon width significantly increased on average by 4.1 mm (17.9 ± 6.1 mm preoperatively to 22.0 ± 7.2 mm postoperatively), P = 0.02. The CSA of the tendon significantly increased on average by 48.4 mm2 (195.1 ± 69.9 mm preoperatively to 243.5 ± 80.3 mm postoperatively), P = 0.001. Finally, there was a trend toward more FI and lower gluteus medius and minimus CSA on postoperative imaging, although not reaching statistical significance (P > 0.05).
Table II.
MRI characteristics preoperatively and 6 months postoperatively
| Preoperative MRI | Postoperative MRI | |||||
|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | ||
| Tear classification | ||||||
| Low-grade (<50%) | 5 | 55.6 | – | – | ||
| High-grade (≥50%) | 4 | 44.4 | – | – | ||
| FI grade | ||||||
| 0 | 9 | 100.0 | 6 | 66.7 | ||
| 1 | 0 | 0.0 | 0 | 0.0 | ||
| 2 | 0 | 0.0 | 2 | 22.2 | ||
| 3 | 0 | 0.0 | 1 | 11.1 | ||
| 4 | 0 | 0.0 | 0 | 0.0 | ||
| Healed | ||||||
| Yes | – | – | 7 | 77.8 | ||
| Partially healed | – | – | 2 | 22.2 | ||
| Mean | SD | Mean | SD | Change | P-value | |
| ML tendon thickness (mm) | 5.9 | 2.1 | 11.7 | 4.3 | 5.9 | <0.001 |
| AP tendon width (mm) | 17.9 | 6.1 | 22.0 | 7.2 | 4.7 | 0.02 |
| Tendon CSA (mm2) | 195.1 | 69.9 | 243.5 | 80.3 | 106.5 | 0.001 |
| Medius CSA (mm2) | 1021.3 | 492.8 | 889.6 | 196.0 | −183.3 | 0.06 |
| Minimus CSA (mm2) | 799.3 | 347.4 | 779.9 | 276.7 | −80.2 | 0.18 |
Fig. 3.
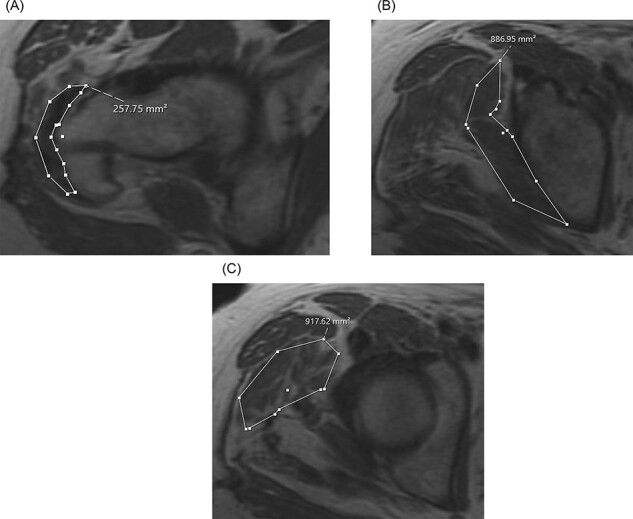
(A–C) Axial magnetic resonance images demonstrating postoperative (A) abductor tendon CSA, (B) gluteus minimus CSA and (C) gluteus medius CSA.
The results for preoperative and 6-month postoperative PRO scores are reported in Table III. Patients demonstrated improvements in mean scores for the HOS ADL (P = 0.002), mHHS (P = 0.01) and iHOT (P = 0.0007) that met defined minimum clinically important differences (MCIDs) (P < 0.05). There was no difference in pre- and postoperative HOS Sport (P = 0.43). There were no complications noted in any patient during the 6-month follow-up period.
Table III.
PRO measures
|
PRO
measure |
n | Preoperative (mean ± SD) | n | 6-month postoperative (mean ± SD) | P-value |
|---|---|---|---|---|---|
| HOS Sport | 7/8 | 10.3 ± 7.3 | 6/8 | 10.3 ± 11.1 | 0.43 |
| HOS ADL | 8/8 | 41.9 ± 8.5 | 7/8 | 66.0 ± 8.0 | 0.002 |
| mHHS | 8/8 | 48.2 ± 12.2 | 8/8 | 72.0 ± 13.7 | 0.01 |
| iHOT | 8/8 | 23.9 ± 9.7 | 8/8 | 72.1 ± 16.9 | 0.0007 |
DISCUSSION
The purpose of the present study was to perform an initial evaluation of safety and postoperative imaging findings 6 months following open repair of chronic, degenerative partial gluteus medius tendon tears with bioinductive collagen patch augmentation. All patients healed their surgical incisions, and there were no serious complications related to the use of the patch or otherwise in this initial cohort. Although a similar augmentation technique has been described previously [16], this is the first report documenting the utilization of a bioinductive collagen patch in chronic, degenerative gluteus medius repairs. The safety demonstrated herein is consistent with what has been reported with the use of the same patch in other applications. Furthermore, significant increases in tendon thickness, width and CSA were observed, suggestive of a robust healing response, and early clinical outcome scores were promising. Thon et al. reported on their experience using this bioinductive patch to augment two- or three-tendon rotator cuff repairs in a cohort of 23 patients and similarly noted no complications related to the graft and high healing rates [15]. The results demonstrated by this analogous study in the shoulder and confirmed in the present study in the hip are a fundamental prerequisite to future comparative studies.
The next major aim of this study was to describe the 6-month postoperative MRI characteristics of the repaired tendons. At 6 months postoperative, 7/9 (77.8%) of tendons were qualitatively classified as completely healed by a fellowship-trained musculoskeletal radiologist. These findings are not surprising, as there have been imperfect healing rates noted in multiple prior clinical studies of gluteus medius repairs [8–10, 19]. The patch serves to improve the biology surrounding the repair and healing environment with enhanced vascularity. Although 2/9 patients demonstrated incomplete (partial) healing, clinical outcomes in these patients were favorable and these findings and their clinical correlation need to be confirmed at longer follow-up and in a larger cohort.
A robust healing response of the repaired tendons was also suggested in this study, as evidenced by a statistically significant increase in the postoperative thickness (ML), width (AP) and CSA. This is in contrast to a previous study of repair without bioinductive patch augmentation performed by McGonagle et al., in which pre- and postoperative MRI characteristics were compared and no major differences in tendon characteristics including thickness were found [20]. A similar study that compared follow-up MRIs between surgically repaired (without a bioinductive patch) and non-operatively treated gluteal tears also showed no significant differences in follow-up MRIs between the two cohorts [21]. In contrast to these previous two studies, the present study suggests that augmentation of gluteus medius repairs with a bioinductive collagen patch may be associated with increased tendon thickness, width and CSA at short-term follow-up.
Although not reaching statistical significance, there was a trend toward more FI and lower gluteus medius and minimus CSA on postoperative imaging in the present study. This phenomenon has also been suggested by previous studies evaluating postoperative imaging characteristics of the hip abductor mechanism [20]. The authors of the present study believe that the atrophy observed here at short-term follow-up may be the result of disuse because of surgery and necessary postoperative restrictions and may resolve with longer follow-up and appropriate rehabilitation and strengthening. Indeed, recovery of muscle mass at longer follow-up has been suggested in previous work [21]. Further follow-up and repeat imaging of this cohort would be beneficial in evaluating this trend.
Given the short-term time frame of this initial safety study and the extensive postoperative rehabilitation period required after gluteus medius repair, defining clinical outcomes was not a primary aim herein; however, the initial findings were surprisingly favorable at this early postoperative timepoint. There were improvements in mean scores for the HOS ADL, mHHS and iHOT outcome measures, and each of these met established MCID criteria [22–24]. Although our sample size was too small to perform linear regression analysis evaluating outcomes based on pre- and postoperative imaging characteristics, there was no observed trend between MRI evidence of postoperative FI and healing and 6-month outcomes. Furthermore, patients with evidence of incomplete healing on postoperative MRI actually scored better or the same on 6-month mHHS, HOS Sport and HOS ADL than the mean. These initial positive results are encouraging and may further improve at future follow-up timepoints after patients have completed their full postoperative rehabilitation and regained full strength.
There are some important limitations to this study. First, the lack of a control group and relatively short follow-up limits the ability to conclude that the imaging findings of increased tendon dimensions and areas are the direct result of bioinductive patch augmentation. The authors believe that the contrast to other published studies of abductor repairs without augmentation provides an initial suggestion that the findings herein are novel but ultimately agree that comparative studies are needed before definitive conclusions can be made. Similarly, comparative studies would help define if augmented repair provides additional clinical improvement over repair alone, as the proportion of clinical improvement observed herein that is attributable directly to the augmentation remains unknown. Finally, the small sample size of this study limits the ability to detect uncommon complications, and expansion of this work into a larger cohort would be beneficial to determine their true incidence.
CONCLUSIONS
Open surgical repair of partial gluteus medius tendon tears with bioinductive collagen patch augmentation is safe as it is not associated with early postoperative complications in a small cohort. On 6-month postoperative imaging, significant increases in tendon thickness, width and CSA are observed with this augmentation technique. Clinical outcome scores at this early postoperative timepoint are encouraging and should be evaluated after patients have completed their full postoperative rehabilitation and regained full strength to measure the whole effect of treatment. Future comparative and prospective studies are warranted to determine clinical efficacy.
Contributor Information
Molly A Day, Sports Medicine Institute, Department of Orthopaedic Surgery, Hospital for Special Surgery, 535 E 70th Street, New York, NY 10021, USA; Department of Orthopedics and Rehabilitation, University of Wisconsin, UW Health East Madison Hospital, 4602 Eastpark Blvd, Madison, WI 53718, USA.
Kyle J Hancock, Sports Medicine Institute, Department of Orthopaedic Surgery, Hospital for Special Surgery, 535 E 70th Street, New York, NY 10021, USA; Desert Orthopaedic Center, 2800 E Desert Inn Rd, Las Vegas, NV 89121, USA.
Ryan S Selley, Sports Medicine Institute, Department of Orthopaedic Surgery, Hospital for Special Surgery, 535 E 70th Street, New York, NY 10021, USA.
Erica L Swartwout, Sports Medicine Institute, Department of Orthopaedic Surgery, Hospital for Special Surgery, 535 E 70th Street, New York, NY 10021, USA.
Matthew Dooley, Sports Medicine Institute, Department of Orthopaedic Surgery, Hospital for Special Surgery, 535 E 70th Street, New York, NY 10021, USA.
Alan G Shamrock, Sports Medicine Institute, Department of Orthopaedic Surgery, Hospital for Special Surgery, 535 E 70th Street, New York, NY 10021, USA; Department of Orthopedics and Rehabilitation, University of Iowa Hospitals & Clinics, 200 Hawkins Dr, Iowa City, IA 52242, USA.
Benedict U Nwachukwu, Sports Medicine Institute, Department of Orthopaedic Surgery, Hospital for Special Surgery, 535 E 70th Street, New York, NY 10021, USA.
Harry G Greditzer, Department of Radiology & Imaging, Hospital for Special Surgery, 535 E 70th Street, New York, NY 10021, USA.
Anil S Ranawat, Sports Medicine Institute, Department of Orthopaedic Surgery, Hospital for Special Surgery, 535 E 70th Street, New York, NY 10021, USA.
DATA AVAILABLITY
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
One or more of the authors report affiliation or involvement in an organization with a financial interest in the subject matter or materials discussed in this manuscript. Anil S. Ranawat is a paid consultant for Smith & Nephew.
Funding
Smith & Nephew, Investigator Initiated with Industry Support [Project # P2019-0752].
REFERENCES
- 1. Segal NA, Felson DT, Torner JC. et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil 2007; 88: 988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams BS, Cohen SP. Greater trochanteric pain syndrome: a review of anatomy, diagnosis and treatment. Anesth Analg 2009; 108: 1662–70. [DOI] [PubMed] [Google Scholar]
- 3. Little H. Trochanteric bursitis: a common cause of pelvic girdle pain. Can Med Assoc J 1979; 120: 456–8. [PMC free article] [PubMed] [Google Scholar]
- 4. Swezey RL. Pseudo-radiculopathy in subacute trochanteric bursitis of the subgluteus maximus bursa. Arch Phys Med Rehabil 1976; 57: 387–90. [PubMed] [Google Scholar]
- 5. Kagan A 2nd. Rotator cuff tears of the hip. Clin Orthop Relat Res 1999; 368: 135–40. [PubMed] [Google Scholar]
- 6. Robertson WJ, Gardner MJ, Barker JU. et al. Anatomy and dimensions of the gluteus medius tendon insertion. Arthroscopy 2008; 24: 130–6. [DOI] [PubMed] [Google Scholar]
- 7. Oehler N, Ruby JK, Strahl A. et al. Hip abductor tendon pathology visualized by 1.5 versus 3. 0 Tesla MRIs. Arch Orthop Trauma Surg 2020; 140: 145–53. [DOI] [PubMed] [Google Scholar]
- 8. Davies H, Zhaeentan S, Tavakkolizadeh A. et al. Surgical repair of chronic tears of the hip abductor mechanism. Hip Int 2009; 19: 372–6. [DOI] [PubMed] [Google Scholar]
- 9. Davies JF, Stiehl JB, Davies JA. et al. Surgical treatment of hip abductor tendon tears. J Bone Joint Surg Am 2013; 95: 1420–5. [DOI] [PubMed] [Google Scholar]
- 10. Walsh MJ, Walton JR, Walsh NA. Surgical repair of the gluteal tendons: a report of 72 cases. J Arthroplasty 2011; 26: 1514–9. [DOI] [PubMed] [Google Scholar]
- 11. McCormick F, Alpaugh K, Nwachukwu BU. et al. Endoscopic repair of full-thickness abductor tendon tears: surgical technique and outcome at minimum of 1-year follow-up. Arthroscopy 2013; 29: 1941–7. [DOI] [PubMed] [Google Scholar]
- 12. Thaunat M, Chatellard R, Noel E. et al. Endoscopic repair of partial-thickness undersurface tears of the gluteus medius tendon. Orthop Traumatol Surg Res 2013; 99: 853–7. [DOI] [PubMed] [Google Scholar]
- 13. Voos JE, Shindle MK, Pruett A. et al. Endoscopic repair of gluteus medius tendon tears of the hip. Am J Sports Med 2009; 37: 743–7. [DOI] [PubMed] [Google Scholar]
- 14. Byrd JW. Gluteus medius repair with double-row fixation. Arthrosc Tech 2013; 2: e247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thon SG, O’Malley L 2nd, O’Brien MJ. et al. Evaluation of healing rates and safety with a bioinductive collagen patch for large and massive rotator cuff tears: 2-year safety and clinical outcomes. Am J Sports Med 2019; 47: 1901–8. [DOI] [PubMed] [Google Scholar]
- 16. Gulledge CM, Makhni EC. Open gluteus medius and minimus repair with double-row technique and bioinductive implant augmentation. Arthrosc Tech 2019; 8: e585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kahlenberg CA, Nwachukwu BU, Jahandar J. et al. Single- versus double-row repair of hip abductor tears: a biomechanical matched cadaver study. Arthroscopy 2019; 35: 818–23. [DOI] [PubMed] [Google Scholar]
- 18. Engelken F, Wassilew GI, Kohlitz T. et al. Assessment of fatty degeneration of the gluteal muscles in patients with THA using MRI: reliability and accuracy of the Goutallier and quartile classification systems. J Arthroplasty 2014; 29: 149–53. [DOI] [PubMed] [Google Scholar]
- 19. Chandrasekaran S, Lodhia P, Gui C. et al. Outcomes of open versus endoscopic repair of abductor muscle tears of the hip: a systematic review. Arthroscopy 2015; 31: 2057-67.e2. [DOI] [PubMed] [Google Scholar]
- 20. McGonagle L, Haebich S, Breidahl W. et al. MRI and clinical analysis of hip abductor repair. Hip Int 2015; 25: 24–7. [DOI] [PubMed] [Google Scholar]
- 21. Lequesne M, Djian P, Vuillemin V. et al. Prospective study of refractory greater trochanter pain syndrome. MRI findings of gluteal tendon tears seen at surgery. Clinical and MRI results of tendon repair. Joint Bone Spine 2008; 75: 458–64. [DOI] [PubMed] [Google Scholar]
- 22. Martin RL, Philippon MJ. Evidence of reliability and responsiveness for the hip outcome score. Arthroscopy 2008; 24: 676–82. [DOI] [PubMed] [Google Scholar]
- 23. Nwachukwu BU, Chang B, Beck EC. How should we define clinically significant outcome improvement on the iHOT-12? HSS J 2019; 15: 103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kemp JL, Collins NJ, Roos EM. et al. Psychometric properties of patient-reported outcome measures for hip arthroscopic surgery. Am J Sports Med 2013; 41: 2065–73. [DOI] [PubMed] [Google Scholar]


