Background:
The risk of aneurysmal subarachnoid hemorrhage (aSAH) is increased in postmenopausal women compared with men of similar age, suggesting a role for sex hormones. We aimed to explore whether sex hormones, and age at menarche/menopause have a causal effect on aSAH risk by conducting a 2-sample MR study (Mendelian randomization).
Methods:
We obtained sex-specific genetic instruments for serum estradiol, bioavailable testosterone (BioT), SHBG (sex hormone-binding globulin), and age at menarche/menopause from genome-wide association studies. The associated sex-specific aSAH risk was estimated with inverse-variance weighted MR analyses with various statistical sensitivity analyses. Multivariable and cluster MR analyses were performed for BioT and SHBG to account for a genetic and phenotypic correlation between the 2 exposures. The clusters represented (1) single-nucleotide polymorphisms primarily increasing SHBG, with secondary decreasing effects on BioT, and (2) single-nucleotide polymorphisms affecting BioT without affecting SHBG.
Results:
Univariable MR analyses showed an 18% increased aSAH risk among women per 1-SD increase in genetically determined SHBG levels (odds ratio, 1.18 [95% CI, 1.05–1.34]; P=0.007). Suggestive evidence was identified for a 27% decreased risk of aSAH among women per 1-SD increase in BioT (odds ratio, 0.73 [95% CI, 0.55–0.95]; P=0.02). The latter association disappeared in cluster analysis when only using SHBG-independent variants. MR analyses with variants from the cluster with primary SHBG effects and secondary (opposite) BioT-effects yielded a statistically significant association (odds ratio, 1.21 [95% CI, 1.05–1.40]; P=0.008). No other causal associations were identified.
Conclusions:
Genetic predisposition to elevated serum levels of SHBG, with secondary lower serum BioT levels, is associated with an increased aSAH risk among women, suggesting that SHBG and BioT causally elevate aSAH risk. Further studies are required to elucidate the underlying mechanisms and their potential as an interventional target to lower aSAH incidence.
Keywords: hormones, menopause, sex hormone-binding globulin, subarachnoid hemorrhage, testosterone
Aneurysmal subarachnoid hemorrhage (aSAH) can have devastating health effects.1 The incidence of aSAH rises with age and remains similar between men and women for the most part of an average lifespan. However, epidemiological data demonstrate that the incidence of aSAH among women disproportionally increases around the start of natural menopause, at the approximate age of 50.2 Since exposure to estrogens decreases substantially after menopause it is often suggested that estradiol, among other sex hormones, may influence the risk on aSAH.3,4 In this respect, SHBG (sex hormone-binding globulin) may also play a role. SHBG is a protein synthesized and secreted by the liver. Once it reaches the bloodstream, it can bind to androgens and estrogens with high affinity. By doing so, it regulates the bioavailability of these steroids, which is usually defined as the percentage of androgens or estrogens not bound to SHGB but either free or loosely bound to albumin.
Until now, most attention has been focused on the role of estradiol (the most potent estrogen), considering its ability to suppress vascular inflammation as a known driver of intracranial aneurysm progression.5,6 However, while experimental studies frequently indicate a substantial role of estradiol on aSAH risk, observational studies on the association between estradiol among other sex hormones and aSAH are still indecisive.
MR studies (Mendelian randomization) use genetic variants (single-nucleotide polymorphisms [SNPs]) associated with an exposure to investigate its effect on an outcome. The random allocation of SNP alleles at conception offers an opportunity to potentially overcome limitations inherent to traditional observational studies, such as residual confounding.7 Analogous to randomized controlled trials, the principle of randomization is exploited to ascertain an unconfounded and therefore causal exposure-outcome relationship. Therefore, we aimed to explore the association between sex hormones and the risk of aSAH using an MR approach. In particular, we assessed whether a genetically determined increase in age at menarche (AAM), age at natural menopause, serum bioavailable testosterone (BioT), SHBG, and estradiol influences the sex-specific risk of aSAH.
Methods
Data Availability
We used summary data from published studies for our analyses, publicly available via the original studies (data sources in the Supplemental Material). All studies obtained relevant ethical approval and participant consent.
Exposure Data
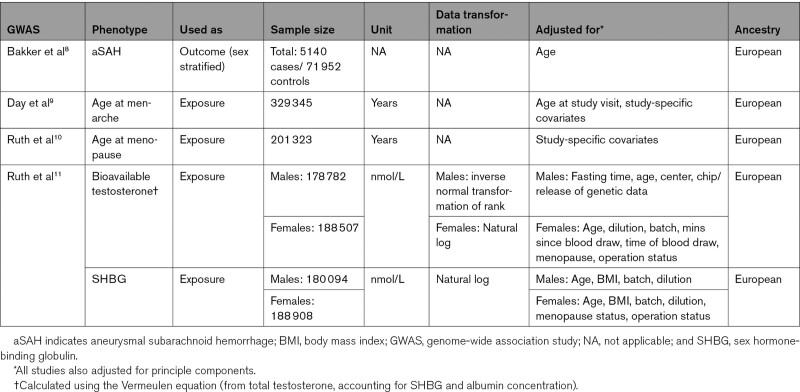
We retrieved publicly available sex-specific genome-wide association study (GWAS) summary-level data of SNPs associated with the exposures (Table).9–11 We selected SNPs associated with the exposure at the genome-wide significance level (P<5×10−8). Independence of SNPs was assessed using stringent criteria (r2, 0.001; clumping window, 10 000 kb). If an instrumental SNP for the exposure was not available in the outcome data set, we replaced it with a suitable proxy SNP (r2>0.8 in the European 1000 Genomes Project reference panel using LDlink [https://ldlink.nci.nih.gov/]) or removed it in the absence of such a proxy. We harmonized the SNP alleles across studies and removed palindromic SNPs with ambiguous allele frequencies (0.42–0.58). Data of all instrumental variables used are shown in Table S1 through S6. For estradiol, we used a single SNP (rs727479) which has been reported in the literature and was found to be associated with serum estradiol levels in both men and postmenopausal women, although with different effect sizes (Table S7).12,13 The SNP is located in the CYP19A1 gene, which encodes aromatase, the enzyme responsible for converting testosterone to estradiol.
Table.
Characteristics of the Used GWAS
Outcome Data
We obtained sex-specific summary-level outcome data from a GWAS on aSAH, comprising 23 cohorts with a total of 5140 cases and 71 934 controls.8 All individuals were of European ancestry. As the calculated potential UK Biobank sample overlap between exposure and outcome GWASs was <4% at maximum, we considered the risk of bias due to sample overlap minimal.14
Statistical Analysis
We performed primary MR analysis among women using a random-effects inverse-variance weighted meta-analysis, if at least 2 SNPs for the exposure were available.15 This comprises a meta-analysis of SNP-specific Wald estimates (ie, SNP-outcome β divided by the SNP-exposure β). This method assumes that all variants are valid instruments.16 Consequently, we performed several statistical sensitivity analyses. We first tested for heterogeneity between variant-specific estimates using Cochran Q statistic in the inverse-variance weighted model. We then explored horizontal pleiotropy via the MR-Egger and MR-Pleiotropy Residual Sum and Outlier methods.17,18 A significant nonzero Egger intercept is suggestive of unbalanced, directional horizontal pleiotropy. It can thereby identify and correct for bias due to directional pleiotropic effects, assuming that the size of the pleiotropic effects is independent of the SNP-exposure effects (InSIDE-assumption). The MR-Egger estimate is however sensitive to outliers and influential data points, which may lead to low statistical power in estimating a causal effect. MR-Pleiotropy Residual Sum and Outlier calculates the effect estimate after identifying and excluding outlier SNPs (ie, potential pleiotropic variants) if present. We additionally performed a weighted median-based MR analysis, which assumes that at least half of the included variants are valid instrumental variables. We repeated the analyses after MR-Steiger filtering which removes SNPs suggestive of a reversed causal direction (ie, those explaining more variance in the outcome than in exposure), a violation of the exclusion restriction assumption.19 In addition, we conducted sensitivity MR analyses for all exposures using the male-specific exposure and outcome summary data. In this respect, AAM and age at natural menopause functioned as negative control outcomes. For estradiol with only one SNP available, we calculated the Wald estimate, as other MR methods require at least 2 SNPs.
A strong genetic correlation (rg=−0.74) was reported between SHBG and BioT among women.11 In additional analyses, the authors were able to identify 2 clusters: (1) a cluster of SNPs mainly affecting SHBG levels, with secondary directionally opposing effects on testosterone and (2) a cluster of SNPs affecting testosterone levels, independent of SHBG. To account for this genetic correlation and to explore the independent effects of each exposure, we used 2 different methods. First, we performed cluster-specific MR analyses. Second, we performed multivariable MR analyses. Here, the SNPs associated with BioT and SHBG are combined, and the direct causal effect on the risk of aSAH is estimated for each exposure while accounting for the other exposure.20 Also, since the SHBG SNPs were all identified after adjusting for body mass index in the exposure GWAS, we performed sensitivity analysis using the same SNPs with their body mass index unadjusted effects on SHBG.
We calculated all effect estimates as odds ratios (OR) with 95% CIs. For AAM and age at natural menopause, we calculated ORs for aSAH per 1-year increment. For all other exposures, we calculated ORs per 1-SD increase in exposure levels. We estimated 1-SD unit using the estimate_trait_sd() function in the TwoSampleMR package, which estimates the trait SD via obtaining the beta estimates from z-scores and finding the ratio with original β values. We set the P value for statistical significance at P<0.01, after correcting for multiple exposures using the Bonferroni method (α=0.05/5 exposures). We considered results with P values between 0.05 and 0.01 suggestive. We performed all MR analyses using the TwoSampleMR (version 0.5.6), Mendelian randomization (version 0.5.1), and MRPRESSO (version 1.0) packages for R.18,21,22
Results
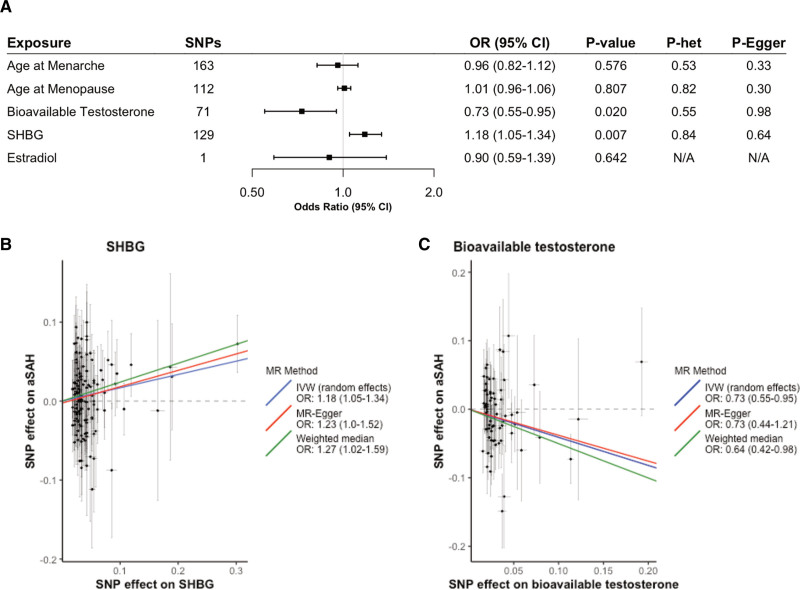
The primary univariable MR analysis showed an 18% increased aSAH risk among women per 1-SD increase in genetically determined SHBG levels (OR, 1.18 [95% CI, 1.05–1.34]; P=0.007) as shown in the Figure. We also found suggestive evidence of a 27% decrease in aSAH risk among women per 1-SD increase in genetically determined BioT (OR, 0.73 [95% CI, 0.55–0.95]; P=0.02). However, when only using SNPs from the cluster of BioT-associated SNPs independent of SHBG, the latter association disappeared (17 SNPs; OR, 1.24 [95% CI, 0.81–1.91]; P=0.313), as shown in Table S8. The MR-estimate after cluster filtering using SNPs with primary SHBG effects and secondary (opposite) effects on testosterone remained significant (126 SNPs; OR, 1.21 [95% CI, 1.05–1.40]; P=0.008). Multivariable MR analyses, however, did not support a direct causal effect of either SHBG (OR, 1.17 [95% CI, 0.94–1.46]; P=0.169) or BioT (OR, 1.02 [95% CI, 0.61–1.69]; P=0.952) on aSAH risk among women (Table S9). However, the multivariable OR estimate for SHBG was almost identical to the univariable estimate, suggesting low power in the multivariable MR analysis. No associations were observed between genetically determined AAM, age at menopause or estradiol levels, and risk of aSAH (Figure and Table S10). MR estimates were similar between body mass index adjusted and unadjusted SHBG, suggesting no body mass index-related collider bias (Table S10).
Figure.
Effect estimates among women. A, Inverse-variance weighted estimates for the association between a genetically determined unit increase in exposure on the risk of aneurysmal subarachnoid hemorrhage (aSAH). B and C, Scatter plots of individual single-nucleotide polymorphism (SNP) effects and estimates from different Mendelian randomization (MR) methods for the effect of (B) sex hormone-binding globulin (SHBG) on aSAH and (C) bioavailable testosterone on aSAH. P het is the P value belonging to the Q statistic for heterogeneity. P Egger is the P value belonging to the Egger intercept. IVW indicates inverse-variance weighted; and OR, odds ratio.
Cochran Q statistic showed no evidence for heterogeneity. The MR-Pleiotropy Residual Sum and Outlier global test and MR-Egger intercepts showed no indication for directional pleiotropic effects. No associations were identified among men (Table S11). MR analyses based on the weighted median method yielded results in the same direction as in the inverse-variance weighted-method, but with lower precision. MR-Steiger filtering was performed for the exposures AAM, age at menopause, SHBG, and BioT to remove SNPs suggestive of reverse causation. Subsequent MR analyses excluding these variants showed comparable results to the main analyses (Table S12).
Discussion
We showed that genetic predisposition to elevated levels of SHBG, with secondary lower levels of BioT, is associated with an increased risk of aSAH among women. Additionally, we found that genetically determined increased AAM or age at menopause is unlikely to have a substantial effect on aSAH risk. Moreover, associations were only found among women, suggesting a smaller role, if any, of sex hormone levels on aSAH risk among men.
Although SHBG has not been investigated before in the context of aSAH, observational studies implicate a role for SHBG in various vascular diseases. For example, serum SHBG levels are inversely associated with the risk of ischemic stroke in postmenopausal women, even after adjusting for potential mediators such as estradiol, testosterone, and diabetes.23 Similarly, higher serum SHBG levels have been associated with optimal cardiovascular health among postmenopausal women.24 A previous MR study has also demonstrated a potential causal association between increasing SHBG levels and decreasing risk of coronary heart disease.25 However, the mechanism of how SHBG directly affects vascular diseases is currently poorly understood.26 Despite the usually protective associations between elevated SHBG and vascular diseases, the direction of effect was the opposite in our study. However, almost all SNPs used for the univariate analyses on SHBG had secondary (opposite) effects on BioT levels. Consequently, the cluster analyses including only SNPs affecting SHBG levels, with secondary (opposite) effects on BioT yielded a similar point estimate.
The effect of SHBG on aSAH risk may be mediated via testosterone. In the literature, it is hypothesized that testosterone deficiency may contribute to vascular aging in both men and women via oxidative stress and inflammation, a process known to affect the risk of aSAH.5,27 However, this is currently unclear, partly due to the high complexity of both protective and detrimental effects of androgens on the cerebral vasculature, which may differ based on sex, dose, and age, among many other factors.28 For example, androgens appear to have proinflammatory effects under basal conditions, but potentially protective anti-inflammatory effects under pathological conditions, such as hypoxia, endotoxin-induced inflammation, or ischemia.28 The vascular protective effects of estradiol, and the potential role of estradiol deficiency in increasing the risk of aSAH among postmenopausal women, have been established in far greater detail. Experimental studies have provided evidence of, for example, endothelial dysfunction and inflammation resulting from estrogen deficiency.6 Clinical studies, mainly retrospective case-control studies, have focused on many estrogen-related factors, such as age at menopause, use of hormone replacement therapy or oral contraceptives, pregnancy, and hysterectomies.3,4 However, these studies are limited by small case numbers, high risk of confounding, and have, in some instances, provided contradicting evidence. Based on our analyses, the AAM or age at menopause is unlikely to have a major effect on aSAH risk. This may reflect a limited effect of estradiol on aSAH risk since menopause, for example, mainly influences estrogens, as opposed to androgens which decline with age shortly after puberty.27 However, although our explorative analysis with 1 SNP for estradiol levels resulted in an inconclusive finding, the substantial (fundamental) evidence behind estradiol and aSAH highlights the importance of further investigation. Overall, our understanding of the mechanisms behind the different sex hormones on the progression of intracranial aneurysms toward rupture remains insufficient and needs further exploration.
Strengths and Limitations
To our knowledge, this is the first MR study on the associations of sex hormones with risk of aSAH. MR studies have benefits over traditional observational studies, for example, by reducing the risk of residual confounding. As such, we were able to provide novel insights that may contribute to a better understanding of the sex difference in aSAH incidence. Also, we used recently published large-scale GWAS-data and performed multiple sensitivity analyses to assess the robustness of our findings.
Our study also has its limitations. First, data were not available for all relevant exposure SNPs in the outcome GWAS, even after searching for potential proxies. A substantial number of exposure SNPs could, therefore, not be used for our MR analyses. Despite affecting the statistical power to detect small effects, we could still include a fair number of SNPs and perform adequate MR analyses. Second, the genetic correlation between SHBG and BioT restricted our ability to estimate their direct effects on aSAH risk. We performed multivariable analyses and cluster analyses to take this into account, despite both having lower power compared to the univariate models. This especially affected the cluster analyses with BioT SNPs independent of SHBG, for which just 17 SNPs could be used. Third, we only used a single SNP related to serum estradiol levels. GWASs of estradiol levels have been complicated by the fluctuations of serum estradiol levels among premenopausal women but also because of difficulties in measuring low serum levels.11 However, we think the use of this single SNP is valid for explorative analyses. Particularly because it is located in a biologically relevant gene (CYP19A1) encoding aromatase, the key enzyme for the aromatization of testosterone to form estradiol. Moreover, the SNP has not been associated with testosterone levels, making it suitable to estimate the independent effect of estradiol.13 Also, the SNP has been validated against known or suspected estradiol-related traits, such as bone mineral density and insulin sensitivity, as well as estrogen receptor positive breast cancer and endometrial cancer.13,29 Finally, MR estimates lifetime effects rather than acute effects. Lifelong exposure typically has a greater effect on an outcome compared with short-term exposure. This is due to the cumulative effects of most exposures on the associated outcome over time.30 Effects of acute hormonal changes, for example, via supplementation, might therefore not be captured.
Conclusions
This MR study provides evidence that a genetically determined increase in serum SHBG levels, with secondary lower BioT levels, is associated with an increased risk of aSAH among women. Our knowledge about this topic is still insufficient, especially about the potential role of androgens. This highlights the need for further (mechanistic) studies on the role of sex hormones on aSAH risk and their potential as an interventional target to lower aSAH incidence.
Article Information
Sources of Funding
None.
Disclosures
Dr Uyttenboogaart received funding from the Dutch Heart Foundation and Health Holland/TKI Public Private partnership program for other research projects not related to the contents of this article. M.K. Bakker has received funding from The Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON2015-08 ERASE. Dr Ruigrok has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 852173). The other authors report no conflicts.
Supplemental Material
Data Sources
Tables S1–S12
ISGC Intracranial Aneurysm Working Group Contributors
STROBE-MR Checklist
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AAM
- age at menarche
- aSAH
- aneurysmal subarachnoid hemorrhage
- BioT
- bioavailable testosterone
- GWAS
- genome-wide association study
- MR
- Mendelian randomization
- SHBG
- sex hormone-binding globulin
- SNPs
- single-nucleotide polymorphisms
A list of the ISGC Intracranial Aneurysm Working Group contributors is available in the Supplemental Material.
This manuscript was sent to Kazunori Toyoda, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.038035.
For Sources of Funding and Disclosures, see page 2875.
Contributor Information
Rob Molenberg, Email: robmolenberg@me.com.
Chris H.L. Thio, Email: c.h.l.thio@umcg.nl.
Marlien W. Aalbers, Email: mwaalbers@gmail.com.
Maarten Uyttenboogaart, Email: m.uyttenboogaart@umcg.nl.
Susanna C. Larsson, Email: Susanna.Larsson@ki.se.
Mark K. Bakker, Email: m.k.bakker-25@umcutrecht.nl.
Ynte M. Ruigrok, Email: ij.m.ruigrok@umcutrecht.nl.
Harold Snieder, Email: h.snieder@umcg.nl.
References
- 1.Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10:349–356. doi: 10.1016/S1474-4422(11)70017-5 [DOI] [PubMed] [Google Scholar]
- 2.Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, Algra A. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76:588–597. doi: 10.1001/jamaneurol.2019.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai M, Wali AR, Birk HS, Santiago-Dieppa DR, Khalessi AA. Role of pregnancy and female sex steroids on aneurysm formation, growth, and rupture: a systematic review of the literature. Neurosurg Focus. 2019;47:E8. doi: 10.3171/2019.4.FOCUS19228 [DOI] [PubMed] [Google Scholar]
- 4.Algra AM, Klijn CJ, Helmerhorst FM, Algra A, Rinkel GJ. Female risk factors for subarachnoid hemorrhage: a systematic review. Neurology. 2012;79:1230–1236. doi: 10.1212/WNL.0b013e31826aace6 [DOI] [PubMed] [Google Scholar]
- 5.Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659–1676. doi: 10.1038/jcbfm.2012.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramesh SS, Christopher R, Indira Devi B, Bhat DI. The vascular protective role of oestradiol: a focus on postmenopausal oestradiol deficiency and aneurysmal subarachnoid haemorrhage. Biol Rev Camb Philos Soc. 2019;94:1897–1917. doi: 10.1111/brv.12541 [DOI] [PubMed] [Google Scholar]
- 7.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163. doi: 10.1002/sim.3034 [DOI] [PubMed] [Google Scholar]
- 8.Bakker MK, van der Spek RAA, van Rheenen W, Morel S, Bourcier R, Hostettler IC, Alg VS, van Eijk KR, Koido M, Akiyama M, et al. ; HUNT All-In Stroke; China Kadoorie Biobank Collaborative Group; BioBank Japan Project Consortium; ICAN Study Group; CADISP Group; Genetics and Observational Subarachnoid Haemorrhage (GOSH) Study investigators; International Stroke Genetics Consortium (ISGC). Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat Genet. 2020;52:1303–1313. doi: 10.1038/s41588-020-00725-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day FR, Thompson DJ, Helgason H, Chasman DI, Finucane H, Sulem P, Ruth KS, Whalen S, Sarkar AK, Albrecht E, et al. ; LifeLines Cohort Study; InterAct Consortium; kConFab/AOCS Investigators; Endometrial Cancer Association Consortium; Ovarian Cancer Association Consortium; PRACTICAL consortium. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49:834–841. doi: 10.1038/ng.3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruth KS, Day FR, Hussain J, Martínez-Marchal A, Aiken CE, Azad A, Thompson DJ, Knoblochova L, Abe H, Tarry-Adkins JL, et al. ; Biobank-based Integrative Omics Study (BIOS) Consortium; eQTLGen Consortium; Biobank Japan Project; China Kadoorie Biobank Collaborative Group; kConFab Investigators; LifeLines Cohort Study; InterAct consortium; 23andMe Research Team. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596:393–397. doi: 10.1038/s41586-021-03779-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, Beaumont RN, Wittemans L, Martin S, Busch AS, et al. ; Endometrial Cancer Association Consortium. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26:252–258. doi: 10.1038/s41591-020-0751-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson DJ, O’Mara TA, Glubb DM, Painter JN, Cheng T, Folkerd E, Doody D, Dennis J, Webb PM, Gorman M, et al. ; Australian National Endometrial Cancer Study Group (ANECS); National Study of Endometrial Cancer Genetics Group (NSECG); for RENDOCAS; AOCS Group. CYP19A1 fine-mapping and Mendelian randomization: estradiol is causal for endometrial cancer. Endocr Relat Cancer. 2016;23:77–91. doi: 10.1530/ERC-15-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson AL, Perry JRB, Coviello AD, Delgado GE, Ferrucci L, Hoffman AR, Huhtaniemi IT, Ikram MA, Karlsson MK, Kleber ME, et al. Genetic determinants of circulating estrogen levels and evidence of a causal effect of estradiol on bone density in men. J Clin Endocrinol Metab. 2018;103:991–1004. doi: 10.1210/jc.2017-02060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40:597–608. doi: 10.1002/gepi.21998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28:30–42. doi: 10.1097/EDE.0000000000000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13:e1007081. doi: 10.1371/journal.pgen.1007081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181:251–260. doi: 10.1093/aje/kwu283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–1739. doi: 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madsen TE, Luo X, Huang M, Park KE, Stefanick ML, Manson JE, Liu S. Circulating SHBG (sex hormone-binding globulin) and risk of ischemic stroke: findings from the WHI. Stroke. 2020;51:1257–1264. doi: 10.1161/STROKEAHA.120.028905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaspers L, Dhana K, Muka T, Meun C, Kiefte-de Jong JC, Hofman A, Laven JS, Franco OH, Kavousi M. Sex steroids, sex hormone-binding globulin and cardiovascular health in men and postmenopausal women: the rotterdam study. J Clin Endocrinol Metab. 2016;101:2844–2852. doi: 10.1210/jc.2016-1435 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Si S, Hou L, Yuan T, Chen X, Liu C, Li W, Li H, Liu Y, Xue F. Causal effect of sex hormone-binding globulin and testosterone on coronary heart disease: a multivariable and network Mendelian randomization analysis. Int J Cardiol. 2021;339:179–184. doi: 10.1016/j.ijcard.2021.06.037 [DOI] [PubMed] [Google Scholar]
- 26.Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. 2015;26:376–383. doi: 10.1016/j.tem.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 27.Moreau KL, Babcock MC, Hildreth KL. Sex differences in vascular aging in response to testosterone. Biol Sex Differ. 2020;11:18. doi: 10.1186/s13293-020-00294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abi-Ghanem C, Robison LS, Zuloaga KL. Androgens’ effects on cerebrovascular function in health and disease. Biol Sex Differ. 2020;11:35. doi: 10.1186/s13293-020-00309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson SC, Kar S, Perry JRB, Carter P, Vithayathil M, Mason AM, Easton DF, Burgess S. Serum estradiol and 20 site-specific cancers in women: Mendelian randomization study. J Clin Endocrinol Metab. 2021;107:e467–e474. doi: 10.1210/clinem/dgab713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ference BA. How to use Mendelian randomization to anticipate the results of randomized trials. Eur Heart J. 2018;39:360–362. doi: 10.1093/eurheartj/ehx462 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We used summary data from published studies for our analyses, publicly available via the original studies (data sources in the Supplemental Material). All studies obtained relevant ethical approval and participant consent.