Polygenic risk scores (PRS) are an increasingly available tool to refine risk prediction for cardiometabolic diseases.1 Favourable lifestyle behaviours might offset increased polygenic risk, but whether frequency of depressed mood stratifies PRS-associated risk is unknown. Here, we calculated individual-level 3-million-variant PRS for coronary artery disease (CAD), type 2 diabetes (T2D), and atrial fibrillation2 among 328,152 genotyped European-ancestry individuals in the UK Biobank. After adjustment for clinical/lifestyle factors and PRS, low versus high frequency of depressed mood was associated with lower risks of incident CAD by 34%, T2D by 33%, and atrial fibrillation by 20%. Frequency of depressed mood stratified risk for CAD and T2D across low (lowest quintile), intermediate (middle 3 quintiles), and high (highest quintile) PRS strata. Depression was associated more strongly with CAD in women compared with men (Pinteraction <0.001). Overall, lower burden of depressed mood was independently associated with lower risk of CAD and T2D across the cardiometabolic polygenic risk spectrum.
While diet, exercise, and smoking have long been recognized as significant behavioural contributors to cardiovascular health,3–6 mounting evidence also implicates depression as an independent risk factor for cardiovascular disease (CVD).7 In the INTERHEART study of approximately 25,000 individuals across 52 countries, depression was independently associated with a 1.55-fold risk of myocardial infarction.8 In a recent study of 563,255 individuals from 22 pooled cohorts, each additional standard deviation (SD) of greater depressive symptoms was independently associated with 1.06- to 1.10-fold increased risk for a composite of CAD and stroke.9
PRS are the quantitative representation of heritable risk for a particular trait or condition conferred by many common genetic variants.1 PRS are predictive of cardiometabolic disease,10,11 may identify individuals most likely to benefit from preventive therapies,10,12 and are increasingly available in clinical and direct-to-consumer settings. PRS may be particularly useful for risk assessment early in life, prior to development of overt cardiometabolic risk factors.1 Although optimal management of high polygenic risk for cardiometabolic diseases remains to be defined, prior observational studies suggest favourable lifestyle behaviours may mitigate heightened polygenic risk,2,11,13 implying lifestyle modification may be an efficient strategy to address elevated polygenic risk. This analysis aimed to assess whether frequency of depressed mood further stratifies polygenic risk of cardiometabolic conditions, independent of lifestyle and conventional CVD risk factors.
Here, we tested the association of frequency of depressed mood with incident CAD, T2D, and atrial fibrillation across the spectrum of polygenic risk, and secondarily in those at high polygenic risk, in the UK Biobank.
Results
Description of the Study Cohort
The study cohort included 328,152 genotyped, unrelated, European-ancestry participants from the UK Biobank (Extended Data Figure 1). Excluded individuals were older and had a greater burden of cardiometabolic risk factors and prevalent CVD (Supplementary Table 1). Mean (SD) age of the study sample was 56.8 (7.9) years, and 173,333 (52.8%) were female. Overall, 255,078 individuals (77.7%) reported no episodes of depressed mood in the past 2 weeks (low frequency of depressed mood), 59,950 (18.3%) reported depression several days in the past 2 weeks (moderate frequency), and 13,124 (4.0%) reported depression more than half of days or nearly every day (high frequency).
Individuals with greater frequency of depressed mood were younger at baseline, more likely to be female, and more likely to be current smokers; reported less vegetable and fresh fruit intake, exercise, and sleep; and had higher body mass index (BMI), higher C-reactive protein, and higher baseline prevalence of CAD, hypertension, hypercholesterolemia, and T2D (Table 1).
Table 1.
Baseline characteristics of study cohort.
| Depression burden | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | High (n=13,124) |
Moderate (n=59,950) |
Low (n=255,078) |
P-value |
| Age, years | 54.4 (7.7) | 55.0 (8.0) | 57.3 (7.9) | <2.2x10−16 |
|
| ||||
| Female sex | 7,762 (59.1%) | 35,870 (59.8%) | 129,701 (50.8%) | <2.2x10−16 |
|
| ||||
| Country of enrolling UK Biobank assessment centre | ||||
| • England | 11,575 (88.2%) | 52,747 (88.0%) | 225,590 (88.4%) | 2.0x10−10 |
| • Scotland | 867 (6.6%) | 4,439 (7.4%) | 18,713 (7.3%) | |
| • Wales | 682 (5.2%) | 2,764 (4.6%) | 10,775 (4.2%) | |
|
| ||||
| Townsend deprivation index (median [IQR]) | −1.4 [−3.3, 1.7] | −2.1 [−3.6, 0.5] | −2.5 [−3.8, −0.4] | <2.2x10−16 |
|
| ||||
| Smoking status | ||||
| • Current | 2,418 (18.4%) | 7,476 (12.5%) | 21,547 (8.4%) | <2.2x10−16 |
| • Former | 4,276 (32.6%) | 20,954 (35.0%) | 90,811 (35.6%) | |
| • Never | 6,430 (49.0%) | 31,520 (52.5%) | 142,720 (56.0%) | |
|
| ||||
| Total pack-years of smoking among current or former smokers (median [IQR]) | 15.9 [0, 31.5] | 11.5 [0, 26.5] | 9.3 [0, 23.6] | <2.2x10−16 |
|
| ||||
| Alcohol intake (median [IQR]) | 1–2 times weekly [1–3 times monthly, daily or almost daily] |
1–2 times weekly [1–3 times monthly, 3–4 times weekly] |
1–2 times weekly [1–3 times monthly, 3–4 times weekly] |
<2.2x10−16 |
|
| ||||
| Total portions of vegetables and fresh fruit daily | 6.6 (4.3) | 6.8 (3.7) | 7.1 (3.7) | <2.2x10−16 |
|
| ||||
| Days of moderate physical activity ≥10 min | 3.3 (2.5) | 3.5 (2.3) | 3.6 (2.3) | <2.2x10−16 |
|
| ||||
| Days of vigorous physical activity ≥10 min | 1.6 (2.0) | 1.7 (1.9) | 1.9 (1.9) | <2.2x10−16 |
|
| ||||
| Average sleep duration, hours | 6.9 (1.3) | 7.1 (1.1) | 7.2 (1.0) | <2.2x10−16 |
|
| ||||
| Body mass index, kg/m2 | 28.4 (5.7) | 27.4 (5.0) | 27.2 (4.5) | <2.2x10−16 |
|
| ||||
| Systolic blood pressure | 136.3 (19.0) | 136.9 (19.1) | 140.9 (19.6) | <2.2x10−16 |
|
| ||||
| Antihypertensive medication use | 2,782 (21.2%) | 11,285 (18.8%) | 51,802 (20.3%) | <2.2x10−16 |
|
| ||||
| Cholesterol-lowering medication use | 2,470 (18.8%) | 9,403 (15.7%) | 43,202 (16.9%) | <2.2x10−16 |
|
| ||||
| Antiplatelet medication use | 2,095 (16.0%) | 8,054 (13.4%) | 36,274 (14.2%) | 3.7x10−14 |
|
| ||||
| Antihyperglycemic medication use | 673 (5.1%) | 2,068 (3.4%) | 7,761 (3.0%) | <2.2x10−16 |
|
| ||||
| Antidepressant medication use | 3,481 (26.5%) | 7,734 (12.9%) | 9,965 (3.9%) | <2.2x10−16 |
|
| ||||
| Non-high-density lipoprotein cholesterol, mg/dL | 165.4 (43.1) | 164.6 (41.7) | 164.9 (41.4) | 0.13 |
|
| ||||
| C-reactive protein, mg/L (median [IQR]) | 1.6 [0.7, 3.4] | 1.3 [0.6, 2.8] | 1.3 [0.6, 2.6] | <2.2x10−16 |
|
| ||||
| Coronary artery disease | 724 (5.5%) | 2,376 (4.0%) | 10,160 (4.0%) | 1.0x10−11 |
|
| ||||
| Hypertension | 4,266 (32.5%) | 17,072 (28.5%) | 72,196 (28.3%) | <2.2x10−16 |
|
| ||||
| Hypercholesterolemia | 2,119 (16.1%) | 8,313 (13.9%) | 37,400 (14.7%) | <2.2x10−16 |
|
| ||||
| Type 2 diabetes mellitus | 501 (3.8%) | 1,396 (2.3%) | 5,007 (2.0%) | <2.2x10−16 |
|
| ||||
| Atrial fibrillation | 197 (1.5%) | 949 (1.6%) | 4,526 (1.8%) | 6.8x10−4 |
Two-sided P-values (unadjusted for multiple comparisons) were calculated using the Pearson chi-squared test for categorical variables and analysis of variance (normally distributed variables) or the Kruskal-Wallis test (non-normally distributed variables).
Participants were followed up for outcomes over a median (interquartile range) of 11.1 (10.4–11.8) years. Among individuals without each condition at baseline in the full cohort, 17,880 (of 314,892 [5.7%]) developed CAD, 14,345 (of 321,248 [4.5%]) developed T2D, and 15,397 (of 322,480 [4.8%]) developed atrial fibrillation.
Polygenic Risk and Incident Outcomes
PRS for CAD, T2D, and atrial fibrillation were derived from genome-wide association studies (GWAS) performed in consortia external to the UK Biobank (CARDIoGRAMplusC4D,14 DIAGRAM,15 and AFGen,16 respectively) using AnnoPred as previously described.2,17 Briefly, AnnoPred is a genome-wide Bayesian method that leverages genomic and epigenomic functional annotations to adjust variant weights.2,17 The AnnoPred method recently yielded superior risk prediction compared with other current state-of-the-art polygenic risk scoring approaches.2
For each condition under study, polygenic risk was independently associated with the corresponding outcome. After complete covariate adjustment including frequency of depressed mood, genetically increased risk was associated with higher risk of CAD (hazard ratio [HR] per SD of CAD polygenic risk: 1.32, 95% 1.30–1.34, P<2.2x10−16), T2D (HR per SD: 1.38, 95% CI 1.35–1.40, P<2.2x10−16), and atrial fibrillation (HR per SD: 1.46, 95% CI 1.44–1.49, P<2.2x10−16).
Greater frequency of depressed mood was modestly associated with higher CAD PRS (higher CAD PRS by 0.012 SD per depression category, 95% CI 0.005–0.019 SD, P=3.6x10−4) and higher T2D PRS (higher T2D by 0.009 SD, 95% CI 0.003–0.016 SD, P=0.006) but not with atrial fibrillation PRS (P=0.29).
Frequency of Depressed Mood and Incident Outcomes
The primary study exposure was self-reported frequency of depressed mood, ascertained at study enrolment. After adjustment for age, age2, sex, the first 20 principal components of ancestry, genotyping array, country of study enrolment (England, Scotland, or Wales), Townsend deprivation index, smoking status, pack-year smoking history, alcohol intake, vegetable and fresh fruit intake, days per week of moderate and vigorous exercise, sleep duration, systolic blood pressure, antihypertensive medication use, non-high-density lipoprotein cholesterol, cholesterol-lowering medication use, antiplatelet medication use, antihyperglycemic medication use, prevalent type 2 diabetes mellitus (models for coronary artery disease and atrial fibrillation only), body mass index, C-reactive protein, and polygenic risk, low frequency of depressed mood (vs. high frequency of depressed mood [reference]) was associated with 34% lower risk of CAD (HR 0.66, 95% CI 0.61–0.71, P<2.2x10−16), 33% lower risk of T2D (HR 0.67, 95% CI 0.62–0.72, P<2.2x10−16), and 20% lower risk of atrial fibrillation (HR 0.80, 95% CI 0.73–0.88, P=1.4x10−6) (Table 2).
Table 2.
Association of low and moderate vs. high frequency of depressed mood with outcomes.
| Condition | Frequency of depressed mood | No. at risk | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|
| Coronary artery disease | High | 12,400 | 1.00 (reference) |
-- |
| Moderate | 57,574 | 0.77 (0.71–0.84) |
6.3x10−10 | |
| Low | 244,919 | 0.66 (0.61–0.71) |
<2.2x10−16 | |
| Type 2 diabetes mellitus | High | 12,623 | 1.00 (reference) |
-- |
| Moderate | 58,554 | 0.79 (0.72–0.86) |
2.5x10−8 | |
| Low | 250,072 | 0.67 (0.62–0.72) |
<2.2x10−16 | |
| Atrial fibrillation | High | 12,927 | 1.00 (reference) |
-- |
| Moderate | 59,001 | 0.85 (0.77–0.94) |
0.001 | |
| Low | 250,553 | 0.80 (0.73–0.88) |
1.4x10−6 |
Two-sided P-values (unadjusted for multiple comparisons) were calculated using multivariable-adjusted Cox proportional hazard models. Models are adjusted for age, age2, sex, PC 1–20, genotyping array, country, Townsend deprivation index, smoking status, pack-year smoking history, alcohol intake, vegetable + fresh fruit intake, days per week of moderate and vigorous exercise, sleep duration, systolic blood pressure, antihypertensive medication use, non-HDL cholesterol, cholesterol-lowering medication use, antiplatelet medication use, antihyperglycemic medication use, prevalent type 2 diabetes mellitus (models for coronary artery disease and atrial fibrillation only), body-mass index, C-reactive protein, and polygenic risk.
Frequency of depressed mood significantly stratified risk among individuals across low (lowest quintile), intermediate (middle 3 quintiles), and high (highest quintile) levels of polygenic risk for incident CAD and T2D, and frequency of depressed mood and polygenic risk conferred additive risk (Figure 1, Extended Data Figure 2, Supplementary Table 2). Absolute risk differences associated with a lower frequency of depressed mood were modestly higher among individuals with high (vs. moderate or low) polygenic risk for CAD and substantially larger among individuals with high polygenic risk for T2D. Among individuals at high polygenic risk without each condition at baseline, low vs. high frequency of depressed mood was associated with a lower incidence rate for CAD by (7.61 vs. 9.72 per 1,000 person-years, incidence rate difference −2.11 [95% CI −3.31 to −0.90] per 1,000 person-years, P=6.1x10−4) and with a lower incidence rate for T2D (6.83 vs. 11.84 per 1,000 person-years, incidence rate difference −5.01 [95% CI −6.34 to −3.68] per 1,000 person-years, P=1.5x10−13). In fully adjusted models (Table 3), the combination of high polygenic risk and high frequency of depressed mood vs. low polygenic risk and low frequency of depressed mood (reference) was associated with 3-fold risk for incident CAD (HR 3.11, 95% CI 2.69–3.59, P<2.2x10−16), 4-fold risk for incident T2D (HR 3.82, 95% CI 3.31–4.40, P<2.2x10−16), and 3.5-fold risk for atrial fibrillation (HR 3.51, 95% CI 2.97–4.15, P<2.2x10−16).
Figure 1. Absolute incidence rates for (a) coronary artery disease, (b) type 2 diabetes mellitus, and (c) atrial fibrillation by polygenic risk tier and frequency of depressed mood.
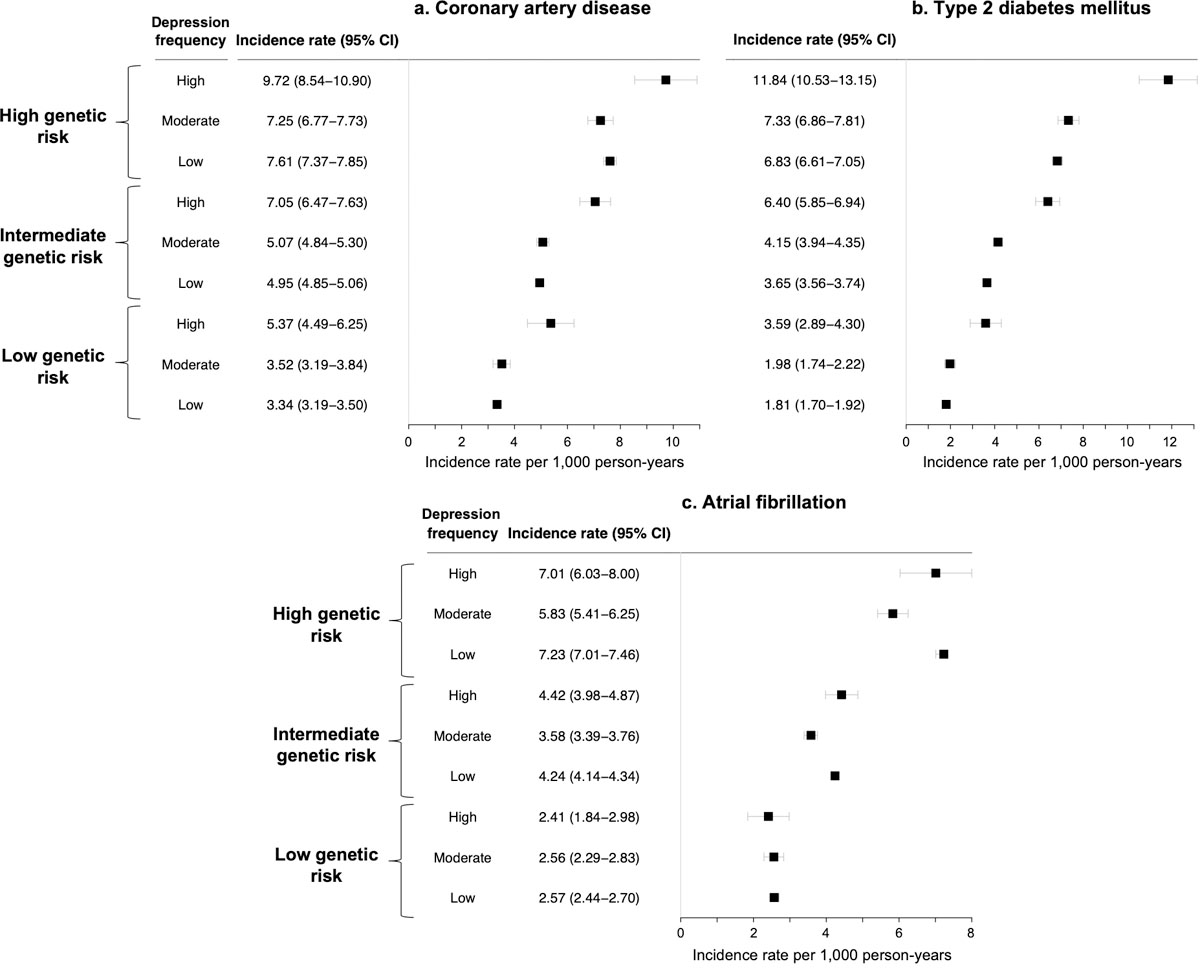
Data are presented as incidence rates (black squares) and 95% confidence intervals (error bars). Two-sided P-values (unadjusted for multiple comparisons) are calculated from the chi-squared statistic for the difference in incidence rates using the ‘fmsb’ package in R version 3.6.0. Absolute crude incidence rates are calculated among individuals without each condition at baseline and are reported per 1,000 person-years of follow-up.
Table 3.
Hazard ratios for incident outcomes associated with polygenic risk tier and frequency of depressed mood.
| Coronary artery disease | Type 2 diabetes mellitus | Atrial fibrillation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | ||||||||
| Poly-genic risk tier | Frequency of depressed mood | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value |
| Low | Low | 1.00 (reference) |
-- | 1.00 (reference) |
-- | 1.00 (reference) |
-- | 1.00 (reference) |
-- | 1.00 (reference) |
-- | 1.00 (reference) |
-- |
| Moderate | 1.34 (1.21–1.49) |
1.8x10−8 | 1.27 (1.13–1.43) |
4.3x10−5 | 1.28 (1.11–1.46) |
4.4x10−4 | 1.18 (1.01–1.38) |
0.03 | 1.31 (1.17–1.48) |
6.5x10−6 | 1.24 (1.08–1.42) |
0.002 | |
| High | 2.12 (1.79–2.51) |
<2x10−16 | 1.81 (1.49–2.19) |
1.0x10−9 | 2.26 (1.84–2.77) |
8.5x10−15 | 1.50 (1.19–1.91) |
7.5x10−4 | 1.33 (1.04–1.69) |
0.02 | 1.20 (0.91–1.58) |
0.19 | |
| Inter-mediate | Low | 1.51 (1.44–1.59) |
<2x10−16 | 1.43 (1.35–1.52) |
<2x10−16 | 2.03 (1.90–2.17) |
<2x10−16 | 1.71 (1.59–1.85) |
<2x10−16 | 1.69 (1.59–1.79) |
<2x10−16 | 1.72 (1.61–1.83) |
<2x10−16 |
| Moderate | 1.97 (1.85–2.10) |
<2x10−16 | 1.70 (1.58–1.83) |
<2x10−16 | 2.71 (2.51–2.94) |
<2x10−16 | 2.02 (1.85–2.20) |
<2x10−16 | 1.87 (1.73–2.01) |
<2x10−16 | 1.81 (1.66–1.96) |
<2x10−16 | |
| High | 2.78 (2.53–3.06) |
<2x10−16 | 2.15 (1.93–2.40) |
<2x10−16 | 4.01 (3.60–4.45) |
<2x10−16 | 2.59 (2.29–2.91) |
<2x10−16 | 2.44 (2.18–2.74) |
<2x10−16 | 2.25 (1.98–2.55) |
<2x10−16 | |
| High | Low | 2.42 (2.29–2.56) |
<2x10−16 | 2.18 (2.05–2.32) |
<2x10−16 | 3.90 (3.64–4.18) |
<2x10−16 | 2.61 (2.41–2.82) |
<2x10−16 | 2.99 (2.81–3.17) |
<2x10−16 | 3.04 (2.84–3.25) |
<2x10−16 |
| Moderate | 2.93 (2.71–3.18) |
<2x10−16 | 2.43 (2.22–2.66) |
<2x10−16 | 4.93 (4.50–5.39) |
<2x10−16 | 3.13 (2.83–3.46) |
<2x10−16 | 3.18 (2.91–3.47) |
<2x10−16 | 3.11 (2.81–3.43) |
<2x10−16 | |
| High | 4.03 (3.54–4.60) |
<2x10−16 | 3.11 (2.69–3.59) |
<2x10−16 | 7.83 (6.90–8.90) |
<2x10−16 | 3.82 (3.31–4.40) |
<2x10−16 | 3.82 (3.28–4.43) |
<2x10−−16 | 3.51 (2.97–4.15) |
<2x10−16 | |
Two-sided P-values (unadjusted for multiple comparisons) were calculated using multivariable-adjusted Cox proportional hazard models. Model 1: Adjusted for age, age2, sex, PC 1–20, genotyping array, country, Townsend deprivation index
Model 2: Adjusted for age, age2, sex, PC 1–20, genotyping array, country, Townsend deprivation index, smoking status, pack-year smoking history, alcohol intake, vegetable + fresh fruit intake, days per week of moderate and vigorous exercise, sleep duration, systolic blood pressure, antihypertensive medication use, non-HDL cholesterol, cholesterol-lowering medication use, antiplatelet medication use, antihyperglycemic medication use, prevalent type 2 diabetes mellitus, body-mass index, C-reactive protein
Among individuals at high polygenic risk, a low vs. high burden of depressed mood was associated with lower risk of all outcomes in sparsely adjusted models, with greatest magnitude of risk reduction observed for T2D (Supplementary Table 3). After further adjustment for lifestyle factors, conventional CVD risk factors, and C-reactive protein, associations of depression with outcomes remained statistically significant for incident CAD (HR for low vs. high frequency of depressed mood: 0.70, 95% CI 0.61–0.81, P=1.2x10−6) and T2D (HR for low vs. high frequency of depressed mood: 0.66, 95% CI 0.57–0.74, P=3.0x10−10), while associations with incident atrial fibrillation did not retain significance (Extended Data Figure 3).
Sensitivity Analyses
Sensitivity analyses excluding all individuals with CAD, T2D, atrial fibrillation, ischemic stroke, peripheral artery disease, or heart failure at baseline were nearly identical to primary analyses (Supplementary Table 4). Similarly, further adjustment for use of antidepressants and exclusion of individuals taking antidepressant medications at baseline each did not materially change findings (Supplementary Table 5). Additional analyses with follow-up truncated prior to the outbreak of the SARS-CoV2 pandemic yielded identical results to those of the primary models (Supplementary Table 6).
In addition, PHQ-2 scores grouped as scores of 0 (low), 1–2 (moderate), and ≥3 (high) were distributed similarly to the primary exposure (72.7%, 22.6%, and 4.7%, respectively) and demonstrated strikingly similar associations compared with primary analyses (Supplementary Table 6). Associations were similar when a low PHQ-2 score was defined as 0–1 (Supplementary Table 7). In fully adjusted models including polygenic risk, each additional point on the PHQ-2 score was associated with greater risk of CAD (HR 1.10, 95% CI 1.08–1.11, P<2.2x10−16), T2D (HR 1.11, 95% CI 1.09–1.13, P<2.2x10−16), and atrial fibrillation (HR 1.05, 95% CI 1.03–1.07, P=5.7x10−9).
Assessment of Interactions
We observed a significant interaction between polygenic risk and frequency of depressed mood for incident CAD (after full covariate adjustment, HR(interaction): 0.95, 95% CI 0.93–0.98, P=0.003; Supplementary Table 8) whereby the association between depression and CAD was slightly smaller among individuals at high vs. low polygenic CAD risk (Supplementary Table 9). To test whether this finding might be driven by higher proportion of individuals with prevalent CAD excluded from analysis among those with high polygenic risk, we tested interactions for total (prevalent and incident) CAD using logistic regression and observed identical interactions (Supplementary Table 10). By contrast, interactions between polygenic risk and frequency of depressed mood were not observed for other outcomes (Supplementary Table 8).
Sex Differences
We observed an interaction between sex and frequency of depressed mood for incident CAD (fully adjusted HR(interaction of depression*female sex): 1.11, 95% CI 1.05–1.18, P<0.001). In fully adjusted models including polygenic risk, a low vs. high burden of depression was independently associated with greater CAD risk reduction in women (HR 0.57, 95% CI 0.51–0.63, P<2.2x10−16) vs. men (HR 0.74, 95% CI 0.67–0.81, P=3.1x10−9, Supplementary Table 11). We did not observe significant sex*depression interactions in fully adjusted models for T2D or atrial fibrillation.
Comparing Depression Frequency and Polygenic Risk
A high frequency of depressed mood was associated with the same crude cumulative incidence as the top 27th percentile of CAD PRS and the top 22nd percentile of T2D PRS. Using the magnitude of associations derived from fully adjusted models, low vs. high frequency of depressed mood was equivalent to having a lower CAD PRS by 1.51 SD, lower T2D PRS by 1.24 SD, and lower atrial fibrillation PRS by 0.57 SD.
Discussion
In a prospective cohort of >325,000 British adults, a lower burden of depressed mood was associated with decreased risk of CAD, T2D, and atrial fibrillation across the polygenic risk spectrum. Among individuals at high polygenic risk, who have highest absolute risk of developing disease, a low vs. high burden of depression was independently associated with lower risk of CAD and T2D by 30–32%. Although absolute risk differences associated with frequency of depressed mood were modest in this relatively low-risk cohort, the association of depressed mood with outcomes was independent of lifestyle factors known to associate with both mental health and CVD (diet, exercise, sleep, tobacco and alcohol use) and other conventional CVD risk factors. In addition, frequency of depressed mood was more strongly linked to incident CAD in women vs. men.
This analysis extends prior work on depression and incident cardiometabolic disease in several respects. Our study now demonstrates additive effects of depression across strata of polygenic risk, as assessed using a recently described PRS approach. Although lifestyle behaviours also stratify polygenic risk of cardiometabolic disease,2,11,13 frequency of depressed mood was associated with cardiometabolic outcomes independent of these lifestyle factors. The large cohort size and detailed phenotyping of UK Biobank participants permitted a more comprehensive set of covariates (including vital signs and laboratory biomarkers) than prior large studies of depression and CVD7–9,18,19 and enabled examination of outcomes beyond atherosclerotic CVD alone.
We identified an interaction between frequency of depressed mood and CAD PRS whereby the association between depression and CAD was slightly smaller among individuals at high vs. low polygenic CAD risk. To our knowledge, no such interaction has been previously reported. Given the correlation we observed between CAD PRS and frequency of depressed mood, one potential explanation for this interaction may be that a genome-wide CAD PRS incorporating millions of variants may be already capturing some of the association between depression and CAD in the PRS itself.
The findings of this study may have implications for improving individual- and population-level cardiometabolic health. First, a reduced burden of depressed mood is associated with lower risk for future development of adverse outcomes independent of other lifestyle factors. This finding is consistent with recent Mendelian randomization analyses suggesting that depression may be a causal risk factor for CVD.20,21 The mechanisms linking depression to cardiometabolic disease, however, remain incompletely understood.22 Prior analyses suggest modest mediating effects of T2D and lipid levels for CAD.20,21 Other proposed mechanisms linking depression to CVD include autonomic dysregulation, alteration of neuroendocrine pathways, and elevated systemic inflammation.22–25
Second, the association of depression with CAD risk may be even stronger among women. Women with established CVD are two-fold more likely than men with established CVD to have depression.26 However, previous data regarding sex differences for prevalent depression and subsequent development of CVD are mixed, suggesting stronger association between depression and incident CVD in women,27,28 men,18,29 or no sex difference.7,30 Here, we observed a stronger association with depression observed in women vs. men. This finding stands seemingly in contrast with that of the PURE study, where a greater number of depressive symptoms associated more strongly with a composite CVD outcome in men vs. women.18 This apparent discrepancy may stem from various differences in experimental design but potentially more so in outcome definition differences; PURE focused on a broad composite CVD outcome whereas we observe an interaction specifically for CAD. A heightened state of systemic inflammation in women vs. men with depression may underlie this observed sex difference,31,32 although further research to elucidate relevant sex-specific pathobiology is needed.
Third, these findings imply that management of depressed mood may reduce cardiometabolic risk. However, high-quality data on the effects of depression treatment on cardiometabolic outcomes, particularly in primary prevention populations, is currently lacking,33 and high-quality randomized trials are necessary to affirm this hypothesis.
This work should also be interpreted in the context of study design. First, the primary exposure was assigned based on a single two-week recall measure. This exposure had strikingly similar associations with cardiometabolic outcomes compared with PHQ-2 scores; indeed, the findings of this study underscore the prognostic significance of this single question, highlighting its potential for incorporation in care settings where depression screening is not routinely performed. Misclassification related to self-reported exposure may bias estimates toward the null. Second, frequency of depressed mood and lifestyle behaviours were assessed only at baseline, and cumulative and time-varying burden of depression, lifestyle risk, and other risk factors were not assessed. Third, data restrictions preclude chart validation of incident events in the UK Biobank; however, genetic analyses using these phenotype definitions have yielded highly consistent results compared with those from validated epidemiologic studies.34,35 Fourth, UK Biobank participants are healthier on average than the broader UK population,36 which may influence absolute risk differences and the magnitude of estimated associations. Fifth, because polygenic risk scores have to date focused on European ancestry individuals, the present study cohort was restricted to white Europeans; further research is needed to establish generalizability to diverse populations. Finally, as in any observational analysis, we cannot exclude the possibility of residual confounding, and causality cannot be established from these results.
In summary, lower frequency of depressed mood was independently associated was decreased risk of CAD and T2D across the spectrum of polygenic risk. Further research is needed to clarify mechanisms and implications for preventive care.
Methods
Study Cohort
The UK Biobank is a prospective, observational, population-based cohort of >500,000 adult residents of the United Kingdom aged 40–69 years at the time of recruitment between 2006–2010.37 The present study was conducted under UK Biobank application number 7089. The UK Biobank was approved by the North West Multi-centre Research Ethics Committee, and all subjects provided written informed consent. Participants were not compensated for study participation, although reimbursement was available for expenses incurred through participation. The Massachusetts General Hospital institutional review board approved secondary analysis of these data.
At enrolment, participants provided detailed information on medical history, medication use, lifestyle factors, and mental health, and underwent physical assessment and phlebotomy for laboratory analysis and genotyping. Participants were followed for the development of incident diagnoses through linkage to national health records and follow-up study visits.37 The present analysis included genotyped, unrelated European ancestry participants with complete available data on self-reported frequency of depressed mood, socioeconomic status, sleep, and health behaviours. Follow-up for the primary analyses occurred through March 2020.
Study Cohort, Genotyping, and Imputation
A subset of 49,950 participants were genotyped using the UK Biobank BiLEVE Axiom Array,38 and the remaining 438,427 were genotyped using the UK Biobank Axiom Array (both arrays by Affymetrix, Santa Clara, CA); these arrays share >95% of marker content.37 Imputation was performed centrally by the UK Biobank using the Haplotype Reference Consortium, UK10K, and 1000Genomes phase 3 reference panels as described previously.37 The present analysis considered individuals with white British ancestry, concordance between genetic and reported sex, and no sex chromosome aneuploidy. Participants within 3 degree of relatedness were identified using the Kinship-Based Inference for Genome-Wide Association Studies tool,39 and one from each pair of related individuals was excluded at random.
Polygenic Risk Scores and Risk Strata
PRS for CAD, T2D, and atrial fibrillation were derived from GWAS performed in consortia external to the UK Biobank (CARDIoGRAMplusC4D,14 DIAGRAM,15 and AFGen,16 respectively) using AnnoPred.2,17 AnnoPred is a genome-wide Bayesian method that leverages genomic and epigenomic functional annotations, all external to the UK Biobank, to adjust variant weights.2,17 Ye et al. recently identified optimal AnnoPred PRS for CAD, T2D, and atrial fibrillation by testing 88 candidate PRS for each trait using 4 levels of functional annotations (including diverse genome annotations,40 GenoCanyon functionality scores, GenoSkyline tissue-specific functionality scores, and cell-specific functionality scores), 2 different assumptions about variant effects, and 11 different tuning parameters, selecting the score which maximized area-under-the-curve in training and testing datasets.2 The AnnoPred method yielded superior risk prediction compared with other current state-of-the-art polygenic risk scoring approaches.2 The optimal AnnoPred-derived polygenic scores (using variants with minor allele frequency ≥0.05) included 2,994,054 variants for CAD, 2,996,76 variants for T2D, and 2,996,792 variants for atrial fibrillation.2 Polygenic risk for each condition was designated to be high (highest quintile of PRS), intermediate (middle 3 quintiles), or low (lowest quintile).
Exposures
The primary study exposure was self-reported frequency of depressed mood in the prior 2 weeks, ascertained at study enrolment. Participants were prompted by touchscreen to complete the Patient Health Questionnaire-2 (PHQ-2) screening instrument for depression, which includes the question: “Over the past two weeks, how often have you felt down, depressed or hopeless?” (UK Biobank field ID 2050). Available answers included “not at all,” “several days”, “more than half of days,” and “nearly every day.” Frequency of depressed mood was categorized as low (no depressed mood in the prior 2 weeks), moderate (several days of depressed mood in the prior 2 weeks), or high (depressed mood more than half of days or nearly every day). In primary analyses, individuals with a high burden of depressed mood constituted the reference group.
Covariates
Medical conditions at baseline were captured by participant self-report, with verification by a trained study nurse, and/or by the presence of qualifying ICD code in the participant’s medical record (Supplementary Appendix). Health behaviours, including dietary intake, exercise frequency, sleep, alcohol and tobacco use, were systematically ascertained by self-report. Pack-years of tobacco use were coded by the UK Biobank as 0 if subject quit smoking before age 16 or total duration of smoking was <6 months. We extracted medication use at baseline, including all cholesterol-lowering medications, antihypertensive medications, antiplatelet medications (aspirin, clopidogrel, dipyridamole), and antihyperglycemic medications (metformin, sulfonylureas, thiazolidinediones, and insulin). In addition, we extracted data on baseline use of antidepressant medications (selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, bupropion, mirtazapine, and trazodone). Participants underwent measurement of anthropomorphic data and vital signs, as well as phlebotomy for laboratory analysis, including total and high-density lipoprotein cholesterol and high-sensitivity C-reactive protein (AU5800 platform, Beckman Coulter, UK).
Outcomes
The 3 co-primary study outcomes were incident (newly diagnosed) CAD, T2D, and atrial fibrillation. Outcomes were ascertained by the appearance of a qualifying International Classification of Diseases or procedure code (Supplementary Appendix) in the patient’s medical record.
Statistical Analysis
Participants’ baseline characteristics were compared across depression categories using analysis of variance or the Kruskal-Wallis test, as appropriate, for continuous variables, and using the Pearson chi-squared test for categorical variables. Absolute incidence rates and incidence rate differences across strata of frequency of depressed mood and polygenic risk were calculated using the ‘fmsb’ package in R version 3.6.0 per 1,000 person-years of follow-up time.
Multivariable Cox proportional hazard models were fitted to test the association of frequency of depressed mood with each cardiometabolic disease outcome. Follow-up began at study enrolment, and time to censoring was determined by the appearance of a qualifying diagnosis in the participant’s medical record or the date of last follow-up. Participants were excluded from each model for which they had an established diagnosis at baseline (e.g., participants with prevalent CAD were excluded from models for incident CAD). The proportional hazards assumption was verified using Schoenfeld residuals. Cox models for each outcome were performed with two levels of covariate adjustment: Model 1: adjusted for age, age2, sex, the first 20 principal components of ancestry, genotyping array, country of UK Biobank enrolment (England, Scotland, or Wales), and the inverse-normalized Townsend deprivation index; and Model 2: Model 1 plus smoking status, pack-years of smoking, alcohol intake, vegetable and fresh fruit intake, days per week of moderate and vigorous exercise, nightly sleep duration, systolic blood pressure, antihypertensive mediation use, non-high-density lipoprotein cholesterol, cholesterol-lowering medication use, antiplatelet medication use, antihyperglycemic medication use, prevalent T2D (models for coronary artery disease and atrial fibrillation only), body-mass index, and log-transformed C-reactive protein. Sensitivity analyses (1) probed possible reverse causation by excluding individuals with any prevalent study outcome (CAD, T2D, and/or atrial fibrillation) as well as ischemic stroke, peripheral artery disease, or heart failure at baseline, (2) further adjusted for antidepressant use and excluded those taking antidepressant medications, (3) tested associations between PHQ-2 score and outcomes, and (4) truncated follow-up at December 31, 2019, to ensure observations were not influenced by the SARS-CoV2 pandemic or associated lockdowns.
We evaluated interactions between frequency of depressed mood, modelled as an ordinal term (0, 1, 2), and polygenic risk as a continuous quantitative variable. To avoid residual confounding, interactions terms were included between frequency of depressed mood and each covariate. We further assessed interactions between frequency of depressed mood, and sex-stratified models were performed for outcomes with significant sex*depression interactions.
Given 3 outcomes studied, two-sided P <0.05/3 = 0.0167 was considered statistically significant. Secondary analyses were considered supportive and hypothesis-generating at P <0.05. Analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing).
Extended Data
Extended Data Fig. 1. Creation of the study cohort.
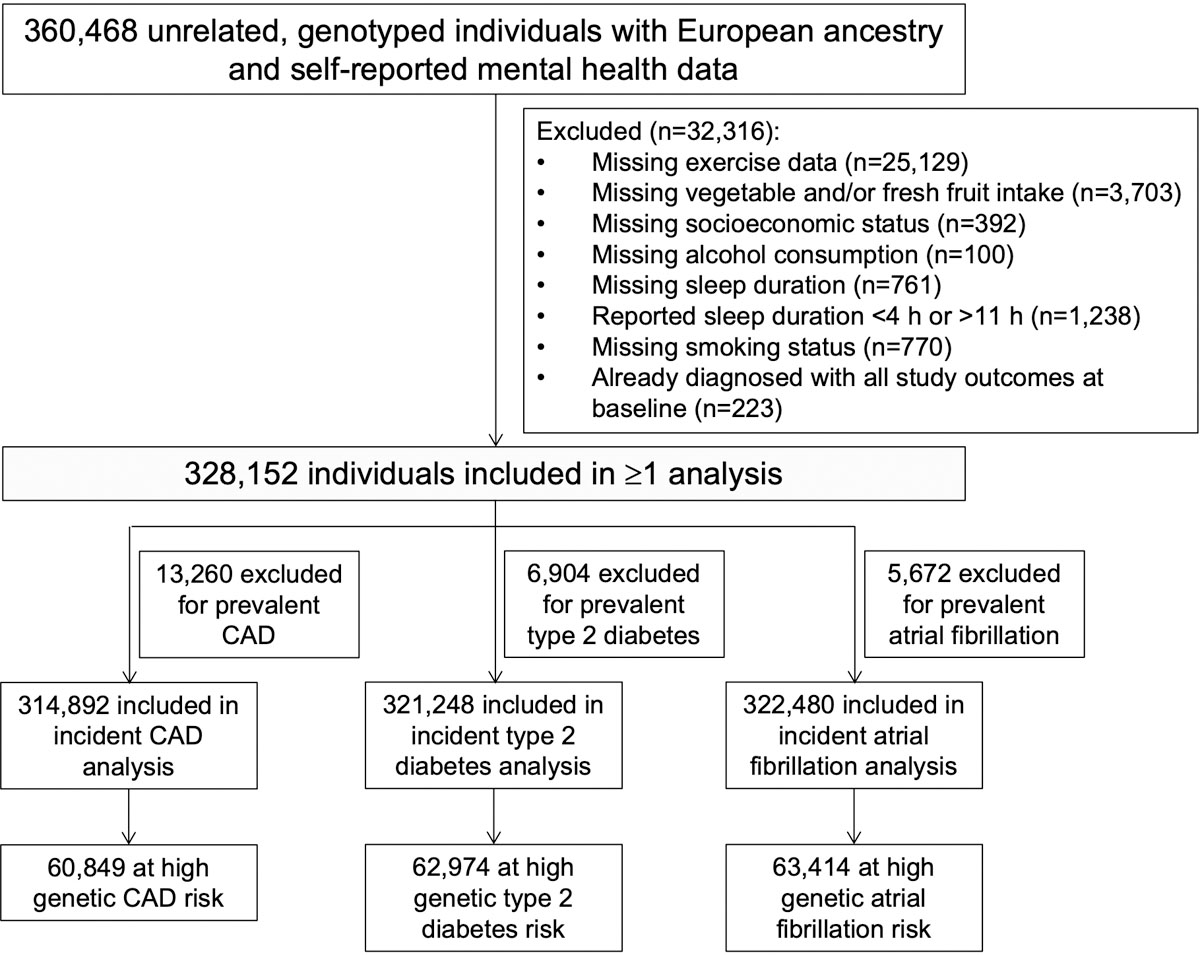
Genotyped, unrelated European ancestry participants in the UK Biobank with complete available data on self-reported frequency of depressed mood, socioeconomic status, sleep, and health behaviors were included. CAD = coronary artery disease.
Extended Data Fig. 2. Additive associations of frequency of depressed mood and polygenic risk for incident coronary artery disease and type 2 diabetes mellitus.
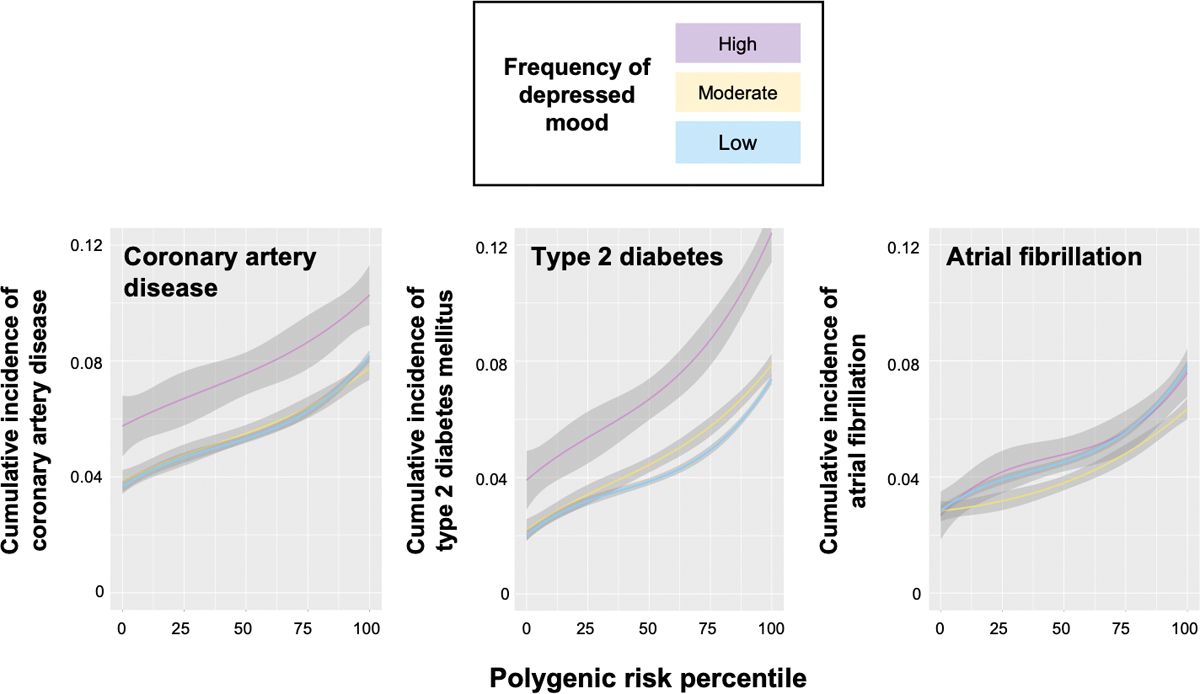
Spline plots of crude cumulative incidence (right) were generated with the ‘ggplot2’ package in R version 3.6.0 using a cubic spline with 3 knots. The colored lines represent the modeled cumulative incidence at each polygenic risk percentile, and the shaded bands represents the modeled 95% confidence bands.
Extended Data Fig. 3. Reduction in cardiometabolic risk associated with lower frequency of depressed mood among individuals at high polygenic risk.
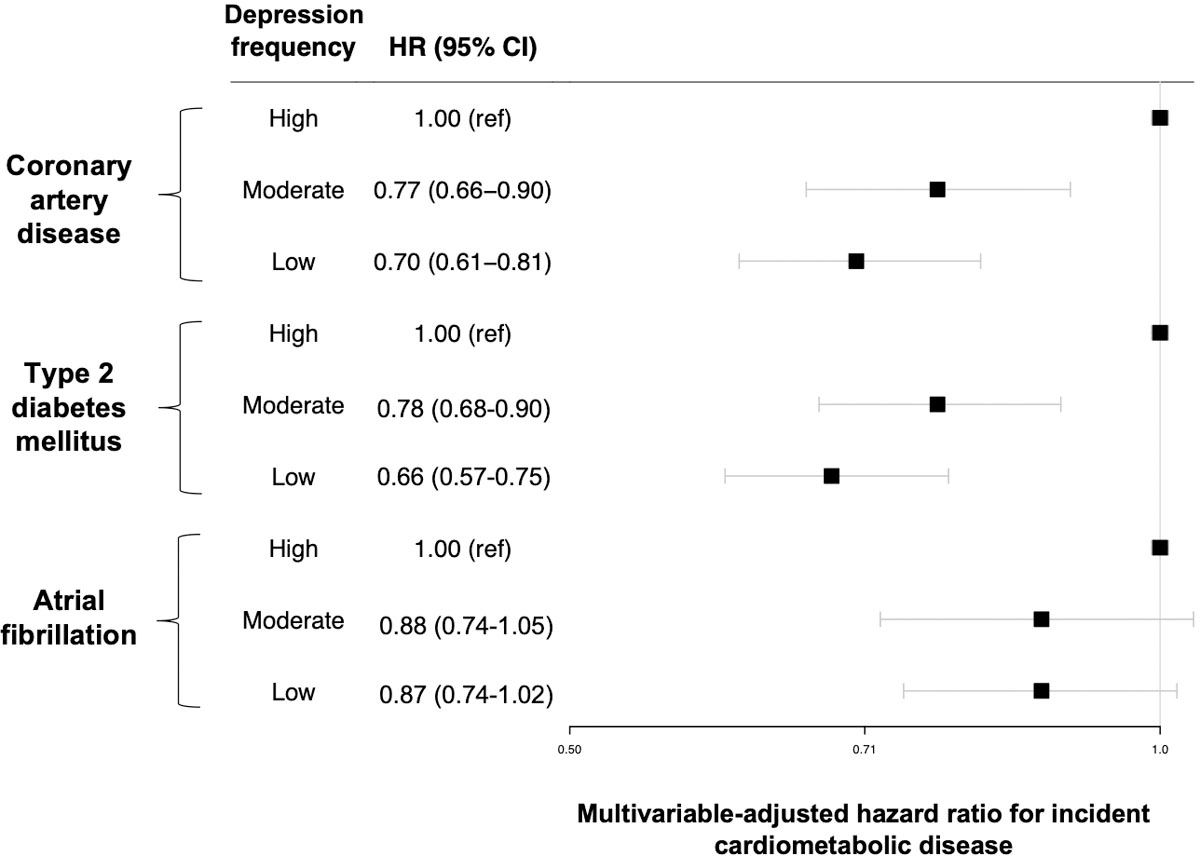
Data are presented as hazard ratios (black squares) and 95% confidence intervals (error bars). Multivariable-adjusted hazard ratios are calculated among 60,849 individuals without prevalent coronary artery disease (2,510 with high, 11,305 with moderate, and 47,034 with low frequency of depressed mood); 62,974 individuals without prevalent type 2 diabetes (2,520 with high, 11,622 with moderate, and 48,832 with low frequency of depressed mood); and 63,414 individuals without prevalent atrial fibrillation (2,573 with high, 11,683 with moderate, and 49,158 with low frequency of depressed mood). Hazard ratios (left) are adjusted for age, age2, sex, the first 20 principal components of ancestry, genotyping array, country, socioeconomic deprivation, smoking status, pack-year smoking history, alcohol intake, vegetable and fresh fruit intake, days per week of moderate and vigorous exercise, sleep duration, systolic blood pressure, antihypertensive medication use, non-HDL cholesterol, cholesterol-lowering medication use, antiplatelet medication use, antihyperglycemic medication use, prevalent type 2 diabetes mellitus (models for coronary artery disease and atrial fibrillation only), body-mass index, and C-reactive protein.
Supplementary Material
Acknowledgements:
This research was conducted under UK Biobank application number 7089. Authors were supported by a CRICO Patient Safety Grant (A.A.S.); the U.S. National Heart, Lung and Blood Institute R01HL133149-04 (A.A.S.), R01HL142711 (P.N.), R01HL148050 (P.N.), R01HL148565 (P.N.), and R01HL151283 (P.N.); Fondation Leducq TNE-18CVD04 (P.N.); and a Hassenfeld Scholar Award from Massachusetts General Hospital (P.N.).
Footnotes
Code Availability: Code for the ‘survival,’ ‘fsmb,’ and ‘ggplot2’ R packages are publicly available (https://cran.r-project.org/).
Competing Interests Statement: P.N. reports grant support from Amgen, Apple, AstraZeneca, Boston Scientific, and Novartis, personal fees from Apple, AstraZeneca, Blackstone Life Sciences, Foresite Labs, Genentech, and Novartis, and spousal employment at Vertex, all unrelated to the present work. All other authors do not report any disclosures.
Data Availability:
UK Biobank data are available to researchers by application (https://www.ukbiobank.ac.uk/). PRS for CAD, T2D, and atrial fibrillation have been submitted to the Polygenic Score Catalog (https://www.pgscatalog.org/).
References
- 1.Aragam KG, Natarajan P. Polygenic Scores to Assess Atherosclerotic Cardiovascular Disease Risk: Clinical Perspectives and Basic Implications. Circ Res 04 2020;126(9):1159–1177. doi: 10.1161/CIRCRESAHA.120.315928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Y, Chen X, Han J, Jiang W, Natarajan P, Zhao H. Interactions between Enhanced Polygenic Risk Scores and Lifestyle for Cardiovascular Disease, Diabetes Mellitus and Lipid Levels. Circ Genom Precis Med Jan 2021;doi: 10.1161/CIRCGEN.120.003128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. New Engl J Med Jul 6 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103 [DOI] [PubMed] [Google Scholar]
- 4.Xanthakis V, Enserro DM, Murabito JM, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation Nov 2014;130(19):1676–83. doi: 10.1161/CIRCULATIONAHA.114.009273 [DOI] [PubMed] [Google Scholar]
- 5.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation Jul 2006;114(2):160–7. doi: 10.1161/CIRCULATIONAHA.106.621417 [DOI] [PubMed] [Google Scholar]
- 6.Corlin L, Short MI, Vasan RS, Xanthakis V. Association of the Duration of Ideal Cardiovascular Health Through Adulthood With Cardiometabolic Outcomes and Mortality in the Framingham Offspring Study. JAMA Cardiol 05 2020;5(5):549–556. doi: 10.1001/jamacardio.2020.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu N, Pan XF, Yu C, et al. Association of Major Depression With Risk of Ischemic Heart Disease in a Mega-Cohort of Chinese Adults: The China Kadoorie Biobank Study. J Am Heart Assoc 12 2016;5(12)doi: 10.1161/JAHA.116.004687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004 Sep 11-17 2004;364(9438):953–62. doi: 10.1016/S0140-6736(04)17019-0 [DOI] [PubMed] [Google Scholar]
- 9.Harshfield EL, Pennells L, Schwartz JE, et al. Association Between Depressive Symptoms and Incident Cardiovascular Diseases. JAMA 12 2020;324(23):2396–2405. doi: 10.1001/jama.2020.23068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aragam KG, Dobbyn A, Judy R, et al. Limitations of Contemporary Guidelines for Managing Patients at High Genetic Risk of Coronary Artery Disease. Journal of the American College of Cardiology Jun 9 2020;75(22):2769–2780. doi: 10.1016/j.jacc.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khera AV, Emdin CA, Drake I, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. The New England journal of medicine Dec 15 2016;375(24):2349–2358. doi: 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natarajan P, Young R, Stitziel NO, et al. Polygenic Risk Score Identifies Subgroup With Higher Burden of Atherosclerosis and Greater Relative Benefit From Statin Therapy in the Primary Prevention Setting. Circulation May 2017;135(22):2091–2101. doi: 10.1161/CIRCULATIONAHA.116.024436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Said MA, Verweij N, van der Harst P. Associations of Combined Genetic and Lifestyle Risks With Incident Cardiovascular Disease and Diabetes in the UK Biobank Study. JAMA Cardiol Aug 1 2018;3(8):693–702. doi: 10.1001/jamacardio.2018.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikpay M, Goel A, Won HH, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet Oct 2015;47(10):1121–1130. doi: 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott RA, Scott LJ, Mägi R, et al. An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes 11 2017;66(11):2888–2902. doi: 10.2337/db16-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christophersen IE, Rienstra M, Roselli C, et al. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet Jun 2017;49(6):946–952. doi: 10.1038/ng.3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y, Lu Q, Powles R, et al. Leveraging functional annotations in genetic risk prediction for human complex diseases. PLoS Comput Biol Jun 2017;13(6):e1005589. doi: 10.1371/journal.pcbi.1005589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajan S, McKee M, Rangarajan S, et al. Association of Symptoms of Depression With Cardiovascular Disease and Mortality in Low-, Middle-, and High-Income Countries. JAMA Psychiatry Oct 2020;77(10):1052–1063. doi: 10.1001/jamapsychiatry.2020.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA Sep 2011;306(11):1241–9. doi: 10.1001/jama.2011.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Wang Z, Georgakis MK, Lin H, Zheng L. Genetic Liability to Depression and Risk of Coronary Artery Disease, Myocardial Infarction, and Other Cardiovascular Outcomes. J Am Heart Assoc Jan 2021;10(1):e017986. doi: 10.1161/JAHA.120.017986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li GH, Cheung CL, Chung AK, et al. Evaluation of bi-directional causal association between depression and cardiovascular diseases: a Mendelian randomization study. Psychol Med Oct 2020:1–12. doi: 10.1017/S0033291720003566 [DOI] [PubMed] [Google Scholar]
- 22.Vaccarino V, Badimon L, Bremner JD, et al. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J May 2020;41(17):1687–1696. doi: 10.1093/eurheartj/ehy913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penninx BW. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev Mar 2017;74(Pt B):277–286. doi: 10.1016/j.neubiorev.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 24.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol Jan 2016;16(1):22–34. doi: 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khandaker GM, Zuber V, Rees JMB, et al. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry 07 2020;25(7):1477–1486. doi: 10.1038/s41380-019-0395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smolderen KG, Strait KM, Dreyer RP, et al. Depressive symptoms in younger women and men with acute myocardial infarction: insights from the VIRGO study. J Am Heart Assoc Apr 2015;4(4)doi: 10.1161/JAHA.114.001424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillespie SL, Anderson CM, Zhao S, et al. Allostatic load in the association of depressive symptoms with incident coronary heart disease: The Jackson Heart Study. Psychoneuroendocrinology 11 2019;109:104369. doi: 10.1016/j.psyneuen.2019.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah AJ, Ghasemzadeh N, Zaragoza-Macias E, et al. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc Jun 2014;3(3):e000741. doi: 10.1161/JAHA.113.000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun WJ, Xu L, Chan WM, Lam TH, Schooling CM. Are depressive symptoms associated with cardiovascular mortality among older Chinese: a cohort study of 64,000 people in Hong Kong? Am J Geriatr Psychiatry Nov 2013;21(11):1107–15. doi: 10.1016/j.jagp.2013.01.048 [DOI] [PubMed] [Google Scholar]
- 30.Bryant KB, Jannat-Khah DP, Cornelius T, et al. Time-Varying Depressive Symptoms and Cardiovascular and All-Cause Mortality: Does the Risk Vary by Age or Sex? J Am Heart Assoc Oct 2020;9(19):e016661. doi: 10.1161/JAHA.120.016661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsey JM, Cooper JD, Bot M, et al. Sex Differences in Serum Markers of Major Depressive Disorder in the Netherlands Study of Depression and Anxiety (NESDA). PLoS One 2016;11(5):e0156624. doi: 10.1371/journal.pone.0156624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labaka A, Goñi-Balentziaga O, Lebeña A, Pérez-Tejada J. Biological Sex Differences in Depression: A Systematic Review. Biol Res Nurs 07 2018;20(4):383–392. doi: 10.1177/1099800418776082 [DOI] [PubMed] [Google Scholar]
- 33.Fernandes N, Prada L, Rosa MM, et al. The impact of SSRIs on mortality and cardiovascular events in patients with coronary artery disease and depression: systematic review and meta-analysis. Clin Res Cardiol Jul 2020;doi: 10.1007/s00392-020-01697-8 [DOI] [PubMed] [Google Scholar]
- 34.Klarin D, Zhu QM, Emdin CA, et al. Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat Genet Sep 2017;49(9):1392–1397. doi: 10.1038/ng.3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 09 2018;50(9):1219–1224. doi: 10.1038/s41588-018-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol Nov 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature Oct 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wain LV, Shrine N, Miller S, et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med Oct 2015;3(10):769–81. doi: 10.1016/S2213-2600(15)00283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics Nov 2010;26(22):2867–73. doi: 10.1093/bioinformatics/btq559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finucane HK, Bulik-Sullivan B, Gusev A, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet Nov 2015;47(11):1228–35. doi: 10.1038/ng.3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
UK Biobank data are available to researchers by application (https://www.ukbiobank.ac.uk/). PRS for CAD, T2D, and atrial fibrillation have been submitted to the Polygenic Score Catalog (https://www.pgscatalog.org/).


