ABSTRACT
Cartilage oligomeric matrix protein (COMP) is an emerging regulator of tumor progression. The aim of this study was to evaluate the expression of COMP in periampullary adenocarcinoma with respect to prognostic value for survival and relapse, levels of fibrosis and infiltrating immune cells. COMP expression was evaluated using immunohistochemistry in primary tumors and subsets of paired lymph node metastases in tissue microarrays including 175 patients with periampullary adenocarcinoma. Collagen content was assessed with Sirius Red-Fast Green staining. High COMP levels were detected in cancer cells and in stroma, in 46% and 57% of the patients, respectively. High COMP expression was strongly associated with more aggressive pancreatobiliary-type (PB-type) compared to intestinal-type tumors (p < .0001). Importantly, high expression of COMP correlated with the exclusion of cytotoxic T-cells from the cancer cell compartment of the tumors, particularly in PB-type tumors. Higher levels of fibrosis measured by the density of collagen fibers correlated with high COMP levels in both cancer cells and stroma. This in turn could lead to exclusion of cytotoxic T-cells from accessing the cancer cells, a recognized immunotherapy resistance mechanism. Targeting COMP could therefore be considered as a novel therapeutic strategy in PB-type periampullary adenocarcinoma.
KEYWORDS: COMP, pancreatic cancer, immune cell exclusion, fibrosis, collagen
Introduction
Cartilage oligomeric matrix protein (COMP) is a large pentameric protein that under normal conditions is almost exclusively expressed in the cartilage. COMP is a regulator of the extracellular matrix (ECM) assembly.1 Fibrosis is characterized by excessive production of ECM proteins, which leads to stiffening of the tissue causing cellular stress and organ malfunction.2 COMP is highly expressed in fibrotic diseases, such as scleroderma,3 idiopathic pulmonary fibrosis,4 and liver fibrosis.5,6 Under fibrotic conditions, COMP plays a vital role in the organization of ECM and regulates type I and II collagen7 production and fibrillogenesis.8 Moreover, in COMP knockout mice, the organization of collagen fibers is unraveled, and development of fibrosis attenuated. Moreover, fibroblasts lacking expression of COMP are unable to secret and deposit collagens into the ECM, leading to intracellular stress due to collagen accumulation in the endoplasmic reticulum.9
COMP is expressed by breast cancer cells and deposited in tumor stroma acting as an independent prognostic marker in breast cancer.10 Additionally, levels of COMP released into serum can independently predict survival of breast cancer patients.11 Functional studies utilizing the COMP knock-out MMTV-PyT spontaneous breast cancer carcinoma model, showed that COMP facilitates the interaction between Notch3 and its ligand, Jag1, which leads to generation of a larger population of cancer stem cells.12 In prostate cancer COMP has a similar detrimental role, where COMP-expressing prostate cancer cells are resistant to various stress-inducing apoptotic agents and docetaxel, likely due to the ability of COMP to regulate intracellular calcium homeostasis.13 Several reports support the hypothesis that COMP can also act as a tumor promoting factor in other types of cancers, such as hepatocellular carcinoma,14 colon cancer,15,16 parotid gland carcinoma,17 and potentially in ovarian cancer.18
Periampullary adenocarcinomas are a heterogeneous group of malignant cancers sharing a common anatomical origin in or around the ampulla of Vater. Periampullary adenocarcinomas are classified clinically into two morphological subtypes; intestinal type (I-type) including duodenal carcinoma and some ampullary carcinomas, and pancreatobiliary type (PB-type) including pancreatic cancer, distal bile duct cancer and some ampullary carcinomas. Stratification into morphological subtypes is important as PB-type tumors have a significantly worse prognosis.19 However, they share a common characteristic of being generally unresponsive to treatment, with most of the patients not responding to chemotherapy.20,21 Moreover, modern therapies such as immunotherapy, immune checkpoint inhibitors, and CAR-T cells display low effectiveness in pancreatic cancer.22 One proposed reason for this failure is the presence of fibrosis leading to immune exclusion.23 The fibrotic, dense ECM tumor microenvironment makes the cancer cells “unreachable” by the immune cells. In various types of cancers, it is well established that the exclusion of cytotoxic T-cells leads to immune checkpoint therapy resistance.24
The present study revealed that COMP expression is tightly correlated with the PB-type of periampullary adenocarcinoma, with over 60% of the included PB-type tumors expressing high COMP levels. We also discovered a novel connection between immune cell infiltration in the tumor microenvironment and COMP levels. Notably, immune exclusion of cytotoxic T cells was associated with a denser collagen matrix in the COMP-containing tumor microenvironment.
Materials and methods
Study cohort
The analyzed material is a retrospective, consecutive series of all primary tumors from 175 patients with periampullary adenocarcinoma who underwent pancreaticoduodenectomy (Table 1) at Skåne University Hospital from 1 January 2001 to 31 December 2011.25,26 Follow-up started at surgery and ended at death or 31 March 2017. The Swedish National Civil Registry was used to obtain data on vital status. Data on neoadjuvant and adjuvant treatment, and disease recurrence were obtained from patient records. All 175 tumors underwent strict histopathological evaluation at the start of the study with 110 tumors classified as PB-type, and 65 as I-type. The anatomical origin of the tumors was in 14 cases duodenal, 46 cases pancreatic, 70 ampullary, and 45 from the distal bile duct. The study was approved by the Ethics committee of Lund University (ref nr 445/07) whereby the committee waived no need for consent other than by the option to opt out. All patient data were anonymized and de-identified prior to analysis.
Table 1.
Associations of clinicopathological characteristics with COMP expression.
| Cancer cells | Stroma cells | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | COMP low |
COMP high |
p-value | COMP low |
COMP high |
p-value | ||||
| |
N |
(%) |
N |
(%) |
|
N |
(%) |
N |
(%) |
|
| All (N = 170) | 92 | 54.1 | 78 | 45.9 | 73 | 42.9 | 97 | 57.1 | ||
| Age at surgery | 0.832b | 0.349b | ||||||||
| <50 | 8 | 4.7% | 4 | 2.4% | 5 | 2.9% | 7 | 4.1% | ||
| 50–70 | 56 | 32.9% | 51 | 30.0% | 43 | 25.3% | 64 | 37.6% | ||
| >70 | 28 | 16.5% | 23 | 13.5% | 25 | 14.7% | 26 | 15.3% | ||
| Sex | 0.420a | 0.139a | ||||||||
| Female | 47 | 27.6% | 35 | 20.6% | 40 | 23.5% | 42 | 24.7% | ||
| Male | 45 | 26.5% | 43 | 25.3% | 33 | 19.4% | 55 | 32.4% | ||
| Morphological subtype | <0.0001a | <0.0001a | ||||||||
| Intestinal | 52 | 30.6% | 11 | 6.5% | 48 | 28.2% | 15 | 8.8% | ||
| Pancreatobiliary | 40 | 23.5% | 67 | 39.4% | 25 | 14.7% | 82 | 48.2% | ||
| Adjuvant vs no adjuvant | 0.002a | 0.001a | ||||||||
| No adjuvant | 61 | 35.9% | 33 | 19.4% | 51 | 30.0% | 43 | 25.3% | ||
| Adjuvant | 31 | 18.2% | 45 | 26.5% | 22 | 12.9% | 54 | 31.8% | ||
| Anatomical tumor origin | <0.0001b | <0.0001b | ||||||||
| Duodenum | 11 | 6.5% | 3 | 1.8% | 11 | 6.5% | 3 | 1.8% | ||
| Papilla-Ampulla Intestinal | 41 | 24.1% | 8 | 4.7% | 37 | 21.8% | 12 | 7.1% | ||
| Papilla-Ampulla Pancreatobiliary | 9 | 5.3% | 10 | 5.9% | 8 | 4.7% | 11 | 6.5% | ||
| Distal Bile Duct | 18 | 10.6% | 26 | 15.3% | 7 | 4.1% | 37 | 21.8% | ||
| Pancreas | 13 | 7.6% | 31 | 18.2% | 10 | 5.9% | 34 | 20.0% | ||
| Tumor size | <0.0001a | <0.0001a | ||||||||
| ≤ 20 mm | 31 | 18.2% | 7 | 4.1% | 28 | 16.5% | 10 | 5.9% | ||
| >20 mm | 61 | 35.9% | 71 | 41.8% | 45 | 26.5% | 87 | 51.2% | ||
| N-stage | 0.005a | 0.004a | ||||||||
| pN0 | 44 | 25.9% | 21 | 12.4% | 37 | 21.8% | 28 | 16.5% | ||
| pN1 | 48 | 28.2% | 57 | 33.5% | 36 | 21.2% | 69 | 40.6% | ||
| T-stage | 0.030a | 0.007a | ||||||||
| pT1-T2 | 21 | 12.4% | 8 | 4.7% | 19 | 11.2% | 10 | 5.9% | ||
| pT3-T4 | 71 | 41.8% | 70 | 41.2% | 54 | 31.8% | 87 | 51.2% | ||
| R-margin status | 0.013a | <0.0001a | ||||||||
| R0 | 18 | 10.6% | 5 | 2.9% | 18 | 10.6% | 5 | 2.9% | ||
| R1 or Rx | 74 | 43.5% | 73 | 42.9% | 55 | 32.4% | 92 | 54.1% | ||
| Perineural growth | <0.0001a | <0.0001a | ||||||||
| No growth | 56 | 32.9% | 12 | 7.1% | 51 | 30.0% | 17 | 10.0% | ||
| Perineural growth | 36 | 21.2% | 66 | 38.8% | 22 | 12.9% | 80 | 47.1% | ||
| Cancer in lymph vessels | 0.272a | 0.084a | ||||||||
| No cancer | 37 | 21.8% | 25 | 14.7% | 32 | 18.8% | 30 | 17.6% | ||
| Cancer | 55 | 32.4% | 53 | 31.2% | 41 | 24.1% | 67 | 39.4% | ||
| Cancer in blood vessels | 0.041a | 0.001a | ||||||||
| No cancer | 75 | 44.1% | 53 | 31.2% | 64 | 37.6% | 64 | 37.6% | ||
| Cancer | 17 | 10.0% | 25 | 14.7% | 9 | 5.3% | 33 | 19.4% | ||
| Growth in peripancreatic fat | <0.0001a | <0.0001a | ||||||||
| No growth | 50 | 29.4% | 14 | 8.2% | 47 | 27.6% | 17 | 10.0% | ||
| Growth | 42 | 24.7% | 64 | 37.6% | 26 | 15.3% | 80 | 47.1% | ||
Abbreviations: COMP, cartilage oligomeric matrix protein; The bold indicates p-values <0.05. aMann–Whitney two-tailed Exact p-value, bKruskal-Wallis p-value.
Immunohistochemical staining
Tissue microarrays (TMA) were constructed as previously described,27 with three 1 mm tissue cores from each resected primary tumor and from 1 to 3 lymph node metastases in 105 cases. Clinicopatological characteristics in cases with or without lymph node metastases are summarized in table S4. Immunohistochemical staining for COMP expression was performed by using 0.47 μg/ml rabbit anti-human COMP antibody (Agrisera) as previously described.10 Stained tissue was scanned for evaluation using an Aperio Scanner (Leica) at 40X. Expression of COMP was evaluated blindly by two experienced observers and scored: 0 for negative staining, 1 for minimal intensity, 2 for moderate intensity, and 3 for high intensity. COMP expression was assessed separately in cancer cells or in stroma (Figure 1a). Additionally, expression of COMP was evaluated with QuPath open-source software for digital pathology image analysis,28 calculating the total amount of positive cells found among the cancer cells and stroma. For majority of patients, two samples were stained and the result was averaged. Median was used as a cut-off to categorize patient samples into low vs. high COMP expression.
Figure 1.
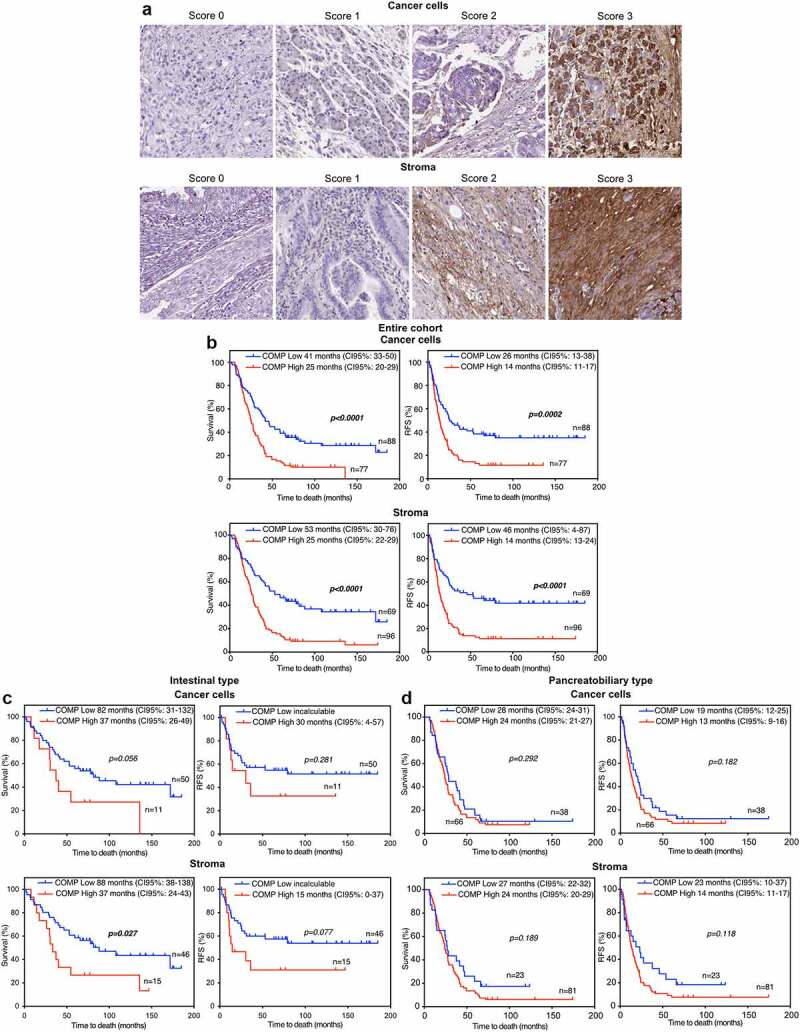
High levels of COMP are associated with a worse prognosis of periampullary adenocarcinoma patient measured as overall survival (OS) and recurrence -free survival (RFS) in whole cohort due to high expression of COMP in morphologic PB-type. (a) Tissue microarrays including biopsies from 165 patients were stained for COMP. The intensity of staining was scored separately for cancer cells and stroma by two independent observers. (b) Patient were stratified in low (score 0–1) and high (score 2–3) COMP expressing groups. Kaplan Meier estimation of OS and RFS showed that high levels of COMP expression in cancer cells or in stroma correlated with shorter OS and RFS of patients. (c, d) OS and RFS survival analyses performed on I-type and PB-type separately. In I-type there was a tendency toward correlation of COMP expression with OS while such correlation was not found in PB-type.
Multiplex immunofluorescence staining
Leucocyte staining and evaluation was performed as described previously.29,30 In short, three immune panels were established to target tumor infiltrating lymphocytes (TIL-panel), natural killer cells, macrophages, myeloid cells (NK/MF-panel) and dendritic, or antigen presenting cells (APC-panel). The TIL panels comprised antibodies against CD4, CD8, CD20, FoxP3, CD45RO; the NK/MF: CD3, CD56, NKp46, CD68, CD163; The APC panel: CD3, CD1a, CD208, CD123, CD68, CD15. Additionally, each panel had pan-cytokeratin antibody to mark epithelial (cancer) cells. Specific antibodies against different markers were used (Table S5).
The staining was developed with Opal fluorophores and scanned with the Phenoimager HT system (Akoya Biosciences) at resolution 2 pixels per um. Spectral deconvolution was performed with inForm (Akoya Biosciences). DAPI staining was used for cell segmentation. Each marker was visually annotated on the random selection of cells (approx. 50–150 annotated cells for each marker) to determine the positivity thresholds, which were further applied for the complete dataset to characterize each cell as positive or negative in terms of each marker expression in a panel. This binary information was then used to classify each cell, as illustrated in Table S6. The examples of different cell phenotypes are shown in Figure 2c.
Figure 2.
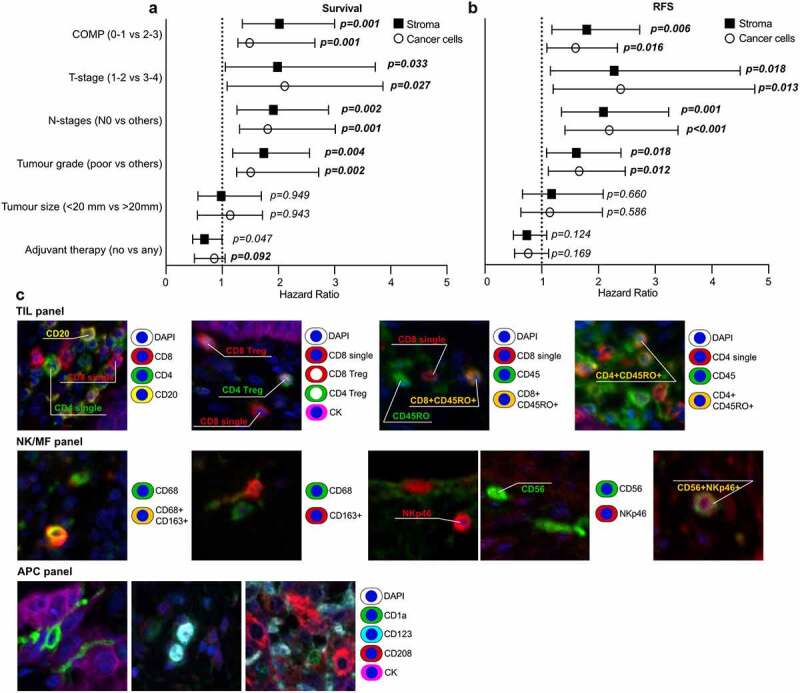
Forest plots illustrating Cox multivariable analyses showing that COMP is independent prognostic of the other analyzed markers of patient overall survival (a) and recurrence free survival (b) in the whole cohort. Plots demonstrate the median hazard ratio and 95% confidence interval for every variable included in the multivariable analysis. (c) Representative images of the multiplex florescence staining used to characterize the various immune cell populations.
The inForm software was used to develop a machine-learning algorithm allowing segmentation of the three classes inside each TMA image: Tumor, Stroma, and Blank. Tumor and Stroma regions were used to compute stroma fraction in the tissue sample, as described.31
Collagen staining
TMA sections were stained for collagen using 0.1% Sirius Red (Sigma-Aldrich) – 0.04% Fast Green (Merck) staining solution in saturated picric acid. Slides were deparaffinised, hydrated, and stained with collagen staining solution for 60 min, rinsing twice in acetic acid, then alcohol dehydration, following Weigert's hematoxylin nuclear staining soluiton (Histolab), and finally mounting (Pertex). Staining intensity of collagen was assessed using QuPath and quantification of matrix characteristics was analyzed by FIJI macro TWOMBLI.32 Serial cuts were used for COMP, collagen, and immune cells staining.
Results
High levels of COMP are detected in half of the patients with periampullary adenocarcinoma and correlate with the morphological PB-type
TMAs were stained for COMP expression and results grouped as a low expression for scores 0–1 and high expression for scores 2–3. Expression levels by the cancer cells and deposits in stroma were assessed separately (Figure 1a). Five patients were excluded from the study due to tissue detachment or lack of cancer cells in the section. We detected high levels of COMP expression from the cancer cells in 45.9% (n = 78) and in the tumor stroma in 57.1% (n = 92) of the patients (Table 1). In support of our results, data extracted from the GEPIA interactive web server containing RNA sequencing data of 9,736 tumors and 8,587 normal samples33 showed that the pancreatic cancers had a COMP expression that was, on average, 70 times higher than in corresponding healthy tissue (Figure S1A).
Importantly, high levels of COMP expression were strongly associated with PB-type tumors (p < .0001) compared to I-type. PB-type tumors had high COMP expression in cancer cells and stroma in 63% and 77% of patients, respectively, compared to 6.8 and 8.8% of I-type tumors (Table S1). In the whole cohort, high levels of COMP expression both by cancer cells and in stroma were associated with larger tumor size (p < .0001), T-stage and N-stage, invasion into perineural structures (p < .0001), and peripancreatic fat (p < .0001). Despite relatively few I-type tumors expressing COMP, most associations remained significant for this type when patients were stratified by morphology (Table S1). In PB-tumors, invasion into perineural structures was significantly associated with high COMP expression by cancer cells and stroma. In addition, expression of COMP in stroma correlated with tumor size and invasion in to peripancreatic fat (Table S2).
COMP expression in both cancer cells and stroma correlates with poor prognosis for patients with periampullary adenocarcinoma
In the survival analyses, five patients were excluded since two received neoadjuvant therapy, one could not be followed due to emigration, and two died of complications within 1-month post-surgery (n = 165). In the analysis of the whole cohort, patients with high levels of COMP expressed by cancer cells had 25 months median overall survival (OS) compared to 41 months for those with low levels (p < .0001, (Figure 1b)). Accordingly, patients expressing high levels of COMP in cancer cells, had median recurrence-free survival (RFS) of 14 months compared to 26 months for patients with low expression of COMP (p = .0002). COMP expression in stroma was shown to have a similar impact on patient outcome. Patients with high levels of COMP in stroma had median OS of 25 months and median RFS of 14 months. Patients with low levels of COMP in stroma had a median OS of 53 months (p < .0001) and median RFS of 46 months (p < .0001). Estimation of OS and RFS at 5-years post-surgery for all the above parameters yielded similar results (Figure S2A&B). In addition, the percentage of COMP positive cells (including both cancer cells and stroma) was determined for each sample with QuPath software and patients stratified as low and high COMP expressing according to median. Calculation of OS and RFS yielded the same results as with the scoring evaluation of COMP expression (Figure S5C&D). In agreement with those results, data generated by the Kaplan-Meier Plotter for Pan-cancer RNA-seq for pancreatic cancer patients (n = 176) demonstrated worse prognosis (p = .024) of patients with high expression of COMP (Figure S1B).
When stratified by morphology, only high COMP expression in stroma was correlated with worse median OS (p = .027, Figure 1c) in I-type tumors and there was similar tendency for high COMP expression in cancer cells. No statistically significant correlations were found in PB-type tumors. In contrast, in multivariable Cox analysis, COMP expression in stroma was independently prognostic from the morphological tumor type in OS and RFS (Figure S3A). This indicates that the group sizes of patients with I-type (n = 61) and PB-type (n = 104) are small. Effects of high levels of expression of COMP on OS and RFS observed in the whole cohort might be driven by its strong association with more aggressive PB-type.
The prognostic value of COMP expression in the whole cohort was assessed using multivariable Cox regression analysis model including variables of adjuvant therapy, tumor, size the tumor grade, the N-stage, and T-stage. High levels of COMP expression in cancer cells were an independent prognostic marker for OS (p = .001, HR = 1.487) or RFS (p = .016, HR = 1.596). The high expression of COMP in the stroma yielded similar results (OS: p = .001, HR = 2.018 and RFS: p = .006, HR = 1.795). The tumor grade, the N-stage, and T-stage were also independent prognostic markers (Figure 2 and Table S3). When patients were stratified according to the morphological type of tumors (Figure S3B), COMP expression was not an independent prognostic marker for OS and RFS.
Evaluation of COMP expression in metastases
Varying levels of COMP were detected in the cancer cells and in stroma of matched lymph node metastases that could be assessed in 81 cases. McNemar analysis evaluating change in COMP expression from primary tumor to the metastases of the same patients revealed that eight tumors retained COMP cancer cell expression at the metastatic site (Table 2). Further, 23 matched pairs of metastatic and primary tumors exhibited COMP expression in stroma. However, most pairs showed a loss of COMP expression in metastatic samples when it was evaluated in cancer cells (60 pairs of tumors, p < .0001) or in stroma (44 pairs of tumors, p < .0001).
Table 2.
Alteration in COMP expression from initial tumor to the metastatic tumor, assessed with McNemar analyses.
| N = 81 | COMP expression in Cancer Cells Metastasis |
p-value | COMP expression in Stroma Metastasis | p-value | ||
|---|---|---|---|---|---|---|
| COMP expression in Cancer Cells | No (score 0) | Yes (score 1–3) | No (score 0) | Yes (score 1–3) | ||
| No (score 0) | 24 | 0 | <0.001 | 20 | 4 | <0.001 |
| Yes (score 1–3) | 49 | 8 | 34 | 23 | ||
| COMP expression in stroma | ||||||
| No (score 0) | 13 | 0 | <0.001 | 10 | 3 | <0.001 |
| Yes (score 1–3) | 60 | 8 | 44 | 24 | ||
Abbreviations: COMP, cartilage oligomeric matrix protein; The bold indicates p-values <0.05.
Levels of COMP expression affect the populations of the immune cells that migrate into tumor and stroma compartments of periampullary adenocarcinoma
The composition of tumor infiltrating immune cells has been previously extensively characterized in the current cohort.29,30 We found now that high levels of COMP expressed either by cancer cells or stroma were associated with lower levels of CD8+CD45RO+ (p < .001) and CD8 Treg cells (p < .001) in the tumor compartment (Figure 3a). Further, CD4+CD45RO+ were less abundant in tumor compartment with high levels of COMP, independent of COMP expression origin (cancer cells: p = .004, stroma: p < .001). High levels of COMP expression by cancer cells and stroma were also associated with lower infiltration of B cells into the tumor compartment (p = .002 and p = .039, respectively). Natural killer (NK, CD56+CD3−) cells were correlated with high levels of COMP expression only when COMP was expressed by the cancer cells (p = .044). Several populations of natural killer T (NKT) cells (CD3+CD56+ and CD3+NKp46+CD56+) were diminished in the tumor compartment only when high levels of COMP were expressed by the stroma (p = .014 and p = .008, respectively). Similarly, high levels of COMP in both the stroma and in cancer cells were associated with significantly lower levels of CD3+NKp46+CD56+ NKT cells in the tumor compartment (p < .001 and p < .001). Tumors with high expression of COMP in both stroma and cancer cells had significantly lower levels of macrophages in the tumor compartment (cancer cells: p = .001, stroma: p < .001). These macrophages are less likely to be M2 phenotype as there was no correlation between COMP expression and CD163+CD68+ population of macrophages. Finally, we examined the populations of infiltrating dendritic cells in the tumor compartment. Plasmacytoid (CD123+) dendritic cells were negatively correlated with the expression of COMP from cancer cells (p = .012) and in the stroma (p = .032).
Figure 3.
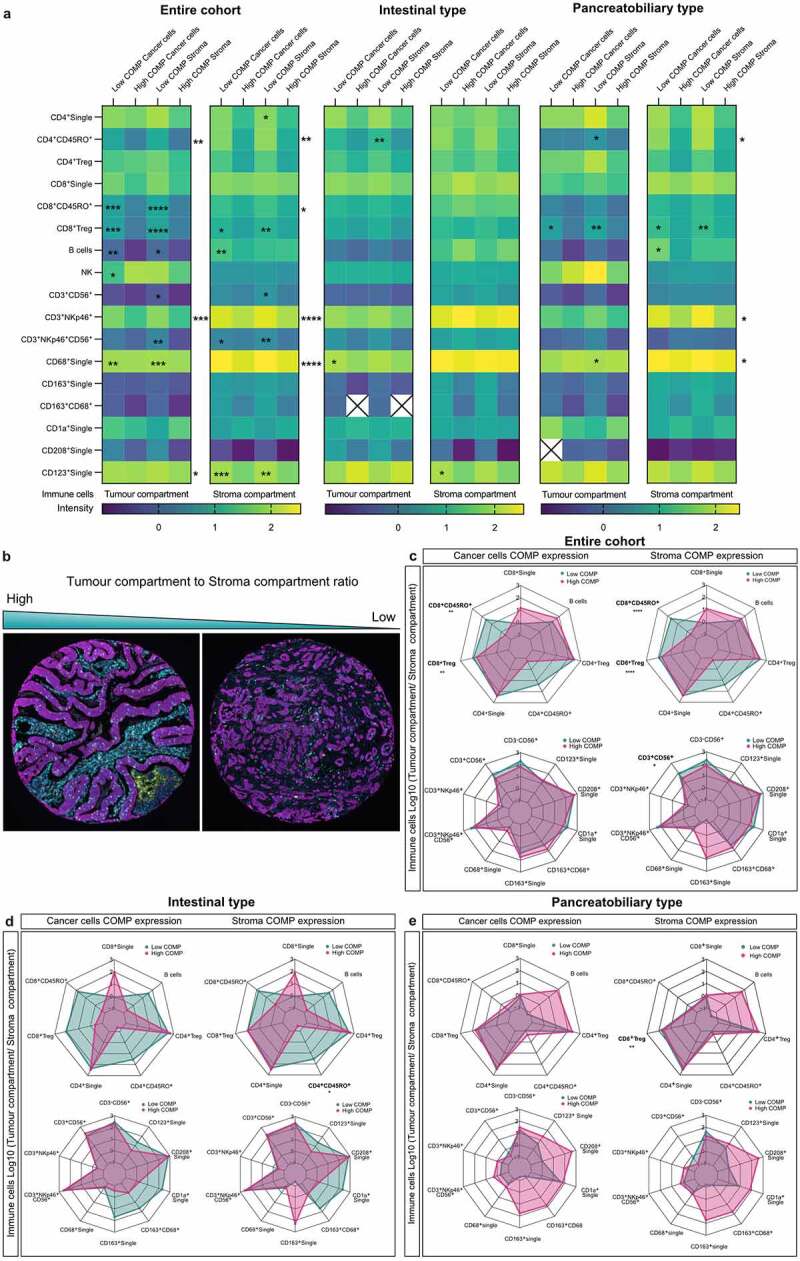
High levels of COMP associate with exclusion of cytotoxic T-cells from the tumor compartment. (a). Correlation between COMP expression and the infiltration of the various immune cell populations (T-cells, B-cells, macrophages, NK cells NKT cells and dendritic cells) was analyzed. (b) Representative TMAs stained for infiltrating immune cells, depicting the difference between the high and low cancer compartment to stroma compartment ratios. Tumor to stroma ratios were calculated for distinct populations of infiltrating cells and are shown as radar plots (each spoke represents one variable, each data point is depicted in every spoke according to the variable value and a line connects the data points) for the entire cohort (c), I-type (d), and PB-type (e). High ratio indicates higher levels of immune cells in the tumor compared to the stroma compartment. Spearman’s non-parametric correlation analysis was used to assess the association between the levels of COMP expression (score 0–3) and immune cell staining and Mann–Whitney two-tailed exact p-value for tumor to stroma ratios (*<0.05, **<0.01, ***<0.001, ****<0.0001).
Proceeding to the analysis of the migrating immune cells into the stroma compartment of the tumor, we noticed a similar phenomenon, that is, high levels of COMP negatively correlated with the presence of CD8+CD45RO+(cancer cells: p = .038, stroma: p = .032), CD8 Treg cells (cancer cells: p = .039, stroma: p = .009) and CD4+CD45RO+ (cancer cells: p = .005, stroma: p = .001; Figure 3a). Also, B cells were found to be less present in the stroma compartment when high levels of COMP were expressed by the cancer cells. The numbers of CD3+NKp46+ (p < .001) or CD3+NKp46+CD56+ NKT cells present in the stroma compartment were higher when COMP was highly expressed in the stroma (p = .005) or the cancer cells (p = .025; Figure 3a). Additionally, the CD3+CD56+ NKT cells infiltrated more when low levels of COMP were expressed by the stroma only (p = .32). Finally, more macrophages (CD68+, p < .001) and plasmacytoid (CD123+) dendritic cells (cancer cells: p = .001, stroma: p = .003) were detected in the stroma compartment when high levels of COMP were expressed by cancer cells or stroma.
Next, we performed analyses stratifying tumors based on morphology. I-type tumors had lower infiltration into the tumor compartment of CD4+CD45RO+ T cells (p = .009) when COMP was present in stroma, and macrophages (CD68+) (p = .049) when COMP was expressed in cancer cells. When high levels of COMP were detected in stroma, fewer CD123+ dendritic cells are present in the stroma compartments (p = .019). When high levels of COMP were found in the cancer cells or stroma, tumor compartment of patients with PB-type tumors were infiltrated with fewer CD8 Treg cells (cancer cells: p = .011, stroma: p = .001). Also, fewer CD4+ CD45RO+ T cells (p = .036) and macrophages (CD68+) (p = .033) were detected when levels of COMP were high in the stroma. In the stroma compartment fewer CD4+CD45RO+ cells (cancer cells: p = .035, stroma: p = .027), CD8 Treg (cancer cells: p = .027, stroma: p = .004), CD3+NKp46+ cells (cancer cells: p = .042, stroma: p = .038) or CD68+ cells (cancer cells: p = .019, stroma: p = .026) could be detected when high levels of COMP were found. Fewer B cells were detected in the stroma compartments when high levels of COMP were expressed by the cancer cells (p = .016). Furthermore, we evaluated the expression of COMP as a percentage of total positive cells per sample using QuPath software. Similar corelation between COMP expression and presence of immune cells in tumor and stroma compartments was observed as described above (Figure S4A).
Conclusively, expression of COMP by the cancer cell or in stroma correlates with the exclusion of immune cells from the tumor compartment. Additionally, some of these immune-cell populations are less infiltrating the stroma, depicting a possible immunosuppressive tumor microenvironment.
COMP expression correlates with exclusion of T-cells from the tumor compartment
The immune cell exclusion specifically from the tumor compartment can be estimated by calculating the tumor to stroma compartment ratio for each immune cell population. A high ratio indicates that the immune cells occur mainly in the tumor compartment and a low ratio shows absence of cells in the tumor compartment (Figure 3b). High levels of COMP expression in cancer cells or in stroma correlated with low tumor-to-stroma ratio of CD8+CD45RO+ T-cells (cancer cells: p = .005, stroma: p < .0001) and CD8+ Treg cells (cancer cells: p = .001, stroma: p < .0001), indicating immune cell exclusion. Also, low cancer to stroma ratios were observed for the CD3+CD56+ NKT cells (p = .023) when high levels of COMP were expressed in the stroma (Figure 3c). After stratification according to morphologic type, I-type tumors had low cancer to stroma ratios of CD4+CD45RO+ T-cells (p = .034) when high levels of COMP were expressed in stroma (Figure 3d). In patients with PB-type tumors high levels of COMP expression in stroma correlated with low cancer to stroma ratios of CD8+ Treg cells (p = .003; Figure 3e). Similar results were obtained when COMP expression in TMAs was evaluated with the QuPath as percentage of total positive cells per sample and corelated using Spearman’s regression analysis with the tumor to stroma compartment ratio for each immune cell population (Figure S4B).
In addition, we used TIMER 2.0, a comprehensive investigation tool with six state-of-the-art algorithms for immune infiltrating cells using mRNA sequencing of patient data from the Cancer Genome Atlas (TCGA).34 Running the algorithms of TIMER 2.0 on samples from patients with pancreatic adenocarcinoma most of the observations concerning immune cell exclusion from tumors expressing COMP were confirmed (Figure S1C).
Fibrosis is associated with COMP expression and immune cold tumors
A main characteristic of fibrotic tissues is the deposition of high quantities of collagens, which are organized in tight fibers. Figure 4a shows representative TMAs used for assessment of fibrosis. Density and organization of collagen fibers were evaluated in unbiased manner with a FIJI macro TWOMBLI. The average collagen density was higher in tumors in which the cancer cells express high levels of COMP, compared with those with low levels of expression (Figure 4b). Moreover, high levels of COMP in stroma were not only correlated with high-density matrix but also with average higher fractal dimension and branch points (Figure 4c). In the metastatic lesions, expression of COMP (score 1–3) was associated with a higher density of collagen fibers compared to the absence of COMP (score 0, Figure 4d). Furthermore, COMP expression by the cancer cells and in stroma was correlated with the presence of a larger fraction of stroma in TMAs.31 Interestingly, collagen density was not correlated with the proportion of stroma (Figure 4g). These data reveal a corelation of COMP expression with elevated levels of fibrosis in primary tumors and at metastatic sites. In accordance, data retrieved from the cBioPortal from patients with pancreatic adenocarcinoma revealed strong correlations (Spearman’s coefficient) between expression of COMP and many types of collagens (Figure S1D).
Figure 4.
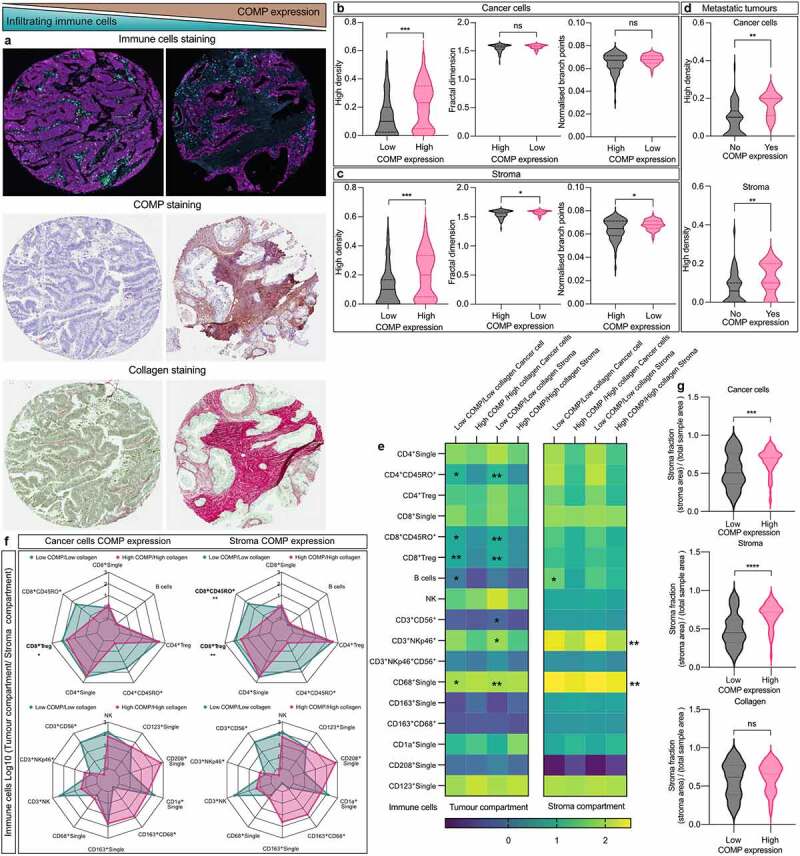
High levels of COMP expression correlate with increased collagen deposits in the stroma. (a) Representative TMAs stained for immune cells, COMP expression and collagen. Collagen deposition was detected using Sirius red staining (red). (b) Collagen characteristics were evaluated with a Fiji macro TWOMBLI. Expression of COMP (score 0–2 vs 2–3) from the cancer cells correlated with higher density of collagen fibers. (c) High stroma deposition of COMP (score 0–2 vs 2–3) correlated with high density, fractal dimension and branch points of collagen fibers. (d) In the metastatic tumors, the positive staining of COMP (score 0 vs 1–3) in the cancer cells or stroma correlated with higher collagen density. Patients were stratified into in two groups based on COMP and collagen levels (low COMP expression/low collagen density and high COMP expression/high collagen density). (e) Correlation between the two aforementioned groups and density of immune cell infiltration. (f) Tumor to stroma ratios were estimated for infiltrating immune cells and correlated with the aforementioned groups. (g) High COMP expression by the cancer cells or in stroma is correlated with larger fraction of stroma. Non-parametric Mann–Whitney two-tailed exact p-value used for plots B, C and D. Spearman’s non-parametric correlation analyses was used for plot E (*<0.05, **<0.01, ***<0.001, ****<0.0001).
To investigate the association of COMP expression and level of fibrosis in reference to immune cell exclusion, we divided the patients in two groups: low COMP expression/low collagen density (n = 41) and high COMP expression/high collagen density (n = 37). Density of CD8+ Treg cells (cancer cells: p = .003, stroma: p < .001), CD8+CD45RO+ T-cells (cancer cells: p = .014, stroma: p = .001), CD4+CD45RO+ T-cells (cancer cells: p = .022, stroma: p = .002), and CD68+ cells (cancer cells: p = .016, stroma: p = .003) were lower in tumor compartment in patients with high COMP expression/high collagen density when COMP was expressed in cancer cells or stroma (Figure 4e). The infiltrations of CD3+CD56+ (p = .011) and CD3+NKp46+ NKT cells (p = .011) were lower in tumor compartments of patients with high COMP expression/high collagen density when COMP was expressed in the stroma. Also, fewer infiltrating B (p = .027) cells were present in the tumor compartment of patients with high COMP expression/high collagen density when COMP was expressed by the cancer cells. In the stroma compartment, fewer CD3+NKp46+ NKT cells (cancer cells: p = .005, stroma: p = .004) and CD68+ macrophages (p < .001) were detected in the group of high COMP expression/high collagen density patients regarding COMP expression both in cancer cells and stroma (Figure 4e). Similarly, fewer B cells (p = .037) infiltrated the stroma compartment of patients with high COMP expression/high collagen density when COMP was expressed by the cancer cells.
In accordance with these findings, a high COMP expression/high collagen density, was associated with low tumor to stroma ratio for CD8+ Treg T-cells (p = .026), when COMP was expressed in cancer cells (figure 4f). Expression of COMP in stroma was associated with the exclusion of CD8+ Treg (p = .002) and CD8+CD45RO+ T cells (p = .009) in patients with high COMP expression/high collagen density (figure 4f).
Discussion
COMP, a major regulator of ECM structure in cartilage, is emerging as an enhancer of the tumor progression in many types of cancer. Unexpected expression of COMP in epithelial cancer cells was first found to correlate with survival and metastases in breast cancer.10 Here we show for the first time, that half of the patients with periampullary adenocarcinoma carry tumors expressing medium to high levels of COMP. High levels of COMP in both cancer cells and stromal cells correlated with the PB-type of tumor morphology. Further, expression of COMP was correlated with higher fibrotic collagen deposits and exclusion of immune cells.
In the cohort used, PB-type tumors had significantly higher COMP levels than I-type tumors and high COMP expression strongly correlated with worsened OS and RFS in the whole cohort. This is concordant with the fact that PB-type tumors have much poorer prognosis compared to I-type tumors and, generally, higher levels of fibrosis. COMP levels in the serum of breast cancer patients as evaluated by ELISA can serve as an independent prognostic factor.11 Thus, if a similar correlation between COMP in blood and in tumor tissues could be found in the future studies in periampullary adenocarcinoma, COMP evaluation in the blood of the patients could perform as a marker of PB-type tumors in irresectable cases, and subsequently have predictive value for the OS and RFS. Furthermore, a study with a larger number of I-type tumors will be needed to assess significance of the correlation of high COMP expression with OS and RFS. Since less than 10% of I-type tumors expressed COMP in our study it was impossible to draw firm conclusions regarding its borderline significant effects in this group of patients.
We observed a strong correlation between high expression of COMP and increased density of collagen fibers. Interestingly, the degree of COMP expression in stroma was not only associated with increased collagen fiber deposition, but also the tighter organization of the fibers, and immune cell exclusion. Collagen density was not a prognostic factor of patient OS and RFS (data not shown). The source of stroma COMP is not well understood. Recent study revealed that 90% of the ECM protein found in pancreatic tumors were produced by the stroma cells. However, ECM proteins produced by cancer cells were more correlated with poor patient survival. In the same study, COMP was predicted to be expressed by the cancer cells.35 Thus, COMP we observe deposited in stroma could be secreted by cancer cells, but we cannot exclude that COMP is also produced by cancer-associated fibroblasts. Presence of COMP could also manipulate the ECM in a complex manner as COMP is able to regulate multiple components of the accumulated ECM in fibrosis.36 Taken together, the data presented herein suggest that COMP plays a role in fibrosis in periampullary adenocarcinoma and particularly in the organization of the accumulated ECM. Accumulation of COMP in ECM, where COMP can bind to collagen fibers, could lead to tissue stiffening, which would mirror the physiological role of COMP in cartilage.8
Adding to this, we revealed that COMP expression in periampullary adenocarcinoma leads to exclusion of cytotoxic T-cells from the tumor compartment. This phenomenon known as immune exclusion is associated with resistance to immunotherapy, such as immune checkpoint blockade and CAR-T cells.22 We hypothesize that the mechanism behind this phenomenon may be relatively simple; when large quantities of ECM are accumulated in the microenvironment of the tumor in the presence of COMP, ECM becomes stiffer and thus harder to migrate through, creating a physical barrier.24 As fibrosis is one of the pathological hallmarks of periampullary adenocarcinoma, it is not unreasonable to hypothesize that COMP expression, a well-known regulator of the ECM assembly, results in increased fibrosis and an increased stiffness of the ECM, leading to immune excluded tumors. A recent study analyzing the prognostic role of the tumor stroma in 16 types of cancer revealed a higher immune cell infiltration in the stroma of I-type tumors compared to PB-type tumors.31 In the same study, the total amount of stroma was also found to be a favorable prognostic factor in patients with PB-type tumors. Of note, the present study evaluated the organization and density of collagen, which is not equal to the total amount of tumor stroma. The tight correlation of the high COMP expression with the PB-type tumors and the subsequent fibrosis could explain this observation. However, the current study cannot rule out if COMP can directly manipulate immune cell infiltration, proliferation, or function. Further studies will be needed to assess such a possibility.
The molecular mechanisms by which COMP may be aggravating periampullary adenocarcinoma remain to be studied. Interestingly, most of metastatic tumors lost the expression of COMP, indicating that COMP expression may play a role in the development of the primary tumor or the intravasation of the metastatic cancer, possibly by affecting the epithelial to mesenchymal transition16 and inducing metalloproteinases and degradation of the ECM.10
In conclusion, COMP was found in a large proportion of the periampullary adenocarcinomas, particularly in tumors with PB-type morphology. COMP expression was associated with increased fibrosis and immune exclusion of cytotoxic T-cells from tumor compartments. COMP could therefore be a promising possible therapeutic target. By targeting COMP with neutralizing peptides mimicking the COMP binding site on collagen, one could potentially be able to reverse its effect on the ECM and fibrosis and manipulate the tumor microenvironment to encourage increased infiltration of immune cells into the tumor.
Supplementary Material
Acknowledgments
We thank Dr. Ben King for language revision of the manuscript and Dr. Emre Can Tuysuz for database data retrieval.
Funding Statement
This study was supported by the Swedish Cancer Society, the Erling-Persson Foundation, Malmö Hospital Cancer Foundation, and the governmental grant for clinical research (ALF); Allmänna Sjukhusets i Malmö Stiftelse för Bekämpande av Cancer; Cancerfonden;Familjen Erling-Perssons Stiftelse.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data needed to evaluate the conclusions in the paper are presented in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2022.2111906
References
- 1.Posey KL, Coustry F, Hecht JT.. Cartilage oligomeric matrix protein: cOMPopathies and beyond. Matrix Biol. 2018;71-72. 161–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey DC, Bell PD, Hill JA, Longo DL. Fibrosis–a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 3.Farina G, Lemaire R, Korn JH, Widom RL. Cartilage oligomeric matrix protein is overexpressed by scleroderma dermal fibroblasts. Matrix Biol. 2006;25:213–222. doi: 10.1016/j.matbio.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, Swigris JJ, Taniguchi H, Wells AU. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 5.Novo E, Cannito S, Paternostro C, Bocca C, Miglietta A, Parola M. Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys. 2014;548:20–37. doi: 10.1016/j.abb.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Udomsinprasert W, Angkathunyakul N, Jittikoon J, Chaikledkaew U, Vejchapipat P, Poovorawan Y, Honsawek S. Cartilage oligomeric matrix protein as a marker of progressive liver fibrosis in biliary atresia. Sci Rep. 2021;11:16695. doi: 10.1038/s41598-021-95805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halasz K, Kassner A, Morgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282:31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal P, Schulz JN, Blumbach K, Andreasson K, Heinegard D, Paulsson M, et al. Enhanced deposition of cartilage oligomeric matrix protein is a common feature in fibrotic skin pathologies. Matrix Biol. 2013;32:325–331. doi: 10.1016/j.matbio.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Schulz JN, Nuchel J, Niehoff A, Bloch W, Schonborn K, Hayashi S, Kamper M, Brinckmann J, Plomann M, Paulsson M, et al. COMP-assisted collagen secretion–a novel intracellular function required for fibrosis. J Cell Sci. 2016;129:706–716. doi: 10.1242/jcs.180216. [DOI] [PubMed] [Google Scholar]
- 10.Englund E, Bartoschek M, Reitsma B, Jacobsson L, Escudero-Esparza A, Orimo A, Leandersson K, Hagerling C, Aspberg A, Storm P, et al. Cartilage oligomeric matrix protein contributes to the development and metastasis of breast cancer. Oncogene. 2016;35:5585–5596. doi: 10.1038/onc.2016.98. [DOI] [PubMed] [Google Scholar]
- 11.Papadakos KS, Darlix A, Jacot W, Blom AM. High Levels of Cartilage Oligomeric Matrix Protein in the Serum of Breast Cancer Patients Can Serve as an Independent Prognostic Marker. Front Oncol. 2019;9:1141. doi: 10.3389/fonc.2019.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadakos KS, Bartoschek M, Rodriguez C, Gialeli C, Jin SB, Lendahl U, Pietras K, Blom AM. Cartilage oligomeric matrix protein initiates cancer stem cells through activation of jagged1-Notch3 signaling. Matrix Biol. 2019;81:107–121. doi: 10.1016/j.matbio.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Englund E, Canesin G, Papadakos KS, Vishnu N, Persson E, Reitsma B, Anand A, Jacobsson L, Helczynski L, Mulder H, et al. Cartilage oligomeric matrix protein promotes prostate cancer progression by enhancing invasion and disrupting intracellular calcium homeostasis. Oncotarget. 2017;8:98298–98311. doi: 10.18632/oncotarget.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Wang C, Wang Y, Sun L, Liu Z, Wang L, Song T, Yao Y, Liu Q, Tu K, et al. HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways. J Exp Clin Cancer Res. 2018;37:231. doi: 10.1186/s13046-018-0908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu TT, Liu XS, Zhang M, Liu XN, Zhu FX, Zhu FM, Ouyang S-W, Li S-B, Song C-L, Sun H-M, et al. Cartilage oligomeric matrix protein is a prognostic factor and biomarker of colon cancer and promotes cell proliferation by activating the Akt pathway. J Cancer Res Clin Oncol. 2018;144:1049–1063. doi: 10.1007/s00432-018-2626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nfonsam VN, Nfonsam LE, Chen D, Omesiete PN, Cruz A, Runyan RB, Jandova J. COMP gene coexpresses with EMT genes and is associated with poor survival in colon cancer patients. J Surg Res. 2019;233:297–303. doi: 10.1016/j.jss.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Meinrath J, Haak A, Igci N, Dalvi P, Arolt C, Meemboor S, Siebolts U, Eischeidt-Scholz H, Wickenhauser C, Grünewald I, et al. Expression profiling on subclasses of primary parotid gland carcinomas. Oncotarget. 2020;11:4123–4137. doi: 10.18632/oncotarget.27797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckert MA, Coscia F, Chryplewicz A, Chang JW, Hernandez KM, Pan S. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature. 2019;569:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang DK, Jamieson NB, Johns AL, Scarlett CJ, Pajic M, Chou A, Pinese M, Humphris JL, Jones MD, Toon C, et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31:1348–1356. doi: 10.1200/JCO.2012.46.8868. [DOI] [PubMed] [Google Scholar]
- 20.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaccaro V, Sperduti I, Milella M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;365:768–769. [DOI] [PubMed] [Google Scholar]
- 22.Schizas D, Charalampakis N, Kole C, Economopoulou P, Koustas E, Gkotsis E, Ziogas D, Psyrri A, Karamouzis MV. Immunotherapy for pancreatic cancer: a 2020 update. Cancer Treat Rev. 2020;86:102016. doi: 10.1016/j.ctrv.2020.102016. [DOI] [PubMed] [Google Scholar]
- 23.Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer. 2018;4:418–428. doi: 10.1016/j.trecan.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel III EE, Koeppen H, Astarita JL, Cubas R, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elebro J, Jirstrom K. Use of a standardized diagnostic approach improves the prognostic information of histopathologic factors in pancreatic and periampullary adenocarcinoma. Diagn Pathol. 2014;9:80. doi: 10.1186/1746-1596-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elebro J, Heby M, Warfvinge CF, Nodin B, Eberhard J, Jirstrom K, St-Pierre Y. Expression and prognostic significance of human epidermal growth factor receptors 1, 2 and 3 in periampullary adenocarcinoma. PLoS One. 2016;11:e0153533. doi: 10.1371/journal.pone.0153533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elebro J, Heby M, Gaber A, Nodin B, Jonsson L, Fristedt R. Prognostic and treatment predictive significance of SATB1 and SATB2 expression in pancreatic and periampullary adenocarcinoma. J Transl Med. 2014;12:289. doi: 10.1186/s12967-014-0289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bankhead P, Loughrey MB, Fernandez JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundgren S, Micke P, Elebro J, Heby M, Hrynchyk I, Nodin B, Leandersson K, Mezheyeuski A, Jirström K. Topographical distribution and spatial interactions of innate and semi-innate immune cells in pancreatic and other periampullary adenocarcinoma. Front Immunol. 2020;11:558169. doi: 10.3389/fimmu.2020.558169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundgren S, Elebro J, Heby M, Nodin B, Leandersson K, Micke P, Jirström K, Mezheyeuski A. Quantitative, qualitative and spatial analysis of lymphocyte infiltration in periampullary and pancreatic adenocarcinoma. Int J Cancer. 2020;146:3461–3473. doi: 10.1002/ijc.32945. [DOI] [PubMed] [Google Scholar]
- 31.Micke P, Strell C, Mattsson J, Martin-Bernabe A, Brunnstrom H, Huvila J, Sund M, Wärnberg F, Ponten F, Glimelius B, et al. The prognostic impact of the tumour stroma fraction: a machine learning-based analysis in 16 human solid tumour types. EBioMedicine. 2021;65:103269. doi: 10.1016/j.ebiom.2021.103269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wershof E, Park D, Barry DJ, Jenkins RP, Rullan A, Wilkins A. A Fiji macro for quantifying pattern in extracellular matrix. Life Sci Alliance. 2021; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–W14. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian C, Clauser KR, Ohlund D, Rickelt S, Huang Y, Gupta M, Mani DR, Carr SA, Tuveson DA, Hynes RO, et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci U S A. 2019;116:19609–19618. doi: 10.1073/pnas.1908626116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acharya C, Yik JH, Kishore A, Van Dinh V, Di Cesare PE, Haudenschild DR. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol. 2014;37:102–111. doi: 10.1016/j.matbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are presented in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from authors.


