Background:
Cerebral venous sinus thrombosis (CVST) secondary to vaccine-induced thrombotic thrombocytopenia is an extremely rare side effect of adenovirus-based COVID-19 vaccines. CVST incidence associated with COVID-19 itself has not been widely reported. We report the incidence of CVST in patients hospitalized with COVID-19 during the first year of the pandemic.
Methods:
We analyzed de-identified electronic medical records of a retrospective cohort of patients admitted with COVID-19 to >200 hospitals between March 2020 and March 2021. We used International Classification of Diseases, Tenth Revision codes and natural language processing extracts to identify patients with a new CVST diagnosis during COVID-19 hospitalization. The primary outcome was CVST incidence in hospitalized, COVID-19-positive patients. Secondary outcomes included CVST incidence and mortality. Incidence rates were calculated using the DerSimonian-Laird estimator method.
Results:
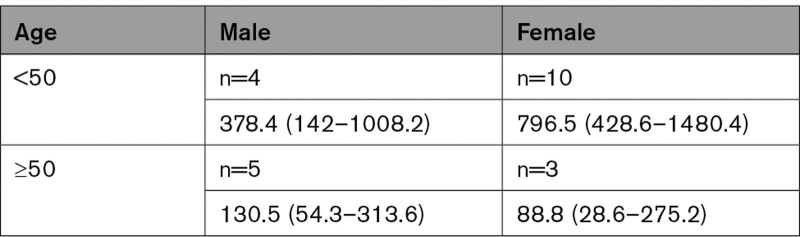
Ninety-one thousand seven hundred twenty-seven patients were evaluated; 22 had new CVST diagnoses by electronic medical record review. CVST incidence in the hospitalized COVID-19 cohort was 231 per 1 000 000 person-years (95% CI, 152.1–350.8). Females<50 had the highest incidence overall (males <50: 378.4 [142–1008.2]; females<50: 796.5 [428.6–1480.4]). In patients ≥50 years old, males had a higher estimated CVST incidence (males≥50: 130.5 [54.3–313.6]; females≥50: 88.8 [28.6–275.2]). Older patients (45.5% of patients ≥50 versus 0% of <50 years of age, P=0.012) and males (44.4% of males versus 7.7% of females, P=0.023) were more likely to die in hospital.
Conclusions:
CVST incidence in COVID-19–positive hospitalized patients is high. Advanced age and male gender were associated with likelihood of death in hospital; further studies are required to confirm these findings.
Keywords: COVID-19; hospitals; incidence; sinus thrombosis, intracranial; thrombosis
COVID-19 is a primary respiratory disease. However, arterial and venous thrombosis influence the morbidity and mortality of COVID-19, with 74% higher odds of mortality among patients who develop thromboembolism.1
Two adenovirus-based COVID-19 vaccinations have been associated with a rare risk of simultaneous acute thrombosis—usually cerebral venous sinus thrombosis (CVST)—and thrombocytopenia in an entity called vaccine-induced thrombotic thrombocytopenia.2,3
CVST is rare in the non-COVID general population. Risk factors for CVST include pregnancy, malignancy, hormone use, and thrombophilia.4 In the pre-COVID US population, reported CVST incidence ranges from 13.9 and 20.2 per million5 and is 3× more frequent in females.6
CVST incidence associated with adenovirus-based COVID-19 vaccination is extremely low and ranges between 7.5 per million after the AstraZeneca vaccine (99% CI, 2.7–4.7)3 to 0.9 per million (99% CI, 0.2–2.3) after the Janssen vaccine.7 The incidence of CVST in unvaccinated COVID-19–infected patients has not been widely reported. The 2 existing publications reporting CVST incidence in COVID-19 patients have very different reported incidence rates, between 42.8 (95% CI, 28.5–64.2)8 and 207.1 per million hospitalized patients (99% CI, 23.3–757.7).7
We report the incidence of CVST in patients hospitalized with COVID-19 during the first year of the pandemic—before vaccines were widely available—and describe patient characteristics and disease course.
Methods
This study had approval from the University of Minnesota Institutional Review Board. A waiver of consent was given as the research was determined to have no more than minimal risk to the subjects, it would not adversely affect the rights and welfare of subjects, and the research could not practically be carried out without the waiver. We reported against STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) criteria as per journal guidelines (Supplemental Material).
Data Source
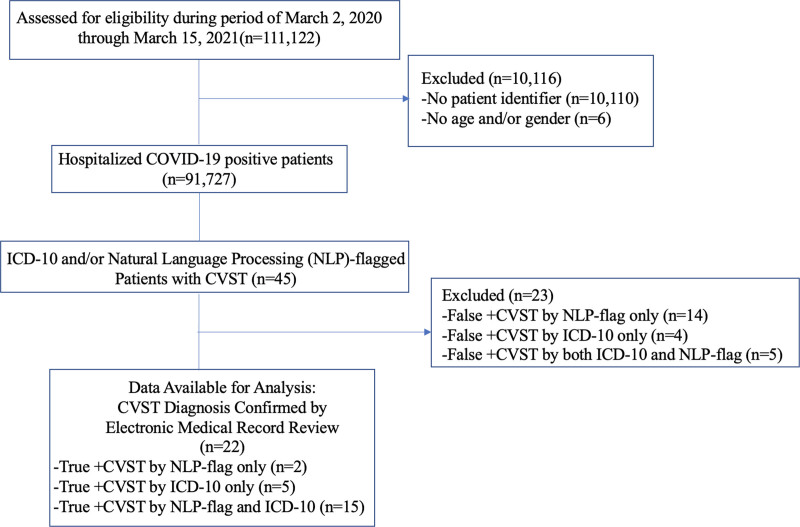
We analyzed retrospective cohort data from >200 US hospitals including all patients hospitalized with COVID-19 between March 2, 2020 and March 15, 2021 (Figure). Anonymized individual electronic medical records for COVID-19-related hospitalizations were fully reviewed. We used the U07.1 International Classification of Diseases, Tenth Revision (ICD-10) code to identify COVID-19 patients. CVST was identified either by validated ICD-10 codes submitted by human coders for CVST diagnosis (I67.6, I63.6, O22.5, O87.3, G08.X)9,10 or natural language processing (NLP) engine extractions. NLP was used as a filter flag for chart review. Our combined method used both claims- and NLP-based searches to narrow the expansive dataset to patients with claims- and/or NLP-flagged diagnosis of CVST. Vascular neurologists (M.E.M.-H., K.L.) confirmed CVST diagnosis by review of patients’ electronic medical records. There was 100% concordance between raters in CVST diagnosis. The data are proprietary and not available for public use but, under certain conditions, may be made available to editors and their approved auditors under a data-use agreement to confirm the findings of this study.
Figure.
Study flow chart. CVST indicates cerebral venous sinus thrombosis; ICD-10, International Classification of Diseases, Tenth Revision; and NLP, natural language processing.
Outcomes and Study Variables
The primary outcome was CVST incidence in hospitalized, COVID-19–positive patients during the defined period presented as per 1 000 000 person-years. Secondary outcomes included CVST incidence and mortality stratified by age and gender and mortality stratified by status of inpatient steroid administration.
Statistical Analysis
Our observation window extended from March 2, 2020 to the patient’s first COVID+ hospitalization during which CVST was diagnosed or when the patient was censored on March 15, 2021 as no CVST during this period. Incidence rates were calculated using the DerSimonian-Laird estimator method (R package).
We used Fisher exact test to examine the independence between in-hospital death versus age, gender, and inpatient steroid use.
Results
One hundred eleven thousand one hundred twenty-two inpatient visits were associated with COVID-19 positivity within the specified period. We excluded 10 110 without an associated patient identifier, which hampered our ability to track diagnoses, and 6 due to missing age or gender. Our final analytic sample consisted of 91 727 distinct patients with at least one COVID-19 inpatient stay.
NLP engine and CVST ICD codes flagged 45 patients as potential CVST cases. After review of clinical notes and radiology reports, we confirmed 22 of 45 had a new CVST diagnosis. The Figure contains NLP versus claims accuracy measures. ICD-10 codes incorrectly identified 9 cases as CVST positive that, after electronic medical record review, were deemed not new CVST diagnoses. ICD-10 codes missed 2 CVST cases that NLP correctly identified. Erroneous CVST flags resulted from history of CVST and mentions of CVST as a differential diagnosis.
Overall CVST incidence rate in the inpatient COVID+ population was 231 per 1,000,000 person-years (95% CI, 152.1–350.8). Table 1 shows age-gender incidence rates; age was dichotomized at 50 as per publication directing CVST research.11
Table 1.
Age-Gender Incidence Rates (95% CI) in 1 000 000 Person-Years
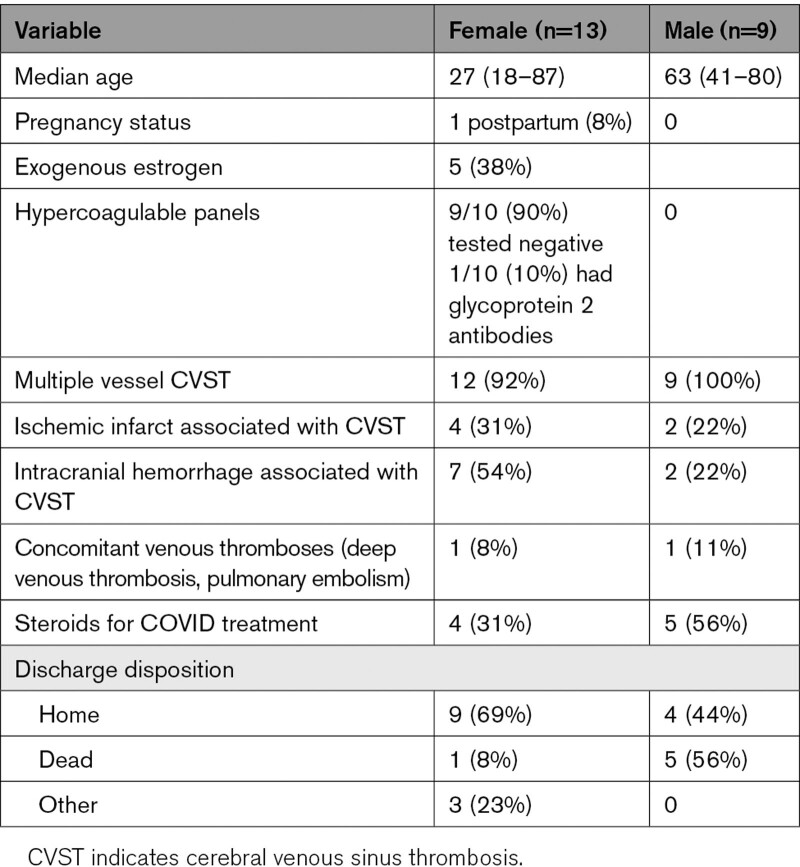
Older patients with CVST were more likely to die in hospital (45.5% of patients over the median age of 46.5 years died versus 0% under the median, P=0.012). Males were more likely to die (44.4% of males versus 7.7% of females; odds ratio, 12.9 [95% CI, 1.03–762.14]; P=0.023). Those treated with inpatient steroids were more likely to die (44.4% who received steroids died versus 7.7% who did not; odds ratio, 12.9 [95% CI, 1.03–762.14]; P=0.023). All steroids were administered for treatment of COVID-19; none were given for treatment of CVST. Table 2 contains pertinent patient characteristics. We used gender, not sex, to describe our patient population because this is how cases were classified in patient charts.
Table 2.
Patient Characteristics, Hospitalization Features
Discussion
We found a high CVST incidence among patients hospitalized with COVID-19. The incidence of CVST in this population appears higher than rates reported in both the general (non–COVID-19) population as well as COVID-19 vaccine-induced thrombotic thrombocytopenia–related CVST. We caution against definitive comparison, as incidence rates for these 3 groups have not been estimated from the same population. We stress that our reported CVST incidence is only among hospitalized COVID-19 patients; the incidence of CVST in the total COVID-19–positive population is likely much lower.8
We observed higher mortality in older patients—particularly male—and in patients treated with steroids for COVID-19 infections with concomitant CVST. COVID-19 mortality is known to increase with age and male sex, and in-hospital steroid administration is typically for severe COVID-19 cases. Our findings may be confounded by known COVID-19 morbidity risk factors. Recently published data suggests that higher COVID-19 male mortality rates may be accounted for by higher risk thrombosis risk in males.12
A limitation of our study is the relatively small number of CVST patients in the hospitalized COVID-19 population. This precludes definitive inference about risk factors for mortality in patients with CVST. Further studies are required to confirm our findings. Due to reporting constraints, we did not have any race or ethnicity data for our cohort, nor did we have sex/gender concordance reporting in this dataset. The study dataset was on a pre–COVID-19 vaccination population; our findings are thus likely most currently relevant to unvaccinated populations. In contrast to traditional retrospective claims-based studies reliant only on ICD-10 codes, our study benefited from NLP-augmented results and full review of patients’ charts. We widened the search for CVST diagnoses using NLP flags in combination with ICD-10 codes. Only 50% of patients flagged for CVST were determined to have a new CVST diagnosis upon full review. We found evidence that claims-based coding both missed some CVST cases and wrongly coded CVST as other diagnoses. The NLP output, by design, is aggressive in identifying and flagging disease for human review. Other estimates of CVST incidence are based on large claims datasets5; based on our experience in this study, claims data may tend to overestimate CVST. Our combined method indicates a potential improvement in accuracy for large dataset, claims-based studies.
Article Information
Sources of Funding
None.
Disclosures
Drs McCullough-Hicks and Lakshminarayan received research funding from Optum Health. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
STROBE Checklist
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CVST
- cerebral venous sinus thrombosis
- EMR
- electronic medical record
- ICD-10
- International Classification of Diseases, Tenth Revision
- NLP
- natural language processing
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.122.038955.
For Sources of Funding and Disclosures, see page e410.
Contributor Information
Daniel J. Halterman, Email: DanHalterman@uhg.com.
David Anderson, Email: sansanjuan@earthlink.net.
Kenneth Cohen, Email: ken.cohen@optum.com.
Kamakshi Lakshminarayan, Email: laksh004@umn.edu.
References
- 1.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, Rampotas A, Ambler G, Makris M. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krzywicka K, van de Munckhof A, Sánchez van Kammen M, Heldner MR, Jood K, Lindgren E, Tatlisumak T, Putaala J, Kremer Hovinga JA, Middeldorp S, et al. Age-stratified risk of cerebral venous sinus thrombosis after SARS-CoV-2 vaccination. Neurology. 2022;98:e759–e768. doi: 10.1212/WNL.0000000000013148 [DOI] [PubMed] [Google Scholar]
- 4.de Freitas GR, Bogousslavsky J. Risk factors of cerebral vein and sinus thrombosis. Handb Cereb Venous Throm. 2008;23:23–54. doi: 10.1159/000111259 [DOI] [PubMed] [Google Scholar]
- 5.Otite FO, Patel S, Sharma R, Khandwala P, Desai D, Latorre JG, Akano EO, Anikpezie N, Izzy S, Malik AM, et al. Trends in incidence and epidemiologic characteristics of cerebral venous thrombosis in the United States. Neurology. 2020;95:e2200–e2213. doi: 10.1212/WNL.0000000000010598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26 [DOI] [PubMed] [Google Scholar]
- 7.Bikdeli B, Chatterjee S, Arora S, Monreal M, Jimenez D, Krumholz HM, Goldhaber SZ, Elkind MSV, Piazza G. Cerebral venous sinus thrombosis in the U.S. population, after adenovirus-based SARS-CoV-2 vaccination, and after COVID-19. J Am Coll Cardiol. 2021;78:408–411. doi: 10.1016/j.jacc.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. eClinicalMedicine. 2021;39:101061.doi: 10.1016/j.eclinm.2021.101061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handley JD, Emsley HC. Validation of ICD-10 codes shows intracranial venous thrombosis incidence to be higher than previously reported. Health Inf Manag. 2020;49:58–61. doi: 10.1177/1833358318819105 [DOI] [PubMed] [Google Scholar]
- 10.Handley JD, Emsley HC. PO256 Validated ICD-10 codes suggest intracranial venous thrombosis incidence may be underestimated. J Neurol Neurosurg Psychiatry. 2017;88:A80–A80. doi: 10.1136/jnnp-2017-ABN.277 [Google Scholar]
- 11.Field TS, Hill MD. Cerebral venous thrombosis. Stroke. 2019;50:1598–1604. doi: 10.1161/STROKEAHA.119.025334 [DOI] [PubMed] [Google Scholar]
- 12.Cohen KR, Anderson D, Ren S, Cook DJ. Contribution of the elevated thrombosis risk of males to the excess male mortality observed in COVID-19: an observational study. BMJ Open. 2022;12:e051624. doi: 10.1136/bmjopen-2021-051624 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.