Abstract
Stigma around hepatitis C virus (HCV) infection is an important and understudied barrier to HCV treatment and elimination. The determinants of HCV-related stigma, including the impacts of stage of HCV treatment (i.e., spontaneously-cleared; diagnosed, untreated; previously treated, not cured; currently being treated; treated, cured) and coinfection with human immunodeficiency virus (HIV), remain unknown.
To address these gaps, we conducted a cross-sectional study among patients with a history of HCV infection (n=270) at outpatient clinics in Philadelphia from July 2018 – May 2019. We evaluated stigma using the validated HCV Stigma Scale, adapted from the Berger HIV Stigma Scale. Associations among HCV-related stigma and hypothesized demographic, behavioral, and clinical risk factors were evaluated by multivariable linear regression.
Most participants (95.5%) experienced HCV-related stigma. Mean stigma scores did not differ significantly between HCV-monoinfected and HIV/HCV-coinfected participants (P=0.574). However, we observed significant interactions between HIV status and multiple determinants; therefore, we stratified analyses by HIV status. Among HIV/HCV-coinfected participants, previous HCV treatment without cure, female gender, Hispanic/Latino ethnicity, and some college education were significantly associated with higher HCV-stigma scores. An annual income of $10,000-$40,000 was associated with significantly lower stigma scores. No significant associations were observed among HCV-monoinfected participants.
We found that most participants experienced stigma associated with HCV diagnosis. While stigma scores were similar between HCV-monoinfected and HIV/HCV-coinfected participants, the determinants associated with HCV stigma differed by HIV status. Understanding how experiences of stigma differs between HCV-monoinfected and HIV/HCV-coinfected patients may aid in the development of targeted interventions to address the HCV epidemic.
Keywords: stigma, determinants, syndemic, Hepatitis C, HIV infection
Hepatitis C virus (HCV) infection is the leading cause of chronic liver disease and infectious disease-related deaths in the United States (US).1–3 HCV affects nearly 4.5 million people in the US, of whom, fewer than half are diagnosed and/or aware of their infection status.4 Despite the advent of highly curative, all-oral HCV treatment regimens and development of national action plans to eliminate HCV infection as a major public health threat by 2030,5–7 the number of HCV infections has increased 3.5-fold over the last decade.1 The increasing incidence and prevalence of HCV infection is associated with the national increase in injection opioid use,8 both of which are syndemic with the ongoing human immunodeficiency virus (HIV) epidemic.9
Syndemics arise when two or more diseases aggregate, exacerbating negative health outcomes within a population. In the US, approximately 25% of people living with HIV are coinfected with HCV; among people living with HIV who have a history of injection drug use (IDU), estimates of HCV coinfection rise to 80%.10 HIV accelerates progression of HCV-related liver fibrosis, and HIV/HCV-coinfected individuals have an increased risk of cirrhosis compared to those with chronic HCV alone.11,12 The use of a syndemic framework can help identify the multi-level clinical, social, and structural factors associated with engagement along the HCV care continuum and determine if there are differences by HIV status.9,13–15
Syndemics frequently emerge in the setting of structural health inequalities, within which disease-related stigma further marginalizes some patient populations.14 Stigma is a complex social phenomenon which occurs when an attribute deviates from “normal” qualities anticipated by society, reducing “a whole and usual person to a tainted, discounted one.”16 The stigmatizing label of a disease can negatively affect a patient’s quality of life by depleting self-esteem, marring individual and social identity, and encouraging isolation and withdrawal.17–20 Disease-related stigma can also adversely impact along the care continuum by creating barriers to diagnostic testing, linkage to care, and treatment.14,20–23 Moreover, disease-related stigmas may intersect with and be compounded by stigmas around other aspects of patient identities that are inherent in the structural inequalities that promote syndemics, such as racism and poverty.24,25 Understanding how different identities intersect upon patients’ experiences of disease-related stigmas allows insights into why patients with the same disease may experience stigma differently.26–28 Moreover, understanding the biopsychosocial interactions among HCV infection, HIV infection, and other marginalizing patient factors is essential to enhancing patient engagement along the healthcare continuum and developing public health initiatives to address the syndemic nature of HCV, HIV, and injection opioid use.14,27–29
The factors associated with HCV-related stigma remain unclear, primarily because, until only recently, no validated instruments existed to measure patient experiences of HCV-related stigma.30 To address this issue, we evaluated demographic, clinical, and behavioral factors associated with HCV-related stigma among patients with a history of HCV infection and determined whether experiences of HCV-related stigma differed between HCV-monoinfected and HIV/HCV-coinfected patients. Regardless of HIV status, we hypothesized that black/African American race, Hispanic ethnicity, history of IDU, and lower socioeconomic status were associated with greater HCV-related stigma, due to existing societal stigmas associated with these attributes (e.g. racism) and disparities in access to healthcare among these patient populations.31–38
MATERIALS & METHODS
Study Design & Setting
We conducted a cross-sectional survey study among patients with a history of HCV infection who presented for care at outpatient infectious diseases clinics across Philadelphia between July 2018 and May 2019. The study setting and procedures have been previously described.30
Briefly, participants were recruited from five infectious disease clinics across two Philadelphia health systems: 1) University of Pennsylvania Health System; and, 2) Philadelphia Field Initiation Group for HIV Trials (FIGHT) Community Health Centers. Patients were recruited from two University of Pennsylvania practices (Center for Viral Hepatitis at Penn Presbyterian Medical Center; Hospital of the University of Pennsylvania MacGregor Infectious Diseases Clinic) and three Philadelphia FIGHT clinics (Jonathan Lax Treatment Center; John Bell Health Center; Clinica Bienestar). These sites offer comparable HCV and HIV treatments and the demographic characteristics of the patients seeking care at these clinics are representative of chronic HCV-infected patients in Philadelphia; the burden of HCV infection by race/ethnicity is representative of Philadelphia’s racially-diverse population and proportion of infections among males compared to females is about 2:1.39-41Upon completion of the survey, participants were reimbursed for their time with a $10 remuneration. This study was approved by the Institutional Review Boards of the University of Pennsylvania and Philadelphia FIGHT.
Participants & Recruitment
Patients were eligible for inclusion if they were: 1) ≥18 years of age, 2) positive for HCV-antibody, and 3) English-speaking. The HCV-antibody criterion was selected to include patients who had ever been diagnosed with HCV infection, including those who had chronic infection, spontaneously cleared their HCV infection, or were cured with antiviral treatment. Across the sites, all eligible patients were identified by their provider and approached by the principal investigator at the end of their outpatient appointment for recruitment. The principal investigator attended each clinic during the afternoons when providers who treat mostly HCV patients were providing care over the course of ten months. We targeted a total of 248 participants in order to have 80% power to detect associations between HCV-related stigma and the hypothesized risk factors of interest.
HCV-Related Stigma
The primary study outcome was perception of HCV-related stigma, measured by HCV Stigma Scale (HCV-SS) Score.30 The HCV-SS is a validated 33-item self-administered questionnaire adapted from the Berger HIV Stigma Scale.42 Previous psychometric evaluation of the HCV-SS produced this unidimensional scale with sufficient reliability (Cronbach α = 0.96) and construct validity (85%).30 Item responses are recorded on a four-point Likert scale ranging from 1 (strongly disagree) to 4 (strongly agree). Scores for each item are summed to achieve the total HCV-SS score, which may range from 33-132, with higher scores indicating greater experiences of stigma.
Data Collection
We collected self-reported demographic characteristics (age, sex/gender identity, race, ethnicity, education, employment, annual income), history of IDU and needle sharing, sexual orientation, sexual contact history, HIV infection status, years since HCV diagnosis, and stage of HCV management (i.e., spontaneously cleared; diagnosed, untreated; previously treated, not cured; currently being treated; treated, cured). Socioeconomic status was separately evaluated by education level, employment status, and income to minimize bias introduced by a composite variable and elucidate granularity in the ways that socioeconomic status may assert its influence on HCV-related stigma.43
All questionnaires were administered using audio-computer assisted self-interview software (ACASI) on laptop computers equipped with headphones. ACASI has been used extensively in research on stigma and disease experience to minimize social desirability bias while evaluating sensitive topics.21,23,44–50 For all questions, response sets included the options “I don’t know the answer” and “I don’t want to answer.” The survey took 15.5 minutes (SD, 8.8 minutes), on average, to complete.
Statistical Analyses
Baseline demographic and clinical characteristics were computed descriptively as counts and proportions. Differences in characteristics by HIV status were assessed using chi-square or Fisher’s exact tests, as indicated, for categorical variables and t tests for continuous variables. Mean HCV-SS scores and standard deviations (SD) were calculated overall and by HIV status.
Linear regression was performed to assess associations between HCV-SS score and hypothesized risk factors for HCV-related stigma. Prior to regression analyses, assumptions of normality, homoscedasticity, and independence of errors were assessed via analysis of histograms, quantile-quantile plots, and the Shapiro-Wilk W-test.22,51 We evaluated the following statistical interactions: race and ethnicity; history of injection drug use and sexual orientation; and all combinations of socioeconomic status covariates. These interactions were found to be non-significant. We observed significant interactions between HIV status and sex/gender identity (P=0.04), years since HCV diagnosis (P=0.01), and employment status (P=0.03). Therefore, we analyzed results stratified by HIV status.
Multivariable linear regression models were fit with all measured covariates in a fully-adjusted model for the overall sample and for the HCV-monoinfected and HIV/HCV-coinfected groups. All analyses were conducting using Stata 15.0 (Stata Corporation, College Station, TX).
RESULTS
Participant Characteristics
We asked 288 patients to participate in the study, of whom 270 (96.4%) agreed and 265/270 (98.1%) completed the entire survey. Participants were predominantly male (68.7%), white (46.8%) or black/African American (39.3%), non-Hispanic (77.4%), had a high school or equivalent degree (49.1%), and earned an annual income of less than $10,000 (58.9%; Table 1), which is consistent with the demographic characteristics of the HCV patients care for at these clinics. The majority of participants reported a history of IDU (73.9%), self-identified as heterosexual (78.9%), and only engaged in heterosexual contact across their lifetime (70.9%). Most participants had been diagnosed with HCV infection for more than one year prior to survey participation (81.1%) and were either currently being treated (29.1%) or cured (41.5%) of chronic HCV infection.
Table 1:
Characteristics of participants, overall and by HIV infection status.
| Characteristics | Overall (n = 265) | HCV-Monoinfected (n = 118) | HCV/HIV-Coinfected (n = 147) | P-Value |
|---|---|---|---|---|
| Age, years (n, %) | ||||
| 18-24 | 3 (1.13%) | 1 (0.85%) | 2 (1.36%) | 0.001 |
| 25-34 | 34 (12.83%) | 22 (18.64%) | 12 (8.16%) | |
| 35-44 | 63 (23.77%) | 36 (30.51%) | 27 (18.37%) | |
| 45-54 | 71 (26.79%) | 30 (25.42%) | 41 (27.89%) | |
| 55+ | 94 (35.47%) | 29 (24.58%) | 65 (44.22%) | |
| Sex/gender identity (n, %) | ||||
| Male | 182 (68.68%) | 87 (73.73%) | 95 (64.63%) | 0.21 |
| Female | 75 (28.30%) | 30 (25.42%) | 45 (30.61%) | |
| Male identifying as female / Transgender / Transsexual | 4 (1.51%) | 0 (0.00%) | 4 (2.72%) | |
| Female identifying as male / Transgender / Transsexual | 2 (0.75%) | 1 (0.85%) | 1 (0.68%) | |
| Queer / Gender non-conforming | 1 (0.38%) | 0 (0.00%) | 1 (0.68%) | |
| Other / Prefer not to answer | 1 (0.38%) | 0 (0.00%) | 1 (0.68%) | |
| Race (n, %) | ||||
| White | 124 (46.79%) | 73 (61.86%) | 51 (34.69%) | <0.001 |
| Black / African American | 104 (39.25%) | 32 (27.12%) | 72 (48.98%) | |
| Hawaiian / Pacific Islander | 14 (5.28%) | 4 (3.39%) | 10 (6.80%) | |
| American Indian / Alaskan Native | 10 (3.77%) | 4 (3.39%) | 6 (4.08%) | |
| Multiracial | 7 (2.64%) | 3 (2.54%) | 4 (2.72%) | |
| Other / Prefer not to answer | 6 (2.26%) | 2 (1.69%) | 4 (2.72%) | |
| Ethnicity (n, %) | ||||
| Not Hispanic / Latino | 205 (77.36%) | 96 (81.36%) | 109 (74.15%) | 0.21 |
| Hispanic / Latino | 57 (21.51%) | 20 (16.95%) | 37 (25.17%) | |
| Other / Prefer not to answer | 3 (1.13%) | 2 (1.69%) | 1 (0.68%) | |
| Education (n, %) | ||||
| Less than high school degree | 59 (22.26%) | 27 (22.88%) | 32 (21.77%) | 0.33 |
| High school degree / GED | 130 (49.06%) | 65 (55.08%) | 65 (44.22%) | |
| Associated degree / Some college | 55 (20.75%) | 19 (16.10%) | 36 (24.29%) | |
| Bachelor’s degree | 13 (4.91%) | 4 (3.39%) | 9 (6.12%) | |
| Graduate / Professional degree | 7 (2.64%) | 3 (2.54%) | 4 (2.72%) | |
| Other / Prefer not to answer | 1 (0.38%) | - | 1 (0.68%) | |
| Employment (n, %) | ||||
| Unemployed | 102 (38.49%) | 52 (44.07%) | 50 (34.01%) | 0.039 |
| Disability | 79 (29.81%) | 26 (22.03%) | 53 (36.05%) | |
| Employed | 65 (24.53%) | 32 (27.12%) | 33 (22.45%) | |
| Retired | 14 (5.28%) | 4 (3.39%) | 10 (6.80%) | |
| Student | 4 (1.51%) | 3 (2.54%) | 1 (0.68%) | |
| Other / Prefer not to answer | 1 (0.38%) | 1 (0.85%) | - | |
| Annual Income (n, %) | ||||
| < $10,000 | 156 (58.87%) | 67 (56.78%) | 89 (60.54%) | 0.17 |
| $10,000 - $49,000 | 74 (27.92%) | 36 (30.51%) | 38 (25.85%) | |
| $50,000 - $99,000 | 11 (4.15%) | 8 (6.78%) | 3 (2.04%) | |
| $100,000 - $140,000 | 2 (0.75%) | - | 2 (1.36%) | |
| > $150,000 | 6 (2.26%) | 1 (0.85%) | 5 (3.40%) | |
| Other / Prefer not to answer | 16 (6.04%) | 6 (5.08%) | 10 (6.80%) | |
| Time since HCV diagnosis (n, %) | ||||
| Diagnosed within previous year | 37 (14.68%) | 22 (19.64%) | 15 (10.71%) | 0.145 |
| 1-5 years | 75 (29.76%) | 37 (33.04%) | 38 (27.14%) | |
| 6-10 years | 45 (17.86%) | 18 (16.07%) | 27 (19.29%) | |
| 11-15 years | 34 (13.49%) | 15 (13.39%) | 19 (13.57%) | |
| 16-20 years | 34 (13.49%) | 11 (9.82%) | 23 (16.43%) | |
| 21-25 years | 16 (6.35%) | 7 (6.25%) | 9 (6.43%) | |
| >25 years | 11 (4.37%) | 2 (1.79%) | 9 (6.43%) | |
| Stage of HCV management (n, %) | ||||
| Spontaneously cleared | 13 (4.91%) | 6 (5.08%) | 7 (4.76%) | <0.001 |
| Diagnosed, Untreated | 48 (18.11%) | 27 (22.88%) | 21 (14.29%) | |
| Previously treated, Not cured | 17 (6.42%) | 8 (6.78%) | 9 (6.12%) | |
| Currently being treated | 77 (29.06%) | 52 (44.07%) | 25 (17.01%) | |
| Treated, Cured | 110 (41.51%) | 25 (21.19%) | 85 (57.82%) | |
| Received HCV treatment (n, %) | 204 (76.98%) | 85 (72.03%) | 119 (80.95) | <0.001 |
| Treated with pegylated interferon-based regimen | 53 (20.00%) | 10 (8.47%) | 43 (29.25%) | |
| Treated with direct-acting antiviral regimen | 151 (56.98%) | 75 (63.56%) | 76 (51.70%) | |
| History of injection drug use (n, %) | 196 (73.96%) | 91 (77.12%) | 105 (71.43%) | 0.29 |
| Self-reported needle-sharing (n, %) | 157 (59.25%) | 70 (59.32%) | 87 (59.18%) | 0.35 |
| Sexual orientation (n, %) | ||||
| Heterosexual | 209 (78.87%) | 107 (90.68%) | 102 (69.39%) | <0.001 |
| Homosexual | 30 (11.32%) | 4 (3.39%) | 26 (17.69%) | |
| Bisexual | 25 (9.43%) | 7 (5.93%) | 18 (12.24%) | |
| Other / Prefer not to answer | 1 (0.38%) | - | 1 (0.68%) | |
| Lifetime sexual contact (n, %) | ||||
| No sexual contact | 3 (1.13%) | 1 (0.85%) | 2 (1.36%) | <0.001 |
| Heterosexual contact, only | 188 (70.94%) | 102 (86.44%) | 86 (58.50%) | |
| Men who have sex with men | 54 (20.38%) | 6 (5.08%) | 48 (32.65%) | |
| Women who have sex with women | 18 (6.79%) | 9 (7.63%) | 9 (6.12%) | |
| Other | 2 (0.75%) | - | 2 (1.36%) |
Abbreviations: GED=General Educational Development; HIV=Human Immunodeficiency Virus; HCV=Hepatitis C Virus
Slightly more than half of participants were coinfected with HIV (147, 55.5%). Compared to HCV-monoinfected participants, HIV/HCV-coinfected participants were older (P=0.001), more frequently black/African American (P<0.001), more often on disability (P=0.039), and more likely to identify as homosexual or bisexual (P<0.001) and have male-male sexual contact (P<0.001). HIV/HCV-coinfected participants had a higher prevalence of HCV treatment and cure (P<0.001) and received previous treatment with pegylated interferon more commonly than did HCV-monoinfected participants (P<0.001; Table 1).
HCV-related Stigma
HCV-SS scores ranged from 33 to 132, with a mean score of 75.57 (SD, 19.13). Mean stigma scores did not differ significantly between HCV-monoinfected and HIV/HCV-coinfected participants (74.83 [SD, 17.00] versus 76.16 [SD, 20.71]; P=0.574; Figure 1).
Figure 1. Distribution of HCV Stigma Scale (HCV-SS) scores.
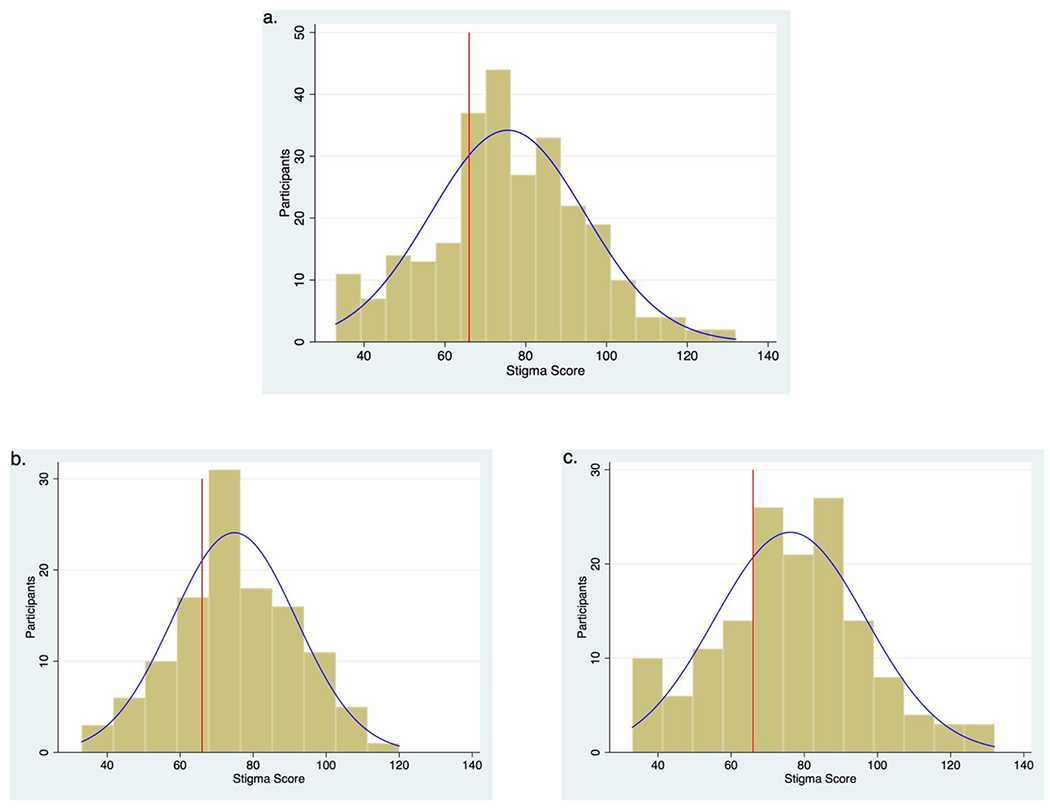
Distribution of HCV-SS scores overall (a) and for HCV-monoinfected (b) and HCV/HIV-coinfected (c) participants. The red vertical line indicates a mid-scale score of 66.
Across HCV-SS scores, the majority of participants (n=253 [95.5%]) endorsed one or more items indicating experiences of HCV-related stigma (Figure 2). Items that participants (>50%) most frequently endorsed related to concerns around disclosure of HCV status, discrimination for having HCV infection, and perceptions that others view those with HCV as dirty or blameworthy. Notably, 76.6% of participants endorsed that they are careful to whom they disclose their HCV diagnosis. Items that participants (>75%) most frequently disagreed with referred to experiences of social distancing by others or internalized stigma of self-blame. HIV/HCV-coinfected participants were more likely to endorse items describing decreased self-worth related to their HCV status and perceptions that others were uncomfortable around them or viewed them as dirty, compared to monoinfected participants.
Figure 2. Heatmap of perceptions of HCV-related stigma reported on HCV Stigma Scale (HCV-SS), sorted by stigma score.
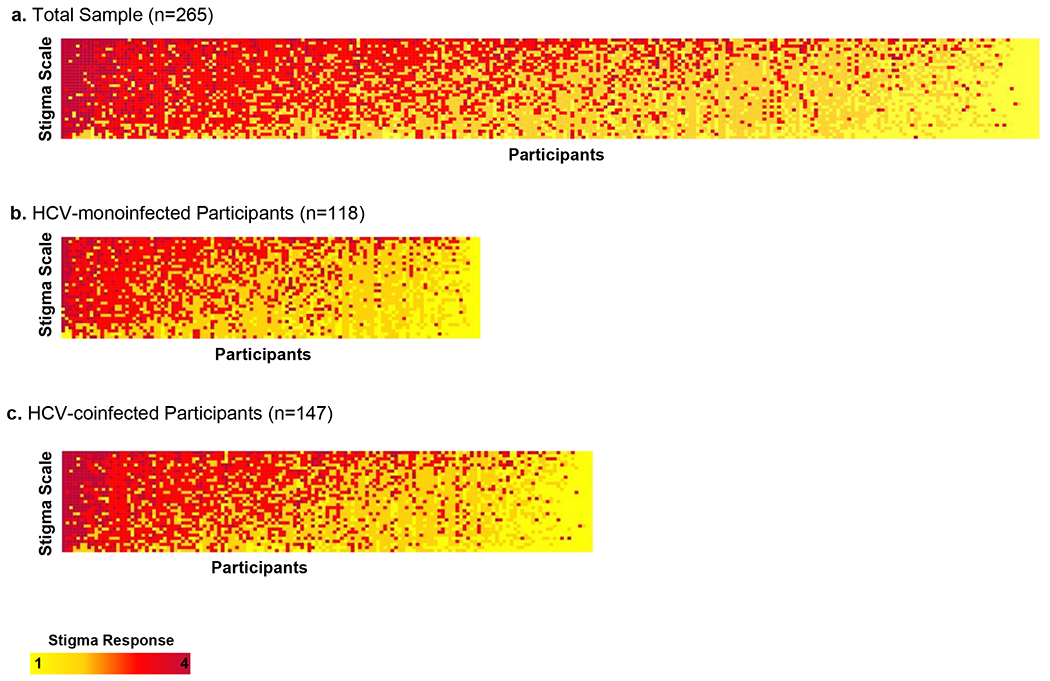
The x-axis represents participants and the y-axis represents HCV-SS items, sorted by HCV-SS score. Each column of squares along the x-axis of this heatmap represents a participant’s HCV-SS response set. Each row of squares represents all responses to a single HCV-SS item. The yellow-red color-scheme for each participant’s answer encodes the Likert scale responses from strongly disagree (yellow) to strongly agree (maroon). Within each heatmap, participants are sorted from highest overall HCV-SS scores at left to lowest overall HCV-SS scores at right. A heatmap of sorted HCV-SS score for the overall sample are presented in Figure 2a. Figure 2b shows the sorted HCV-SS scores for HCV-monoinfected participants. Figure 2c presents results for HIV/HCV-coinfected participants.
Factors Associated with HCV Stigma
In the fully adjusted multivariable model among HIV/HCV-coinfected participants, significantly higher HCV-SS scores were associated with female sex/gender identity, Hispanic ethnicity, educational-attainment of an associate degree or some college, and previous HCV treatment without cure. In addition, significantly lower stigma scores were also associated with an annual income level of $10,000-$40,000 (Table 2).
Table 2: Multivariable fully adjusted models evaluating hypothesized determinants of HCV-related stigma.
Analyses are stratified by HIV status. Beta coefficients for each characteristic refer to the mean difference in HCV Stigma Scale (HCV-SS) scores compared to the reference category for that variable. Bolded results refer to statistically significant difference in stigma scores.
| Characteristics | HCV-Monoinfected | HIV/HCV-Coinfected |
|---|---|---|
| β (95% CI) | β (95% CI) | |
| Age, years | ||
| 18-34 | Ref | Ref |
| 35-44 | 3.46 (−7.26, 14.19) | 0.23 (−14.74, 15.20) |
| 45-54 | −0.73 (−11.97, 10.52) | −0.51 (−15.42, 14.41) |
| 55+ | −4.70 (−17.69, 8.29) | 3.99 (−11.02, 18.99) |
| Sex/gender identity | ||
| Male | Ref | Ref |
| Female | 0.65 (−8.13, 9.43) | 11.66 (2.87, 20.45) |
| Other | - | 16.37 (−3.50, 36.25) |
| Race | ||
| White | Ref | Ref |
| Black / African American | 1.87 (−8.38, 12.13) | 3.65 (−5.09, 12.40) |
| Other | 6.22 (−6.52, 18.96) | −10.70 (−23.25, 1.84) |
| Hispanic / Latino Ethnicity | ||
| Not Hispanic / Latino | Ref | Ref |
| Hispanic / Latino | 1.65 (−8.69, 11.99) | 15.04 (5.23, 24.85) |
| Education | ||
| Less than High school degree | Ref | Ref |
| High school degree / GED | 2.38 (−6.74, 11.49) | 0.30 (−8.86, 9.47) |
| Associated degree / some college | 6.58 (−6.25, 19.41) | 12.11 (1.14, 23.07) |
| College / graduate degree | −0.69 (−18.22, 16.83) | 8.57 (−7.33, 24.48) |
| Employment | ||
| Unemployed | Ref | Ref |
| Disability | 9.24 (−0.65, 19.12) | 3.85 (−5.49, 13.18) |
| Employed | 19.58 (−1.74, 40.89) | 1.86 (−9.48, 13.21) |
| Retired | 4.51 (−5.11, 14.12) | 4.97 (−11.80, 21.74) |
| Annual Income | ||
| < $10,000 | Ref | Ref |
| $10,000 - $49,000 | −2.77 (−11.67, 6.14) | −9.37 (−18.51, −0.24) |
| ≥$50,000 | 4.03 (−9.38, 17.44) | −2.16 (−17.68, 13.35) |
| Other / prefer not to answer | −1.85 (−22.39, 18.69) | −4.68 (−22.55, 13.19) |
| Sexual orientation | ||
| Heterosexual | Ref | Ref |
| Homosexual | 17.87 (−8.77, 44.51) | 3.34 (−10.41, 17.09) |
| Bisexual | −4.17 (−21.39, 13.06) | −6.87 (−21.02, 7.27) |
| Lifetime sexual contact | ||
| Heterosexual contact, only | Ref | Ref |
| Non-heterosexual contact | −2.05 (−15.37, 11.28) | 0.53 (−11.36, 12.41) |
| History of injection drug use | ||
| No | Ref | Ref |
| Yes | 0.32 (−9.90, 10.53) | 7.28 (−1.96, 16.53) |
| Time since HCV diagnosis | ||
| Diagnosed within previous year | Ref | Ref |
| 1-5 years ago | 8.28 (−2.02, 18.38) | −4.89 (−19.09, 9.30) |
| >5 years ago | −1.62 (−11.43, 8.19) | 1.73 (−11.58, 15.04) |
| Stage of HCV Management | ||
| Treated, cured | Ref | Ref |
| Diagnosed, untreated | −1.09 (−11.94, 9.76) | 8.38 (−3.12, 19.89) |
| Previously treated, not cured | 4.88 (−10.85, 20.60) | 19.81 (4.70, 34.92) |
| Currently being treated | 1.82 (−8.39, 12.03) | 10.62 (−0.72, 21.95) |
| Prior PEGylated interferon treatment | ||
| No | Ref | Ref |
| Yes | −1.34 (−15.42, 85.43) | −3.50 (−13.02, 6.01) |
Abbreviations: CI=confidence interval; GED=General Educational Development; HIV=Human Immunodeficiency Virus; HCV=Hepatitis C Virus
We observed numerous differences in the associations between hypothesized determinants and HCV-SS scores by HIV infection status. Among HCV-monoinfected participants, HCV-SS score was not significantly associated with any measured covariates in the fully adjusted model (Table 2).
DISCUSSION
This is the first study to measure HCV-related stigma among HCV-monoinfected and HIV/HCV-coinfected patients and to evaluate determinants of HCV-related stigma. We found that most participants experienced stigma associated with HCV diagnosis. Interestingly, mean HCV-SS stigma scores were similar between monoinfected and coinfected participants. However, the determinants associated with stigma differed by HIV status. Among HCV-monoinfected participants, we observed no significant associations between the measured determinants and HCV-SS score. In contrast, among HIV/HCV-coinfected participants, we found that female gender, Hispanic/Latino ethnicity, an associate degree or some college, and stage of HCV management were significantly associated with higher HCV-stigma scores.
Over 95% of patients in this study endorsed at least one HCV-SS item, consistent with qualitative study reports that an overwhelming proportion HCV patients have experienced HCV-related stigma.17,52 Participants more frequently endorsed questions describing a fear of discrimination, blame for their HCV status, being treated as dirty, and concerns about disclosing their HCV status. Qualitative studies have similarly described patient experiences of stigma and discrimination in private and healthcare environments, including being treated with suspicion and blamed for their disease by healthcare providers, and being told by family members to keep their HCV diagnosis secret.17,20,28,52 Our results demonstrate that HCV-related stigma is prevalent.
We observed important differences in the determinants of HCV-related stigma according to HIV status. Our finding of higher HCV-SS scores among HIV/HCV-coinfected Hispanic/Latino and female participants are consistent with analyses by Loutfy et al., which found similar gender and ethnicity differences in HIV-related stigma among a cohort of 1,026 HIV-positive individuals living in Ontario, Canada.53 Alternatively, while Loutfy et al. (2012) found lower stigma scores among patients who attended college 53, we observed higher HCV-SS scores among participants who attended some college or earned an associate degree. Several studies among people living with HIV have similarly found increased stigma among those with higher educational attainment.22,54,55 It is possible that those with higher education may be more attuned to the health-related and social consequences of their HCV status, and therefore experience greater internalized or perceived stigma. These findings support arguments that stigma layering due to HIV-coinfection may augment experiences of stigma associated with HCV infection.27
Interestingly, although pegylated interferon-based therapy has been associated with an increased side-effect profile and decreased cure rates, compared to DAA-based therapies, prior treatment with pegylated interferon was not associated with HCV-related stigma. However, stage of HCV management, specifically previous treatment without cure, was associated with significantly higher HCV-SS scores among the HIV/HCV-coinfected group. This association may support the role of a stigma-trajectory, which informs patients’ experiences with their disease at different stages of care.28,56 Research on patient-level barriers to HCV-treatment previously found that stigma and shame were motivators for the uptake and completion HCV-treatment.57 Successful HCV treatment with sustained virologic response may possibly diminish these negative feelings, while patients who undergo treatment but fail to cure the virus may develop amplified concerns about the potential success of future treatments, reinforcing perceptions of stigma.
One particularly interesting finding that points to syndemicity between HCV, HIV, and IDU, was the association between HCV-SS scores and IDU history. Patients with history of IDU had significantly higher HCV-SS scores overall (mean, 77.00 vs 71.51) and in the HIV/HCV-coinfected group (mean, 78.69 vs 69.86). However, among HCV-monoinfected patients, HCV-SS scores were not significantly associated with history of IDU (mean, 75.05 vs 74.07). Previous studies have reported that patients often feel stigmatized by the association of HCV with IDU, including assumptions by healthcare providers that a patient presenting with HCV must have a history of IDU.17,28,29,52 Monoinfected patients may experience HCV-related stigma associated with IDU, regardless of the method by which they acquired HCV. Likewise, HIV/HCV-coinfected patients without a history of IDU may experience less stigma from IDU due to the association of HIV with sexual means of transmission, rather than IDU. It is of note that sexual orientation and sexual contact, which are widely associated with HIV stigma,22 were not statistically significant in any of the analyses. Finally, the highest mean HCV-SS scores were among HIV/HCV-coinfected participants with a history of IDU, which supports the theory that patients at the center of the intersecting HCV, HIV, and opioid epidemics may be most susceptible to the negative health consequences of stigma.
The differences observed between HCV-monoinfected and HIV/HCV-coinfected groups support the need for intersectionality frameworks that expand on theories of layered stigma among patients with more than one disease. Intersectionality frameworks are essential in evaluating how multiple patient identities intersect in individual micro-level experiences with diseases such as HCV and HIV, and how these experiences are shaped by the macro-level social understandings of marginalized behaviors and identities associated with these diseases.27,58 More research is needed to incorporate an intersectionality framework into understandings of HCV-related stigma and to develop appropriate public health interventions that address social processes, such as power and knowledge production, which foster stigma by reproducing inequalities and social exclusion.59
Our study had several limitations. First, since the HCV-SS has only been validated in English, our study included only English-speaking patients who presented for outpatient HCV care in Philadelphia. The results may not be generalizable to non-English-speaking individuals or those who are not engaged in healthcare. Second, while our analyses identified several factors associated with stigma, quantitative models are unlikely to fully capture the complexity of how these factors intersect on patients’ experiences of disease related stigma. Qualitative research that complements these models may allow additional contextualization of the findings. Third, participants self-reported on determinants of stigma, which may be subject to social desirability bias. However, we attempted to minimize this through the use of anonymous computerized questionnaires. Finally, due to the cross-sectional nature of our study, we could not determine causal relationships or evaluate how perceptions of HCV-related stigma changed over time or across stage of care. Longitudinal research is needed to better understand how stigma changes as a patient progresses through the HCV care cascade.
This study had several strengths. This is the first study to evaluate determinants of HCV-related stigma using a validated HCV stigma scale. Our study represents an initial important step in identifying the factors associated with HCV stigma. Additional studies are needed to identify other determinants of HCV-related stigma and develop and test interventions to reduce this stigma across the steps of the HCV care cascade. Moreover, we were able to capture a more representative patient population and increase generalizability by using ACASI software across several clinics, including within a syringe-service program. Generalizability was also increased by including patients across all stages of HCV-management.
In conclusion, our findings demonstrate that most HCV-infected patients experience some degree of disease-related stigma. However, the determinants of HCV-stigma are multi-faceted and differ by HIV status. This finding may have important implications for the development of targeted interventions to reduce negative health-related consequences of stigma and combat the HCV, HIV, and opioid syndemic. Future studies are needed to identify determinants of stigma among HCV-monoinfected patients and develop interventions to decrease HCV-related stigma.
ACKNOWLEDGEMENTS
The authors would like to thank the providers and staff at the clinical sites for their support of this study. This study was funded by a 2018 LDI Pilot Grant (to MES), PCOR Pilot Study Award (to VLR), National Institute of Allergy and Infectious Diseases (R01-AI-136626 to VLR), and National Institutes on Aging grant funding (T32 AG051090 supporting MES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis – United States, 2016. 2016. [Google Scholar]
- 2.Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising Mortality Associated With Hepatitis C Virus in the United States, 2003–2013. Clinical Infectious Diseases. 2016;62(10):1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The Increasing Burden of Mortality From Viral Hepatitis in the United States Between 1999 and 2007. Annals of Internal Medicine. 2012;156(4):271–278. [DOI] [PubMed] [Google Scholar]
- 4.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013-2016. Hepatology (Baltimore, Md). 2019;69(3):1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Academies of Sciences E, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: The National Academies Press;2017. [PubMed] [Google Scholar]
- 6.Grebely J, Dore GJ, Morin S, Rockstroh JK, Klein MB. Elimination of HCV as a public health concern among people who inject drugs by 2030 - What will it take to get there? J Int AIDS Soc. 2017;20(1):22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030: Advocacy Brief. MAy 2016. [Google Scholar]
- 8.Increase in hepatitis C infections linked to worsening opioid crisis [press release]. Atlanta, GA: 2017. [Google Scholar]
- 9.Perlman DC, Jordan AE. The Syndemic of Opioid Misuse, Overdose, HCV, and HIV: Structural-Level Causes and Interventions. Current HIV/AIDS reports. 2018;15(2):96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). HIV/AIDS and Viral Hepatitis. 2018; https://www.cdc.gov/hepatitis/populations/hiv.htm. Accessed 21 June 2018, 2018.
- 11.Lo Re V 3rd, Kostman JR, Amorosa VK. Management complexities of HIV/hepatitis C virus coinfection in the twenty-first century. Clinics in liver disease. 2008;12(3):587–609, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo Re VI, Kallan MJ, Tate JP, et al. Hepatic Decompensation in Antiretroviral-Treated Patients Co-Infected With HIV and Hepatitis C Virus Compared With Hepatitis C Virus–Monoinfected Patients: A Cohort Study. Annals of Internal Medicine. 2014;160(6):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer M. Introduction to syndemics : a critical systems approach to public and community health. 1st ed. San Francisco, CA: Jossey-Bass; 2009. [Google Scholar]
- 14.Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet (London, England). 2017;389(10072):941–950. [DOI] [PubMed] [Google Scholar]
- 15.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9(7):e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goffman E Stigma: Notes on the Management of Spoiled Identitiy. New York: Simon & Schuster, Inc.; 1963. [Google Scholar]
- 17.Zickmund S, Ho EY, Masuda M, Ippolito L, LaBrecque DR. “They Treated Me Like a Leper”: Stigmatization and the Quality of Life of Patients with Hepatitis C. Journal of General Internal Medicine. 2003;18(10):835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowsett LE, Coward S, Lorenzetti DL, MacKean G, Clement F. Living with Hepatitis C Virus: A Systematic Review and Narrative Synthesis of Qualitative Literature. Can J Gastroenterol Hepatol. 2017;2017:3268650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong AR, Herrmann SE, Chassany O, et al. The International development of PROQOL-HCV: An instrument to assess the health-related quality of life of patients treated for Hepatitis C virus. BMC Infectious Diseases. 2016;16(1):443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinho RT, Barreira DP. Hepatitis C, stigma and cure. World J Gastroenterol. 2013;19(40):6703–6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacQueen KM, Chen M, Jolly D, et al. HIV testing experience and risk behavior among sexually active Black young adults: a CBPR-based study using respondent-driven sampling in Durham, North Carolina. American journal of community psychology. 2015;55(0):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golub SA, Gamarel KE. The impact of anticipated HIV stigma on delays in HIV testing behaviors: findings from a community-based sample of men who have sex with men and transgender women in New York City. AIDS patient care and STDs. 2013;27(11):621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-Related Stigma on Health Behaviors and Psychological Adjustment Among HIV-Positive Men and Women. AIDS Behav. 2006;10(5):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore TM, Scott JC, Reise SP, et al. Development of an abbreviated form of the Penn Line Orientation Test using large samples and computerized adaptive test simulation. Psychological assessment. 2015;27(3):955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sengupta S, Banks B, Jonas D, Miles MS, Smith GC. HIV interventions to reduce HIV/AIDS stigma: a systematic review. AIDS Behav. 2011;15(6):1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reidpath DD, Chan KY. A method for the quantitative analysis of the layering of HIV-related stigma. AIDS care. 2005;17(4):425–432. [DOI] [PubMed] [Google Scholar]
- 27.Lekas HM, Siegel K, Leider J. Felt and enacted stigma among HIV/HCV-coinfected adults: the impact of stigma layering. Qual Health Res. 2011;21(9):1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butt G, Paterson BL, McGuinness LK. Living with the stigma of hepatitis C. Western journal of nursing research. 2008;30(2):204–221; discussion 222-233. [DOI] [PubMed] [Google Scholar]
- 29.Day C, Ross J, Dolan K. Hepatitis C-related discrimination among heroin users in Sydney: drug user or hepatitis C discrimination? Drug and alcohol review. 2003;22(3):317–321. [DOI] [PubMed] [Google Scholar]
- 30.Saine ME, Moore TM, Szymczak JE, et al. Validation of a modified Berger HIV stigma scale for use among patients with hepatitis C virus (HCV) infection. PLOS ONE. 2020;15(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcus J, Hurley L, Chamberland S, et al. Racial/Ethnic and Socioeconomic Disparities in Initiation of Direct-Acting Antiviral Agents for Hepatitis C Virus in an Insured Population. Open Forum Infectious Diseases. 2017;4(Suppl 1):S198–S198. [Google Scholar]
- 32.El-Serag H, McGlynn KA, Graham GN, et al. Achieving health equity to eliminate racial, ethnic, and socioeconomic disparities in HBV- and HCV-associated liver disease. J Fam Pract. 2010;59(4 Suppl):S37–S42. [PMC free article] [PubMed] [Google Scholar]
- 33.Sims OT, Pollio DE, Hong BA, North CS. Racial Disparities in Hepatitis C Treatment Eligibility. Annals of hepatology. 2017;16(4):530–537. [DOI] [PubMed] [Google Scholar]
- 34.Lo Re V, Gowda C, Urick PN, et al. Disparities in Absolute Denial of Modern Hepatitis C Therapy by Type of Insurance. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14(7):1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omland LH, Osler M, Jepsen P, et al. Socioeconomic status in HCV infected patients - risk and prognosis. Clinical epidemiology. 2013;5:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones L, Atkinson A, Bates G, et al. Views and experiences of hepatitis C testing and diagnosis among people who inject drugs: systematic review of qualitative research. Int J Drug Policy. 2014;25(2):204–211. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe D, Luhmann N, Harris M, et al. Human rights and access to hepatitis C treatment for people who inject drugs. Int J Drug Policy. 2015;26(11):1072–1080. [DOI] [PubMed] [Google Scholar]
- 38.Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis. 2013;207 Suppl 1(Suppl 1):S19–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philadelphia Fight. Philadelphia FIGHT Community Health Centers Annual Report 2018. Philadelphia: Philadelphia FIGHT;2018. [Google Scholar]
- 40.2017 Division of Disease Control (DDC) Annual Report. Philadelphia: Philadelphia Department of Public Health, Division of Disease Control;2017. [Google Scholar]
- 41.University of Pennsylvania Health System (UPHS). 2016. COMMUNITY HEALTH NEEDS ASSESSMENT. In. Philadelpha, Pa: 2016. [Google Scholar]
- 42.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24(6):518–529. [DOI] [PubMed] [Google Scholar]
- 43.Shavers VL. Measurement of socioeconomic status in health disparities research. Journal of the National Medical Association. 2007;99(9):1013–1023. [PMC free article] [PubMed] [Google Scholar]
- 44.Okumu E, Jolly DH, Alston LM, Eley NT, Laws M, MacQueen KM. Relationship between Human Immunodeficiency Virus (HIV) Knowledge, HIV-Related Stigma, and HIV Testing among Young Black Adults in a Southeastern City. Frontiers in Public Health. 2017;5(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason S, Berger B, Ferrans CE, Sultzman V, Fendrich M. Developing a Measure of Stigma by Association With African American Adolescents Whose Mothers Have HIV. Research on Social Work Practice. 2009;20(1):65–73. [Google Scholar]
- 46.Clum G, Chung SE, Ellen JM. Mediators of HIV-related stigma and risk behavior in HIV infected young women. AIDS care. 2009;21(11):1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Estes LJ, Lloyd LE, Teti M, et al. Perceptions of audio computer-assisted self-interviewing (ACASI) among women in an HIV-positive prevention program. PLoS One. 2010;5(2):e9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, Dubowitz H, Hudson-Martin E, Lane W. Comparison of 3 data collection methods for gathering sensitive and less sensitive information. Ambulatory pediatrics : the official journal of the Ambulatory Pediatric Association. 2008;8(4):255–260. [DOI] [PubMed] [Google Scholar]
- 49.Wu Y, Newfield SA. Comparing data collected by computerized and written surveys for adolescence health research. The Journal of school health. 2007;77(1):23–28. [DOI] [PubMed] [Google Scholar]
- 50.Ghanem KG, Hutton HE, Zenilman JM, Zimba R, Erbelding EJ. Audio computer assisted self interview and face to face interview modes in assessing response bias among STD clinic patients. Sexually transmitted infections. 2005;81(5):421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevens JP. Applied Multivariate Statistics for the Social Sciences, 4th edn. 2002, Lawrence Erlbaum Associates, Mahwah, NJ, USA. [Google Scholar]
- 52.Paterson BL, Backmund M, Hirsch G, Yim C. The depiction of stigmatization in research about hepatitis C. International Journal of Drug Policy. 2007;18(5):364–373. [DOI] [PubMed] [Google Scholar]
- 53.Loutfy MR, Logie CH, Zhang Y, et al. Gender and Ethnicity Differences in HIV-related Stigma Experienced by People Living with HIV in Ontario, Canada. PLoS ONE. 2012;7(12):e48168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z, Morano JP, Khoshnood K, Hsieh E, Sheng Y. HIV-related stigma among people living with HIV/AIDS in rural Central China. BMC health services research. 2018;18(1):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nobre N, Pereira M, Roine RP, Sutinen J, Sintonen H. HIV-Related Self-Stigma and Health-Related Quality of Life of People Living With HIV in Finland. The Journal of the Association of Nurses in AIDS Care : JANAC. 2018;29(2):254–265. [DOI] [PubMed] [Google Scholar]
- 56.Alonzo AA, Reynolds NR. Stigma, HIV and AIDS: an exploration and elaboration of a stigma trajectory. Social science & medicine (1982). 1995;41(3):303–315. [DOI] [PubMed] [Google Scholar]
- 57.Sublette VA, Smith SK, George J, McCaffery K, Douglas MW. The Hepatitis C treatment experience: Patients’ perceptions of the facilitators of and barriers to uptake, adherence and completion. Psychology & health. 2015;30(8):987–1004. [DOI] [PubMed] [Google Scholar]
- 58.Bowleg L The Problem With the Phrase Women and Minorities: Intersectionality—an Important Theoretical Framework for Public Health. American Journal of Public Health. 2012;102(7):1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Social Science & Medicine. 2003;57(1):13–24. [DOI] [PubMed] [Google Scholar]


